Abstract
Mercury (Hg) toxicity is governed by cellular thiol compounds and its capacity to generate reactive oxygen radicals and oxidative stress. Selenium (Se) plays a key role in the prevention of the toxic effects of Hg by modulating the activity of several Se-dependent enzymes, including glutathione peroxidase (GSH-Px). In addition, dietary Se can reduce Hg toxicity by directly interacting with either Hg(II) or methylmercury (MeHg) to form inert products, such as HgSe complexes.. Although experimental and environmental data have indicated a protective role for selenium against Hg toxicity, human data are more limited and somewhat conroversial In the Amazon Region of Brazil, Hg pollution is rampant as a result of gold (Au) mining and other anthropogenic factors, leading to pervasive release of large quantities of metallic Hg0 into the environment. Exposure to Hg in this region is associated with direct occupational exposure in the gold mining industry, as well as consumption by in inhabitants of riverside communities of a diet rich in MeHg-contaminated fish. Human exposure to MeHg in the Amazon through the diet has been monitored by measuring Hg and MeHg in hair samples. In this paper, we review the environmental contamination of Hg in the Amazon and detail human exposures in populations of this region. We conclude with a brief synopsis on Se levels in the Amazon population and provide a brief review of data available on the interaction between Hg and Se in this region. Overall, the literature supports the notion that low environmental Se is linked to susceptibility to Hg toxicity and that Se levels could be used as a bioindicator to monitor the health of Hg exposed subjects. However, in light of the limited human data on this subject, further epidemiological studies are needed to clarify how changes in Se levels modify the toxicity of environmental Hg.
Keywords: mercury, exposure, methylmercury, selenium, Tapajós river basin, Amazon
MERCURY
Mercury (Hg) is a chemical element found in the environment in several different forms. Elemental Hg (Hg0) is a silvery liquid metal at room temperature and pressure that easily evaporates with increasing temperature. Mercury is used in the manufacture of industrial chemicals for electrical and electronic applications, in a limited number of thermometers and as a gas in fluorescent lamps; its use for most other applications has been largely discontinued over the last several decades due to health and safety regulations. Mercury forms inorganic compounds with other elements, such as chlorine, sulfur, selenium and oxygen. The formation of insoluble Hg salts with sulfur and, particularly with selenium can have a profound effect of the fate of this toxic element. In fact, HgSe (the analogous selenium salt of cinnabar) is extremely insoluble and its formation both outside the living cell (abiota) and in the cellular environment can reduce the bioavaibility and toxicity of mercury. Experimental and environmental data clearly indicate that selenium status can considerably affect the availability and, consequently, the toxicity of different forms of mercury (Berry and Ralston 2008). The majority of inorganic Hg compounds are white powders or crystals with the exception of cinnabar (mercuric sulfide), which is red, turning black upon exposure to light. Some Hg compounds have been used as fungicides, antiseptics, vaccine preservatives, disinfectants, laxatives, diuretics, nasal sprays, cosmetics and many other biomedical applications; however, due to the propensity of mercurials to cause health effects in humans and ecological systems, their usage has been greatly curtailed (ATSDR, 1998).
Mercury is a global pollutant with no environmental boundaries. Even the most stringent control of Hg pollution from man-made sources will not eliminate human exposure to potentially toxic quantities, given its ubiquitous presence in the environment. The largest global repository for Hg is found in ocean sediments, estimated to contain a total of about 1017 g of Hg, mainly in the form of HgS, which is a relatively inert form of this toxic element. Ocean waters contain around approximately 1013 g, soils and freshwater sediments 1013 g, the biosphere 1011 g (mostly in land biota), the atmosphere 108 g, and freshwater 107 g of Hg. This budget excludes “unavailable” Hg in mines and other subterranean repositories. According to the 1997 EPA report on Hg to the US Congress (http://www.epa.gov/hg/report.htm), recent estimates of total annual natural and anthropogenic Hg emissions are about 4,400 to 7,500 metric tons, with Asia accounting for 53% of the total emissions, followed by Africa (18%), Europe (11%), North America (9%), Australia (6%), and South America (4%). Roughly 2/3 of the total emissions are anthropogenic, mainly from coal combustion and industrial uses.
Mercury is preferentially released into the environment as Hg0 vapor. In the atmosphere, it undergoes several transformations, the first of which is its oxidation to Hg ions (mainly Hg2+). It exists in nature mainly as three different chemical species: elemental (Hgo), ionic (Hg2+), and organic (MeHg). Hg released into the environment from both natural and anthropogenic sources is sustained in the marine ecosphere, where it methylates in the upper sedimentary layers of sea and lake beds by the action of microorganisms (Clarkson and Magos 2006). The methylated form, MeHg, is rapidly taken up by living organisms in the aquatic environment and biomagnified through the food chain reaching concentrations in fish 10,000–100,000 times greater than the surrounding water. Once methylated, MeHg reaches humans predominantly from fish consumption. Nearly all fish contain detectable amounts of MeHg. However, the enrichment of MeHg in the aquatic food chain is not uniform and is dependent upon the Hg content in the water and bottom sediments, pH and redox potential of the water, species and age, as well as the size of the fish. In addition, environmental conditions, such as anoxia, favor the growth of microorganisms, increasing the methylation rate of Hg, and by inference its accumulation in fish (WHO 1990; ATSDR 1998; Lacerda and Malm 2008; Clarkson and Magos 2006).
Human Exposure to Mercury Vapor
Human exposure to Hg0 occurs through the occasional inhalation of Hg0 vapor or, more often, continuous chronic exposure associated with occupational activities. Monitoring Hg levels in the environment and urine of exposed subjects is essential for assessing risk for human health. According to the World Health Organization (WHO), 25 μg/m3 air represents the maximum allowed Hg concentration allowed for chronic occupational exposure, while 500 μg/m3 air is the maximum Hg concentration acceptable for acute exposure (WHO 1991; WHO 2003). In addition, the threshold for Hg concentration in the urine associated with the onset of subclinical toxic effects in exposed humans is 30 μg/g creatinine, while the maximal permissible Hg concentration in the urine is 50 μg/g creatinine (WHO 1991; WHO 2003). Dentists represent one such occupational group of workers chronically exposed to low levels of Hg0 (Magro et al. 1994). However, the importance of this type of exposure as a health risk for these and similar populations has yet to be definitively confirmed by appropriate quantitative evaluation methods of physiological functions (Silveira et al. 2003; Ventura et al. 2004; 2005; do Canto Pereira et al. 2005; Rodrigues et al. 2007; da Costa et al. 2008).
Human Exposure to Methylmercury from Contaminated Food
Several environmental accidents have been associated with ingestion of food contaminated with organic Hg compounds. Fish and seafood contaminated with MeHg constitute the most frequent source of non-occupational human exposure to this metal. Vegetables and cereals treated with organomercurial fungicides have also been associated with episodes of human intoxication (Bakir et al. 1973; Davis et al. 1994). The Minamata Bay disaster (Harada 1982) and similar epidemics in Iraq, Canada (Wheatley 1994) and China (Soong et al. 1994), just to name a few, were caused by the ingestion of MeHg contaminated food originating from industrial plants or the use of organic mercurials in crop protection, a practice that has been abandoned.
Mercury Toxicity
All Hg forms are toxic. Acute exposure to Hg0 causes lung, kidney, and brain damage, while long lasting exposure to low levels of Hg0 causes a neurological and psychiatric syndrome – the so called “mad hatter’s disease” (Faintuch and Rocha 1990; Rodrigues et al. 2007; Silveira et al. 2003; WHO 1991; WHO 2003).
Methylmercury formed in aquatic systems (see above) where methylation of inorganic Hg by methanogenic bacteria leads to its release into water and bioaccumulation in the food chain. When ingested, MeHg readily binds to cysteine, forming a methylmercuric-cysteinyl complex. This complex is recognized by amino acid transporting proteins, such as the neutral amino acid transporter LAT1; (Yin et al. 2008), which allows for its transport across membranes. The mobility of the methylmercuric-cysteinyl complex allows it to cross not only the blood-brain barrier (BBB), but also the placenta. Chronic exposure to MeHg is characterized by severe and irreversible neurological impairment (Bakir et al. 1973; Harada 1982; WHO 1990). Adult patients with the classical form of MeHg intoxication, called Hunter-Russell syndrome or Minamata disease, show somatic sensory disturbances with paresthesia, affecting upper and lower limb extremities, deafness, peripheral visual field blindness, disturbed gait and lack of coordination, motor impairment and tremor (WHO 1990). Minamata disease takes an extremely severe form in pregnant women exposed to large amounts of MeHg (Harada 1982; 1995; WHO 1990). High MeHg exposure early in pregnancy leads to embryo-lethality, whereas at later stages exposure to MeHg causes profound impairment in neural development, resulting in a form of newborn cerebral palsy. Mothers giving birth to children with severe symptoms may remain asymptomatic or show mild forms of the disease, establishing the heightened sensitivity of the developing brain to MeHg exposure (Harada 1995; 1997). Chronic exposure to low levels of MeHg during pregnancy is of concern since MeHg can readily reach the fetus. In general, MeHg levels in the fetus exceed maternal levels, reflecting an incomplete blood-brain barrier and lack of MeHg excretion in the fetus. The most significant difference in organ retention (neonates > adults) is associated with MeHg brain concentrations. The developing fetus is 5 to 10 times more sensitive to MeHg than the adult due, in part, to the high sensitivity of developmental processes (i.e., cellular division, differentiation, and migration) to disruption by MeHg, as well as increased MeHg concentrations in fetal brains (referred to as a “sink” for MeHg). Thus, in view of the exacerbated sensitivity of developing brain to chemicals, there is a great concern over a silent pandemic (Grandjean 2008), and MeHg can be considered one of the most significant of these environmentally-derived neurotoxic agents.
MERCURY IN THE AMAZON
Anthropogenic activities in the Amazon are an important source of environmental Hg contamination. Gold mining is the main source of Hg0 emission into the atmosphere and it is responsible for the contamination of various Amazon ecosystems. In the rudimentary form of gold mining, still used in the Amazon, it is estimated that for every kilogram of gold, 1–2 kg of Hg0 are released in the environment and that at the peak of gold mining activity, two decades ago, as much as 1,500 t of Hg0 per year were released into the Amazon environment (Pfeiffer and Lacerda 1988). Since 1989, Amazon gold production has decreased, but the global Hg load released in the environment during all these years continues to represent a considerable risk for the local population living in this region. An additional anthropogenic source of Hg pollution in the Amazon is the continuous burning of the tropical forest biomass (releasing Hg into the environment) and soil erosion in the deforested regions that have been transformed into human settlements (Roulet et al. 1999; Veiga et al. 1994).
