Abstract
Background.
Right ventricular (RV) pacing-induced cardiomyopathy (PICM) occurs in ~30% of patients with RV leads. Here, we evaluate the long-term effects of restoring antegrade conduction with a biological pacemaker in a porcine model of RV PICM.
Objectives.
To determine if antegrade biological pacing can attenuate RV PICM
Methods.
In pigs with complete atrioventricular (AV) block, TBX18 was injected into the His bundle region in either of two experimental protocols: protocol A sought to prevent PICM, while protocol B sought to reverse PICM. In protocol A, we injected adenoviral vectors expressing TBX18 (or the reporter construct GFP) after AV node ablation, and followed the animals for 8 weeks. In protocol B, PICM was established by AV node ablation and 4 weeks of electronic RV pacing, at which point TBX18 was injected in the His bundle region.
Results.
In protocol A, TBX18 biological pacing led to superior chronotropic support (62.4±3 bpm vs 50.4±0.4 bpm, p=0.01), lower back-up pacemaker utilization (45±2.6% vs 94.6±1.4%, p=0.001), and greater ejection fraction (EF; 58.5±1.3% vs 46.7±2%, p=0.001). In protocol B, full-blown RV PICM was evident 4 weeks after complete AV block in both groups; subsequent intervention led to higher mean HR(56±2 bpm vs 50.10±0.4bpm, p=0.05), less backup pacemaker utilization(53±8.2% vs 95±1.6%, p=0.003), and a greater EF(61.7±1.3 vs 49±1.6%, p=0.0003) in TBX18-injected animals vs. GFP controls.
Conclusions.
In a preclinical model, pacemaker-induced cardiomyopathy can be prevented, and reversed, by restoring antegrade conduction with TBX18 biological pacing.
Keywords: Biological Pacemaker, Gene therapy, His Bundle pacing
Condensed Abstract
Right ventricular (RV) pacing-induced cardiomyopathy (PICM) occurs in ~30% of RV-paced patients. Bi-ventricular pacing and His-bundle pacing have been used to treat these patients and those with Heart Failure. Here we evaluated the efficacy of delivery of TBX18 biological pacemaker (BioP) into the His-bundle region in a swine model of PICM. With restoration of anterograde conduction by TBX18 BioP, and reducing electronic RV pacing dependency, we observed improvement in mechanical dyssynchrony, and systolic function. Additionally, we observed improvement in QRS duration, fibrosis, and adverse molecular remodeling associated with PICM. These findings indicate that restoring antegrade conduction byTBX18 BioP can ameliorate the adverse changes induced by PICM.
For the past 6 decades, conduction system disorders, including high-grade atrioventricular (AV) block, have been treated with electronic pacemakers. The most common configuration includes a pacing electrode in the right ventricle (RV) apex for ventricular excitation (1). The electrical impulse thus created travels in a retrograde manner through the myocardium, producing a left bundle branch block electrocardiographic (ECG) pattern reflective of dyssynchronous activation (2). Long-term RV apical pacing has been associated with an increased risk of pacing-induced cardiomyopathy (PICM), which in turn predisposes to overt heart failure [HF] and atrial fibrillation (AF) (3–6). PICM is not only associated with contractile deficits, but also adverse left ventricular (LV) remodeling, LV dilatation, asymmetrical hypertrophy, left atrial enlargement and decreased exercise capacity (3–5,7–10). The concept of His bundle pacing originated in 1967(11) but was used only sporadically until recently, when interest has resurged. Normal antegrade conduction through the His-Purkinje system results in physiological synchronous activation of the ventricles, avoiding RV pacing-induced dyssynchrony (12), and preserving LV function (13). Additionally, antegrade pacing can reverse the decline in LV systolic function seen in patients with PICM(14). The incidence of LV dysfunction associated with pacing-induced cardiomyopathy varies, depending on currently accepted clinical definitions. Most recently, three different definitions of PICM have been established (15). Kiehl et al. noted that a pacing burden greater than 20% as well as a lower pre-pacemaker implantation LVEF were important factors in predicting the development of PICM, whereas other studies suggest >40% pacing burden as a relevant predictor (16–19). In the Mode Selection Trial (MOST) an increase in pacing burden of 10% translated to an increased hospitalization rate of 20% (5). An increased burden of ventricular pacing therefore appears to be a major risk factor for developing PICM.
Here, we tested if PICM could be prevented, or reversed, by long-term (1–2 months) restoration of antegrade conduction with TBX18 biological pacemaker (BioP) delivered to the His bundle region in a preclinical model. Ectopic expression of the human embryonic transcription factor T-box 18 (TBX18) can convert ventricular myocytes into induced sino-atrial node (iSAN) cells, creating a BioP(20). We used a minimally-invasive delivery technique to achieve in vivo somatic reprogramming of ventricular myocytes in the para-Hissian region, in a pig model of complete heart block(20). Two experimental protocols were tested sequentially. First, using an early intervention protocol we evaluated the long-term safety and efficacy of adenoviral TBX18 gene delivery into the His bundle region in pacemaker-dependent pigs. The overall goal was to prevent PICM. In a second protocol, we tested His bundle region BioP in pigs with established PICM, to “rescue” the induced LV dysfunction. In both protocols, randomized control pigs received injections of an adenoviral GFP vector. Phenotyping included ambulatory monitoring for heart rate, rhythm and physical activity, as well as post-injection evaluations of structure and function by cardiac magnetic resonance imaging (MRI).
Materials and Methods
Study design
20 Yorkshire-SPF farm pigs were utilized to evaluate the long-term safety and efficacy of human transcription factor TBX18, as a suitable candidate for a BioP. We implemented a porcine model of complete chronic AV block. In protocol A, we injected adenoviral vectors expressing TBX18 (or the reporter construct GFP) immediately after AV node ablation and followed the animals for 8 weeks. In protocol B, PICM was established by AV node ablation and 4 weeks of electronic RV pacing, at which point TBX18, GFP, or vehicle was injected in the His bundle region. Animals were phenotyped by MRI, by electrocardiographic monitoring and by endpoint histology and QPCR. Please see online data supplement for methodological details.
Statistical analysis.
Data are presented as means ± SEM. A two-tailed t test was used to compare animals that received TBX18 vs animals that received GFP or PBS as a control. For all time points evaluated by repeated-measures, analysis of variance (ANOVA) was used. A rank sum test was performed to confirm results from data that was not normally distributed.
