Abstract
Background
Platelet membranes are extremely susceptible to peroxidation, forming a variety of lipid peroxides, including malondialdehyde (MDA), which has been implicated in the etiology of cardiovascular diseases. Moreover, platelet-leukocyte aggregates (PLAs) are known to contribute to advanced endothelial injury and atherogenesis.
Material/Methods
Fatty acid (FA) methyl esters of the platelet membranes of 79 apparently healthy men without any acute clinical condition at the time of the study were identified by GC/MS. MDA was measured by HPLC in blood serum, and PLAs were analyzed by whole-blood flow cytometry. Individuals were divided into quartiles according to MDA concentration and percentage of PLAs formation. The composition of platelet membrane FAs was compared to MDA concentration and the percentage of PLAs formation in apparently healthy individuals.
Results
In quartiles (Q) with higher MDA concentration, percentage of C 16: 1ω7 (Q1 vs. Q3, p=0.021), C 20: 1ω9 (Q2 vs. Q4, p=0.028) and C 20: 5ω3 (Q2 vs. Q4, p=0.046) was lower. However, C 22: 5ω3 (Q1 vs. Q4, p=0.038) and total ω3 (Q1 vs. Q2, p=0.024) were higher.
Conclusions
MDA and the formation of platelet-monocyte aggregates stimulate the incorporation of monounsaturated fatty acids and polyunsaturated fatty acids in platelet phospholipid membranes, which may be a hallmark for a changed level of biologically active compounds required for the activation of future platelets.
MeSH Keywords: Malondialdehyde, Oxidative Stress, Platelet Activation
Background
Oxidative stress and lipid peroxidation are closely associated with a large number of pathophysiological processes. Formed during oxidative stress, reactive oxygen species (ROS) attack biomolecules, disrupt cellular functions, and cause inflammation or even cell apoptosis [1,2]. Research shows that oxidative stress is associated with increased platelet activation, thrombosis [3,4], and cardiovascular diseases [5]. One of the main targets for ROS is phospholipids of the cell membrane, especially polyunsaturated fatty acids (PUFAs) [6].
During lipid peroxidation, especially of PUFAs, malondialdehyde (MDA) is formed as a degradation product of lipid oxidation. MDA is considered an important biomarker of oxidative stress [7]. MDA is also associated with carcinogenic and cytotoxic effects on the cell, as well as the pathogenesis of diabetes mellitus and neurodegenerative and cardiovascular diseases [8–10].
In vivo, MDA can be produced as a by-product from omega 3 (ω3) or omega 6 (ω6) PUFAs by enzymatic processes during the biosynthesis of thromboxane A2 or generated from bicycle endoperoxides by nonenzymatic processes during lipid peroxidation [11,12]. The number of MDA molecules that can maximally be formed depends on the number of double bonds (since the methylene group between the double bonds is used to form MDA), e.g., arachidonic acid (C 20: 4ω6) could provide 3 MDA molecules, while eicosapentaenoic acid (C 20: 5ω3) could provide a maximum of 4 MDA molecules per PUFA molecule [13]. Moreover, biological MDA exists primarily in 2 forms, i.e., free or covalently bound to/conjugated with proteins and nucleic acids, lipoproteins, and certain amino acids [14].
Human platelets are known to a main source of MDA formation in human blood [13]. According to scientific data, platelets play a key role in protecting against haemorrhage, as well as in inflammatory processes associated with atherosclerosis, homeostasis, and thrombosis [15].
Phospholipids account for 65% of all platelet lipids [16]. Therefore, platelet function and activity are closely related to the composition of the phospholipid membrane. Due to the changes in platelet membrane FAs, the synthesis of biologically active eicosanoids with pro-inflammatory or anti-inflammatory effects may increase [17].
Vascular inflammation plays an essential role in endothelial injury and activation of atherogenesis. Platelets and platelet-leucocyte aggregates (PLAs) are known to contribute to this ongoing endothelial injury, resulting in platelet-dependant thrombosis, especially in acute coronary syndromes [18–20]. Therefore, platelets activated by oxidative stress and accompanied by lipid peroxidation of the phospholipid membrane are closely associated with the risk of cardiovascular diseases.
To explore how processes of lipid peroxidation and platelet activation might be modified when the composition of platelet membrane FA changes, we designed our study to determine the relationship between the changes in the composition of platelet membrane FAs, blood serum MDA concentration, and PLA formation. This research could be useful in evaluating platelet preparation for the next activation phase and assessing the synthesis intensity of biologically active compounds (e.g., eicosanoids/docosanoids).
Material and Methods
Study design
This study (duration: 2 years) was carried out on a group of 79 volunteers (men) aged 36.5 years ±10.8 years, who were apparently healthy (without any acute clinical condition) and who gave their written consent to participate in the study. Individuals with any cardiac and chronic diseases or prior stroke or venous thromboembolism were excluded from the study. Female subjects were not included in this study, as males usually have earlier onset of the disease than their female counterparts [21]. The research was carried out at the laboratory of the Department of Physiology, Biochemistry, Microbiology, and Laboratory Medicine of the Institute of Biomedical Sciences at the Faculty of Medicine of Vilnius University. The study protocol was approved by the Vilnius Regional Bioethics Committee (Approval No. 15820-15-807-319) and was supported by the Research Council of Lithuania (Grant No. MIP-050/2015).
Platelet extraction
Blood samples were collected in a sodium heparin Vacutainer tube and centrifuged immediately at 3000 g for 10 min. Then, ¾ of the plasma was removed without touching the cell and foam layer. The remaining portion (¼ of the plasma), rich in thrombocytes, was extracted and mixed with freezing media (BI, Israel) in a ratio 2: 1 and frozen at −80°C.
Extraction and determination of platelet membrane FAs
Methyl esters of platelet membrane FAs were prepared using the Folch method [22]. Thin-layer chromatography (Sil G-25 UV254) was then performed to extract platelet phospholipids [23]. After FA transesterification, the FA spectrum was determined by gas chromatography/mass spectrometry with a GCMS-QP2010 Ultra manufactured by Shimadzu. Data were collected and processed using LabSolutions software (Shimadzu). Table 1 shows the FAs investigated in this study expressed as a percentage of total FAs.
Table 1.
