Abstract
Background & Aims:
Diet may be an important factor in progression of Crohn’s disease (CD). We performed a randomized controlled trial to determine whether reduced consumption of red and processed meats decreases the risk of symptomatic relapse of CD, analyzing results from the Food and Crohn’s Disease Exacerbation Study (FACES) trial.
Methods:
Adults with CD were recruited into the FACES trial from IBD Partners, an internet-based cohort of IBD patients, from November 2013 through June 2015. Individuals who were in remission (CD activity index (sCDAI) scores of 150 or less), had completed a biannual survey, and reported consumption of red meat at least once weekly were randomly assigned to groups that consumed a minimum of 2 servings/week of red or processed meat (high meat, n=118) or not more than 1 serving per month (low meat, n=96) for 49 weeks. The primary outcome was relapse of CD, defined as increase in sCDAI score by ≥70 points and to >150 or a need for CD surgery or new CD medication. A secondary outcome, moderate or severe relapse, was based on an increase in sCDAI to >219
Results:
During the trial, the high-meat groups reported consumption of 2 or more servings of red or processed meat during 98.5% of observed weeks compared 18.8% of weeks for the low-meat group. Any and moderate to severe relapse occurred in 62% of participants in the high-meat group and 42% of participants in the low-meat group. There were no significant differences in time to any (P=.61) or moderate/severe (P=.50) relapse.
Conclusions:
In an analysis of data from the FACES trial, we found that among patients with CD in remission, level of red and processed meat consumption was not associated with time to symptomatic relapse. ClinicalTrials.gov Identifier:
Keywords: Inflammatory bowel disease, IBD, prevention, quiescent disease
Background
Crohn’s disease (CD) is an inflammatory disorder of the intestines, where host genetics, environmental factors, intestinal microbiome, epithelial barrier, the gut-brain axis, and innate and adaptive immune system contribute to the pathophysiology. Current evidence suggests that environmental factors, including diet, may be important in the development and progression of CD (1). Given that diet is modifiable, it has become an attractive potential target for both prevention and treatment of CD.
The role of diet in the management of CD is one of the most common questions that patients ask their physicians, yet high quality data to answer this question are limited. Defined formula-based diets are well established to be effective for the induction of remission in CD (2, 3). Several small trials of extreme restriction diets using regular food have also demonstrated improved disease activity and prolonged time to relapse (4–6). Additionally, two small studies suggest that a semi-vegetarian diet and a diet that restricts predominantly meat and eggs may prolong CD remission (7, 8). However, these have not been adequately tested in randomized controlled trials. These data, along with epidemiologic studies linking high dietary intake of total fats, PUFAs, omega-6 fatty acids, and meat with an increased risk of CD (9), led us to hypothesize that a diet characterized by lower meat intake would be associated with a more quiescent disease course. We sought to test the hypothesis that reduced consumption of red and processed meats decreases the risk of relapse of CD in the Food and Crohn’s Exacerbation Study (FACES), a prospective randomized trial.
Methods
Study Setting and Participants
Between 11/5/2013 and 6/30/2015 participants in the Crohn’s and Colitis Foundation Partners Study (which has since been renamed IBD Partners), an internet-based cohort of more than 15,600 participants with inflammatory bowel disease (IBD), who self-identified as having CD were recruited into FACES (10). See supplemental methods for additional details. Briefly, individuals with inflammatory bowel disease (IBD) who are older than 18 years of age were recruited to join IBD Partners using foundation e-mail rosters, social media, educational and fundraising events, and the Crohn’s & Colitis Foundation website. Each participant completed a baseline survey that contained questions about demographic characteristics, treatments, disease duration, and disease activity. Follow-up surveys were completed every 6 months after baseline to capture changes in disease activity and treatment since the prior survey.
To be included in the FACES Study, subjects must have been in symptomatic remission at the time of the most recent IBD Partners survey. Remission was defined as a short Crohn’s Disease Activity Index (sCDAI) of ≤150 (11) measured based on the patient’s estimate of his/her symptoms over the prior week. The sCDAI has been previously demonstrated to closely correlate with the original CDAI (11).
Randomization, Consent, and Exclusions
Randomization took place prior to recruitment so that the consent process could be tailored to the specific treatment arm, thus avoiding contamination by allowing the subject to know what the alternative diet entailed. Anticipating a higher participation rate in the high meat arm, we utilized a 3:2 randomization schedule with a target of achieving a 1:1 participation rate. Randomization was stratified by use of anti-TNFα medications. The randomization sequence was generated by the Biostatistical Analysis Center at the University of Pennsylvania.