Results
Protocol A: Prevention of PICM
Electrophysiology
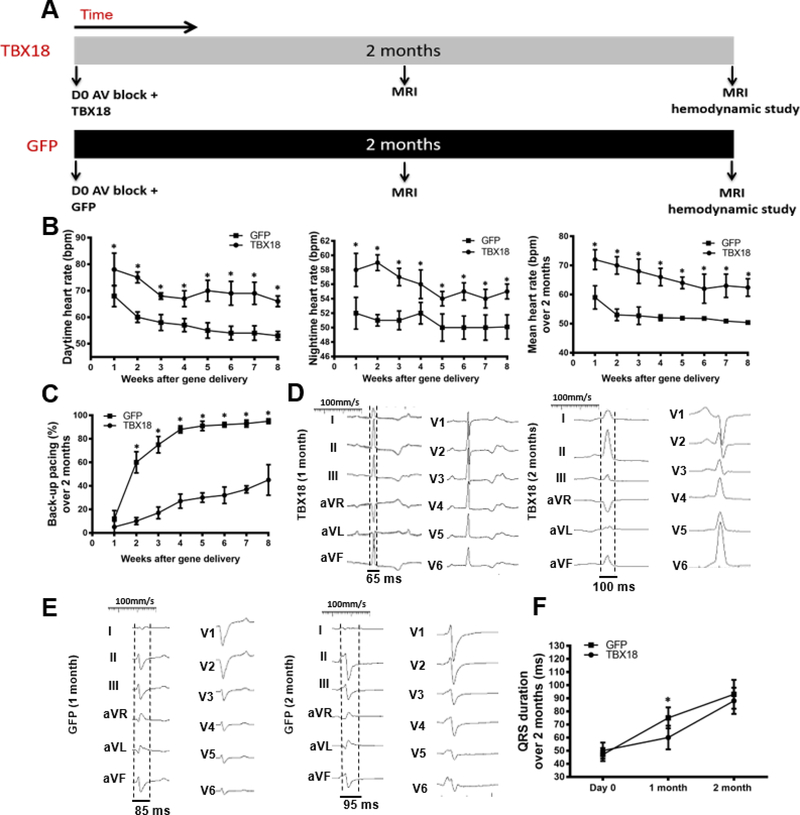
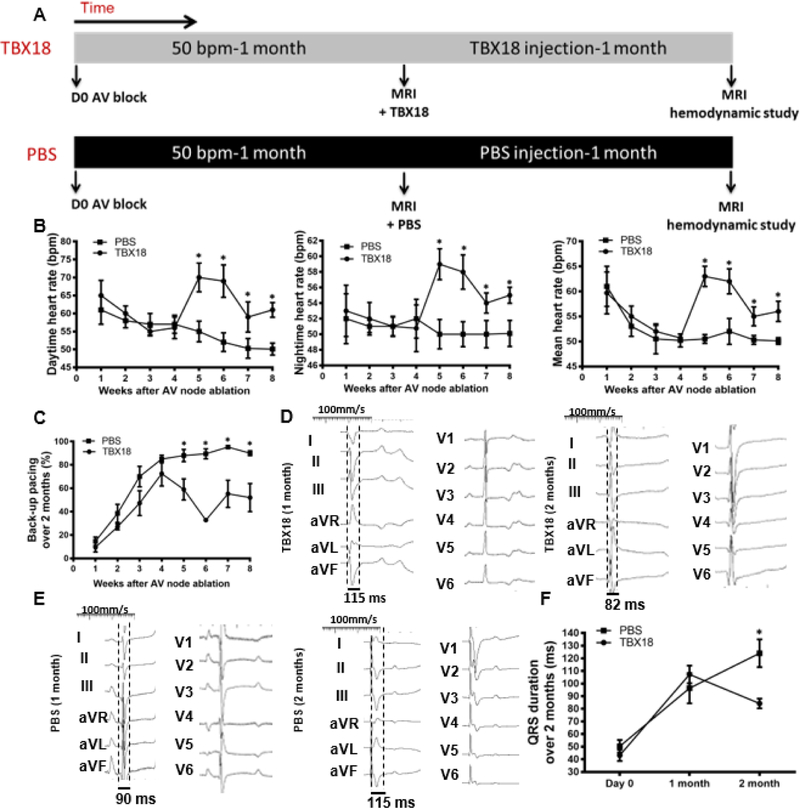
Figure 1A depicts schematically the schedule of events in the early intervention protocol (protocol A). Following implantation of a backup electronic pacemaker, complete heart block was induced by RF ablation of the AV node. Animals were then injected with either TBX18 or GFP and followed for 8 weeks. As soon as week one following TBX18 gene transfer, diurnal (day/night) as well as mean heart rate (HR) was higher in animals injected TBX18 compared to GFP-injected controls, and remained so over the entire course of the study (Day: 70.3±1.5bpm vs 57.4±1.7bpm, p<0.0001; Night: 56.1±0.6bpm vs 50.7±0.4bpm, p=0.01; Mean: 62.4±3bpm vs 50.4±0.4bpm at 8 weeks, p=0.01) (Figure 1B). Likewise, TBX18-injected animals required backup electronic pacing less frequently than did GFP controls during the study and at endpoint (45%±2.6% vs 94.6±1.4%. p=0.001) (Figure 1C, Online Figure 1–group A). Backup pacemaker utilization trended upward over the course of 8 weeks in the TBX18 group but remained significantly lower than in GFP controls. Thus, TBX18 creates robust BioP activity, minimizing the need for back-up electronic pacing(20). Above and beyond prior demonstrations of BioP activity for 2 weeks, here we find sustained chronotropic benefit of TBX18 injection for the duration of the 2-month study. To index overall electrical synchrony, we measured electrocardiographic QRS duration monthly. One month following AV block and gene transfer, GFP-treated pigs had wider QRS complexes, on average, compared to TBX18 animals, but there was no difference in QRS duration at the end of the study (Figure 1D–F).
Figure 1: Group A - Early intervention protocol: changes in heart rate, backup electronic pacemaker utilization, and electrical remodeling in TBX18 antegrade biological pacemaker-treated animals compared to controls.
(A) Early intervention protocol comparing TBX18 gene transfer vs. vector control labeled with GFP. 10 animals received AV node ablation followed by delivery of either TBX18, n=6, or GFP n=4 with a functional evaluation at 2 months by MRI. (B) Diurnal and mean 24-hr heart rate over the course of the study. Endpoint heart rate was superior in TBX18 pigs (Day: 57.4±1.7bpm, GFP, vs 70.3±1.5bpm TBX18, p<0.0001, night: 50.7±0.4bpm GFP, vs 56.1±0.6bpm TBX18, p=0.01, mean: 50.4±0.4bpm GFP, vs 62.4±3bpm TBX18 p=0.01 (C) Electronic pacemaker usage over 2 months following gene transfer, endpoint pacing ratio was higher in control pigs (94.6±1.4% GFP, 45± 2.6% TBX18, p<0.001). (D-E) Representative 12-lead QRS morphology in both groups at endpoint. (F) Group A QRS duration over the 2-month study (75±8ms, GFP, vs. 60±9ms TBX18 p=0.05), and at endpoint (93±11ms GFP, 88±10ms TBX18, p=0.4). The increase in QRS duration is consistent with at junctional escape rhythm at endpoint. *P < 0.05 for all time points by repeated-measures analysis of variance (ANOVA).
