Abstract
From an early age, individuals with autism spectrum disorder (ASD) spend less time engaged in social interaction compared to typically developing peers (TD). One reason behind this behavior may be that the brains of children diagnosed with ASD do not attribute enough value to potential social exchanges as compared to the brains of typically developing children; thus, potential social exchanges are avoided because other environmental stimuli are more highly valued by default. Neurobiological investigations into the mechanisms underlying value-based decision-making has shown that the ventral medial prefrontal cortex (vmPFC) is critical for encoding the expected outcome value of different actions corresponding to distinct environmental cues. Here, we tested the hypothesis that the responsiveness of the vmPFC in children diagnosed with ASD (compared to TD controls) is diminished for visual cues that represent highly valued social interaction. Using a passive picture viewing task and functional magnetic resonance imaging (fMRI) we measured the response of an a priori defined region of interest in the vmPFC in children diagnosed with ASD and an age-matched TD cohort. We show that the average response of the vmPFC is significantly diminished in the ASD group. Further, we demonstrate that a single-stimulus and less than 30s of fMRI data are sufficient to differentiate the ASD and TD cohorts. These findings are consistent with the hypothesis that the brains of children with ASD do not encode the value of social exchange in the same manner as TD children. The latter finding suggests the possibility of utilizing single-stimulus fMRI as a potential biologically based diagnostic tool to augment traditional clinical approaches.
Keywords: autism spectrum disorders, functional MRI, reward, ventromedial prefrontal cortex, favorite face, favorite object, value-based decision making
Introduction
As early as infancy, humans express preference for faces over other types of stimuli (Farroni et al., 2005; Johnson et al., 1991; Zion-Golumbic et al., 2008) – a preference that likely contributes to the foundation of healthy social development (Brooks and Meltzoff, 2002). Behavioral studies have shown that typically developing (TD) children inherently value and pursue social stimuli such as a hug or smile from a parent (Dawson et al., 2001; Dawson et al., 2004). In contrast, individuals diagnosed with autism spectrum disorders (ASD) appear indifferent to faces and social interactions (Baron-Cohen et al., 1995; Gliga and Csibra, 2007; Golarai et al., 2006; Nation and Penny, 2008; Schultz, 2005). Clinical observations and previous studies suggest the hypothesis that early developmental dysfunction of brain pathways linking social stimuli and reward (Dawson et al., 1998; Dawson et al., 2001) lead to autistic individuals’ deficits in social and emotional reciprocity (Kanner, 1968; Mundy et al., 1986; Rutter, 1978).
Dopaminergic projections from the ventral tegmental area (VTA) to corticolimbic regions are important in mediating the effects of reward on behavior (Berridge and Robinson, 1998; McClure et al., 2003), and neuroimaging studies have shown that neural activity in regions of the brain where dopaminergic neurons project, including the ventral medial prefrontal cortex, ventral striatum, posterior cingulate and precuneus, are modulated by eye contact, a social reward signal (Dawson et al., 2005a; Fliessbach et al., 2007; Izuma et al., 2008; Kampe et al., 2001). Dysfunctions in this pathway that may contribute to the lack of social motivation in ASD have also been previously explored using behavioral (Dawson et al., 1998; Dawson et al., 2001; Dawson et al., 2004; Dawson et al., 2005a), event-related potential (ERP) (Dawson et al., 2002; Kohls et al., 2011; Larson et al., 2011), and structural imaging studies (Courchesne and Pierce, 2005; McAlonan et al., 2005). Results from blood-oxygen level dependent (BOLD) functional magnetic resonance imaging (fMRI) studies of reward processing in ASD are mixed (Dichter et al., 2012a; Dichter et al., 2012b; Kohls et al., 2013; Schmitz et al., 2008; Scott-Van Zeeland et al., 2010). Scott-Van Zeeland et al (Scott-Van Zeeland et al., 2010) reported pronounced corticostriatal hypoactivation to social compared to monetary rewards, but Dichter et al (Dichter et al., 2012b) and Kohls et al (Kohls et al.,2013) described hypoactive reward responses to both social and monetary rewards. One study reported increased response in the ventromedial prefrontal cortex (vmPFC) to salient objects in individuals with ASD using fMRI (Dichter et al., 2012a).
In the present study we used a picture-viewing paradigm with fMRI to probe reward-related responses in TD children and children with ASD. This task imposed minimal cognitive demands on participants as opposed to the more complex tasks used previously, such as the monetary incentive delay; (Knutson et al., 2001). The goal was to create a task that younger children and those with more severe forms of ASD could perform, but would still be useful to assess the differences in valuation responses across social and non-social stimuli.
We hypothesized that images of a favorite person and object (self-selected by each participant) would elicit valuation-related responses in TD participants’ corticostriatal reward-processing regions (Haber and Knutson, 2010; Knutson et al., 2003; McClure et al., 2004b; Rangel et al., 2008) compared to control images of non-favored people and objects. We were particularly interested in the vmPFC where previous studies showed activity linearly correlated with reward value across multiple kinds of motivating stimuli (Goel and Dolan, 2001; Harvey et al., 2010; Knutson et al., 2003; McClure et al., 2004a; O’Doherty et al., 2003). In addition, we focused on the vmPFC because it has been shown to be more active when rewards are received (i.e. viewing a favored image); in contrast, the ventral striatum is strongly activated when rewards are anticipated (Knutson et al., 2001; Knutson et al., 2003). We expected children with ASD to have weaker responses to images of favorite people and stronger responses to images of favorite objects (Lam et al., 2008; Sasson et al., 2011). We found that the vmPFC response in TD children increases with increasing subjective ratings of pleasantness of images of objects; and, this response was maximal for images of the participants’ self-selected favorite person and object. We show that the association between the vmPFC response and self-reported preferences in children diagnosed with ASD is disrupted for objects and people, including the response to the individuals’ self-selected favorites. Finally, we demonstrate that a single presentation of an image of the participants’ self-selected favorite person is sufficient to distinguish age- and gender matched cohorts of TD (n = 16) and ASD children (n = 16), while the single stimulus response to the favorite object fails to differentiate these two groups.
Methods and Materials
Participants
Fifty-four (n = 54) typically developing (TD) children and 31 (n = 31) children diagnosed with ASD participated in this study. None of the TD children were diagnosed with any developmental disorder including ADHD or ASD. This was confirmed by parent report at the time of recruitment and consent. Children recruited to the ASD cohort were included based on initial diagnosis and current treatment clinically or behaviorally for ASD. Four TD children (n = 50) were excluded from all analyses: one due to technical problems during scanning, one because the subject fell asleep while being scanned and two because of participants’ decision to discontinue with the study. Of the 50 TD children, 28 were included in the General Linear Model (GLM) and full experiment region-of-interest (ROI) analyses. These participants completed the full experiment with head motion ≤ ±3.5 mm. We chose this threshold because (1) we expected children to move more, (2) in particular children diagnosed with ASD, and (3) observed movements in our child participants were large when they occurred. In those participants whose movements were less than this threshold we observed no significant difference in movements across the TD and ASD groups.
For the ROI analysis of single stimulus response to favorites, 44 TD children were included. These were participants who viewed a single presentation of each stimulus type (1) with head motion within the set limit of ± 3.5 mm 6s pre and 20s post presentation of stimulus and (2) with vmPFC response variability of less than 1% signal change (standard deviation) to the first presentation. The second criterion was set to ensure the outlier signals do not skew the measured BOLD responses.
Seven children with ASD were excluded from all analysis: five because of excessive head movement beginning early in the experiment rendering their data unusable, one because the subject decided to discontinue with the study, and one because of gross anatomical abnormality noted after the initial structural brain scan. Of the remaining 24 ASD participants, 12 were included in the GLM and full experiment ROI analysis and 16 were included in the single stimulus ROI analysis. See Table 1 for TD and ASD demographic and diagnostic assessments data.
Table 1.
