Abstract
Background
This is the fourth update of a Cochrane Review first published in 2002 and last updated in 2016. It is common clinical practice to follow patients with colorectal cancer for several years following their curative surgery or adjuvant therapy, or both. Despite this widespread practice, there is considerable controversy about how often patients should be seen, what tests should be performed, and whether these varying strategies have any significant impact on patient outcomes.
Objectives
To assess the effect of follow‐up programmes (follow‐up versus no follow‐up, follow‐up strategies of varying intensity, and follow‐up in different healthcare settings) on overall survival for patients with colorectal cancer treated with curative intent. Secondary objectives are to assess relapse‐free survival, salvage surgery, interval recurrences, quality of life, and the harms and costs of surveillance and investigations.
Search methods
For this update, on 5 April 2109 we searched CENTRAL, MEDLINE, Embase, CINAHL, and Science Citation Index. We also searched reference lists of articles, and handsearched the Proceedings of the American Society for Radiation Oncology. In addition, we searched the following trials registries: ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform. We contacted study authors. We applied no language or publication restrictions to the search strategies.
Selection criteria
We included only randomised controlled trials comparing different follow‐up strategies for participants with non‐metastatic colorectal cancer treated with curative intent.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. Two review authors independently determined study eligibility, performed data extraction, and assessed risk of bias and methodological quality. We used GRADE to assess evidence quality.
Main results
We identified 19 studies, which enrolled 13,216 participants (we included four new studies in this second update). Sixteen out of the 19 studies were eligible for quantitative synthesis. Although the studies varied in setting (general practitioner (GP)‐led, nurse‐led, or surgeon‐led) and 'intensity' of follow‐up, there was very little inconsistency in the results.
Overall survival: we found intensive follow‐up made little or no difference (hazard ratio (HR) 0.91, 95% confidence interval (CI) 0.80 to 1.04: I² = 18%; high‐quality evidence). There were 1453 deaths among 12,528 participants in 15 studies. In absolute terms, the average effect of intensive follow‐up on overall survival was 24 fewer deaths per 1000 patients, but the true effect could lie between 60 fewer to 9 more per 1000 patients.
Colorectal cancer‐specific survival: we found intensive follow‐up probably made little or no difference (HR 0.93, 95% CI 0.81 to 1.07: I² = 0%; moderate‐quality evidence). There were 925 colorectal cancer deaths among 11,771 participants enrolled in 11 studies. In absolute terms, the average effect of intensive follow‐up on colorectal cancer‐specific survival was 15 fewer colorectal cancer‐specific survival deaths per 1000 patients, but the true effect could lie between 47 fewer to 12 more per 1000 patients.
Relapse‐free survival: we found intensive follow‐up made little or no difference (HR 1.05, 95% CI 0.92 to 1.21; I² = 41%; high‐quality evidence). There were 2254 relapses among 8047 participants enrolled in 16 studies. The average effect of intensive follow‐up on relapse‐free survival was 17 more relapses per 1000 patients, but the true effect could lie between 30 fewer and 66 more per 1000 patients.
Salvage surgery with curative intent: this was more frequent with intensive follow‐up (risk ratio (RR) 1.98, 95% CI 1.53 to 2.56; I² = 31%; high‐quality evidence). There were 457 episodes of salvage surgery in 5157 participants enrolled in 13 studies. In absolute terms, the effect of intensive follow‐up on salvage surgery was 60 more episodes of salvage surgery per 1000 patients, but the true effect could lie between 33 to 96 more episodes per 1000 patients.
Interval (symptomatic) recurrences: these were less frequent with intensive follow‐up (RR 0.59, 95% CI 0.41 to 0.86; I² = 66%; moderate‐quality evidence). There were 376 interval recurrences reported in 3933 participants enrolled in seven studies. Intensive follow‐up was associated with fewer interval recurrences (52 fewer per 1000 patients); the true effect is between 18 and 75 fewer per 1000 patients.
Intensive follow‐up probably makes little or no difference to quality of life, anxiety, or depression (reported in 7 studies; moderate‐quality evidence). The data were not available in a form that allowed analysis.
Intensive follow‐up may increase the complications (perforation or haemorrhage) from colonoscopies (OR 7.30, 95% CI 0.75 to 70.69; 1 study, 326 participants; very low‐quality evidence). Two studies reported seven colonoscopic complications in 2292 colonoscopies, three perforations and four gastrointestinal haemorrhages requiring transfusion. We could not combine the data, as they were not reported by study arm in one study.
The limited data on costs suggests that the cost of more intensive follow‐up may be increased in comparison with less intense follow‐up (low‐quality evidence). The data were not available in a form that allowed analysis.
Authors' conclusions
The results of our review suggest that there is no overall survival benefit for intensifying the follow‐up of patients after curative surgery for colorectal cancer. Although more participants were treated with salvage surgery with curative intent in the intensive follow‐up groups, this was not associated with improved survival. Harms related to intensive follow‐up and salvage therapy were not well reported.
Plain language summary
Follow‐up strategies for participants treated for non‐metastatic colorectal cancer
What is the issue?
Colorectal cancer affects about 1 in 20 people in high‐income countries. Most patients (about two thirds) have curable disease. Follow‐up after surgical resection +/‐ chemotherapy treatment usually means visits to the doctor as well as having some tests. Many people believe that follow‐up saves lives, but we are not sure how often the patient should see the doctor and what tests they should have, and when.
Why is it important?
Follow‐up is expensive, it can make patients anxious around the time of their visit, and can be inconvenient. Tests are expensive and can have side effects. If tests find that cancer has come back in a person who feels well, but treatment cannot cure them, finding the recurrent cancer may not have helped that person or their family.
We asked...
We asked if follow‐up (i.e. tests and doctor visits) after colorectal cancer has been treated curatively is helpful. We looked at all different kinds of follow‐up: some versus none; more tests versus fewer tests; and follow‐up done by surgeons, general practitioners (GPs), or nurses.
We found...
We found 19 studies, including 13,216 participants. Our results are presented along with a judgement of quality which reflects how certain we are about the results. We found that follow‐up did not improve overall survival (high‐quality evidence), colorectal cancer‐specific survival (moderate‐quality evidence), or relapse‐free survival (high‐quality evidence). If patients have follow‐up, they are much more likely to have surgery if the cancer is detected again (high‐quality evidence). With follow‐up, more asymptomatic 'silent' cancer relapses are likely to be found at planned visits (moderate‐quality evidence). Harmful side effects (harms) from tests were not common, but more intensive follow‐up may increase harms (reported in two studies; very low‐quality evidence). Costs may be increased with more intensive follow‐up (low‐quality evidence). More intensive follow‐up probably makes little or no difference for quality of life (moderate‐quality evidence).
This means...
The information we have now suggests that there is little benefit from intensifying follow‐up, but there is also little evidence about quality of life, harms, and costs. We do not know what is the best way to follow patients treated for non‐metastatic (no secondaries) colorectal cancer, or if we should at all. We know little about the costs of follow‐up in this setting. Consumer needs and concerns with respect to the value of follow‐up require further research.
Summary of findings
Summary of findings for the main comparison. Intensive follow‐up compared to less intensive follow‐up for patients treated for colorectal cancer with curative intent.
| Intensive follow‐up compared to less intensive follow‐up for patients treated for colorectal cancer with curative intent | |||||
|
Participants: people with non‐metastatic colorectal cancer treated with curative intent Setting: primary and secondary care Interventions: more intensive follow‐up (clinic visits and investigations aimed at detecting recurrence) Comparison: less intensive follow‐up | |||||
| Outcomes | № of participants (studies) | Quality of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with less intensive follow‐up | Risk difference with intensive follow‐up | ||||
| Overall survival Follow‐up: range 8 months to 134 months | 12,528 (15 RCTs) | ⊕⊕⊕⊕ Higha,b | HR 0.91 (0.80 to 1.04) | Study population | |
| 107 per 1000 | 22 fewer per 1000 (60 fewer to 9 more)c | ||||
| Colorectal cancer‐specific survival Follow‐up: range 8 months to 134 months | 11,771 (11 RCTs) | ⊕⊕⊕⊝ Moderatea,b,d | HR 0.93 (0.81 to 1.07) | Study population | |
| 73 per 1000 | 15 fewer per 1000 (47 fewer to 12 more) | ||||
| Relapse‐free survival Follow‐up: range 48 months to 120 months | 8047 (16 RCTs) | ⊕⊕⊕⊕ Higha,b | HR 1.05 (0.92 to 1.21) | Study population | |
| 283 per 1000 | 17 more per 1000 (30 fewer to 66 more) | ||||
| Salvage surgery (surgery at relapse with curative intent) Follow‐up: range 2 months to 134 months | 5157 (13 RCTs) | ⊕⊕⊕⊕ Highe | RR 1.98 (1.53 to 2.56) | Study population | |
| 62 per 1000 | 60 more per 1000 (33 more to 96 more) | ||||
| Interval recurrences (recurrence CRC diagnosed between scheduled follow‐up visits) Follow‐up: range 2 months to 134 months | 3933 (7 RCTs) | ⊕⊕⊕⊝ Moderatee,f | RR 0.59 (0.41 to 0.86) | Study population | |
| 127 per 1000 | 52 fewer per 1000 (75 fewer to 18 fewer) | ||||
|
Harms Colonoscopy complications ‐ bowel perforation |
326 (1 RCT) |
⊕⊝⊝⊝ Very Lowg | RR 6.83 (0.36 to 131.21) |
Study population | |
| 0 per 1000 | 0 fewer per 1000 (0 fewer to 0 fewer) | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; CRC: colorectal cancer; HR: hazard ratio; RCT: randomised controlled trial; RR: risk ratio; OR: Peto odds ratio | |||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | |||||
aNot downgraded for imprecision because there were more than 300 events. bConfidence intervals include 1 and exclude clinically meaningful benefit or harm. cWe used the number of events in the control arms of the included studies to derive absolute values. dDowngraded because we deemed 192/468 (41%) events from studies at high risk of bias because of incomplete follow‐up. eNot downgraded because prespecified sensitivity analysis explained heterogeneity on basis of study age. fDowngraded because we deemed 95/178 (58%) events from studies at high risk of bias because of incomplete follow‐up. gDowngraded for risk of bias related to lack of blinding and imprecision.
Background
Description of the condition
Colorectal cancer is a commonly diagnosed malignancy affecting about one person in 20 in most westernised countries (DevCan 2005). Approximately two‐thirds of patients will present with potentially curable disease (by surgery plus or minus adjuvant therapies). Of these, 50% to 60% will relapse with metastatic disease (Lee 2007; Van Cutsem 2006; Yoo 2006). Surgeons, oncologists, and other health professionals caring for people with colorectal cancer have pursued a number of strategies to try to improve clinical outcomes. These have included population screening, better diagnostic testing, improved surgical and anaesthetic techniques, and more widespread utilisation of effective adjuvant therapies.
After definitive treatment is completed, clinician attention turns to follow‐up strategies designed to detect recurrence at a stage when further curative procedures can be used. Follow‐up strategies have also been developed in order to detect new curable metachronous (i.e. occurring at different times) primary tumours. There is overlap between these two strategies; this current review focuses on the former issue of strategies designed to detect curable recurrences of the original cancer. These include recurrences that are localised in either the lung, liver, abdomen, or pelvis and can be completely resected or ablated with curative intent.
Following patients after definitive treatment for cancer has become a traditional component of medical care (Edelman 1997). It is likely that clinicians follow patients after curative treatment for colorectal cancer at least in part to provide positive feedback on their management but also to assess the toxicities of treatment and to provide more accurate outcome data (Audisio 1996). Patients and their clinicians develop relationships during treatment and follow‐up that can make it hard to discharge patients and return responsibility for care back to their primary physician in the community (Audisio 2000). As a result, a practising clinician can accumulate a large number of follow‐up patients, and the surveillance of this cohort consumes significant resources.
The opportunity cost of the resources involved is considerable, limiting the care that the clinician can provide for other individuals. Few clinicians restrict colorectal cancer follow‐up visits to clinical examination only, and the temptation to order routine investigations is often reinforced by patients who desire tests to 'prove' that their disease is under control (Audisio 2000; Kievit 2000). Clinicians justify this approach by claiming that recurrences are being detected earlier than would otherwise occur and that patient outcomes are improved as a result (Kievit 2000).
Description of the intervention
Follow‐up programmes in colorectal cancer should be based on the anatomic and temporal patterns of recurrence (Audisio 2000; Edelman 1997). The most important phase of follow‐up is the first two to three years after primary resection, as during this time, the majority of recurrences will become apparent (Böhm 1993; Ovaska 1989). The liver is the most common site of metastases from colorectal cancer. A small proportion of these patients (10% to 20%) will have liver metastases that are distributed within the liver in such a fashion that makes them amenable to surgical resection or ablation (Alberts 2005; Muratore 2007). Published series of patients undergoing such surgical interventions (with significant numbers of long‐term survivors) encourage this approach (Choti 2002; Kanas 2012; Pawlik 2005). A number of strategies have been proposed to detect liver metastases at an early stage in order to identify such patients; these include the monitoring of blood tests (liver function, level of serum carcinoembryonic antigen (CEA)), and routine imaging of the liver and lung (Fleischer 1989; Sugarbaker 1987).
The psychological outcomes of follow‐up programmes for people with cancer can be positive or negative. Positive outcomes include reassurance and support. The negative outcomes include false reassurance, increased anxiety, fear and disappointment associated with early detection of an incurable recurrence, morbidity and mortality associated with procedures performed as a result of abnormal results, and distress caused by false‐positive results. Appropriate quality‐of‐life measurements could provide information about these outcomes.
Follow‐up can comprise clinic visits, examinations, and tests (blood tests and endoscopic and radiological examinations). More intensive follow‐up may consist of an increased frequency of clinic visits, tests, and examinations in comparison with none or fewer clinic visits, tests, and examinations.
How the intervention might work
Follow‐up programmes in colorectal cancer are thought to increase the early detection of recurrence at a stage when further curative procedures can be used, as well as new curable metachronous primary tumours, thereby, improving survival outcomes (GILDA 1998).
Why it is important to do this review
Whether systematic follow‐up can alter long‐term clinical outcomes for colorectal cancer remains controversial (Pfister 2004). Whilst some commentators have concluded that follow‐up is worthwhile (Gerdes 1990), others have questioned its effectiveness (Kievit 2000; McArdle 2000). The variation in follow‐up programmes, in terms of timing and frequency of clinician visits and the investigations undertaken by clinicians, is considerable (Collopy 1992; Connor 2001; Vernava 1994; Virgo 1995). Routine follow‐up has the potential to create psychological harm in patients, and any such disadvantages need to be outweighed by improved clinical outcomes (such as overall survival) that matter to patients. Data from follow‐up studies in other cancers (e.g. breast cancer and overall survival) is not encouraging in this regard (Rojas 2005).
Therefore, we conducted a systematic review of randomised controlled trials exploring questions relating to the effectiveness of follow‐up strategies in colorectal cancer patients treated with curative intent.
Objectives
To assess the effect of follow‐up programmes (follow‐up versus no follow‐up, follow‐up strategies of varying intensity, and follow‐up in different healthcare settings) on overall survival for patients with colorectal cancer treated with curative intent. Secondary objectives are to assess relapse‐free survival, salvage surgery, interval recurrences, quality of life, and the harms and costs of surveillance and investigations.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing different follow‐up strategies for participants with colorectal cancer. These included comparisons of follow‐up versus no follow‐up, follow‐up strategies of varying intensity (differing frequency or quantity of testing, or both), and follow‐up in different healthcare settings (e.g. primary care versus hospital). Cluster‐RCTs were eligible.
Types of participants
Male and female patients of any age with histologically proven adenocarcinoma of the colon or rectum, staged as T1‐4N0‐2M0 (Edge 2010), treated surgically with curative intent (plus or minus adjuvant treatment).
Types of interventions
Follow‐up visits with any health professionals in any setting, including symptom enquiry, clinical examination, and procedures and investigations (including but not limited to colonoscopy, blood tests, faecal analysis, and radiological examinations).
Types of outcome measures
Primary outcomes
Overall survival (measured from the time of randomisation in the study)
Secondary outcomes
Colorectal cancer‐specific survival (measured from the time of randomisation in the study)
Relapse‐free survival (measured from the time of randomisation in the study)
Salvage surgery (surgery performed with curative intent for relapse of colorectal cancer)
Interval recurrences (relapse of colorectal cancer detected between follow‐up visits)
Quality of life (using study‐specific instruments, including but not limited to FACT (Functional Assessment of Cancer Therapy), EORTC QLQ‐C30 (European Organization for Research and Treatment of Cancer Quality of Life), and EORTC‐CRC (Cella 1993; Sprangers 1993; Whistance 2009)
Harms, including but not limited to psychological harms, investigation‐related complications, and waste of resources
Costs of surveillance (including investigations)
Search methods for identification of studies
Electronic searches
We searched the following electronic databases with no language restriction:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 4) in the Cochrane Library using the strategy in Appendix 1;
MEDLINE Ovid (from 1950 to 5 April 2019) using the strategy in Appendix 2;
Embase Ovid (from 1974 to 5 April 2019) using the strategy in Appendix 3;
CINAHL EBSCO (Cumulative Index to Nursing and Allied Health Literature; from 1981 to April 2019) using the strategy in Appendix 4; and
Science Citation Index (from 1900 to April 2019) using the strategy in Appendix 5.
For the Review first published in the Cochrane Library 2002 issue 1, we also searched the electronic database CANCERLIT, which stopped existing in 2003.
Searching other resources
Trials registries
We searched the following trials registries:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov; www.clinicaltrials.gov; (searched April 2019); and
World Health Organization International Clinical Trials Registry Platform; apps.who.int/trialsearch; (searched April 2019).
Handsearching
We searched the following journals and conference proceedings:
American Society for Clinical Oncology (ASCO) (1995 to 2010, 2012‐2018);
European Society for Therapeutic and Radiation Oncology (1990, 1993, 2000 to 2010, 2012 to 2018); and
International Journal of Radiation Oncology Biology Physics: proceedings of the American Society for Radiation Oncology (ASTRO) (2011 to 2018).
We searched reference lists of published articles and previous systematic reviews and made personal contact with experts. We identified non‐English and unpublished studies.
Grey literature
We searched OpenGrey (www.opengrey.eu; 8 April 2019).
Data collection and analysis
Selection of studies
Two review authors (BEH and GMJ) checked the titles and abstracts identified from the databases. The review authors obtained the full text of all studies of possible relevance for independent assessment, decided which studies met the inclusion criteria, and graded their methodological quality. Discussion between the review authors resolved any disagreement. We contacted authors of primary studies for clarification where necessary: we contacted the authors for Barillari 1996 on 10 November 2018 but have had no response. We did not use the reported outcomes as criteria for including studies. We included studies irrespective of their publication status. We documented the selection process using Covidence and presented the details of the search in a PRISMA diagram (Moher 2009). Reasons for exclusion are presented in the 'Characteristics of excluded studies' table. We collated multiple reports of the same study so that each study, rather than the report, was the unit of interest in the review, and we identified the primary source.
Data extraction and management
Two review authors (BEH and GMJ) independently performed data extraction; we contacted the authors of studies to provide missing data where possible. We entered data into a previously piloted data form then into Covidence. One review author (BEH) entered data into Review Manager 5 (RevMan 5), which a second author (AS) checked (Review Manager 2014). We resolved any disagreements by discussion. We extracted the following data when available:
number of participants;
the age and status of the participants;
inclusion and exclusion criteria;
setting;
treatment regimen;
follow‐up details; and
survival, adverse events, and quality‐of‐life indices.
We collected data that were sufficient to populate a table of characteristics of included studies. For studies where only a subset of the participants recruited were eligible for inclusion, we included them if they reported data for that subgroup separately. Where a study had more than one study arm, such as FACS 2014, we combined those intervention study arms that met the inclusion criteria and compared them with the control arm; this ensured we did not double‐count data. When we performed subgroup analysis for FACS 2014, we combined the two arms with measured carcinoembryonic antigen (CEA) and the two arms that used computerised tomography (CT). We compared the magnitude and direction of effects reported by studies with how they were presented in the review.
In order to report time‐to‐event data, we used the RevMan 5 calculator (Review Manager 2014), and a spreadsheet developed by Matthew Sydes (Tierney 2007), to derive observed (O) and log‐rank expected events (E) (O‐E) and variance. Tierney 2007 presents 11 methods for calculating a hazard ratio (HR) or associated statistics, or both, from published time‐to‐event‐analyses into a practical, less statistical guide. The methods we used to do so were dependent on the available information in the texts, and we report them as follows.
Reports presenting HRs and 95% confidence intervals allowed application of method 3 in Tierney 2007 and were available for analysis as follows:
overall survival (FACS 2014; ONCOLINK; Strand 2011; Treasure 2014; Wang 2009 (please note that for Wang 2009, we used the RevMan 5 calculator to derive the HR, because this agreed with the P value given in the text));
colorectal cancer‐specific survival (FACS 2014; ONCOLINK; Rodríguez‐Moranta 2006); and
relapse‐free survival (FACS 2014; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Strand 2011; Treasure 2014).
In reports with a P value, events in each arm, and where the randomisation ratio was 1:1, we used method 7 in Tierney 2007 to derive O‐E and variance. Such studies contributed to the following analyses:
overall survival (GILDA 1998; Kjeldsen 1997; Ohlsson 1995; Rodríguez‐Moranta 2006; Schoemaker 1998 (for Rodríguez‐Moranta 2006, we used the RevMan 5 calculator to derive the HR, because the statistic presented in the text was adjusted for confounding; this was also the approach that the systematic review Pita‐Fernández 2014 used for this study));
colorectal cancer‐specific survival (Kjeldsen 1997; Ohlsson 1995); and
relapse‐free survival (GILDA 1998; Kjeldsen 1997; Rodríguez‐Moranta 2006).
In reports where we extracted data from the survival curve, assuming constant censoring, we used method 10 in Tierney 2007 for two studies contributing to the outcome of overall survival (Mäkelä 1995; Pietra 1998).
For relapse‐free survival, we extracted curve data with numbers at risk (method 11) for Sobhani 2018.
Assessment of risk of bias in included studies
Two study authors (BEH and GMJ) constructed and presented 'Risk of bias' tables using the Cochrane 'Risk of bias' tool, resolving any disagreements by discussion (Higgins 2017). We evaluated the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete follow‐up (exclusions, attrition);
selective reporting; and
other bias, including but not limited to early stopping, inadequate duration of follow‐up, or baseline imbalances.
We graded domains as at low risk of bias, high risk of bias, or unclear risk of bias (using the criteria in the Cochrane Handbook for Systematic Reviews of Interventions, Table 8.5d) (see Appendix 6), with our reasons and supporting evidence detailed in the tables (Higgins 2017). We summarised the risk of bias for each of the key study outcomes (overall survival, disease‐specific survival, relapse‐free survival, salvage surgery, interval recurrences, and complications of colonoscopy).
Measures of treatment effect
Where possible, we conducted time‐to‐event analyses for overall survival, colorectal cancer‐specific survival, and relapse‐free survival. We expressed the results as hazard ratios (HR) with 95% confidence intervals (CI) when the relevant information was available in the text or could be derived. Where necessary, we derived the HR using the RevMan 5 calculator (Review Manager 2014), and calculated associated statistics using an Excel spreadsheet developed by Matthew Sydes (Cancer Division of the Medical Research Council (MRC) Clinical Trials Unit) in collaboration with the Meta‐analysis Group of the MRC Clinical Trials Unit, London (Tierney 2007). We reported risk ratios (RR) and 95% CIs for dichotomous outcomes and would use the mean difference and 95% CI to report continuous outcomes. In the case of rare events (Analysis 1.6) we used the Peto odds ratio (OR; Deeks 2017). We interpreted a statistically non‐significant result (P value larger than 0.05) as a finding of uncertainty unless the confidence intervals were sufficiently narrow to rule out a potentially important magnitude of effect. We defined confidence intervals between 0.75 and 1.25 as excluding clinically meaningful benefits or harms.