Once randomized, subjects received an email invitation describing the study. Those who clicked “yes” to participate were led to a screening survey that asked additional questions to assess inclusion/exclusion criteria and also baseline dietary habits. Subjects were excluded if they reported consumption of red meat less than one time per week. See supplemental methods for additional exclusion criteria. If eligibility was confirmed, subjects were directed to an online, treatment arm-specific, consent form.
Treatments
Participants randomized to the intervention diet (referred to hereafter as low meat group) were instructed to follow their usual diet with the additional criteria, 1) To consume not more than 1 serving per month of red meat or processed meat and 2) To consume a minimum of 16 oz. of water per day. Participants randomized to the control diet (referred to hereafter as high meat group) were instructed to follow their usual diet with the additional criteria, 1) To consume a minimum of two servings of red meat or processed meat each week and 2) To consume a minimum of 16 oz. of water per day. Red meat was defined as all meat from livestock (12) and processed meats were any red or white meat that was prepared with smoking, salting, curing, or addition of preservatives. The definition of a serving was 3 oz., equivalent to the size of a small, lean hamburger. We included consumption of 16 oz. of water per day in both groups in order to provide a “placebo-like” intervention to the participants assigned to the control diet. Participants were instructed to follow their assigned diet for 49 weeks. Concomitant medications were continued at the discretion of the treating physician.
Assessment of Participants
We relied on participant self-report and IBD Partners records for demographic information, CD history, non-IBD related medical history, and medication history (see supplemental methods for additional details). We collected information on the participant’s usual diet, in the past month, at baseline and at week 20 using the Diet History Questionnaire II (DHQ II) from the National Cancer Institute.
Disease activity at baseline and throughout the trial was measured by the sCDAI (11). Every week, participants received one email with a link to a web-based survey that asked questions about disease status and adherence to study diet. At baseline and during weeks 9, 17, 25, 33, 41, and 49, instead of one survey, participants received a daily email for 7 days with a link to a web-based survey where they reported disease activity and current medications. At week 20, a subset of the participants was emailed a consent form to provide one stool sample that they collected and shipped from home directly to Genova Diagnostics for measurement of fecal calprotectin.
The primary outcome was symptomatic relapse of CD, defined as an increase in the sCDAI by >=70 points and to >150 or self-reported initiation or increase dose of an IBD medication (mesalamine, thiopurine, methotrexate, corticosteroid, anti-TNF-alpha, natalizumab) or surgery for a flare of CD. A secondary outcome of moderate to severe relapse of disease was defined the same but required an increase in the sCDAI to >219 in the absence of undergoing CD surgery or starting any new CD medication. A persistent relapse required participants to meet the definition of symptomatic relapse on two consecutive weeks.
In planning the sample size for this study, we considered a therapeutic benefit of 20% or greater with a dietary intervention to be clinically significant (13–15). We conservatively estimated the sample size requirements by using a dichotomous outcome of continued remission at all time points vs. relapse at any time point prior to the end of follow-up. Under this assumption, 97 patients per group provides a minimum of 80% power to detect a 20% absolute difference in relapse rates across the full range of possible relapse rates.
Statistical Analysis
Analyses were conducted using modified intention to treat, such that participants were analyzed according to the diet that they were assigned, even if they were non-adherent to the diet, except that we did not include subjects who refused participation once they learned of their assigned diet or failed to complete any follow-up surveys. Descriptive analyses utilized mean, standard deviation, median, interquartile range, counts, and percentages. Continuous and categorical variables were compared using the Wilcoxon rank sum test and the χ2 test, respectively. Principal component analysis was utilized to define overall dietary patterns. PERMANOVA using Euclidian distances was used to compare overall dietary composition at baseline between the treatment arms. Energy-adjusted linear regression with total calorie intake as the independent variable and raw nutrient or food intake as the dependent variable was used to compare intake between treatment arms. P-values were generated by comparing the residuals using Wilcoxon rank sum.
Statistical analysis of the primary outcome, time to symptomatic relapse, utilized Kaplan-Meier survival curves to display the relapse rate among the two study groups. Stratified Cox regression was used to determine the association of the study diet with the outcome, with use of anti-TNF therapy as the stratification factor. Participants who were lost to follow-up were censored at the time of last contact. Identical methods were used for the secondary outcomes.
Subgroup analyses, using Cox regression, were conducted to further explore the potential efficacy of the dietary intervention. These included the following subgroups: treatment at enrollment with an immunomodulator (azathioprine, mercaptopurine, or methotrexate) without an anti-TNF medication, treatment at enrollment with an anti-TNF therapy, treatment at enrollment with neither an immunomodulator or anti-TNF therapy, prior CD surgery, age <18 and age 18 or older at diagnosis with CD, and baseline red meat consumption above and below the median for the study population.