Mercury in the Soil
In terrestrial ecosystems, Hg originating from gold mining is estimated to be less than 3% of the total amount found in the surface of horizontal soils. In cities where gold commerce is economically vibrant, such as Poconé (Mato Grosso), samples from residues and soil obtained from the city center and periphery showed high Hg concentrations: 1.91 and 0.09 μg/g in the city center, 63.3 and 3.2 μg/g in the city periphery for residues and soil, respectively. The values in residues from the city center and periphery, as well as the value in the soil of city periphery are significantly higher than those reported in a control region located outside the city limits at 0.25 and 0.27 μg/g for residues and soil, respectively, where no activity related to gold mining and gold commerce has been reported (Câmara et al. 1997).
Mercury in the Water
The aquatic biota are the main Hg transfer route from the contaminated environment to humans (Veiga et al. 1994). Hg found in water and sediments, either as inorganic Hg or MeHg, accumulates in the phytoplankton (see above) and progressively biomagnifies from herbivorous, detrivorous, omnivorous, planktivorous, carnivorous-omnivorous, to ictiophagous carnivorous (Bidone et al. 1997).
Several studies in the Amazon have demonstrated that the Hg concentrations in different fish species vary across a large range of values, but is specifically elevated in predator species, those most likely to be consumed by the “ribeirinhos” (riverines) (Lodenius et al. 1992; Akagi et al. 1994; Malm et al. 1995, 1997; Eve et al. 1996; Bidone et al. 1997; Lebel et al. 1997; Dorea et al. 1998; Kehrig et al. 1998; Brabo et al. 1999; Lima et al. 2000; Faial et al. 2003; Santos et al. 2005). In addition to the riverines, the entire population of the Amazon (and “pantanal”) may be considered at risk of exposure to potentially toxic levels of MeHg via consumption of contaminated carnivorous fish. Of additional concern is the fact that some Amazonian fish products (like salted fish, etc.) are also sold in other regions of Brazil. Thus, Hg derived from anthropogenic and natural sources in the Amazon may also be reaching distant centers in Brazil and the world.
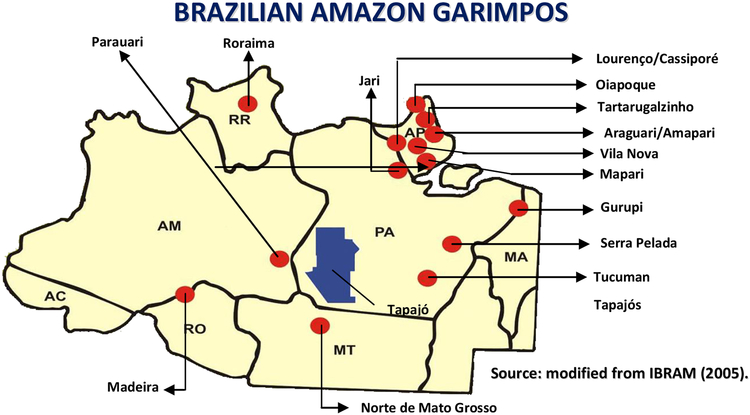
Several fish species in the Tapajós River (Figure 1) that are regularly consumed by the riverines contain high Hg concentrations, well in excess of the permissible limits for human food allowed by the Brazilian Health Regulatory Agencies (Ministério da Saúde do Brasil 1975). The most contaminated fish species are tucunaré (Cichla ocellaris), pescada (Plagioscion surinamensis), filhote (Brachysplatistoma filamentosum), dourada (Brachysplatistoma flovicans), peixe-cachorro (Hydrolycus scomberoides), and traíra (Hoplias malabarilus) (Bidone et al. 1997; Malm et al. 1997) (Tables 1–2). In other locations in the Amazon (Figure 1), such as the Madeira River and the Negro River, a similar situation has been found. Generally, fish with high Hg content are found in locations with intense gold mining activity. However, this is not the case for the Negro River where there is no significant gold mining activity and fish with high Hg levels have been consistently found, suggesting another source of Hg in this region or conditions that promote higher uptake rates. Tables 1–2 show the mean Hg concentrations in fish consumed by Amazon riverines (Lodenius et al. 1992; Akagi et al. 1994; Malm et al. 1995, 1997; Eve et al. 1996; Bidone et al. 1997; Lebel et al. 1997; Dorea et al. 1998; Kehrig et al. 1998; Brabo et al. 1999; Lima et al. 2000; Faial et al. 2003).
Figure 1.
Gold mines, called Garimpos in Portuguese, are located throughout several states of the Brazilian Amazon: Amapá (AP), Amazonas (AM), Maranhão (MA), Mato Grosso (MT), Pará (PA), Rondônia (RO), and Roraima (RR). In these locations, gold mining is very often performed using artisanal processes that release large amounts of elemental mercury (Hg0) in the environment.
Table 1.
Total Hg concentration in fish consumed by Amazon populations living in communities located in the Tapajós River basin, 1994–2000.
| Location | Region | Habits | N | Hg-total (min-max) (μg / g) (*) |
Reference |
|---|---|---|---|---|---|
| Various | Tapajós | C | 21 | (0.28 – 3.82) | Akagi et al. 1994 |
| Alta Floresta | Teles Pires | C, NC | 24 | (0.80 – 3.82) | Akagi et al. 1995 |
| Various | Tapajós | C | 16 | 0.55 (0.04 – 3.77) | Malm et al. 1995 |
| Various | Tapajós | C | 98 | 0.42 ± 0.23 | Bidone et al. 1997 |
| Various | Tapajós | NC | 140 | 0.06 ± 0.05 | Bidone et al. 1997 |
| Brasília Legal | Tapajós | C | 73 | 0.51 ± 0.22 (0.06 – 1.35) | Lebel et al. 1997 |
| Brasília Legal | Tapajós | NC (O) | 32 | 0.46 ± 0.24 | Lebel et al. 1997 |
| Brasília Legal | Tapajós | NC (H) | 68 | 0.07 ± 0.07 | Lebel et al. 1997 |
| Various | Tapajós | C | 122 | 0.48 ± 0.00 | Malm et al. 1997 |
| Jacareacanga | Tapajós | C | - | 0.29 ± 0.10 | Brabo et al. 1999 |
| Jacareacanga | Tapajós | NC | - | 0.11 ± 0.04 | Brabo et al. 1999 |
| Jacareacanga | Tapajós | C | 32 | 0.30 (0.65 – 0.55) | Brabo et al. 2000 |
| Jacareacanga | Tapajós | NC | 48 | 0.10 (0.03 – 0.20) | Brabo et al. 2000 |
| Santarém | Tapajós | C | 69 | 0.22 (0.08 – 0.89) | Lima et al. 2000 |
| Santarém | Tapajós | NC | 40 | 0.04 (0.00 – 0.14) | Lima et al. 2000 |
C, carnivorous. NC, non-carnivorous. NC (O), non-carnivorous (omnivorous). NC (H), non-carnivorous, herbivorous.
Original values rounded to the second decimal.
Table 2.
Total Hg concentration in fish consumed by Amazon populations living in locations outside the Tapajós River basin, 1994–2000.
| Location | Region | Habits | N | Hg-total (min-max) (μg / g) (*) |
References |
|---|---|---|---|---|---|
| Rondônia | Madeira | C | 284 | 0.85 (− 0.39) | Malm et al. 1997 |
| Amazonas | Madeira | C | - | 0.33 | Eve et al. 1996 |
| Madeira | C | - | 4.00 | Dorea et al. 1998 | |
| Madeira | NC | - | 0.90 | Dorea et al. 1998 | |
| Balbina | - | C | 14 | 0.30 ± 0.20 (0.06 – 0.70) | Kehrig et al. 1998 |
| Balbina | - | NC | 10 | 0.06 ± 0.03 (0.03 – 0,10) | Kehrig et al. 1998 |
| Tucuruí | - | C | 230 | 1.10 | Lodenius et al. 1992 |
| Trombetas | C | 103 | 0.22 (0.05 – 0.87) | Faial et al. 2003 | |
| Trombetas | P | 40 | 0.35 (0.04 – 1.34) | Faial et al. 2003 | |
| São Gabriel | Negro | C | 140 | 0.45 ± 0.51 (0.04 – 3.92) | Santos et al. 2005 |
| São Gabriel | Negro | NC | 76 | 0.10 ± 0.08 (0.00 – 0.55) | Santos et al. 2005 |
| Barcelos | Negro | C | 126 | 0.46 ± 0.30 (0.06 – 1.93) | Santos et al. 2005 |
| Barcelos | Negro | NC | 147 | 0.10 ± 0.08 (0.02 – 0.61) | Santos et al. 2005 |
C, carnivorous. NC, non-carnivorous. P, piscivorous.
Original values rounded to the second decimal.
Human Exposure to Mercury in the Amazon
There are two pathways for Hg exposure in the Amazon region, namely, occupational exposure to inhaled Hg0 and to a lesser extent via cutaneous absorption, and dietary exposure to MeHg-contaminated fish. In the next section we will detail the available information on these exposure modalities within the Amazon region.
Occupational Exposure to Mercury Vapor
Inhalation of Hg0 vapor is the most common form of human Hg exposure and it is generally associated with occupational activities (Branches et al. 1993; Faintuch and Rocha 1990). In the Amazon, occupational Hg0 exposure is inherent to the artisanal process of gold mining and gold commerce, where large amounts of Hg0 are used to amalgamate the gold during its commercial processing (Figure 1). As a result of this process, gold miners in the “garimpos” (gold mines) and gold dealers in the gold shops, as well as other professionals working in the same environment and industry are commonly exposed to excessive levels of Hg (Akagi et al. 1995; Branches et al. 1993; Câmara et al. 1997; Couto et al. 1988; Souza et al. 2003). Several studies have reported total-Hg concentrations in hair samples obtained from gold miners from different garimpos zones: Cachoeiro do Piriá (Pará), mean total-Hg concentration 11.49 μg/g, range 1.97–68.98 μg/g (Couto et al. 1988); Cumarú (Pará), mean total-Hg concentration 5.18 μg/g, range 1.50–13.68 μg/g (Couto et al. 1988). Despite the relatively high Hg levels in gold miner hair samples, the degree of exposure to Hg0 vapor remains controversial as no data exist on the contribution of fish-derived Hg from dietary sources to the overall body-burden of Hg in these individuals. In addition, the Hg amount in hair samples is not a good indicator of body Hg levels in cases of occupational exposure to Hg0 vapor due to possible external contamination of the samples (Couto et al. 1988).
