Watch a video presentation of this article
Watch the interview with the author
Answer questions and earn CME
Abbreviations
- AE
adverse event
- AFP
α‐fetoprotein
- BCLC
Barcelona Clinic Liver Cancer
- CCR4
chemokine receptor 4
- CI
confidence interval
- CTLA‐4
cytotoxic T lymphocyte‐associated antigen 4
- DLTs
dose limiting toxicity
- ECOG PS
Eastern Cooperative Oncology Group performance status
- EHS
extrahepatic spread
- FDA
US Food and Drug Administration
- FGFR
fibroblast growth factor receptor
- FGFR4
fibroblast growth factor receptor 4
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- Hsp90
heat shock protein 90
- IDO1
indolamine2,3‐dioxygenase 1
- IHC
immunohistochemistry
- mRECIST
modified response evaluation criteria in solid tumors
- MTD
maximum tolerated dose
- ORR
objective response rate
- OS
overall survival
- PD‐1
programmed death‐1
- PDGFR
platelet‐derived growth factor receptor
- PDGFRB
platelet‐derived growth factor receptor β
- PD‐L1
programmed death ligand 1
- RP2D
recommended phase II dose
- TGF‐βR1
transforming growth factor‐β receptor 1
- VEGFA
vascular endothelial growth factor A
- VEGFR
vascular endothelial growth factor receptor
- VEGFR2
vascular endothelial growth factor receptor 2
- VEGFR3
vascular endothelial growth factor receptor 3
Liver cancer is a major health problem, being the second leading cause of cancer‐related death worldwide,1 with an annual incidence of more than 850,000 new cases globally.2 Hepatocellular carcinoma (HCC) represents 85% to 90% of all primary liver cancers and occurs mainly in the setting of chronic inflammatory liver diseases.2 Only 40% to 50% of patients with HCC are diagnosed at early stages (Barcelona Clinic Liver Cancer [BCLC] 0‐A) amenable to potentially curative approaches.2 However, up to 70% of patients present with disease recurrence within 5 years,2 and no adjuvant therapies to prevent this complication are available to date. Patients diagnosed at an intermediate stage (BCLC B) are treated with transarterial chemoembolization,2, 3 whereas 40% of patients are diagnosed at an advanced stage (BCLC C) and can benefit from systemic therapies.3 In this scenario, the approval of sorafenib in 2007 was followed by several unsuccessful phase III trials assessing novel targeted therapies and locoregional therapies, such as radioembolization,4, 5 that did not fulfill the primary overall survival (OS) endpoints. From 2016 to 2018, five new drugs (lenvatinib, regorafenib, cabozantinib, ramucirumab, and nivolumab) showed clinical efficacy and have been adopted by guidelines3, 6 (Table 1; Figs. 1 and 2). We herein review the systemic treatments available for advanced HCC that have revolutionized the management of this devastating cancer.
Table 1.
Successful Phase III Trials in Advanced HCC
| Trial | Arms | N | OS | |
|---|---|---|---|---|
| Median (months) | HR (95% CI) | |||
| First‐line | ||||
| SHARP | Sorafenib versus placebo | 299 vs. 303 | 10.7 vs. 7.9 | 0.69 (0.55‐0.87) |
| Asian‐Pacific | Sorafenib versus placebo | 150 vs. 76 | 6.5 vs. 4.2 | 0.68 (0.50‐0.93) |
| REFLECT* | Lenvatinib versus sorafenib | 478 vs. 476 | 13.6 vs. 12.3 | 0.92* (0.79‐1.06) |
| Second‐line | ||||
| RESORCE | Regorafenib versus placebo | 379 vs. 194 | 10.6 vs. 7.8 | 0.63 (0.50‐0.79) |
| CELESTIAL | Cabozantinib versus placebo | 470 vs. 237 | 10.2 vs. 8 | 0.76 (0.63‐0.92) |
| REACH‐2 | Ramucirumab versus placebo | 197 vs. 95 | 8.5 vs. 7.3 | 0.71 (0.53‐0.95) |
Positive study for noninferiority design.