FAs analyzed by gas chromatography/mass spectrometry.
| SFAs* | MUFAs** | PUFAs*** |
|---|---|---|
| 14: 0# | 16: 1ω%7 | 18: 2ω6 |
| Myristic acid | 9-hexadecenoic/Palmitoleic acid | 9,12-octadecadienoic/Linoleic acid |
|
| ||
| 16: 0# | 18: 1ω%9 | 18: 3ω3 |
| Palmitic acid | 9-octadecenoic/Oleic acid | 9,12,15-octadecatrienoic/α-Linolenic acid |
|
| ||
| 18: 0 | 18: 1ω7 | 20: 4ω6 |
| Stearic acid | 11-octadecenoic/Vaccenic acid | 5,8,11,14-eicosatetraenoic/Arachidonic acid |
|
| ||
| 20: 1ω9 | 20: 5ω3 | |
| 11-eicosenoic/Gondoic acid | 5,8,11,14,17-eikosapentaenoic/Timnodonic acid | |
|
| ||
| 22: 5ω3 | ||
| 7,10,13,16,19-docosapentaenoic/Clupanodonic acid | ||
|
| ||
| 22: 6ω3 | ||
| 4,7,10,13,16,19-docosahexaenoic/Cervonic acid | ||
Saturated fatty acids;
monounsaturated fatty acids;
polyunsaturated fatty acids;
number of carbon atoms and double bonds;
position of double bond between carbon atoms.
Determination of MDA concentration in blood serum
Blood serum MDA concentration was measured using a method published by Khoschsorur et al. [24] with minor modifications. The sample preparation serves for the sample purge and for the derivatisation of the analyte with thiobarbituric acid (TBA) into a detectable form, i.e., the MDA-TBA adduct. MDA concentration was determined by a Shimadzu Nexera X2 UHPLC system (Shimadzu). Data were collected and processed using LabSolutions software (Shimadzu).
Determination of platelet activation markers
Flow cytometric analysis was performed on platelet functional activity in agonist non-stimulated EDTA anticoagulated blood not later than 10 min after blood collection (BD FACS Canto, BD Biosciences, USA). Data analysis was carried out using BD FACS Diva software (version 6.1.2). Leukocyte populations (neutrophils, monocytes, and lymphocytes) were identified according to CD45/CD14 expression: neutrophils (CD45+, CD14−, high side scattered light), monocytes (CD45+, CD14+, mean side scattered light) and lymphocytes (CD45++, CD14−, low side scattered light). Then, the percentage of neutrophils, monocytes and lymphocytes expressing the CD42a marker was calculated. This combination of markers is characteristic for PLAs and was considered an indicator of adhesion phase. The data that were obtained were expressed in absolute numbers (the number of studied platelets tagged with the marker), percentages (a part of the studied population tagged with the marker), and mean of fluorescence intensity (fluorescence intensity of platelet population with tagged marker).
Distribution of volunteers
First, individuals participating in this study were grouped into quartiles according to the concentration of blood serum MDA. Blood serum MDA concentration was 62.47–77.58 μg/l (n=20) in the first quartile (Q1), 77.79–97.07 μg/l (n=20) in the second quartile (Q2), 97.22–117.61 μg/l (n=20) in the third quartile, and 118.10–169.32 μg/l (n=19) in the fourth quartile (Q4). Then, the spectrum of platelet membrane FA was compared with the concentration of blood serum MDA in quartiles, and the correlation between the platelet membrane FA spectrum and blood serum MDA concentration was calculated.
Second, volunteers were grouped into quartiles according to the percentage of PLA formation. The percentage of PLAs formation ranged from 3.7 to 8.3 (n=20) in the first quartile (Q1), 8.4 to 9.5 (n=19) in the second quartile (Q2), 9.6 to 10.8 (n=21) in the third quartile (Q3), and 10.9 to 14.5 (n=19) in the fourth quartile (Q4). The quartiles of PLAs formation were then compared with the spectrum of platelet membrane FA, and the correlation between the variables was measured.
Statistical analysis
Data analysis was carried out using IBM SPSS software (version 24) and Microsoft Excel 2016. Data are expressed as median, minimum, and maximum. Differences between the groups were tested for significance using the Mann-Whitney U test and the Spearman’s rank correlation coefficient for assessing the correlation between variables. P<0.05 was considered statistically significant.
Results
According to our data, there was a tendency for a higher level of C 14: 0 to be found in the first quartile, which had a lower concentration of blood serum MDA than the third and fourth quartiles, where blood serum MDA concentration was higher (Q1 and Q3, p=0.05; Q1 and Q4, p=0.089) (Figure 1).
Figure 1.
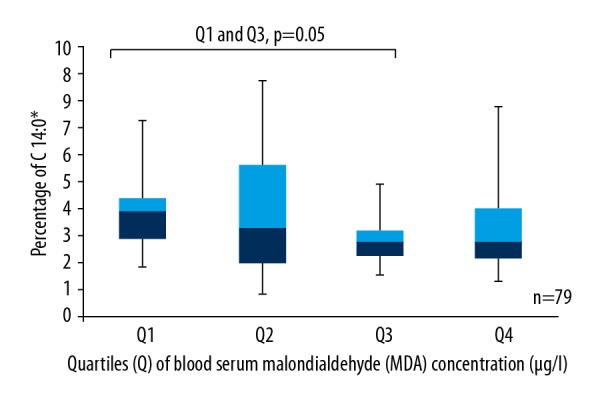
Box plots represent a comparison of the percentage of C 14: 0 between quartiles of blood serum MDA concentration. Q1 and Q3, p=0.05; Q1 and Q4, p=0.089. N=79. * Number of carbon atoms and double bonds.
C 16: 0 made up the highest percentage of total FAs in the platelet phospholipid membrane (47%). The distribution of C 16: 0 was slightly higher in the first quartile of blood serum MDA concentration than in the second, third, and fourth quartiles. Although there was no statistically significant difference between Q1 and Q4 of C 16: 0 (p=0.728), it was observed that the highest percentage of C 16: 0 in the first quartile increases the total percentage of saturated fatty acids (SFAs).
Our results showed that the highest level of C 16: 1ω7 was found in the first quartile, which also had a lower blood serum MDA concentration than the third quartile (p=0.021). It was also noticed that the higher the level of C 18: 1ω7, the higher the concentration of blood serum MDA (Q1 and Q4, p=0.070).
The highest percentage of MUFAs in platelet phospholipid membrane consisted of C18: 1ω9 (46.5%), but the differences were not statistically significant. However, our data showed that significantly more C 20: 1ω9 was found in the second quartile than in the fourth quartile (p=0.028), where the concentration of blood serum MDA was the highest (Figure 2).
Figure 2.