Blood Hg concentrations were measured in 55 patients with history and symptoms of Hg exposure between 1986 and 1991 (Branches et al. 1993). Among them, 33 patients (60%) had been occupationally directly exposed to Hg0 vapor either in Garimpos or gold shops. The mean total-Hg concentration in the blood was 30.5 μg/l and the values ranged between 4–130 μg/l. The reported symptoms were consistent with elemental Hg intoxication, namely impairment of cerebellar function, lack of movement coordination, gait imbalance and tremor (Branches et al. 1993).
A survey of 121 gold miners and 102 other residents of Garimpo do Rato (Pará), a gold mining settlement, revealed that 25 subjects had urine total-Hg ≥ 10 μg/l and 22 of them had symptoms of chronic intoxication by Hg0 vapor (Santos et al. 1995). In another survey in Cachoeiro do Piriá (Pará), 20 gold miners had total-Hg concentration in the urine ranging between 1.3–300 μg/g creatinine, differing significantly from control subjects living in areas located at remote distances from gold mines (Pinheiro et al. 2004).
Câmara and colleagues (Câmara et al. 1997) evaluated the effects of gold commerce in the city environment and in the health of residents of Poconé (Mato Grosso). They found that the environment contamination and human exposure in the city center was due to Hg0 emissions in gold shops, while in the city periphery Hg contamination was associated with the burning of gold-mercury (Au-Hg) amalgam inside residences. The mean total-Hg concentration in urine collected from subjects in the city center and periphery were 4.35 and 4.89 μg/l, respectively, compared to 1.25 μg/l in a control area. Anxiety, irritability, memory loss, insomnia, asthenia, all of which are characteristic symptoms of mercurial erethism, were noted among subjects with high urinary Hg (Câmara et al. 1997).
Total-Hg concentration in the urine upon exposure to Hg0 vapor was also reported in 46 workers of 17 gold shops of Itaituba. The mean total-Hg0 concentration in the gold shop atmosphere during 15 days of assessment was approximately one hundred times higher compared with the control shops, where Au-Hg amalgam burning was absent. The mean total-Hg concentration in the gold shop workers’ urine was 15.5 μg/l. In 51% of them the mean total-Hg concentration was higher than the limit of 10 μg/l, and in 3% of them higher than 50 μg/l (Souza et al. 2003).
Occupational exposure to Hg0 vapor was also evaluated in São Chico and Crepurizinho, two Garimpos from the region of Itaituba (Pará) (de Jesus 2005). In São Chico, the mean total-Hg concentration was 9.3 ± 13.7 μg/g creatinine (range 0.08–78.5 μg/g creatinine) and 28.1 ± 23.5 μg/l (range 3.9–141.0 μg/l) in the urine and blood, respectively. In Crepurizinho it was 6.1 ± 9.6 μg/g creatinine (range 0.01–61.6 μg/g creatinine) and 17.6 ± 18.3 μg/l (range 0.7–119.5) μg/l.
These data indicate that gold miners and gold shop workers represent Amazonian groups at high risk for occupational exposure to Hg0 vapor and that they should be carefully monitored for the potential chronic health effects of exposures. As mentioned above, Se can have protective effects against Hg toxicity (Ralston et al. 2008a). Consequently, it would be important in future studies to determine the whether Se can serve as a biodicator of susceptibility to Hg exposure in Amazonian exposed population. In addition, a vigorous educational campaign accompanied by improvement in their labor conditions should be considered as a top priority to reduce the potential for excessive Hg0 vapor exposures.
Methylmercury Exposure through the Diet
Populations living in regions polluted by Hg and consuming a diet rich in fish are particularly at risk to the noxious effects of MeHg (WHO 1990). Risks associated with exposure to MeHg-adulterated fish have been also recognized in the Amazon region, where gold mining activity leads to pervasive contamination of the environment with MeHg and by inference consumption of MeHg from contaminated fish. Several studies performed in different Amazon riverside communities have revealed high total-Hg levels in hair samples of the native populations (Tables 3–5).
Table 3.
Total Hg concentration in hair samples from Amazon populations living in communities located in the Tapajós River basin, 1994–2003.
| Location | Region | N | Hg-total (min-max) (μg / g) (*) |
Reference |
|---|---|---|---|---|
| Itaituba | Tapajós | 125 | 20.1 (0.8 – 151.2) | Akagi et al. 1994 |
| Itaituba | Tapajós | 51 | 30.3 ± 15.9 | Akagi et al. 1995 |
| Jacareacanga Brasília Legal |
Tapajós | - | 25.0 (− 151.0) | Malm et al. 1995 |
| Brasília Legal | Tapajós | 96 | 12.9 | Lebel et al. 1997 |
| Tapajós | 432 | 17.0 (− 176.0) | Malm et al. 1997 | |
| Cametá | Tapajós | 68 | 10.8 ± 6.1 | Dolbec et al. 2000 |
| São Luiz do Tapajós | Tapajós | 256 | 20.9 ± 12.2 (0.10 – 94.5) | Santos et al. 2000 |
| Brasília Legal | Tapajós | 152 | 11.3 ± 7.3 (0.70 – 37.2) | Santos et al. 2000 |
| São Luiz do Tapajós | Tapajós | 32 | 14.7 ± 7.2 (1.9 – 29.9) | Pinheiro et al. 2003 |
| Barreiras | Tapajós | 37 | 15.3 ± 9.3 (3.3 – 35.6) | Pinheiro et al. 2003 |
Original values rounded to the first decimal.
Table 5.
Total Hg concentration in hair samples from indigenous populations from the Brazilian Amazon.
| Indigenous Groups | Region | N | Hg-total (min-max) (μg / g) (*) |
References |
|---|---|---|---|---|
| Kayapó-Gorotire | Xingu | - | 5.18 (1.50 – 13.68) | Couto et al. 1988 |
| Yanomami | Rondônia | - | (1.40 – 8.14) | Castro et al. 1991 |
| Kayapó | Xingu | - | 7.36 (5.20 – 13.30) | Ferrari et al. 1993 |
| Parakanã | Tucuruí | 12 | 8.50 ± 2.80 (3.30 – 12.00) | Leino & Lodenius 1995 |
| Apiacás | Mato Grosso | 55 | 34.20 (− 128,00) | Barbosa et al. 1997 |
| Pacaanovos | Madeira | - | 6.05 (1.41 – 11.70) | Campos et al. 2002 |
| Pacaanovos | Madeira | 910 | 8.37 (0.52 – 83.89) | Santos et al. 2003 |
Original values rounded to the second decimal.
Studies performed in the riverside communities of the Tapajós River (Figure 1) in the State of Pará, an area encompassing a large gold mining reserve, have shown high total-Hg levels in hair samples, in some instances above the biological tolerance limit and indicating a possible health risk to local individuals (Table 3). Over the years, Hg exposure in this region has gradually diminished, reflecting a decrease in gold mining activities. Consistent with the environmental reduction in hg levels, a decrease in mean total-Hg level in hair samples in the population living in the Upper Tapajós (in comparison with those living in the Lower Tapajós) has been documented (Table 3). The reduction in the level of Hg in people living in this region likely reflects decreased Hg exposure from Hg0 vapor and does not appear to be due to decreased MeHg exposure from fish consumption.
Riverside communities of the Madeira River (Figure 1) living in the proximities of Garimpos and with a diet rich in fish also showed high total-Hg levels in hair samples (Table 4). A correlation was found between total-Hg levels in hair samples and the species of consumed fish. Populations with high consumption of carnivorous fish showed higher mean total-Hg concentration in hair samples than populations with high consumption of non carnivorous fish: 38.6 ± 14.4 μg/g versus 5.6 ± 3.1 μg/g, respectively (Eve et al. 1996).
Table 4.
Total Hg concentration in hair samples from Amazon populations living in locations outside the Tapajós River basin, 1996–2003.
| Location | Region | N | Hg-total (min-max) (μg / g) (*) |
Reference |
|---|---|---|---|---|
| Carajás | 29 | (0.25 – 15.70) | Fernandes et al. 1990 | |
| Iranduba | Madeira | 86 | 5.60 ± 3.10 | Eve et al. 1996 |
| Barreirinha | Madeira | 15 | 38.60 ± 14.40 | Eve et al. 1996 |
| Madeira | 169 | 9.00 (− 71.00) | Malm et al. 1997 | |
| Santana de Ituqui | Madeira | 167 | 4.28 ± 1.85 (0.50 – 10.90) | Santos et al. 2000 |
| Negro | 76 | 21.40 ± 12.60 (1.50 – 59.00) | Barbosa et al. 2001 | |
| Panacauera | Tocantins | 23 | 8.13 ± 5.89 (1.40 – 25.50) | Pinheiro et al. 2003a |
| Pindobal | Tocantins | 43 | 3.30 ± 1.60 (0.80 – 7.20) | Pinheiro et al, 2003a |
| São Gabriel | Negro | 157 | 13.02 (0.30 – 83.11) | Santos et al. 2003 |
| Barcelos | Negro | 242 | 9.67 (0.07 – 52.04) | Santos et al. 2003 |
Original values rounded to the second decimal.
In communities living in areas distant from gold mining activities, total-Hg concentrations in hair samples were relatively low (Table 4). For instance, in Santana de Ituqui (Amazonas), the mean total-Hg concentration in hair samples from 167 subjects was 4.2 ± 1.8 μg/g, ranging between 0.5–10.9 μg/g (Santos et al. 2000). In Iranduba (Amazonas), mean total-Hg concentrations in hair samples of 86 subjects were 5.6 ± 3.1 μg/g (Eve et al. 1996). In Panacauera and Pindobal (Pará), two communities in the Tocantins River, which are distant from gold mining activities, but having a diet rich in fish, total-Hg levels were low, indicating limited exposure to Hg (Pinheiro et al. 2006). On the other hand, studies performed in riverside communities of the Negro River have found relatively high Hg levels in hair samples despite the absence of significant gold mining activity in this region (Barbosa et al. 2001; Santos et al. 2005) (Table 4). Thus, these results suggest that additional Hg sources are likely responsible for the environmental contamination and human exposure in the Negro River basin (Barbosa et al. 2001; Santos et al. 2003).
Human Hg exposure was also studied in areas located near the hydroelectric dams built over the last few decades in the Amazon region. In one study performed in fishermen from Caraipé, near the Tucuruí Hydroelectric (Pará), the mean total-Hg concentrations in hair samples was 47 μg/g with values ranging between 4–240 μg/g (Fernandes et al. 1990). In another study performed in the same location, the mean total-Hg in hair samples was 65 ± 5.8 μg/g (Leino and Lodenius 1995). In the Balbina Hydroelectric (Amazonas), despite the low levels of MeHg in fish, the mean Me-Hg concentrations in hair samples was 8.7 ± 5.2 μg/g, ranging between 2.0–21.6 μg/g (Kehrig et al. 1998).