Unit of analysis issues
All of the RCTs were parallel in design, with participants being the unit of randomisation; therefore, we had no unit of analysis issues.
Dealing with missing data
We contacted the authors of Secco 2002 to request the raw data. We were informed on 20 April 2015 that because of personnel changes, the authors were unable to retrieve the data, which meant we were not able to report overall survival data for this study. Because they plotted two curves for each study arm, it was not possible to extract data for use in time‐to‐event analysis. We were in contact with the study authors for GILDA 1998 on 18 February 2016, who kindly provided us with unpublished data, which we were able to include for the outcomes 'Interval recurrences' and 'Salvage surgery' (Fossati 2015). Therefore, all analyses were by intention‐to‐treat.
Assessment of heterogeneity
We assessed heterogeneity both visually and statistically using the Chi² test of heterogeneity (Altman 1992; Walker 1988), and I² statistic (Deeks 2017; Higgins 2002). The criterion for identification of heterogeneity is a P value less than 0.10 for the Chi² test (acknowledging the limitations of this process) and an I² statistic value of greater than 50%. Where we identified significant heterogeneity, we first checked the data to ensure it was not due to error, explored the potential causes of it, and made a cautious attempt to explain the heterogeneity.
Assessment of reporting biases
We assessed the potential impact of reporting biases by the use of a funnel plot for the four outcomes that included data from 10 or more studies (overall survival, cancer‐specific survival, relapse‐free survival, and salvage surgery). Including 15 studies allowed us to visually assess whether small‐study effects were present or not.
Data synthesis
We calculated a weighted treatment effect (using a random‐effects model) across studies using the Cochrane statistical package in RevMan 5 (Review Manager 2014). Where O‐E and variance were available, we used a log‐rank approach and a fixed‐effect model to synthesise data. We summated data where we judged the participants, interventions, and outcomes to be sufficiently similar to ensure a clinically meaningful answer.
Subgroup analysis and investigation of heterogeneity
We used subgroup analyses to investigate possible differences in participant outcomes according to study variables that we believed could be effect modifiers. These included the use of CEA, CT, and PET‐CT (positron emission tomography–computed tomography) in the intensive follow‐up strategy when compared with no use or less frequent use (twice at most) in the control arm, and setting for follow‐up (general practitioner (GP)‐ or nurse‐led follow‐up compared with hospital follow‐up and 'dose' of follow‐up, that is, studies that compared the use of more visits and tests with fewer visits and tests). These subgroup analyses may help identify which investigations are useful in follow‐up for colorectal cancer and allow us to give specific guidance to clinicians. We used a formal statistical test to compare subgroups.
Sensitivity analysis
We performed prespecified sensitivity analyses to test the strength of our conclusions by excluding studies judged to be at high risk of bias for the particular outcome concerned (Kjeldsen 1997; Mäkelä 1995; Pietra 1998; Schoemaker 1998; Wang 2009), and by study age (excluding those studies that completed accrual by 1996) (Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Schoemaker 1998; Treasure 2014).
We performed post hoc sensitivity analysis in response to reviewer suggestion by excluding one study (Ohlsson 1995), where the intensity of follow‐up in the intensive arm was comparable with the intensity of follow‐up in the control arm of other studies.
'Summary of findings' tables
We evaluated the quality of evidence using the GRADE approach for the following outcomes (Schünemann 2013):
overall survival;
colorectal cancer‐specific survival;
relapse‐free survival;
salvage surgery;
interval recurrences; and
harms associated with surveillance.
We used GRADEpro GDT to present the quality of evidence for the aforementioned outcomes in 'Summary of findings' tables. We could downgrade the quality of the evidence by one (serious concern) or two levels (very serious concern) for the following reasons: risk of bias, inconsistency (unexplained heterogeneity, inconsistency of results), indirectness (indirect population, intervention, control, outcomes), imprecision (wide confidence intervals, single study), and publication bias. We could also downgrade the quality by one level due to a large summary effect.
Results
Description of studies
Results of the search
For this update, we screened 7571 references, of which we assessed 33 references in full. We identified four new studies for inclusion (CEAwatch 2015; COLOFOL 2018; Sobhani 2018; SurvivorCare 2013), and we identified 9 additional references for four previously included studies (FACS 2014; ONCOLINK; Rodríguez‐Moranta 2006; Treasure 2014). In this update, we moved nine references referring to three studies that were previously either ongoing or awaiting assessment to included studies. We identified four new ongoing studies (COLOPEC; FURCA; ProphyloCHIP; SCORE).
In summary, this updated version of the review now includes a total of 82 references:
63 references refer to 19 included studies (CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Sobhani 2018; Strand 2011; SurvivorCare 2013; Treasure 2014; Wang 2009; Wattchow 2006);
five references refer to four excluded studies (Kronborg 1981; NCT00182234; Sano 2004; Serrano 2018);
three references refer to three studies awaiting assessment (Barillari 1996; NCT00199654; UMN000001318); and
11 references refer to six ongoing studies (COLOPEC; FURCA; HIPEC; ProphyloCHIP; SCORE; SURVEILLANCE).
There was considerable variation in the follow‐up strategies employed by the 19 studies; both the frequency of, the setting for, and the investigations that were performed during follow‐up visits were different in each study (see the 'Characteristics of included studies' tables and Figure 1).
1.

Study flow diagram
Included studies
Similarities and differences between the included studies
Thirteen of the 19 studies were multicentred (CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Ohlsson 1995; ONCOLINK; Pietra 1998; Schoemaker 1998; Sobhani 2018; SurvivorCare 2013; Treasure 2014; Wattchow 2006). We identified one cluster‐RCT (CEAwatch 2015).
Participants
Eleven of the 19 studies included Dukes' stage A, B, and C colon and rectal cancer (CEAwatch 2015; FACS 2014; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Schoemaker 1998; SurvivorCare 2013; Treasure 2014; Wang 2009; Wattchow 2006). Six studies excluded Dukes' A participants (COLOFOL 2018; GILDA 1998; Pietra 1998; Rodríguez‐Moranta 2006; Secco 2002; Sobhani 2018), two studies excluded participants with rectal cancer (Pietra 1998; Wattchow 2006), and one study included only rectal cancer participants (Strand 2011). Sobhani 2018 included some participants with completely resected Stage IV disease.
Interventions
The studies can be grouped into the following areas of assessment:
'dose' of follow‐up: more visits and tests versus fewer visits and tests (CEAwatch 2015; COLOFOL 2018; Kjeldsen 1997; Mäkelä 1995; Pietra 1998; Rodríguez‐Moranta 2006; Secco 2002; Sobhani 2018; Treasure 2014; Wang 2009);
formal follow‐up versus minimal/no follow‐up (COLOFOL 2018; FACS 2014; Ohlsson 1995; Schoemaker 1998; Secco 2002);
more liver imaging versus less liver imaging (COLOFOL 2018; FACS 2014; GILDA 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2018);
CEA versus no CEA (CEAwatch 2015; COLOFOL 2018; FACS 2014; Kjeldsen 1997; Ohlsson 1995; Sobhani 2018; Treasure 2014);
setting for follow‐up (where frequency of visits and tests were identical in both arms): GP‐led follow‐up (ONCOLINK; Wattchow 2006), or nurse‐led follow‐up (Strand 2011), compared with surgeon‐led follow‐up;
usual follow‐up care was supplemented by provision of survivorship educational materials, a survivorship care plan, end of treatment consultation and subsequent phone calls (SurvivorCare 2013).
The included studies did not assess the quality of histopathology.
Outcomes
Fifteen RCTs reported overall survival (COLOFOL 2018; CEAwatch 2015; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2018; Strand 2011; Treasure 2014; Wang 2009).
Eleven RCTs reported colorectal cancer‐specific survival, measured from the time of randomisation in the study ((CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Sobhani 2018; Wang 2009).
Sixteen RCTs reported relapse‐free survival, measured from the time of randomisation in the study (COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Sobhani 2018; Strand 2011; Treasure 2014; Wang 2009).
Thirteen RCTs reported salvage surgery, that is, surgery performed with curative intent for relapse of colorectal cancer (FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Treasure 2014; Wang 2009).
Seven RCTs reported interval recurrences, relapse of colorectal cancer detected between follow‐up visits or symptomatic recurrences (FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Secco 2002; Sobhani 2008; Wang 2009).
-
Seven RCTs assessed quality of life (CEAwatch 2015; COLOFOL 2018; GILDA 1998; Kjeldsen 1997; ONCOLINK; SurvivorCare 2013; Wattchow 2006).
CEAwatch 2015 used a Dutch version of the Cancer Worry Scale (Custers 2014), measured participant attitudes to follow‐up using a validated tool (Stiggelbout 1997), and reported using the Hospital Anxiety Depression Scale (HADS; Zigmond 1983).
COLOFOL 2018 used SF‐36 and validated scales (EORTC QLQ C‐30 (European Organization for Research and Treatment of Cancer quality of life questionnaire; Aaronson 1993)) and reported using the HADS (Zigmond 1983).
GILDA 1998 used the 12‐item short version (SF‐12) of SF‐36 (Apolone 1998; Gandek 1998), which was validated in the Italian population, and the Psychological General Well‐Being (PGWB) Index (Dupuy 1984).
Kjeldsen 1997 used the Nottingham Health Profile (Anderson 1996; Hunt 1980).
ONCOLINK used validated scales EORTC QLQ C‐30 (European Organization for Research and Treatment of Cancer quality of life questionnaire; Aaronson 1993) and EQ‐5D (EuroQol five dimensions questionnaire; Dolan 1997);
SurvivorCare 2013 assessed psychological distress using the Brief Symptom Inventory 18 (BSI‐18; Zabora 2001), survivors' unmet needs using Cancer Survivors' Unmet Need measure (CaSUN; Hodgkinson 2007), health‐related quality of life using EORTC QLQ C‐30 (Aaronson 1993), and the EORTC QLQ CR‐29 (Whistance 2009).
Wattchow 2006 reported the short form (SF)‐12 Physical and Mental Health component (Ware 1995), and reported using the HADS (Zigmond 1983).
Schoemaker 1998 and Wang 2009 reported harms.
Six studies evaluated costs of surveillance including investigations (CEAwatch 2015; ONCOLINK; Rodríguez‐Moranta 2006; Secco 2002; Sobhani 2018; Strand 2011). ONCOLINK and Rodríguez‐Moranta 2006 performed cost‐minimisation analyses.
See Characteristics of included studies.
Study accrual dates spanned over three decades. Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Schoemaker 1998; Secco 2002; and Treasure 2014 accrued in the 1980s and 1990s. GILDA 1998; Rodríguez‐Moranta 2006; Sobhani 2008; and Wang 2009 accrued participants in the 1990s and early 2000s. CEAwatch 2015; COLOFOL 2018; FACS 2014; ONCOLINK; and Sobhani 2018 accrued participants from 2003 to 2012.
The variety of investigations used across the studies may affect the applicability of results. For example, Kjeldsen 1997; ONCOLINK; and Treasure 2014 did not use CT scanning, while Sobhani 2018 used PET/CT.
Excluded studies
For this update, we applied the current recommendations from the Cochrane Handbook for Systematic Reviews of Interventions with respect to excluded studies and only classified studies as excluded if they were those that one might reasonably expect could have been eligible for inclusion.
We excluded four studies (see the 'Characteristics of excluded studies' tables). Sano 2004 was not eligible because participants did not have colorectal cancer. NCT00182234 included participants with both breast and colorectal cancer and did not analyse them separately. Participants in Serrano 2018 had liver metastases, so were not eligible for inclusion in this review.
Risk of bias in included studies
There was complete concordance between review authors regarding the evaluation of study methodology (Figure 2).
2.

'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study
Allocation
Although all of the studies were reported to be randomised, only two explicitly reported that they concealed the allocation of participants to study groups (Mäkelä 1995; Wattchow 2006). We found that none of the studies were at high risk of bias with respect to allocation; we judged them all to be at low (COLOFOL 2018; CEAwatch 2015; FACS 2014; ONCOLINK; Rodríguez‐Moranta 2006; Schoemaker 1998; Treasure 2014) or unclear risk of bias (GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Secco 2002; Sobhani 2008; Sobhani 2018; Strand 2011; SurvivorCare 2013; Wang 2009; Wattchow 2006).
Blinding
Participant or clinician blinding was not possible. We judged one study to be at high risk of bias for blinding of participants ( Wang 2009). One study used independent radiologists who were blinded to study group allocation to assess CT scans (Schoemaker 1998). We judged three studies to be at high risk of bias for blinding of outcome assessors (Kjeldsen 1997; Sobhani 2018; Wang 2009). Most studies were at low risk of bias for blinding Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Schoemaker 1998;Secco 2002; Sobhani 2008; Strand 2011; Treasure 2014; Wattchow 2006 and five were at unclear risk of bias (CEAwatch 2015; COLOFOL 2018; GILDA 1998; Rodríguez‐Moranta 2006; SurvivorCare 2013).
Incomplete outcome data
Eight studies ensured that they obtained outcome data from more than 80% of the participants. Wattchow 2006 obtained outcome data for 77% of the participants. All studies conducted intention‐to‐treat analyses. We judged one study to be at high risk of bias for incomplete outcome data (Pietra 1998). Two studies examined compliance with the follow‐up regimen (Rodríguez‐Moranta 2006; Schoemaker 1998), but no study fully assessed contamination. Most studies (CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2008; Sobhani 2018; SurvivorCare 2013; Wattchow 2006) were at low risk of attrition bias, four studies were unclear risk of attrition bias (Secco 2002; Strand 2011; Treasure 2014; Wang 2009).
Selective reporting
We did not have access to the protocols for most studies (CEAwatch 2015; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Strand 2011; Wang 2009; Wattchow 2006), so we judged them to be at unclear risk of bias. With more information available we judged COLOFOL 2018; FACS 2014; SurvivorCare 2013 and Treasure 2014 to be at low risk of bias for this domain (see Characteristics of included studies). We judged one study at high risk of bias for selective reporting (Sobhani 2018).
Other potential sources of bias
We did not find other sources of bias (including inadequate follow‐up duration and baseline imbalances on study populations). One study was stopped early (Treasure 2014), but we did not feel this was likely to introduce bias.
Overall survival
We judged four studies contributing data to this outcome to be at high risk of bias because they did not mention blinding (Kjeldsen 1997; Mäkelä 1995; Secco 2002; Wang 2009). We did not feel this represented any risk of bias for this objective outcome. We deemed three studies at high risk of bias for allocation concealment, Mäkelä 1995; Schoemaker 1998, and attrition bias, Pietra 1998, but because they contributed in total 17.2% of study weight, we did not feel that we needed to downgrade for risk of bias.
Colorectal cancer‐specific survival
We judged Pietra 1998 to be at high risk of attrition bias because the study authors potentially excluded 15% of the participants randomised without explaining to which study arm they belonged. Sobhani 2018 was at high risk of bias for lack of blinding of outcome assessors. These two studies contributed in total 11.9% of study weight for this outcome. Kjeldsen 1997 and Pietra 1998 did not mention blinding and probably did not ensure it (this represented 41% of the events contributing to this outcome). Because this could cause ascertainment bias for cause of death, we downgraded evidence quality for colorectal cancer‐specific survival for risk of bias.
Relapse‐free survival
For this outcome, Kjeldsen 1997; Mäkelä 1995 and Secco 2002 (which contributed 383/1340 (28%) of the events) did not mention blinding. We judged both Sobhani 2018 and Schoemaker 1998 at high risk of bias for lack of blinding, but we did not downgrade, because fewer than 30% of events were contributed from studies deemed at high risk of bias.
Salvage surgery
We judged Schoemaker 1998 to be at high risk of bias for allocation concealment. We also deemed three other studies contributing to this outcome to be at high risk of bias: Kjeldsen 1997 and Wang 2009 for lack of blinding of outcome assessment, Pietra 1998 for incomplete outcome reporting, and Wang 2009 further did not blind participants and personnel. We did not downgrade for risk of bias despite these limitations, because Schoemaker 1998 contributed only 11/526 (0.05%) of the events for this outcome, lack of blinding was unlikely to have affected the outcome reporting of salvage surgery, and the incomplete outcome reporting in Pietra 1998 was related to other outcomes.
Interval recurrences
We did not downgrade this outcome for risk of bias. We judged FACS 2014 to be at low risk of bias for all domains. We judged both Secco 2002 and Wang 2009 to be at high risk of bias for the domain of blinding, but this was because blinding was not mentioned in Wang 2009, and in Secco 2002, there were prespecified follow‐up schedules. We did not downgrade this outcome for risk of bias (Risk of bias in included studies and Table 1).
Effects of interventions
See: Table 1
1. Primary outcome
1.1 Overall survival
We report on 1453 deaths in 12,528 participants in 15 studies. Intensive follow‐up versus less intense follow‐up for participants treated with curative intent for colorectal cancer makes little or no difference to overall survival (HR 0.91, 95% CI 0.80 to 1.04; 15 studies, 12,528 participants). We found no evidence of heterogeneity: I² = 18%, P = 0.25. In absolute terms, the average effect of intensive follow‐up on overall survival was 24 fewer deaths per 1000 participants, but the true effect could lie between 60 fewer to 9 more per 1000 participants. The GRADE assessment of evidence quality for this outcome was high. The funnel plot did not show evidence of small study effect (COLOFOL 2018; CEAwatch 2015; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2018; Strand 2011; Treasure 2014; Wang 2009; Figure 3).
3.

Funnel plot of comparison 1. Intensive follow‐up versus minimalist follow‐up, outcome: 1.1 overall survival
Subgroup analyses
We compared studies comparing follow‐up provided by different health professionals. Formal testing for subgroup differences was negative (Chi² = 0.40; P = 0.53; I² = 0%) when we compared those studies that used different settings with GP‐ or nurse‐led follow‐up (ONCOLINK; Strand 2011), with those set in hospitals (CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2018; Treasure 2014; Wang 2009; Analysis 1.7).
In studies that compared more visits and tests with fewer visits and tests (Kjeldsen 1997; Mäkelä 1995; Pietra 1998; Rodríguez‐Moranta 2006; Sobhani 2018; Treasure 2014; Wang 2009), versus studies that compared follow‐up with minimal or no follow‐up (COLOFOL 2018; FACS 2014; Ohlsson 1995; Schoemaker 1998), formal testing for subgroup differences was negative (Chi² = 0.34; P = 0.56 ; I² = 0 %; Analysis 1.8).
In studies using CEA in the intensive follow‐up regimen (CEAwatch 2015; COLOFOL 2018; FACS 2014; Ohlsson 1995; Sobhani 2018; Treasure 2014) versus those studies that did not use CEA (Kjeldsen 1997), formal testing revealed no evidence of differences between the subgroups (Chi² = 0.15 ; P = 0.7, I² = 0% (Analysis 1.9).
We compared studies using CT in the intensive follow‐up regimen (COLOFOL 2018; FACS 2014; Mäkelä 1995; Ohlsson 1995; Rodríguez‐Moranta 2006; Schoemaker 1998; Sobhani 2018) versus those that did not use CT (Kjeldsen 1997; ONCOLINK; Treasure 2014). Formal statistical testing revealed no evidence of differences between the subgroups (Chi² = 0.31; P = 0.58; I² = 0% (Analysis 1.10)
We compared studies using frequent CT scans in the intervention arm (FACS 2014; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998) versus the use of two or fewer CT scans in the control arm (Kjeldsen 1997; ONCOLINK; Treasure 2014) (Analysis 1.11). Formal statistical testing revealed no evidence of differences between the subgroups (Chi² = 0.99; P = 0.32: I² = 0%).
1.7. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 7 Overall survival SG Healthcare professional.
1.8. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 8 Overall survival SGA "dose" of follow‐up.
1.9. Analysis.
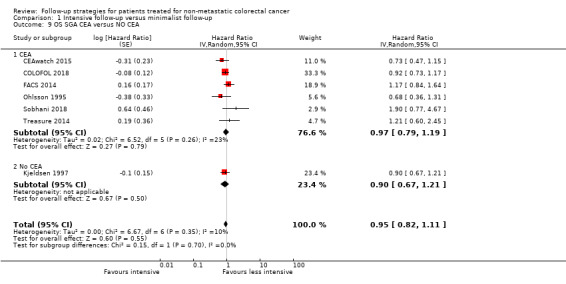
Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 9 OS SGA CEA versus NO CEA.
1.10. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 10 OS CT versus no CT.
1.11. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 11 OS CT versus < 2 or no CT.
Sensitivity analyses
Our findings for the outcome of overall survival were robust to sensitivity analyses.
Excluding studies at high risk of bias for this outcome (Schoemaker 1998; Pietra 1998), we found no statistical evidence of a survival advantage for the comparison of intensive versus less intensive follow‐up (HR 0.97, 95% CI 0.86 to 1.08). We found no heterogeneity: I² = 0%; P = 0.68, The GRADE assessment of evidence quality for this outcome was high.
Excluding six studies on the basis of study age (Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Schoemaker 1998; Treasure 2014), we found no statistical evidence of a survival advantage for the comparison of intensive versus less intensive follow‐up (HR 0.98, 95% CI 0.84 to 1.15; Analysis 1.1). We found little evidence of heterogeneity: I² = 18%; P = 0.28. The GRADE assessment of evidence quality for this outcome was high.
Excluding one study where the intensity of the follow‐up in the 'intensive' arm was similar to that in the control arm of other studies (Ohlsson 1995), we found no evidence of a clinically meaningful effect on overall survival (HR 0.92, 95% CI 0.81 to 1.05). We found no evidence of heterogeneity: I² = 20%; P = 0.24. The GRADE assessment of evidence quality for this outcome was high.
1.1. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 1 Overall survival.
2. Secondary outcomes
2.1 Colorectal cancer‐specific survival
We were able to report on 925 colorectal cancer deaths in 11,771 participants enrolled in 11 studies; 99.6% had a median follow‐up of greater than 48 months. We found that intensive versus less intensive follow‐up probably makes little or no difference to colorectal cancer‐specific survival (HR 0.93, 95% CI 0.81 to 1.07). We found no evidence of heterogeneity: I² = 0%, P = 0.57 (CEAwatch 2015; COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Sobhani 2018; Wang 2009; Analysis 1.2).
1.2. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 2 Colorectal cancer‐specific survival.
In absolute terms, the average effect of intensive follow‐up on colorectal cancer‐specific survival was five fewer colorectal cancer‐specific deaths per 1000 participants, but the true effect could lie between 14 fewer to five more per 1000 participants. The GRADE assessment of evidence quality for this outcome was moderate, we downgraded once for risk of bias. The funnel plot did not show evidence of small study effect (Figure 4).
4.

Funnel plot of comparison 1. Intensive follow‐up versus minimalist follow‐up, outcome: 1.2 colorectal cancer‐specific survival
Sensitivity analyses
Our findings for the outcome of colorectal cancer‐specific survival were robust to the following sensitivity analyses.
Excluding studies at high risk of bias for this outcome (Kjeldsen 1997; Sobhani 2018; Wang 2009), we found no statistical evidence of an effect on colorectal cancer‐specific survival (HR 0.90, 95% CI 0.76 to 1.06) and no evidence of heterogeneity: I² = 0%; P = 0.43.
We found no statistical evidence of study age having an effect (HR 0.91, 95% CI 0.67 to 1.23) and no evidence of heterogeneity: I² = 30%; P = 0.22 (excluding GILDA 1998; Kjeldsen 1997; Ohlsson 1995).
2.2 Relapse‐free survival
We were able to report on 2254 relapses in 8047 participants enrolled in 16 studies, with a median follow‐up of greater than 48 months for 97.9% of participants studied (COLOFOL 2018; FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Sobhani 2018; Strand 2011; Treasure 2014; Wang 2009; Analysis 1.3).