Ventricular function 2 months post AV block
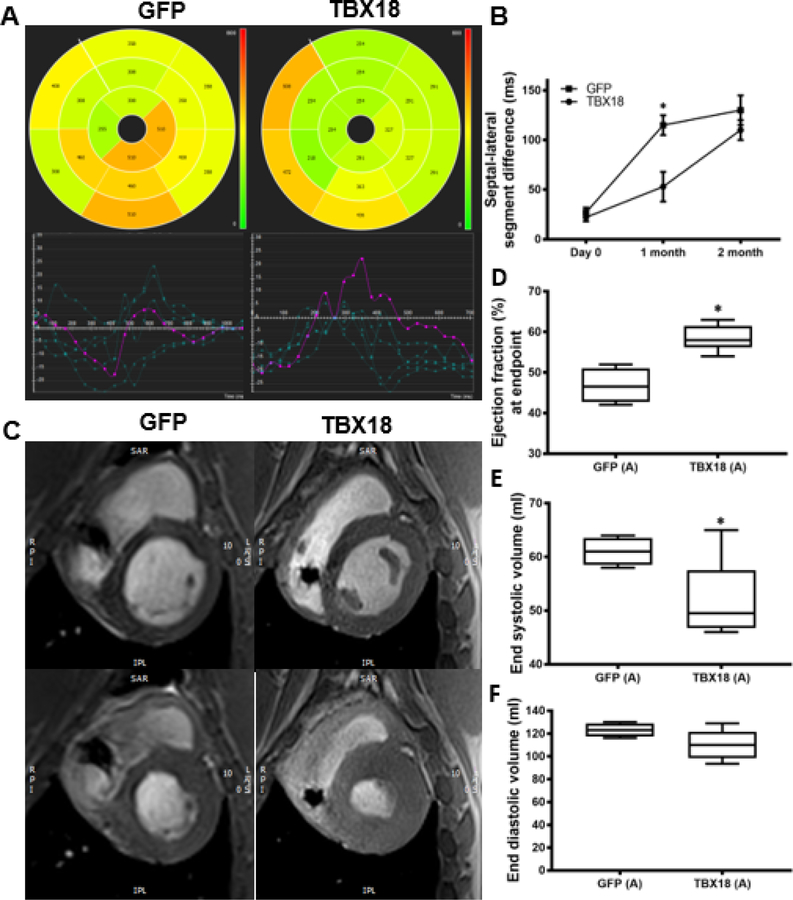
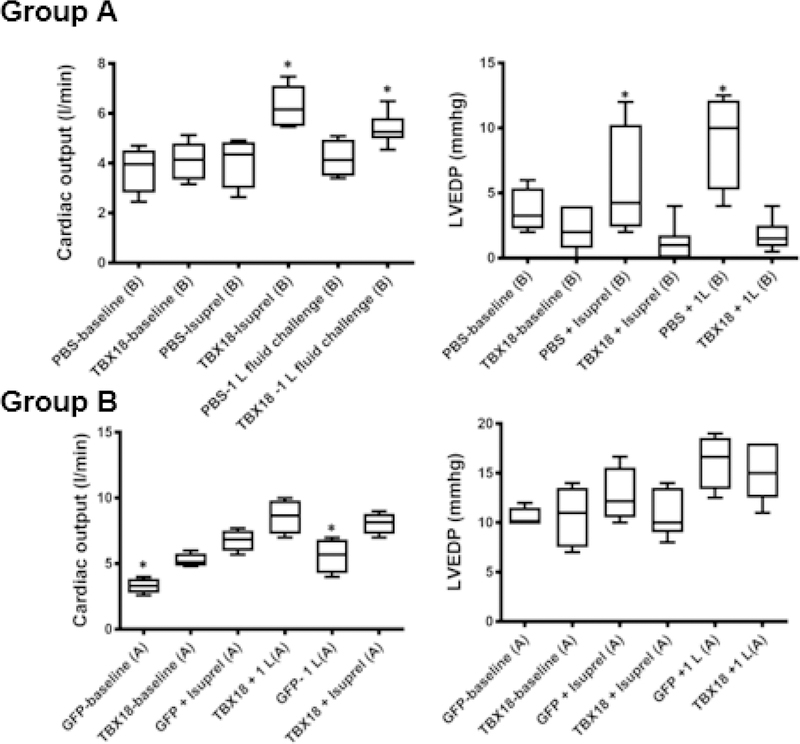
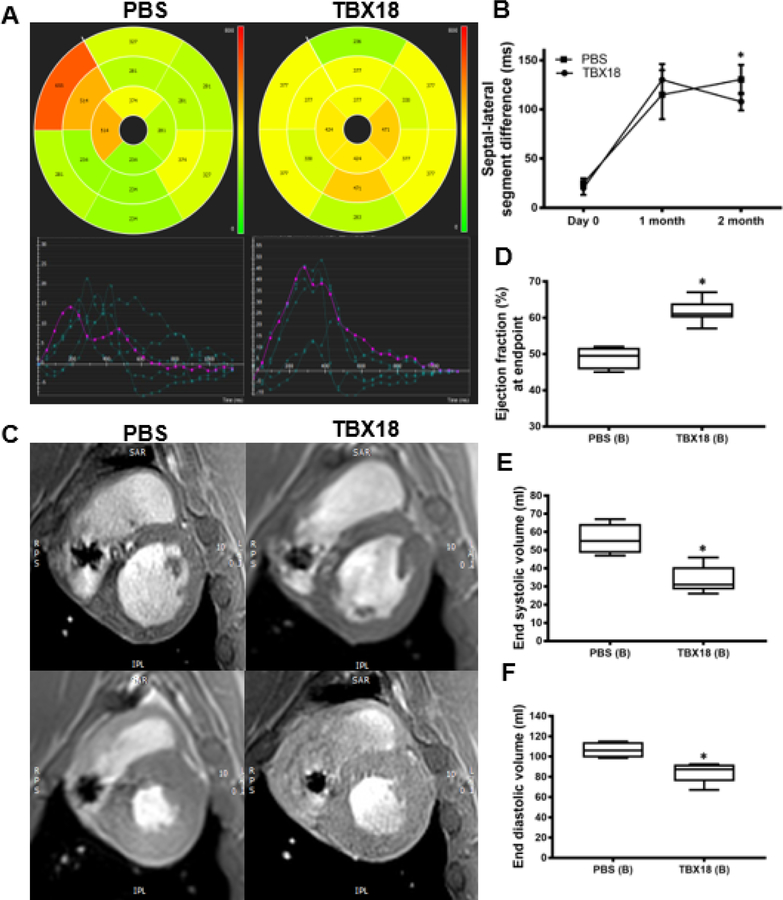
To assess functional and structural changes, each animal underwent cardiac MRI 1 and 2 months post AV block. In addition to standard MRI quantification of left ventricular ejection fraction (LVEF) and left ventricular volumes, myocardial tissue tracking was used to evaluate the time to peak radial strain of the interventricular septum and lateral free wall. This parameter reports dyssynchrony as septo-lateral segment differences. After one month of AV block, TBX18-treated pigs demonstrated more synchronous contraction as reflected by smaller septolateral segment differences in TBX18 animals (53±15ms) than in GFP controls (115±10ms, p=0.001). Representative polar maps were created to provide a 2D interpretation of the 3D curvilinear endocardial/epicardial surface of the left ventricle short-axis using AHA segmentation with corresponding values (Figure 2A–B). Although differences in mechanical dyssynchrony faded subsequently, there was nevertheless a sustained increase in LVEF at endpoint: animals injected with TBX18 maintained a higher LVEF (58.5±1.3%) compared to GFP controls (46.7±2%, p=0.001). This is demonstrated by representative short-axis MR images at end systole, and end-diastole in both TBX18 and GFP injected pigs (Figure 2C–D). Meanwhile, TBX18 injection conferred salutary reductions in end systolic volume (52±3ml vs 61.1±1.2ml, p=0.04) and a similar trend for end diastolic volume (110±5ml vs 123±3, p=0.1) (Figure 2E–F). At endpoint, cardiac output (CO) under general anesthesia measured by thermodilution was higher in TBX18 pigs (3.3±0.28l/min GFP vs 5.25±2.6l/min TBX18 p=0.0025), with further increases during isoproterenol infusion (6.77±0.5 l/min GFP vs 8.1±0.42 l/min TBX18 p=0.06) or after saline infusion (5.6±0.68 l/min GFP vs 8.6±0.51 l/min TBX18 p=0.02). There were no significant differences in left ventricular end-diastolic pressure (LVEDP) at endpoint during baseline (10.5±.350mmhg, GFP vs 10.6±1.36mmhg, TBX18 p=0.95), following isoproterenol (12.74±1.4mmhg GFP vs 11±1.1mmhg TBX18 p=0.3), or after the 1 L fluid challenge (16.2±1.4mmhg, GFP vs 15.2±5.31mmhg TBX18 p=0.6) (Figure 3).