Mercury exposure in indigenous Amazon populations has also been studied (Table 5). High exposure levels have been noted in one such group, the Apiacás (Mato Grosso; Figure 1; Barbosa et al. 1997), but, in general, indigenous populations show exposure levels lower than other riverside communities living in the same region. The high Hg levels found in the Apiacás are likely due to several factors, namely (1) the heavy mining activities in the region and the contamination of local rives, upstream the Teles Pires River and downstream the Tapajós River; (2) the location of the Alta Floresta (Mato Grosso) gold mine in the region, and (3) the Apiacás diet is rich in fish (90% of the Apiacás report a minimum of six fish meals per week). Nevertheless, it is noteworthy, that, the majority of indigenous populations thus far studied in the Amazon show Hg exposure levels lower than other riverside communities both indigenous and non-indigenous. This is likely to be a reflection of their diets, which are predominantly based on game and farming products.
Maternal Exposure to Methylmercury
In the majority of environmental accidents, maternal Hg exposure has occurred as a result of consumption of fish contaminated with MeHg. The toxic effects of MeHg caused nervous system lesions in children, varying from mild disturbances in neuropsychomotor development to severe degrees of cerebral palsy (Harada 1982). Studies of women at reproductive age in the Amazon have shown a range of total-Hg concentrations in hair samples that seem to be dependent upon the region, diet type and frequency of fish in their meals (Barbosa et al. 1997; 2001; 1998; Eve et al. 1996).
Women from riverside communities located along the Tapajós River, near the area of gold mining, have high levels of Hg exposure as assessed by total-Hg concentration in hair samples (Eve et al. 1996; Malm et al. 1997). A recent study in women in the reproductive age from this region found total-Hg concentrations in hair samples ranging from 1.51 to 19.43 μg/g during pregnancy and from 5.25 to 21.00 μg/g in non-pregnant women (Pinheiro et al. 2005).
In the Madeira River, two groups of women at reproductive age and with high frequency of fish consumption had high total-Hg concentration in hair samples (Eve et al. 1996). One group comprising women of 18 years-old or older had a mean total-Hg concentration of 34.28 ± 13.75 μg/g, while the younger group of 11–17 years-old had mean total-Hg concentration of 31.5 ± 6.01 μg/g (Eve et al. 1996). In another study in the same region, it was found that the mean total-Hg concentrations in hair samples of riverside women in reproductive age was 14.08 μg/g, ranging between 0.8–94.7 μg/g, and 67.4% of them had total-Hg concentration above 10 μg/g (Barbosa et al. 1998). Again in the Madeira River, total-Hg concentrations in hair samples have been measured during pregnancy and the observed values ranged between 12.2–41.0 μg/g and 4.0–33.5 μg/g, while during lactation they ranged between 21.2–84.4 μg/g (Boischio and Cernichiari 1998). A study in the Negro River found that women in the reproductive age, 15–40 years-old, showed a mean hair total-Hg concentrations of 18.32 μg/g and values ranging from 1.65 to 32.63 μg/g, of which 65% were above 10 μg/g (Barbosa et al. 1998).
Among the indigenous mothers that have been studied in the Amazon, those from Apiacás (Mato Grosso) showed the highest total-Hg concentration in hair samples. This indigenous group is located near the gold mine of Alta Floresta (Mato Grosso). Thus, the high Hg exposure observed among the members of this group is related to an elevated consumption of fish contaminated with Me-Hg (Barbosa et al. 1998).
These data indicate that inhabitants of villages with robust gold mining activities along the Amazon are additionally exposed to MeHg accumulating in fish. Pregnant women and their children are especially susceptible groups due to the propensity of MeHg to accumulate in the developing nervous system. Careful monitoring of these populations is recommended to prevent hazardous MeHg toxic effects. In addition, educational campaigns oriented towards nutritional habits and risks associated with consumption of MeHg-adulterated fish should be conducted to expose the local population to the risks associated with MeHg (Pinheiro et al. 2008).
SELENIUM
Selenium (Se) is an essential trace element for most biological organisms and its physiological role in vertebrates is carried out after its complex cotranslational incorporation into the amino acid selenocysteine. The selenol group of selenocysteine, which is analogous to the thiol group of cysteine, is one of the most powerful nucleophile centers in living cells and functions in important anti-oxidant roles in several selenoproteins (Farina et al. 2009 in Press). For instance, it is an important component of the active center of the antioxidant enzymes glutathione peroxidase (GSH-Px), thioredoxin reductase, and three thyroid hormone deiodinases (Burk and Levander 1999). In addition, Se can be found in proteins after its incorporation into selenomethionine in bacteria, yeast and higher plants. In vertebrates, exogenous selenomethione residues can be non-specifically incorporated into proteins during translation as surrogates of methionine residues. However, selenomethione has no physiological role in these proteins, because its Se atom is not biologically reactive. Consequently, these proteins, which have randomly incorporated selenomethione residues in their structure, are not classified as selenoproteins (Burk and Levander 1999; Schrauzer 2000).
Dietary Se is important in cancer prevention (Combs et al. 2001), immune system enhancement (Ursini et al. 1999), aging (Martin-Romero et al. 2001), male reproduction (Ursini et al. 1999) and other physiological and pathophysiological processes, such as maintenance of the P450 cytochrome system, DNA repair and enzyme activation (Burk and Levander 1999; Rayman 2000). As discussed above, Se also plays an important role in the antioxidant defense system as an essential component of the family of GSH-Px and thioredoxin reductase enzymes (Burk and Levander 1999; Flohe 2009 in press; Rayman 2000).
Selenium: Sources, Maximum Safe Dietary Intake, and Deficiency
Some soils are naturally rich in Se and certain plants can readily bioaccumulate it. Anthropogenic sources of Se include coal burning and sulfide ore mining and smelting. Dietary Se is mainly derived from nuts, cereals, meat, fish and eggs. High levels are found in kidney, tuna, crab, lobster, and the Brazil nuts. The Se recommended daily intake (RDA) for both men and women is 55 μg/day (National Academies Press 2000). The maximum safe dietary Se intake is currently established as 550 μg/day (National Academies Press 2000), but there is emerging evidence that daily consumption should be kept below 400 μg/day to avoid symptoms associated with selenosis. On the other hand, several studies have shown that approximately 40 μg/day of Se are necessary to maximize GSH-Px activity and that a daily intake below this level may lead to clinical symptoms of Se deficiency (Burk and Levander 1999; Gropper et al. 2009).
Se deficiency is a rare condition in most locations, but may occur in regions where the soil is poor in this trace element. Dietary Se deficiency may induce a wide range of effects in humans and can be fatal in some circumstances. Its general symptoms include muscle pain, myasthenia, as well as hair and skin pigmentation loss (Gropper et al. 2009). Se deficiency causes severe miocardiopathy that could evolve to cardiogenic shock and in certain cases to congestive cardiac insufficiency or failure. Children may also suffer an acute form of Se deficiency, the so called Keshan disease. Another form of acute Se deficiency is referred to as Kashin-Becks disease; it occurs in pre-adolescents and presents with simultaneous iodine deficiency, leading to severe osteoarthritis, chondrocyte and peripheral nerve degeneration, as well as joint deformities (Burk and Levander 1999). Chronic Se deficiency evolves to cardiomegaly, myocardiac necrosis and calcification, and variable degree of cardiac insufficiency (Burk and Levander 1999; Gropper et al. 2009).
Selenium and Mercury Toxicity
The interactions of MeHg or Hg2+ with thiols dictate their fate and toxicity (Clarkson and Magos 2006). Theoretically, any bimolecule containing sulfhydryl groups can be considered a potential target for mercurials and it has been extensively reported that the interaction of MeHg with low molecular weight thiols, particularly cysteine and reduced GSH plays a fundamental role in determining MeHg toxicity. After entering the circulation, MeHg can bind free cysteine and get transported into different tissues by molecular mimicry (i.e., MeHg can be transported as a surrogate of methionine (Aschner 1989; Aschner and Clarkson 1988; Mokrzan et al. 1995). However, details about how MeHg and Hg2+ detach from their low molecular complexes to interact with proteins is not well understood. Ample evidence suggests that selenoproteins can be important targets of Hg toxicity. It has been demonstrated in different experimental models that inorganic Hg, MeHg and phenylmercury (PheHg) can inhibit the activity of GSH-Px (Bem et al. 1985; de Freitas et al. 2009; Farina et al. 2003; 2004; Hirota et al. 1980; Lucena et al. 2007; Stringari et al. 2006; 2008; Watanabe 2002). Inhibition of seleno-enzymes by inorganic and organic Hg compounds may be at least partially due to a direct interaction between mercurials and the selenol/selenolate groups at the active site of these seleno-enzymes (Wada et al. 1976; Carvalho et al. 2008; Farina et al. 2009 in press).
Given the above, early assumptions that mercurials could be toxic by directly depleting cellular thiols should be revised to include the possibility that mercurials can be toxic via disruption of the seleno-antioxidant enzymes, which could initiate an overproduction of reactive oxygen species (ROS) that ultimately could deplete the cellular thiol homeostasis. Support for this can be found in various experimental models, where both the inorganic and organic Hg compounds have been shown to deplete cellular thiol levels (Gstraunthaler et al. 1983; Lund et al. 1993; Stringari et al. 2008; de Freitas et al. 2009). In addition, Hg compounds cause cellular lesions by increasing the cell load of toxic ROS (Stacey and Kappus 1982) and that MeHg neurotoxicity is associated with their overproduction (Aschner et al. 2007; Hirayama and Yasutake 2001; LeBel et al. 1992). The death of cerebellar neurons by apoptosis induced by MeHg, for example, is related to increased intracellular oxidative stress (Kunimoto et al. 2001).