Demographic and diagnostic measures for TD and ASD groups
| Variables | TD, n = 44a Mean ± SEM |
ASD, n = 17b Mean ± SEM |
t-Test, P-Value |
||
|---|---|---|---|---|---|
| Agec (Mean) | 13.61 ± 0.49 | 11.98 ± 3.42 |
t59
= 1.69, P= 0.095 |
||
| Age (Range) | 7.62 – 18.00 y | 6.56 – 16.48 y | --- | ||
| Sex (M:F) | 22 M:22 F | 14M:3F | --- | ||
| IQd | |||||
| Composite | 104.43 ± 2.36 | 100.27 ± 7.35 |
t39
= 0.71, P = 0.48 |
||
| Verbal | 102.10 ± 2.48 | 94.36 ± 7.53 |
t44 = 1.27, P = 0.21; |
||
| Nonverbal | 105.10 ± 2.20 | 104.67 ± 5.58 |
t40
= 0.088, P=0.93 |
||
| ADOSe | 17.82 ± 1.23 | --- | |||
| Communication | --- | 4.91 ± 0.46 | --- | ||
| Reciprocal social interaction | --- | 9.54 ± 0.68 | --- | ||
| Imagination/creativity | --- | 1.00 ± 0.19 | --- | ||
| Stereotyped behaviors and restricted interests | --- | 2.36 ±0.51 | --- | ||
| ADI-Rf | |||||
| Reciprocal social interaction | --- | 23.33 ± 1.86 | --- | ||
| Communication | --- | 17.17 ± 1.81 | --- | ||
| Restricted, repetitive, and stereotyped patterns of behavior | --- | 7.50 ± 0.67 | --- | ||
| SRSg | Total SRS score | 27.93 ± 3.30 | 120.92 ± 7.62 |
t40
= −11.40, p = 3.84 × 10−14 |
|
| Social awareness | 6.00 ± 0.60 | 14.75 ± 1.29 |
t40 = −7.01, p = 1.82 × 10−8 |
||
| Social cognition | 5.77 ± 0.80 | 24.42 ± 1.32 |
t40
= −12.33, p = 3.30 × 10−155 |
||
| Social communication | 10.1 ± 1.60 | 41.17 ± 3.36 |
t40
= −9.42, p = 1.05 × 10−11 |
||
| Social motivation | 6.40 ± 0.81 | 17.92 ± 1.35 |
t4o
= −7.46, p = 4.39 × 10−9 |
||
| Autistic mannerisms | 3.63 ± 0.68 | 22.67 ± 1.44 |
t46= −13.52, p = 1.67 × 10−16 |
||
All tests are thresholded at p < 0.05 (t-test, two-tailed)
There are 44 total unique TD subjects: 28 subjects included full experiment analyses (Figures 2 and 3, Supplemental Figure 1); 16 in matched single stimulus ROI analysis (Figure 4); and all 44 for non-matched comparison (Supplemental Figure 2).
There are 17 total unique ASD subjects: 12 subjects included full experiment analyses (Figures 2 and 3); and, 16 in matched single stimulus ROI analysis (Figure 4). One participant was excluded, please see methods for details.
No significant difference between ages of two groups
Assessed using Kaufmann Brief Intelligence Test, Second Edition; no significant difference between IQ scores of each group; n = 11/17 (12 for non-verbal) ASD, n = 30/44 TD
Autism Diagnostic Observation Schedule; n = 11/17 ASD, 1 subject assessed using Module (Mod) 1, 1 using Module 2, 7 using Module 3, 2 using Module 4; ADOS scores were not available for 3 ASD subjects diagnosed with ADOS.
Autism Diagnostic Interview – Revised; n = 6/17 ASD
Social Responsiveness Scale (raw-scores); Total SRS score and subscale scores are significantly higher in ASD children compared to TD; n = 12/17 ASD, n = 30/44 TD
Advertisements and word of mouth were used to recruit participants. ASD participants were also recruited from the Texas Children’s Hospital’s Autism Center. Participants who passed initial assessment using safety related fMRI exclusion criteria (e.g., metallic implants, claustrophobia) were invited to Baylor College of Medicine (BCM) for familiarization with the scanning environment, scanning and assessments. For fourteen ASD participants, clinical diagnosis of autism was confirmed with the Autism Diagnostic Observation Schedule (ADOS, Table 1) (Lord et al., 2000). For three participants, psychiatrists in tertiary hospitals and/or licensed school psychologist diagnosed ASD based on clinical presentation and developmental history. Scores from the Social Responsiveness Scale (SRS) (Constantino and Todd, 2003) and Kaufman Brief Intelligence Test, Second Edition (KBIT-2) (Kaufman and Kaufman, 1990) were also obtained for a subset of participants (Table 1). No significant difference was found comparing the ASD and TD groups’ IQ (mean composite IQ scores were 104.43 and 100.27, for TD and ASD groups, respectively; also see Table 1 for breakdown of verbal and non-verbal scores). BCM’s institutional review board approved the research protocol. Parents and children gave informed consent and assent, respectively.
MRI Data Acquisition
Experiments were conducted using two 3 tesla Siemens MR scanners (Allegra and Trio). Both ASD and TD participants were randomly assigned to one of the two scanners to counterbalance differences in scanner characteristics. The same scanning protocols were used in both scanners: (1) A localizer image was acquired first followed by high-resolution T1-weighted anatomical images using the following parameters: 192 volumes; in-plane resolution: 256 × 256; field of view: 245 mm; slice thickness: 1 mm. (2) Continuous whole-brain imaging was performed while participants viewed the experiment stimuli. BOLD fMRI signals were recorded using an echo-planar imaging (EPI) sequence: echo-planar imaging, gradient recalled echo; repetition time = 2000ms; echo time = 30ms; flip angle = 90; 64 × 64 matrix (in-plane resolution); 34 4 mm axial slices positioned 30° to the anterior commissure/posterior commissure line.
Statistical Parametric Mapping 8 (SPM8) software (Friston et al., 1994)(http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used to preprocess and analyze data. During preprocessing, functional brain images were: (1) temporally realigned using linear interpolation to address variability in the timing of slice acquisition, (2) spatially realigned using a six-parameter rigid-body transformation to correct for head movements, (3) co-registered onto high-resolution/high-contrast structural images, (4) segmented and spatially normalized using a customized T1 template and tissue probability maps (generated using the SPM8 toolbox Template-O-Matic (Wilke et al., 2008) to facilitate inter-subject comparison and (5) spatially smoothed using an 8 mm Gaussian kernel and temporally high-pass filtered using a cutoff period of 128s.
Functional MRI task.
Participants viewed pictures of four faces and four objects on a back-projection screen viewed through a mirror during scanning (Figure 1A). Images (Figure 1B) of either a face or object in four different valence categories: favorite, pleasant, neutral and unpleasant were presented through a back-projected computer screen. “Favorite” images were an image each of participants’ self-selected favorite person and thing. The rest of the images were selected from the International Affective Picture System or IAPS database (Lang et al., 1999) and were designated as pleasant, neutral, or unpleasant based on their IAPS emotional valence scores.
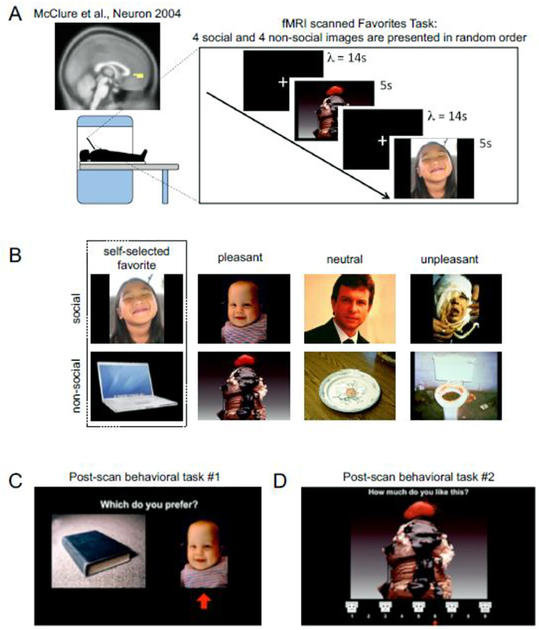
Figure 1. Experimental design.
(A) FMRI task. Pictures were shown for 5 s each in six random permutations of the eight images. A blank screen with a white crosshair in the middle was shown between images (inter-stimulus time interval, λ = 14s). Inset: vmPFC ROI determined from a previous study examining valuation responses and choice behavior McClure et al., Neuron 2004 [39]. We hypothesized that this ROI would show diminished valuation responses to images of favorite person in children with ASD. (B) Sample image set for ‘Favorites’ Task. The images were categorized into social (i.e. faces, top row) and non-social (i.e. objects, bottom row) groups and further classified as ‘favorites’, pleasant, neutral and unpleasant. Pleasant, neutral and unpleasant valence categories are based on previously published IAPS ratings. (C,D) Post-scan Behavioral Tasks. After the fMRI task, participants answered two computer-based assays to determine their subjective preferences, a self-paced forced-choice task (C) and a modified Self-Assessment Manikin (D; Bradley & Lang, 1994).