1.3. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 3 Relapse‐free survival.
We found intensive follow‐up versus less intense follow‐up makes little or no difference to relapse‐free survival (HR 1.05, 95% CI 0.92 to 1.21). The CIs excluded both clinically meaningful benefits and harms. We found no evidence of heterogeneity: I² = 41%, P = 0.05.
The funnel plot did not show evidence of small‐study effect (Figure 5).
5.

Funnel plot of comparison 1. Intensive follow‐up versus minimalist follow‐up, outcome: 1.3 relapse‐free survival
The average effect of intensive follow‐up on relapse‐free survival was 12 more relapses per 1000 participants, but the true effect could lie between 19 fewer and 48 more per 1000 participants. The GRADE assessment of evidence quality for this outcome was high.
Sensitivity analyses
Our findings for the outcome of relapse‐free survival were robust to the following sensitivity analyses.
Excluding studies at high risk of bias for this outcome (Kjeldsen 1997; Pietra 1998; Schoemaker 1998; Sobhani 2018; Wang 2009), we found no statistical evidence of an effect (HR 1.13, 95% CI 0.96 to 1.33) and no clear evidence of heterogeneity: I² = 40%; P = 0.08.
With regard to study age (excluding GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Schoemaker 1998; Treasure 2014), we found no statistical evidence of an effect (HR 1.05, 95% CI 0.79 to 1.40) and little evidence of heterogeneity: I² = 30%; P = 0.21.
2.3 Salvage surgery
We were able to report on 457 episodes of salvage surgery in 5157 participants enrolled in 13 studies, with a follow‐up duration of greater than 48 months in 90.6% of participants studied (FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; ONCOLINK; Pietra 1998; Rodríguez‐Moranta 2006; Schoemaker 1998; Secco 2002; Sobhani 2008; Treasure 2014; Wang 2009; Analysis 1.4).
1.4. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 4 Salvage surgery.
We found the use of salvage surgery increased with intensive follow‐up for colorectal cancer (RR 1.98, 95% CI 1.53 to 2.56). The CIs included a range of clinically significant increases in salvage surgery. We found some non‐significant evidence of heterogeneity: I² = 31%; P = 0.14.
The funnel plot did not show evidence of small‐study effect (see Figure 5).
In absolute terms, the effect of intensive follow‐up on salvage surgery was 60 more episodes of salvage surgery per 1000 participants, but the true effect could lie between 33 to 96 more episodes per 1000 participants. The GRADE assessment of evidence quality for this outcome was high.
Sensitivity analyses
Our findings for the outcome of salvage surgery were robust to the following sensitivity analyses.
Excluding studies at high risk of bias for this outcome (Pietra 1998; Schoemaker 1998; Wang 2009), we found some non‐significant heterogeneity (RR 2.03, 95% CI 1.53 to 2.69; I² = 33%; P = 0.15).
With regard to study age we found that when we excluded the older studies (GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Ohlsson 1995; Pietra 1998; Schoemaker 1998; Treasure 2014), there was less heterogeneity (RR 2.04, 95% CI 1.35 to 3.09; I² = 25%; P = 0.25).
2.4 Interval (symptomatic) recurrences
We found 376 interval recurrences reported in 3933 participants enrolled in seven studies, with a median follow‐up duration of greater than 48 months for 100% of participants studied (FACS 2014; GILDA 1998; Kjeldsen 1997; Mäkelä 1995; Secco 2002; Sobhani 2008; Wang 2009; Analysis 1.5). There was an appreciable decrease in the number of interval recurrences (RR 0.59, 95% CI 0.41 to 0.86). The CIs included a range of clinically significant decreases in interval recurrences (Analysis 1.5). We detected heterogeneity: I² = 66%; P = 0.007.
1.5. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 5 Interval recurrences.
Intensive follow‐up was associated with fewer interval recurrences: 52 fewer per 1000 participants; the true effect is between 18 and 75 fewer per 1000 participants. The GRADE assessment of quality of evidence was moderate, we downgraded once for risk of bias.
Sensitivity analyses
Our findings were robust to the following sensitivity analyses.
Excluding studies at high risk of bias for this outcome (Kjeldsen 1997; Wang 2009), we found evidence of heterogeneity (RR 0.61, 95% CI 0.37 to 1.02; I²= 75%; P = 0.003).
With regard to study age (excluding GILDA 1998; Kjeldsen 1997; Mäkelä 1995), we found no evidence of heterogeneity (RR 0.42, 95% CI 0.32 to 0.56; I² = 0%; P = 0.81).
2.5 Quality of life
CEAwatch 2015 found no significant differences between the two groups in terms of attitude to follow‐up, fear of recurrence, HADS score and Cancer Worry score, and there was no detectable burden or improvement in psychological burden associated with intensification of follow‐up.
GILDA 1998 found no clinically significant differences among the three main quality‐of‐life scales (SF‐12 mental component, SF‐12 physical component, and PGWB Index) between the two study arms.
Kjeldsen 1997 reported the influence of different follow‐up strategies on quality of life for 350 out of 597 Danish participants. They reported a small increase in quality of life (P < 0.05), as measured by the Nottingham Health Profile, associated with more frequent follow‐up visits compared with virtually no follow‐up.
ONCOLINK reported no significant effect on quality of life for the main outcome measures. They reported significant effects in favour of GP‐led follow‐up for EORTC QLQ‐C30 for role functioning (P = 0.02), emotional functioning (P = 0.01), and pain (P = 0.01). They did not report any significant differences in global health status.
SurvivorCare 2013 reported that psychological distress was similar between the two groups (difference 0.2, 95% CI −2.5 to 2.9) with the prespecified clinically meaningful difference of 0.42 (figures from the text). DIfferences between unmet needs, information needs and health‐related quality of life were not clinically significant. The intervention group were more satisfied with their care, but the median scores were similar (see Characteristics of included studies).
Wattchow 2006 assessed depression and anxiety, quality of life, and participant satisfaction in a cohort of participants randomised to follow‐up of their colon cancer in different settings (see Included studies). They found that the study participants remained in the normal range for depression and anxiety with no difference between the two groups at either 12 months or 24 months. Study participants (in each arm) had reduced physical quality of life at baseline, which improved as the study progressed, but there were no significant differences between the two groups. There were no differences between the two groups on the participant satisfaction scale, and both groups reported high levels of satisfaction with their care.
More intensive follow‐up probably makes little or no difference to quality of life (moderate‐quality evidence). The data were not available in a form that allowed analysis.
2.6 Harms (colonoscopy complications)
Two studies reported adverse events associated with follow‐up (Schoemaker 1998; Wang 2009). Three perforations and four gastrointestinal haemorrhages (requiring transfusion) were reported from a total of 2292 (0.3%) colonoscopies. Data from Schoemaker 1998 were not available in a form that allowed analysis. Intensive follow‐up may increase the complications (perforation or haemorrhage) from colonoscopies (RR 7.30, 95% CI 0.75 to 70.69); one study, 326 participants) (Wang 2009) Analysis 1.6; Table 1). GRADE evidence quality was very low, downgraded for risk of bias related to lack of blinding, reporting bias and imprecision.
2.7 Costs of surveillance
ONCOLINK found the cost per participant for 24 months' follow‐up was GPB 9889 for surgeon‐led follow‐up and GBP 8233 for GP‐led follow‐up (P < 0.001 (figures from text)).
Rodríguez‐Moranta 2006 demonstrated that although the cost of intensive follow‐up was higher, when resectability of recurrences was considered, the cost per resectable recurrence was lower in the intensively followed group.
Secco 2002 provided risk‐adapted follow‐up based on prognostic factors prospectively identified, and the study authors commented that risk‐adapted follow‐up reduced costs for those with a better prognosis.
In Sobhani 2018 the intensive arm cost significantly more than the control arm (P < 0.0033 (figure from text)).
Strand 2011 found no difference in costs.
Costs were assessed in CEAwatch 2015, but have not yet been reported.
In summary, limited data suggests that the cost of more intensive follow‐up may be increased in comparison with less intense follow‐up, but the cost of surgery for resectable recurrence may be reduced. GRADE evidence quality was low, being downgraded for imprecision. The data were not available in a form that allowed analysis.
Discussion
The results of our review suggest that there is no overall survival benefit for intensifying the follow‐up of participants after curative surgery for colorectal cancer. The analyses did not show a significant difference in the incidence of recurrence between the participants in the intensively followed groups and the control groups. However, significantly more surgical procedures for recurrence were performed in the experimental arms of the studies. Recurrences in the more intensively followed groups may have been detected earlier allowing for effective salvage treatments, but this did not lead to better overall survival.
Each study follow‐up strategy combined a number of different components, including frequency of visits, type of clinical assessment, types and frequency of tests, and the setting in which follow‐up was conducted (see Table 2). No study compared the addition of one specific intervention, and the feasibility of comparing strategies with a variety of components and varying complexity becomes problematic. The use of liver imaging does not appear to be associated with improved survival. A specific variation across the studies was the intensity of follow‐up. For example, the follow‐up intensity in the intensively followed group in Ohlsson 1995 was similar to the intensity of follow‐up in the control groups of other studies in the review (Mäkelä 1995; Pietra 1998; Schoemaker 1998). Therefore, it was not possible to extract from these data a precise indication of the optimal combinations of frequency, type, and setting for follow‐up investigations for these participants. Our findings were robust to sensitivity analysis when excluding Ohlsson 1995.
1. Interventions in included studies.
| Study | More intensive follow‐up | Less intensive follow‐up |
| Studies comparing more visits and tests versus fewer visits and tests | ||
| CEAwatch 2015 | CEA 2‐monthly year 1‐3, then 3‐monthly year 4‐5 Clinic visits 12‐monthly year 1‐3 CT CAP 12 monthly for 3 years CXR and liver US annually year 1‐3 |
CEA 3‐6‐monthly year 1‐3, 12 monthly year 4‐5 Clinic visits 6‐monthly year 1‐3, 12 monthly year 4‐5 CXR and liver US 6‐monthly year 1‐3, 12‐monthly year 4‐5 |
| GILDA 1998 | Office visits at 4, 8, 12, 16, 20, 24, 30, 36, 42, 48, and 60 months and history and clinical examination, FBC, CEA, and CA 19‐9 Colonoscopy and CXR at 12, 24, 36, 48, and 60 months Liver US at 4, 8, 12, 16, 24, 36, 48, and 60 months For rectal participants, pelvic CT at 4, 12, 24, and 48 months |
Office visits at 4, 8, 12, 16, 20, 24, 30, 42, 48, and 60 months, including history, examination, and CEA Colonoscopy at 12 and 48 months Liver US at 4 and 16 months Rectal cancer participants in addition had rectoscopy at 4 months, CXR at 12 months, and liver US at 8 and 16 months. A single pelvic CT was allowed if a radiation oncologist required it as baseline following adjuvant treatment |
| Kjeldsen 1997 | Clinic visits at 6, 12, 18, 30, 36, 48, 60, 120, 150, and 180 months after radical surgery Examinations included medical history, clinical examination, DRE, gynaecological examination, Haemoccult‐II test, colonoscopy, CXR, haemoglobin level, erythrocyte sedimentation rate, and liver enzymes | Clinic visits at 60, 120, and 180 months Examinations included medical history, clinical examination, DRE, gynaecological examination, Haemoccult‐II test, colonoscopy, CXR, haemoglobin level, erythrocyte sedimentation rate, and liver enzymes |
| Mäkelä 1995 | Participants with rectal or sigmoid cancers had flexible sigmoidoscopy with video imaging every 3 months, colonoscopy at 3 months (if it had not been done preoperation), then annually. Liver US and primary site at 6 months, then annually | Participants who had rectal and sigmoid cancers had rigid sigmoidoscopy and barium enema annually |
| Pietra 1998 | Clinic visits at 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, and 60 months, then annually thereafter. At each visit: clinical examination, US, CEA, and CXR. Annual CT liver and colonoscopy were performed |
Seen at 6 and 12 months, then annually. At each visit, clinical examination, CEA, and US were performed. They had annual CXR, yearly colonoscopy, and CT scan. |
| Rodríguez‐Moranta 2006 | History, examination, and bloods (including CEA), US/CT, CXR, and colonoscopy | history, examination, and bloods (including CEA) |
| Sobhani 2008 | PET performed at 9 and 15 months and conventional follow‐up | Conventional follow‐up |
| Sobhani 2018 | FDG PET/CT at 6, 12, 18, 24, 30 and 36 months pus conventional follow‐up | Conventional follow‐up: physical examination and laboratory tests (FBC, tumour markers) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36 months, liver US and CXR at 3, 9, 15, 21, 27, 33 months, CT CAP at 6, 12, 18, 24, 30 and 36 months, colonoscopy at 12 and 36 months |
| SurvivorCare 2013 | Nurse‐led survivorship care package:
|
Usual care defined as: "care according to the treating cancer centre or practitioner's usual practice" |
| Treasure 2014 | CEA rise triggered 'second‐look' surgery, with intention to remove any recurrence discovered | CEA rise did not trigger 'second‐look' surgery |
| Wang 2009 | Colonoscopy at each visit | Colonoscopy at 6 months, 30 months, and 60 months from randomisation |
| Studies comparing tests and visits with minimal or no follow‐up | ||
| COLOFOL 2018 | CEA 1 month postoperatively then CEA, CT chest and abdomen 6, 12, 18, 24, and 36 months | CEA 1 month postoperatively then CEA, CT chest and abdomen 12 and 36 months after surgery |
| FACS 2014 |
|
No scheduled follow‐up except a single CT scan of the CAP if requested at study entry by a clinician |
| Ohlsson 1995 | 3‐, 6‐, 9‐, 12‐, 15‐, 18‐, 21‐, 24‐, 30‐, 36‐, 42‐, 48‐, and 60‐month clinic visits. Performed at each visit were clinical exam, rigid proctosigmoidoscopy, CEA, alkaline phosphatase, gamma‐glutaryl transferase, faecal haemoglobin, and CXR. Examination of anastomosis (flexible sigmoidoscopy or colonoscopy, as dictated by the lesion) was performed at 9, 21, and 42 months. Colonoscopy was performed at 3, 15, 30, and 60 months. CT of the pelvis was performed at 3, 6, 12, 18, and 24 months. | No planned follow‐up visits. Participants received written instructions recommending that they leave faecal samples with the district nurse for examination every third month during the first 2 years after surgery then once a year. They were instructed to contact the surgical department if they had any symptoms. |
| Schoemaker 1998 | Yearly CXR, CT of the liver, and colonoscopy | These investigations were only performed in the control group if indicated on clinical grounds or after screening test abnormality, and at 5 years of follow‐up, to exclude a reservoir of undetected recurrences |
| Secco 2002 | Clinic visits and serum CEA, abdomen/pelvic US scans, and CXR. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR. | "minimal follow‐up programme performed by physicians" |
| Studies that assessed effect of setting for follow‐up | ||
| ONCOLINK | Surgeon‐led follow‐up Frequency of follow‐up equal |
GP‐led follow‐up Frequency of follow‐up equal |
| Strand 2011 | Surgeon‐led follow‐up | Nurse‐led follow‐up |
| Wattchow 2006 | Primary care setting and environment for follow‐up Follow‐up guidance was based on current clinical practice, and guidance was provided that suggested follow‐up visits every 3 months for the first 2 years postoperatively, then every 6 months for the next 3 years. Each visit incorporated asking a list of set questions about symptoms, physical examination, annual faecal occult blood testing, and colonoscopy every 3 years | Secondary care setting for follow‐up |
| CA 19‐9: cancer antigen 19‐9; CEA: carcinoembryonic antigen; CAP: chest, abdomen, pelvis; CT: computed tomography; CXR: chest X‐ray; DRE: digital rectal examination; FBC: full blood count; FDG: fluorodeoxyglucose; PET: positron emission tomography; US: ultrasound | ||
Most recurrences (about 90%) occur within the initial 36 months after initial therapy for colorectal cancer (Ryuk 2014), so to detect recurrences, follow‐up duration should be at least 36 months for colorectal cancer. Patients with rectal cancer should have longer follow‐up because liver and lung recurrences may be delayed. The use of adjuvant chemotherapy may further delay recurrence (Sadahiro 2003). For the outcomes included in this study, median follow‐up duration was greater than 48 months for more than 90% of the participants studied.
We presented substantially altered conclusions in the 2016 update of the review (Jeffery 2016). Where we previously reported that "there was evidence that an overall survival benefit at five years exists for patients undergoing more intensive follow up", Jeffery 2016 did not confirm these findings. The four new studies included in this updated version of the review (CEAwatch 2015; COLOFOL 2018; Sobhani 2018; SurvivorCare 2013), with an additional 6238 participants, reinforced our findings in Jeffery 2016 that there is no overall survival benefit for intensifying the follow‐up of patients after curative surgery for colorectal cancer.
Summary of main results
Overall survival: the use of intensive versus less intensive follow‐up makes little or no difference after curative treatment (HR 0.91, 95% CI 0.80 to 1.04; Analysis 1.1).
Colorectal cancer‐specific survival: the use of intensive versus less intensive follow‐up makes little or no difference after curative treatment (HR 0.93, 95% CI 0.81 to 1.07; Analysis 1.2).
Relapse‐free survival: intensive versus less intensive follow‐up makes little or no difference after curative treatment (HR 1.05, 95% CI 0.92 to 1.21; Analysis 1.3).
The use of salvage surgery was increased with intensive follow‐up after curative treatment for colorectal cancer (RR 1.98, 95% CI 1.53 to 2.56; Analysis 1.4).
Interval (symptomatic) recurrences were probably slightly reduced with intensive follow‐up after curative treatment for colorectal cancer (RR 0.59, 95% CI 0.41 to 0.86; Analysis 1.5).
Quality of life
CEAwatch 2015 found no significant differences between the two groups in terms of attitude to follow‐up, fear of recurrence, HADS score and Cancer Worry score, and there was no detectable burden or improvement in psychological burden associated with intensification of follow‐up. GILDA 1998 found no clinically significant differences among the three main quality‐of‐life scales (SF‐12 mental component, SF‐12 physical component, and PGWB Index) between the two study arms. Kjeldsen 1997 reported a small increase in quality of life associated with more frequent follow‐up visits compared with virtually no follow‐up. ONCOLINK reported no significant effect on quality of life main outcome measures: for EORTC QLQ‐C30, they reported significant effects in favour of GP‐led follow‐up for pain, role functioning, and emotional functioning; they reported no differences in global health status, with intensive follow‐up compared with less intense follow‐up. SurvivorCare 2013 found no clinically meaningful differences in psychological distress, unmet needs, information needs and health‐related quality of life. Wattchow 2006 found that the study participants remained in the normal range for depression and anxiety with no difference between the two groups at either 12 or 24 months.
Harms and costs of surveillance (including investigations)
The studies reported three bowel perforations and four gastrointestinal haemorrhages (requiring transfusion) from a total of 2292 (0.3%) colonoscopies (Schoemaker 1998; Wang 2009).
ONCOLINK found the cost per participant for 24 months' follow‐up was GBP 9889 for surgeon‐led follow‐up and GBP 8233 for GP‐led follow‐up (P < 0.001 (figures from text)). Rodríguez‐Moranta 2006 demonstrated that although the cost of intensive follow‐up was higher, when they considered resectability of recurrences, the cost per resectable recurrence was lower in the intensively followed group. Secco 2002 reported that risk‐adapted follow‐up reduced costs for those with a better prognosis. Strand 2011 showed no difference in costs.
Overall completeness and applicability of evidence
The evidence we report is directly relevant to the study question.
The studies have been accrued over a prolonged time period, during which time there have been significant changes in cancer staging procedures, operative techniques (surgical metastasectomy or ablation of liver metastases), postoperative care, adjuvant therapies, and the investigations available to detect recurrence. Systemic adjuvant therapies and effective palliative chemotherapy drugs are now widely utilised, with significant prolongation of survival rates. All of these factors question the validity of applying the results of early studies to the modern surgical and oncological setting. A sensitivity analysis excluding studies that commenced accrual before 1996 did not however reveal any effect of study age on the outcome of overall survival (HR 0.97, 95% CI 0.74 to 1.28). The lack of benefit from intensive follow‐up persisted despite the inclusion of modern studies that used modern surgical techniques for resection of liver metastases, (FACS 2014), and more sensitive investigations to detect recurrence, such as PET scanning (Sobhani 2008).
The argument for intensive follow‐up has been up to now based on observational data that reports improved survival after hepatic metastasectomy. We did not find an improvement in overall survival with the use of liver imaging (HR 0.92, 95% CI 0.81 to 1.21).
The identified studies are sufficient to address our objectives; future updates of this review are likely to address those outcomes that we cannot yet address in detail, when ongoing studies detail quality of life ( SURVEILLANCE), costs (SURVEILLANCE; NCT00199654), and the effects of the addition of PET scanning into follow‐up for these participants (NCT00199654).
We were unable to obtain data for overall survival for the Secco 2002 study (despite contacting the authors) because of personnel changes at the institution concerned.
The studies included the relevant participant population. The interventions assessed were very inclusive, addressing a variety of 'doses' or intensities of follow‐up, ranging from no follow‐up to intense follow‐up, and evaluated multiple investigations including the use of CT and PET scanning (see Table 2). We recognise that there is considerable variation in the intensity of both the intervention and the control arms in the included studies. We feel our decision to pool the studies is vindicated by the lack of important heterogeneity for overall survival (I² = 18%, P = 0.25) and the lack of subgroup effect on overall survival when we explored follow‐up 'dose' and composition.
No study addressed any potential psychological harms, anxiety, or distress that may be associated with follow‐up after treatment for colorectal cancer.
The studies included in this review did not well report the potential harms (physical, psychological) and costs of follow‐up strategies. Two studies reported harms related to colonoscopy (Schoemaker 1998; Wang 2009). The rate of perforation (4/2292) 0.17% and haemorrhage (3/2292) 0.13 was consistent with other published series (Araghizadeh 2001; Bowles 2004; Rabenek 2008). None of the study reports included specific details of any harms (mortality or morbidity) resulting from investigation or treatment of recurrences. These outcomes should be available in order to fully assess any net benefit or harm of follow‐up.
Some researchers have investigated the psychological effects of follow‐up (Kjeldsen 1997; ONCOLINK; Stiggelbout 1997; Wattchow 2006). These studies have reported mixed effects on quality‐of‐life measures, but no study has found a deterioration in quality of life. Some form of follow‐up appeared superior to virtually no follow‐up in terms of quality of life (Kjeldsen 1997). Different settings for follow‐up (GP‐ versus surgeon‐led) did not appear to affect anxiety or depression; both groups had a high and similar level of participant satisfaction (Wattchow 2006). Ongoing studies will address the effects of intensifying follow‐up on quality of life in this population (SURVEILLANCE).
Further research is required into the value that participants place on follow‐up after their curative surgery. Any survival benefit (or lack of benefit) of follow‐up would have to be considered along with the views of participants so that follow‐up programmes are accessible, acceptable, and address all participants' needs and concerns.
Little useful data are available from the studies in this review on the cost‐effectiveness of follow‐up in this group of patients treated for non‐metastatic colorectal cancer. It appears that GP‐led follow‐up is cheaper than surgeon‐led follow‐up (ONCOLINK); risk‐adapted follow‐up is cheaper for those with better prognosis disease (Secco 2002), and although the cost of intensive follow‐up is higher, it makes the cost per resectable recurrence lower (Rodríguez‐Moranta 2006). Without a better understanding of which of the specific follow‐up interventions is responsible for the improvement in outcomes, it is not possible to even speculate on the potential cost‐effectiveness of any one approach. Investigators have previously tried to project the costs of a single intervention such as CEA testing (Audisio 1996; Moertel 1993), and the reported costs have appeared prohibitively large. In contrast, an incremental cost‐effectiveness analysis based on five randomised controlled trials has reported costs of intensive follow‐up, which appear acceptable in the setting of the National Health Service in the UK (Renehan 2004), although the study authors do acknowledge a number of limitations of their study. The ongoing PRODIGE study will address the issue of costs so that the relative cost‐effectiveness of follow‐up can be viewed from an economic perspective as well as a clinical one (SURVEILLANCE).