Adjusted Cox regression models were used to assess for confounding by differences in baseline dietary patterns. Missing data on baseline confounders were accounted for using multiple imputation. A sensitivity analysis was conducted assuming that all participants who agreed to participate but did not return any surveys (n=11) were considered to have relapsed at week 1. All authors had access to the study data and reviewed and approved the final manuscript.
Results
Participation rates and comparison of participants and non-participants
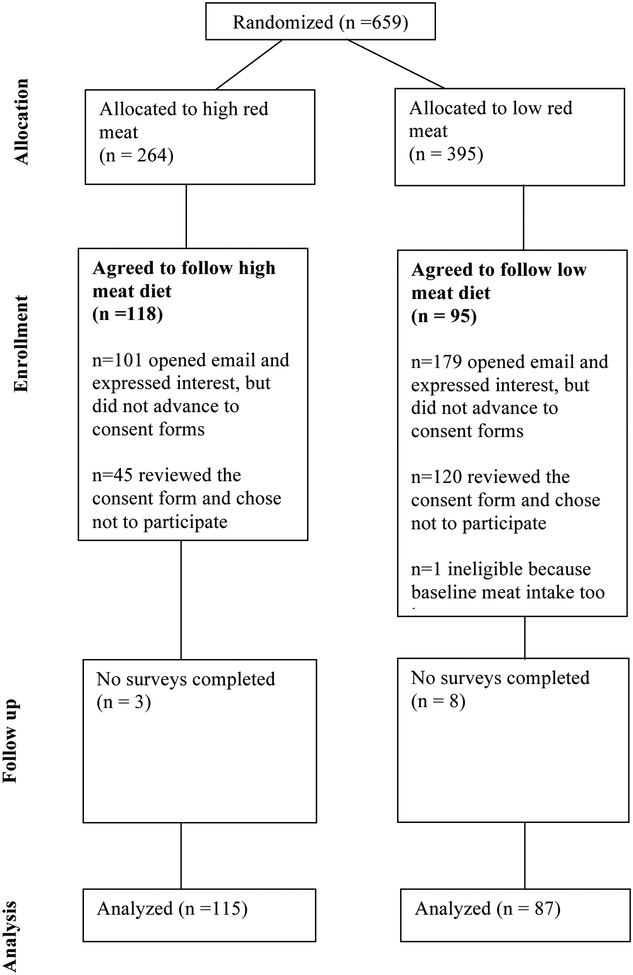
In total, 659 eligible subjects were randomized and invited to participate in the study, of whom 214 signed the consent form - 118 in the high meat arm and 96 in the low meat arm, one of whom was ineligible in the low meat arm due to baseline meat consumption that was too low, leaving 213 participants for analysis (Figure 1). Participants were more likely to be female, were young, had an early age at diagnosis, and were more commonly from the northeastern and less commonly from the western United States (Supplemental Table 1).
Figure 1.
FACES CONSORT diagram
Baseline characteristics of participants
The baseline characteristics were generally well balanced (Table 1). The median sCDAI was 75.5 (IQR 44.0 – 107.0) in the high meat group and 79.0 (44.0 – 121.0) in the low meat group. The median short IBDQ quality of life score was 5.8 (5.2 – 6.3) in both groups. Similar proportions of participants reported rarely active or absence of symptoms over the prior 6 months on the Manitoba index (48.7% of the high meat group and 49.5% of the low meat group). There was also a slightly higher proportion of participants who had ever been hospitalized in the low red meat group, but the median number of hospitalizations did not differ between the two groups. Medication use patterns were very similar between the groups.
Table 1.