The possible role of Se in modulating Hg toxicity has been recognized since the 60s, where it was shown that selenite administration decreased the toxicity of sublimate (an ionic inorganic form of Hg) (Parizek and Ostadalova 1967). One of the first observations that Se could protect vertebrates from the toxicity of MeHg was recognized in 1972 by Ganther and collaborators (Ganther et al. 1972), who demonstrated protection by dietary Se against MeHg-induced toxicity in Japanese quail. After these pioneering studies, the in vitro and in vivo protective effects of inorganic Se and selenomethionine against the toxicity of Hg have been confirmed by different laboratories (Choi et al. 2008; Fredriksson et al. 1993; Kaur et al. 2009; Nishikido et al. 1987; Ralston et al. 2007; 2008a; Roos et al. 2009; Folven et al. 2009) and field studies (see Belzile et al., this issue). As summarized by Ralston (Ralston 2008b), data in the literature have also reported that the developmental effects of MeHg are more pronounced in children born to mothers consuming fish with low compliments of Se. Furthermore, Se deficiency was shown to be associated with increased MeHg toxicity (Nishikido et al. 1987). Nevertheless, the mechanisms associated with the protective effect of Se are still not well understood, but possibly, they are associated with diversion of Hg from target molecules to high molecular weight Hg-Se-containing proteins found in plasma (Burk et al. 1974) and other tissues (Agarwal and Behari 2007; Chen et al. 1974). Ralston and colleagues (Ralston et al. 2007) have determined the effect of dietary Se on toxicity in weanling male rats exposed to MeHg, showing that growth of rats fed high-MeHg and adequate-Se diets was impaired relative to their control group, whereas rats fed high-MeHg, rich-Se diets were indistinguishable from controls. The authors emphasized that the Hg to Se molar ratios provide a reliable criterion for assessing risks associated with MeHg exposure. Furthermore, they demonstrated that low-MeHg exposure had no effect on rats’ growth at any dietary Se level. Hg toxicity also directly correlated to the Hg to Se molar ratios, thus providing direct support that Hg-dependent sequestration of Se is a primary mechanism of Hg toxicity. Additional studies have also noted the protective properties of Se. High Se concentrations in fresh lake trout (in Argentina) were associated with low Hg content in tissues (muscle), suggesting detoxification of Hg by a Se-rich diet (Arribere et al. 2008) or the formation of insoluble salts between Se and Hg in the aquatic environment that are non-bioavailable to the living organisms (Belzile et al. 2006; see below for additional discussion).
Taken together, the experimental data from laboratory animal studies and the environmental studies indicating a protective role of selenium against Hg emphasize the potential utility of Se as a bioindicator of susceptibility to Hg intoxication. The exact mechanism/s by which Se decreases Hg toxicity is not well understood. One additional plausible explanation can be found in the metabolism of inorganic Se and selenomethione. For instance, during their metabolism (rendering them extractable or incorporated into selenoproteins via incorporation into selenocysteine) different selenohydryl intermediates can be formed (Figure 2). Selenohydryl groups are analogous to sulfhydryl groups; however, as discussed above, they are more powerful nucleophiles than sulfhydryl groups and possess a higher affinity for mercurials than sulfhydryl groups (Sugiura et al. 1976). Thus, selenohydryl intermediates formed during the metabolism of Se compounds can bind to MeHg or Hg2+ and change their toxicokinetics (Choi et al. 2008; Ganther et al. 1972; Ralston et al. 2007; 2008). Consequently, the formation of inert complex(es) between Se and Hg can be suggested as an important mechanism to explain the protective effect of Se against mercurial toxicity. In fact, the formation of inert complexes of the type HgSe can explain the co-accumulation or co-deposition of Se and Hg in different tissues of exposed animals.
Figure 2.
Interaction of inorganic selenium [Se(IV)] and selenomethionine with MeHg or Hg(II) via formation of H2Se (selenidric acid) or “selenohydryl pathway”. Selenite [Se(IV)] and selenomethione can be metabolized to H2Se, which can be incorporated into selenocysteine that is incorporated in selenoproteins. H2Se can react with inorganic mercury forming HgSe. H2Se can also react with methylmercury forming a complex that can be decomposed to form the insoluble mercury selenide salt (HgSe). Hypothetically, CH3Se− (monomethylselenolate) can also react with Hg(II) or methylmercury to form complexes that can decompose to form HgSe. Adapted and modified from (Farina et al. 2009 in Press).
For the case of environmental contamination, Se has also been successfully added to some lakes to remediate high levels of Hg. After Se addition, Hg levels decreased in water and in pike and perch, indicating that the Hg was removed from the aquatic environment via formation of HgSe, which is more insoluble than the analog HgS, and possibly less bioavailable to the microbiota from lakes sediments (Paulsson and Lundbergh 1991, Belzile et al. 2006; Yang et al. 2008). From an immediate toxicological or environmental point of view, the reaction of Hg with selenohydryl forming insoluble and relatively inert salts can be highly desirable; however, little is know about the toxicological properties and long-term fate of this insoluble compound(s) both within living organism and in the environment (see Belzile et al. in this issue for more discussion on this aspect).
Selenium in Amazonian Food
Staples which are widely consumed by poor or low income populations in Brazil such as rice, beans, wheat, cassava and corn have low Se levels. Se levels in these foods are lower than 0.05 μg/g and thus do not meet the minimum dietary daily intake for human adults in Brazil. Se levels are higher in food from animal origin, especially fish – tuna, dogfish, merluza and sardines (Ferreira et al. 2002).
A limited number of studies have evaluated Se concentration in Amazon fish. Dorea et al. (1998) have measured Hg and Se concentrations in Amazon fish species regularly consumed by the population living in riverside communities. Their results have shown a Hg:Se molar ratio that increases in accordance with the trophic level of the fish. The mean Se concentration in the piscivorous fish species was 4 μg/g and was much lower than at 0.9 μg/g in the herbivorous fish species. The molar ratio of Hg:Se has important practical consequences for populations that rely heavily upon fish consumption. Hg and Se in fish may occur as a bis-methyl-mercury selenide complex turning Hg less toxic. As discussed above, the interaction of MeHg or Hg2+ with Se occurs preferentially with the selenohydryl intermediates. However there is scant experimental data to support interaction of MeHg with reduced forms of Se (mainly HSe- and possibly CH3Se-), resulting in the formation of an intermediate, such as CH3HgSe, which may spontaneously dissociate to HgSe (Iwata et al. 1982). An analogous phenomenon occurs in the aquatic environment (see above), where Se can considerably reduce the bioavailability of Hg. This likely occurs as a result of decomposition of bis-methyl-mercury selenide to a more stable and insoluble salt, i.e., HgSe, which has reduced absorption from edible fish (Iwata et al. 1982).
In addition to fish, selected Amazon populations have another source that is rich in Se, the Brazil nuts (called Pará nuts in the Amazon). The Brazil nut is the fruit of the gigantic Bertholletia excelsa confined to the tropical forest of the Amazonas River basin. Brazil nuts have the highest Se levels in human diet (per g of consumed food) though this is soil-dependent, as the Bertholletia excelsa itself does not require high levels of Se. Se levels up to 53 μg/g have been found in the Brazil nuts. Se in the Brazil nut is found in the protein fraction of the fruit mostly as the amino acids selenocysteine and selenomethionine (Chunhieng et al. 2004). Furthermore, the bioavaibility of Se in Brazil nuts was similar to that of selenomethione, but Se from Brazil nuts caused a two-fold increase in GSH-Px compared to human subjects supplemented with selenomethionine (Thomson et al. 2008), suggesting that selenocysteine is more easily metabolized than selenomethionine and more readily re-incorporated into selenoproteins. Similarly, it has been shown in animal experiments, that adding Brazil nuts to the diet significantly increased blood Se levels and the activity of GSH-Px (Chansler et al. 1986) and I-5´-deiodinase (IDI) (Ip and Lisk 1994).
Selenium Levels in Amazon Populations: Blood Levels
In Rio de Janeiro, Brazil, Se concentration in the serum of healthy subjects was estimated at 73.2 μg/l, ranging between 56.5–94.5 μg/l (da Cunha et al. 2003), values similar to those found in many other countries (Safaralizadeh et al. 2005).
In the Amazon, a study performed on 236 inhabitants of riverside communities of the Tapajós River, mean blood Se was 284.3 μg/l, ranging between 142.1–2029.0 μg/l (Lemire et al. 2006). In healthy subjects living outside the Amazon, in Rio de Janeiro, there were no correlations between Se levels and age, sex or smoking, but in the Amazon populations were significantly higher in alcoholics and farmers (Lemire et al. 2006). Independent of fish consumption, it was observed that a positive correlation exists between Se and Hg in the blood (Lemire et al. 2006). In addition, Se levels increased with consumption of piscivorous fish, a finding in agreement with previous studies that showed high Se levels in piscivorous fish (Dorea et al. 1998).
Se and Hg levels have been measured in hair samples from indigenous and control populations in the Amazon. In control individuals a near 1:1 molar ratio between Hg and Se was noted, while in indigenous populations the Se levels were higher, especially among subjects with high Hg levels (Vasconcellos et al. 2000). In another study of Amazon indigenous populations, a molar relation between hair Hg and Se at low Hg levels (Campos et al. 2002) was reported. These findings are in agreement with studies performed in other locations (e.g., autopsied Hg miners), showing high Hg and Se concentrations in their brains (Kosta et al. 1975). In another study, Hg and Se levels were measured in hair samples from women living in riverside communities along the Tapajós River. In women of reproductive age the mean Se concentrations were 0.61 μg/g in pregnant and 2.46 μg/g in non-pregnant women, respectively; in 68.4% of the women of reproductive age, Se levels were below the detection limit (Pinheiro et al. 2005).
Conclusions
Although the studies in the Amazon region are relatively limited in scope, it is apparent that the fate of Hg in the environment of the tropical forest is similar to that found in other regions of the world. For instance, the bioaccumulation of Hg in Amazonian piscivorous fish is similar to that found in swordfish and shark, reported elsewhere. Regarding human exposure, the Hg levels in hair samples indicate that Amazon riverside communities are exposed to MeHg possibly as a consequence of the release of Hg0 vapor used in the gold mining industry. The measurements of Se levels in fish and exposed human subjects of the current study were insufficient to establish a conclusive protective effect of Se against the toxic effects of MeHg. Nevertheless, the available evidence suggests that Se, in addition to serving as an antioxidant, as a consequence of its metabolism to generate Se intermediates may react with mercurials, transforming them into more inert compounds, and by inference reducing their toxicity. In fact, data in the literature has clearly indicated the potential role of Se to serve as a bioindicator of susceptibility to Hg exposure (Ralston 2008a; 2008b). Accordingly, assessing and ensuring adequate dietary Se intake for the riverside communities of the Amazon as means of mitigating Hg-induced toxicity should be a priority of the Brazilian Health Authorities. This is particularly important, in view of the fact that poor or low income Amazonian populations that are at risk of exposure to Hg are known to consume a diet poor in Se. This is paradoxical in view of the abundance of one of best sources of Se in the world in the Amazonian region, namely, the Brazil nuts. Furthermore, since the bioavaibility of Se from Brazil nuts seems to be superior to that of selenomethione (which can afford considerable protection against Hg toxicity), as reflected by the activity of GSH-Px, it is plausible to expect that Se derived from Pára nuts can be superior to inorganic Se or selenomethione in attenuating Hg toxicity. However, further experimental and epidemiological studies will be required to clarify this supposition.
