Abstract
Objective
Paraneoplastic neurologic syndrome (pns) is a rare condition indirectly caused by an underlying malignancy. In many cases, the malignancy is occult at the time of the pns diagnosis, and the optimal diagnostic modality to detect the underlying tumour is unclear. In the present study, we aimed to assess the utility of 18F-fluorodeoxyglucose positron-emission tomography (fdg-pet) or pet integrated with computed tomography (pet/ct) in the investigation of these patients.
Methods
We retrospectively analyzed data from the PET Access Program (pap) database in the province of Ontario to identify patients who underwent fdg-pet/ct imaging as part of a workup for pns. In all patients, prior conventional imaging was negative or indeterminate. To determine the diagnostic accuracy of fdg-pet/ct, data about demographics, presenting symptoms, and biochemical and radiologic workup, including fdg-pet/ct imaging results, were compared with data collected by the Ontario Cancer Registry (ocr). A systematic review of the literature and meta-analysis using our study inclusion criteria were performed for studies of fdg-pet accuracy.
Results
Of 29 patients identified in the pap database, 9 had fdg-pet/ct results suspicious for malignancy. When correlated with data from the ocr, 5 fdg-pet/ct results were informative, resulting in a detection rate of 17%. Local sensitivity and specificity were 0.83 and 0.83 respectively. Two studies meeting our criteria were identified in the literature. The pooled sensitivity and specificity from the literature and local data were 0.88 and 0.90 respectively.
Conclusions
When investigating for underlying malignancy in patients with suspected pns and negative conventional imaging, pet has high sensitivity and specificity.
Keywords: Paraneoplastic neurologic syndrome, positron-emission tomography, malignancy, sensitivity, specificity
INTRODUCTION
Paraneoplastic neurologic syndrome (pns) is the term for a group of rare nervous system disorders that are associated with the presence of a malignancy, but that are not a direct effect of the primary tumour or its metastases1. Most of these disorders are likely immune-mediated, either by antibody-related or T cell–related mechanisms2. Antibodies called “paraneoplastic antibodies” can be generated in response to a tumour antigen and can then also cross-react with a target in the nervous system. Paraneoplastic neurologic syndrome can affect various components of the central and peripheral nervous systems, including the neuromuscular junction. Certain antibodies are associated with particular types of pns and also with specific underlying malignancies3. In many cases, the presentation of pns precedes clinical manifestation of the malignancy itself. Treatment of pns varies depending on the syndrome and can include immunosuppression, treatment of the underlying tumour, or both4. Early diagnosis of the underlying malignancy is therefore vital to achieving optimal treatment and better outcomes for patients.
Given the rarity of the condition, a diagnosis of pns requires a high degree of clinical suspicion. Initial investigations include testing for paraneoplastic antibodies in serum and cerebrospinal fluid and, depending on the clinical presentation, brain magnetic resonance imaging (mri) and electrophysiology. If well-characterized paraneoplastic antibodies are detected or if pns is suspected based on clinical presentation, image-based screening for an underlying tumour can be performed5. Conventional imaging such as computed tomography (ct), ultrasonography, and mammography are commonly used modalities; however, those techniques have limitations in detecting occult malignancies because they are based on structural changes and usually focus on limited parts of the body. 18F-Fluorodeoxyglucose positron-emission tomography (fdg-pet) provides nuclear tomographic information related to the metabolic activity of cells based on uptake and use of glucose, and integration with ct (pet/ct) provides greater anatomic localization6. Tumours associated with pns that are otherwise missed by conventional imaging might be detectable with pet/ct.
In Ontario, pet is not currently routinely funded for the evaluation of patients suspected to have pns. Access is obtained through a PET Access Program (pap) in which cases are adjudicated by a panel of experts. The current standard for patients suspected to have pns is initially to evaluate with conventional imaging (ct, mri) and to pursue fdg-pet only when results are negative or inconclusive. The purpose of the present study was to review the Ontario experience in combination with a systematic review of the literature to determine the diagnostic utility of fdg-pet imaging in the workup of patients with suspected pns and negative conventional imaging.
METHODS
Study Design
This is a retrospective population-based study with a systematic review of the literature and meta-analysis. All cases reviewed through the pap are recorded in a database. To identify patients referred for fdg-pet imaging as a part of a workup for suspected pns, the pap database was searched from its inception in 2011 to 2015 using the keywords “paraneoplastic” and “pns” as a part of the indication data. To identify additional patients who might not have been captured in the database search, a search of the e-mail correspondence between the pap expert review panel members was performed using the keywords “paraneoplastic” and “pns.” All data were anonymized, with patient identifiers, service providers, and institution names censored. Each case was then individually reviewed to determine eligibility and to extract relevant data. To be eligible, all patients had to have been more than 18 years of age, to have been referred for suspected pns, and to have undergone ct imaging whose results for a primary tumour were negative or indeterminate.