Figure 2: Group A – Early intervention protocol: changes in mechanical dyssynchrony, and, and left ventricular systolic function in TBX18 antegrade biological pacemaker treated-animals compared to controls.
(A) 2D polar maps of radial strain with AHA segmentation representing time to peak of each segment with plots (below) depicting the time to peak radial strain of individual chords (cvi42®). (B) Septal-lateral segment difference was better in TBX18 pigs one month following AV block (115±10ms GFP, 53±15ms TBX18, P=0.001) (C) Representative post processed MRI (cvi42®) identifying end diastolic (top) and end systolic (bottom) phases (D) Endpoint ejection fraction was higher in TBX18 animals (46.75±2% GFP, 58.5±1.3% TBX18, p=0.001). (E-F) Additionally, TBX18 pigs demonstrated lower end systolic (61.1±1.2ml GFP, 52±3ml TBX18 p=0.04) and end diastolic volume (123±3ml GFP, 110±5ml TBX18, p=0.1)
Figure 3: Changes in cardiac output and left ventricular end-diastolic pressure in TBX18 antegrade biological pacemaker-treated animals compared to controls:
(A) Cardiac output and LV end-diastolic pressure at baseline, following β adrenergic stimulation with isoproterenol, and following a 1L fluid challenge, TBX18 vs control. Measured by thermodilution, CO was better in TBX18 pigs in protocol A at baseline (3.3±0.28l/min GFP vs 5.25±2.6l/min TBX18, p=0.0025). Following β adrenergic stimulation with isoproterenol, TBX18 pigs showed a trend of an increased adrenergic response compared to controls (6.77±0.5 l/min GFP vs 8.1±0.42 l/min TBX18 p=0.06). Following isoproterenol animals recovered and were then given a 1L fluid bolus of saline. CO measurements were taken again showing superior ventricular function in TBX18 pigs (5.6±0.68 l/min GFP vs 8.6±0.51 l/min TBX18 p=0.02). There were no differences in LV EDP in protocol A, baseline (10.5±.350mmhg, GFP vs 10.6±1.36mmhg, TBX18, p=0.95), after isoproterenol (12.74±1.4mmhg GFP vs 11±1.1mmhg TBX18, p=0.3) and the 1L fluid challenge (16.2±1.4mmhg, GFP vs 15.2±5.31mmhg TBX18, p=0.6) (B). In protocol B, cardiac output under was similar at baseline (3.77±0.47l/min PBS vs 4.11±0.53l/min TBX18, p=0.5), however after 10 minutes of β adrenergic stimulation, CO in TBX18 pigs was significantly increased from baseline, relative a modest increase in control animals (4.1±0.51l/min PBS vs 6.30±0.33l/min TBX18 p=0.004). β adrenergic stimulation was discontinued, 1 L of normal saline was infused. After the 1L fluid challenge, BioP animals showed a higher CO compared to controls (4.1±0.37l/min PBS vs 5.4±0.26l/min TBX18 p=0.02). LVEDP was the same in both groups at baseline (3.6±0.8mmhg PBS vs 4±0.3mmhg TBX18, p=0.6), however was increased in controls following β adrenergic stimulation (5.62±2.21mmhg PBS vs 3.91±0.27mmhg TBX18, p=0.3), and the 1L fluid challenge (9.12±1.85mmhg PBS vs 3.75±0.41mmhg TBX18 p=0.008).
Activity
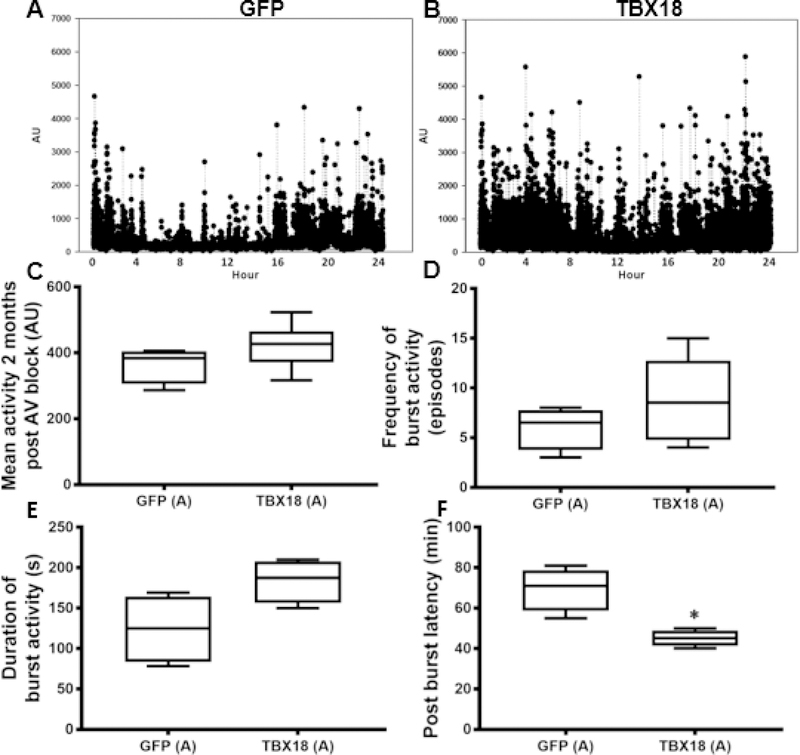
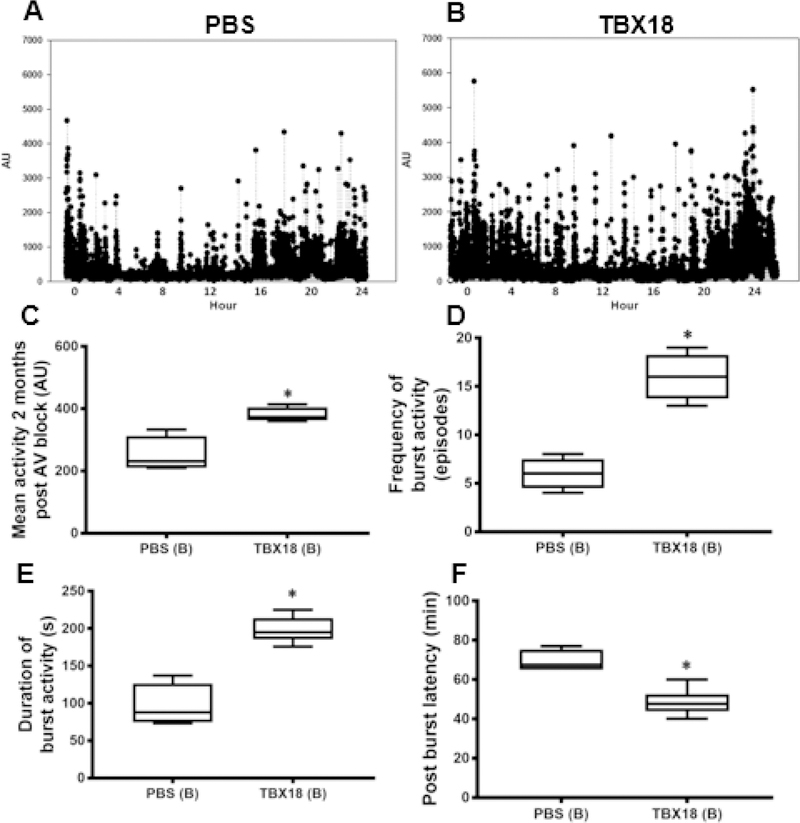
Chronotropic support, if systemically impactful, is often associated with improved mobility. Figure 4A and 4B show examples of activity recordings over a 24-hour period at endpoint. Analysis of such data revealed a trend towards greater spontaneous activity in TBX18 pigs (Figure 4C) (365±27au GFP, 421.5±27.7au TBX18 p=ns). Bursts of high activity (>2000a.u.) were more frequent and lasted longer in TBX18 pigs (Figure 4 D, E). Perhaps the most telling indication of increased perfusion is the observed decrease in the latency period, i.e. the recovery time between bursts of activity, in the TBX18 group relative to controls (69.53min GFP, 45±2min TBX18 p=0.005) (Figure 4F). In group A, at the one-month timepoint following injection where the QRS is narrow, there was elevated activity observed in the TBX18 pigs compared to controls, however the data was not significantly different (GFP= 323.8±25 a.u.,TBX18= 399±31.90 a.u., p=0.14) Taken together, these findings indicate some systemic functional benefit of restoring antegrade conduction with TBX18 BioP.