Figure 1.
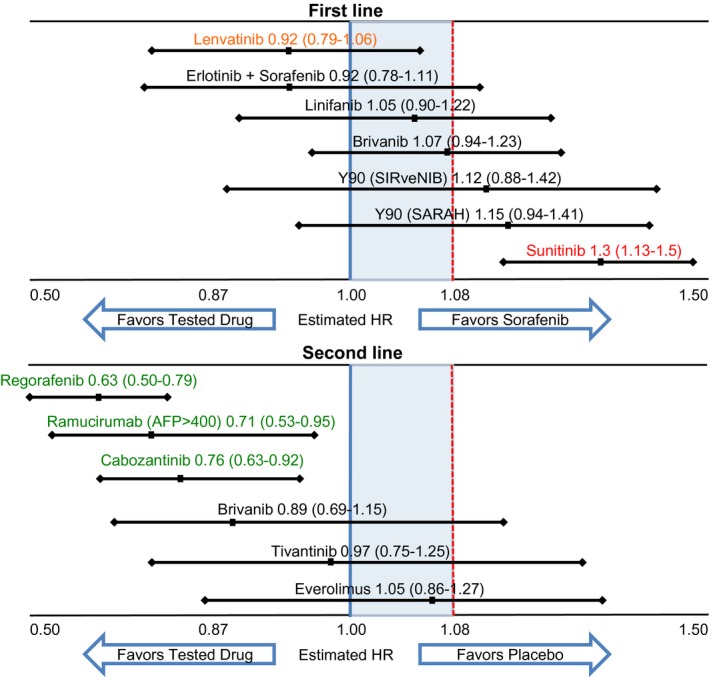
Overall survival outcomes of phase III clinical trials testing molecular targeted therapies or radioembolization in patients with advanced‐stage hepatocellular carcinoma. Adapted from Llovet JM, et al. Nat Rev Clin Oncol 2018.3 The figure illustrates the estimated overall survival hazard ratios (HRs) and 95% confidence intervals for the experimental drug (or combination) versus either sorafenib in the first‐line setting or placebo in the second‐line setting. Green text indicates positive results from trials with a superiority design. Orange text indicates positive results from trials with a non‐inferiority design. Black text and red text represent negative results with a HR confidence interval crossing or not crossing 1, respectively. Blue and red lines refer to the upper limit for superiority and non‐inferiority, respectively.
Figure 2.
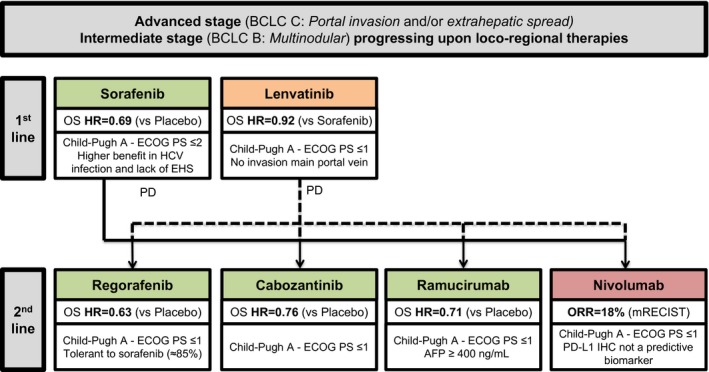
Treatment strategy for advanced hepatocellular carcinoma. Adapted from Llovet JM, et al. Nat Rev Clin Oncol 2018.3 Drugs in green have positive results from phase III trials with a superiority design (sorafenib in the first‐line setting and regorafenib, cabozantinib and ramucirumab in the second‐line setting). Drugs in orange have positive results from phase III trials with a non‐inferiority design (lenvatinib in the first‐line setting). Drugs in red have received accelerated approval from the FDA on the basis of promising efficacy results in phase II trials (nivolumab in the second‐line setting). Key details of the patient populations are provided.