Demographics and Baseline Characteristics
| High meat (n=118) | Low meat (n=95) | ||
|---|---|---|---|
| Sex | Female | 88 (74.6%) | 76 (80.0%) |
| Age at baseline | Median (Q1–Q3) | 37.0 (30.0–46.0) | 35.0 (28.0–50.0) |
| Age at IBD diagnosis | Median (Q1–Q3) | 24.0 (19.0–30.0) | 24.0 (17.0–33.0) |
| Race | White | 110 (97.3%) | 81 (92.0%) |
| Black/African American | 0 (0.0%) | 4 (4.5%) | |
| Asian | 1 (0.9%) | 1 (1.1%) | |
| More than one race | 2 (1.8%) | 2 (2.3%) | |
| US Census Bureau region | Northeast | 36 (31.6%) | 26 (28.3%) |
| Midwest | 35 (30.7%) | 22 (23.9%) | |
| South | 31 (27.2%) | 29 (31.5%) | |
| West | 12 (10.5%) | 15 (16.3%) | |
| Education level | Less than 12th grade | 1 (0.9%) | 1 (1.1%) |
| 12th grade | 4 (3.5%) | 1 (1.1%) | |
| Some college | 20 (17.4%) | 14 (15.1%) | |
| College | 54 (47.0%) | 41 (44.1%) | |
| Graduate school | 36 (31.3%) | 36 (38.7%) | |
| Saw GI doctor in past year | Never | 17 (15.9%) | 11 (12.4%) |
| 1 or 2 times | 60 (56.1%) | 51 (57.3%) | |
| 3 or 4 times | 20 (18.7%) | 17 (19.1%) | |
| 5 or more times | 10 (9.3%) | 10 (11.2%) | |
| Manitoba disease activity | Constantly active | 0 (0.0%) | 2 (2.1%) |
| Often active | 10 (8.5%) | 7 (7.4%) | |
| Sometimes active | 25 (21.4%) | 18 (18.9%) | |
| Occasionally active | 25 (21.4%) | 21 (22.1%) | |
| Rarely active | 26 (22.2%) | 27 (28.4%) | |
| remission/absence of symptoms | 31 (26.5%) | 20 (21.1%) | |
| Smoking history | never | 83 (70.3%) | 67 (70.5%) |
| former | 34 (28.8%) | 21 (22.1%) | |
| current | 1 (0.8%) | 7 (7.4%) | |
| Hx of IBD surgery | Yes | 54 (45.8%) | 45 (47.4%) |
| Ever hospitalized for IBD | Yes | 74 (62.7%) | 73 (76.8%) |
| Number of times hospitalized for IBD | Median (Q1–Q3) | 1.0 (0.0–2.0) | 1.0 (1.0–3.0) |
| Short CD Activity Index | Median (Q1–Q3) | 75.5 (44.0–107.0) | 79.0 (44.0–121.0) |
| Short IBD QOL score | Median (Q1–Q3) | 5.8 (5.2–6.3) | 5.8 (5.2–6.3) |
| Current use of aminosalicylates | Yes | 33 (28.0%) | 26 (27.7%) |
| Current use of steroids | Yes | 3 (2.5%) | 0 (0.0%) |
| Current use of immunosuppressants | Yes | 43 (36.4%) | 32 (34.0%) |
| Current use of biologics | Yes | 59 (50.0%) | 47 (50.0%) |
| Current use of antibiotics for IBD | Yes | 3 (2.5%) | 1 (1.1%) |
| Current use of narcotics for IBD | Yes | 7 (5.9%) | 7 (7.4%) |
| Current use of probiotics for IBD | Yes | 28 (23.7%) | 23 (24.5%) |
| PROMIS anxiety t-score | Median (Q1–Q3) | 48.0 (40.3–55.8) | 48.0 (40.3–55.8) |
| PROMIS depressive symptoms t-score | Median (Q1–Q3) | 41.0 (41.0–53.9) | 49.0 (41.0–51.8) |
| PROMIS fatigue t-score | Median (Q1–Q3) | 49.8 (46.0–57.0) | 48.6 (46.0–57.0) |
| PROMIS pain interference t-score | Median (Q1–Q3) | 41.6 (41.6–52.0) | 41.6 (41.6–53.9) |
| PROMIS sleep disturbance t-score | Median (Q1–Q3) | 52.4 (50.5–54.3) | 52.4 (50.5–54.3) |
| PROMIS social satisfaction t-score | Median (Q1–Q3) | 51.8 (48.2–64.4) | 51.8 (48.2–64.4) |
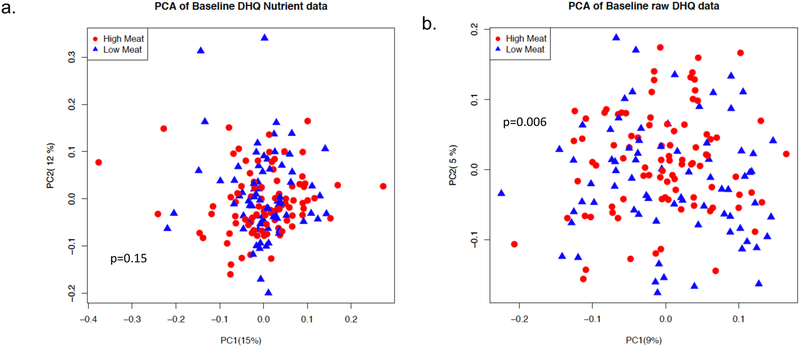
Of the 213 participants, 190 completed a DHQ II questionnaire at week 1 to assess baseline dietary pattern. From these, 16 participants were not included in the analysis because the DHQ II questionnaire was incomplete and 9 because of implausible caloric intake estimates. Figure 2a represents a 2-dimensional principal components analysis (PCA) with each data point representing a summary of the dietary pattern of a participant using all of the raw nutrient variables within the DHQ II, showing almost complete overlap of the two treatments groups (PERMANOVA p=0.15). A similar analysis using whole foods (i.e. how often the participant consumed a particular food in the last month regardless of quantity), while generally overlapping, demonstrated somewhat more divergence between the groups (PERMANOVA p=0.006) (Figure 2b). Further exploration of these data was conducted by comparing specific nutrients and foods of interest (Supplemental Table 2). The dietary patterns were quite similar, but differed in baseline red meat intake (mean red meat intake in ounces per day of 1.28 and 0.65 in the high meat and low meat groups, respectively, p=0.0002) (Supplemental Figure 1).