Figure 4: Group A – Early intervention protocol: changes in physical activity in TBX18 antegrade biological pacemaker-treated animals compared to controls.
(A-B) Representative 24-hour activity plots at endpoint from an implanted accelerometer. (C) Mean 24-hour activity at endpoint (365±27au GFP, 421.5±27.7au TBX18 p=0.1). (D-E) Burst activity was considered spontaneous high levels of activity (>2000 a.u.). TBX18 animals demonstrated higher frequency (6 ±1 episodes GFP, 8.8±1 episodes TBX18, p=NS) and duration of burst activity (124±21.2s GFP, 183.8±13.4s TBX18, p=0.06). (F) Describes the period between high levels of burst activity. The latency period between bursts of activity was superior in TBX18 pigs (69.53min GFP, 45±2min TBX18, p=0.005).
Histological analysis of remodeling
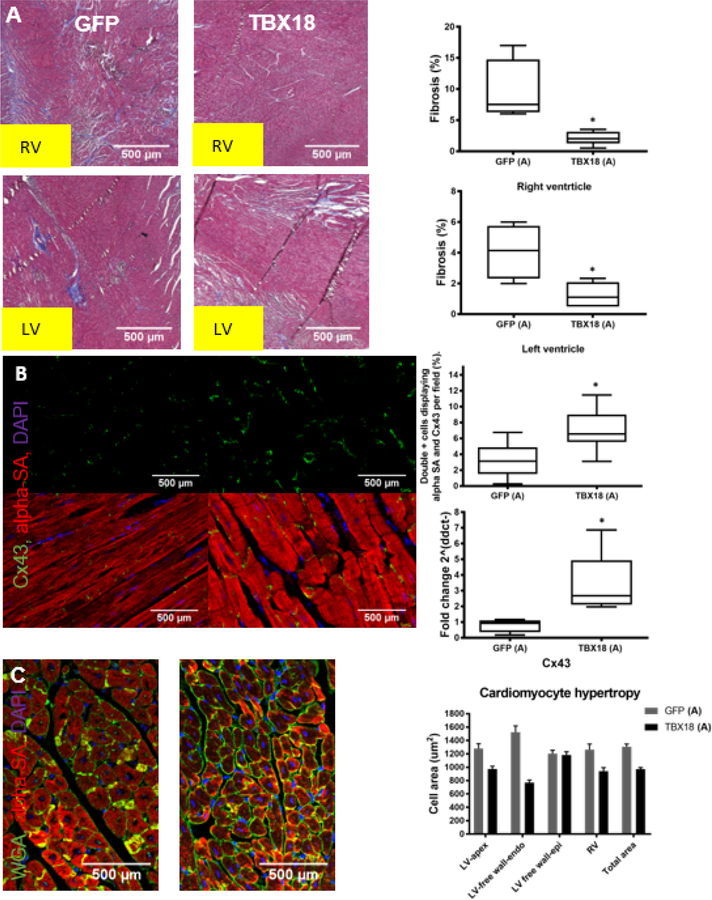
At study endpoint, tissue was collected and evaluated histologically. Masson’s trichrome staining showed substantially less fibrosis in TBX18 pig hearts relative to controls, both in the RV (2.1±0.43% vs 9.5±2.5% p=0.007) and the LV (1.25±0.31% vs 4.08±0.88% p=0.008) (Figure 5A). Immunohistochemistry (IHC) further revealed greater preservation of gap junction protein Cx43 in the LV of TBX18 animals (LV: 6.98±0.68 vs 3.22±0.52%, p=0.0001), matched by directionally-appropriate changes in Cx43 mRNA levels (3.35±0.89 vs 0.79±0.21, p=0.04) (Figure 5B). As an index of hypertrophy, we also quantified cardiomyocyte cross-sectional area in various representative ventricular samples. Cell area in GFP animals was significantly larger in 2 of the 3 LV regions evaluated (LV apex, p=0.001; LV free wall-endocardium, p<0.001; LV free wall-epicardium, p=0.7) and larger in the total LV when pooled (p=0.0001). RV cardiomyocytes were hypertrophied in GFP relative to TBX18 (p=0.002) (Figure 5C).
Figure 5: Group A – Early intervention protocol: changes in fibrosis, connexin-43, and cardiomyocyte hypertrophy in TBX18 antegrade biological pacemaker-treated animals compared to controls.
(A) Masson’s trichrome staining evaluating fibrosis present in the right and left ventricles of TBX18 pig’s vs control. 2 months following transduction with TBX18 both right and left ventricular fibrosis was reduced in BioP animals, when compared to control animals which were primarily electronically paced (RV: 9.5±2.5% GFP, 2.1±0.43% TBX18, p=0.007), (LV:4.08±0.88% GFP, 1.25±0.31% TBX18, p=0.008). (B) Immunohistochemistry also revealed a decrease in gap junction protein Cx43 in the left ventricle of GFP control animals (LV: 3.22±0.52% GFP, 6.98±0.68% TBX18, p=0.0001). (C) Cell area in GFP animals was significantly larger in 2 of the 3 LV regions evaluated (LV apex, p=0.001; LV free wall-endocardium, p<0.001; LV free wall-epicardium, p=0.7) and larger in the total LV when pooled (p=0.0001). RV cardiomyocytes were hypertrophied in GFP relative to TBX18 (p=0.002).