Imaging was considered indeterminate if an abnormality was present, but was not highly suspicious for malignancy. Positive paraneoplastic antibodies were not a requirement. Imaging by fdg-pet was performed in 1 of 13 locations across the province of Ontario. Scanner protocols are likely to vary slightly between institutions, and detailed information about each protocol was not collected. The procedure standards for fdg-pet/ct disseminated by the Society of Nuclear Medicine and Molecular Imaging are adhered to in Ontario7. Integrated fdg-pet/ct was performed in all cases.
Each fdg-pet/ct imaging examination was reviewed and classified as either highly suspicious for malignancy, indeterminate, or negative. Indeterminate imaging was defined as areas of abnormal hypermetabolic activity that were not consistent with malignancy. The Ontario Cancer Registry (ocr), a provincial database that compiles all information about newly diagnosed cancer in the province of Ontario was then searched to determine if a patient had a record of malignancy. If a suspicious or indeterminate fdg-pet/ct imaging examination could be clinically and temporally correlated with a diagnosis of cancer in the ocr, then that examination was deemed to be informative.
Systematic Review
Literature Search Strategy
A search for existing systematic reviews was conducted. If no eligible systematic reviews were identified, a primary search of the literature was conducted. The Ovid search of the primary literature used the medline (1946 to September 2016) and embase (1974 to 2016 week 39) databases. In addition, reference lists from relevant systematic reviews and primary literature were scanned for potentially useful studies.
Study Selection Criteria and Process
Publications were included if they met these criteria:
■ Published as a full article in a peer-reviewed journal
■ Evaluated the use of pet or pet/ct with fdg
■ Post-biopsy or postmortem histology, or clinical or radiologic follow-up, used as the reference standard for final diagnosis
■ Previous investigation with conventional imaging (ct, ultrasonography, mri, mammography, as appropriate)
failed to identify an underlying malignancy Publications were excluded if they met these criteria:
■ Were case reports, conference abstracts, literature or narrative reviews, letters, editorials, historical articles, or commentaries
■ Provided insufficient information to calculate the number of true-positive, false-positive, false-negative, and true-negative results
■ Were reported in a language other than English
The foregoing criteria were selected to best reflect the current practices of fdg-pet/ct selection for patients with suspected pns in Ontario, which requires negative ct imaging and does not select for antibody status. A review of the titles and abstracts that resulted from the search was conducted independently by 1 reviewer. Items that warranted full-text review were evaluated by 2 reviewers independently, after which a consensus was reached.
Data Extraction and Assessment of Study Quality and Potential for Bias
Data were extracted from the included studies by 1 reviewer. For each article, the principal author, country of origin, publication year, study design, number of patients, patient age and sex, the types of pet and conventional imaging performed, and the numeric data for diagnostic performance were recorded. All extracted data and information were audited by an independent auditor. The Quality Assessment of Diagnostic Accuracy Studies tool8 was used to evaluate the risk of bias and applicability concerns for each eligible study.
Statistical Analysis
Data were summarized in evidence tables and are described in the text. When clinically homogenous results from two or more studies and sufficient data were available to reassess the sensitivity and specificity of fdg-pet or fdg-pet/ct, a random-effects model was used to produce summary estimates with 95% confidence intervals (cis). The I2 percentage was calculated as a measure of heterogeneity. Statistical analyses were performed using the Meta-DiSc software application (version 1.4: Unit of Clinical Biostatistics, Ramón y Cajal Hospital, Madrid, Spain) which uses a generalization of the Littenberg and Moses linear model to implement meta-regression9,10.
RESULTS
Patient Demographics and Initial Investigations
From the pap database, we identified 29 patients (12 men, 17 women) with suspected pns who met the inclusion criteria for the study (Table I). One patient had 2 pap entries because of undergoing 2 separate fdg-pet/ct exams. Median age in this group was 55.5 years (range: 19–80 years). The most common presentations of pns were encephalitis and cerebellar degeneration (Table I). Positivity for paraneoplastic antibodies was found in 12 patients (41%, Table I). The most common antibodies were anti-Hu (n = 3) and anti-nmda [N-methyl-d-aspartate (n = 3)]. Antibody status was negative in 8 patients, and 9 patients had no reported antibody status.