New biomarkers such as circulating tumour DNA are likely be investigated in future follow‐up studies to detect earlier colorectal cancer metastatic spread and recurrence (Oellerich 2017).
Quality of the evidence
The findings of this review allow robust conclusions, with minimal heterogeneity and low risk of publication bias (based on the use of funnel plots).
Overall survival
For the outcome of overall survival, we studied 12,528 participants in 15 studies. We did not downgrade for risk of bias. Outcome assessment was standard in the included studies. We did not downgrade for inconsistency (I² = 18%; P = 0.25) or indirectness: 9054/12,528 (72 %) participants contributing to this outcome were accrued after 2003 (so used modern investigations and surgical salvage techniques). We did not downgrade for imprecision (there were more than 300 events (1453), and the confidence intervals excluded clinically meaningful benefit or harm). We did not downgrade for publication or other bias. The GRADE assessment of evidence quality for this outcome was high.
Colorectal cancer‐specific survival
For the outcome colorectal cancer‐specific survival, we studied 11,771 participants in 11 studies. We downgraded because 40% of the events were from studies deemed at high risk of bias for incomplete outcome reporting or lack of blinding. We did not downgrade for inconsistency (I² = 0%; P = 0.57). We did not downgrade for indirectness, despite the long time period over which studies accrued participants; 34% of the participants included in this outcome were enrolled in studies that accrued in the 2000s. We did not downgrade for imprecision (there were more than 300 events (925), and the confidence intervals excluded clinically meaningful benefit or harm). We did not downgrade for publication or other bias. The GRADE assessment of evidence quality for this outcome was moderate.
Relapse‐free survival
For the outcome of relapse‐free survival, we studied 8047 participants in 16 studies. We did not downgrade for risk of bias, because fewer than 30% of the events were from studies deemed at high risk of bias from lack of blinding. We did not downgrade for inconsistency (I² = 41 %; P = 0.05); we felt there was unlikely to be meaningful heterogeneity: analysis using the fixed‐effect model gave similar effect magnitude, direction, and 95% CI (HR 1.15, 95% CI 1.05 to 1.25). There was no funnel plot asymmetry, which also helped exclude heterogeneity. We did not downgrade for indirectness, or imprecision (there were more than 300 events (2254), and the confidence intervals excluded clinically meaningful benefit or harm). We did not downgrade for publication or other bias. The GRADE assessment of evidence quality for this outcome was high.
Salvage surgery
For the outcome of salvage surgery, we studied 5157 participants in 13 studies. We did not downgrade for risk of bias. We did not downgrade for indirectness, because 1988 of 5854 (33%) of participants contributing to this outcome were enrolled in studies done in the 2000s. There was evidence of precision (with more than 300 events (457) and optimum information size met). While there was some evidence of non‐significant inconsistency (I² = 31%; P = 0.14), we felt that the variation in the intensity of follow‐up across the studies and the prolonged time period for accrual (which allowed for varied surgical techniques and degrees of surgical aggression) explained this because of clinical heterogeneity between the studies, so we did not downgrade for this. Prespecified sensitivity analysis based on study age supported this (I² = 25%; P = 0.25). We did not downgrade for publication bias. The GRADE assessment of evidence quality for this outcome was high.
Interval (symptomatic) recurrences
For the outcome of interval recurrences, we studied 3933 participants in seven studies. We downgraded for risk of bias, because 58% of the events came from studies at high risk of bias because of lack of blinding. We did not downgrade for inconsistency (I² = 66%; P = 0.007), because prespecified sensitivity analysis based on study age explained this heterogeneity (I² = 0%; P = 0.81). We did not downgrade for indirectness or imprecision (there were more than 300 events (376)) or publication bias. The GRADE assessment of quality of evidence was moderate.
Quality of life
Quality‐of‐life data were not available in a form that allowed analysis, so we did not pool these data. Studies comparing intensive follow‐up to less intensive follow‐up used a variety of scales to assess multiple outcomes. GRADE evidence quality was moderate, downgraded for risk of bias.
Harms
For the outcome of harms, we studied 651 participants in two studies. These were rare events (seven events reported in 2292 colonoscopies in two studies). We downgraded for lack of blinding, reporting bias and imprecision, so the GRADE assessment of quality of evidence was very low.
Costs
Limited data suggests the cost of more intensive follow‐up is increased in comparison with less intense follow‐up but the cost of surgery for resectable recurrence may be reduced. GRADE evidence quality was low, being downgraded for imprecision (there were fewer than 300 events, OIS was not met and 95% CI could not be estimated).
Potential biases in the review process
The studies included in this review did not report the potential harms of either investigations or salvage treatments. It is possible that investigators excluded participants from study enrolment whom they felt to be at high risk of recurrence; if this did occur, it may have diluted the effect of follow‐up strategies on survival.
Agreements and disagreements with other studies or reviews
We identified six published systematic reviews relevant to our question. Our findings differ from those reported by Mokhles 2016; Pita‐Fernández 2014; Renehan 2002; and Tjandra 2007: they found that intensive follow‐up for participants treated with curative intent for colorectal cancer improved survival. The other two systematic reviews did not report a quantitative meta‐analysis (Augestad 2014; Baca 2011). See Table 3.
2. Systematic reviews on topic.
| Study | Number of studies included | Search date | Outcomes for overall survival for comparison intensive versus less intensive follow‐up for participants treated with curative intent for colorectal cancer |
| Augestad 2014 | Unclear | No search date given | Did not present quantitative meta‐analysis |
| Baca 2011 | 15 | January 2000 to 2001 | Did not present quantitative meta‐analysis |
| Pita‐Fernández 2014 | 11 | June 2014 | HR 0.75 (95% CI 0.66 to 0.86) |
| Renehan 2002 | 5 | April 2001 | RR 0.81 (95% CI 0.70 to 0.94) |
| Tjandra 2007 | 8 | June 2007 | OR 0.74 (95% CI 0.59 to 0.93) |
| CI: confidence interval; HR: hazard ratio; OR: odds ratio; RR: risk ratio | |||
Augestad 2014 searched PubMed and reference lists of published studies (no search date given). They reported no quantitative meta‐analysis for the five studies they included. They commented that recent data did not report a survival advantage (FACS 2014), and they suggested that the potential survival benefits of surveillance should be weighed against possible negative effects.
Baca 2011 searched PubMed and reference lists (search date: June 2000 to June 2010). They included GILDA 1998 (Grossman data), Secco 2002 and Wang 2009 but did not present a quantitative meta‐analysis. They included both randomised (n = 5) and non‐randomised (n = 11) studies. They concluded that recent literature is inconclusive with respect to the benefit of surveillance for colorectal cancer after curative treatment. The difference in our findings was likely to relate to our more recent search and inclusion of 10 more RCTs.
Mokhles 2016 included seven studies and 3325 participants. Their search was systematic (search date not reported). They excluded non‐English studies. They reported no improvement in survival with intensive follow‐up (HR 0.98, 95% CI 0.87 to 1.11). They excluded Schoemaker 1998 and the review was published before the results of CEAwatch 2015 and COLOFOL 2018 were available. Their findings support those we report in this update.
Pita‐Fernández 2014 included 11 RCTs, with 4055 participants. Their search was limited to four bibliographic databases (search date: June 2014). They reported that overall survival increased with intensive follow‐up (HR 0.75, 95% CI 0.66 to 0.86; see Table 3). They included data relating to the GILDA 1998 study from an earlier paper with 14 months' median follow‐up, published while the study was still accruing participants (Grossman 2004). In addition, they included data from Secco 2002 and Wattchow 2006, which we were unable to extract. It appears that the data for overall survival reported for Secco 2002 may have been derived from the actuarial survival at five years (reported as percentages). We contacted the study authors for Secco 2002 (detailed in the Methods section), who informed us that they could not give us any more information than was in the text due to personnel changes. As stated above, it appears that the review authors incorrectly derived the overall survival data from the actuarial survival percentages reported.
Renehan 2002 included five RCTs and 1342 participants. Their search was systematic (search date: April 2001). They reported improved survival with intensive follow‐up in this setting. Again, the difference in our findings was likely to relate to our more recent search and inclusion of 10 more RCTs.
Tjandra 2007 included eight RCTs and 2923 participants. Their search was systematic (search date: June 2007). They reported improved survival with intensive follow‐up. Once again, the difference in our findings was likely to relate to our more recent search and inclusion of seven more RCTs.
The first iteration of this Cochrane Review also found improved survival in this setting (Jeffery 2007). The 2016 updated version of the review (Jeffery 2016), contradicted the previously reported effects of surveillance on survival for participants treated with curative intent for colorectal cancer. This updated version of the review, which now includes data from 19 studies with 13,216 participants, consolidates these conclusions.
Authors' conclusions
Implications for practice.
The results of this review suggest that intensifying clinical follow‐up for participants with colorectal cancer after curative treatment does not improve survival outcomes. The exact details of the optimal follow‐up regimen still need clarification, but limiting follow‐up intensity does not seem to be disadvantageous.
Implications for research.
Clinicians are encouraged to enrol their participants in any ongoing studies in this field. Such studies may reflect advances in imaging and surgical technique and the use of adjuvant therapies. All investigators are encouraged to explicitly document any harms relating to follow‐up and subsequent interventions.
Separate research programmes should explore patient needs and concerns relating to the value of follow‐up, incorporating other study designs, using qualitative as well as quantitative methods.
What's new
| Date | Event | Description |
|---|---|---|
| 28 June 2019 | New citation required but conclusions have not changed | Search updated, four new studies added and conclusion updated |
| 28 June 2019 | New search has been performed | Search updated, four new studies added and conclusion updated |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 22 June 2016 | New citation required and conclusions have changed | We have updated the review: new studies added and conclusions changed. We have updated inconsistencies between the text and abstract present in the first publication of the review. |
| 23 July 2008 | Amended | Converted to new review format |
| 27 August 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Adrienne See who ran the current search strategy and was an author on previous versions of the review.
Susan Bidwell, who both designed and performed the original search.
New Zealand Health Technology Assessment and Cochrane Colorectal Cancer assisted with retrieval of articles and careful proof reading.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane CENTRAL
Database: EBM Reviews ‐ Cochrane Central Register of Controlled Trials <April 2016>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 (colorectal or colon$ or rectal or rectum or sigmoid).ti,ab,sh. (24231)
2 (cancer$ or neoplasm$ or or tumor or carcinoma or adenoma or adenocarcinoma).ti,ab,sh. (81443)
3 1 and 2 (10445)
4 random$.af. (713326)
5 double blind.mp. (184812)
6 single blind.mp. (24374)
7 or/4‐6 (739346)
8 recurr$.ti,ab,sh. (31008)
9 metastas$.ti,ab,sh. (6552)
10 8 or 9 (36060)
11 3 and 7 and 10 (1697)
12 limit 11 to yr=2006‐2016 (906)
13 (follow‐up or follow up).ti,ab,sh. (88787)
14 longitudinal.ti,ab,sh. (7264)
15 survival.ti,ab,sh. (38629)
16 mortality.ti,ab,sh. (28096)
17 prognosis.ti,ab,sh. (16514)
18 quality of life.ti,ab,sh. (34855)
19 Treatment Outcome/ (97141)
20 (treatment adj3 outcome).ti,ab. (8454)
21 or/13‐20 (232471)
22 12 and 21 (758)
Appendix 2. MEDLINE search strategy
MEDLINE
Database: Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations <May 20, 2016>, Ovid MEDLINE(R) <1996 to Present with Daily Update>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 exp colorectal neoplasms/ (116407)
2 randomized controlled trial.pt. (322284)
3 random allocation/ (52293)
4 random$.af. (854660)
5 double blind method/ (91088)
6 single blind method/ (19369)
7 controlled clinical trial.pt. (45443)
8 or/2‐7 (905129)
9 recurrence/ (97196)
10 neoplasm recurrence, local/ (64834)
11 neoplasm metastasis/ (43469)
12 recurr$.ti,ab,sh. (376234)
13 or/9‐12 (431795)
14 follow up studies/ (376887)
15 follow up.ti,ab,sh. (577775)
16 exp longitudinal studies/ (82818)
17 exp survival analysis/ (204612)
18 exp mortality/ (243407)
19 exp prognosis/ (1064148)
20 office visits/ (4839)
21 "Episode of Care"/ (1387)
22 exp population surveillance/ (48291)
23 Practice Patterns, Physicians'/ (42538)
24 exp treatment outcome/ (725188)
25 "Outcome Assessment (Health Care)"/ (51554)
26 quality of life.mp. or "Quality of Life"/ (214742)
27 or/14‐26 (2025787)
28 1 and 8 and 13 (2176)
29 1 and 8 and 27 (5301)
30 28 or 29 (5809)
31 limit 30 to humans (5736)
32 limit 31 to yr="2006 ‐Current" (3795)
Appendix 3. EMBASE search stategy
Embase
Database: Embase <1980 to 2016 May 23>, Embase Classic <1947 to 1979>
Search Strategy:
‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐
1 colon tumor/ (24596)
2 colon cancer/ (54320)
3 colon carcinoma/ (21376)
4 colon adenocarcinoma/ (8481)
5 colorectal tumor/ (18557)
6 sigmoid carcinoma/ (926)
7 rectum carcinoma/ (12139)
8 rectum cancer/ (25073)
9 rectum tumor/ (16280)
10 rectum adenoma/ (1907)
11 colorectal carcinoma/ (19399)
12 colorectal cancer/ (98996)
13 or/1‐12 (262185)
14 randomization/ (70575)
15 randomized controlled trial/ (404026)
16 double blind procedure/ (133057)
17 single blind procedure/ (22126)
18 random$.af. (1263540)
19 or/14‐18 (1292538)
20 metastasis/ (258705)
21 cancer recurrence/ (96706)
22 tumor recurrence/ (45501)
23 recurrent disease/ (145450)
24 (recur$ or metastas$).ti,ab. (975672)
25 or/20‐24 (1161930)
26 13 and 19 and 25 (5349)
27 (rat or rats or mouse or mice).ti,ab,sh. (3468071)
28 (monkey$ or rabbit$ or hamster$).ti,ab,sh. (639841)
29 (bovine or sheep).ti,ab,sh. (331074)
30 animal/ or experimental animal/ (1787198)
31 or/27‐30 (5244594)
32 26 not 31 (5113)
33 longitudinal study/ (88575)
34 follow up/ (1057646)
35 (follow‐up or follow up).ti,ab. (1064657)
36 prospective study/ (334785)
37 treatment outcome/ (720484)
38 cancer survival/ (205796)
39 quality of life/ (316649)
40 prognosis/ (516100)
41 mortality/ (662941)
42 morbidity/ (273023)
43 exp survival/ (803758)
44 or/33‐43 (3736259)
45 13 and 19 and 44 (10019)
46 45 not 31 (9818)
47 46 not 32 (5790)
48 case report/ (2137256)
49 letter/ or letter.pt. (941176)
50 48 or 49 (2894625)
51 32 or 47 (10903)
52 51 not 50 (10718)
53 limit 52 to yr=2006‐2016 (7843)
Appendix 4. CINAHL search strategy
CINAHL‐ EBSCOhost
Note: search has been rekeyed from the original printout to enhance readability
| 1 | MH “Rectal Neoplasms+ | 1802 |
| 2 | MH Colonic Neoplasms+ | 4298 |
| 3 | MH Colorectal Neoplasms+ | 15,461 |
| 4 | S1 OR S2 OR S3 | 15,461 |
| 5 | “follow up” | 80,974 |
| 6 | MH Recurrence OR recur* | 44,486 |
| 7 | MH Neoplasm recurrence, local | 5399 |
| 8 | MH Prospective Studies OR longitudinal | 194,415 |
| 9 | S5 OR S6 OR S7 OR S8 | 274,290 |
| 10 | S4 AND S9 | 2714 |
| 11 | MW meta‐analysis | 17,460 |
| 12 | TX meta analy* OR metaanaly* | 27,334 |
| 13 | TX Cochrane* | 22,935 |
| 14 | PT nursing interventions | 1379 |
| 15 | MH literature review | 3584 |
| 16 | MH literature searching | 835 |
| 17 | MH computerized literature searching | 4940 |
| 18 | MH reference databases | 1726 |
| 19 | TX review* OR overview* | 2,887,898 |
| 20 | TX pooled data OR pooled analy* | 1951 |
| 21 | PT review | 106,455 |
| 22 | TX systematic* OR methodologic* OR quantitative OR research* OR literature OR studies OR trial* OR effective* | 1,683,194 |
| 23 | S21 AND S22 | 65,969 |
| 24 | TX (synthesis* AND (literature OR studies OR data)) | 11,542 |
| 25 | TX ((hand OR manual*) AND search*) | 5121 |
| 26 | TX ((electronic* OR bibliography*) AND (database* OR data base*)) | 12,971 |
| 27 | S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 | 2,890,087 |
| 28 | S23 OR S24 OR S25 OR s26 | 90,915 |
| 29 | S27 or S28 | 2,891,877 |
| 30 | S10 and S29 | 2657 |
| 31 | MW randomized | 26,597 |
| 32 | MW random | 60,954 |
| 33 | TX random* | 177,131 |
| 34 | S31 OR S32 OR S33 | 177,131 |
| 35 | S10 AND S34 | 562 |
| 36 | S30 OR S35 | 2,664 |
| 37 | PT letter OR case study | 325,844 |
| 38 | S36 NOT S37 | 2479 |
| 39 | Limiters Published Date: 20060101‐20161231 | 1916 |
| 40 |
Appendix 5. Science Citation Index search strategy
Science Citation Index and Conference Abstracts
| # 15 | 3,292 | #7 AND #12 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 14 | 11,791 | #7 OR #13 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 13 | 11,050 | #5 AND #12 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 12 | 2,049,980 | #11 OR #10 OR #9 OR #8 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 11 | 163,800 | TS="quality of life" Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 10 | 955,659 | TS=(prognosis or outcome) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 9 | 877,979 | TS=(survival or mortality or morbidity) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 8 | 661,485 | TS=(follow up or follow‐up or longitudinal) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 7 | 4,033 | #5 AND #6 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 6 | 404,566 | TS=(recur* or metastas*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 5 | 17,480 | #3 AND #4 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 4 | 849,999 | TS=(random* or double blind* or single blind*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 3 | 166,220 | #1 AND #2 Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 2 | 1,263,394 | TS=(cancer or tumor* or * or carcinoma* or adenoma* or adenocarcinoma*) Indexes=SCI‐EXPANDED, CPCI‐S, ESCI Timespan=2006‐2016 |
|||
| # 1 | 364,015 | TS=(colorectal or colon* or rectal or rectum or sigmoid) |
Data and analyses
Comparison 1. Intensive follow‐up versus minimalist follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall survival | 15 | 12528 | Hazard Ratio (Random, 95% CI) | 0.91 [0.80, 1.04] |
| 2 Colorectal cancer‐specific survival | 11 | 11771 | Hazard Ratio (Random, 95% CI) | 0.93 [0.81, 1.07] |
| 3 Relapse‐free survival | 16 | 8047 | Hazard Ratio (Random, 95% CI) | 1.05 [0.92, 1.21] |
| 4 Salvage surgery | 13 | 5157 | Risk Ratio (M‐H, Random, 95% CI) | 1.98 [1.53, 2.56] |
| 5 Interval recurrences | 7 | 3933 | Risk Ratio (M‐H, Random, 95% CI) | 0.59 [0.41, 0.86] |
| 6 Harms (colonoscopic complications) | 1 | 326 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.30 [0.75, 70.69] |
| 7 Overall survival SG Healthcare professional | 15 | Odds Ratio (Random, 95% CI) | Subtotals only | |
| 7.1 Community | 2 | Odds Ratio (Random, 95% CI) | 1.39 [0.38, 5.12] | |
| 7.2 Hospital | 13 | Odds Ratio (Random, 95% CI) | 0.91 [0.80, 1.03] | |
| 8 Overall survival SGA "dose" of follow‐up | 11 | Hazard Ratio (Random, 95% CI) | 0.89 [0.77, 1.02] | |
| 8.1 More visits and tests versus fewer visits and tests | 7 | Hazard Ratio (Random, 95% CI) | 0.85 [0.69, 1.05] | |
| 8.2 VIsits and tests versus minimal or no follow‐up | 4 | Hazard Ratio (Random, 95% CI) | 0.93 [0.76, 1.12] | |
| 9 OS SGA CEA versus NO CEA | 7 | Hazard Ratio (Random, 95% CI) | 0.95 [0.82, 1.11] | |
| 9.1 CEA | 6 | Hazard Ratio (Random, 95% CI) | 0.97 [0.79, 1.19] | |
| 9.2 No CEA | 1 | Hazard Ratio (Random, 95% CI) | 0.90 [0.67, 1.21] | |
| 10 OS CT versus no CT | 10 | Hazard Ratio (Random, 95% CI) | 0.93 [0.81, 1.08] | |
| 10.1 CT | 7 | Hazard Ratio (Random, 95% CI) | 0.93 [0.79, 1.10] | |
| 10.2 No CT | 3 | Hazard Ratio (Random, 95% CI) | 1.31 [0.40, 4.23] | |
| 11 OS CT versus < 2 or no CT | 9 | Hazard Ratio (Random, 95% CI) | 0.87 [0.73, 1.05] | |
| 11.1 CT | 6 | Hazard Ratio (Random, 95% CI) | 0.82 [0.65, 1.04] | |
| 11.2 < 2 or no CT | 3 | Hazard Ratio (Random, 95% CI) | 1.04 [0.70, 1.54] |
1.6. Analysis.

Comparison 1 Intensive follow‐up versus minimalist follow‐up, Outcome 6 Harms (colonoscopic complications).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CEAwatch 2015.
| Methods | RCT, multicentred stepped‐wedge cluster with a unidirectional cross‐over design Accrual: 1 October 2010‐1 October 2012 Follow‐up: Setting: non‐academic teaching hospitals (n = 11) |
|
| Participants | 5604 participants registered 2791 eligible for study inclusion 3223 participants randomised Inclusion criteria: AJCC Stage I‐III colorectal cancer with R0 resection between 2007‐July 2012 Exclusion criteria: not medically fit for metastasectomy, concurrent malignancy, metachronous metastases Country: Netherlands |
|
| Interventions |
Intervention (n = 2041):
Control ( n = 2907):
|
|
| Outcomes |
QoL: Study evaluated 3 dimensions of quality of life were evaluated:
Costs: evaluated, but not reported |
|
| Notes | Trial Registry Number NTR2182 Funding source: Netherlands Organization for Health Research and Development |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "hospitals were randomly grouped into five clusters" "with randomisation used to allocate clusters to different switch moments" 2017 page 1070 Quote: "patients entering the study before the switch were followed using the control protocol and switched to the intervention after their hospital's switch. Patients entering the study after the randomised switch of a hospital were followed using the intervention protocol only" 2015, page 1190. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed independently by Trial Coordination Centre (TCC) Groningen" 2015, page 1189. Comment: this describes allocation concealment, so we judged this domain to be at low risk of bias. |
| Blinding of participants and personnel | Unclear risk | No information reported, so unable to make a judgement, so we judged this domain at unclear risk of bias |
| Blinding of outcome assessment | Unclear risk | No information reported, so unable to make a judgement, so we judged this domain at unclear risk of bias |
| Incomplete outcome data (attrition and exclusions) | Low risk | 1591/2016 (78.9%) and 1848/2156 (84.2%) of participants completed all quality‐of‐life forms. We deemed this domain to be at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the protocol so deemed this domain to be at unclear risk of bias Costs evaluated, but not reported yet. |
COLOFOL 2018.