First‐Line Therapies
Sorafenib
Sorafenib is a multikinase inhibitor that exerts antiproliferative (RAF1, BRAF, and KIT), antiangiogenic (vascular endothelial growth factor receptor [VEGFR] and platelet‐derived growth factor receptor β [PDGFRB]), and proapoptotic effects. Sorafenib has shown antitumor activity in phase III trials in patients with advanced HCC. Both the SHARP trial7 conducted in western countries and the Asian‐Pacific trial8 demonstrated survival benefits of sorafenib versus placebo (median OS 10.7 versus 7.9 months; SHARP trial: hazard ratio [HR] 0.69; 95% confidence interval [CI]: 0.55‐0.87; P < 0.001; Asian‐Pacific trial: 6.5 versus 4.2 months; HR 0.68; 95% CI: 0.50‐0.93; P = 0.014). This represented a breakthrough in the management of advanced stage HCC. Sorafenib is indicated as a first‐line treatment option for patients with advanced tumors (BCLC C) or tumors at an intermediate stage (BCLC B) with a well‐preserved liver function (Child‐Pugh A) that progressed upon locoregional therapies. A greater magnitude of benefit is obtained in patients without extrahepatic spread and with hepatitis C virus etiology.8
Lenvatinib
Lenvatinib is an oral multikinase inhibitor of VEGFRs 1 to 3, fibroblast growth factor receptors (FGFRs) 1 to 4, RET, KIT, and PDGFRα. A recent phase III trial in HCC (REFLECT9) demonstrated noninferiority OS benefit of lenvatinib versus sorafenib (median OS 13.6 versus 12.3 months; HR 0.92; 95% CI: 0.79‐1.06) and showed an improvement in secondary efficacy endpoints, including objective response (24% lenvatinib versus 9% sorafenib as per modified response evaluation criteria in solid tumors [mRECIST]). Of note, patients with 50% of liver occupation, clear invasion into the bile duct, and main portal vein invasion were not included in this trial.
Second‐Line Therapies
Regorafenib
Regorafenib is an oral multikinase inhibitor that has a similar mechanism of action to sorafenib but with a greater potency against the VEGFR kinases and a broader activity, for example, against angiopoietin 1 receptor (TIE2), KIT, and RET. A phase III trial (RESORCE) in patients with advanced HCC that progressed and were tolerant to sorafenib demonstrated an impact on survival with regorafenib (7.8 months with placebo to 10.6 months with regorafenib; HR 0.63; 95% CI: 0.50‐0.79; P < 0.0001). Median treatment duration was 3.6 months with regorafenib and 1.9 months with placebo.10 The precise mechanism by which regorafenib improves survival after sorafenib progression is unknown.
Cabozantinib
Cabozantinib is a small‐molecule multitarget tyrosine kinase inhibitor that inhibits VEGFRs with a potent inhibitory effect against MET and AXL. CELESTIAL11 was a global, randomized, placebo‐controlled phase III trial of cabozantinib in patients who had HCC progression on prior sorafenib. The trial revealed a median OS of 10.2 months for cabozantinib versus 8.0 months in the placebo arm (HR 0.76; 95% CI: 0.63‐0.92; P = 0.0049) and hence was stopped after a second interim analysis. The most frequent grade 3/4 adverse event (AE) observed in the cabozantinib group was palmar‐plantar erythrodysesthesia (17% versus 0% in the placebo arm).
Ramucirumab
Ramucirumab is an anti‐VEGFR2 monoclonal antibody. A phase III trial (REACH12) involving patients with advanced stage HCC after prior treatment with sorafenib showed negative results for its primary endpoint of OS, although a subgroup of patients with a baseline serum alpha‐fetoprotein (AFP) level ≥400 ng/mL demonstrated a significant improvement in median OS from 4.2 months with placebo to 7.8 months with ramucirumab. Based on this observation, a second phase III trial of ramucirumab in the second‐line setting (REACH‐213) was performed incorporating biomarker‐based enrichment for patients with baseline AFP concentrations ≥400 ng/dL. Results of this trial have demonstrated an improvement in OS of 8.5 months with ramucirumab versus 7.3 months for placebo (HR 0.710; 95% CI: 0.531‐0.949; P = 0.0199). Therefore, ramucirumab represents the first agent with a demonstrated clinical benefit in a biomarker‐selected population of patients with HCC.