TABLE I.
Characteristics of the 29 study patients
| Characteristic | Value |
|---|---|
| Age (years) | |
| Median | 55.5 |
| Range | 19–80 |
|
| |
| Sex [n (%)] | |
| Women | 17 (59) |
| Men | 12 (41) |
|
| |
| Reason to suspect PNS [n (%)] | |
| Encephalitis, othera | 8 (28) |
| Cerebellar degeneration | 7 (24) |
| Limbic encephalitis | 4 (14) |
| Encephalitis with peripheral neuropathy | 3 (10) |
| Axonal polyneuropathy | 2 (7) |
| Sensory neuropathy | 2 (7) |
| Autonomic Neuropathy | 2 (7) |
| POEMS | 1 (3) |
|
| |
| Paraneoplastic antibodies [n (%)] | |
| Anti-Hu | 3 (10) |
| Anti-NMDA | 3 (10) |
| Anti-Ma2, Anti-Yo | 1 (3) |
| Anti-Yo | 1 (3) |
| Anti-GAD | 1 (3) |
| Anti-amphiphysin | 1 (3) |
| Anti-recoverin | 1 (3) |
| Anti-Ma2 | 1 (3) |
| Negative | 8 (28) |
| Not reported | 9 (31) |
Anti-NMDA (n= 3), progressive encephalomyelitis with rigidity and myoclonus (n = 1), anti-Ma2 (n = 1), nonspecific (n = 3).
PNS = paraneoplastic neurologic syndrome; POEMS = polyneuropathy, organomegaly, endocrinopathy, monoclonal plasma-proliferative disorder, skin changes; NMDA= N-methyl-D-aspartate; GAD= glutamic acid decarboxylase.
All patients had undergone ct imaging of chest and abdomen with or without pelvis before their fdg-pet/ct imaging. Imaging by ct was negative in 18 patients (62%) and indeterminate in 11 (38%). The most common finding in indeterminate ct imaging was suspicious hilar or mediastinal lymph nodes, seen in 6 patients. Of the 29 patients, 24 (83%) also underwent mri of brain or spine (or both), with findings in 11 of those patients being reported as abnormal. Abnormalities included cortical or cerebellar atrophy, changes observed during T2-weighted fluid-attenuated inversion recovery (most commonly in the temporal lobes), spinal nerve root enhancement, and in 1 case, suspected myelomatous involvement of the spine.
FDG-PET/CT Imaging Results
Of 30 fdg-pet/ct examinations, 19 (63%) were abnormal. After expert review, 9 of those examinations were determined to be highly suspicious, and 10 were indeterminate.
Highly Suspicious FDG-PET/CT Imaging
Of the 9 patients with highly suspicious fdg-pet/ct imaging, 7 had a record of malignancy in the ocr (Table II). In those cases, the fdg-pet/ct examination was determined to have been informative in the workup of the paraneoplastic condition in 5 cases. In 3 of those 5 cases, patients had no prior history of malignancy recorded in the ocr. Subsequently, 1 patient was diagnosed with cancer of unknown primary, 1 with lung cancer, and 1 with myelodysplastic syndrome. In the 5 patients with informative fdg-pet/ct imaging, paraneoplastic antibody testing was positive for anti-Hu antibodies in 2 patients, negative for antibodies in 1 patient, and not reported in 2 patients.
TABLE II.