Figure 2.
Dietary pattern before and after the intervention
a. Principle component analysis of baseline dietary patterns using nutrient variables
b. Principle component analysis of baseline dietary patterns using whole foods variables
Primary and secondary outcomes
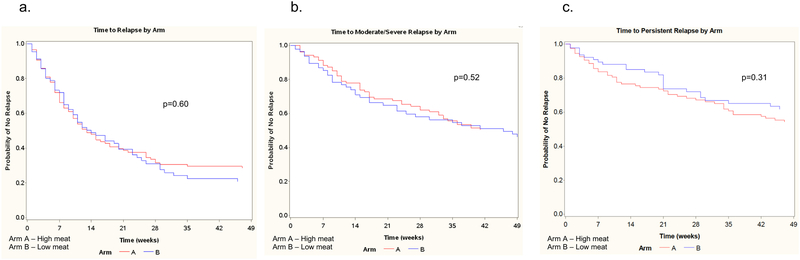
Overall, 78% of participants reached either the end of the study (week 49) or experienced an outcome. Of the 213 participants who signed consent, 11 did not complete any follow-up surveys and thus contributed no data to the analyses. The primary outcome, symptomatic relapse of CD, occurred in 62% of participants during the course of the study, while 42% and 35% had moderate-severe or persistent recurrence, respectively. Figure 3 shows the comparison of unadjusted time to symptomatic relapse (3a), time to moderate to severe symptomatic relapse (3b) and time to persistent relapse (3c) by arm. There were no significant differences in time to relapse for any of the outcomes (p>0.3 for all outcomes). Additionally, a sensitivity analysis, assuming that participants who did not complete any surveys relapsed at week 1, did not impact the results (data not shown).
Figure 3.
Comparison of time to symptomatic relapse by arm
a. Time to any symptomatic relapse
b. Time to moderate to severe symptomatic relapse
c. Time to persistent symptomatic relapse
Stratified Cox regression was used to determine the association of the baseline diet (using principle components and baseline red meat intake above and below the median) with the outcome and to assess for confounding, with use of anti-TNF-alpha therapy as the stratification factor. While this did not change the results in terms of the relationship between the study diets and any of the outcomes (Supplemental Table 3), baseline diet pattern in terms of nutrient intake, as measured by PC1, was strongly associated with the risk of symptomatic relapse with a hazard ratio of 13.46 (95% confidence interval 1.22 to 148.45, p=0.03). Analyses adjusted for baseline red meat consumption provided similar results to the primary analyses (data not shown). The relationship between the dietary intervention and the time to symptomatic relapse was generally similar to the primary analysis in each subgroup tested (Supplemental Table 4).
Adherence to the study diets
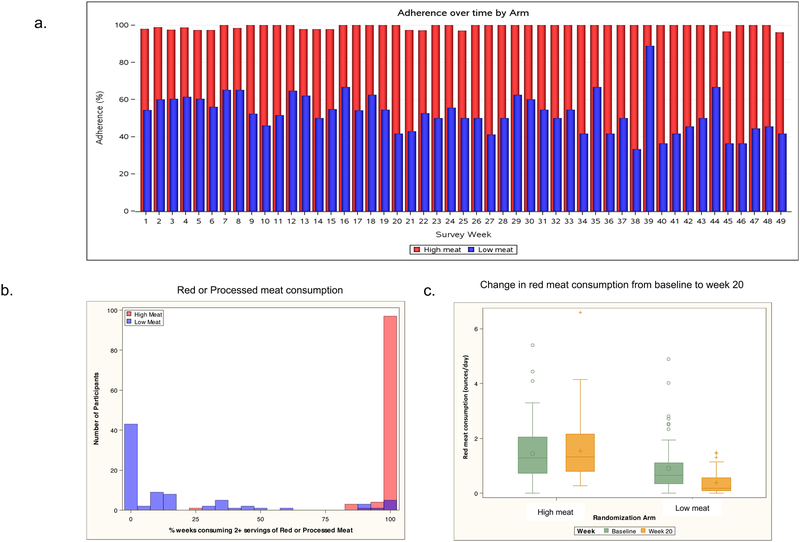
Adherence to the high meat diet, estimated by mean percentage of weeks consuming 2 or more servings of red or processed meat, was 98.5%; adherence to the low meat diet defined more rigorously as consuming no red or processed meat in the prior week averaged 57.3% (Figure 4a). However, consumption of red or processed meat differed substantially between the two groups during follow-up. The median percent of weeks that participants in the low meat group reported consuming 2 or more portions of red or processed meat was far lower than that for the high meat group (2.1%, IQR 0.0 – 30.4% vs 100%, IQR 100 – 100%) (Figure 4b). We fit a logistic regression model with generalized estimating equations, clustering on patient, for predicting consuming 2 or more servings of red/processed meat in the last week. Individuals in the high meat group were much more likely to consume 2 or more servings of red/processed meat in the last week (OR=340, 95% CI 130–886, p=<0.0001). Adherence to water consumption was 91.7% and 89.0% in the high meat and low meat groups, respectively.