Each of the eight pictures were shown for once for 5 s in a block. Each block was repeated six times. In each block, each of the eight images were shown in a random order. Inter-image time intervals were determined by randomly sampling a Poisson inter-interval distribution, with lambda (λ = mean = variance), set to 14s. The random order and Poisson inter-interval distribution for the inter-image time intervals were chosen to maximize the uncertainty of the onset times of each image; this minimizes potential anticipatory brain responses from cross-contaminating the response to each image of interest. During each of the inter-image time intervals, a black screen with a white fixation cross in the middle was displayed. Apart from viewing the pictures and the fixation cross, there were no additional task demands. The passive version of the experiment was used to ensure that brain signals related to valuation were not confounded by activations related to action planning or execution (Aharon et al., 2001; Berridge and Robinson, 1998; Farroni et al., 2005; Harvey et al., 2010; Johnson et al., 1991; McClure et al., 2003; Zion-Golumbic et al., 2008). This simple task also increased the possibility of scanning younger children and individuals with neurodevelopmental disorders who may present with difficulties in completing complex tasks or who may tend to move excessively and prohibit task completion (Brooks and Meltzoff, 2002; Fliessbach et al., 2007; Freilich and Gaillard, 2010; Izuma et al., 2008; Kampe et al., 2001; Yerys et al., 2009).
Post-Scan Behavioral Assays.
After the fMRI experiment, participants completed two post-scan behavioral tasks. Each task was a computer-based preference assay (Figures 1C and 1D) to measure participants’ individual behavioral preferences for the previously viewed images in the scanner and confirm each of the respective valence ratings (e.g., favorite, pleasant, neutral, and unpleasant). Two tasks were used to minimize potential effects of context (Dawson et al., 1998; Dawson et al., 2001; Dawson et al., 2004; Dawson et al., 2005a; Tversky and Kahneman, 1981). Task #1 was a forced-choice task (example screenshot shown in Figure 1C) and Task #2 was a modified Self-Assessment Manikin (example screenshot shown in Figure 1D) (Baron-Cohen et al., 1995; Bradley and Lang, 1994; Gliga and Csibra, 2007; Golarai et al., 2006; Nation and Penny, 2008; Schultz, 2005). In the forced-choice task, participants were asked to choose between two images simultaneously shown on the screen. All unique combinations of the 8 (28 pairs) images were presented. In the Self-Assessment Manikin task, participants were asked to rate how much they liked each of the eight pictures using a modified Self-Assessment Manikin (Bradley and Lang, 1994; Dawson et al., 1998; Dawson et al., 2001), which is a visual affective rating system used to rate the pictures in the IAPS database. The order of these two tasks were counterbalanced by randomly assigning which of the two tasks would be completed first.
Data Analysis
Post-Scan Behavior.
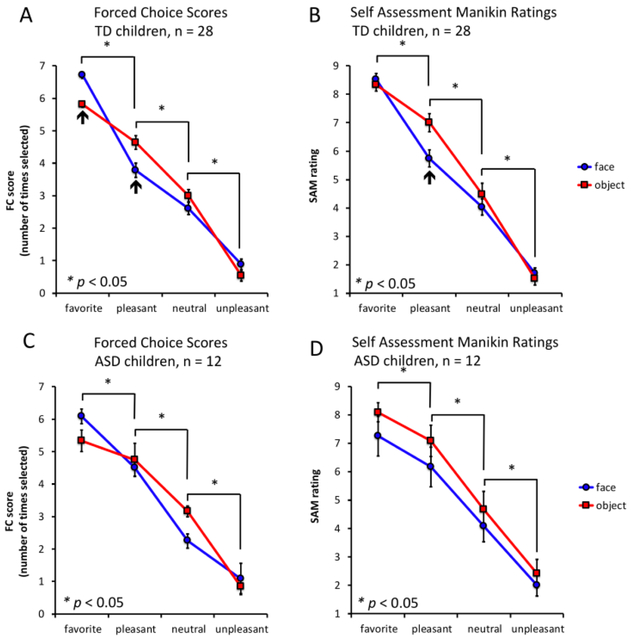
Individual preference rankings in the forced-choice task determined the ranking scores for each of the image sets. The order of preference for each image (faces and objects) was first determined for all images. Then the rank scores for each valence category (favorite, pleasant, neutral, and negative) were averaged within either the object or face grouping and across individuals in the TD (Figure 2A) and ASD (Figure 2C) cohorts. Similarly, average Self-Assessment Manikin scores for each image valence category, within either the object or face grouping, and across individuals in the TD (Figure 2B) and ASD (Figure 2D) cohorts were determined, plotted, and statistically compared.
Figure 2. Forced choice and modified Self-Assessment Manikin results in TD (n = 28) and ASD children (n = 12).
Mean forced choice (FC) scores, TD (A) and ASD (C) groups. (A and C) Vertical axes: mean forced-choice score = number of times the image was chosen over all pairwise comparisons; horizontal axes: image categories; blue: face images; red: object images. Favorite images were selected more times than pleasant, neutral and unpleasant pictures in TD children and children diagnosed with ASD who completed the entire task. Mean Self-Assessment Manikin (SAM) ratings for TD (B) and ASD (D) groups. (B and D) Vertical axes: mean SAM rating; horizontal axes: image categories; blue: face images; red: object images. (B) TD children and (D) children diagnosed with ASD rated favorite images higher than pleasant, pleasant more than neutral and neutral more than unpleasant images. For all panels: *p < 0.05, k = 10; black arrows indicate valence categories where there is a significant difference between faces and objects.)
ROI analyses.
Two ROI time series analyses were performed. In the first ROI timeseries analysis, only those participants in both the TD (n= 28) and ASD (n=12) groups who were able to complete the entire fMRI session without excessive head movement were included (Figure 3). In the second ROI timeseries analysis, we focused on the first stimulus presentation for each of the image categories (Figure 4, and data not shown). In the second, “single-stimulus”, ROI analysis participants who viewed a single presentation of favorite face and favorite object with (1) translational head motion within a threshold of 3.5 mm and (2) less than 1% signal standard deviation of the vmPFC BOLD response to the first presentation of favorites were included (TD, n = 44; ASD, n = 16). This allowed us to increase the sample size from 12 to 16 participants in the ASD group and from 28 to 44 participants in the TD group. The results of this analysis also demonstrate that sufficient information about the responsivity of a brain region can be assessed with minimal fMRI data.
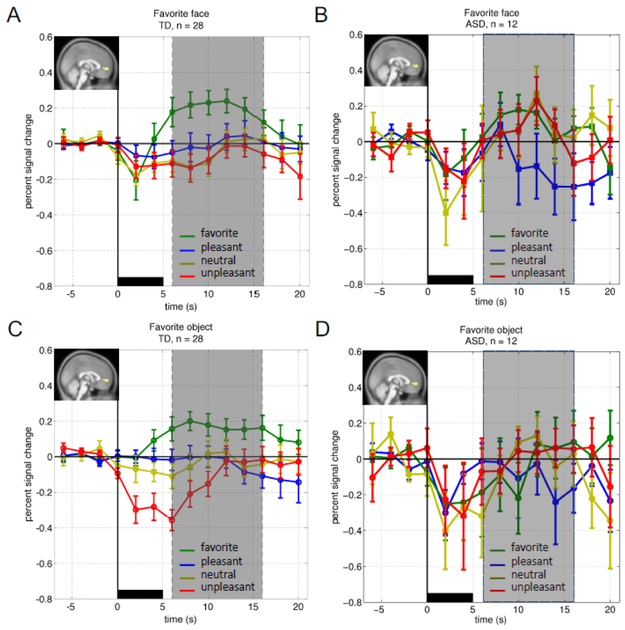
Figure 3. Time courses of the BOLD response in the vmPFC ROI over the full experiment.
For all panels: Vertical axes: percent signal change from baseline (BOLD response −6s to 0s prior to image presentation); horizontal axis: time(s) peri-stimulus; black bar: image presentation (images were shown for 5s); green: mean (+/− SEM) BOLD response to favorite image; blue: mean (+/− SEM) BOLD response to pleasant image; yellow: mean (+/− SEM) BOLD response to neutral image; red: mean (+/− SEM) BOLD response to unpleasant image; inset: a priori determined region of interest mask covering the vmPFC as defined in McClure et al. 2004. (A and C) TD children’s response to faces (A) and objects (C). vmPFC BOLD responses to favorite images were significantly greater than the BOLD response to any IAPs image (paired t-test, pFDR < 0.05; gray area). (B and D) ASD children’s vmPFC response to faces (B) and objects (D). In the ASD group favorite images failed to elicit increased BOLD responses in the vmPFC compared to any IAPS image.