Characteristics of 9 patients with highly suspicious integrated 18F-fluorodeoxyglucose positron-emission tomography (PET) and computed tomography imaging
| Pt | Age (years) | Sex | Paraneoplastic neurologic syndrome | Antibody | Area of abnormality on imaging | Malignancy in OCR | Informative (yes or no) |
|---|---|---|---|---|---|---|---|
| 1 | 60 | Female | Cerebellar ataxia | Anti-Hu | Mediastinal node, left hilar node | Lung malignancy, polycythemia vera | Yes |
| 2 | 76 | Female | Encephalitis, sensory neuropathy | Anti-Hu | Mediastinal node | Not present | No (no identified malignancy) |
| 3 | 62 | Male | Sensory neuropathy | Not tested | Supraclavicular node, gastrohepatic node | Brain NOS, lung malignancy | Yes |
| 4 | 76 | Female | Cerebellar ataxia | Not tested | Nasopharynx, thyroid lobe, LUL nodule, LLL nodule | Not present | No (no identified malignancy) |
| 5 | 44 | Male | Limbic encephalitis | Negative | Global increased uptake in axial skeleton | Myelodysplastic syndrome | Yes |
| 6 | 53 | Female | Limbic encephalitis, sensory neuropathy | Anti-Hu | Bilateral hilar nodes, mediastinal nodes, low level uptake in atelectasis, LLL subpleural node | Cancer of unknown primary | Yes |
| 7 | 80 | Male | Limbic encephalitis | Not reported | Paratracheal node | Cancer of unknown primary | No (biopsy preceded PET imaging) |
| 8 | 62 | Female | Peripheral neuropathy, encephalitis | Not tested | LUL consolidation, peripancreatic node, external iliac node, pubic bone, sacrum | Lung cancer | Yes |
| 9 | 52 | Female | Ataxia, tremor | Negative | Diffuse activity in liver, spleen; focal activity in hepatic hilum, para-aortic node; increased marrow activity | Previous diagnosis of kidney, thyroid cancer | No (biopsy preceded PET imaging) |
Pt = patient; OCR = Ontario Cancer Registry; NOS = not otherwise specified; LUL = left upper lobe, LLL = left lower lobe
Of the patients with informative fdg-pet/ct imaging, 2 had a record of malignancy before the imaging examination. One patient had a cerebellar mass that had been resected and had demonstrated small-cell carcinoma. Imaging by ct of chest, abdomen, and pelvis was negative, but imaging by fdg-pet/ct revealed disseminated disease. The other patient had a previous diagnosis of limited-stage small-cell lung cancer. Imaging by ct demonstrated radiation changes and consolidation in the lung, but no definite evidence of disease recurrence. Imaging by fdg-pet/ct was suggestive of recurrence in the lung and demonstrated lymph node and bone metastases.
Of the highly suspicious fdg-pet/ct examinations, 4 were ultimately deemed to be non-informative. In 2 cases, no malignancy was diagnosed. One examination demonstrated a hypermetabolic paratracheal lymph node with a standardized uptake value of 3.4; the other demonstrated several hypermetabolic pulmonary nodules, a right thyroid nodule, and hypermetabolism in the nasopharynx with no ct correlate. In another case, a biopsy had confirmed squamous cell carcinoma in a mediastinal lymph node before the fdg pet/ct examination. In the final case, the fdg-pet/ct imaging demonstrated findings consistent with a hematologic malignancy, but a plasma-cell neoplasm had already been diagnosed on a bone marrow biopsy.
Indeterminate FDG-PET/CT Imaging
Of the 10 indeterminate fdg-pet/ct examinations, none was deemed to be informative. A diagnosis of cancer pre-dated the fdg-pet/ct imaging in 3 patients; however, the fdg-pet/ct imaging did not demonstrate findings consistent with disease recurrence. As mentioned, 1 patient underwent 2 separate fdg-pet/ct examinations, the first being indeterminate, and the second being highly suspicious, leading a diagnosis of cancer of unknown primary. That patient initially presented with positive anti-Hu antibodies and a sensory neuropathy, but no malignancy was detected in the initial fdg-pet/ct imaging. The patient’s symptoms progressed, and the development of a limbic encephalopathy led to the repeat, highly suspicious, fdg-pet/ct imaging approximately 2 years later. In 3 patients with indeterminate imaging, antibody positivity had been found. A patient with anti-nmda antibodies and a patient with both anti-Yo and anti-Ma2 antibodies had no subsequent diagnosis of a malignancy recorded in the ocr.
Negative FDG-PET/CT Imaging
Imaging by fdg-pet/ct was negative in 11 patients. Of those 11 patients, 6 tested positive for paraneoplastic antibodies, with 2 being positive for anti-nmda antibodies. Antibodies were well characterized in 3 patients (anti-Ma2, anti-amphiphysin, anti-Yo, anti-recoverin); the remaining patients had anti-gad (glutamic acid decarboxylase) antibodies. Of the patients with negative fdg-pet/ct imaging, only 2 had a diagnosis of cancer in the ocr. In both patients, the diagnosis of cancer pre-dated the fdg-pet/ct imaging. No patient with a negative fdg-pet/ct examination was later diagnosed with a malignancy.