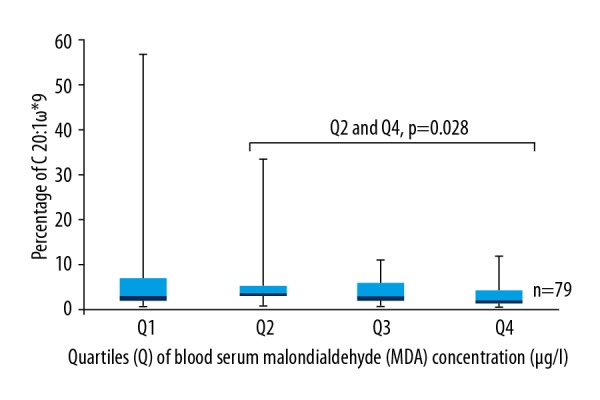
Box plots represent a comparison of the percentage of C 20: 1ω9 between quartiles of blood serum MDA concentration. Q2 and Q4, p=0.028. N=79. * Position of double bond between carbon atoms.
Assessing the total sums of ω3 and ω6 PUFAs separately, we observed that with the highest concentration of blood serum MDA (Q4), the total sums of ω3 and ω6 increase, and with the lowest concentration of blood serum MDA (Q1), the total sums decrease (ω3 p=0.184, ω6 p=0.813). However, a statistically significant difference was found only between the amount of ω3 PUFAs (p=0.024) in the first and second quartiles.
Statistically significantly less C 20: 5ω3 was found in the first quartile, which had a lower blood serum MDA concentration than the second quartile (p=0.028), but statistically significantly more C 20: 5ω3 was observed in the second quartile than in the third quartile, where blood serum MDA concentration was higher (p=0.046) (Figure 3).
Figure 3.
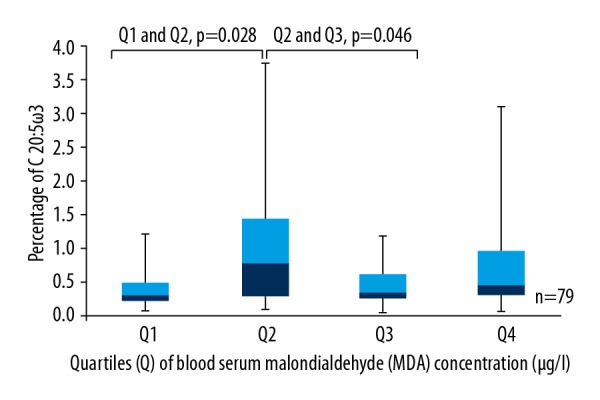
Box plots represent a comparison of the percentage of C 20: 5ω3 between quartiles of blood serum MDA concentration. Q1 and Q2, p=0.028; Q2 and Q3, p=0.046. N=79.
Statistically significantly less C 22: 5ω3 was observed in the first quartile, which had the lowest blood serum MDA concentration, than in the second (p=0.037) and fourth quartiles, which had higher levels of blood serum MDA concentration (p=0.038) (Figure 4).
Figure 4.
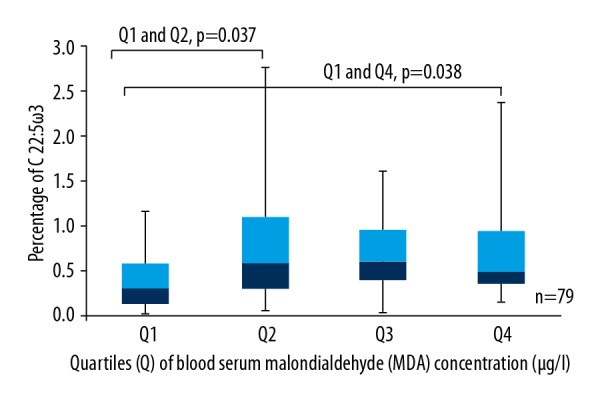
Box plots represent a comparison of the percentage of C 22: 5ω3 between quartiles of blood serum MDA concentration. Q1 and Q2, p=0.037; Q1 and Q4, p=0.038. N=79.
Our study results also showed that at the highest concentration of blood serum MDA (Q4), the ratio of C 18: 2ω6/C 20: 4ω6 was statistically significantly lower than the lowest blood serum MDA concentration (Q1) (p=0.038) (Table 2).
Table 2.
Comparison of the composition of platelet membrane FA between quartiles of blood serum MDA concentration.
| FA@ (provided by percentage of total amount) | Median, minimum, maximum | Quartiles of MDA& concentration | P value | |||
|---|---|---|---|---|---|---|
| Q1* (n=20) | Q2$ (n=20) | Q3# (n=20) | Q4% (n=19) | |||
| C 14: 0$$ | Med. | 3.93 | 3.30 | 2.81 | 2.81 |
*,$p=0.529 *,#p=0.005 *,%p=0.089 $,#p=0.231 $,%p=0.428 #,%p=0.627 |
| Min. | 1.88 | 0.81 | 1.54 | 1.34 | ||
| Max. | 7.28 | 8.75 | 4.92 | 7.79 | ||
| C 16: 0 | Med. | 48.99 | 45.39 | 48.49 | 43.81 |
*,$p=0.718 *,#p=0.659 *,%p=0.728 $,#p=0.253 $,%p=0.857 #,%p=0.247 |
| Min. | 20.53 | 32.72 | 37.43 | 29.32 | ||
| Max. | 65.11 | 59.53 | 63.15 | 63.39 | ||
| C 18: 0 | Med. | 20.73 | 19.97 | 22.00 | 18.45 |
*,$p=0.925 *,#p=0.231 *,%p=0.792 $,#p=0.060 $,%p=0.627 #,%p=0.026 |
| Min. | 8.76 | 11.52 | 16.96 | 11.89 | ||
| Max. | 26.65 | 27.54 | 43.87 | 28.60 | ||
| C 16: 1ω**7 | Med. | 1.63 | 1.37 | 1.12 | 1.59 |
*,$p=0.327 *,#p=0.021 *,%p=0.444 $,#p=0.383 $,%p=0.879 #,%p=0.134 |
| Min. | 0.39 | 0.22 | 0.19 | 0.18 | ||
| Max. | 12.44 | 15.70 | 4.44 | 13.29 | ||
| C 18: 1ω7 | Med. | 1.06 | 1.01 | 1.27 | 1.38 |
*,$p=0.989 *,#p=0.718 *,%p=0.070 $,#p=0.904 $,%p=0.158 #,%p=0.247 |
| Min. | 0.15 | 0.13 | 0.20 | 0.19 | ||
| Max. | 4.09 | 2.30 | 2.64 | 6.90 | ||
| C 18: 1ω9 | Med. | 7.31 | 5.69 | 6.79 | 9.79 |
*,$p=0.841 *,#p=0.968 *,%p=0.283 $,#p=0.862 $,%p=0.120 #,%p=0.204 |
| Min. | 0.46 | 1.62 | 1.61 | 0.69 | ||
| Max. | 15.43 | 12.65 | 14.52 | 22.05 | ||
| C 20: 1ω9 | Med. | 2.65 | 3.56 | 2.92 | 1.98 |
*,$p=0.512 *,#p=0.799 *,%p=0.204 $,#p=0.277 $,%p=0.028 #,%p=0.224 |
| Min. | 0.58 | 0.72 | 0.56 | 0.36 | ||
| Max. | 56.77 | 33.26 | 10.90 | 11.72 | ||