Figure 4. Single-stimulus vmPFC response to favorite faces differentiates TD from ASD across age- and gender- matched groups.
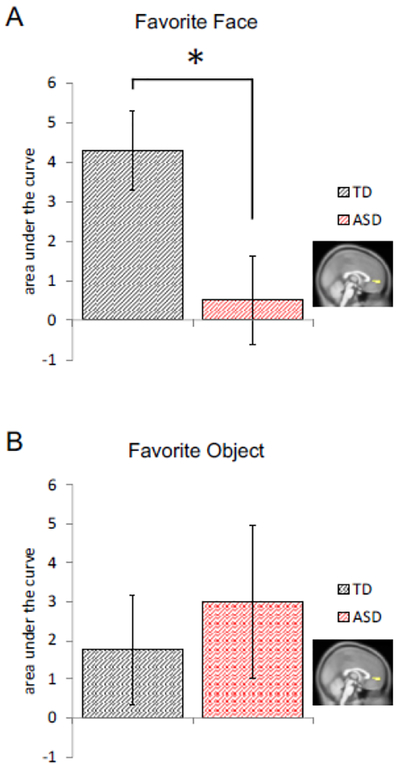
For both panels: Vertical axes: mean (+/− SEM) area under the curve (sum of the BOLD response 6s to 16s after image presentation, see shaded region in Figure 3); horizontal axis: diagnosis category: red: ASD, black: TD; inset brain image: Region of interest mask covering the vmPFC (as determined in McClure et al. 2004). (A) Comparison of AUC for the vmPFC BOLD response showed diminished single stimulus responses to favorite faces in children diagnosed with ASD (n = 16) compared to TD children (n = 16; *p < 0.05; left). (B) There was no significant difference in the single-stimulus responses to favorite objects.
To perform the ROI analyses, a mask identifying which vmPFC voxels would be included in our ROI analyses (MNI coordinates [8 60 0], cluster size k = 26 voxels) was determined by using a previously published study where participants tasted culturally familiar drinks preceded by visual (i.e., brand) cues (see (McClure et al., 2004a). An image of the mask is shown and labeled “McClure et al., Neuron 2004” in the inset of Figure 1A). McClure and colleagues demonstrated that these voxels in the vmPFC activated in a manner that consistently correlated with individual participants’ preferences for the drinks tasted. Further, subsequent work by other groups has consistently identified the vmPFC as critical in the valuation process (Brooks and Meltzoff, 2002; Fliessbach et al., 2007; Izuma et al., 2008; Kampe et al., 2001; McClure et al., 2004a). Thus, we hypothesized that we could use the BOLD response in this region during our passive picture viewing task as a neural bioassay for individuals’ brain response to preferences for the images shown. To do this analysis, raw time courses of the BOLD signal from the vmPFC ROI were extracted and plotted as peri-stimulus BOLD timeseries data (Figure 3). The four valence categories for each of the face and object image sets were plotted for the TD (Figure 3A and 3B) and ASD (Figure 3C and 3D) participants. After examining the vmPFC BOLD responses to favorites and IAPS images for the full experiment, we then focused our attention to the vmPFC response to a single presentation of each stimulus type. In Figure 4, we calculated the average BOLD response in the vmPFC during the shaded timepoints shown in Figures 3A and 3B for each of the image categories. In Figure 4 we compare the BOLD response in the vmPFC to participants’ favorite person (Figure 4, left) and favorite object (Figure 4, right) in sample-size-, age-, and gender-matched ASD (n=16) and TD (n=16) individuals.
Whole brain, general linear model (GLM).
An event-related, general linear model was used to determine areas within the whole brain that responded to each image valence category at the subject level. Contrasts generated at this first-level analysis were forwarded to a second-level group analysis. Neural activity associated with the onset of each stimulus was modeled with an impulse-response function and convolved with a hemodynamic response function (HRF) to yield the hypothesized BOLD signal. To determine what areas of participants’ brains showed increased activity to favorite pictures, linear model parameter estimates for favorite images were compared to linear model parameter estimates for IAPS images. The following GLM contrasts were performed: favorites > pleasant, favorites > neutral and favorites > unpleasant. Images generated from these contrasts were forwarded to a second-level group analysis (random effects analysis) using a one sample t-test. Conjunction analysis was then performed to determine which brain regions commonly activated to favorite images. Resulting SPM t-maps from the group analyses were thresholded at false discovery rate (FDR) corrected p < 0.05 and converted into binary images using SPM8’s ImCalc function. These binary images were then combined to obtain regions of overlap across the three contrasts. Only participants who completed the full experiment (six presentations) with head motion less than or equal to 3.5 mm were included in the whole brain GLM analyses (TD, n = 28; ASD, n = 12; Supplemental Figure 1).
Results
Preference ratings match anticipated valence rankings.
We chose three images of faces and three images of objects from the IAPS database of images based on their published valence scores such that we would have a canonical set of images that all participants saw and that fell into “pleasant”, “neutral”, and “unpleasant” valence categories (Figure 1B). Both TD and ASD participants ranked our canonical set of images in a manner consistent with our goal (Figure 2). We also asked participants to provide an image of their self-selected favorite object and person in order to have a social and non-social image of their ‘most valued’ person and thing (Figure 1B). All participants consistently ranked their self-selected favorite pictures over our canonical image set (Figure 2A and 2C, forced choice task); and, all participants also rated their designated favorite objects the highest on the Self-Assessment Manikin (Figure 2B and 2D).
We hypothesized that social stimuli (i.e., images of faces) would be valued less by the brains of children diagnosed with ASD compared to TD children. Consistent with our hypothesis, at the behavioral level, analysis of post-scanning behavioral tasks showed that TD children ranked their self-selected favorite face higher than the ASD group (Figure 2) as measured by both the forced-choice task and the Self-Assessment Manikin.
vmPFC activated strongly to favorite pictures in TD children but not in children with ASD.
To directly test the hypothesis that the responsiveness of the vmPFC in children diagnosed with ASD (compared to TD controls) is diminished for visual cues that represent what should be highly valued social interactions we performed a ROI analysis on BOLD imaging data. We compared the BOLD response over time within an a priori determined region of interest (vmPFC (McClure et al., 2004a), Figure 1A, inset). Time courses of BOLD responses from the vmPFC ROI were averaged across six presentations of each of the valence categories for the face and object stimuli in only those individuals who completed the full fMRI scanning procedure with sub-threshold movements (Figure 3). Consistent with our hypothesis, vmPFC responses in TD children to favorite face stimuli were significantly greater than vmPFC responses to favorite face stimuli in the ASD group (Figure 3A and 3B).
Further, we performed repeated measures analysis of variance (ANOVA) to assess the effects of image category (i.e. face or object), image valence (i.e. favorite, pleasant, neutral, and unpleasant) and time (i.e. 12 time points from −6 to 20 s) on the vmPFC BOLD response. In 28 typically developing (TD) children, there was a statistically significant interaction between valence and time (Figure 3A: faces; and Figure 3C: objects), F(39,1053) = 3.52, p = 6.30 × 10−12). Post-hoc pairwise comparisons (FDR-corrected p < 0.05) performed to explore this interaction showed that vmPFC BOLD responses to favorite faces and objects at 6 to 16 seconds after onset of picture presentation were significantly greater than responses to pleasant, neutral and unpleasant pictures at corresponding time points. Strongly significant main effects of valence (F(3,81) = 7.08, p = 2.75 × 10−4) and time (F(13,351) = 4.30, p = 1.05 × 10−6) were also noted. There was no significant difference between vmPFC response to faces and objects in the TD children (F(1,27) = 0.061, p = 0.81).
Unlike the TD group, where the response to favorite pictures were significantly greater than IAPS images around the peak of the hemodynamic response (Dawson et al., 1998; Dawson et al., 2001; Dawson et al., 2004; Dawson et al., 2005a; Miezin et al., 2000; Richter and Richter, 2003), there was no interaction between valence and time observed in the ASD group (Figure 3B). Further, in the ASD group, a significant main effect of time (F(13,143) = 3.83, p = 2.74 × 10−5; Figure 3B and 3D) was noted, likely caused by a dip in the hemodynamic response at 2–4s after stimulus onset. Subsequent FDR correction, however, did not reveal any significantly different time points.