Biodistribution and systemic safety
To assess biodistribution and persistence of intramyocardial Ad.TBX18 delivery, samples of heart, lung and spleen were acquired at weeks 2, 4, and 12 post Ad.TBX18 gene therapy in a subset of animals. (Online Figure 2A) shows that, 2 weeks following injection of Ad.TBX18, ample viral DNA can be detected at the injection site (894±460.4 copies/µg genomic DNA), but not in off-target organs (lung, 2.89, and spleen, 18.2 copies/µg genomic DNA). At 4 weeks, a minimal number of viral particles are located at the injection site with none in off-target organs (Online Figure 2A). By week 8, no trace of Ad.TBX18 could be found either at the injection site or in the off-target organs evaluated. Complete blood counts and serum biochemistry profiles were normal at study endpoint in both groups (Online Figure 2B).
Protocol B: Reversal of PICM
Having demonstrated that restoring antegrade conduction by TBX18 BioP was safe in effective in preventing PICM, we went on to determine whether the detrimental effects of RV pacing could be reversed or attenuated after PICM was already evident. Figure 6A schematically depicts protocol B. Here we injected vehicle Phosphate Buffered Saline(PBS) as the control, to ascertain the total effects of vector plus transgene on rescue efficacy. One month after AV node ablation, pigs underwent a second percutaneous procedure to deliver TBX18 or PBS into the His bundle region, with phenotyping like that described for protocol A. At week 4, just before injection, both groups had mean heart rates ~50bpm (Figure 6B–D) with ~70% reliance on the backup electronic pacemaker (Figure 6E). Within one week of gene delivery, TBX18 pigs had significantly higher diurnal and mean heart rates (week 5, Figure 6B–D), and lower pacemaker utilization (week 5, Figure 6E), than PBS controls. At study endpoint, heart rate remained significantly higher in TBX-injected pigs (Day: 62±2bpm vs 55±1.4bpm, p=0.01; Night: 54±1.1bpm vs 50.8±0.4bpm; p=0.03, Mean 56±2bpm vs 50.1±.0.6bpm, p=0.05) (Figure 6B), and electronic back-up pacing remained much lower (95±1.6% PBS, 53±8.2% TBX18,p=0.003) (Figure 6E, Online Figure 1–group B).
Figure 6: Group B – Rescue protocol: changes in heart rate, backup electronic pacemaker utilization, and electrical remodeling in TBX18 antegrade biological pacemaker-treated animals compared to controls.
(A) Rescue protocol comparing TBX18 vs. PBS control. Like group A, AV block was induced in 10 animals. Following a functional evaluation by MRI after 1 month, animals received a His bundle region injection of either PBS as a control (n=4), or TBX18 (n=6). (B) Diurnal and mean 24-hr heart rate over the course of the study, endpoint heart rate was superior in TBX18 pigs (Day: 55±1.4bpm PBS, vs 62±2bpm TBX18, p=0.01; Night: 50.8±0.4bpm PBS, vs 54±1.1bpm,TBX18, p=0.03; Mean: 50.1±.0.6bpm PBS, 56±2bpm TBX18, p=0.05) (C) Electronic pacemaker usage over 2 months following gene transfer was lower in TBX18 pigs in control pigs (95±1.6% PBS, 53±8.2% TBX18, p=0.003) (D-E) Representative 12-lead QRS morphology in both groups at endpoint. (F) Group B QRS duration over the 2-month study. At endpoint (105±2.1 PBS, vs 54.8±1.3 TBX18, P<0.001).
Restoration of anterograde conduction and ventricular function
In addition to the chronotropic effect, TBX18 injection reversed, at least partially, adverse electrical and mechanical remodeling associated with PICM. TBX18 BioP restored antegrade conduction as evidenced by reduction in QRS duration (54.8±1.3ms vs 105±2.1ms, p<0.001) (Figure 6D–F). Meanwhile, TBX18 injection led to significant improvements in mechanical synchrony relative to PBS controls (septo-lateral segment difference assessed by MRI: 108.5±4ms vs 130.4±10ms, p=0.05; Figure 7A–B), as well as a superior LVEF (61.6±1.3% vs 49.1±5%,p<0.001; Figure 7C–D). Structural remodeling was also attenuated by TBX18 injection: at endpoint, ESV and EDV were both significantly smaller relative to controls (p=0.002 and p=0.006, respectively; Figure 7 E–F). The magnitude of the differences in ESV seen here is comparable to that reported in human responders to cardiac resynchronization therapy (CRT)(21).
Figure 7: Group B – Rescue protocol: changes in mechanical dyssynchrony, and, and left ventricular systolic function in TBX18 antegrade biological pacemaker treated-animals compared to controls.
(A) AHA segmented chords showing time to peak radial strain (cvi42®). (B) Septal-lateral segment difference was significantly different at endpoint (130.4±10ms PBS, 108.5±4ms TBX18, p=0.05). (C) Representative MRI identifying end diastolic (top) and end systolic (bottom) phases. (D) Endpoint ejection fraction was higher in TBX18 pigs (49.1±1.5% PBS, 61.6±1.3% TBX18, p<0.001). (E-F) There were favorable end systolic (56.1±4.2ml PBS, 33.6±3ml TBX18, p=0.002) and end diastolic volumes (104.4±4.1ml PBS, 84.1±4.2 TBX18, p=0.006) in TBX18 pigs.
In protocol B, cardiac output was similar at baseline (3.77±0.47l/min PBS vs 4.11±0.53l/min TBX18,p=0.5), however after 10 minutes of β-adrenergic stimulation with isoproterenol, CO in TBX18-treated animals was significantly increased from baseline relative to a modest increase following β-adrenergic stimulation in the control animals (4.1±0.51l/min PBS vs 6.30±0.33l/min TBX18,p=0.004). Beta stimulation was discontinued, and the animal could recover for 10 minutes when 1 L of normal saline was infused rapidly. CO was then revaluated as well as additional measurements of including end-diastolic-pressure (LVEDP). After the fluid challenge, BioP animals showed a significant increase in CO compared to controls (4.1±0.37l/min PBS vs 5.4±0.26l/min TBX18, p=0.02). Although there were no apparent differences in LVEDP at baseline, there was an increase in chamber pressure in the control group following β-adrenergic stimulation (5.62±2.21mmhg PBS vs 3.91±0.27mmhg TBX18 p=0.3) and a significant increase after the 1L fluid challenge (9.12±1.85mmhg PBS vs 3.75±0.41mmhg TBX18 p=0.008) (Figure 3).
Activity
Figure 8 shows implanted accelerometry data for protocol B; panel A-B shows examples of raw data. At endpoint, overall levels of activity were higher (250.9±28.3au PBS, 380.1±11.8au TBX18 p=0.006; Figure 8C), and bursts of activity were more frequent 6±0.8 episodes PBS, 16±0.9 episodes TBX18 (p<0.001; Figure 8D) in TBX18 animals than in controls. Likewise, bursts of activity lasted longer in the TBX18 group than in controls (96.2±14.3s PBS, 198.3±7s TBX18 p<0.001; Figure 8E), and the post-burst latency was shorter (69.2±2.8min PBS, 48.3±2.7min TBX18 p=0.001; Figure 8F).
Figure 8: Group B – Rescue protocol: changes in physical activity in TBX18 antegrade biological pacemaker-treated animals compared to controls.