| Methods | RCT Multicentred (n = 24) Accrual: January 2006 ‐ December 2010 Setting: hospitals Median follow‐up: 5 years |
|
| Participants | 13,718 participants screened, 2555 participants randomised Inclusion criteria: ≤ 75 years (mean 63.5 years) Radical surgery (R0 resection) for colorectal adenocarcinoma ‐ with or without adjuvant treatment "Clean colon" verified by perioperative barium enema or colonoscopy in the last 3 months post surgery Stage II‐III (T2, N1‐2, M0, T3‐4, any N, M0; Edge 2010). Baseline imaging: (US MR or CT liver, CT or CXT lungs preoperatively). CEA at 1 month post‐operatively Exclusion criteria: hereditary CRC, familial adenomatous polyposis, life expectancy < 2 years because of concomitant disease, local excision, other or previous malignancies, inability to comply with protocol, participation in other clinical studies interfering with COLOFOL. Country: Denmark, Sweden, Uruguay |
|
| Interventions |
Intervention: (n = 1253) CEA 1 month postoperatively then CEA, CT chest and abdomen 6, 12, 18, 24, and 36 months Control: (n = 1256) CEA 1 month postoperatively then CEA, CT chest and abdomen 12 and 36 months after surgery NB: detailed instructions to ensure uniform quality of investigations were provided and benchmarking of investigation quality was performed. Clear instructions were available for interpretation of investigations and management of recurrence. |
|
| Outcomes |
OS: death any cause measured at 5 years C‐SS: CRC deaths, measured at 5 years R‐FS: CRC‐specific recurrence rate, measured at 5 years. If recurrence was suspected the case was discussed a the local multi‐disciplinary meeting and further investigation and treatment was recommended. SS: IR: QoL: SF‐36 (Ware 1993), EORTC‐QLQ‐C30 (Aaronson 1993), and HADS (Zigmond 1983) Harms: not recorded (as per protocol) Costs: |
|
| Notes | Study ID: NCT00225641 Quality assurance: Quote: "Quality of examinations Before entering a trial, the individual centre should prove the high quality of their control‐examinations. The central steering group’s diagnostic board should approve five consecutive examples." Protocol Funding: The Nordic Cancer Union, The Danish Cancer Society Management of recurrence: Quote: "If recurrence is suspected or verified, the patient‐case should be evaluated in a local (country‐ or county separated) MDT conference, in order to decide and offer the best available treatment for the patient (salvage surgery, palliative chemotherapy and/or radiotherapy, no treatment). These groups also form the local steering‐groups for the study. They should preferably include a surgeon, a radiologist, and an oncologist". Protocol |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "computerised, in blocks, stratified by centre and cancer stage. The size of the blocks was variable and unknown to the participating centres" 2016, page 16, paragraph 6. Comment: we judged this domain at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation will take place by Internet from a central randomisation unit (appendix 12) placed at Department of Clinical Epidemiology in Århus Denmark. Randomisation will be stratified according to tumour stage and clinical centre. Randomisation will be blocked in variable groups of which the size will be kept secret for the participating centres. The allocation procedure should be concealed to the deliverers of treatment." Protocol Quote: "The randomisation will take place over the Internet via a server placed at Aarhus University Hospital, Denmark. The server contains a randomisation programme, which each centre will gain access to via a username and password. When logging on to the randomisation site, each centre must key patient data on all patients, who have given their consent and who fulfil the inclusion criteria for the study. After keying the data, a randomisation code will be provided. The data is encrypted before the information is transmitted over the Internet." Protocol Comment: we judged this domain at low risk of bias. |
| Blinding of participants and personnel | Unclear risk | Not mentioned: inadequate information to form a judgement, deemed at unclear risk of bias |
| Blinding of outcome assessment | Unclear risk | Quote: "At each follow‐up visit, data were collected on symptoms, CT scans and CEA tests per protocol and additional examination preformed for suspected recurrence". Quote: "If a recurrence was suspected...the case was discussed at a meeting of the local multidisciplinary team and further diagnostic assessment ..and treatment was undertaken as recommended ". Comment: not mentioned; inadequate information to form a judgement, deemed at unclear risk of bias |
| Incomplete outcome data (attrition and exclusions) | Low risk |
OS: 2509/2555 (analysed/randomised) C‐SS: 2498/2555 (analysed/randomised) R‐FS: 2498/2555 (analysed/randomised) 71 participants in each arm excluded because of protocol violation. We deemed the risk of bias for these outcomes low for attrition bias Harms: not recorded |
| Selective reporting (reporting bias) | Low risk | We had the opportunity to review the protocol, so deemed this domain to be at low risk of bias. |
FACS 2014.
| Methods | RCT (1:1:1:1:1 minimisation algorithm, 2 x 2 randomised study) Accrual: 2003‐2009 Stratified for adjuvant chemotherapy, age, and sex Mean follow‐up: 40.8 months Setting: tertiary centres |
|
| Participants | 1202 participants (736 men and 466 women) treated with curative surgery for primary CRC Dukes' A: 254 Dukes' B: 553 Dukes' C: 354 Colon primary: 811 Rectal primary: 359 Country: UK |
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Eligible participants were those with no residual disease (confirmed by a CT scan of the chest and liver or a MRI of the liver), microscopically clear margins, and postoperative CEA ≤ 10 μ/L following surgery or completion of adjuvant therapy as indicated. All participants had colonoscopy at study entry to ensure there was no residual intraluminal disease and were offered an end‐of‐study colonoscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 265): "Randomization to 1 of 4 groups (Figure 1) on a 1:1:1:1 ratio was performed centrally at the Oxford Clinical Trials Unit using a minimization algorithm to balance patient characteristics within each centre based on 3 variables: adjuvant chemotherapy, sex, and age group." Comment: we judged this domain to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 265): "Study nurses contacted the Oxford Clinical Trials Unit by telephone to enter a patient in the trial, reporting the relevant patient characteristics; they were then told the trial group to which the patient had been allocated." Comment: we judged this domain to be at low risk of bias. |
| Blinding of participants and personnel | Unclear risk | Quote (page 265): "Because this was a pragmatic open trial, it was not possible to conceal the allocation group from either participants or clinicians." Comment: we judged this domain to be at unclear risk of bias. |
| Blinding of outcome assessment | Low risk | Quote: "However, the research staff who abstracted outcome data from clinical notes were employed by the local National Cancer Research Network teams independent of the investigators. The analysis program was undertaken first using dummy variables for the allocation groups and the code was not broken until the precise procedures for analysis were agreed on." Comment: we judged this domain to be at low risk of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (page 265): "Exclusions:
Comment: there was complete information given to explain the exclusions, and they were reported by arm and given the 3:1 randomisation ratio; the numbers were similar in each arm. We therefore judged this domain to be at low risk of bias (figure 1). Attrition was reported; it did not occur. |
| Selective reporting (reporting bias) | Low risk |
Primary outcome measures (recorded on isctrn.org) Current primary outcome measure amended as of 11 February 2009: number of recurrences in each group treated surgically with curative intent, analysed at study end (5 years) Previous primary outcome measure: OS by ITT analysis Secondary outcome measures Current secondary outcome measures as of 11 February 2009:
Outcome measures reported Primary outcome:
Secondary outcomes:
We assumed that this was the initial publication and that subsequent publications would present the QoL data, so judged this to be at low risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
GILDA 1998.
| Methods | RCT Accrual dates: 1998‐2006 Multi‐centred, international study Median follow‐up: 62 months Setting: not stated (presumed hospital) |
|
| Participants | 1228 participants (746 men and 482 women) with histopathologic diagnosis of adenocarcinoma of the colon or rectum, Dukes Astler‐Coller stage B2‐C, treated with curative intent (radical excision plus or minus adjuvant radio/chemotherapy)
Participant must be free of known cancer prior to entry, attested by normal endoscopy, US, CXR, and CEA
Exclusion criteria
|
|
| Interventions |
Intervention
Control
|
|
| Outcomes |
Principal endpoints
Secondary endpoints
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (abstract, page 274): "Colon cancer patients were randomised." Comment: as no details were given, we deemed this domain to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | Quote (paragraph 5, page 6): "Randomisation was performed centrally via telephone at the Mario Negri Institute, Milan, Italy." Comment: this implied that randomisation was remote and potentially concealed, but the details were not given. |
| Blinding of participants and personnel | Unclear risk | There was no mention of blinding of either participants or personnel, so this was probably not done. |
| Blinding of outcome assessment | Unclear risk | There was no mention of blinding with regard to the outcome assessors, so this was probably not done. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (figure 2, Rosati 2016): "3 patients from 3 centres that ceased collaboration soon after randomisation, four patients not eligible..." Comment: the study gave details of reasons for exclusions and attrition by arm, so we judged this domain as not at high risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | The study reports on the primary outcome and selected secondary outcomes (QoL measures). We did not have access to the study protocol, so we judged this domain to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Kjeldsen 1997.
| Methods | RCT (random numbers)
Accrual: 1983‐1994
Stratified for Dukes' stage and location Country: Denmark Setting: Odense University Hospital, Odense Follow‐up: not stated |
|
| Participants | 597 participants, (326 men and 271 women) treated with primary radical surgery for CRC, no residual neoplasia
Inclusion criteria
Dukes' A 138 Dukes' B: 293 Dukes' C: 166 Colon primary: 314 Rectal primary: 283 |
|
| Interventions |
Intervention Follow‐up examinations at 6, 12, 18, 30, 36, 48, 60, 120, 150, and 180 months after radical surgery. Control Examinations at 60, 120, and 180 months Examinations included medical history, clinical examination, DRE, gynaecological examination, Haemoccult‐II test, colonoscopy, CXR, haemoglobin level, erythrocyte sedimentation rate, and liver enzymes. |
|
| Outcomes |
|
|
| Notes | Definition of radical surgery: no residual neoplasia detected by the following examinations: complete colonoscopy or incomplete colonoscopy plus double‐contrast barium enema, CXR (2 views), histological evaluation of all surgical margins, biopsy of suspicious lesions (lymph nodes), inspection and palpation of liver during surgery LR was defined as growth in the region of the primary radical operation, including the surgical wound, and demonstrated clinically or by imaging techniques, but not necessarily verified by biopsy. New lesions were called metachronous when diagnosed at least 12 months after primary cancer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 666): "After surgery, the patients were allocated to one of two follow‐up programmes (groups 1 and 2) by random numbers." Comment: the use of random numbers may be adequate, but there is not enough description to be certain; therefore, we graded this as unclear. |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 666): "After surgery, the patients were allocated to one of two follow‐up programmes (groups 1 and 2) by random numbers." Comment: there was no description of concealment of allocation, so it was probably not done. |
| Blinding of participants and personnel | Unclear risk | Quote (page 666): "All patients were also instructed to visit their general practitioner if they developed abdominal pain or changing bowel habits lasting more than 2 weeks after the immediate postoperative period." Comment: Participants: not mentioned, although it is possible that those participants in the minimal follow‐up group may have put more weight on their symptoms in the knowledge that they had fewer planned investigations. We judged that this domain was probably not at risk of bias. Assessors: not mentioned, but there is a risk that in the minimal follow‐up group, personnel may have put more weight on reported symptoms in the knowledge that they had fewer planned investigations. However, there were prespecified follow‐up schedules. |
| Blinding of outcome assessment | High risk | Quote (page 666): "Local recurrence was defined as growth in the region of the primary radical operation, including the surgical wound, and demonstrated clinically or by imaging techniques, but not necessarily verified by biopsy." Quote (page 666): "Group 1 had follow‐up examinations at 6, 12, 18, 24, 30, 36, 48, 60, 120, 150 and 180 months after radical surgery, while group 2 had examinations at 60, 120 and 180 months."
|
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (page 668): "In all, 88 of 290 patients in group 1 and 100 of 307 in group 2 have died." Comment: this implies that they had followed all participants. |
| Selective reporting (reporting bias) | Unclear risk | Quote (page 666): "The main purpose of the present randomised study was to evaluate the possible influence of follow‐up upon survival." "Recurrence (and/or distant spread)" Comment: the primary outcome of survival was reported on in the results. There were multiple outcomes reported, but as we were not able to review the protocol, we judged the risk of bias for this domain as "unclear". Outcomes reported included the following:
|
| Other sources of bias | Unclear risk | We detected no other bias. |
Mäkelä 1995.
| Methods | RCT
Accrual: 1988‐1990
Single‐centre study Follow‐up: 60 months Setting: tertiary centre |
|
| Participants | 106 participants (52 men, 54 women) who had "radical primary surgery for CRC" at the Oulu University Hospital (1988‐1990)
Dukes' A: 28
Dukes' B: 48
Dukes' C: 30
Colon primary: 75
Rectal primary: 31 Country: Finland |
|
| Interventions | Intervention: participants who had rectal or sigmoid cancers had flexible sigmoidoscopy with video imaging every 3 months, colonoscopy at 3 months (if it had not been done pre operation), then annually. They also had US of the liver and primary site at 6 months, then annually. Control: participants who had rectal and sigmoid cancers had rigid sigmoidoscopy and barium enema annually. | |
| Outcomes |
|
|
| Notes | Radical resection: macroscopic removal of, with microscopically negative margins
LR: restricted to anastomosis and its surrounds
Regional recurrence: invasion beyond the site of the primary without distant metastases All participants reviewed at 3, 6, 9, 12, 15, 21, 24, 30, 36, 42, 48, 54, and 60 months At each visit: history, examination, FBC, faecal occult blood test, CEA, CXR performed |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The study provided no more details regarding sequence allocation; therefore, we judged this domain to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | The study reported no details of allocation concealment, so we judged this domain to be at unclear risk of bias. |
| Blinding of participants and personnel | Low risk | Participants: not mentioned, but the clear instructions to the participants (see paragraph 4, page 620) about when they were to contact the surgical department meant we judged this domain to be low risk of bias. Assessors: not mentioned, but the protocol for intensive follow‐up was prespecified, as was the process for the minimal group. This would have reduced the risk of bias. |
| Blinding of outcome assessment | Unclear risk | Participants: not mentioned, but probably not done. This could have been done relatively easily, but was unlikely to have introduced bias. Assessors: not mentioned, but probably not done. This may have introduced bias because the personnel were aware that there were few planned investigations in the minimal group; this may have meant greater weight was placed in reported symptoms in this group. This could be a source of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | The study did not report incomplete outcome data, so we judged this domain to be at low risk of bias (both exclusions and attrition). |
| Selective reporting (reporting bias) | Unclear risk | Specified in the methods: recurrence (regional and anastomotic), time to detection of recurrence, method of detection of recurrence, surgery for recurrence, survival, synchronous adenomas detected during surveillance
Actually reported: time to recurrence, recurrences (local, regional, and distant), method that detected recurrence most frequently, presence of symptoms at recurrence, method of detection of recurrence, surgery for recurrence, survival, survival after radical surgery of recurrence, adenomas detected during surveillance We did not have access to the protocol, so judged this domain to be an unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Ohlsson 1995.
| Methods | RCT
Accrual: 1983‐1986
Multi‐centre study (Lund & Helsingborg) Setting: tertiary Follow‐up: 66‐105.6 months |
|
| Participants | 107 participants (51 men, 56 women) undergoing resection with curative intent for CRC at the departments of surgery in Lund and Helsingborg, Sweden, from 1983‐1986
Exclusion criteria
Dukes' A: 19 Dukes' B: 47 Dukes' C: 41 Colon primary: 71 Rectal primary: 36 Country: Finland Setting: hospital |
|
| Interventions | Intervention: the experimental group were seen at 3‐, 6‐, 9‐, 12‐, 15‐, 18‐, 21‐, 24‐, 30‐, 36‐, 42‐, 48‐, and 60‐month intervals. Performed at each visit were clinical exam, rigid proctosigmoidoscopy, CEA, alkaline phosphatase, gamma‐glutaryl transferase, faecal haemoglobin, and CXR. Examination of anastomosis (flexible sigmoidoscopy or colonoscopy, as dictated by the lesion) was performed at 9, 21, and 42 months. Colonoscopy was performed at 3, 15, 30, and 60 months. CT of the pelvis was performed at 3, 6, 12, 18, and 24 months. Control: the control group had no follow‐up visits planned. They received written instructions recommending that they leave faecal samples with the district nurse for examination every 3rd month during the first 2 years after surgery then once a year. They were instructed to contact the surgical department if they had any symptoms. | |
| Outcomes |
|
|
| Notes | LR: recurrence within the initial bed, operative field, anastomosis, or structures contiguous or adherent to the primary (included relapse in the abdominal wound, drain site, pelvis, or perineum) Anastomotic recurrence: intraluminal recurrence within 5 cm of the anastomosis Symptomatic: when symptoms could be related to the participant's initial illness and when they resulted in or would have resulted in the participant seeking advice Resection with curative attempt: all visible tumour removed, microscopic‐free margins Time to first recurrence: interval between primary surgery and unequivocal demonstration of recurrence at laparotomy, imaging, or autopsy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised to..." Comment: the study provided no details about sequence generation; therefore, we judged this as unclear. |
| Allocation concealment (selection bias) | Unclear risk | The paper provided no details, so we judged this as unclear. |
| Blinding of participants and personnel | Low risk | Quote (page 620): "No follow‐up visits were planned for patients in the control group. They received written instruction, recommending they leave fecal samples with the district nurse for examination of haemoglobin every third month during the two first years after surgery and then once a year. They were also instructed to contact the surgical department as soon as they experienced any problems with the colostomy, abdominal or perineal pain, altered bowel movements, change in fecal colour, micturition problems, or weight loss. Protocol for active follow‐up is given in Table 1." Comment: although it was not mentioned, participants received clear instructions about when they were to contact the surgical department, and the follow‐up protocols for both groups were prespecified, which would have reduced the risk of bias. |
| Blinding of outcome assessment | Unclear risk | The study did not mention blinding of outcome assessment; it would have been possible to blind those reporting the investigation's treatment arm, but not having done so is unlikely to have introduced bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (page 623): "Twenty‐two of 54 patients in the control group and 15 of 53 patients in the F‐U [follow‐up] group were dead at the end of the study..." Comment: all participants reported on attrition; there was no attrition. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol, so we judged this domain at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
ONCOLINK.
| Methods | RCT Accrual: 2007‐2011 Multi‐centre study (3 local hospitals and 1 University hospital) Median follow‐up: 24 months |
|
| Participants | 110 participants (65 men and 45 women) surgically treated for colon cancer Dukes' A: 24 Dukes' B: 55 Dukes' C: 32 Country: Norway Setting: hospital and community |
|
| Interventions |
Intervention: surgeon follow‐up Control: GP follow‐up The follow‐up intervals were the same. |
|
| Outcomes |
|
|
| Notes | National follow‐up guidelines were applied in both study arms, and participants were followed for 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients are randomised to follow‐up either by their GP (intervention) or at the surgical clinic (controls)." Comment: the study did not report a description of the method used to generate randomisation sequence. |
| Allocation concealment (selection bias) | Low risk | Quote: "...using a web‐based randomisation service and managed by the Norwegian University of Science and Technology." Comment: because the study used a web‐based method, we assumed that it was truly concealed. |
| Blinding of participants and personnel | Low risk | Quote (page 3): "Recruited patients were not informed about the other patients recruited in the same trial. Similarly, no information regarding trial progress and allocation was revealed to the participating GPs or surgeons. However, as GP‐organised follow‐up represented a new practice, blinding was not possible in the intervention arm." Comment: we judged this domain to be at low risk of bias. |
| Blinding of outcome assessment | Low risk | Quote (page 3): "The local trial investigator was not involved in the subsequent follow‐up appointments in any way." Comment: this indicated that the assessors were blinded to the treatment arm; therefore, we judged this domain to be at a low risk of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | The study reported no exclusions, but detailed information with respect to attrition (detailed by arm and with reasons given) ensured that we judged this domain to be at low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes specified in the objectives
The paper reported on all of these. We did not have access to the protocol, so judged this outcome to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Pietra 1998.
| Methods | RCT
Accrual: 1987‐1990
Single‐centre study Setting: university hospital Follow‐up: 60 months |
|
| Participants | 207 consecutive participants (111 men, 96 women) who had curative resections for large bowel cancer; all had colonoscopy at 3 months post‐operation if had not been done preoperatively
Exclusion criteria
Dukes' A: 0 Dukes' B: 122 Dukes' C: 85 Colon primary: 139 Rectal primary: 68 Country: Italy Setting: university hospital |
|
| Interventions | The experimental group were seen at 3, 6, 9, 12, 15, 18, 21, 24, 30, 36, 42, 48, 54, and 60 months, then annually thereafter. There was clinical examination, US, CEA, and CXR at each visit. Annual CT of the liver and colonoscopy were performed. The control group were seen at 6 and 12 months, then annually. At each visit, clinical examination, CEA, and US were performed. They had annual CXR, yearly colonoscopy, and CT scan. | |
| Outcomes |
|
|
| Notes | LR: all local disease detectable at follow‐up, either alone or in conjunction with generalised recurrence
LRs were divided into extramural recurrences, where regrowth was located in and around the bed, including the pericolic fat, adjoining mesentery, or lymph nodes. Intramural recurrence: regrowth involving only the anastomosis A LR was considered resected when no macroscopic/microscopic disease remained after surgery. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (paragraph 3, page 1128): "were randomly assigned" Comment: the study reported no details of the method of sequence generation, which makes this domain at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | The study reported no details, so we judged this domain to be at unclear risk of bias. |
| Blinding of participants and personnel | Low risk | Participants: not mentioned, unlikely to have introduced bias Assessors: quote (paragraph 3, page 1128): "The same clinical and instrumental tests CT included were performed whenever a patient of either group had symptoms suggestive of a possible recurrence of the disease (abdominal or perineal pain, altered bowel movements, change in fecal colour, or weight loss)." Comment: the prespecified follow‐up schedules and the lists of symptoms to be investigated make this domain at low risk of bias. |
| Blinding of outcome assessment | Unclear risk | Blinding of outcome assessment was not mentioned. LR was the primary outcome measure so susceptible to bias. |
| Incomplete outcome data (attrition and exclusions) | High risk | Quote (page 1128): "Nine patients (3.8 per cent with Stage A were excluded from the study because our previous reports demonstrated a low rate of recurrences in these cases. Other exclusion criteria were the presence of liver metastases (4 patients), even though these had been removed in apparently radical fashion during surgery on the primary, and the presence of severe illness that precluded intense follow‐up or treatment of recurrent disease (10 patients). The remaining 207 patients were enrolled in this study..." Exclusions: the exclusions were not reported by study arm. It is not clear from the report whether these exclusions occurred before randomisation. This means that the study potentially excluded 37/239 (15%) of those randomised. As little information has been provided, we have judged this to be at high risk of bias. Attrition: none reported, so we judged this domain at low risk of bias |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes specified in the methods
Outcomes actually reported in the paper
We did not have access to the protocol, so judged this outcome to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Rodríguez‐Moranta 2006.