From the environmental point of view, the studies presented here are intriguing as they indicate low level of hair Hg in several communities (Panacaura and Pindobal) with high fish consumption that are distant from Hg contaminated areas. These observations imply that the toxicological impact of Hg released by anthropogenic activities may be restricted to the proximity of contaminated site. However, given the limited number of studies on this issue, future research will be needed to better understand the fate and the environmental consequences of human derived Hg. Since the Amazonian region is vast, the long-term follow-up of Hg in different parts of this important ecosystem, will furnish timely information on the persistence and the potential health hazards of one of the most toxic elements in the periodic table. Furthermore, the paradoxical findings of high levels of Hg in humans living in regions that are considered to be uncontaminated indicate that the fate and kinetics of Hg in Amazonian environment is complex. The intricate interactions between geochemical and the intense biological activities in this region of the world may also involve genetic variations, reinforcing the necessity of more detailed and continued studies in this vulnerable sanctuary of the planet.
There is little doubt that the main source of MeHg exposure in the Amazon is associated with consumption of adulterated fish. In the presence of toxic levels of Hg, equal parts of Se and Hg form compounds that are poorly transported into the brain and other tissue (Yoneda and Suzuki 1997). There is evidence that low environmental Se is linked to susceptibility to Hg toxicity and that Se levels could be used as a bioindicator to monitor the health of Hg exposed subjects.
ACKNOWLEDGEMENTS
This research was supported by grants from CNPq-PRONEX / FAPESPA #2268, CNPq #486351/2006–8, CNPq #620037/2008–3, and CAPES-PROEQUIP #1649/2007. LCLS and JBTR are CNPq research fellows. Supported by the FINEP research grant “Rede Instituto Brasileiro de Neurociência (IBN-Net)” #01.06.0842–00. MA was supported in part by a grant from the National Institute of Environmental Health Sciences (NIEHS 07331).
REFERENCES
- Agarwal R, Behari JR. 2007. Effect of selenium pretreatment in chronic mercury intoxication in rats. Bull Environ Contam Toxicol 79:306–10. [DOI] [PubMed] [Google Scholar]
- Akagi H, Kinjo Y, Branches P, Malm O, Harada M, Pfeffer WC, Kato H. 1994. Methylmercury pollution in Tapajós River Basin, Amazon. Environ Sci 3:25–32. [Google Scholar]
- Akagi H, Kinjo Y, Branches FJP, Malm O, Harada M, Pfeiffer WC. 1995. Methylmercury pollution in the Amazon, Brazil. Sci Total Environ 5:85–95. [DOI] [PubMed] [Google Scholar]
- Arribere MA, Ribeiro Guevara S, Bubach DF, Arcagni M, Vigliano PH. 2008. Selenium and mercury in native and introduced fish species of patagonian lakes, Argentina. Biol Trace Elem Res 122:42–63. [DOI] [PubMed] [Google Scholar]
- Aschner M 1989. Brain, kidney and liver 203Hg-methyl mercury uptake in the rat: relationship to the neutral amino acid carrier. Pharmacol Toxicol 65:17–20. [DOI] [PubMed] [Google Scholar]
- Aschner M, Clarkson TW. 1988. Uptake of methylmercury in the rat brain: effects of amino acids. Brain Res 462:31–9. [DOI] [PubMed] [Google Scholar]
- Aschner M, Syversen T, Souza DO, Rocha JB, Farina M. 2007. Involvement of glutamate and reactive oxygen species in methylmercury neurotoxicity. Braz J Med Biol Res 40:285–91. [DOI] [PubMed] [Google Scholar]
- ATSDR (Agency for Toxic Substances and Disease Registry). 1998. Toxicological Profile for Mercury U. S. Department of Health and Human Services. Division of Toxicology and Environmental Medicine. [Google Scholar]
- Bakir F, Damluji SF, Amin-Zaki L, Murtadha M, Khalidi A, al-Rawi NY, Tikriti S, Dahahir HI, Clarkson TW, Smith JC and others. 1973. Methylmercury poisoning in Iraq. Science 181:230–41. [DOI] [PubMed] [Google Scholar]
- Barbosa AC, Garcia AM, Souza J. 1997. Mercury contamination in hair of riverine of Apiacás Reserve in the Brazilian Amazon. Water, Air and Soil Pollution 97:1–8. [Google Scholar]
- Barbosa AC, Jardim W, Dorea JG, Fosberg B, Souza J. 2001. Hair mercury speciation as a function of gender, age, and body mass index in inhabitants of the Negro River basin, Amazon, Brazil. Arch Environ Contam Toxicol 40:439–44. [DOI] [PubMed] [Google Scholar]
- Barbosa AC, Silva SR, Dorea JG. 1998. Concentration of mercury in hair of indigenous mothers and infants from the Amazon basin. Arch Environ Contam Toxicol 34:100–5. [DOI] [PubMed] [Google Scholar]
- Belzile N, Chen YW, Gunn JM, Tong J, Alarie Y, Delonchamp T, Lang CY. 2006. The effect of selenium on mercury assimilation by freshwater organisms. Can J Fish Aquat Sci 63:1–10. [Google Scholar]
- Bem EM, Mailer K, Elson CM. 1985. Influence of mercury (II), cadmium (II), methylmercury, and phenylmercury on the kinetic properties of rat liver glutathione peroxidase. Can J Biochem Cell Biol 63:1212–6. [DOI] [PubMed] [Google Scholar]
- Berry MJ, Ralston NVC. 2008. Mercury Toxicity and the Mitigating Role of Selenium. Ecohealth 5:456–459. [DOI] [PubMed] [Google Scholar]
- Bidone ED, Castilhos ZC, Cid de Souza TM, Lacerda LD. 1997. Fish contamination and human exposure to mercury in the Tapajós River, Pará State, Amazon, Brazil: a screening approach. Bull Environ Contam Toxicol 59:194–201. [DOI] [PubMed] [Google Scholar]
- Boischio AA, Cernichiari E. 1998. Longitudinal hair mercury concentration in riverside mothers along the Upper Madeira river (Brazil). Environ Res 77:79–83. [DOI] [PubMed] [Google Scholar]
- Branches FJ, Erickson TB, Aks SE, Hryhorczuk DO. 1993. The price of gold: mercury exposure in the Amazonian rain forest. J Toxicol Clin Toxicol 31:295–306. [DOI] [PubMed] [Google Scholar]
- Burk RF, Foster KA, Greenfield PM, Kiker KW, Hannon JP. 1974. Binding of simultaneously administered inorganic selenium and mercury to a rat plasma protein. Proc Soc Exp Biol Med 145:782–5. [DOI] [PubMed] [Google Scholar]
- Burk RF, Levander OA. 1999. Selenium In: Shil SM, Olson J, Shike M, Ross AC, editors. Modern Nutrition in Health and Disease, 9th edition, Baltimore, Maryland: Williams and Wilkins; p 265–276. [Google Scholar]
- Camara Vde M, Filhote MI, Lima MI, Alheira FV, Martins MS, Dantas TO, Luiz RR. 1997. Strategies for preventing adolescent mercury exposure in Brazilian gold mining areas. Toxicol Ind Health 13:285–97. [DOI] [PubMed] [Google Scholar]
- Campos MS, Sarkis JES, Muller RCS, Brabo EA, Santos EO. 2002. Correlation between mercury and selenium concentrations in Indian hair from Rondônia State, Amazon region, Brazil. Sci Total Environ 287:155–161. [DOI] [PubMed] [Google Scholar]
- Carvalho CM, Chew EH, Hashemy SI, Lu J, Holmgren A. 2008. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J Biol Chem 283:11913–23. [DOI] [PubMed] [Google Scholar]
- Chansler MW, Mutanen M, Morris VC, Levander OA. 1986. Nutritional bioavailability to rats of selenium in Brazil nuts and Mushrooms. Nutr Res 6:1419–1428. [Google Scholar]
- Chen RW, Whanger PD, Fang SC. 1974. Diversion of mercury binding in rat tissues by selenium: a possible mechanism of protection. Pharmacol Res Commun 6:571–9. [DOI] [PubMed] [Google Scholar]
- Choi AL, Budtz-Jorgensen E, Jorgensen PJ, Steuerwald U, Debes F, Weihe P, Grandjean P. 2008. Selenium as a potential protective factor against mercury developmental neurotoxicity. Environ Res 107:45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chunhieng T, Petritis K, Elfakir C, Brochier J, Goli T, Montet D. 2004. Study of selenium distribution in the protein fractions of the Brazil nut, Bertholletia excelsa. J Agric Food Chem 52:4318–22. [DOI] [PubMed] [Google Scholar]
- Clarkson TW, Magos L. 2006. The toxicology of mercury and its chemical compounds. Crit Rev Toxicol 36:609–62. [DOI] [PubMed] [Google Scholar]
- Combs GF Jr., Clark LC, Turnbull BW. 2001. An analysis of cancer prevention by selenium. Biofactors 14:153–9. [DOI] [PubMed] [Google Scholar]
- Couto RCS, Camara VM, Sabrosa PC. 1988. Intoxicação mercurial: resultados preliminares em duas áreas garimpeiras - PA. Cadernos de Saúde Pública 4:301–315. [Google Scholar]
- da Costa GM, dos Anjos LM, Souza GS, Gomes BD, Saito CA, Pinheiro Mda C, Ventura DF, da Silva Filho M, Silveira LC. 2008. Mercury toxicity in Amazon gold miners: visual dysfunction assessed by retinal and cortical electrophysiology. Environ Res 107:98–107. [DOI] [PubMed] [Google Scholar]
- da Cunha S, Filho FM, Antelo DS, de Souza MM. 2003. Serum sample levels of selenium and copper in healthy volunteers living in Rio de Janeiro city. Sci Total Environ 301:51–4. [DOI] [PubMed] [Google Scholar]
- Davis LE, Kornfeld M, Mooney HS, Fiedler KJ, Haaland KY, Orrison WW, Cernichiari E, Clarkson TW. 1994. Methylmercury poisoning: long-term clinical, radiological, toxicological, and pathological studies of an affected family. Ann Neurol 35:680–8. [DOI] [PubMed] [Google Scholar]
- de Freitas AS, Funck VR, Rotta Mdos S, Bohrer D, Morschbacher V, Puntel RL, Nogueira CW, Farina M, Aschner M, Rocha JB. 2009. Diphenyl diselenide, a simple organoselenium compound, decreases methylmercury-induced cerebral, hepatic and renal oxidative stress and mercury deposition in adult mice. Brain Res Bull 79:77–84. [DOI] [PubMed] [Google Scholar]
- de Jesus IM. 2005. Exposição Ocupacional ao Mercúrio Metálico na Região do Tapajós. Aspectos Clínicos e Epidemiológicos Trabalho de Conclusão de Curso de Medicina. Belém, Pará, Brasil: Universidade Federal do Pará. [Google Scholar]
- Do Canto Pereira LHM, Lago M, Costa MF, Rodrigues AR, Saito CA, Silveira LCL, Ventura DF. 2005. Visual impairment related to occupational exposure in dentists. Environmental Toxicology and Pharmacology 19:517–522. [DOI] [PubMed] [Google Scholar]
- Dorea JG, Moreira MB, East G, Barbosa AC. 1998. Selenium and mercury concentrations in some fish species of the Madeira River, Amazon Basin, Brazil. Biol Trace Elem Res 65:211–20. [DOI] [PubMed] [Google Scholar]
- Environmental Protection Agency. 1997. Mercury study report to congress. http://www.epa.gov/hg/report.htm.