(A-B) 24-hour activity plots at endpoint from an implanted accelerometer (C) Mean 24-hour activity at endpoint (250.9±28.3au PBS, 380.1±11.8au TBX18,p=0.006) (D) Frequency of burst activity (6±0.8 episodes PBS, 16±0.9 episodes TBX18, p<0.001) (E) the duration of burst activity (96.2±14.3s PBS, 198.3±7s TBX18, p<0.001), and (F) and the latency period between bursts of activity was superior in TBX18 pigs (69.2±2.8min PBS, 48.3±2.7min TBX18, p<0.001).
Taken together, these findings indicate that restoring antegrade conduction with TBX18 BioP could decrease back-up pacemaker utilization while improving electrical and mechanical synchrony, cardiac function (assessed both by MRI and invasive hemodynamics), and activity in our model of PICM.
Discussion
Antegrade biological pacing would offer a hardware-free alternative to His bundle pacing (Central Illustration). This would benefit patients in which indwelling hardware would be contraindicated due to complications such as device-related infections, lead complications, generator malfunction. Such therapy may also benefit pediatric pacemaker candidates as the biological pacemaker reprograms ventricular myocytes and responds to changes in growth and nervous system stimulation over time (20). In the Dual Chamber and VVI Implantable Defibrillator (DAVID) trial, programming that promotes right ventricular pacing was associated with increased mortality and hospitalizations for heart failure in patients with a standard ICD indication(4). During antegrade pacing, since activation occurs via the normal conduction system, it does not result in ventricular dyssynchrony(22). Compared with right ventricular apical pacing, antegradepacing results in improved LV function both acutely(23) and with chronic pacing(24). Here we demonstrated that restoring antegrade conduction with BioP created by minimally invasive somatic reprogramming with the human transcription factor TBX18 can provide superior chronotropic support (20) for a period of 2 months when compared to RVP controls. The higher heart rate obtained by delivery of TBX18-BioP to the His-bundle region, reduced the need for back-up electronic pacing while reducing and attenuating some of the detrimental effects of PICM attributed to long term RVP(2). This led animals in the BioP group(s) to show decreased signs of electro-mechanical dyssynchrony as indicated by QRS duration during non-paced beats, and global septo-lateral segment differences by cardiac MRI. The reduced incidence of back-up pacing (preventing the deleterious effects of RVP) resulted in a superior LVEF, and improved chamber volumes in TBX18 BioP animals. These differences were observed despite modest differences in backup pacemaker utilization in this model. Comparatively, we believe that early intervention (Group A) had improved response likely due to a preserved function and absence of adverse remodeling. In group B, BioP animals during the first 4 weeks had a high burden of ventricular pacing and potential adverse LV remodeling like controls. Consequently, in this group TBX18-treated animals had a lower decrease in backup pacing utilization compared to group A. Additionally, BioP animals showed improved invasive hemodynamics compared to RVP controls. The lack of hemodynamic difference at baseline between treatment groups may be associated with CNS depression associated with general anesthesia. However, following β-adrenergic stimulation, or an intravenous fluid challenge, animals which received BioP showed increased CO and significantly lower LVEDP compared to RVP controls. Heart rate during periods of high activity, frequency of bursts of activity, and the time between high levels of activity all suggest improved hemodynamic support in BioP animals compared to controls. Decreased expression in gap junction proteins (Cx43, Cx45) is associated with chronic RVP(25) and adverse ventricular remodeling.
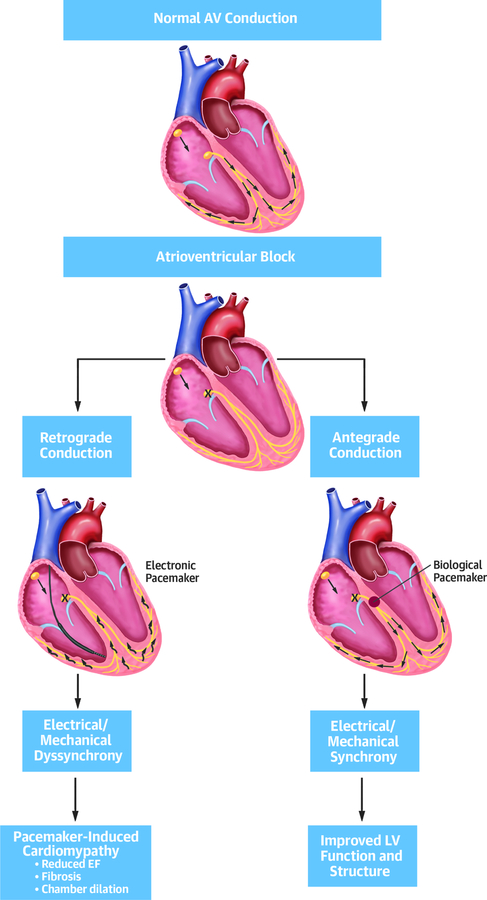
Central Illustration: Proposed Mechanism of Pacemaker-induced Cardiomyopathy.
In complete atrioventricular heart block, the impulse is not propagated from the atria to the ventricles. Electronic pacemakers (left) provide an electric impulse with retrograde conduction through slow conducting myocardium (black arrows). This can lead to electrical and mechanical dyssynchrony, decreased systolic function, fibrosis and adverse remodeling. A biological pacemaker (right) provides antegrade conduction via His-purkinje network and can prevent or rescue the deleterious changes seen in pacing induced cardiomyopathy.
Preservation of gap junction protein Cx43 may be associated with more uniform activation of the ventricular myocardium as suggested by QRS duration of BioP animals relative to RVP controls. However, direct investigation of changes in ventricular conduction velocity between groups was not performed.
Study limitations
Our experimental study has the following limitations. First, we delivered our TBX18-BioP to the His bundle region but we have not confirmed selective His-bundle pacing (as opposed to His-bundle and surrounding myocardium)(26). However, we observed improvement in QRS duration and function by MRI consistent with restoration of antegrade conduction through the His-Purkinje system. Second, we delivered our BioP using an adenovirus-based vector system which is known for having transient expression of the transgene. To achieve permanent antegrade or His-bundle biopacing, other strategies will have to be implemented such as non-viral delivery systems or long-term expression vectors (i.e. Adeno-associated virus). Third, many patients with complete AV block and PICM have conduction disease below the AV node (infranodal disease)(27). The potential utility of antegrade BioP to improve PICM in that population needs to be thoroughly investigated. The operators were not blinded to group allocations. Normal control pigs were not evaluated to further demonstrate LV dysfunctional and cellular remodeling associated with RV single chamber pacing.
Conclusions
In summary, restoration of antegrade conduction and resynchronization by TBX18 biological pacemaker contributes to reverse molecular remodeling while attenuating detrimental clinical effects associated with RV single chamber pacing and pacing induced cardiomyopathy.
Supplementary Material
Acknowledgments
Funding: This study was funded by NIH 1K01HL133510-01A1 and NIH RO1 HL135866.
Abbreviations.