| Methods | RCT
Accrual: 1997‐2001
Multicentred, stratified for centre, location, and stage Setting: hospital Follow‐up: 48 months |
|
| Participants | 259 participants (161 men and 98 women), stage II and III colon and rectal cancer Country: Spain |
|
| Interventions | Intervention: the experimental group were seen with history, examination, and bloods (including CEA), US/CT, CXR, and colonoscopy. Control: the control group were seen with history, examination, and bloods (including CEA) | |
| Outcomes |
|
|
| Notes |
Experimental group
Control group
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were stratified according to centre, location (colon/rectum), and TNM stage (II/III); thereafter, patients were randomly allocated to either simple or intensive surveillance strategies by means of sealed envelopes containing computer‐generated random numbers." Comment: we judged this method of sequence generation to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were randomly allocated to either simple or intensive surveillance strategies by means of sealed envelopes containing computer‐generated random numbers. Random assignment was centralized at the Hospital Clinic." Comment: sequence generation was reported to be remote, but as the study gave no further details, we rated this domain as at unclear risk of bias. |
| Blinding of participants and personnel | Unclear risk | Participants: blinding to treatment arm was not mentioned, but this would have been difficult to do. As history and examination were performed for both arms, it is unlikely to have been a cause of bias for objective outcomes. The study reported no subjective outcomes. Personnel: not mentioned, unlikely to have been done. The follow‐up schedule was specified (see Table 3, page 387). This would reduce the risk of bias. Knowledge of study arm could influence clinical decisions made on the basis of history and clinical findings, to influence further investigations, which could introduce potentially introduce bias. We therefore judged this domain to be at high risk of bias. |
| Blinding of outcome assessment | Unclear risk | The study did not mention blinding of outcome assessment, but it is unlikely to have been a source of bias. Therefore, we judged this domain to be at low risk of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote: "During the study period, 270 patients were included. Eleven patients (4%) were excluded after random assignment because of inadequate initial assessment of tumour stage (eight had distant metastases and three had a stage I tumour). Consequently, 259 participants constitute the basis of this study." Exclusions: these exclusions were reported by arm:
This is unlikely to have introduced bias, as the reasons are similar for exclusions in each arm and the numbers excluded in each arm are similar. Attrition: quote (page 388): "No patient was lost to follow‐up" Comment: this is unlikely to have introduced bias. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes specified in the methods
Outcomes actually reported in the paper
We were not able to review the protocol, so we judged this domain to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias |
Schoemaker 1998.
| Methods | RCT
Accrual: 1984‐1990
2 centres in the study
Stratified according to site (colon or rectum) and Dukes' stage Setting: tertiary centres Follow‐up: 60 months |
|
| Participants | 325 participants (207 men and 118 women) who had curative resection of newly diagnosed CRC
Exclusion criteria
Dukes' A: 71 Dukes' B: 153 Dukes' C: 101 Colon primary: 238 Rectal primary: 87 Country: Australia |
|
| Interventions | Intervention: participants in the experimental arm underwent yearly CXR, CT of the liver, and colonoscopy. Control: these investigations were only performed in the control group if indicated on clinical grounds or after screening test abnormality, and at 5 years of follow‐up, to exclude a reservoir of undetected recurrences. | |
| Outcomes |
|
|
| Notes | Both groups had regular clinical review, including history, examination, and screening investigation at 3, 6, 9, 12, 15, 21, 24, 30, 36, 42, 48, 54, and 60 months or until a major endpoint was reached. A nurse research assistant performed a review at each visit, and a consultant surgeon, on at least alternate visits. Clinical signs and symptoms were recorded on a structured ProForma. Screening investigations at each visit comprised FBC, LFTs, CEA, and faecal occult blood testing using the Haemoccult‐II test (without hydration) on 3 faecal samples. All screening or clinical abnormalities were investigated on merit. The only exception was CEA ‐ an isolated rise in CEA was not used to trigger further investigations. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 8): "The patients were then randomized to either standard or intensive follow‐up by choosing the next card from a box of cards indicating the type of follow‐up. The cards had been previously randomized using random tables." Comment: this is an adequate method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 8): "The patients were then randomized to either standard or intensive follow‐up by choosing the next card from a box of cards indicating the type of follow‐up." Comment: the study did not report details about how this was done, so we conclude that it makes this domain at unclear risk of bias. |
| Blinding of participants and personnel | Low risk | Quote (page 8): "Review was performed by a nurse research assistant at each visit and by a consultant surgeon on at least alternate visits. Clinical symptoms and signs were obtained and recorded on a structured pro forma." Comment: the study did not mention blinding of participants and personnel, but it would have been difficult to do and unlikely to have introduced bias. The use of the pro‐forma for data collection on symptoms and signs would reduce the risk of bias from lack of personnel and participant blinding. |
| Blinding of outcome assessment | Low risk | Quote (page 8): "CXR and CT scans were interpreted by an independent senior radiologist. Colonoscopies were performed or supervised by recognized accredited colonoscopists and aimed to examine the entire residual colon to identify recurrence, metachronous carcinoma, and polyps." Comment: blinding of outcome assessors was likely to reduce the risk of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (page 8): "Eighteen patients withdrew from the study (standard, 8; intensive, 10). Three patients from each group were lost to follow‐up at intervals ranging from 9 to 54 months because they moved to another state. Five patients in the standard group and 7 in the intensive group withdrew at intervals ranging from 3 to 54 months because of development of other medical illnesses that precluded further structured follow‐up." Comment: the study did not report post randomisation exclusions. This attrition has been reported by study arm and the reasons are similar for the 2 arms, it is thus unlikely to be a source of bias. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes specified in the methods
Outcomes actually reported in the paper
We did not have access to the study protocol, so we judged the risk of bias for this domain to be unclear. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Secco 2002.
| Methods | RCT (2 'studies' within 1 publication)
Accrual: 1988‐1996
Single‐centre study Setting: not stated Follow‐up: 61.5 months (high‐risk group) and 48 months (low‐risk group) |
|
| Participants | 337 participants (163 men and 174 women) who had curative surgery alone for CRC
Participants were stratified into the following:
Country: Italy |
|
| Interventions | Intervention: 108 high‐risk participants were randomised to "intensive follow‐up" (experimental arm); they had clinic visits and serum CEA, abdomen/pelvic US scans, and CXR. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR. Control: 84 high‐risk participants were randomised to a "minimal follow‐up programme performed by physicians". | |
| Outcomes |
|
|
| Notes | Curative surgery: "macroscopic excision of primary, peri‐rectal tissues and nodes" Experimental arm: clinic visits and serum CEA measured at 3, 6, 9, 12, 15, 18, 21, 24, 28, 32, 36, 42, 48, 54, and 60 months; abdomen/pelvic US scans at 6, 12, 18, 24, 30, 36, 48, and 60 months; CXR at 12, 24, 36, 48, and 60 months. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR at 12, 24, 36, 48, and 60 months. Participants with rectal carcinoma had rigid sigmoidoscopy and CXR at 12, 24, 36, 48, and 60 months. All participants received education regarding follow‐up and the signs and symptoms of possible recurrence. All were expected to phone the surgical team every 6 months. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 419): "Patients of each group were randomly included..." Comment: the study provided no details regarding sequence generation; therefore, we judged this domain to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | The paper provided no description of allocation concealment. |
| Blinding of participants and personnel | Unclear risk | The study did not mention blinding of participants and personnel. As participants in each arm were educated about signs and symptoms of a possible recurrence, it is possible that those allocated to the minimal arm might be more likely to report symptoms in the knowledge that they would not have any investigations performed in the absence of symptoms. This may have introduced bias; therefore, we judged this at high risk of bias. The lack of blinding for personnel is less likely to have caused bias, because the follow‐up schedule was prespecified for both arms. |
| Blinding of outcome assessment | Low risk | The study did not mention blinding of outcome assessment; it would have been possible to do so, but unlikely to have introduced bias. |
| Incomplete outcome data (attrition and exclusions) | Unclear risk | Quote (paragraph 2, page 419): "Of the initial 358 patients...definitive randomisation of 337 patients" Comment: it is not clear whether these were prerandomisation or post‐randomisation exclusions; we judged this domain to be at unclear risk of bias. Attrition: quote (paragraph 1, page 419): "Twenty‐one (5.8%) patients dropped out over the first 13 months: eight cases from group 1 and 13 from Group 2." Comment: although the reasons for attrition were not reported, those who dropped out were reported by study arm, and as the numbers were similar, we judged this domain to be at low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk | We did not have access to the study protocol, so we judged this domain to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Sobhani 2008.
| Methods | Randomised, single institution study, which enrolled participants from 7 French teaching hospitals Follow‐up: 24 months or until death |
|
| Participants | N = 130 participants who had R0 (complete resections) surgery for colon or rectal cancer | |
| Interventions |
Intervention: PET performed at 9 and 15 months and conventional follow‐up Control: conventional follow‐up |
|
| Outcomes |
|
|
| Notes | All participants followed the same schedule: 6 visits that included physical examination; CEA or CA 19‐9, or both; US scan every 3 months (except at 9 and 15 months' follow‐up); CXR every 6 months; and abdominal CT at 9 and 15 months' follow‐up. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (paragraph 2, page 876): "Patients were randomly divided..." Comment: the study reported no details of the process of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | The paper gave no details about allocation concealment, so we judged this domain to be at unclear risk of bias. |
| Blinding of participants and personnel | Low risk | The paper did not mention blinding of participants and personnel; it was probably not done, but because of prespecified protocols, we deemed this domain to be at low risk of bias. |
| Blinding of outcome assessment | Low risk | Quote (paragraph 3, page 876): "Physicians were unaware of the findings of the CT scan" Comment: the blinding of outcome assessors was not described, although because the finding of recurrence was dependent on biopsy and determined in a multidisciplinary clinic, we deemed this domain to be at low risk of bias. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (paragraph 2, page 877): "One hundred and thirty patients (65 in each group) were evaluated in a ITT analysis." Comment: because the paper reported that all randomised participants were included in an ITT analysis, we deemed this domain to be at low risk of bias. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes in the objectives
Reported outcomes
We did not have access to the study protocol, so deemed this outcome to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Sobhani 2018.
| Methods | Multicentered, open‐label RCT Accrual dates: March 2008‐ November 2012 Country: France Follow‐up: 3 years (not further defined) |
|
| Participants | 257 participants screened, 239 randomised Control arm: (n = 119) Intervention arm: (n = 120) Inclusion criteria: ≥ 18 years, "high risk" histologically proven colorectal cancer, in "remission" at study entry. High risk defined as: Stage II perforated, Stage III or Stage IV CRC after curative surgery (remission confirmed at 4‐5 months post‐surgery based on negative physical examination, FBC, liver function tests and tumour markers, CT (CAP) and liver magnetic resonance imaging (MRI)). |
|
| Interventions | All participants had physical examination and laboratory tests (FBC, tumour markers) at 3, 6, 9, 12, 15, 18, 21, 24, 27, 30, 33, 36 months, liver US and CXR at 3, 9, 15, 21, 27, 33 months, CT (CAP) at 6, 12, 18, 24, 30 and 36 months, colonoscopy at 12 and 36 months. Intervention: FDG PET/CT at 6, 12, 18, 24, 30 and 36 months |
|
| Outcomes | Primary outcome: treatment failure defined as unresectable recurrence or death any cause: median follow‐up not reported Secondary outcomes: OS: median follow‐up not reported Disease‐free survival: median follow‐up not reported OS: measured at 3 years C‐SS: R‐FA: not defined, measured at 3 years SS: IR: QoL: Harms: Costs: measured at 3 years, assessed in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement, discounting was not performed |
|
| Notes | Salvage: treated with surgery if feasible and with chemotherapy otherwise. "Patients with one or few foci of recurrent disease underwent potentially curative surgery. Patients with metastatic dissemination received chemotherapy and/or palliative care". Protocols for imaging techniques were predefined. Funding sources: French Ministry of Health Conflicts of interests: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly allocated" Methods, Sobhani 2018. Insufficient detail provided to allow judgement, so this domain deemed at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided to allow judgement, so this domain deemed at unclear risk of bias. |
| Blinding of participants and personnel | Unclear risk | No detail provided to allow judgement, so this domain deemed at unclear risk of bias. |
| Blinding of outcome assessment | High risk | Quote: "Patients were examined between 3‐monthly follow‐up scheduled visits if their usual physicians felt the symptoms suggested recurrent disease" Sobhani 2018 Follow‐up Quote: "The images were reviewed without blinding during the multidisciplinary meetings.." Sobhani 2018 Follow‐up Quote: "a Multi‐disciplinary committee reviewed each patient's data every three months and classified the recurrence status as yes/no/doubtful" Sobhani 2018 Abstract. The outcome of R‐FS was deemed at high risk of bias for lack of blinding. |
| Incomplete outcome data (attrition and exclusions) | Low risk |
OS: intervention arm 119/119 and control arm 120/120 analysed Costs: reported on 92/120 intervention arm and 96/119 control arm For these two outcomes, this domain was deemed at low risk of attrition bias |
| Selective reporting (reporting bias) | High risk | We did not have access to the study protocol, but the paper does not report time to detection of resectable recurrence or number of recurrences as stated in the methods, so we deemed the study at high risk of selective reporting |
Strand 2011.
| Methods | RCT Accrual: 2002‐2005 Setting: tertiary centre Follow‐up: 60 months |
|
| Participants | 110 participants (59 men and 51 women) curatively operated on for CRC Country: Sweden Setting: hospital |
|
| Interventions |
Intervention: surgeon‐led follow‐up Control: nurse‐led follow‐up |
|
| Outcomes |
|
|
| Notes | A nurse and surgeon performed follow‐up in the same way: 6‐monthly visits for 3 years, then annually up to 5 years. Symptom enquiry occurred at each visit (bloods and CEA as indicated) Abdomen US and CXR (replaced by CT in latter half of the study) at 1 and 3 years If 'clean' colon was established preoperatively, colonoscopy at 5 years |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization to nurse (ES) or surgeon (KS) was performed using closed envelopes in blocks of four." Comment: the paper reported no more details, so we judged this domain to be at unclear risk of bias. |
| Allocation concealment (selection bias) | Unclear risk | The stoma therapist (IN) provided written and verbal information and conducted the randomisation. We judged this domain to be at unclear risk of bias. |
| Blinding of participants and personnel | Low risk | The study did not mention blinding of participants and personnel; it was probably not done, but unlikely to have introduced bias. |
| Blinding of outcome assessment | Low risk | The study did not mention blinding of outcome assessment, but it was unlikely to have been done. |
| Incomplete outcome data (attrition and exclusions) | Unclear risk | Quote (page 1001): "All patients completed the questionnaires." Comment: the paper reports that there was no attrition. Quote (page 1001): "One hundred and thirteen (113) consecutive patients were asked to participate in the study. Of these, three patients refused to participate, 56 were allocated to surgeon follow‐up and 54 to nurse‐led follow‐up." Comment: it is not clear if these were post‐randomisation or prerandomisation exclusions. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes prespecified
Actually reported
As we did not have access to the study protocol, we judged this domain to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
SurvivorCare 2013.
| Methods | RCT Multicentre study (18 sites) Country: Australia Setting: metropolitan, rural and regional, pubic and private Accrual dates: not stated |
|
| Participants | N = 221 Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | SurvivorCare intervention (nurse‐led survivorship care package) to usual post‐treatment care, for patients with potentially cured CRC Intervention (n = 110): SurvivorCare (SC), comprised of the following:
Control (n = 111):
|
|
| Outcomes | Primary outcome Psychological distress (measured by BSI (Zabora 2001), assessed at baseline, 2 and 6 months' follow‐up) Seondary outcomes:
|
|
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote; "patients were randomly assigned 1:1 to either usual care or SurvivorCare" (primary reference for SurvivorCare 2013, page 1015). Inadequate information provided to allow judgement, so deemed at unclear risk of bias |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Allocation was balanced by site using a minimization method and participants were randomly assigned and notified of allocation after completion of consent" (primary reference for SurvivorCare 2013, page 1015). Inadequate information provided to allow judgement, so deemed at unclear risk of bias |
| Blinding of participants and personnel | Unclear risk | Quote: "Intervention nurses received training in all aspects of the protocol, including prevention of diffusion into usual care" (primary reference for SurvivorCare 2013, page 1015). Inadequate information provided to allow judgement, so deemed at unclear risk of bias, blinding of participants and personnel is not mentioned. |
| Blinding of outcome assessment | Unclear risk | Blinding of outcome assessors not mentioned, so this domain was deemed at unclear risk of bias |
| Incomplete outcome data (attrition and exclusions) | Low risk | Primary outcome:
Secondary outcomes: There were minimal missing data
|
| Selective reporting (reporting bias) | Low risk | All outcomes listed in the protocol are reported on in the publication, so we deemed this domain at low risk of bias |
Treasure 2014.
| Methods | RCT Accrual: 1982‐1993 Multicentered study Setting: not stated Follow‐up: not stated |
|
| Participants | 216 (128 men and 88 women) participants who have had potentially curative resection of CRC Dukes' A: 10 Dukes' B: 95 Dukes' C: 74 Country: UK Setting: hospital |
|
| Interventions | If a CEA rise occurred, the participants were randomised to the "aggressive" arm or "conventional" arm. In the "aggressive" arm, a CEA rise triggered the "second‐look" surgery, with intention to remove any recurrence discovered. | |
| Outcomes |
|
|
| Notes | All participants had identical clinical follow‐up: every 3 months for the first 2 years, then every 6 months for the next 3 years. CEA was monthly for 3 years, then every 3 months for 2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 5): "Patients were randomised equally between the two arms (1:1). Patients whose compliance was between 50% and 70% or whose immediate postoperative sample had not been received within the 4–6‐week guideline were randomised in a separate stratum. Randomisation was also stratified by participating clinician. A block size of two was used in order to maintain as close a balance as possible between the two treatment arms." Comment: while the report did not describe the method of sequence generation, the authors who wrote this publication (not the original investigators) stated that the study was well performed, so we judged this domain to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 4): "The trial was coordinated (initially) from the Cancer Research Campaign Clinical Trials Centre at King’s College Hospital." Comment: while the method of allocation concealment was not described, the study was co‐ordinated remotely, so we judged this domain to be at low risk of bias. |
| Blinding of participants and personnel | Low risk | Quote (page 6): "By the nature of the trial design, the clinician was blind as to whether such patients had been randomised to the 'Conventional' arm of the trial or had not been randomised because the CEA had failed to denote the presence of recurrent disease." Participants were blinded to treatment allocation. With regard to personnel, clinicians were blinded to which arm their participant had been allocated to; we judged this domain to be at low risk of bias. |
| Blinding of outcome assessment | Low risk | The study did not mention blinding of outcome assessment. It was most likely not done. but is at little risk of introducing bias because of the blinding of participants and personnel. |
| Incomplete outcome data (attrition and exclusions) | Unclear risk | Exclusions: not reported Attrition: not reported We judged this domain to be at unclear risk of bias. |
| Selective reporting (reporting bias) | Low risk |
Outcomes stated in the protocol
Outcomes reported in the paper
The third and fourth outcomes were thought to be preplanned subanalyses, but problems with data formatting by the original study authors meant they were not able to be reported in the final publication. We judged this domain to be at low risk of bias. |
| Other sources of bias | Low risk | Quote (page 8): "A Data Monitoring Sub‐Committee (DMSC) composed of Working Party members not entering patients into the trial was asked to review the data after the first 100 patients had been randomised, which occurred in January 1988, and again after 200 patients had been randomised in February 1993. At this point it was recommended by the Data Monitoring Committee that the trial be stopped since it was very unlikely that any clinically important advantage would be demonstrated for patients undergoing second‐look surgery." Comment: early stopping occurred; this was recommended by the study monitoring committee, and was unlikely to have introduced bias. |
Wang 2009.
| Methods | RCT Accrual dates: January 1995‐March 2001 Setting: teaching hospital Follow‐up: 64‐79 months |
|
| Participants | 326 participants (177 men and 149 women) who had curative resection for CRC Stratified for location (colon or rectum) and Dukes' stage. Dukes' A: 53 Dukes' B: 186 Dukes' C: 93 Country: China |
|
| Interventions |
Intervention: colonoscopy at each visit Control: colonoscopy at 6 months, 30 months and 60 months from randomisation |
|
| Outcomes |
|
|
| Notes | All participants had clinic visits 3/12 for 12/12, 6/12 for 24/12, then 12/12 for 24/12. At each visit, history and examination were performed, as well as CEA, CXR, and liver imaging (CT or US). In each group, more investigations and examinations were performed if symptomatic. Curative resection: no macroscopic residual and clear pathological margins All recurrences were confirmed histologically. LR were divided into anastomotic (intraluminal recurrence within 5 cm of the initial primary) and extraluminal. Metachronous: second primary CRC after exclusion of a synchronous primary by a preoperative colonoscopy or within 6 months postoperatively Salvage surgery was considered curative when all macroscopic was removed and pathological margins were clear. doi: 10.1016/j.gie.2008.05.017 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote (page 610): "The patients were then randomized to either the Routine Colonoscopic Surveillance (RCS) group or the Intensive Colonoscopic Surveillance (ICS) group by means of sealed envelopes containing cards printed with ICS or RCS within each stratum." Comment: the paper gave insufficient details of the randomisation process. |
| Allocation concealment (selection bias) | Unclear risk | Quote (page 610): "Sealed envelopes containing cards printed with ICS or RCS within each stratum..." Comment: there is insufficient detail given to be sure that allocation concealment was truly concealed; it is not stated who performed the randomisation and if the envelopes were opaque. |
| Blinding of participants and personnel | High risk | The study did not mention blinding of participants or personnel. Participants in the control arm may have been more likely to report symptoms knowing that they were not having colonoscopies. |
| Blinding of outcome assessment | High risk | Quote (page 610): "More complete and systematic examinations were performed whenever a patient in either group had symptoms suggestive of a possible recurrence of the disease (e.g. abdominal or perineal pain, altered bowel movements, change in fecal colour, weight loss)." Comment: lack of blinding may have introduced bias with assessment of reported symptoms or signs, perhaps making further investigation more likely in the control group. |
| Incomplete outcome data (attrition and exclusions) | Unclear risk | Quote (page 611): "Seven patients (ICS, 4; RCS, 3) were lost during the follow‐up period." Comment: the paper gave no details about why they were lost to follow‐up, which may introduce bias. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes stated in the paper
Outcomes reported
We did not have access to the study protocol, so rated the risk of bias for this outcome as unclear. |
| Other sources of bias | Unclear risk | We detected no other bias. |
Wattchow 2006.