Favorite images show stronger activation in valuation regions in the brains of TD participants
We hypothesized that in TD participants, pictures of participants’ favorite person’s face and favorite object would elicit a greater response in the reward-processing areas in the brain compared to pleasant, neutral and unpleasant images. To test this hypothesis, we performed a conjunction analysis across the contrasts favorites > pleasant, favorites > neutral and favorites > unpleasant (contrast images thresholded at FDR corrected p < 0.05, cluster size k = 10; Figure 2 A). Consistent with our hypothesis regions that were significantly more active when TD children viewed their favorites compared to IAPS pictures included the ventromedial prefrontal cortex (vmPFC), ventral striatum (VS), anterior cingulate cortex (ACC), posterior cingulate cortex (PCC), and precuneus (Supplemental Figure 1). In contrast, ASD children’s favorite images failed to elicit responses in the VS (not shown) or vmPFC (Figure 3).
ASD children show diminished single-stimulus vmFPC response to favorite face compared to TD children.
A significant portion of ASD participants were excluded from initial analyses because of excessive head movement during the course of the fMRI scan, a problem inherent to imaging children and individuals with neurodevelopmental disorders such as ASD (Dawson et al., 2002; Kohls et al., 2011; Larson et al., 2011; Yerys et al., 2009). Prior work by Lu and colleagues has shown that single-stimulus experiments in BOLD imaging with ASD children may provide a biomarker that correlates with certain features of clinically-assessed symptom severity in ASD (Lu et al., 2015). Given this, and prior work, we assessed participants’ single-stimulus vmPFC response to favorite faces and objects and determined whether this was sufficient to differentiate age- and gender-matched TD and ASD groups (n = 16; TD, 11.99 ± 0.69, mean age ± SEM; Figure 4). We predicted diminished responses to favorite pictures in the vmPFC in children with ASD compared to children in the TD group. To test this, we compared the single stimulus vmPFC BOLD response to favorite face and object across the two groups using the area under the curve (AUC) of the BOLD signal (Figure 4). Specifically, we looked at time points 6s to 16s after stimulus onset (shaded area in Figure 3) - a period where the responses to favorite pictures were shown to be significantly different from IAPS images. We observed (Figure 4A) a significantly diminished vmPFC response (t30 = 2.47, p = 0.026) to favorite faces in children with ASD (0.53 ± 1.12, mean AUC ± SEM) compared to TD controls (4.31 ± 1.01). The responses of TD and ASD groups to favorite objects were comparable (t30 = −0.44, p = 0.66; TD, 1.77 ± 1.41; ASD, 3.00 ± 1.97). Comparison of single stimulus vmPFC response between all TD (n = 44) and ASD (n = 16) participants who successfully viewed a single presentation of favorite face and object yielded similar results (Supplementary Figure 2). Comparison of faces and objects for the pleasant, neutral and unpleasant valence categories showed no significant differences between ASD and TD cohorts (not shown).
Discussion
We hypothesized that diminished valuation-related brain responses in the vmPFC to stimuli that portend social interaction may contribute to deficits in social exchange observed in individuals diagnosed with ASD. To test this hypothesis, we used a passive picture-viewing task with fMRI to evaluate BOLD responses associated with reward processing of stimuli depicting self-selected favorite persons and objects in TD children and children diagnosed with ASD. Additionally, we used two post-scan behavioral tasks to control for potential differences in valuation rankings across self-selected favorites and a canonical set of images consisting of pleasant, neutral, and unpleasant images.
Analysis of our post-scan behavioral tasks provided results consistent with our expectations about the subjective nature of the images shown. On average, participants consistently rated their favorite images higher than the most pleasant image in our canonical image set. Within the canonical image set the pleasant, neutral, unpleasant images were rated ordinally as expected. Interestingly, within the forced-choice ranking task, participants with ASD ranked images of self-selected favorite people lower than their TD counterparts (Figure 2A and 2C). This behavioral result is consistent with the idea that children with ASD undervalue cues associated with social exchange; however, it is unclear to what extent this behavior is due to under-valuation at the level of brain encoding of value or some other mechanism that may interfere with the behavioral efficacy of intact valuation signals. Thus, we tested our primary hypothesis that the value-related vmPFC responses to self-selected favorite social images (i.e., faces or their favorite person) would be decreased in children diagnosed with ASD compared to TD children.
ROI time-course analyses were used to assess vmPFC BOLD signals averaged across the full experiment within those participants who were able to complete the entire fMRI task without violating maximum head movement thresholds (n=28 TD and n=12 ASD). Across the TD and ASD groups, a clear difference in the vmPFC BOLD response to the different image valence categories was observed. TD children show a clear differentiation of the valenced objects with the lowest valued (unpleasant) image showing the lowest response and the favorite image showing the highest response. Across unpleasant, neutral, pleasant, and favorite object images the vmPFC response showed a parametric relationship with increasingly positive valence (Figure 3C). This was not the case for the social category of images in the TD group. For these images, little differentiation in the vmPFC response in the TD brain can be seen for the three face images from our canonical set, but the vmPFC response to the TD participants’ favorite people is clear and robust (Figure 3A). In the twelve participants diagnosed with ASD who were able to complete the task, no differentiation of valence categories was observed for either the social or non-social images (Figure 3B and 3D). This would be consistent with there being no valuation responses to either social or non-social images in the ASD cohort. However, this is in contrast to what we observe for early single stimulus responses to favorite objects in the same group (Figure 4B). Prior work by our lab has shown that over the course of passive picture viewing experiments like this one, brain responses from participants with ASD habituate much more quickly compared to TD children (Lu et al., 2015). This effect would result in overall diminished responses when considering an average over repeated trials. This possibility and the challenges we faced with excessive head movement in the scanner motivated us to examine the earliest image presentations to determine whether the vmPFC response at those time points (prior to any habituation response) would show a specific difference in the ASD versus TD groups.
Using a single-stimulus vmPFC BOLD response from the early phases of the task, we assessed vmPFC responses to favorite faces and objects in sample-size, age- and gender-matched TD and ASD groups. By only requiring an early single stimulus, we were able to capture four more individuals diagnosed with ASD who were able to meet scanning head movement thresholds; thus, we examined these responses in 16 children with the diagnosis of ASD and 16 matched TD children. Single stimulus vmPFC responses to favorite faces in TD children were consistent with full experiment findings: favorite faces strongly activated the vmPFC relative to IAPS images (Figures 3A and 4A). Also, children with ASD showed significantly diminished vmPFC response to favorite faces compared to TD children (Figures 3B and 4A). This is consistent with our primary hypothesis; however, we were concerned about the specificity of this difference and sought to compare TD versus ASD vmPFC responses to other image types. Unlike the results from the full experiment, single-stimulus responses to favorite objects were comparable across the two groups; and, no difference between the ASD and TD single-stimulus vmPFC response was observed for any other ASD vs TD comparison (not shown). These results suggest that the diminished single-stimulus response to favorite people’s faces in the ASD group is specific to this category. Finally, by examining the BOLD response to a single stimulus we were able to increase the number of participants who could be included in the analysis. This approach actually allows us to examine 44 TD children at the single-stimulus level. The results of examining the full 44 TD versus 16 ASD were completely consistent with our smaller matched sample-size 16 vs 16 comparison (Supplemental Figure 2).
Our primary hypothesis was focused on examining the vmPFC due to its central role in valuation processes during decision-making. However, there are brain regions other than the vmPFC that are also critical for choice behavior driven by value and reward. Thus, we performed a whole brain search to determine if other brain responses to images of favorite people showed differences in the ASD and TD cohorts. In those individuals that were able to complete the entire fMRI task, whole brain analysis revealed that in TD children, images of favorites activated brain regions associated with reward processing: the vmPFC, VS, posterior cingulate and precuneus (Courchesne and Pierce, 2005; May et al., 2004; McAlonan et al., 2005). No significant differences were found when we compared across the TD and ASD groups. However, 28 TD children were able to complete the fMRI task whereas only 12 children diagnosed with ASD were able to complete the task within head movement thresholds. Thus, the null result in our GLM contrast analyses comparing the TD and ASD groups may be due to the small sample size of the ASD group.