| C 18: 2ω6 | Med. | 5.08 | 4.33 | 6.19 | 6.75 |
*,$p=0.738 *,#p=0.862 *,%p=0.901 $,#p=0.758 $,%p=0.967 #,%p=0.999 |
| Min. | 2.01 | 0.54 | 1.68 | 0.40 | ||
| Max. | 20.12 | 19.82 | 17.03 | 21.46 | ||
| C 18: 3ω3 | Med. | 1.10 | 1.96 | 1.58 | 1.82 |
*,$p=0.369 *,#p=0.289 *,%p=0.569 $,#p=0.659 $,%p=0.607 #,%p=0.792 |
| Min. | 0.35 | 0.28 | 0.65 | 0.03 | ||
| Max. | 5.43 | 6.08 | 5.71 | 21.66 | ||
| C 20: 4ω6 | Med. | 0.63 | 0.92 | 0.96 | 1.08 |
*,$p=0.201 *,#p=0.478 *,%p=0.235 $,#p=0.620 $,%p=0.923 #,%p=0.569 |
| Min. | 0.09 | 0.21 | 0.02 | 0.07 | ||
| Max. | 7.84 | 8.31 | 5.84 | 9.01 | ||
| C 20: 5ω3 | Med. | 0.30 | 0.78 | 0.34 | 0.46 |
*,$p=0.028 *,#p=0.698 *,%p=0.141 $,#p=0.046 $,%p=0.365 #,%p=0.309 |
| Min. | 0.06 | 0.10 | 0.05 | 0.06 | ||
| Max. | 1.21 | 3.75 | 1.18 | 3.10 | ||
| C 22: 5ω3 | Med. | 0.31 | 0.57 | 0.59 | 0.48 |
*,$p=0.037 *,#p=0.052 *,%p=0.038 $,#p=0.820 $,%p=0.923 #,%p=0.923 |
| Min. | 0.02 | 0.05 | 0.02 | 0.15 | ||
| Max. | 1.15 | 2.76 | 1.61 | 2.37 | ||
| C 22: 6ω3 | Med. | 0.37 | 0.97 | 0.68 | 0.70 |
*,$p=0.265 *,#p=0.602 *,%p=0.141 $,#p=0.289 $,%p=0.923 #,%p=0.513 |
| Min. | 0.02 | 0.06 | 0.03 | 0.13 | ||
| Max. | 2.40 | 3.58 | 2.24 | 3.95 | ||
| Total SFAs (C 14: 0+C 16: 0+C 18: 0) | Med. | 73.42 | 68.98 | 74.56 | 65.95 |
*,$p=0.820 *,#p=0.529 *,%p=0.607 $,#p=0.253 $,%p=0.687 #,%p=0.141 |
| Min. | 31.17 | 49.60 | 57.28 | 45.78 | ||
| Max. | 93.00 | 87.77 | 90.51 | 92.59 | ||
| Total MUFAs | Med. | 14.54 | 15.23 | 13.33 | 16.99 |
*,$p=0.883 *,#p=0.383 *,%p=0.771 $,#p=0.341 $,%p=0.687 #,%p=0.158 |
| Min. | 3.69 | 5.15 | 5.04 | 5.05 | ||
| Max. | 62.80 | 36.57 | 22.50 | 32.99 | ||
| Total PUFAs | Med. | 9.80 | 13.89 | 11.15 | 13.09 |
*,$p=0.314 *,#p=0.314 *,%p=0.336 $,#p=0.659 $,%p=0.945 #,%p=0.569 |
| Min. | 3.31 | 4.04 | 3.83 | 1.33 | ||
| Max. | 34.22 | 33.89 | 26.42 | 34.97 | ||
| Σ*** ω3 | Med. | 2.81 | 4.53 | 3.60 | 4.20 |
*,$p=0.024 *,#p=0.201 *,%p=0.184 $,#p=0.076 $,%p=0.728 #,%p=0.687 |
| Min. | 0.59 | 0.79 | 1.99 | 0.84 | ||
| Max. | 9.37 | 13.00 | 6.83 | 25.46 | ||
| Σ ω6 | Med. | 5.72 | 7.12 | 7.42 | 7.53 |
*,$p=0.565 *,#p=0.779 *,%p=0.813 $,#p=0.947 $,%p=0.901 #,%p=0.879 |
| Min. | 2.25 | 1.14 | 1.84 | 0.49 | ||
| Max. | 27.96 | 28.13 | 21.70 | 28.28 | ||
| Ratio of ω3/ω6 | Med. | 0.43 | 0.67 | 0.53 | 0.45 |
*,$p=0.149 *,#p=0.301 *,%p=0.296 $,#p=0.547 $,%p=0.771 #,%p=0.945 |
| Min. | 0.08 | 0.18 | 0.17 | 0.18 | ||
| Max. | 2.57 | 5.15 | 2.03 | 8.21 | ||
| Ratio of PUFAs/SFAs | Med. | 0.15 | 0.19 | 0.15 | 0.20 |
*,$p=0.429 *,#p=0.820 *,%p=0.428 $,#p=0.547 $,%p=0.967 #,%p=0.444 |
| Min. | 0.04 | 0.06 | 0.04 | 0.01 | ||
| Max. | 0.70 | 0.67 | 0.46 | 0.70 | ||
| Ratio of C 18: 2ω6/C 20: 4ω6 | Med. | 7.49 | 6.30 | 7.75 | 5.08 |
*,$p=0.091 *,#p=0.659 *,%p=0.038 $,#p=0.192 $,%p=0.749 #,%p=0.204 |
| Min. | 2.57 | 0.54 | 2.29 | 0.99 | ||
| Max. | 26.78 | 14.48 | 118 | 43.57 | ||
| Ratio of C 18: 3ω3/C 20: 5ω3 | Med. | 4.20 | 1.62 | 4.46 | 3.15 |
*,$p=0.076 *,#p=0.659 *,%p=0.531 $,#p=0.108 $,%p=0.531 #,%p=0.296 |
| Min. | 1.20 | 0.26 | 0.83 | 0.04 | ||
| Max. | 14.68 | 49.00 | 50.44 | 83.31 | ||
| Ratio of C 20: 4ω6/C 20: 5ω3 | Med. | 2.16 | 1.62 | 2.00 | 2.71 |
*,$p=0.583 *,#p=0.820 *,%p=0.728 $,#p=0.398 $,%p=0.247 #,%p=0.813 |
| Min. | 0.39 | 0.58 | 0.29 | 0.09 | ||
| Max. | 8.50 | 7.23 | 17.70 | 13.50 | ||
SFAs – saturated fatty acids; MUFAs – monounsaturated fatty acids; PUFAs – polyunsaturated fatty acids;
fatty acids;
malondialdehyde;
quartile 1 (Q1);
quartile 2 (Q2);
quartile 3 (Q3);
quartile 4 (Q4);
number of carbon atoms and double bonds;
position of double bond between carbon atoms in the molecule;
total sum.