| Methods | RCT Accrual: 1998‐2001 Multicentred study Follow‐up: 24 months Randomisation method: remote and concealed (random numbers) Single‐blinded (researchers) Baseline characteristics: balanced other than trend to higher education levels in surgeon follow‐up group Power calculation: power of 80% (2‐sided) significant at 0.05, based on primary outcome measures (QoL, anxiety and depression, and participant satisfaction). Number of participants required was 64, set target of 100 participants in each arm | |
| Participants | 203 participants (117 men and 86 women) who had undergone curative surgery for Dukes' A, B, or C colon cancer who had completed any postsurgical chemotherapy (rectal cancer excluded because of requirement for sigmoidoscopy in follow‐up) Follow‐up by GPs and surgeons had to be available, and informed consent given. Participants were randomised at either postsurgical visit or at completion of chemotherapy. Country: Australia Setting: primary vs secondary care |
|
| Interventions | Setting and environment of follow‐up (primary vs secondary care) Follow‐up guidance was based on current clinical practice, and guidance was provided that suggested follow‐up visits every 3 months for the first 2 years postoperatively, then every 6 months for the next 3 years. Each visit incorporated asking a list of set questions about symptoms, physical examination, annual faecal occult blood testing, and colonoscopy every 3 years. | |
| Outcomes |
Primary
Other
|
|
| Notes | QoL assessment was based on SF‐12 physical and mental health component scores at baseline and 12 and 24 months. Depression and anxiety assessment was based on the HADS, measured at baseline and 12 and 24 months. Participant satisfaction was based on PSVQ measured at 24 months. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote (page 1117): "Consenting patients were then randomly allocated to either 'GP‐led' or 'surgeon‐led' follow‐up using an Excel random number generator." Comment: we judged this domain to be at low risk of bias. |
| Allocation concealment (selection bias) | Low risk | Quote (page 1117): "Randomisation was conducted by the study researchers, who were not involved in the design of the study or the clinical care of the patients, and was concealed until the interventions were assigned." Comment: we judged this domain to be at low risk of bias. |
| Blinding of participants and personnel | Low risk | Quote (page 1117): "The study was single‐blinded. Patients were reviewed by GPs in their practice rooms and surgeons in their surgical clinics." Comment: we assumed this means that they were blinded to treatment arm. |
| Blinding of outcome assessment | Low risk | Quote (page 1117): "Researchers at all times were unaware of the patient allocation until after the randomisation process." Quote (page 1118): "Analysis was blinded..." Comment: we assumed this means outcome assessors were blinded. |
| Incomplete outcome data (attrition and exclusions) | Low risk | Quote (page 1118): "Withdrawal was viewed as non‐completion of questionnaires (primary outcome measures) – data on deaths were still collected. Reasons given for withdrawing were participant commitment (10), concern over the time involved (4), lack of understanding of the study (1) and one did not 'wish to be reminded of their illness'. The remaining patients gave no explanation, but the withdrawals were equally distributed between the groups. There were 76 patients in the GP group, and 81 in the surgical group after 24 months of follow‐up, meeting the numbers required for statistical validity. Analysis was on an 'intention to treat' basis." Comment: reasons for withdrawal were reported by study arm and reasons were given. |
| Selective reporting (reporting bias) | Unclear risk |
Outcomes in the methods
Outcomes reported
We did not have access to the protocol, so judged this outcome to be at unclear risk of bias. |
| Other sources of bias | Unclear risk | We detected no other bias. |
AJCC: American Joint Committee on Cancer; BSI: Brief Symptom Inventory; CA 19‐9: cancer antigen 19‐9; CAP: chest, abdomen, pelvis; CaSUN: Cancer Survivors Unmet Needs Measure; CEA: carcinoembryonic antigen; CIS: carcinoma in situ; C‐SS: colorectal cancer‐specific survival; CRC: colorectal cancer; CT: computerised tomography; CXR: chest X‐ray; DFS: disease‐free survival; DRE: digital rectal examination; EORTC QLQ‐C30: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for Cancer patients; EQ VAS: EuroQol visual analogue scale; EQ‐5D: EuroQol‐5D; EuroQol five dimensions questionnaire; FBC: full blood count; FDG: fluorodeoxyglucose; GP: general practitioner; HADS: Hospital Anxiety and Depression Scale; HR: hazard ratio; HRQoL: health‐related quality of life; IR: interval recurrences; ITT: intention‐to‐treat; LFTs: liver function tests; LR: local recurrence; MDT: multidisciplinary team; MR(I): magnetic resonance (imaging); MCS: mental component summary; NHS: National Health Service; OS: overall survival; PCS: physical component summary; PET: positron emission tomography; PSVQ: Patient Visit‐Specific Questionnaire; QoL: quality of life; RCT: randomised controlled trial; R‐FS: relapse‐free survival SF‐12: short form‐12; SF‐36: short form 36; SS: salvage surgery; TNM: tumour, node, metastasis; US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Kronborg 1981 | Participants with polyps and invasive cancers included. Those with polyps were randomised to 6‐monthly or 24‐monthly colonoscopies, those with cancer were not included in the randomisation and all had 6‐monthly colonoscopies. |
| NCT00182234 | Particpants with breast and CRC were analysed together and data for those with CRC were not reported separately. |
| Sano 2004 | The study was ineligible; participants did not have colorectal cancer. |
| Serrano 2018 | Population was participants with liver metastases |
CRC: colorectal cancer
Characteristics of studies awaiting assessment [ordered by study ID]
Barillari 1996.
| Methods | RCT |
| Participants | Participants had curative resection for CRC, margin negative, no distant metastases, preoperative colonoscopy and baseline clear post‐operative colonoscopy. Exclusion criteria: familial polyposis and ulcerative colitis |
| Interventions |
Intervention: coloscopy at 12‐month intervals plus 3‐monthly clinic visits and CEA Control: colonoscopy at 24‐month intervals plus 3‐monthly clinic visits and CEA |
| Outcomes | Anastomotic recurrences Metachronous primaries OS at 5 years Distant metastases |
| Notes | Dr. Barillari Via della Camilluccia 589/c, 00135 Rome, Italy |
NCT00199654.
| Methods | Phase III, open‐labelled, multicentre, multidisciplinary, randomised study, comparing 2 arms of 188 participants (i.e. 376 total participants). |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes |
|
| Notes | Study start date: February 2004 Study completion date: April 2013 No study results are published yet. |
UMN000001318.
| Methods | Parallel RCT, unblinded |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | Intensive post‐operative follow‐up schedule vs standard post‐operative follow‐up schedule |
| Outcomes | OS at 5 years Rate of curative resection after recurrence Total costs |
| Notes | This study was supported in part by a Grant‐in‐Aid for Cancer Research from the Ministry of Health and Welfare and Labor of Japan. Contact: Yoshihiro Moriya, Department of colorectal and pelvic surgery, National Cancer Center Hospital, 6‐5‐1, Kashiwanoha, Kashiwa, Chiba, Japan |
CEA: carcinoembryonic antigen; CRC: colorectal cancer; CT: computerised tomography; CXR: chest X‐ray; OS: overall survival; PET: positron emission tomography; RCT: randomised controlled trial; TNM: tumour, node, metastases
Characteristics of ongoing studies [ordered by study ID]
COLOPEC.
| Trial name or title | Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases (COLOPEC‐II) |
| Methods | Allocation: randomised Intervention model: parallel assignment Masking: none (open‐label) Masking description: in order to prevent caretakers from being influenced by the assigned follow‐up strategy, the randomisation outcome will remain unknown to everyone involved in the patient's care until the 18‐month CT‐scan is reported by the radiologist. |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | |
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | |
| Contact information | Vivian P Bastiaenen, MD |
| Notes | Contact: Pieter J Tanis, MD, PhD |
FURCA.
| Trial name or title | Follow‐up after rectal cancer |
| Methods | RCT Multicenter (n = 4) Sweden Accrual dates: February 2016‐? end 2017 Median follow‐up: planned for 3 years |
| Participants | Inclusion criteria
Exclusion criteria
|
| Interventions | |
| Outcomes | OS C‐SS R‐FS IR SS Toxicity QoL: symptom burden (PROMS and HRQoL, measured using FACT‐C, assessed at baseline, 1 and 3 years Costs |
| Starting date | February 2016 |
| Contact information | Jakobsen IH idajakob@rm.dk |
| Notes |
HIPEC.
| Trial name or title | Second look laparoscopy in colorectal cancer (HIPEC) |
| Methods | Randomised phase II |
| Participants | Participants who have had radical resection of mucinous CRC Inclusion criteria
Exclusion criteria
|
| Interventions | Second‐look laparoscopy, followed by peritonectomy, hyperthermic intraperitoneal chemotherapy (HIPEC), or systemic chemotherapy in case of peritoneal carcinosis |
| Outcomes |
Primary outcome measures
Secondary outcome measures
|
| Starting date | Study start date: April 2012 Estimated study completion date: 2017 |
| Contact information | Francesco Perrone, MD, PhD
+39 081 5903571
francesco.perrone@usc‐intnapoli.net Mariliana Piccirillo, MD +39 081 5903383 marilina.piccirolli@usc‐intnapoli.net |
| Notes | This study is currently recruiting participants. |
ProphyloCHIP.
| Trial name or title | Multicentric phase III trial comparing simple follow‐up to exploratory laparotomy plus "in principle" HIPEC (hyperthermic intraperitoneal chemotherapy) in colorectal patients initially treated with surgery and adjuvant chemotherapy who have a high risk of developing colorectal peritoneal carcinomatosis |
| Methods | RCT |
| Participants |
|
| Interventions |
Intervention: laparotomy plus HIPEC Control: no intervention, surveillance |
| Outcomes | D‐FS at 3 years OS at 3 years Peritoneal D‐FS at 3 years OS at 5 years |
| Starting date | April 2010 |
| Contact information | Diane GOERE, MD |
| Notes |
SCORE.
| Trial name or title | SCORE: shared care of colorectal cancer survivors: protocol for a randomised controlled trial |
| Methods | RCT |
| Participants |
Inclusion criteria
Exclusion criteria
|
| Interventions |
Intervention: shared follow‐up care between specialist and PCP (shared care intervention) for 12 months plus standard specialist‐based follow‐up care Control: standard specialist‐based follow‐up care, comprising: 3‐monthly follow‐up for first 12 months (patient history, physical examination, CEA), CT at 12 months if recommended by the specialist |
| Outcomes | Primary outcome: QoL (measured using EORTC QLQ‐C30 and EORTC QLQ‐CR29 Secondary outcomes:
Outcome measures assessed at baseline, 6 and 12 months. |
| Starting date | 24 February 2017 |
| Contact information | score@petermac.org |
| Notes | ACTRN12617000004369p |
SURVEILLANCE.
| Trial name or title | Follow‐up care with or without CEA assessments in patients who have undergone surgery for stage II or stage III colorectal cancer |
| Methods | RCT n = 1925 |
| Participants |
|
| Interventions | Other: diagnostic laboratory biomarker analysis Procedure: CT Procedure: diagnostic colonoscopy Procedure: standard follow‐up care Procedure: US imaging |
| Outcomes |
|
| Starting date | Study start date: September 2009 Estimated primary completion date: April 2018 |
| Contact information | Come Lepage, MD Centre Hospitalier Universitaire de Dijon |
| Notes | This study is ongoing, but not recruiting participants. |
CEA: carcinoembryonic antigen; C‐SS: colorectal cancer‐specific survival; CT: computerised tomography;D‐FS: disease‐free survival; DLS: diagnostic laparoscopy; ECOG: Eastern Cooperative Oncology Group; EORTC QLQ‐C30/CR29: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire for Cancer patients; FACT‐C: Functional assessment of cancer treatment; HIPEC: hyperthermic intraperitoneal chemotherapy; HRQoL: health‐related quality of life; IR: interval recurrences; MRI: magnetic resonance imaging; OS: overall survival; PC: peritoneal carcinomatosis; PCP: primary care physician; PM: peritoneal metastases; PROMS: patient‐reported outcome measures; QoL: quality of life; RCT: randomised controlled trial; R‐FS: relapse‐free survival; SS: salvage surgery; US: ultrasound; WHO: World Health Organization
Differences between protocol and review
We have simplified the wording of the Objectives. We have deleted the reference to secondary endpoints as they are itemised under Types of outcome measures.
Types of participants: we have simplified cancer staging.
Types of interventions: we have added the phrase "investigations including but not limited to" for clarity.
-
Types of outcome measures > Secondary outcomes:
we have renamed 'Disease‐specific survival' as 'Colorectal cancer‐specific survival'.
We changed 'Time to diagnosis of recurrence' to 'Relapse‐free survival'.
We changed 'Incidence of surgery (with curative intent for recurrence)' to 'Salvage surgery (surgery performed with curative intent for relapse of colorectal cancer)'.
We replaced 'Interval (between planned visits) recurrences' with 'Interval recurrences (relapse of colorectal cancer detected between follow‐up visits)'.
We clarified the quality of life outcome to indicate that we permitted the use of study‐specific quality‐of‐life instruments.
Harms and costs of surveillance now specifically include that of investigations.
We defined colorectal cancer‐specific survival and relapse‐free survival.
We moved the search strategy to Appendices.
Our reporting of the search strategy is now consistent with MECIR (Methodological Expectations of Cochrane Intervention Review) guidelines.
We updated our reporting of the processes relating to selection of studies and data extraction and management in accordance with the MECIR guidelines.
We modified our assessment of risk of bias to ensure consistency with the current Cochrane Handbook for Systematic Reviews of Interventions recommendations.
We modified our measures of treatment effect to incorporate time‐to‐event data where possible.
We have added a 'Summary of findings' table.
We now present time‐to‐event data with hazard ratios, rather than risk ratios.
We have deleted the multiple post hoc subgroup analyses we performed in the earlier version of the review (clinic visits and tests versus no clinic visits and tests, more clinic visits versus fewer clinic visits, more tests versus fewer tests, community versus hospital, liver imaging versus no liver imaging, time to recurrence with intensive follow‐up versus time to recurrence with less intensive follow‐up).These were included in previous versions of the review, but we now recognise that large numbers of undirected sub‐group analyses may lead to spurious explanations of heterogeneity (Higgins 2018). In response to a reviewer suggestion, we included post hoc subgroup analysis of follow‐up "dose" and the use of different settings (specialist versus GP‐ or nurse‐led follow‐up).
We used a random‐effects model for meta‐analysis based on reviewer input.
In response to reviewer input, we performed sensitivity analysis by excluding one study (Ohlsson 1995), where the intensity of follow‐up in the intensive arm was comparable with the intensity of follow‐up in the control arm of other studies.
Contributions of authors
All authors read through all abstracts, appraised the potential papers, appraised the included studies, and extracted the data. The text of the review was a collaborative effort by all four authors. AS ran the searches, and BH checked that they were correct.
Sources of support
Internal sources
-
Princess Alexandra Hospital Cancer Collaborative Group, Australia.
Supported AS (who ran search strategies for the review)
External sources
No sources of support supplied
Declarations of interest
Mark Jeffery was an international member of the Follow‐up After Colorectal Surgery (FACS) trial management committee. Brigid E Hickey: nothing to declare Phil N Hider: nothing to declare Adrienne M: nothing to declare
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
CEAwatch 2015 {unpublished data only}
- Verberne C, Doornbos PM, Grossmann I, Bock GH, Wiggers T. Intensified follow‐up in colorectal cancer patients using frequent carcino‐embryonic antigen (CEA) measurements and CEA‐triggered imaging. European Journal of Cancer. 2013; Vol. 49:S480. [DOI] [PubMed]
- Verberne CJ, Wiggers T, Grossmann I, Bock GH, Vermeulen KM. Cost‐effectiveness of a carcinoembryonic antigen (CEA) based follow‐up programme for colorectal cancer (the CEA Watch trial). Colorectal Disease 2016;18(3):O91‐o96. [DOI] [PubMed] [Google Scholar]
- Verberne CJ, Zhan Z, Bock G, Wiggers T. Intensifying colorectal cancer follow‐up survival analysis of the randomized multicenter CEAwatch trial. European Journal of Surgical Oncology. 2016; Vol. 42:9 S106.
- Verberne CJ, Zhan Z, Heuvel E, Grossmann I, Doornbos PM, Havenga K, et al. Intensified follow‐up in colorectal cancer patients using frequent carcino‐embryonic antigen (CEA) measurements and CEA‐triggered imaging: results of the randomized "CEAwatch" trial. European Journal of Surgical Oncology 2015;41(9):1188‐96. [DOI] [PubMed] [Google Scholar]
- Verberne CJ, Zhan Z, Heuvel ER, Oppers F, Jong AM, Grossmann I, et al. Survival analysis of the CEAwatch multicentre clustered randomized trial. British Journal of Surgery 2017;104(8):1069‐77. [DOI: 10.1002/bjs.10535] [DOI] [PubMed] [Google Scholar]
- Zhan Z, Heuvel ER, Doornbos PM, Burger H, Verberne CJ, Wiggers T, et al. Strengths and weaknesses of a stepped wedge cluster randomized design: its application in a colorectal cancer follow‐up study. Journal of Clinical Epidemiology 2014;67(4):454‐61. [DOI] [PubMed] [Google Scholar]
- Zhan Z, Verberne CJ, Hauvel ER, Grossman I, Ranchor AV, Wiggers T, et al. Psychological effects of the intensified follow‐up of the CEAwatch trial after treatment for colorectal cancer. PLOS One 2017;12(9):e0184740. [DOI: 10.1371/journal.pone.0184740] [DOI] [PMC free article] [PubMed] [Google Scholar]
COLOFOL 2018 {unpublished data only}
- Hansdotter P, Wille‐Jørgensen, Horváth‐Puhó E, Petersen SH, Martling A, Sørenson HT. The COLOFOL trial: study design and comparison of the study population with the source cancer population. Clinical Epidemiology 2016;8:15‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00225641. Assessment of frequency of surveillance after curative resection in patients with stage II and III colorectal cancer [COLOFOL ‐ a pragmatic study to assess the frequency of surveillance tests after curative resection in patients with stage II and III colorectal cancer ‐ a randomised multicentre trial]. clinicaltrials.gov/ct2/show/NCT00225641?term=NCT00225641&rank=1 (first received 14 November 2012).
- Wille‐Jorgensen P, Syk I, Smedh K, Laurberg S, Nielsen DT, Petersen SH, et al. Effect of more vs less frequent follow‐up testing on overall and colorectal cancer‐specific survival mortality in patients with stage II or III colorectal cancer. The COLFOL randomized clinical trial. JAMA 2018;319(20):2095‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wille‐Jørgensen P, Laurberg S, Påhlman L, Carriquiry L, Lundqvist N, Smedh K, et al. An interim analysis of recruitment to the COLOFOL trial. Colorectal Disease 2009;11(7):756‐8. [DOI] [PubMed] [Google Scholar]
- Wille‐Jørgensen P, Laurberg S, Toft Sørensen H, Påhlman L, on behalf of the COLOFOL study group. COLOFOL study protocol a pragmatic study to assess the frequency of surveillance tests after curative resection in patients with stage II and III colorectal cancer– a randomised multicentre trial. www.colofol.com/html/download/COLOFOL_7‐5.pdf (accessed 30 April 2008).
FACS 2014 {published data only}
- Corkhill A, Primrose JN, Mant D, The FACS Study Group. Follow up after colorectal cancer (the FACS trial). British Journal of Cancer 2004;91(Suppl 1):S78. [Google Scholar]
- Mant D, Gray A, Pugh S, Campbell H, George S, Fuller A, et al. A randomised controlled trial to assess the cost‐effectiveness of intensive versus no scheduled follow‐up in patients who have undergone resection for colorectal cancer with curative intent. Health Technology Assessment 2017; Vol. 21, issue 32. [DOI: 10.3310/hta21320] [DOI] [PMC free article] [PubMed]
- Mant D, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3‐5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: FACS randomized controlled trial. Journal of Clinical Oncology. 2013; Vol. 31S:3500. [DOI] [PubMed]
- NCT00560365. Follow‐up study of patients who have undergone surgery for stage I, stage II, or stage III colorectal cancer [A randomised controlled trial to assess the cost‐effectiveness of intensive versus no scheduled follow‐up in patients who have undergone resection for colorectal cancer with curative intent (FACS ‐ Follow‐up After Colorectal Surgery)]. clinicaltrials.gov/ct2/show/NCT00560365?term=NCT00560365&rank=1 (first received 14 November 2012).
- Primrose J, Mant D, Rose P, George S, Nugent K, Gray A, et al. A randomised controlled trial to assess the cost‐effectiveness of intensive versus no scheduled follow‐up in patients who have undergone resection for colorectal cancer with curative intent (The FACS Trial). www.nets.nihr.ac.uk/projects/hta/991099. Winchester: John Wiley & Sons, Ltd., (accessed 23 July 2018). [DOI] [PMC free article] [PubMed]
- Primrose JN, Fuller A, Rose P, Perera‐Salazar R, Mellor J, Corkhill A, et al. Follow‐up after colorectal cancer surgery: preliminary observational findings from the UK FACS trial. Journal of Clinical Oncology, 2011 ASCO Annual Meeting Abstracts Part 1 2011;29, No 15(Suppl (May 20)):3521. [Google Scholar]
- Primrose JN, Perera R, Gray A. Effect of 3 to 5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. Diseases of the Colon and Rectum 2014;57:e421‐2. [DOI] [PubMed] [Google Scholar]
- Primrose JN, Perera R, Gray A, Rose P, Fuller A, Corkhill A, et al. Effect of 3 to 5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA 2014;311(3):263‐70. [DOI] [PubMed] [Google Scholar]
- Primrose JN, Perera R, Gray A, et al. FACS Trial Investigators. Supplementary Online Content. Effect of 3 to 5 years of scheduled CEA and CT follow‐up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. cdn.jamanetwork.com/ama/content_public/journal/jama/929670/joi130124supp1_prod.pdf?Expires=2147483647&Signature=tqyZRUZOZ3TvJrZ2‐4iAyWuXfpwCyW6kMQChZEFbH6˜NvVywPoTOsHcY9POszrRepxg˜7n˜FIzHUIhSz4yTen‐CqgWp0xYUlfJ˜VwEheI24XC00OX9x9eM3NaTrfnrXIjSFu‐TPHuevJ1fyp8UyLkFLFxQ2mnFaP1PzOSnH‐twxhWsyS6iIL9‐Kuza7G70XLwnAwQk‐CkWyOxSgXmxBTW1Hwdihn8BsAJpCWTdPD7HoK1CcUb5uCUMozUTHxRyXOvL˜oQJZy78j˜QxigPperxRFqctIyYEomfj9Y3vsgcWVcLeoORQvGWhHlvMUZpbm2AJ4ivAT0xVWkR‐7pKOBsfg__&Key‐Pair‐Id=APKAIE5G5CRDK6RD3PGA (accessed 10th August 2019).
- Pugh SA. Site and stage of colorectal cancer influences the likelihood and distribution of disease recurrence and post‐recurrence cancer survival. Data from FACS randomised controlled trial. Annals of Surgery 2016;263(6):1143. [DOI] [PubMed] [Google Scholar]
- Pugh SA, Mant B, Shinkins B, Mellor R, Perrera R, Primrose J. Scheduled use of CEA and CT follow‐up to detect recurrence of colorectal cancer: 6‐12 year results from the FACS randomised controlled trial. Annals of Oncology. 2016; Vol. 27:S6.