Figure 4.
a. Comparison of adherence to the diet by treatment arm. The high meat group consumed 2+ servings of red or processed meat in 98.5% of weeks. The low meat group consumed 0 servings red or processed meat in 57.3% of weeks.
b. Percent of weeks that participants in each treatment group reported consuming two or more portions of red or processed meats
c. Change in consumption of red meat from baseline to week 20 by treatment arm
There were 24 participants (12 in each arm) who did not provide adequate adherence data, completing no or very few adherence surveys. 106 participants provided both baseline and week 20 DHQII questionnaires with plausible caloric intake estimates. There was no significant change in red meat consumption in the high meat group (median change -0.10 ounces per day, IQR -0.37 – 0.65, p=0.97), while in the low meat group there was a significant decrease in red meat consumption (median change 0.26 ounces per day, IQR 0.08 – 0.72, p<0.0001) (Figure 4c).
Supplemental Table 5 compares the nutrient data from the week 20 DHQII questionnaires between the two groups. The differences were generally consistent with the dietary interventions prescribed. Additionally, we examined within subject change in nutrient intake from baseline to week 20 by arm in the 106 participants (66 in the high meat arm and 40 in the low meat arm) who completed both baseline and week 20 DHQII measures and whose caloric intake was in a plausible range at both time points. Using the Wilcoxon rank sum test, the low meat arm had statistically significantly larger decreases in intake of calories (p=0.049), carbohydrates (p=0.038), protein (p=0.049), lean meat from meat, poultry, fish (p=0.007), meat from beef, pork, veal, lamb, and game (p=0.003), and meat from franks, sausage and luncheon meats (p=0.02).
In an exploratory analysis, we fit a Cox regression model examining time-updating adherence on time to relapse in the individuals randomized to the low meat arm. The predictor was a time-updating variable with a one-week lag. For example, when looking at outcome in week 20, the predictor included percent of weeks that the individual consumed zero servings of red or processed meat during weeks 1–19. We excluded week 1 and weeks with missing adherence data were considered “non-adherent.” We found that for every 10% increase in adherence to the low meat diet, the risk of relapse increased by 8% (HR=1.075, 95% CI 0.99–1.17, p=0.0864).
Fecal calprotectin levels
At week 20, 18 participants in each arm submitted a stool sample for fecal calprotectin. The high meat arm had a higher median (74.5 mcg/g, IQR 37 – 133) fecal calprotectin compared to the low meat arm (36.0 mcg/g, IQR 17 – 78), but this was not statistically significant by Wilcoxon rank sum (p=0.13) (supplemental Figure 2). Additionally, there was no significant difference in the proportion of participants who had a fecal calprotectin >150 or >250 by arm (Fisher’s exact p=1.0 for both).
Discussion
In the FACES randomized controlled trial, we sought to determine whether a diet that reduces red and processed meat consumption decreases the risk of symptomatic CD relapse. In this study, participants in the low meat group reported consuming 2 or more servings of red and/or processed meat far less frequently than the high meat group. Additionally, the low meat group significantly decreased their average weekly red meat consumption during the study. Despite these clear differences in diets, there were no statistically significant differences in time to relapse for any of the outcomes suggesting that reduction of red and processed meats does not reduce the risk of symptomatic CD relapse in patients with quiescent disease.