Spearman’s test showed a weak but statistically significant inverse correlation between C 14: 0 and blood serum MDA concentration (r=−0.255; p=0.023) and between the concentration of blood serum MDA and the ratio of C 18: 2ω6/C 20: 4ω6 (r=−0.244; p=0.034) (Table 3).
Table 3.
The correlation of platelet membrane FA spectrum with blood serum MDA concentration and percentage of PMA formation.
| FAs@ (provided by percentage of total amount) | Spearman’s rho | P value | ||
|---|---|---|---|---|
| MDA* concentration | PMAs** | MDA concentration | PMAs | |
| C 14: 0$ | −0.255 | −0.222 | 0.023 | 0.050 |
| Ratio of C 18: 2ω$$6/C 20: 4ω6 | −0.244 | – | 0.034 | – |
Fatty acids;
malondialdehyde;
platelet-monocyte aggregates;
number of carbon atoms and double bonds;
position of double bond between carbon atoms in the molecule.
Comparing the composition of platelet membrane FAs with the percentage of PMA formation, it was observed that with the increase in the formation of aggregates, the total sums of MUFAs and PUFAs were higher separately and the total sum of SFAs was lower, but the differences were not statistically significant. However, the tendency was observed for an increased level of C 14: 0 and an increased ratio of C 18: 3ω3/C 20: 5ω3 in the first and the fourth quartiles of the formation of PMAs (p=0.093). The same tendency was observed in the comparison of the ratio of C 18: 3ω3/C 20: 5ω3 between the first and the third quartiles of the formation of PMAs (p=0.055) (Table 4). In terms of the mutual differences between the percentage of the formation of other PLAs (granulocytes and lymphocytes) and platelet membrane FA spectrum, the differences were not statistically significant.
Table 4.
Comparison of certain platelet membrane FAs between quartiles of PMA formation.
| FAs@ (provided by percentage of total amount) | Median, minimum, maximum | Quartiles of percentage of PMA& formation | P value | |||
|---|---|---|---|---|---|---|
| Q1* (n=20) | Q2$ (n=19) | Q3# (n=21) | Q4% (n=19) | |||
| C 14: 0$$ | Med. | 3.39 | 2.93 | 2.81 | 2.76 |
*,$p=0.204 *,#p=0.197 *,%p=0.093 $,#p=0.936 $,%p=0.822 #,%p=0.791 |
| Min. | 1.34 | 1.42 | 0.81 | 1.54 | ||
| Max. | 7.79 | 6.48 | 7.70 | 8.75 | ||
| Total SFAs (C 14: 0+C 16: 0+C 18: 0) | Med. | 77.45 | 63.57 | 70.64 | 73.04 |
*,$p=0.194 *,#p=0.291 *,%p=0.696 $,#p=0.872 $,%p=0.298 #,%p=0.512 |
| Min. | 45.78 | 49.06 | 31.17 | 49.11 | ||
| Max. | 87.77 | 93.00 | 92.59 | 92.46 | ||
| Total MUFAs | Med. | 13.33 | 16.91 | 15.40 | 15.16 |
*,$p=0.336 *,#p=0.449 *,%p=0.942 $,#p=0.649 $,%p=0.233 #,%p=0.606 |
| Min. | 5.15 | 3.69 | 5.04 | 5.05 | ||
| Max. | 32.99 | 36.57 | 62.80 | 23.83 | ||
| Total PUFAs | Med. | 9.51 | 15.30 | 10.63 | 11.47 |
*,$p=0.283 *,#p=0.648 *,%p=0.633 $,#p=0.555 $,%p=0.599 #,%p=0.856 |
| Min. | 4.28 | 3.31 | 1.33 | 2.49 | ||
| Max. | 24.84 | 34.97 | 33.89 | 34.22 | ||
| Ratio of C 18: 3ω**3/C 20: 5ω3 | Med. | 2.21 | 3.91 | 4.06 | 4.16 |
*,$p=0.184 *,#p=0.055 *,%p=0.093 $,#p=0.294 $,%p=0.799 #,%p=0.587 |
| Min. | 0.04 | 0.26 | 0.67 | 1.04 | ||
| Max. | 49.00 | 50.44 | 83.31 | 17.83 | ||
SFAs – saturated fatty acids; MUFAs – monounsaturated fatty acids; PUFAs – polyunsaturated fatty acids;
fatty acids; & platelet-monocyte aggregates;
quartile 1 (Q1);
quartile 2 (Q2);
quartile 3 (Q3);
quartile 4 (Q4);
number of carbon atoms and double bonds;
position of double bond between carbon atoms in the molecule.
Calculations demonstrated that the correlation between the platelet membrane FA spectrum and the percentage of the formation of PMAs had a weak but statistically significant inverse correlation between the percentage of the formation of PMAs and C 14: 0 (r=−0.222; p=0.050) (Table 3).
Discussion
According to our data, the highest percentage of FAs in the platelet phospholipid membrane consisted of SFA. Other authors have obtained very similar results [25–28]. Moreover, C16: 0 is reported to be the main FA of the platelet membrane [29], as was observed in our study. According to recent scientific studies, SFA higher levels were detected in those cell membranes that are closely related to signalling mechanisms. C 14: 0 and C 16: 0 can covalently modify proteins associated with signal transmission [30].