- RV
Right ventricular
- PICM
Pacemaker induced cardiomyopathy
- CRT
Cardiac resynchronization therapy
- LVEF
Left ventricular ejection Fraction
- iSAN
induced sinoatrial node cells
- BioP
Biological pacemaker
- ESV
End systolic volume
- EDV
End diastolic volume
- HF
Heart failure
- GFP
Green fluorescent protein
- MRI
Magnetic resonance imaging
Footnotes
Disclosures: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.López-Villegas A 1 C-MD, Martín-Saborido C 3, Villegas-Tripiana I 4, Robles-Musso E 5. A Systematic Review of Economic Evaluations of Pacemaker Telemonitoring Systems. Rev Esp Cardiol 2016;69(2):125–33. [DOI] [PubMed] [Google Scholar]
- 2.Dreger HMK, Bondke H, Baumann G, Melzer C. Pacing-induced cardiomyopathy in patients with right ventricular stimulation for >15 years. Europace 2012:238–42. [DOI] [PubMed]
- 3.Lamas GA, Lee KL, Sweeney MO, et al. Ventricular pacing or dual-chamber pacing for sinus-node dysfunction. N Engl J Med 2002;346(24):1854–62. [DOI] [PubMed] [Google Scholar]
- 4.Wilkoff BL, Cook JR, Epstein AE, et al. Dual-chamber pacing or ventricular backup pacing in patients with an implantable defibrillator: the Dual Chamber and VVI Implantable Defibrillator (DAVID) Trial. JAMA 2002;288(24):3115–23. [DOI] [PubMed] [Google Scholar]
- 5.Sweeney MO, Hellkamp AS, Ellenbogen KA, Greenspon AJ, Freedman RA, Lee KL, Lamas GA, MOde Slection Trial Investigators. Adverse effects of ventricular pacing on heart failure and atrial fibrillation among patients with normal baseline QRS duratin in a clinical trial of pacemaker therapy for sinus node dysfunction. Circulation 2003;107. [DOI] [PubMed]
- 6.Sharma Parikshit S.; Ellenbogen Kenneth A. & Trohman Richard G.. Permanent his bundle pacing: the Past, Present and Future. J Cardiovasc Electr 2016. [DOI] [PubMed]
- 7.Thambo JBBP, Garrigue S, Lafitte S, et al. Detrimental ventricular remodeling in patients with congenital complete heart block and chronic right ventricular apical pacing. Circulation 2004:3766–72. [DOI] [PubMed]
- 8.Connolly SJKC, Gent M, Roberts RS, et al. Effects of phsyiologic pacing versus ventricular pacing on the risk of stroke and death due to cardiovascular causes. N Engl J Med 2000;342:1385–91. [DOI] [PubMed] [Google Scholar]
- 9.Nielsen JC, Kristensen L, Andersen HR, Mortensen PT, Pedersen OL, Pedersen AK. A randomized comparison of atrial and dual-chamber pacing in 177 consecutive patients with sick sinus syndrome: echocardiographic and clinical outcome. J Am Coll Cardiol 2003;42(4):614–23. [DOI] [PubMed] [Google Scholar]
- 10.Pastore G, Aggio S, Baracca E, et al. Hisian area and right ventricular apical pacing differently affect left atrial function: an intra-patients evaluation. Europace 2014;16(7):1033–9. [DOI] [PubMed] [Google Scholar]
- 11.Scherlag BJ, Kosowsky BD, Damato AN. A technique for ventricular pacing from the His bundle of the intact heart. J Appl Physiol 1967;22(3):584–7. [DOI] [PubMed] [Google Scholar]
- 12.Sharma PS, Dandamudi G, Naperkowski A, et al. Permanent His-bundle pacing is feasible, safe, and superior to right ventricular pacing in routine clinical practice. Heart Rhythm 2015;12(2):305–12. [DOI] [PubMed] [Google Scholar]
- 13.Alberti LM, Pieragnoli P, Ricciardi G, Padeletti L. Hemodynamics of his bundle pacing. Journal of electrophysiology 2017;50:161–5. [DOI] [PubMed] [Google Scholar]
- 14.Iida YM, Izawa T, Kobari C, Yatsuhashi T, Makishima N. Successful resynchronization by permanent His-bundle pacing in a patient with pacing-induced cardiomyopathy. J Arrhythmia 2016;32:499–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaye G, Ng JY, Ahmed S, Valencia D, Harrop D, Ng ACT. The Prevalence of Pacing-Induced Cardiomyopathy (PICM) in Patients With Long Term Right Ventricular Pacing - Is it a Matter Of Definition? Heart Lung Circ 2018;27. [DOI] [PubMed]
- 16.Kiehl EL, Makki T, Kumar R, et al. Incidence and predictors of right ventricular pacing-induced cardiomyopathy in patients with complete atrioventricular block and preserved left ventricular systolic function. Heart Rhythm 2016;13(12):2272–8. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XH, Chen H, Siu CW, et al. New-onset heart failure after permanent right ventricular apical pacing in patients with acquired high-grade atrioventricular block and normal left ventricular function. J Cardiovasc Electr 2008;19(2):136–41. [DOI] [PubMed] [Google Scholar]
- 18.Shimony A, Eisenberg MJ, Filion KB, Amit G. Beneficial effects of right ventricular non-apical vs. apical pacing: a systematic review and meta-analysis of randomized-controlled trials. Europace 2012;14(1):81–91. [DOI] [PubMed] [Google Scholar]
- 19.Hussain MA, Furuya-Kanamori L, Kaye G, Clark J, Doi SAR. The Effect of Right Ventricular Apical and Nonapical Pacing on the Short- and Long-Term Changes in Left Ventricular Ejection Fraction: A Systematic Review and Meta-Analysis of Randomized-Controlled Trials. Pace 2015;38(9):1121–36. [DOI] [PubMed] [Google Scholar]
- 20.Hu YF, Dawkins JF, Cho HC, Marban E, Cingolani E. Biological pacemaker created by minimally invasive somatic reprogramming in pigs with complete heart block. Sci Transl Med 2014;6(245):245ra94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung ES, Leon AR, Tavazzi L, et al. Results of the Predictors of Response to CRT (PROSPECT) trial. Circulation 2008;117(20):2608–16. [DOI] [PubMed] [Google Scholar]
- 22.Catanzariti D, Maines M, Manica A, Angheben C, Varbaro A, Vergara G. Permanent His-bundle pacing maintains long-term ventricular synchrony and left ventricular performance, unlike conventional right ventricular apical pacing. Europace 2013;15(4):546–53. [DOI] [PubMed] [Google Scholar]
- 23.Kronborg MB, Poulsen SH, Mortensen PT, Nielsen JC. Left ventricular performance during para-His pacing in patients with high-grade atrioventricular block: an acute study. Europace 2012;14(6):841–6. [DOI] [PubMed] [Google Scholar]
- 24.Kronborg MB, Mortensen PT, Poulsen SH, Gerdes JC, Jensen HK, Nielsen JC. His or para-His pacing preserves left ventricular function in atrioventricular block: a double-blind, randomized, crossover study. Europace 2014;16(8):1189–96. [DOI] [PubMed] [Google Scholar]
- 25.Akar FG, Nass RD, Hahn S, et al. Dynamic changes in conduction velocity and gap junction properties during development of pacing-induced heart failure. Am J Physiol Heart Circ Physiol 2007;293(2):H1223–30. [DOI] [PubMed] [Google Scholar]
- 26.Ajijola OA, Macias C, Shivkumar K, Tung R. Permanent His-bundle pacing for cardiac resynchronization therapy: Initial feasibility stud in lieu of left ventricular lead. Heart Rhythm 2017;14(9):1353–61. [DOI] [PubMed] [Google Scholar]
- 27.Katz MGFA, Wever T, Hajjar RJ, Bridges CR. Use of Adeno-Associated Virus vector for cardiac gene deliver in Large animal surgical models of heart failure. Hum Gene Ther Clin Dev 2017;28(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.