Sensitivity and Specificity Testing
Overall, of the 30 fdg-pet/ct imaging examinations in 29 patients, 5 (17%) were determined to be informative. Sensitivity and specificity were determined under 2 separate conditions:
■ Abnormal but indeterminate fdg-pet/ct imaging considered to be negative
■ Abnormal but indeterminate fdg-pet/ct imaging considered to be positive
When indeterminate fdg-pet/ct imaging was considered negative, the sensitivity of fdg-pet/ct examinations in patients with suspected pns and indeterminate conventional imaging was 0.83, and the specificity was 0.83. When indeterminate fdg-pet/ct imaging was considered positive, then the sensitivity increased to 1.00 and the specificity declined to 0.44.
Literature Search Results
Search for Existing Systematic Reviews
A search for systematic reviews did not yield an appropriate source document on which to build an evidence base. Thus, the amstar tool was not used.
Search for Primary Literature
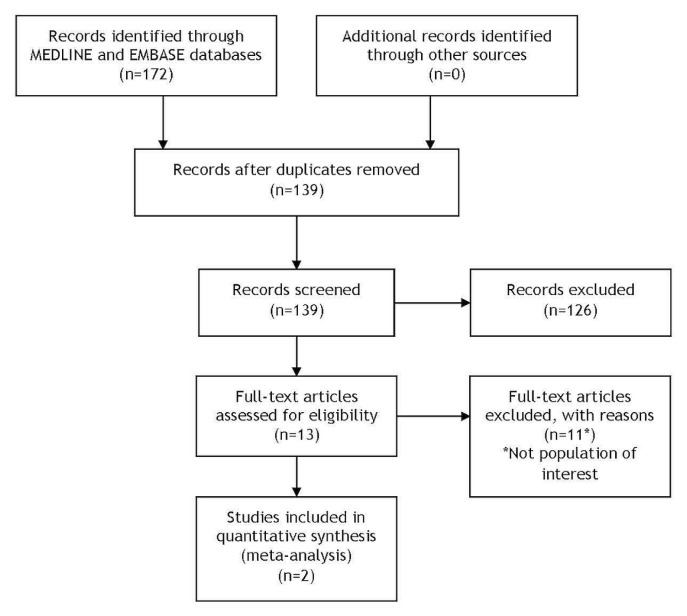
The electronic search for primary literature identified 139 unique citations, of which 126 were excluded after a review of titles and abstracts (Figure 1). Thirteen citations were considered candidates, but upon full-text review, eleven did not meet the inclusion criteria. The remaining two studies were included in the systematic review.
FIGURE 1.
Modified PRISMA flow diagram outlining the search results of the systematic literature review.
Study Design and Quality
Both studies were observational in nature: one study used a prospective design11; the other retrospectively reviewed the case records of patients12. Hadjivassiliou et al.11 performed pet imaging using a modified gamma camera equipped with a low-dose ct system for anatomic localization and attenuation correction. Rees et al.12 used a standalone fdg-pet scanner. The duration of follow-up was noted in Rees et al.12 and ranged from 2 months to 44 months (mean: 18.1 months; median: 16 months). Hadjivassiliou et al.11 did not specify the timing or length of follow-up. The risk of bias for each study was assessed according to the 4 Quality Assessment of Diagnostic Accuracy Studies domains. No concerns about applicability arose; however, both studies were assessed as having a high risk of bias for flow and timing. In particular, a definite diagnosis of malignancy was not made in all cases because some patients died without histology confirmation, which could have been a study limitation, given that obtaining a histology diagnosis in all patients is impractical. Furthermore, both studies lacked information about whether interpretation of the pathology results and extended follow-up were blinded to the fdg-pet findings. According to the grade (Grading of Recommendations Assessment, Development and Evaluation) criteria, the results were consistent in both studies, but suffered from imprecision because of low patient numbers. Taken as a whole, the quality of the evidence was judged to be moderate.
Paraneoplastic Antibodies
Serology testing for paraneoplastic antibodies was performed in 91.9% of the patients in the two studies (113 of 123)11,12. Tests showed that 1 patient had anti-Yo antibodies, 2 patients had voltage-gated calcium antibodies, 10 patients had anti-Hu antibodies, 38 patients were antibody-negative, and 62 patients had an unspecified antibody status. Of the 34 patients with a positive pet or pet/ct examination, 10 were positive for paraneoplastic antibodies, and 22 were negative (2 patients were not tested).
Diagnostic Accuracy
Our meta-analysis examining the diagnosis of malignancy in patients clinically suspected of having pns excluded 9 patients because their findings on fdg-pet could not be confirmed, leaving 114 patients from the combined cohort for the analysis11,12. The prevalence of malignancy was 12.0% (9 of 75) in the Hadjivassiliou et al.11 study and 25.6% (10 of 39) in the Rees et al.12 study. Of the 19 patients overall with a proven malignancy, fdg-pet was positive in 17 (7 with small-cell lung cancer, 4 with colon cancer, 2 with non-small-cell lung cancer, 1 with non-Hodgkin lymphoma, 1 with thymoma, 1 with endometrial cancer, 1 with an unknown primary) and negative in 2 patients (1 with small-cell lung cancer, 1 with breast cancer).