One of the MUFAs we analyzed, C 16: 1ω7, was at its highest level when blood serum MDA concentration was at the lowest. This was probably due to intensified C 16: 1ω7 synthesis from SFA C 16: 0 by stearoyl-CoA desaturase – 1 during FA desaturation [31], and/or C16: 1ω7 was obtained from vegetable food and oils. Such a diet contains a number of antioxidants, e.g., fat-soluble vitamin E (tocopherol), leading to a higher level of C 16: 1ω7 at a lower concentration of blood serum MDA.
C 18: 1ω9 accounts for the highest percentage of FA compared to other MUFAs [25,26,28]. The same tendency was observed in our data. This increase in C 18: 1ω9 at the highest concentration of blood serum MDA can be interpreted as an intention to reduce blood serum MDA level or as compensation by using PUFA for active blood serum MDA synthesis. The absence of ω3 and ω6 PUFAs may intensify C 18: 1ω9 synthesis, as it is the precursor of other ω9 PUFAs required for cell membranes.
According to our data, statistically significantly less C 20: 5ω3 was found at the lowest level of blood serum MDA concentration, but statistically significantly more C 20: 5ω3 was observed when the concentration of blood serum MDA was at its higher level. This result shows that increased oxidation stimulates platelets to synthesize more PUFAs (e.g., C 20: 5ω3) and therefore increases the production of biologically active compounds and platelet activation. C 20: 5ω3 is associated with a lower incidence of major coronary events. This effect of C 20: 5ω3 in reducing the risk of cardiovascular diseases could be mediated by increased production of prostaglandin I3 (PGI3), inhibiting platelet aggregation; promoting vasodilatation, myocardial ischemic injury, and arteriosclerosis; and inducing neoangiogenesis. Furthermore, an increased level of PGI3 decreases thromboxane A2 (TXA2) production, which is known to have the opposite effect on the cardiovascular system: TXA2 causes platelet activation, coronary spasms, and vascular smooth muscle cell proliferation that can result in arteriosclerosis and, subsequently, cardiovascular events. [32]. C 20: 4ω6 and C 20: 5ω3 antagonize each other. C 20: 5ω3 competes with C 20: 4ω6 in the cyclooxygenase (COX) pathway, leading to the formation of eicosanoids that are less pro-thrombotic and inflammatory and may also directly inhibit platelet aggregation to a greater degree than the eicosanoids derived from C 20: 4ω6 [33–35].
Our data showed that the total sums of individual ω3 and ω6 were higher when blood serum MDA concentration was at the highest level, when the total sums of individual ω3 and ω6 were lower, and when blood serum MDA concentration was at the lowest level. However, a statistically significant difference was found only in the first and second quartiles of the total sum of ω3 FA (p=0.024). Similar results were obtained by Li et al. [36]. The increased level of PUFAs, with a higher blood serum MDA concentration, could be explained as a platelet response to prepare for the future activation process. Therefore, the oxidation process and the increased blood serum MDA concentration are factors that stimulate platelets to synthesize more PUFAs (e.g., C 20: 4ω6, C 20: 5ω3, and C 22: 6ω3) from essential FAs: C 18: 2ω6 and C 18: 3ω3 by a series of desaturase and elongase enzymes, leading to intensified synthesis of biologically active compounds, e.g., pro- or anti-inflammatory eicosanoids and docosanoids.
According to our data, with the rise of blood serum MDA concentration, the level of C 22: 5ω3 increases in platelet phospholipid membranes. This result could be explained by intensified metabolism of FA, synthesizing more eicosanoic and docosanoic FA, which will be later used for platelet activation. The tendency of docosahexaenoic FA (C 22: 6ω3) distribution was similar to that of C 22: 5ω3, but there were no statistically significant differences. The experiment in which C 22: 6ω3 was incorporated into the platelet membrane and the blood serum MDA concentration was measured showed that when the platelet membrane contains more C 22: 6ω3, the concentration of blood serum MDA is higher. It was previously reported that a higher level of ω3 PUFA in platelet phospholipid membrane, particularly in C 22: 6ω3, could be related to lipid peroxidation [37]. Moreover, other researchers also studied the effect of C 22: 6ω3 on platelets. Dietary supplements with C 22: 6ω3 were given to healthy men. Then, the incorporation of C 22: 6ω3 into the platelet phospholipid membrane and platelet activity were monitored. The study results showed that a higher level of C 22: 6ω3 in the platelet membrane statistically significantly reduces platelet activity and induces an antioxidant effect, increasing platelet vitamin E concentration. Accordingly, it could be regarded as a protective factor against platelet-related cardiovascular events [38, 39]. Our study, which included only healthy individuals, did not show any significant oxidation effect. Only a study carried out with a markedly higher blood serum MDA concentration could confirm the significant effect of oxidation.
We also found that the ratio of C 18: 2ω6/C 20: 4ω6 at the highest blood serum MDA concentration was statistically significantly lower than it was at the lowest blood serum MDA concentration. According to this result, more intensive conversion of C 18: 2ω6 to C 20: 4ω6 occurs with a higher concentration of blood serum MDA. This conversion could be considered as the preparation of platelets for the synthesis of pro-inflammatory eicosanoids. Moreover, the thromboxanes that are produced activate platelets, allowing them to start the process of blood coagulation more quickly. C 20: 4ω6, incorporated in platelet membrane, is used for the synthesis of TXA2, which is involved in the pathogenesis of cardiovascular diseases in that it promotes platelet aggregation and vasoconstriction, acting through specific receptors coupled with the G-protein Gq [40,41]. Therefore, a higher concentration of blood serum MDA could be a factor in the formation of a higher level of TXA2.
In our study, an increased ratio of C 18: 3ω3/C 20: 5ω3 was noted when the lowest and the highest percentage of the formation of PMAs were compared. Since C 18: 3ω3 is not synthesized in the human body and is obtained only with food (vegetable oils), it reflects the diet of a particular person and eventually will be converted to C 20: 5ω3. An experimental study showed that when the platelet membrane is saturated with C 20: 5ω3, platelet procoagulative properties can be reduced. Moreover, C 20: 5ω3 in combination with C 22: 6ω3 has a protective effect against cardiovascular diseases, since anti-inflammatory biologically active compounds are synthesized from these FAs [42]. Consequently, ω3 PUFA can regulate processes associated with inflammation through multiple mechanisms: platelet activation and aggregation and vasoconstriction [43]. By changing C 20: 4ω6 as a substrate in the production of eicosanoids, ω3 PUFA can act directly, thereby inhibiting the metabolism of C 20: 4ω6, or indirectly through gene expression, activating peroxisome proliferator-activated receptors (PPARs) α and γ [44–46]. Furthermore, ω3 PUFAs inhibit the secretion of monocyte/macrophage inflammatory cytokines (interleukins, TNF) [47].