Immune Checkpoint Inhibitors
Immunotherapies have changed the landscape of cancer treatment and have provided hope to patients with advanced tumors. Regulatory agencies have approved immune checkpoint inhibitors in several solid tumors including melanoma, lung cancer, renal cancer, and bladder cancer. These drugs enhance antigen‐specific T cell responses, unleashing the potential of the preexisting antitumor immune response.
Nivolumab
Nivolumab is a monoclonal antibody directed against the negative immunoregulatory human cell surface receptor programmed death‐1 (PD‐1). A large phase I/II study14 of nivolumab including 262 patients with HCC with or without previous exposure to sorafenib showed an objective response rate (ORR) of 14% by RECIST (18% by mRECIST). The median OS for patients in second‐line therapy was 15.6 months.15 Based on these results, the US Food and Drug Administration (FDA) granted accelerated approval to nivolumab for patients with advanced stage HCC previously treated with sorafenib. A confirmatory open‐label, randomized phase III trial comparing sorafenib versus nivolumab in the front‐line setting is ongoing (CheckMate 459; NCT02576509); patient accrual is complete, and the results are eagerly awaited.
Pembrolizumab
Pembrolizumab is another anti–PD‐1 monoclonal antibody that has shown antitumor activity with a safe profile in several cancers.16 The efficacy and safety of this drug has been evaluated in a phase II clinical trial (KEYNOTE‐224) in 104 patients with advanced HCC previously treated with sorafenib. This trial showed an overall response rate of 17% (according to RECIST version 1.1) with a median time to progression and progression‐free survival of 4.9 months and a median OS of 12.9 months. However, a randomized phase III clinical trial (KEYNOTE‐240; NCT02702401) that will assess pembrolizumab versus placebo as a second‐line therapy in advanced HCC is ongoing in several countries.16 Longer‐term follow‐up data on the former phase II as well as the results of the phase III trial are awaited.
Final Conclusions and Future Perspectives
There has been a revolution in the management of patients with advanced HCC. The efficacy of the seven drugs described in this review increases the antitumor armamentarium, which is being translated in encouraging outcomes with sequential therapies. However, up to date, apart from patients with high AFP level (>400 ng/dL) who could benefit from ramucirumab, there are no other available biomarkers to predict patient response or allow the choice of one drug over another for the treatment of HCC. In addition, programmed death ligand 1 (PD‐L1) expression by immunohistochemistry did not show to be useful in HCC.
In the future and considering the possibility that the results of the phase III CheckMate‐459 trial assessing nivolumab versus sorafenib are positive, this anti–PD‐1 monoclonal antibody will become the standard of care in advanced HCC, causing the current front‐line sorafenib and the recently approved lenvatinib to move to the second line, leaving, in this hypothetical scenario, the current second‐line drugs as a third option for the treatment of this dismal disease.
Currently, combinations of kinase inhibitors and immunotherapies are emerging as tools to boost responses of the immune system against HCC‐derived neoantigens (Table 2). These unprecedented outcomes (objective responses >45%) resulted in breakthrough therapy designation for bevacizumab and atezolizumab by the FDA.17 Overall, major improvements are expected in the coming years, particularly if biomarkers of response or resistance to therapies are identified.
Table 2.