Combined Analysis with Literature
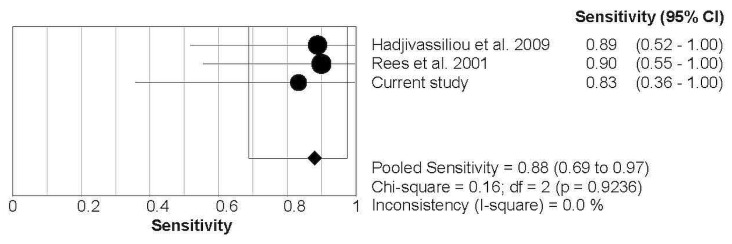
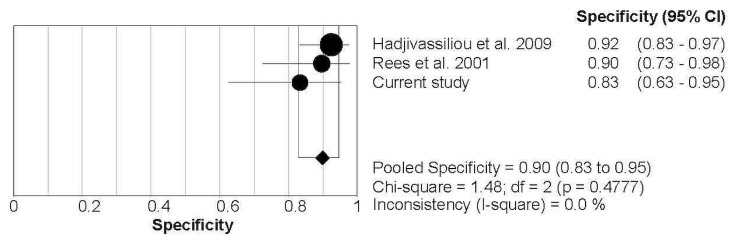
In a per-patient analysis, with inclusion of the data from the local analysis, the pooled sensitivity and specificity were 0.88 (95% ci: 0.69 to 0.97) and 0.90 (95% ci: 0.83 to 0.95) respectively. Forest plots (Figures 2 and 3) showed no significant heterogeneity between the studies (I2 = 0%).
FIGURE 2.
Forest plot demonstrating the pooled sensitivity from the studies identified in the systematic review and the local data. CI = confidence interval.
FIGURE 3.
Forest plot demonstrating the pooled specificity from the studies identified in the systematic review and the local data. CI = confidence interval.
DISCUSSION
Identification of an underlying malignancy is a crucial component in the management of pns. The optimal approach to diagnosis is unclear. Our study attempted to determine whether the use of fdg-pet imaging in the workup of pns is a justifiable use of a limited resource. Our analysis of local data identified 29 patients with a suspicion of pns and negative conventional imaging. The detection rate in our study was 17.2%. That result is consistent with the 12.0% reported in the Hadjivassiliou et al.11 study and the 25.3% reported in the Rees et al.12 study. Considering the importance of detecting an underlying malignancy, our result can be interpreted as clinically significant. Two separate analyses for sensitivity and specificity were conducted, the first considering only fdg-pet/ct imaging with a high degree of suspicion for malignancy to be positive, and the second considering abnormal but indeterminate fdg-pet/ct imaging also to be positive. In the first analysis, the sensitivity and specificity were moderately high at 0.83 and 0.83 respectively. In the second analysis, the sensitivity remained high at 1.00, but the specificity dropped to 0.44. Given that the indeterminate fdg-pet/ct imaging had a low suspicion of malignancy, the first analysis is likely more accurate and was therefore used in the subsequent calculation of pooled sensitivity and specificity.
The literature review located two studies meeting our inclusion criteria. There were several underlying differences in the methods used in the two studies and in our local analysis. Although both published studies were observational, Hadjivassiliou et al.11 was prospective and Rees et al.12 was retrospective. Hadjivassiliou et al. performed fdg-pet imaging using a modified gamma camera equipped with a low-dose ct system for anatomic localization and attenuation correction. Rees et al. used a standalone fdg-pet scanner. We used a combined fdg-pet/ct scanner. Differences in fdg-pet imaging methods is a limitation of our analysis. But despite those differences, the results were similar across the studies. When data from the two published studies were combined with the results from our analysis, the final pooled sensitivity and specificity were 0.88 and 0.90 respectively. No significant heterogeneity between the studies was noted. Those results suggest that fdg-pet represents a reliable and useful diagnostic technique in the workup of paraneoplastic syndromes.