Activation of reward-processing regions in TD children to visual stimuli of favorite people and objects is consistent with the notion that viewing these images is intrinsically rewarding. This is also consistent with previous work that reported viewing pictures of personally relevant faces, such as one’s own child or romantic partner, activated reward-associated brain regions such as the ventral striatum and ventromedial prefrontal cortex (Bartels and Zeki, 2000; Dichter et al., 2012a; Dichter et al., 2012b; Kohls et al., 2013; Noriuchi et al., 2008; Schmitz et al., 2008; Scott-Van Zeeland et al., 2010; Strathearn et al., 2008). Previous studies have also shown that viewing socially relevant stimuli (i.e. beautiful faces) activated the reward circuitry (Aharon et al., 2001; O’Doherty et al., 2003; Scott-Van Zeeland et al., 2010). Similarly, highly valued cultural objects have been shown to increase activity in the ventral striatum (Dichter et al., 2012b; Erk et al., 2002). While images of favorite faces and objects have not previously been used to assess reward responses in the brain, other categories of favorites (music, for example), have been used to differentiate emotional and reward responses in ASD and TD children (Caria et al., 2011; Kohls et al., 2013). Here, using both whole brain analysis and vmPFC ROI analysis, we showed that we can likewise elicit reward valuation responses in children using pictures of favorite people and objects. Unlike previous studies that evaluated reward circuitry dysfunction in ASD, here we assessed reward responses of TD and ASD children to faces and objects within the same experimental task. Previous fMRI studies compared either social or non-social incentives against monetary rewards (Dichter et al., 2012a; Dichter et al., 2012b; Scott-Van Zeeland et al., 2010), but none, to the best of our knowledge, have looked at both faces and objects within the same paradigm. This allowed us to directly compare vmPFC BOLD signals to these stimulus types.
We focused on the vmPFC because of its hypothesized role in encoding the value of different types of reward into a ‘common currency’ (Knutson et al., 2001; Montague and Berns, 2002; Kishida and Montague, 2012; Levy and Glimcher, 2012; Bartra et al., 2013), thereby allowing a person to compare different types of rewards (i.e. social versus non-social) and make choices accordingly. Since participants in the present study were allowed to specify pictures of their favorite objects and people it is possible that visual features of the self-selected images were different compared to the canonical set. To this point, there were no obvious differences in the image sets for ASD and TD groups; however, future work would do well to characterize this more explicitly. Also, it is possible that the vmPFC brain response differences we report are due to the familiarity versus novel content of the presented images and differences across TD and ASD processing of this information. This cannot be ruled out in the present study and an important direction of investigation for future studies; however, activation in the vmPFC has been shown to correlate with the positive subjective or reward value of a variety of stimuli (Haber and Knutson, 2010; Knutson et al., 2003; McClure et al., 2004a; Peters and Buchel, 2009; Rangel et al., 2008). This is in contrast to other brain regions, such as the anterior insula, dorsomedial prefrontal cortex, dorsal and posterior striatum, and thalamus, that have been shown to encode arousal and salience without specifically differentiating positive and negative value (Bartra et al., 2013; Goel and Dolan, 2001; Harvey et al., 2010; Knutson et al., 2003; McClure et al., 2004a; O’Doherty et al., 2003).
It has also been shown that the vmPFC is activated during social exchange with a partner whose economic value to the participant is learned over multiple rounds of interaction (King-Casas et al., 2005). Interestingly, this adaptive learning signal showed high correlation with the caudate (King-Casas et al., 2005) during phases of the game where one would plan to express trust (an ‘intention to trust’ response) with the expectation of future benefit from doing so. Notably, the same social exchange task used by King-Casas et al., has also been used to show diminished perspective-taking related responses in the middle cingulate cortex in adolescents diagnosed with ASD (Chiu et al., 2008; Kishida et al., 2012). King-Casas and colleagues showed that these three regions (vmPFC, caudate, and middle cingulate cortex) form a functional network that adapts over multiple rounds of interaction as partners learn to trust one another. Altogether, this background focused our attention on the vmPFC as a candidate region for us to investigate differential valuation responses of TD and ASD children to social (i.e. faces) and non-social (i.e. objects) stimuli. Future work should aim to better understand the role of the vmPFC in social exchange and how it coordinates with other brain regions to execute successful social exchange.
This vmPFC-caudate (cortico-striatal) circuitry is a critical component of reward processing and decision-making in humans. Both the caudate and the vmPFC are densely innervated by dopamine-releasing nerve terminals that are believed to be activated by events that signal errors in expected rewards (Montague et al., 1996; Montague et al., 2004; Schultz et al., 1997). Recent intracranial work investigating the mechanisms underlying reward-processing and adaptive behavioral control in the human brain has shown that learning signals about actual outcomes and counterfactual alternative possible outcomes (e.g., ‘what could have been’) are integrated in sub-second dopamine fluctuations in the human caudate (Kishida et al., 2016); moreover, coincident serotonin fluctuations in the same location and on the same time-scale coordinate with dopamine fluctuations to modulate risky behaviors in humans (Moran et al., 2018). Further work will be needed to determine the role that sub-second dopamine and serotonin fluctuations play in modulating human social exchange. With the advent of new technology that permits direct real-time recordings of dopamine, serotonin, and norepinephrine throughout the human brain (Montague and Kishida, 2018) these questions may be answered. Whether changes in learning signals carried by these neuromodulators underlie value representations in the vmPFC that promote or hinder the development of social interaction should be explored. Further, the ability to directly monitor these kinds of signals should motivate investigations into the impact medications targeting serotonin, dopamine, and norepinephrine release and reuptake may have on adaptive social exchange since these medications are often used in patients diagnosed with ASD and comorbid psychiatric conditions.
We demonstrated an attenuated vmPFC response to a single presentation of favorite face stimulus in ASD children compared to TD children. Diminished reward circuitry activation to social incentives in ASD is consistent with findings reported by Scott-Van Zeeland et al (2010). Using an implicit learning task, they showed that children with ASD had reduced anterior cingulate cortex (ACC), vmPFC and NAcc activation to social rewards (i.e. smiling faces) compared to control children (Scott-Van Zeeland et al., 2010). Recent work by Kohls et al., (2013) also showed depressed reward circuitry response to social rewards in the ventral ACC in ASD children. In contrast to our findings, Dichter and colleagues (Dichter et al., 2012b) reported comparable vmPFC response to social incentives in ASD and TD adults. Differences in the social reward used in both studies may account for this discrepancy. The use of subject-relevant, favorite faces possibly elicited a greater reward response in TD children and enabled detection of differential vmPFC response between the two groups. Faces with neutral expressions were used as social rewards in Dichter’s study.
Single stimulus vmPFC response to favorite objects in ASD children were comparable to the TD group. In contrast, a previous study (Dichter et al., 2012a) reported greater vmPFC response to objects in adults with ASD compared to control participants during the reward outcome phase of a monetary incentive delay task. The objects used in this experiment were shown, using eye-tracking, to be visually salient and highly interesting to individuals with ASD. This initial bias towards objects preferred by ASD participants could account for the significantly greater vmPFC response in the ASD group in this study. Here, the use of favorite objects - objects that were relevant to and liked by each individual subject – may have contributed to diminishing the difference between ASD and TD cohorts’ responses to favorite objects.
Notably, the ability to probe reward responses using a single-stimulus fMRI paradigm provides a useful tool for better understanding neuropsychiatric disorders such as ASD, where reward dysfunction has been speculated to play a role. Such an assay is especially useful for settings (i.e. pediatric population, neurodevelopment disorders, cognitive impairment) where prolonged scanning is not ideal or possible (O’Shaughnessy et al., 2008; Wilke et al., 2003). Moreover, our findings suggest the possibility of using fast and simple fMRI-based assays to diagnose and identify ASD subtypes.
In the present study, we recruited participants who had an initial diagnosis of ASD and were currently being treated clinically and behaviorally for ASD. These individuals represent a complex heterogeneous population. The process of providing the research “gold standard” characterization is a complex emotionally strenuous process for both patients and caregivers. Further these measures (i.e., ADOS) require trained practitioners to spend multiple hours with each patient. This precludes many individuals (in non-research settings) from acquiring such characterization, because of the time commitment required and practitioner availability; indeed, many centers that offer this level of characterization are back-logged with waiting lists that extend out for several months. Thus, we did not include or exclude potential ASD participants based on what is commonly referred to as “gold standard” research criteria – though most of our participants diagnosed with ASD did have this assessment performed. That three of our participants were missing this assessment may be seen by some as a limitation of our study; however, we sought to include as heterogenous a population of ASD participants as possible in order to begin to characterize this population as they are seen clinically and in society. Our analyses are thus constrained to ask questions about categorical differences between the TD and ASD cohorts, which we report. Future work could incorporate a more complete behavioral phenotyping profile for each participant to determine if any of the behavioral measures contained in those assessments may map directly to the brain response effects we report here. Additionally, we sought to fully characterize participants using the SRS and KBIT exams for completeness; however, our study suffered from incomplete characterization on these scales due to participants not returning to the lab to complete these tests. However, we propose that individuals are diagnosed and treated with Autism Spectrum Disorder based on physicians’ opinions about whether patients meet DSM criteria (not research gold standards); thus, in order to transition to more objective brain-based measure for diagnosis, such brain-based measures should be characterized in the whole population of those carrying the diagnosis of ASD and not just those that can endure research gold standard assessments.