Our results show a statistically significant inverse correlation between C 14: 0 and blood serum MDA concentration. The same correlation was observed between the ratio of C 18: 2ω6/C 20: 4ω6 and the concentration of blood serum MDA. A statistically significant inverse correlation was also found between the percentage of the formation of PMAs and C 14: 0. Some researchers reported a direct correlation between MUFAs, PUFAs, and blood serum MDA concentration and an inverse correlation between SFAs and concentration of blood serum MDA. They also noticed that lipid peroxidation was much more intensive in healthy individuals. This data could be explained by an increased amount of PUFAs in the platelet phospholipid membrane [48]. Although we did not find a statistically significant correlation between blood serum MDA concentration and SFAs, MUFAs, and PUFAs in the platelet phospholipid membrane, we found that with a higher blood serum MDA concentration, the level of MUFA and PUFAs was slightly higher, while the level of SFAs was lower. Moreover, with a higher percentage of PMA formation, the level of SFAs was lower as well.
Conclusions
The results of our study in healthy men showed that increasing levels of blood serum MDA concentration and percentage of PMA formation are factors that stimulate the incorporation of MUFA and PUFA into the platelet phospholipid membrane and may play a role in increasing the level of biologically active compounds (eicosanoids/docosanoids) required for further platelet activation. Though we hypothesize that the alteration of cell membrane composition caused by oxidative stress may modify platelet response to activation, further human studies are needed to confirm the significant effect of oxidation on platelets.
Footnotes
Source of support: This study was supported by the Research Council of Lithuania (Grant No. MIP-050/2015)
Conflicts of interest
None.
References
- 1.Yoshikawa T, Naito Y. What is oxidative stress? JMAJ. 2002;45(7):271–76. [Google Scholar]
- 2.Ito K, Suda T. Metabolic requirements for the maintenance of self-renewing stem cells. Nat Rev Mol Cell Biol. 2014;15(4):243–56. doi: 10.1038/nrm3772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Violi F, Pignatelli P. Platelet oxidative stress and thrombosis. Thromb Res. 2012;129(3):378–81. doi: 10.1016/j.thromres.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Fuentes E, Palomo I. Role of oxidative stress on platelet hyperreactivity during aging. Life Sci. 2016;148:17–23. doi: 10.1016/j.lfs.2016.02.026. [DOI] [PubMed] [Google Scholar]
- 5.Vichova T, Motovska Z. Oxidative stress: Predictive marker for coronary artery disease. Exp Clin Cardiol. 2013;18(2):e88–91. [PMC free article] [PubMed] [Google Scholar]
- 6.Gaschler MM, Stockwell BR. Lipid peroxidation in cell death. Biochem Biophys Res Commun. 2017;482(3):419–25. doi: 10.1016/j.bbrc.2016.10.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grotto D, Maria LS, Valentini J, et al. Importance of the lipid peroxidation biomarkers and methodological aspects for malondialdehyde quantification. Quim Nova. 2009;32:169–74. [Google Scholar]
- 8.Cai F, Dupertuis YM, Pichard C. Role of polyunsaturated fatty acids and lipid peroxidation on colorectal cancer risk and treatments. Curr Opin Clin Nutr Metab Care. 2012;15(2):99–106. doi: 10.1097/MCO.0b013e32834feab4. [DOI] [PubMed] [Google Scholar]
- 9.Garcia SC, Grotto D, Bulcão RP, et al. Evaluation of lipid damage related to pathological and physiological conditions. Drug Chem Toxicol. 2013;36(3):306–12. doi: 10.3109/01480545.2012.720989. [DOI] [PubMed] [Google Scholar]
- 10.Negre-Salvayre A, Auge N, Ayala V, et al. Pathological aspects of lipid peroxidation. Free Radic Res. 2010;44(10):1125–71. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- 11.Massey KA, Nicolaou A. Lipidomics of oxidized polyunsaturated fatty acids. Free Radic Biol Med. 2013;59(100):45–55. doi: 10.1016/j.freeradbiomed.2012.08.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayala A, Muñoz MF, Argüelles S. Lipid Peroxidation: Production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal. Oxid Med Cell Longev. 2014;2014 doi: 10.1155/2014/360438. 360438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsikas D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal Biochem. 2017;524:13–30. doi: 10.1016/j.ab.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 14.Tsikas D, Rothmann S, Schneider JY, et al. Development, validation and biomedical applications of stable-isotope dilution GC-MS and GC-MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2α and nitric oxide (NO) J Chromatogr B Analyt Technol Biomed Life Sci. 2016;1019:95–111. doi: 10.1016/j.jchromb.2015.10.009. [DOI] [PubMed] [Google Scholar]
- 15.Colkesen Y, Muderrisoglu H. The role of mean platelet volume in predicting thrombotic events. Clin Chem Lab Med. 2012;50(4):631–34. doi: 10.1515/CCLM.2011.806. [DOI] [PubMed] [Google Scholar]
- 16.Dolegowska B, Lubkowska A, De Girolamo L. Platelet lipidomic. J Biol Regul Homeost Agents. 2012;26(2 Suppl 1):23S–33S. [PubMed] [Google Scholar]
- 17.Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog Lipid Res. 2008;47(2):147–55. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Ashman N, Macey MG, Fan SL, et al. Increased platelet–monocyte aggregates and cardiovascular disease in end-stage renal failure patients. Nephrol Dial Transplant. 2003;18:2088–96. doi: 10.1093/ndt/gfg348. [DOI] [PubMed] [Google Scholar]
- 19.Ross R. Atherosclerosis – an inflammatory disease. N Engl J Med. 1999;340:115–26. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20.McEver RP. Adhesive interactions of leukocytes, platelets and the vessel wall during hemostasis and inflammation. Thromb Haemostasis. 2000;86:746–56. [PubMed] [Google Scholar]
- 21.Fairweather DL. Sex differences in inflammation during atherosclerosis. Clinical Medicine Insights: Cardiology. 2014;8(Suppl 3):49–59. doi: 10.4137/CMC.S17068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Stanley G. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Touchstone JC. Thin-layer chromatographic procedures for lipid separation. J Chromatogr B: Biomed Sci Apls. 1995;671(1–2):169–95. doi: 10.1016/0378-4347(95)00232-8. [DOI] [PubMed] [Google Scholar]