Existing data have led to a hypothesis that diet, and particularly red meat consumption, may be associated with relapse of CD. However, nearly all of the prior data are from observational rather than interventional studies. In a study from the IBD Partners cohort, red meat was one of the foods that patients with CD reported to worsen symptoms and it was commonly avoided (16). However, dietary pattern is a complex construct since certain foods tend to be consumed together and foods also contain additives, contaminants, chemical products of preparation, etc. (17). It is possible that in the FACES study, the level of adherence to the low meat diet led to a dietary intervention which was less extreme than what is required to demonstrate a difference in time to relapse. Perhaps a diet completely devoid of red and processed meat is required to reduce the rate of CD flares and that simply reducing one’s intake is not enough. Similarly, we did not include an intervention arm without any meat as most patient directed recommendations focus on consumption of lean meats (18) and reducing only red and processed meats is more practical for patients. Indeed, although diet is hypothesized to be an environmental risk factor for IBD pathogenesis through its effects on the gut microbiome, existing studies of dietary interventions and the gut microbiome have generally shown modest effects on gut microbiota composition, particularly in the short-term, with the exception of very extreme elimination diets (19). Alternatively, when patients reduce red and processed meat in their diet, it must be replaced with some other food. It is possible that the participants in the trial tended to replace red and processed meat with another food item that has a deleterious effect on CD.
A unique aspect of this trial was the implementation within an internet-based cohort. The growing use of the internet and social media provides investigators with an opportunity to conduct pragmatic trials in larger populations at a fraction of the cost. This study took advantage of IBD Partners, an online cohort, to identify, recruit, enroll and follow-up patients. The patients were recruited from the entire country, not from the vicinity of a major medical center and there was no direct contact with the treating physicians. Behavioral interventions, such as dietary modification, are likely the most well suited to this design, as it does not entail changing the patient’s medication regimen. Similarly, because the study design did not involve direct contact with the patient or the treating physician, we focused on prevention of relapse. Studies of interventions for active disease would require a more complex to design. This study can serve as a model for future research on diet and other environmental factors suspected of influencing IBD relapse or other chronic relapsing diseases.
Our study has several limitations. Only one third of individuals who were randomized signed the consent form. However, unlike most trials, the use of IBD Partners allowed us to demonstrate that the characteristics of the participants and non-participants were very similar. Participants were not blinded to which arm they were assigned. Another limitation was the potential for misclassification of IBD diagnosis, baseline disease activity and/or dietary pattern. We used validated measures, when possible, such as the sCDAI and FFQ. Additionally, CD diagnosis has previously been shown to be highly valid in the IBD Partners cohort (10). Another limitation is the potential for enrollment bias due to methods of recruitment, interest in participating, requirement for reading English, and the technology required to join the e-cohort (10). Finally, the outcomes were based on symptoms rather than endoscopy. Fecal calprotectin was measured in a small subset and there was no significant difference between the groups; this would be expected since calprotectin has been consistently demonstrated to predict future symptomatic relapse (20).
In conclusion, we have demonstrated the feasibility of executing a randomized controlled trial of a dietary intervention to prevent relapse of symptoms in patients with CD using IBD Partners, an internet-based cohort. This randomized controlled trial demonstrated that substantial reduction of red and processed meat consumption among patients with asymptomatic CD was not efficacious in reducing time to symptomatic relapse. Based on these results, there is insufficient evidence to recommend reduction of red and processed meat consumption solely for the purpose of improving CD outcomes, although there may be some benefit for other health conditions.
Supplementary Material
Acknowledgments
This research was supported by grants from the Crohn’s and Colitis Foundation and from the NIH (P30 DK034987, K24-DK078228, and K23 DK109136)
Footnotes
Potential Conflicts of Interest:
Dr. Albenberg has received research funding from Seres Therapeutics.
Dr. Lewis has served as a consultant for: Nestle Health Science, Johnson & Johnson Consumer Inc, Janssen Pharmaceuticals, Takeda, AbbVie, Merck, Celgene, Eli Lilly and Company, Samsung Bioepis, Pfizer, Gilead, UCB, Arena Pharmaceuticals and Bridge Biotherapeutics. He has received research funding from Nestle Health Science, Takeda, and Janssen.
Dr. Kappelman has received research funding from and serves as a consultant for Johnson & Johnson Consumer Inc, Abbvie, Pfizer, and Eli Lilly. He is a shareholder to Johnson & Johnson Consumer Inc.