ASD disproportionately affects male versus female patients, which is reflected in our sample study population with 3 female out of 17 total ASD participants. While truer to the greater ASD population, this ratio is not found in the TD sample or population, with both closer to an equal sex divide. This unequal comparison is a limitation of the study, though no differences across gender is anticipated for the TD population. Future work may address this concern with specific study designs powered to investigate sex differences in ASD and TD populations.
Individuals with excessive head movements had to be excluded from the final analyses due to the current limitations of fMRI technology. This has the potential to bias the study population towards individuals who do not move and may impact the results. This is true for all brain imaging studies; however, by demonstrating in the present study that early BOLD responses are sufficient measurements, we provide a potential experimental design innovation, which would allow future investigations to capture BOLD imaging data from participants who cannot endure the length of a typical BOLD imaging experiment.
In our present work, single stimulus results showed vmPFC dysfunction related to processing of favorite faces, but not favorite objects, in ASD children. Our results suggest that perturbed valuation of social rewards in the vmPFC contributes to the impairments observed in autism: social stimuli are devalued, leading to decreased motivation to seek such rewards (Dawson et al., 1998; Dawson et al., 2005a). A behavioral consequence of this may be the lack of motivation to pursue social reinforcers - a core characteristic of individuals with ASD (Dawson et al., 2005b; Demurie et al., 2011). While the present neural data showed intact vmPFC response to favorite objects, the diminished appeal of social rewards may still manifest as extreme attachment to objects observed in ASD. Since only stimuli perceived as valuable by the brain would be preferentially pursued, typically rewarding social stimuli may be neglected while highly preferred objects may be intensely pursued (Lam et al., 2008; Sasson et al., 2011). Future therapeutic interventions may be able to use these conclusions to develop individual difference biomarkers based on specific changes in brain responses to favorite images and images of one’s self (Lu et al., 2015). Such biologically based objective measures will become important for augmentation of clinical opinions and stratifying the spectrum of individuals that comprise Autism Spectrum Disorder.
Supplementary Material
Highlights.
Some social deficits in ASD are hypothesized to be due to neural under-valuation of social vs. non-social interactions.
The vmPFC is known to encode value across a variety of dimensions including actions.
ASD children show diminished single-stimulus responses to pictures of people, but not favorite objects.
Differences between ASD and TD children can be captured with single-stimulus fMRI using less than 30s of BOLD data.
Acknowledgements
This work was funded by a Wellcome Trust Principal Research Fellowship (PRM), Kane Family Foundation (PRM), Autism Speaks (PRM), the Charles A. Dana Foundation (PRM) and the National Institutes of Health (RO1 DA11723 (PRM), RO1 MH085496 (PRM), T32 NS43124 (KTK), UL1TR001420-KL2 (KTK). The authors would like to thank the NEMO software development team for their assistance in programming the stimulus presentation scripts, and the Human Neuroimaging Laboratory’s technological staff for their assistance with recruitment and scanning.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Disclosures
We report no financial conflicts of interest. We report no conflicts of interest relevant to this article.
References
- Aharon I, Etcoff N, Ariely D, Chabris CF, O’Connor E, Breiter HC, 2001. Beautiful faces have variable reward value: fMRI and behavioral evidence. Neuron 32, 537–551. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S, Campbell R, Karmiloff-Smith A, Grant J, Walker J, 1995. Are children with autism blind to the mentalistic significance of the eyes? British Journal of Developmental Psychology 13, 379–398. [Google Scholar]
- Bartels A, Zeki S, 2000. The neural basis of romantic love. Neuroreport 11, 3829–3834. [DOI] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW, 2013. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage 76, 412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE, 1998. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev 28, 309–369. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ, 1994. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. J Behav Ther Exp Psychiatry 25, 49–59. [DOI] [PubMed] [Google Scholar]
- Brooks R, Meltzoff AN, 2002. The importance of eyes: how infants interpret adult looking behavior. Dev Psychol 38, 958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caria A, Venuti P, de Falco S, 2011. Functional and dysfunctional brain circuits underlying emotional processing of music in autism spectrum disorders. Cereb Cortex 21, 2838–2849. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Kayali MA, Kishida KT, Tomlin D, Klinger LG, Klinger MR, Montague PR, 2008. Self responses along cingulate cortex reveal quantitative neural phenotype for high-functioning autism. Neuron 57, 463–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, Todd RD, 2003. Autistic traits in the general population: a twin study. Arch Gen Psychiatry 60, 524–530. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Pierce K, 2005. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol 15, 225–230. [DOI] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff AN, Panagiotides H, McPartland J, Webb SJ, 2002. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Dev 73, 700–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J, Brown E, 1998. Children with autism fail to orient to naturally occurring social stimuli. J Autism Dev Disord 28, 479–485. [DOI] [PubMed] [Google Scholar]
- Dawson G, Osterling J, Rinaldi J, Carver L, McPartland J, 2001. Brief report: Recognition memory and stimulus-reward associations: indirect support for the role of ventromedial prefrontal dysfunction in autism. J Autism Dev Disord 31, 337–341. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J, 2004. Early social attention impairments in autism: social orienting, joint attention, and attention to distress. Dev Psychol 40, 271–283 [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, McPartland J, 2005a. Understanding the nature of face processing impairment in autism: insights from behavioral and electrophysiological studies. Dev Neuropsychol 27, 403–424. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb SJ, Wijsman E, Schellenberg G, Estes A, Munson J, Faja S, 2005b. Neurocognitive and electrophysiological evidence of altered face processing in parents of children with autism: implications for a model of abnormal development of social brain circuitry in autism. Dev Psychopathol 17, 679–697. [DOI] [PubMed] [Google Scholar]
- Demurie E, Roeyers H, Baeyens D, Sonuga-Barke E, 2011. Common alterations in sensitivity to type but not amount of reward in ADHD and autism spectrum disorders. J Child Psychol Psychiatry 52, 1164–1173. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Green SR, Rittenberg AM, Sasson NJ, Bodfish JW, 2012a. Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci 7, 160–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dichter GS, Richey JA, Rittenberg AM, Sabatino A, Bodfish JW, 2012b. Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord 42, 147–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erk S, Spitzer M, Wunderlich AP, Galley L, Walter H, 2002. Cultural objects modulate reward circuitry. Neuroreport 13, 2499–2503. [DOI] [PubMed] [Google Scholar]
- Farroni T, Johnson MH, Menon E, Zulian L, Faraguna D, Csibra G, 2005. Newborns’ preference for face-relevant stimuli: effects of contrast polarity. Proc Natl Acad Sci U S A 102, 17245–17250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fliessbach K, Weber B, Trautner P, Dohmen T, Sunde U, Elger CE, Falk A, 2007. Social comparison affects reward-related brain activity in the human ventral striatum. Science 318, 1305–1308. [DOI] [PubMed] [Google Scholar]
- Freilich ER, Gaillard WD, 2010. Utility of functional MRI in pediatric neurology. Curr Neurol Neurosci Rep 10, 40–46. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worsley KJ, Poline JP, Frith CD, Frackowiak RSJ, 1994. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp 2, 189–210. [Google Scholar]
- Gliga T, Csibra G, 2007. Seeing the face through the eyes: a developmental perspective on face expertise. Prog Brain Res 164, 323–339. [DOI] [PubMed] [Google Scholar]
- Goel V, Dolan RJ, 2001. The functional anatomy of humor: segregating cognitive and affective components. Nat Neurosci 4, 237–238. [DOI] [PubMed] [Google Scholar]
- Golarai G, Grill-Spector K, Reiss AL, 2006. Autism and the development of face processing. Clin Neurosci Res 6, 145–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Knutson B, 2010. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35, 4–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AH, Kirk U, Denfield GH, Montague PR, 2010. Monetary favors and their influence on neural responses and revealed preference. J Neurosci 30, 9597–9602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Saito DN, Sadato N, 2008. Processing of social and monetary rewards in the human striatum. Neuron 58, 284–294. [DOI] [PubMed] [Google Scholar]
- Johnson MH, Dziurawiec S, Ellis H, Morton J, 1991. Newborns’ preferential tracking of face-like stimuli and its subsequent decline. Cognition 40, 1–19. [DOI] [PubMed] [Google Scholar]
- Kampe KK, Frith CD, Dolan RJ, Frith U, 2001. Reward value of attractiveness and gaze. Nature 413, 589. [DOI] [PubMed] [Google Scholar]
- Kanner L, 1968. Autistic disturbances of affective contact. Acta Paedopsychiatr 35, 100–136. [PubMed] [Google Scholar]
- Kaufman A, Kaufman N, 1990. K-BIT: Kaufman Brief Intellignece Test: Manual. American Guidance Service. [Google Scholar]
- King-Casas B, Tomlin D, Anen C, Camerer CF, Quartz SR, Montague PR, 2005. Getting to know you: reputation and trust in a two-person economic exchange. Science 308, 78–83. [DOI] [PubMed] [Google Scholar]
- Kishida KT, Li J, Schwind J, Montague PR, 2012. New approaches to investigating social gestures in autism spectrum disorder. J Neurodev Disord 4, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Montague PR, 2012. Imaging models of valuation during social interaction in humans. Biol Psychiatry 72, 93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida KT, Saez I, Lohrenz T, Witcher MR, Laxton AW, Tatter SB, White JP, Ellis TL, Phillips PE, Montague PR, 2016. Subsecond dopamine fluctuations in human striatum encode superposed error signals about actual and counterfactual reward. Proc Natl Acad Sci U S A 113, 200–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Adams CM, Varner JL, Hommer D, 2001. Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 12, 3683–3687. [DOI] [PubMed] [Google Scholar]
- Knutson B, Fong GW, Bennett SM, Adams CM, Hommer D, 2003. A region of mesial prefrontal cortex tracks monetarily rewarding outcomes: characterization with rapid event-related fMRI. Neuroimage 18, 263–272. [DOI] [PubMed] [Google Scholar]
- Kohls G, Peltzer J, Schulte-Ruther M, Kamp-Becker I, Remschmidt H, Herpertz-Dahlmann B, Konrad K, 2011. Atypical brain responses to reward cues in autism as revealed by event-related potentials. J Autism Dev Disord 41, 1523–1533. [DOI] [PubMed] [Google Scholar]
- Kohls G, Schulte-Ruther M, Nehrkorn B, Muller K, Fink GR, Kamp-Becker I, Herpertz-Dahlmann B, Schultz RT, Konrad K, 2013. Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 8, 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam KS, Bodfish JW, Piven J, 2008. Evidence for three subtypes of repetitive behavior in autism that differ in familiality and association with other symptoms. J Child Psychol Psychiatry 49, 1193–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang P, Bradley M, Cuthbert B, 1999. International affective picture system (IAPS): Technical manual and affective ratings.