Since the completion of our analysis, two meta-analyses examining the role of fdg-pet in the diagnosis of malignancy in patients with suspected pns have been published. The first, authored by García Vicente et al.13, focused only on patients with pns. The other, authored by Sheikhbahaei et al.14, included studies of both pns and non-neurologic paraneoplastic syndromes, although a subset analysis of patients with pns was performed. García Vicente et al. included sixteen studies in their analysis and reported a pooled sensitivity of 0.91 (95% ci: 0.82 to 0.97), a pooled specificity of 0.87 (95% ci: 0.80 to 0.92), and a detection rate of 14.9% (95% ci: 11.5% to 18.7%)13. Subset analyses of fdg-pet performed as a part of the initial investigation and, as in our study, after negative conventional imaging were also performed. The sensitivity, specificity, and detection rates were similar in both analyses13. In the subset analysis of pns in Sheikhbahaei et al., the pooled sensitivity and specificity of fdg-pet were 0.89 (95% ci: 0.81 to 0.94) and 0.83 (95% ci: 0.79 to 0.87; I2 = 76.9%) respectively14.
Our study differs from the recently published meta-analyses in several ways. First, our study focused only on pns, whereas Sheikhbahaei et al. included studies focusing on other paraneoplastic syndromes. Second, our study focused on a specific scenario of patients with pre-existing negative imaging and non-specified antibody status, in keeping with current practice in Ontario. Finally, our study provides new data concerning patients with suspected pns. The two studies included in our analysis were also included in the recently published meta-analyses; however, despite selecting studies for a specific clinical scenario and adding new data, the results emerging from all the analyses remain congruent. That observation adds validity to the current practice in Ontario.
A detailed analysis of the role of paraneoplastic antibodies was limited. Although 13 patients (43%) were antibody-positive, 9 patients (31%) had no reported antibody status. In our pooled analysis of Hadjivassiliou et al. and Rees et al., 68 patients had an unspecified antibody status. In addition, neither study performed a comprehensive panel for known paraneoplastic antibodies. Hadjivassiliou et al.11 tested for anti-Hu, anti-Yo, and anti-Ri; Rees et al.12 tested only for anti-Hu and anti-Yo. The limited testing curtailed our ability to perform a subgroup analysis based on antibody status. Other studies examining the utility of pet imaging in the workup of pns used positive paraneoplastic antibodies as an inclusion criterion15,16. The latter trials demonstrated higher detection rates of 70% and 90%—findings that are supported by García Vicente et al.13, who found that the subgroup with positive paraneoplastic antibodies showed the highest prevalence and pet detection rate, although the sensitivity and specificity were similar in the antibody-positive and antibody-negative subgroups. In our local analysis, 1 patient with an informative fdg-pet/ct imaging examination and confirmed malignancy had negative antibody testing, and another 2 patients were untested. In the pooled analysis of Hadjivassiliou et al.11 and Rees et al.12, only 10 of 34 patients with a positive fdg-pet imaging examination were positive for paraneoplastic antibodies. In the diagnostic criteria proposed by Graus et al.3, positive paraneoplastic antibodies are not required for a possible or definite pns. Despite a potential increase in the detection rate, we therefore do not recommend limiting pet imaging to patients positive for paraneoplastic antibodies because there is then a risk of missing patients with an underlying malignancy.
Our study has several limitations. The sample size of our local analysis was small, with only 29 patients meeting the inclusion criteria. Because of the nature of our data source, we could not access pathology results to confirm the histology diagnosis of malignancy. As a surrogate, we used linked data from the ocr, which captures information from all patients diagnosed with cancer in Ontario. Given the reliability of the ocr, we do not feel that using linked data significantly affected our results. The pap database was started in 2011, and we do not have access to long-term follow-up data. A proportion of the patients positive for paraneoplastic antibodies had negative fdg-pet/ct imaging, and it remains unclear whether long-term follow-up with serial imaging—as suggested in 2011 by a Task Force of the European Federation of Neurological Societies5 (dissolved in 2014)—would have yielded additional malignancies.
Several questions remain unanswered. It is unclear whether fdg-pet should be performed as part of the initial pns workup or after negative conventional imaging. García Vicente et al.13 demonstrated a minimal difference in the sensitivity, specificity, and detection rates when fdg-pet was part of the initial workup or after negative imaging; either approach might therefore be reasonable, with the choice depending more on resource availability. In the setting of negative fdg-pet, but ongoing suspicion of pns, the optimal frequency, duration, and modality of repeat screening is unclear. The European Federation of Neurological Societies Task Force recommended repeating investigations every 6 months for up to 4 years; however, the evidence to support that recommendation is poor, and the necessity of ongoing fdg-pet imaging is uncertain.