References
- 1.Ananthakrishnan AN. 2013. Environmental risk factors for inflammatory bowel disease. Gastroenterol Hepatol (N Y) 9:367–74. [PMC free article] [PubMed] [Google Scholar]
- 2.Lochs H, Dejong C, Hammarqvist F, Hebuterne X, Leon-Sanz M, Schutz T, van Gemert W, van Gossum A, Valentini L, Lubke H, Bischoff S, Engelmann N, Thul P. 2006. ESPEN Guidelines on Enteral Nutrition: Gastroenterology. Clin Nutr 25:260–74. [DOI] [PubMed] [Google Scholar]
- 3.Sandhu BK, Fell JM, Beattie RM, Mitton SG, Wilson DC, Jenkins H. 2010. Guidelines for the Management of Inflammatory Bowel Disease in Children in the United Kingdom. J Pediatr Gastroenterol Nutr doi: 10.1097/MPG.0b013e3181c92c53. [DOI] [PubMed] [Google Scholar]
- 4.Riordan AM, Hunter JO, Cowan RE, Crampton JR, Davidson AR, Dickinson RJ, Dronfield MW, Fellows IW, Hishon S, Kerrigan GN, et al. 1993. Treatment of active Crohn’s disease by exclusion diet: East Anglian multicentre controlled trial. Lancet 342:1131–4. [DOI] [PubMed] [Google Scholar]
- 5.Bartel G, Weiss I, Turetschek K, Schima W, Puspok A, Waldhoer T, Gasche C. 2008. Ingested matter affects intestinal lesions in Crohn’s disease. Inflamm Bowel Dis 14:374–82. [DOI] [PubMed] [Google Scholar]
- 6.Jones VA, Dickinson RJ, Workman E, Wilson AJ, Freeman AH, Hunter JO. 1985. Crohn’s disease: maintenance of remission by diet. Lancet 2:177–80. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran N, Kumar D. 2011. Food-specific IgG4-guided exclusion diets improve symptoms in Crohn’s disease: a pilot study. Colorectal Dis 13:1009–13. [DOI] [PubMed] [Google Scholar]
- 8.Chiba M, Abe T, Tsuda H, Sugawara T, Tsuda S, Tozawa H, Fujiwara K, Imai H. 2010. Lifestyle-related disease in Crohn’s disease: relapse prevention by a semi-vegetarian diet. World J Gastroenterol 16:2484–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hou JK, Abraham B, El-Serag H. 2011. Dietary intake and risk of developing inflammatory bowel disease: a systematic review of the literature. Am J Gastroenterol 106:563–73. [DOI] [PubMed] [Google Scholar]
- 10.Long MD, Kappelman MD, Martin CF, Lewis JD, Mayer L, Kinneer PM, Sandler RS. 2012. Development of an internet-based cohort of patients with inflammatory bowel diseases (CCFA Partners): methodology and initial results. Inflamm Bowel Dis 18:2099–106. [DOI] [PubMed] [Google Scholar]
- 11.Thia K, Faubion WA Jr., Loftus EV Jr., Persson T, Persson A, Sandborn WJ. 2011. Short CDAI: development and validation of a shortened and simplified Crohn’s disease activity index. Inflamm Bowel Dis 17:105–11. [DOI] [PubMed] [Google Scholar]
- 12.Moubarac JC, Parra DC, Cannon G, Monteiro CA. 2014. Food Classification Systems Based on Food Processing: Significance and Implications for Policies and Actions: A Systematic Literature Review and Assessment. Curr Obes Rep 3:256–72. [DOI] [PubMed] [Google Scholar]
- 13.Colombel JF, Sandborn WJ, Rutgeerts P, Enns R, Hanauer SB, Panaccione R, Schreiber S, Byczkowski D, Li J, Kent JD, Pollack PF. 2007. Adalimumab for maintenance of clinical response and remission in patients with Crohn’s disease: the CHARM trial. Gastroenterology 132:52–65. [DOI] [PubMed] [Google Scholar]
- 14.Ford AC, Achkar JP, Khan KJ, Kane SV, Talley NJ, Marshall JK, Moayyedi P. 2011. Efficacy of 5-aminosalicylates in ulcerative colitis: systematic review and meta-analysis. Am J Gastroenterol 106:601–16. [DOI] [PubMed] [Google Scholar]
- 15.Rutgeerts P, Sandborn WJ, Feagan BG, Reinisch W, Olson A, Johanns J, Travers S, Rachmilewitz D, Hanauer SB, Lichtenstein GR, de Villiers WJ, Present D, Sands BE, Colombel JF. 2005. Infliximab for induction and maintenance therapy for ulcerative colitis. N Engl J Med 353:2462–76. [DOI] [PubMed] [Google Scholar]
- 16.Cohen AB, Lee D, Long MD, Kappelman MD, Martin CF, Sandler RS, Lewis JD. 2013. Dietary patterns and self-reported associations of diet with symptoms of inflammatory bowel disease. Dig Dis Sci 58:1322–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis JD, Albenberg L, Lee D, Kratz M, Gottlieb K, Reinisch W. 2017. The Importance and Challenges of Dietary Intervention Trials for Inflammatory Bowel Disease. Inflamm Bowel Dis 23:181–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hou JK, Lee D, Lewis J. 2014. Diet and inflammatory bowel disease: review of patient-targeted recommendations. Clin Gastroenterol Hepatol 12:1592–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ. 2014. Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heida A, Park KT, van Rheenen PF. 2017. Clinical Utility of Fecal Calprotectin Monitoring in Asymptomatic Patients with Inflammatory Bowel Disease: A Systematic Review and Practical Guide. Inflamm Bowel Dis 23:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.