- Larson MJ, South M, Krauskopf E, Clawson A, Crowley MJ, 2011. Feedback and reward processing in high-functioning autism. Psychiatry Res 187, 198–203. [DOI] [PubMed] [Google Scholar]
- Levy DJ, Glimcher PW, 2012. The root of all value: a neural common currency for choice. Curr Opin Neurobiol 22, 1027–1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, Pickles A, Rutter M, 2000. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord 30, 205–223. [PubMed] [Google Scholar]
- Lu J, Kishida K, De Asis Cruz J, Lohrenz T, Deering DT, Beauchamp M, Montague PR, 2015. Single stimulus fMRI produces a neural individual difference measure for Autism Spectrum Disorder. Clin Psychol Sci 3, 422–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May JC, Delgado MR, Dahl RE, Stenger VA, Ryan ND, Fiez JA, Carter CS, 2004. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biol Psychiatry 55, 359–366. [DOI] [PubMed] [Google Scholar]
- McAlonan GM, Cheung V, Cheung C, Suckling J, Lam GY, Tai KS, Yip L, Murphy DG, Chua SE, 2005. Mapping the brain in autism. A voxel-based MRI study of volumetric differences and intercorrelations in autism. Brain 128, 268–276. [DOI] [PubMed] [Google Scholar]
- McClure SM, Daw ND, Montague PR, 2003. A computational substrate for incentive salience. Trends Neurosci 26, 423–428. [DOI] [PubMed] [Google Scholar]
- McClure SM, Li J, Tomlin D, Cypert KS, Montague LM, Montague PR, 2004a. Neural correlates of behavioral preference for culturally familiar drinks. Neuron 44, 379–387. [DOI] [PubMed] [Google Scholar]
- McClure SM, York MK, Montague PR, 2004b. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist 10, 260–268. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL, 2000. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage 11, 735–759. [DOI] [PubMed] [Google Scholar]
- Montague PR, Kishida KT. 2018. Computational underpinnings of neuromodulation in humans. Cold Spring Harb Symp Quant Biol doi: 10.1101/sqb.2018.83.038166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Berns GS, 2002. Neural economics and the biological substrates of valuation. Neuron 36, 265–284. [DOI] [PubMed] [Google Scholar]
- Montague PR, Dayan P, Sejnowski TJ, 1996. A framework for mesencephalic dopamine systems based on predictive Hebbian learning. J Neurosci 16, 1936–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montague PR, Hyman SE, Cohen JD, 2004. Computational roles for dopamine in behavioural control. Nature 431, 760–767. [DOI] [PubMed] [Google Scholar]
- Moran RJ, Kishida KT, Lohrenz T, Saez I, Laxton AW, Witcher MR, Tatter SB, Ellis TL, Phillips PE, Dayan P, Montague PR, 2018. The Protective Action Encoding of Serotonin Transients in the Human Brain. Neuropsychopharmacology 43, 1425–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy P, Sigman M, Ungerer J, Sherman T, 1986. Defining the social deficits of autism: the contribution of non-verbal communication measures. J Child Psychol Psychiatry 27, 657–669. [DOI] [PubMed] [Google Scholar]
- Nation K, Penny S, 2008. Sensitivity to eye gaze in autism: is it normal? Is it automatic? Is it social? Dev Psychopathol 20, 79–97. [DOI] [PubMed] [Google Scholar]
- Noriuchi M, Kikuchi Y, Senoo A, 2008. The functional neuroanatomy of maternal love: mother’s response to infant’s attachment behaviors. Biol Psychiatry 63, 415–423. [DOI] [PubMed] [Google Scholar]
- O’Doherty J, Winston J, Critchley H, Perrett D, Burt DM, Dolan RJ, 2003. Beauty in a smile: the role of medial orbitofrontal cortex in facial attractiveness. Neuropsychologia 41, 147–155. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy ES, Berl MM, Moore EN, Gaillard WD, 2008. Pediatric functional magnetic resonance imaging (fMRI): issues and applications. J Child Neurol 23, 791–801. [DOI] [PubMed] [Google Scholar]
- Peters J, Buchel C, 2009. Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci 29, 15727–15734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangel A, Camerer C, Montague PR, 2008. A framework for studying the neurobiology of value-based decision making. Nat Rev Neurosci 9, 545–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter W, Richter M, 2003. The shape of the fMRI BOLD response in children and adults changes systematically with age. Neuroimage 20, 1122–1131. [DOI] [PubMed] [Google Scholar]
- Rutter M, 1978. Diagnosis and definition of childhood autism. J Autism Child Schizophr 8, 139–161. [DOI] [PubMed] [Google Scholar]
- Sasson NJ, Elison JT, Turner-Brown LM, Dichter GS, Bodfish JW, 2011. Brief report: Circumscribed attention in young children with autism. J Autism Dev Disord 41, 242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz N, Rubia K, van Amelsvoort T, Daly E, Smith A, Murphy DG, 2008. Neural correlates of reward in autism. Br J Psychiatry 192, 19–24. [DOI] [PubMed] [Google Scholar]
- Schultz RT, 2005. Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area. Int J Dev Neurosci 23, 125–141. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR, 1997. A neural substrate of prediction and reward. Science 275, 1593–1599. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY, 2010. Reward processing in autism. Autism Res 3, 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR, 2008. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 122, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tversky A, Kahneman D, 1981. The framing of decisions and the psychology of choice. Science 211, 453–458. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Altaye M, Gaser C, 2008. Template-O-Matic: a toolbox for creating customized pediatric templates. Neuroimage 41, 903–913. [DOI] [PubMed] [Google Scholar]
- Wilke M, Holland SK, Myseros JS, Schmithorst VJ, Ball WS Jr., 2003. Functional magnetic resonance imaging in pediatrics. Neuropediatrics 34, 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yerys BE, Jankowski KF, Shook D, Rosenberger LR, Barnes KA, Berl MM, Ritzl EK, Vanmeter J, Vaidya CJ, Gaillard WD, 2009. The fMRI success rate of children and adolescents: typical development, epilepsy, attention deficit/hyperactivity disorder, and autism spectrum disorders. Hum Brain Mapp 30, 3426–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zion-Golumbic E, Golan T, Anaki D, Bentin S, 2008. Human face preference in gamma-frequency EEG activity. Neuroimage 39, 1980–1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.