- 24.Khoschsorur GA, Winklhofer-Roob BM, Rabl H, et al. Evaluation of a sensitive HPLC method for the determination of Malondialdehyde, and application of the method to different biological materials. Chromatographia. 2000;52(3–4):181–84. [Google Scholar]
- 25.De Castro J, Hernández-Hernández A, Rodríguez MC, et al. Comparison of changes in erythrocyte and platelet phospholipid and fatty acid composition and protein oxidation in chronic obstructive pulmonary disease and asthma. Platelets. 2007;18(1):43–51. doi: 10.1080/09537100600800776. [DOI] [PubMed] [Google Scholar]
- 26.Lin S, Li T, Liu X, et al. Abnormal octadeca-carbon fatty acids distribution in erythrocyte membrane phospholipids of patients with gastrointestinal tumor. Medicine (Baltimore) 2017;96(24):e7189. doi: 10.1097/MD.0000000000007189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vognild E, Elvevoll EO, Brox J, et al. Effects of dietary marine oils and olive oil on fatty acid composition, platelet membrane fluidity, platelet responses, and serum lipids in healthy humans. Lipids. 1998;33(4):427–36. doi: 10.1007/s11745-998-0224-8. [DOI] [PubMed] [Google Scholar]
- 28.Walker CG, West AL, Browning LM, et al. The pattern of fatty acids displaced by EPA and DHA following 12 months supplementation varies between blood cell and plasma fractions. Nutrients. 2015;7(8):6281–93. doi: 10.3390/nu7085285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skeaff CM, Hodson L, McKenzie JE. Dietary-induced changes in fatty acid composition of human plasma, platelet, and erythrocyte lipids follow a similar time course. J Nutr. 2006;136(3):565–69. doi: 10.1093/jn/136.3.565. [DOI] [PubMed] [Google Scholar]
- 30.Calder PC. Functional roles of fatty acids and their effects on human health. JPEN J Parenter Enteral Nutr. 2015;39(1 Suppl):18S–32S. doi: 10.1177/0148607115595980. [DOI] [PubMed] [Google Scholar]
- 31.Cruz MM, Lopes AB, Crisma AR, et al. Palmitoleic acid (16: 1n7) increases oxygen consumption, fatty acid oxidation and ATP content in white adipocytes. Lipids Health Dis. 2018;17:55. doi: 10.1186/s12944-018-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Onishi H, Saito Y. Eicosapentaenoic acid (EPA) reduces cardiovascular events: Relationship with the EPA/arachidonic acid ratio. J Ateroscler Thromb. 2013;20:861–77. doi: 10.5551/jat.18002. [DOI] [PubMed] [Google Scholar]
- 33.Li D, Wahlqvist ML, Sinclair AJ. Advances in n-3 polyunsaturated fatty acids. Asia Pac J Clin Nutr. 2019;28(1):1–5. doi: 10.6133/apjcn.201903_28(1).0001. [DOI] [PubMed] [Google Scholar]
- 34.Cottin SC, Alsaleh A, Sanders TAB, Hall WL. Lack of effect of supplementation with EPA or DHA on platelet-monocyte aggregates and vascular function in healthy men. Nutr Metab Cardiovasc Dis. 2016;26:743–51. doi: 10.1016/j.numecd.2016.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Lagarde M, Liu M, Véricel E, et al. Docosahexaenoic acid, protectin synthesis: Relevance against atherothrombogenesis. Poc Nutr Soc. 2014;73:186–89. doi: 10.1017/S0029665113003704. [DOI] [PubMed] [Google Scholar]
- 36.Li D, Turner A, Sinclair AJ. Relationship between platelet phospholipid FA and mean platelet volume in healthy men. Lipids. 2002;37(9):901–6. doi: 10.1007/s11745-002-0977-0. [DOI] [PubMed] [Google Scholar]
- 37.Véricel E, Polette A, Bacot S, et al. Pro- and antioxidant activities of docosahexaenoic acid on human blood platelets. J Thromb Haemost. 2003;1(3):566–72. doi: 10.1046/j.1538-7836.2003.00076.x. [DOI] [PubMed] [Google Scholar]
- 38.Véricel E, Colas R, Calzada C, et al. Moderate oral supplementation with docosahexaenoic acid improves platelet function and oxidative stress in type 2 diabetic patients. Thromb Haemost. 2015;114(2):289–96. doi: 10.1160/TH14-12-1003. [DOI] [PubMed] [Google Scholar]
- 39.Guillot N, Caillet E, Laville M, et al. Increasing intakes of the long-chain omega-3 docosahexaenoic acid: Effects on platelet functions and redox status in healthy men. FASEB J. 2009;23(9):2909–16. doi: 10.1096/fj.09-133421. [DOI] [PubMed] [Google Scholar]
- 40.Guichardant M, Bernoud-Hubac N, Calzada C, Véricel E. Oxygenation of polyunsaturated fatty acids and oxidative stress within blood platelets. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863(6):651–56. doi: 10.1016/j.bbalip.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 41.Chen H. Role of thromboxane A2 signaling in endothelium-dependent contractions of arteries. Prostaglandins Other Lipid Mediat. 2018;134:32–37. doi: 10.1016/j.prostaglandins.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Larson MK, Tormoen GW, Weaver LJ, et al. Exogenous modification of platelet membranes with the omega-3 fatty acids EPA and DHA reduces platelet procoagulant activity and thrombus formation. Am J Physiol Cell Physiol. 2013;304(3):C273–79. doi: 10.1152/ajpcell.00174.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Molfino A, Gioia G, Rossi Fanelli F, Muscaritoli M. The role for dietary omega-3 fatty acids supplementation in older adults. Nutrients. 2014;6(10):4058–73. doi: 10.3390/nu6104058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calder C. Mechanisms of action of (n-3) fatty acids. J Nutr. 2012;142(3):592S–99S. doi: 10.3945/jn.111.155259. [DOI] [PubMed] [Google Scholar]
- 45.Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. PNAS. 1997;94(9):4318–23. doi: 10.1073/pnas.94.9.4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 47.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: Nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60(9):502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 48.Klvanová J, Beno I, Ondreicka R, et al. Relation between fatty acid composition, vitamin E and malondialdehyde levels, and activity of antioxidant enzymes in the blood. Bratisl Lek Listy. 1998;99:245–49. [PubMed] [Google Scholar]