- Eve E, Oliveira EF, Eve C. 1996. The mercury problem and diets in the Brazilian Amazon: planning a solution. Environmental Conservation. Environ Conserv 23:133–139. [Google Scholar]
- Faintuch JJ, Rocha AS. 1990. Intoxicação por mercúrio no Brasil. Revista Brasileira de Medicina 47:505–508. [Google Scholar]
- Farina M, Frizzo ME, Soares FA, Schwalm FD, Dietrich MO, Zeni G, Rocha JB, Souza DO. 2003. Ebselen protects against methylmercury-induced inhibition of glutamate uptake by cortical slices from adult mice. Toxicol Lett 144:351–7. [DOI] [PubMed] [Google Scholar]
- Farina M, Rocha JBT, Aschner M. 2009. in Press. Oxidative Stress and Methyl Mercury-Induced Neurotoxicity In: Developmental Neurotoxicology Research: Principles, Models, Techniques, Strategies and Mechanisms, Wang C and Slikker W Jr, editors. John Wiley and Sons, Inc. [Google Scholar]
- Farina M, Soares FA, Zeni G, Souza DO, Rocha JB. 2004. Additive pro-oxidative effects of methylmercury and ebselen in liver from suckling rat pups. Toxicol Lett 146:227–35. [DOI] [PubMed] [Google Scholar]
- Fernandes HM, Guimaraes AF, Bidone ED. 1990. Monitoramento do mercúrio na área do Projeto Carajás. Saneamento Ambiental 6:34–41. [Google Scholar]
- Ferreira KS, Gomes JC, Bellato CR, Jordao CP. 2002. [Selenium concentration in food consumed in Brazil]. Rev Panam Salud Publica 11:172–7. [DOI] [PubMed] [Google Scholar]
- Flohe L 2009. in press. The labour pains of biochemical selenology: The history of selenoprotein biosynthesis Biochim Biophys Acta. [DOI] [PubMed] [Google Scholar]
- Folven KI, Glover CN, Malde MK, Lundebye AK. 2009. Does selenium modify neurobehavioural impacts of developmental methylmercury exposure in mice? Environ. Toxicol Pharmacol 28:111–119. [DOI] [PubMed] [Google Scholar]
- Fredriksson A, Gardlund AT, Bergman K, Oskarsson A, Ohlin B, Danielsson B, Archer T. 1993. Effects of maternal dietary supplementation with selenite on the postnatal development of rat offspring exposed to methyl mercury in utero. Pharmacol Toxicol 72:377–82. [DOI] [PubMed] [Google Scholar]
- Ganther HE, Goudie C, Sunde ML, Kopecky MJ, Wagner P. 1972. Selenium: relation to decreased toxicity of methylmercury added to diets containing tuna. Science 175:1122–4. [DOI] [PubMed] [Google Scholar]
- Grandjean P 2008. Late insights into early origins of disease. Basic Clin Pharmacol Toxicol 102:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gropper SS, Smith JL, Groff JL. 2009. Advanced Nutrition and Human Metabolism, 5th edition Belmont, California: Wadsworth. [Google Scholar]
- Gstraunthaler G, Pfaller W, Kotanko P. 1983. Glutathione depletion and in vitro lipid peroxidation in mercury or maleate induced acute renal failure. Biochem Pharmacol 32:2969–72. [DOI] [PubMed] [Google Scholar]
- Harada M 1982. Minamata disease. Organic mercury poisoning caused by ingestion of contaminated fish methylmercury In: Jelliffe P, Jelliffe DB, editors. Adverse Effects of Foods. Kumamoto, Japan: Plenum Publishing; p 135–147. [Google Scholar]
- Harada M 1995. Minamata disease: methylmercury poisoning in Japan caused by environmental pollution. Crit Rev Toxicol 25:1–24. [DOI] [PubMed] [Google Scholar]
- Harada M 1997. Neurotoxicity of methylmercury, Minamata and the Amazon In: Yasui M, Strong MJ, Ota KK, Verity MA,editors. Mineral and Metal Neurotoxicology, New York, New York: CRC Press; p 177–187. [Google Scholar]
- Hirayama K, Yasutake A. 2001. In vivo degradation of methylmercury. Its mechanisms and significance in methylmercury induced neurotoxicity In: Takizawa Y, Osame M, editors. Methylmercury Poisoing in Minamata and Niigata, Japan, Japan Public Health Association; p 103–110. [Google Scholar]
- Hirota Y, Yamaguchi S, Shimojoh N, Sano KI. 1980. Inhibitory effect of methylmercury on the activity of glutathione peroxidase. Toxicol Appl Pharmacol 53:174–6. [DOI] [PubMed] [Google Scholar]
- Ip C, Lisk DJ. 1994. Bioactivity of selenium from Brazil nut for cancer prevention and selenoenzyme maintenance. Nutr Cancer 21:203–12. [DOI] [PubMed] [Google Scholar]
- Iwata H, Masukawa T, Kito H, Hayashi M. 1982. Degradation of methylmercury by selenium. Life Sci 31:859–66. [DOI] [PubMed] [Google Scholar]
- Kaur P, Evje L, Aschner M, Syversen T. 2009. The in vitro effects of selenomethionine on methylmercury-induced neurotoxicity. Toxicol In Vitro 23:378–85. [DOI] [PubMed] [Google Scholar]
- Kehrig HA, Malm O, Akagi H, Guimaraes JR, Torres JP. 1998. Methylmercury in fish and hair samples from the Balbina Feservoir, Brazilian Amazon. Environ Res 77:84–90. [DOI] [PubMed] [Google Scholar]
- Kosta L, Byrne AR, Zelenko V. 1975. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature 254:238–9. [DOI] [PubMed] [Google Scholar]
- Kunimoto M, Takanaga H, Adachi T. 2001. Factors controling the cell death of cerebellar neurons induced by methylmercury In: Proceedings of the 6th International Conference on Mercury as a Global Pollutant. Abstract HE-153. Minamata, Japan: National Institute for Minamata Disease (NIMD). [Google Scholar]
- LeBel CP, Ali SF, Bondy SC. 1992. Deferoxamine inhibits methyl mercury-induced increases in reactive oxygen species formation in rat brain. Toxicol Appl Pharmacol 112:161–5. [DOI] [PubMed] [Google Scholar]
- Leino T, Lodenius M. 1995. Human hair mercury levels in Tucurui area, State of Para, Brazil. Sci Total Environ 175:119–25. [DOI] [PubMed] [Google Scholar]
- Lemire M, Mergler D, Fillion M, Passos CJ, Guimaraes JR, Davidson R, Lucotte M. 2006. Elevated blood selenium levels in the Brazilian Amazon. Sci Total Environ 366:101–11. [DOI] [PubMed] [Google Scholar]
- Lucena GM, Franco JL, Ribas CM, Azevedo MS, Meotti FC, Gadotti VM, Dafre AL, Santos AR, Farina M. 2007. Cipura paludosa extract prevents methyl mercury-induced neurotoxicity in mice. Basic Clin Pharmacol Toxicol 101:127–31. [DOI] [PubMed] [Google Scholar]
- Lund BO, Miller DM, Woods JS. 1993. Studies on Hg(II)-induced H2O2 formation and oxidative stress in vivo and in vitro in rat kidney mitochondria. Biochem Pharmacol 45:2017–24. [DOI] [PubMed] [Google Scholar]
- Magro AC, Bastos PAM, Navarro MFL. 1994. Segurança no uso de Hg em restaurações de amálgamas. Revista Odontológica da Universidade de São Paulo. 8:1–6. [Google Scholar]
- Malm O, Branches FJP, Akagi H, Castro MB, Pfeiffer WC, Harada M, Bastos WR, Kato H. 1995. Mercury and methylmercury in fish and human hair from the Tapajós river basin, Brazil. Sci Total Environ 175:141–150. [DOI] [PubMed] [Google Scholar]
- Malm O, Guimararaes JRD, Castro MB, Bastos WR, Viana JP, Branches FJP, Silveira EG, Pfeiffer WC. 1997. Follow-up of mercury levels in fish, human hair and urine in the Madeira and Tapajós basins, Amazon, Brazil. Water, Air, Soil and Pollution 97:45–51. [Google Scholar]
- Martin-Romero FJ, Kryukov GV, Lobanov AV, Carlson BA, Lee BJ, Gladyshev VN, Hatfield DL. 2001. Selenium metabolism in Drosophila: selenoproteins, selenoprotein mRNA expression, fertility, and mortality J Biol Chem 276:29798–804. [DOI] [PubMed] [Google Scholar]
- da Saú Ministério de do Brasil. 1975. Resolução n° 18/75 da Comissão Nacional de Normas e Padrões para Alimentos Diário Oficial da União (Brasília), Seção 1, p. 16378, 9 de dezembro de [Google Scholar]
- Mokrzan EM, Kerper LE, Ballatori N, Clarkson TW. 1995. Methylmercury-thiol uptake into cultured brain capillary endothelial cells on amino acid system L. J Pharmacol Exp Ther 272:1277–84. [PubMed] [Google Scholar]
- National Academies Press. 2000. Food and Nutrition Board, Institute of Medicine. Selenium In: Dietary reference intakes for vitamin C, vitamin E, selenium, and carotenoids, p. 284–324. Washington, District of Columbia: National Academies Press. [Google Scholar]
- Nishikido N, Furuyashiki K, Naganuma A, Suzuki T, Imura N. 1987. Maternal selenium deficiency enhances the fetolethal toxicity of methyl mercury. Toxicol Appl Pharmacol 88:322–8. [DOI] [PubMed] [Google Scholar]
- Parizek J, Ostadalova I. 1967. The protective effect of small amounts of selenite in sublimate intoxication. Experientia 23:142–3. [DOI] [PubMed] [Google Scholar]
- Paulsson K, Lundbergh K. 1991. Treatment of mercury contaminated fish by selenium addition. Water Air Soil Pollution 56:833–841. [Google Scholar]
- Pfeiffer WC, Lacerda LD. 1988. Mercury Inputs into the Amazon Region, Brazil. Environ Technol Lett 9:325–330. [Google Scholar]
- Pinheiro MCN, Vieira JLF, Bacelar MDR. 2004. Exposição humana ao mercúrio na atividade garimpeira. Revista Paraense de Medicina 18:19–22. [Google Scholar]