Ongoing Trials of Targeted and Immune Therapies Combinations for HCC*
| Drugs | Targets | Clinical Stage | Enrichment | Phase (Comparator) | Endpoint | NCT |
|---|---|---|---|---|---|---|
| Atezolizumab + bevacizumab | PD‐L1/VEGFA | Advanced first line | No | III (sorafenib) | OS | NCT03434379 |
| Durvalumab +/− tremelimumab | PD‐L1/CTLA‐4 | Advanced first line | No | III (sorafenib) | OS | NCT03298451 |
| Nivolumab +/− ipilimumab | PD‐1/CTLA‐4 | Neoadjuvant | No | II | AEs | NCT03222076 |
| Galunisertib + sorafenib | TGF‐βR1/VEGFRs, C‐KIT, PDGFRB, RAF | Advanced first line | No | II | OS | NCT02178358 |
| Mogamulizumab + nivolumab | CCR4/PD‐1 | Advanced second line | No | I‐II | MTD | NCT02705105 |
| Pembrolizumab + epacadostat | PD‐1/IDO1 | Advanced second line | No | I‐II | DLTs | NCT02178722 |
| Galunisertib + nivolumab | TGF‐βR1/PD‐1 | Advanced second line | AFP > 200 ng/mL | I‐II | MTD | NCT02423343 |
| Apatinib + SHR1210 | VEGFR2/PD‐1 | Advanced second line | No | I‐II | OS | NCT02942329 |
| Spartalizumab +/− capmatinib | PD‐1/MET | Advanced second line | No | I‐II | DLTs | NCT02795429 |
| FGF401 +/− spartalizumab | FGFR4/PD‐1 | Advanced second line | FGFR4+ KLB+ | I‐II | DLTs | NCT02325739 |
| Pembrolizumab + sorafenib | PD‐1/VEGFRs, C‐KIT, PDGFRB, RAF | Advanced first line | No | I‐II | ORR | NCT03211416 |
| Pembrolizumab + lenvatinib | PD‐1/VEGFR2, VEGFR3 | Advanced second line | No | I | DLTs | NCT03006926 |
| Spartalizumab + sorafenib | PD‐1/VEGFRs, C‐KIT, PDGFRB, RAF | Advanced first line | No | I | AEs | NCT02988440 |
| Regorafenib + pembrolizumab | VEGFRs, FGFRs, C‐KIT, PDGFRs, RAF/PD‐1 | Advanced first line | No | I | AEs | NCT03347292 |
| Cabozantinib + nivolumab | MET, VEGFRs/PD‐1 | Neoadjuvant | No | I | AEs | NCT03299946 |
| Avelumab + axitinib | PD‐L1/VEGFRs, C‐KIT, PDGFRs | Advanced first line | No | I | AEs | NCT03289533 |
| Ramucirumab + durvalumab | VEGFR2/PD‐L1 | Advanced second line | AFP > 1.5× upper limit of normal | I | DLTs | NCT02572687 |
| XL888 + pembrolizumab | Hsp90/PD‐1 | Advanced second line | No | I | RP2D | NCT03095781 |
Data were accessed in January 2018 on the ClinicalTrials.gov database. Keyword searches for “hepatocellular carcinoma” were used to identify active clinical trials that started in the last 5 years investigating combination systemic targeted therapies.
J.M.L. was supported by grants from the European Commission Horizon 2020 Program (HEPCAR, proposal number 667273‐2), US Department of Defense (CA150272P3), National Cancer Institute (P30 CA196521), Spanish National Health Institute (SAF 2016‐76390), Cancer Research UK–Asociación Española Contra el Cáncer (Accelerator Award‐HUNTER‐A26813), Samuel Waxman Cancer Research Foundation, and Generalitat de Catalunya (AGAUR, SGR‐1358). R.M. is supported by a Fundación Sociedad Española de Oncología Médica (FSEOM)‐Boehringer Ingelheim grant. C.M. is a recipient of a Josep Font grant from Hospital Clinic de Barcelona.