CONCLUSIONS
When investigating for underlying malignancy in patients with suspected pns and negative conventional imaging, fdg-pet has reasonably high sensitivity and specificity. Our results are congruent with recently published meta-analyses examining a broader patient population. In patients with suspected pns, fdg-pet is a useful tool and should play a role in the investigation of occult malignancy.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349:1543–54. doi: 10.1056/NEJMra023009. [DOI] [PubMed] [Google Scholar]
- 2.Dalmau J, Gultekin HS, Posner JB. Paraneoplastic neurologic syndromes: pathogenesis and physiopathology. Brain Pathol. 1999;9:275–84. doi: 10.1111/j.1750-3639.1999.tb00226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75:1135–40. doi: 10.1136/jnnp.2003.034447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the cns. Lancet Neurol. 2008;7:327–40. doi: 10.1016/S1474-4422(08)70060-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Titulaer MJ, Soffietti R, Dalmau J, et al. on behalf of the European Federation of Neurological Societies (efns) Screening for tumours in paraneoplastic syndromes: report of an efns Task Force. Eur J Neurol. 2011;18:19–e3. doi: 10.1111/j.1468-1331.2010.03220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinilla I, Rodríguez-Vigil B, Gómez-León N. Integrated fdg pet/ct: utility and applications in clinical oncology. Clin Med Oncol. 2008;2:181–98. doi: 10.4137/cmo.s504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boellaard R, Delgado-Bolton R, Oyen WJ, et al. fdg pet/ct: eanm procedure guidelines for tumour imaging: version 2.0. Eur J Nucl Med Mol Imaging. 2015;42:328–54. doi: 10.1007/s00259-014-2961-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AWS, Westwood ME, et al. Research and reporting methods accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Moses LE, Shapiro D, Littenberg B. Combining independent studies of a diagnostic test into a summary roc curve: data-analytic approaches and some additional considerations. Stat Med. 1993;12:1293–316. doi: 10.1002/sim.4780121403. [DOI] [PubMed] [Google Scholar]
- 10.Lijmer JG, Bossuyt PM, Heisterkamp SH. Exploring sources of heterogeneity in systematic reviews of diagnostic tests. Stat Med. 2002;21:1525–37. doi: 10.1002/sim.1185. [DOI] [PubMed] [Google Scholar]
- 11.Hadjivassiliou M, Alder SJ, Van Beek EJ, et al. pet scan in clinically suspected paraneoplastic neurological syndromes: a 6-year prospective study in a regional neuroscience unit. Acta Neurol Scand. 2009;119:186–93. doi: 10.1111/j.1600-0404.2008.01089.x. [DOI] [PubMed] [Google Scholar]
- 12.Rees JH, Hain SF, Johnson MR, et al. The role of 18Ffluoro-2-deoxyglucose–pet scanning in the diagnosis of paraneoplastic neurological disorders. Brain. 2001;124:2223–31. doi: 10.1093/brain/124.11.2223. [DOI] [PubMed] [Google Scholar]
- 13.García Vicente AM, Delgado-Bolton RC, Amo-Salas M, et al. 18F-Fluorodeoxyglucose positron emission tomography in the diagnosis of malignancy in patients with paraneoplastic neurological syndrome: a systematic review and meta-analysis. Eur J Nucl Med Mol Imaging. 2017;44:1575–87. doi: 10.1007/s00259-017-3722-4. [DOI] [PubMed] [Google Scholar]
- 14.Sheikhbahaei S, Marcus CV, Fragomeni RS, Rowe SP, Javadi MS, Solnes LB. Whole-body 18F-fdg pet and 18F-fdg pet/ct in patients with suspected paraneoplastic syndrome: a systematic review and meta-analysis of diagnostic accuracy. J Nucl Med. 2017;58:1031–6. doi: 10.2967/jnumed.116.183905. [DOI] [PubMed] [Google Scholar]
- 15.Younes-Mhenni S, Janier MF, Cinotti L, et al. fdg-pet improves tumour detection in patients with paraneoplastic neurological syndromes. Brain. 2004;127:2331–8. doi: 10.1093/brain/awh247. [DOI] [PubMed] [Google Scholar]
- 16.Linke R, Schroeder M, Helmberger T, Voltz R. Antibody-positive paraneoplastic neurologic syndromes: value of ct and pet for tumor diagnosis. Neurology. 2004;63:282–6. doi: 10.1212/01.WNL.0000129983.06983.4E. [DOI] [PubMed] [Google Scholar]