- Pinheiro MCN, Muller RCS, Sarkis JE, Vieira JLF, Oikawa T, Gomes MS, Guimarães GA, do Nascimento JLM, Silveira LCL. 2005. Mercury and selenium concentrations in hair samples of women in fertile age from Amazon riverside communities. Sci Total Environ 349:284–8. [DOI] [PubMed] [Google Scholar]
- Pinheiro MC, Oikawa T, Vieira JL, Gomes MS, Guimaraes GA, Crespo-Lopez ME, Muller RC, Amoras WW, Ribeiro DR, Rodrigues AR and others. 2006. Comparative study of human exposure to mercury in riverside communities in the Amazon region. Braz J Med Biol Res 39:411–4. [DOI] [PubMed] [Google Scholar]
- Pinheiro MCN, Macchi BM, Vieira JLF, Oikawa T, Amoras WW, Guimarães GA, Costa CA, Crespo López ME, Herculano AM, Silveira LCL, do Nascimento JLM. 2008. Mercury exposure and antioxidant defenses in women: a comparative study in the Amazon. Environ Res 107:53–9. [DOI] [PubMed] [Google Scholar]
- Ralston NV, Blackwell JL 3rd, Raymond LJ. 2007. Importance of molar ratios in selenium-dependent protection against methylmercury toxicity. Biol Trace Elem Res 119:255–68. [DOI] [PubMed] [Google Scholar]
- Ralston NVC, Ralston CR, Blackwell JL 3rd, Raymond LJ. 2008a. Dietary and tissue selenium in relation to methylmercury toxicity. Neurotoxicology 29:802–11. [DOI] [PubMed] [Google Scholar]
- Ralston NVC 2008b. Selenium health benefit values as seafood safety criteria, Ecohealth 5:442–455. [DOI] [PubMed] [Google Scholar]
- Rayman MP. 2000. The importance of selenium to human health. Lancet 356:233–41. [DOI] [PubMed] [Google Scholar]
- Rodrigues AR, Souza CR, Braga AM, Rodrigues PS, Silveira AT, Damin ET, Cortes MI, Castro AJ, Mello GA, Vieira JL and others. 2007. Mercury toxicity in the Amazon: contrast sensitivity and color discrimination of subjects exposed to mercury. Braz J Med Biol Res 40:415–24. [DOI] [PubMed] [Google Scholar]
- Roos DH, Puntel RL, Santos MM, Souza DO, Farina M, Nogueira CW, Aschner M, Burger ME, Barbosa NB, Rocha JB. 2009. Guanosine and synthetic organoselenium compounds modulate methylmercury-induced oxidative stress in rat brain cortical slices: involvement of oxidative stress and glutamatergic system. Toxicol In Vitro 23:302–7. [DOI] [PubMed] [Google Scholar]
- Roulet M, Lucotte M, Farella N, Serique G, Coelho H, Sousa Passos CJ, Jesus da Silva E, Scavone De Andrade P, Mergler D, Guimaraes JRD and others. 1999. Effects of recent human colonization on the presence of Mercury in Amazonian ecosystems. Water, Air and Soil Pollution 112:297–313. [Google Scholar]
- Safaralizadeh R, Kardar GA, Pourpak Z, Moin M, Zare A, Teimourian S. 2005. Serum concentration of selenium in healthy individuals living in Tehran. Nutr J 4:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos EC, Camara Vde M, Brabo Eda S, Loureiro EC, de Jesus IM, Fayal K, Sagica F. 2003. [Mercury exposure evaluation among Pakaanova Indians, Amazon Region, Brazil]. Cad Saude Publica 19:199–206. [DOI] [PubMed] [Google Scholar]
- Santos EC, Jesus IM, Brabo ES, Loureiro EC, Mascarenhas AF, Weirich J, Camara VM, Cleary D. 2000. Mercury exposures in riverside Amazon communities in Para, Brazil. Environ Res 84:100–7. [DOI] [PubMed] [Google Scholar]
- Santos EO, Loureiro EC, Jesus IM, Brabo E, Silva RS, Soares MC, Camara VM, Souza MR, Branches F. 1995. [Diagnosis of health conditions in a pan-mining community in the Tapajós River Basin, Itaituba, Par, Brazil, 1992]. Cad Saúde Publica 11:212–25. [DOI] [PubMed] [Google Scholar]
- Santos EO, Sá GC, de Jesus IM, Brabo ES, Câmara VM, Lima MO, Faial KF, Mendes RAM, Mascarenhas SAF. 2005. Mercúrio no Rio Negro, Amazonas, Brasil. Estudo preliminar de indicadores de exposição no pescado e em populações humanas. Cad Saúde Coletiva 3:225–36. [Google Scholar]
- Schrauzer GN. 2000. Selenomethionine: A review of its nutritional significance, metabolism and toxicity. J Nutr 130:1653–1656. [DOI] [PubMed] [Google Scholar]
- Silveira LCL, Damin ETB, Pinheiro MCN, Rodrigues AR, Moura ALA, Cortes MIT, Mello GA. 2003. Visual dysfunction following mercury exposure by breathing mercury vapour or by eating mercury-contaminated food In: Mollon JD, Pokorny J, Knoblauch K, editors. Normal and Defective Colour Vision. Oxford, England: Oxford University Press; p 407–417. [Google Scholar]
- Soong TR, Chu FR, Fun ZT. 1994. Epidemiological study on the health of residents along the Sunhua River, polluted by methylmercury. Environ Sci 3:41–54. [Google Scholar]
- Souza TMC, Anjos JR, Barros FC, Bastos WR, Silva GS, Oliveira RC. 2003. Mercury exposure in the gold shops of Itaituba, Amazon, Brazil In: Eto K, Nakamura K, Silveira LCL, Tezuka H, Nagai K, Horiike H, editors. Proceedings of the International Workshop on Health and Environmental Effects of Mercury - Impacts of Mercury in South and Central America, Minamata, Japan: National Institute of Minamata Disease; p 77–83. [Google Scholar]
- Stacey NH, Kappus H. 1982. Cellular toxicity and lipid peroxidation in response to mercury. Toxicol Appl Pharmacol 63:29–35. [DOI] [PubMed] [Google Scholar]
- Stringari J, Meotti FC, Souza DO, Santos AR, Farina M. 2006. Postnatal methylmercury exposure induces hyperlocomotor activity and cerebellar oxidative stress in mice: dependence on the neurodevelopmental period. Neurochem Res 31:563–9. [DOI] [PubMed] [Google Scholar]
- Stringari J, Nunes AK, Franco JL, Bohrer D, Garcia SC, Dafre AL, Milatovic D, Souza DO, Rocha JB, Aschner M and others. 2008. Prenatal methylmercury exposure hampers glutathione antioxidant system ontogenesis and causes long-lasting oxidative stress in the mouse brain. Toxicol Appl Pharmacol 227:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura Y, Hojo Y, Tamai Y, Tanaka H. 1976. Letter: Selenium protection against mercury toxicity. Binding of methylmercury by the selenohydryl-containing ligand. J Am Chem Soc 98:2339–41. [DOI] [PubMed] [Google Scholar]
- Thomson CD, Chisholm A, McLachlan SK, Campbell JM. 2008. Brazil nuts: an effective way to improve selenium status. Am J Clin Nutr 87:379–84. [DOI] [PubMed] [Google Scholar]
- Ursini F, Heim S, Kiess M, Maiorino M, Roveri A, Wissing J, Flohe L. 1999. Dual function of the selenoprotein PHGPx during sperm maturation. Science 285:1393–6. [DOI] [PubMed] [Google Scholar]
- Vasconcellos MBA, Bode P, Paletti G, Catharino MGM, Ammerlaan AK, Saiki M, Favaro DIT, Byrne AR, Baruzzi R, Rodrigues DA. 2000. Determination of Mercury and selenium in hair samples of Brazilian Indian populations living in the Amazonic region by NAA. J. Radioanal Nucl Chem 244:81–85. [Google Scholar]
- Veiga MM, Meech JA, Onate N. 1994. Mercury pollution from deforestation. Nature 368:816–7. [DOI] [PubMed] [Google Scholar]
- Ventura DF, Costa MT, Costa MF, Berezovsky A, Salomao SR, Simoes AL, Lago M, Pereira LH, Faria MA, De Souza JM and others. 2004. Multifocal and full-field electroretinogram changes associated with color-vision loss in mercury vapor exposure. Vis Neurosci 21:421–9. [DOI] [PubMed] [Google Scholar]
- Ventura DF, Simoes AL, Do Canto Pereira LHM, Tomaz S, Lago M, Costa MT, Costa MF, De Souza JM, Faria MA, Silveira LC. 2005. Colour vision and contrast sensitivity losses of mercury contaminated industry workers in Brazil. Environ Toxicol Pharmacol 19:523–529. [DOI] [PubMed] [Google Scholar]
- Wada O, Yamaguchi N, Ono T, Maganashi M, Morinuma T. 1976. Inhibitory effect of mercury on kidney glutathione peroxidade and its prevention by selenium. Environ Res 12:75–80.954710 [Google Scholar]
- Watanabe C 2002. Modification of mercury toxicity by selenium: practical importance? Tohoku J Exp Med 196:71–7. [DOI] [PubMed] [Google Scholar]
- Wheatley B 1994. Exposure of Canadian aboriginal peoples to methylmercury. Environ Sci 3:33–40. [Google Scholar]
- WHO. 1990. International Program in Chemical Safety (IPCS) Environmental Health Criteria 101: Methylmercury. Geneva, Switzerland. [Google Scholar]
- WHO. 1991. International Program in Chemical Safety (IPCS) Environmental Health Criteria 118: Inorganic Mercury. Geneva, Switzerland. [Google Scholar]
- WHO. 2003. Concise International Chemical Assessment Document 50: Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Geneva, Switzerland. [Google Scholar]
- Yang DY, Chen YW, Gunn JM, Belzile N. 2008. Selenium and mercury in organisms: Interactions and mechanisms. Env Rev 16:71–92. [Google Scholar]
- Yin Z, Jiang H, Syversen T, Rocha JB, Farina M, Aschner M. 2008. The methylmercury-L-cysteine conjugate is a substrate for the L-type large neutral amino acid transporter. J Neurochem 107:1083–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoneda S, Suzuki KT. 1997. Detoxification of mercury by selenium by binding of equimolar Hg-Se complex to a specific plasm protein. Toxicol Appl Pharmacol 143:274–80. [DOI] [PubMed] [Google Scholar]