Potential conflict of interest: J.M.L. receives research support from Bayer HealthCare Pharmaceuticals, Eisai Inc., Bristol‐Myers Squibb, and Ipsen, and consulting fees from Eli Lilly, Bayer HealthCare Pharmaceuticals, Bristol‐Myers Squibb, Eisai Inc., Celsion Corporation, Exelixis, Merck, Ipsen, Glycotest, Navigant, Leerink Swann LLC, Midatech Ltd., Fortress Biotech, Sprink Pharmaceuticals, and Nucleix
References
- 1. Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin 2015;65:87‐108. [DOI] [PubMed] [Google Scholar]
- 2. Llovet JM, Zucman‐Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- 3. Llovet JM, Montal R, Sia D, et al. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599‐616. [DOI] [PubMed] [Google Scholar]
- 4. Vilgrain V, Pereira H, Assenat E, et al.; SARAH Trial Group . Efficacy and safety of selective internal radiotherapy with yttrium‐90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open‐label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624‐1636. [DOI] [PubMed] [Google Scholar]
- 5. Chow PKH, Gandhi M, Tan SB, et al.;Asia‐Pacific Hepatocellular Carcinoma Trials Group . SIRveNIB: selective internal radiation therapy versus sorafenib in Asia‐Pacific patients with hepatocellular carcinoma. J Clin Oncol 2018;36:1913‐1921. [DOI] [PubMed] [Google Scholar]
- 6. Zucman‐Rossi J, Villanueva A, Nault JC, et al. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology 2015;149:1226‐1239. [DOI] [PubMed] [Google Scholar]
- 7. Llovet JM, Ricci S, Mazzaferro Z, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378‐390. [DOI] [PubMed] [Google Scholar]
- 8. Bruix J, Cheng AL, Meinhardt G, et al. Prognostic factors and predictors of sorafenib benefit in patients with hepatocellular carcinoma: analysis of two phase III studies. J Hepatol 2017;67:999‐1008. [DOI] [PubMed] [Google Scholar]
- 9. Kudo M, Finn RS, Qin S, et al. Lenvatinib versus sorafenib in first‐line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non‐inferiority trial. Lancet 2018;391:1163‐1173. [DOI] [PubMed] [Google Scholar]
- 10. Bruix J, Qin S, Merle P, et al.; RESORCE Investigators . Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet 2017;389:56‐66. [DOI] [PubMed] [Google Scholar]
- 11. Abou‐Alfa GK, Meyer T, Cheng AL, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med 2018;379:54‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second‐line treatment in patients with advanced hepatocellular carcinoma following first‐line therapy with sorafenib (REACH): a randomised, double‐blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859‐870. [DOI] [PubMed] [Google Scholar]
- 13. Zhu AX, Kang Y‐K, Yen C‐J, et al. REACH‐2: a randomized, double‐blind, placebo‐controlled phase 3 study of ramucirumab versus placebo as second‐line treatment in patients with advanced hepatocellular carcinoma (HCC) and elevated baseline alpha‐fetoprotein (AFP) following first‐line sorafe. J Clin Oncol 2018;36(Suppl. 15):4003. [Google Scholar]
- 14. El‐Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open‐label, non‐comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492‐2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El‐Khoueiry AB, Melero I, Yau TC, et al. Impact of antitumor activity on survival outcomes, and nonconventional benefit, with nivolumab (NIVO) in patients with advanced hepatocellular carcinoma (aHCC): subanalyses of CheckMate‐040. J Clin Oncol 2018;36(Suppl. 4):475. [Google Scholar]
- 16. Zhu AX, Finn RS, Edeline J, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE‐224): a non‐randomised, open‐label phase 2 trial. Lancet Oncol 2018;19:940‐952. [DOI] [PubMed] [Google Scholar]
- 17. Genentech . FDA grants breakthrough therapy designation for Genentech’s TECENTRIQ in combination with avastin as first‐line treatment for advanced or metastatic hepatocellular carcinoma (HCC). Available at: https://www.gene.com/media/press-releases/14736/2018-07-17/fda-grants-breakthrough-therapy-designat. Published July 17, 2018.