- Reibetanz J, Germer CT. Influence of structured CEA determination and CT‐scan to detect recurrence of colorectal cancer: results of the randomized clinical FACS study. Der Chirurg; Zeitschrift für alle Gebiete der operativen Medizen 2014;85(7):642. [DOI] [PubMed] [Google Scholar]
- Shinkins B, Nicholson BD, James T, Pathiraja I, Pugh S, Perera R, et al. What carcinoembryonic antigen level should trigger further investigation during colorectal cancer follow‐up? A systematic review and secondary analysis of a randomised controlled trial. Health Technology Assessment 2017; Vol. 21, issue 32:1‐60. [DOI] [PMC free article] [PubMed]
- Shinkins B, Primrose JN, Pugh SA, Nicholson BD, Perera R, James T, et al. Serum carcinoembryonic antigen trends for diagnosing colorectal cancer recurrence in the FACS randomized clinical trial. British Journal of Surgery 2018;6:658‐62. [DOI] [PubMed] [Google Scholar]
- Shrinkins B, Nicholson BD, Primrose J, Perera R, James RT, Pugh S, et al. The diagnostic accuracy of a single CEA blood test in detecting colorectal cancer recurrence: results from the FACS trial. PLOS One 2017; Vol. 12, issue 3:e0171810. [DOI] [PMC free article] [PubMed]
GILDA 1998 {unpublished data only}
- Fossati R, Andreoni A, Costanzo B, Fossati D, Johnson G, Liberati L, et al. Preliminary results of the Italian study "GILDA" [Preliminari dello studio I risultati preliminari dello studio italiano GILDA]. 2005. [3026272]
- Grossmann EM, Johnson FE, Virgo KS, Longo WE, Fossati R. Follow‐up of colorectal cancer patients after resection with curative intent‐the GILDA trial. Surgical Oncology 2004;13(2‐3):119‐24. [DOI] [PubMed] [Google Scholar]
- Johnson FE, Virgo KS, Grossmann EM, Longo WE, Fossati R. Colorectal cancer patient follow‐up following surgery with curative intent: the GILDA trial. Journal of Clinical Oncology, 2004 ASCO Annual Meeting Proceedings (Post‐meeting Edition) 2004;22:14S. [Google Scholar]
- Rosati G, Ambrosini G, Barni S, Andreoni B, Corradini G, Luchena G, et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2‐C colorectal carcinoma. Annals of Oncology 2016;27(2):274‐80. [DOI: 10.1093/annonc/mdv541] [DOI] [PubMed] [Google Scholar]
Kjeldsen 1997 {published data only}
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. A prospective randomised study of follow‐up after radical surgery for colorectal cancer. British Journal of Surgery 1997;84(5):666‐9. [PubMed] [Google Scholar]
- Kjeldsen BJ, Kronborg O, Fenger C, Jørgensen OD. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. International Journal of Colorectal Disease 1997;12(6):329‐34. [DOI] [PubMed] [Google Scholar]
- Kjeldsen BJ, Thorsen H, Whalley D, Kronborg O. Influence of follow‐up on health‐related quality of life after radical surgery for colorectal cancer. Scandinavian Journal of Colorectal Diseases 1999;34(5):509‐15. [DOI] [PubMed] [Google Scholar]
Mäkelä 1995 {published data only}
- Mäkelä J, Laitinen S, Kairaluoma MI. Early results of follow‐up after radical resection for colorectal cancer. Preliminary results of a prospective randomized trial. Surgical Oncology 1992;1(2):157‐61. [DOI] [PubMed] [Google Scholar]
- Mäkelä JT, Laitinen SO, Kairaluoma MI. Five‐year follow‐up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Archives of Surgery 1995;130(10):1062‐7. [DOI] [PubMed] [Google Scholar]
Ohlsson 1995 {published data only}
- Kronborg O, Fenger C, Deichgräber E, Hansen L. Follow‐up after radical surgery for colorectal cancer. Design of a randomized study. Scandinavian Journal of Gastroenterology 1988;23(Suppl 149):159‐62. [DOI] [PubMed] [Google Scholar]
- Ohlsson B, Breland U, Ekberg H, Graffner H, Tranberg KG. Follow‐up after curative surgery for colorectal carcinoma. Randomized comparison with no follow‐up. Diseases of the Colon and Rectum 1995;38(6):619‐26. [DOI] [PubMed] [Google Scholar]
ONCOLINK {published data only}
- Augestad KM, Norum J, Dehof S, Aspevik R, Ringberg U, Nestvold T, et al. Cost‐effectiveness and quality of life in surgeon versus general practitioner‐organised colon cancer surveillance: a randomised controlled trial. BMJ Open 2013;3(4):e002391. [NCT00572143] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augestad KM, Norum J, Rose J, Lindsetmo RO. A prospective analysis of false positive events in a National Colon Cancer Surveillance Program. BMC Health Services Research 2014;14:137. [DOI: 10.1186/1472-6963-14-137] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augestad KM, Vonen B, Aspevik R, Nestvold T, Ringberg U, Johnsen R, et al. Should the surgeon or the general practitioner (GP) follow up patients after surgery for colon cancer? A randomized controlled trial protocol focusing on quality of life, cost‐effectiveness and serious clinical events. BMC Health Services Research 2008;8:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00572143. Follow‐up after surgery for colon cancer. General practice vs. surgical‐based follow‐up? (ONKOLINK). clinicaltrials.gov/ct2/show/NCT00572143?term=follow‐up&type=Intr&cond=Colorectal+Cancer&rank=6 (accessed 9 Janury 2019).
Pietra 1998 {published data only}
- Pietra N, Sarli L, Costi R, Ouchemi C, Grattarola M, Peracchia A. Role of follow‐up in management of local recurrences of colorectal cancer: a prospective, randomized study. Diseases of the Colon and Rectum 1998;41(9):1127‐33. [DOI] [PubMed] [Google Scholar]
Rodríguez‐Moranta 2006 {published data only}
- Bessa X, Saló J, Arcusa A, Elizalde J, Boadas J, Alentorn E, et al. Effectiveness of postoperative follow‐up in patients with colorectal cancer to detect curable recurrences. Preliminary analysis of a multicenter, randomized controlled trial. Gastroenterology and Hepatology. 2001; Vol. 24:2.
- Rodríguez‐Moranta F, Saló J, Arcusa A, Boadas J, Piñol V, Bessa X, et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. Journal of Clinical Oncology 2006;24(3):386‐93. [DOI] [PubMed] [Google Scholar]
Schoemaker 1998 {published data only}
- Schoemaker D, Black R, Giles L, Toouli J. Yearly colonoscopy, liver CT, and chest radiography do not influence 5‐year survival of colorectal cancer patients. Gasteroenterology 1998;114(1):7‐14. [DOI] [PubMed] [Google Scholar]
Secco 2002 {published data only}
- Secco GB, Fardelli R, Gianquinto D, Bonfante P, Baldi E, Ravera G, et al. Efficacy and cost of risk adapted follow‐up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. European Journal of Surgical Oncology 2002;28(4):418‐23. [DOI] [PubMed] [Google Scholar]
Sobhani 2008 {published data only}
- Sobhani I, Tiret E, Labtahi R, Aparicio T, Itti E, Montravers F, et al. Early detection of recurrence by 18FDG‐PET in the follow‐up of patients with colorectal cancer. British Journal of Cancer 2008;98(5):875‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sobhani 2018 {unpublished data only}
- NCT00624260. Impact of PET scan on the curative strategy of colo‐rectal cancers : a randomized study (ITEP). clinicaltrials.gov/ct2/show/record/NCT00624260?term=NCT00624260&rank=1 (accessed 27 May 2018).
- Sobhani I, Baumgaertner I, Tounigand C, Ette E, Brunetti F, Gagniere C, et al. option19 Follow‐up of colorectal cancer (CRC) patients including 18FDGPET‐CT (PET‐CT): an open‐label multicenter randomized trial (clinical trial: NCT 00624260). United European Gastroenterology Journal. 2017; Vol. 5:S1 A13.
- Sobhani I, Baumgaertner I, Itti E, Luciani A, Layese R, Natella PA, et al. Colorectal cancer (CRC) patients surveyed by 18FDGPET‐CT (PET‐CT): an open label multicenter randomized trial (NCT 00624260). Journal of Clinical Oncology. 2018; Vol. 35:15S1.
- Sobhani I, Itti E, Baumgaertner I, Layese R, Andre T, Ducreux M, et al. Colorectal cancer (CRC) Monitoring by Six‐Monthly 18 FDG‐PET/CT: An Open‐Label Multicientre Randomised Trial. Annals of Oncology 2018;29(4):931‐937. [DOI: 10.1093/annonc/mdy031] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I, Itti E, Baumgaertner I, Layese R, Andre T, Ducreux M, et al. Colorectal cancer (CRC) monitering by six‐monthly 18. academic‐oup‐com.ezproxy.library.uq.edu.au/annonc/article/29/4/931/4819113#supplementary‐data (accessed 20 June 2018) 2018; Vol. 29, issue 4.
Strand 2011 {published data only}
- Strand E, Nygren I, Bergkvist L, Smedh K. Nurse or surgeon follow‐up after rectal cancer: a randomized trial. Colorectal Disease 2011;13(9):999‐1003. [DOI] [PubMed] [Google Scholar]
SurvivorCare 2013 {published data only}
- Jefford M, Aranda S, Gough K, Lotfi‐Jam K, Butow P, Krishnasamy M, et al. Evaluating a nurse‐led survivorship care package (SurvivorCare) for bowel cancer survivors: study protocol for a randomized controlled trial. Trials 2013;14(1):260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow P, et al. A randomized controlled trial of a nurse‐led supportive care package (SurvivorCare) for survivors of colorectal cancer. Oncologist 2016;21(8):1014‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefford M, Gough K, Drosdowsky A, Russell L, Aranda S, Butow PN, et al. A randomized controlled trial (RCT) of a supportive care package (SurvivorCare, SC) for survivors of colorectal cancer (CRC). Journal of Clinical Oncology 2016;34(3):3S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Treasure 2014 {published data only}
- ISRCTN76694943. A multicentre trial to evaluate the use of serial carcinoembryonic antigen (CEA) assay as the prime indicator for second‐look surgery in recurrent colorectal cancer. www.isrctn.com/ISRCTN76694943?q=&filters=&sort=&offset=14345&totalResults=14626&page=287&pageSize=50&searchType=basic‐search (accessed 6 April 2019).
- Lennon T, Houghton J, Northover J, on behalf of CRC/NIH CEA Trial Working Party. Post‐operative CEA monitoring and second‐look surgery in colorectal cancer: trial results. British Journal of Cancer. Proceedings of Ninth Annual Scientific Meeting of the British Oncological Association. New Jersey: Macmillan Publishers Ltd, 1994:16.
- Northover J, Houghton J, Lennon T. CEA to detect recurrence of colon cancer. JAMA 1994;272(1):31. [PubMed] [Google Scholar]
- Treasure T, Monson K, Fiorentino F, Russell C. The CEA Second‐Look Trial: a randomised controlled trial of carcinoembryonic antigen prompted reoperation for recurrent colorectal cancer. BMJ open 2014;4(5):e004385. [DOI: 10.1.1136/bmjopen-2013-004385] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2009 {published data only}
- Wang T, Cui Y, Huang WS, Deng YH, Gong W, Li CJ, et al. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: a prospective, randomized clinical study. Gastrointestinal Endoscopy 2009;69(3 Pt 2):609‐15. [DOI] [PubMed] [Google Scholar]
Wattchow 2006 {published data only}
- Wattchow DA, Weller D, Pilotto L, Estermann A. Randomized controlled trial (RCT) comparing hospital and general practice based follow‐up of patients with colon cancer. Australian and New Zealand Journal of Surgery 2002;72 Suppl:A22. [Google Scholar]
- Wattchow DA, Weller DP, Esterman A, Pilotto LS, McGorm K, Hammett Z, et al. General practice vs surgical‐based follow‐up for patients with colon cancer: randomised controlled trial. British Journal of Cancer 2006;94(8):1116‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Kronborg 1981 {published data only}
- Kronborg O, Hage E, Deichgraeber E. A prospective, partly randomized study of the effectiveness of repeated examination of the colon after polypectomy and radical surgery for cancer. Scandinavian Journal of Gasteroenterology 1981;16(7):879‐84. [DOI] [PubMed] [Google Scholar]
NCT00182234 {published data only}
- NCT00182234. SONICS ‐ Effectiveness of Specialist Oncology Nursing. clinicaltrials.gov/ct2/show/study/NCT00182234?term=NCT00182234&rank=1 (accessed 23 July 2018).
- Sussman J, Bainbridge D, Whelan TJ, Brazil K, Parpia S, Wiernikowski J, et al. Evaluation of a specialized oncology nursing supportive care intervention in newly diagnosed breast and colorectal cancer patients following surgery: a cluster randomized trial. Supportive Care in Cancer 2018;26(5):1533‐41. [DOI] [PubMed] [Google Scholar]
Sano 2004 {published data only}
- Sano Y, Fujii T, Oda Y, Matsuda T, Kozu T, Kudo S, et al. A multicenter randomized controlled trial designed to evaluate follow‐up surveillance strategies for colorectal cancer: the Japan Polyp Study. Digestive Endoscopy 2004;16(4):376‐8. [Google Scholar]
Serrano 2018 {published data only}
- Serrano P, Gu C, Husien M, Jalink D, Martel G, Tsang ME, et al. Effect of PET‐CT on disease recurrence and its management in patients with potentially resectable colorectal cancer liver metastases: the long‐term results of a randomized controlled trial. Annals of Surgical Oncology. 2018; Vol. 25:1S. [DOI] [PubMed]
References to studies awaiting assessment
Barillari 1996 {published data only}
- Barillari P, Ramacciato G, Manetti G, Bovino A, Sammartino P, Stipa V. Surveillance of colorectal cancer: effectiveness of early detection of intraluminal recurrences on prognosis and survival of patients treated for cure. Diseases of the Colon and Rectum 1996;39(4):388‐93. [DOI] [PubMed] [Google Scholar]
NCT00199654 {unpublished data only}
- NCT00199654. Follow‐up care with or without CEA assessments in patients who have undergone surgery for stage II or stage III colorectal cancer [Follow‐up of fully resected stage II or III colorectal cancer. Phase III multicentric prospective randomised study]. clinicaltrials.gov/ct2/show/NCT00995202?term=NCT00995202&rank=1 (first received 14 November 2012).
UMN000001318 {unpublished data only}
- UMN000001318. Randomized control trial of follow‐up schedule after curative resection for colorectal cancer. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000001417 (accessed 6 April 2019).
References to ongoing studies
COLOPEC {unpublished data only}
- NCT03413254. Second and third look laparoscopy in pT4 colon cancer patients for early detection of peritoneal metastases (COLOPEC‐II). clinicaltrials.gov/ct2/show/NCT03413254?term=follow‐up&type=Intr&cond=Colorectal+Cancer&draw=2&rank=11 (accessed 9 January 2019).
FURCA {published data only}
- Jakobsen IH, Juul T, Bernstein I, Christensen P, Jensen FS, Johansen C, et al. Follow‐up after rectal cancer: developing and testing a novel patient‐led follow‐up program. Study protocol. Acta Oncologica 2017;56(2):307. [DOI] [PubMed] [Google Scholar]
- NCT03622437. Individual follow‐up after rectal cancer ‐ focus on the needs of the patient (FURCA). clinicaltrials.gov/ct2/show/NCT03622437?id=NCT03622437&rank=1 (accessed 09 January 2019).
HIPEC {unpublished data only}
- NCT01628211. Second look laparoscopy in colorectal cancer (HIPEC) [Randomized phase 2 study comparing second look laparoscopy to standard follow up in patients with no radiologic evidence of disease at 6 months after complete resection of colorectal mucinous carcinoma]. clinicaltrials.gov/ct2/show/NCT01628211?term=NCT01628211&rank=1 (first received 20 August 2014).
ProphyloCHIP {unpublished data only}
- Goere D, Glehen O, Quenet F, Ducreux M, Guilloit J, Texier M, et al. Results of a randomized phase 3 study evaluating the potential benefit of a secondlook surgery plus HIPEC in patients at high risk of developing colorectal peritoneal metastases (PROPHYLOCHIP‐ NTC01226394). Journal of Clinical Oncology 2018;36(15S):3531. [Google Scholar]
- NCT01226394. Multicentric phase III trial comparing simple follow‐up to exploratory laparotomy plus "in principle" HIPEC (hyperthermic intraperitoneal chemotherapy) in colorectal patients initially treated with surgery and adjuvant chemotherapy who have a high risk of developing colorectal peritoneal carcinomatosis. clinicaltrials.gov/ct2/show/NCT01226394?id=NCT01226394&rank=1 (accessed 6 June 2018).
SCORE {published data only}
- ACTRN126170000004369p. Shared care of colorectal cancer survivors ‐ a randomised controlled trial of hospital‐based follow up versus shared hospital / community follow up for survivors of colorectal cancer. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=371925 (accessed 01 August 2018).
- Jefford M, Emery J, Grunfeld E, Martin A, Rodger P, Murray AM, et al. SCORE: shared care of colorectal cancer survivors: protocol for a randomised controlled trial. Trials 2017;18(1):506. [DOI] [PMC free article] [PubMed] [Google Scholar]
SURVEILLANCE {unpublished data only}
- Lepage C, Phelip J‐M, Cany L, Faroux R, Manfredi S, Ain J‐F. Effect of 5 years of imaging and CEA follow‐up to detect recurrence of colorectal cancer: the FFCD PRODIGE 13 randomised phase III trial. Digestive and Liver Disease 2015;47(7):529‐31. [DOI] [PubMed] [Google Scholar]
- Lepage C, Phelip JM, Cany L, Maillard E, Lievre A, Chatellier T, et al. Effect of 5 years of imaging and CEA follow‐up to detect recurrence of colorectal cancer‐PRODIGE 13 a FFCD and Unicancer phase III trial: baseline characteristics. Annals of Oncology 2016.
- NCT00995202. Follow‐up care with or without CEA assessments in patients who have undergone surgery for stage II or stage III colorectal cancer [Follow‐up of fully resected stage II or III colorectal cancer. Phase III multicentric prospective randomised study]. clinicaltrials.gov/ct2/show/NCT00995202?term=NCT00995202&rank=1 (first received 14 November 2012).
Additional references
Aaronson 1993
- Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute 1993;85:365‐76. [DOI] [PubMed] [Google Scholar]
Alberts 2005
- Alberts SR, Horvath WL, Sternfeld WC, Goldberg RM, Mahoney MR, Dakhil SR, et al. Oxaliplatin, fluorouracil, and leucovorin for patients with unresectable liver‐only metastases from colorectal cancer: a North Central Cancer Treatment Group phase II study. Journal of Clinical Oncology 2005;23(36):9243‐9. [DOI] [PubMed] [Google Scholar]
Altman 1992
- Altman DG. Practical statistics for medical research. London: Chapman and Hall, 1992. [Google Scholar]
Anderson 1996
- Anderson RT, Aaronson NK, Bullinger M, McBee WL. A review of the progress towards developing health‐related quality of life instruments for international clinical studies and outcomes research. PharmacoEconomics 1996;10(4):336‐55. [DOI] [PubMed] [Google Scholar]
Apolone 1998
- Apolone G, Mosconi P. The Italian SF‐36 Health Survey: translation, validation and norming. Journal of Clinical Epidemiology 1998;51(11):1025‐36. [DOI] [PubMed] [Google Scholar]
Araghizadeh 2001
- Araghizadeh FY, Timmcke AE, Opelka FG, Hicks TC, Beck DE. Colonoscopic perforations. Diseases of the Colon and Rectum 2001;44(5):713‐6. [DOI] [PubMed] [Google Scholar]
Audisio 1996
- Audisio RA, Setti‐Carraro P, Segala M, Capko D, Andreoni B, Tiberio G. Follow‐up in colorectal cancer patients: a cost‐benefit analysis. Annals of Surgical Oncology 1996;3(4):349‐57. [DOI] [PubMed] [Google Scholar]
Audisio 2000
- Audisio RA, Robertson C. Colorectal cancer follow‐up: perspectives for future studies. European Journal of Surgical Oncology 2000;26(4):329‐37. [DOI] [PubMed] [Google Scholar]
Augestad 2014
- Augestad KM, Rose J, Crawhsaw B, Cooper G, Delaney C. Do the benefits outweigh the side effects of colorectal cancer surveillance? A systematic review. World Journal of Gastrointestinal Oncology 2014;6(5):104‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baca 2011
- Baca B, Beart RW, Etzioni DA. Surveillance after colorectal cancer resection: a systematic review. Diseases of the Colon and Rectum 2011;54(8):1036‐48. [DOI] [PubMed] [Google Scholar]
Bowles 2004
- Bowles CJ, Leicester R, Romaya C, Swarbrick E, Williams CB, Epstein O. A prospective study of colonoscopy practice in the UK today: are we adequately prepared for national colorectal cancer screening tomorrow?. Gut 2004;53(2):277‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Böhm 1993
- Böhm B, Schwenk W, Hucke HP, Stock W. Does methodic long‐term follow‐up affect survival after curative resection of colorectal carcinoma?. Diseases of the Colon and Rectum 1993;36(3):280‐6. [DOI] [PubMed] [Google Scholar]
Cella 1993
- Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. Journal of Clinical Oncology 1993;11(3):570‐9. [DOI] [PubMed] [Google Scholar]
Choti 2002
- Choti MA, Sitzmann JV, Tiburi MF, Sumetchotimetha W, Rangsin R, Schulick RD, et al. Trends in long‐term survival following liver resection for hepatic colorectal metastases. Annals of Surgery 2002;235(6):759‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Collopy 1992
- Collopy BT. The follow‐up of patients after resection for large bowel cancer, May 1992. Colorectal Surgical Society of Australia. Medical Journal of Australia 1992;157(9):633‐4. [PubMed] [Google Scholar]
Connor 2001
- Connor S, Frizelle FA, Bagshaw PF. Follow‐up after attempted curative surgery for colorectal cancer; postal survey of New Zealand surgeons' practice. New Zealand Medical Journal 2001;114(1129):151‐3. [PubMed] [Google Scholar]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 23 October 2015. Melbourne, Australia: Veritas Health Innovation.
Custers 2014
- Custers J, Berg SW, Laarhoven HW, Beliker EM, Gielissen MF, Prins JB, et al. The Cancer Worry Scale: detecting fear of recurrence in breast cancer survivors. Cancer Nursing 2014;37(1):E44‐50. [DOI] [PubMed] [Google Scholar]
Deeks 2017
- Deeks JJ, Higgins JP, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
DevCan 2005 [Computer program]
- Statistical Research and Applications Branch National Cancer Institute. DevCan: probability of developing or dying of cancer software. Version 6.7.0. Statistical Research and Applications Branch National Cancer Institute, 2005.
Dolan 1997
- Dolan P. Modeling valuations for EuroQol health states. Medical Care 1997;35:1095‐108. [DOI] [PubMed] [Google Scholar]
Dupuy 1984
- Dupuy H. The Psychological General Well‐Being (PGWB) Index. In: Wenger N, Mattson M, Furberg D, Elinson J editor(s). Assessment of Quality of Life in Clinical Trials of Cardiovascular Therapies. New York: Le Jacq Publishing, 1984. [DOI] [PubMed] [Google Scholar]
Edelman 1997
- Edelman MJ, Meyers FJ, Siegel D. The utility of follow‐up testing after curative cancer therapy: a critical review and economic analysis. Journal of General Internal Medicine 1997;12(5):318‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Edge 2010
- Edge SB, Byrd SR, Compton CC. In: Edge SB, Byrd SR, Compton CC, et al. editor(s). AJCC Cancer Staging Manual. 7th Edition. New York: Springer Verlag, 2010. [Google Scholar]
Fleischer 1989
- Fleischer DE, Goldberg SB, Browning TH, Cooper JN, Friedman E, Goldner FH, et al. Detection and surveillance of colorectal cancer. JAMA 1989;261(4):580‐5. [PubMed] [Google Scholar]
Fossati 2015
- Fossati R. Colorectal cancer follow‐up review update overdue [personal communication]. Email to: GM Jeffery 4 May 2015.
Gandek 1998
- Gandek B, Ware JE, Aaronson NK, Apolone G, Bjorner JB, Brazier JE, et al. Cross‐validation of item selection and scoring for the SF‐12 Health Survey in nine countries: results form the IQOLA Project. International Quality of Life Assessment. Journal of Clinical Epidemiology 1998;51(11):1171‐8. [DOI] [PubMed] [Google Scholar]
Gerdes 1990
- Gerdes H. Surveillance after colon cancer: is it worthwhile?. Gastroenterology 1990;99(6):1849‐51. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version (accessed 07th August 2019). Hamilton (ON): McMaster University (developed by Evidence Prime).
Grossman 2004
- Grossmann EM, Johnson FE, Virgo KS, Longo WE, Fossati R. Follow‐up of colorectal cancer patients after resection with curative intent‐the GILDA trial. Surgical Oncology 2004;13(2‐3):119‐24. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA (editors). Chapter 8:Assessing risk of bias in included studies. In: Higgins JPT, Churchill R, Chandler J, Cumpston MS (editors), Cochrane Handbook for Systematic Reviews of Interventions version 5.2.0 (updated June 2017), Cochrane, 2017. Available from www.training.cochrane.org/handbook.
Higgins 2018
- Higgins JP, Lasserson T, Chandler J, Tovey D, Churchill R. Standards for the conduct and reporting of new Cochrane Intervention Reviews, reporting of protocols and the planning, conduct and reporting of updates. Methodological Expectations of Cochrane Intervention Reviews (MECIR). Available from community.cochrane.org/mecir‐manual 2018.
Hodgkinson 2007
- Hodgkinson K, Butow P, Hunt GE, Pendlebury S, Hobbs KM, Lo SK, et al. The development and evaluation of a measure to assess cancer survivor's unmet supportive care needs; the CaSUN (Cancer Survivors Unmet Needs Measure). Psycho‐oncology 2007;16:796‐804. [DOI] [PubMed] [Google Scholar]
Hunt 1980
- Hunt SM, McKenna SP, McEwen J, Backett EM, Williams J, Papp E. A quantitative approach to perceived health status: a validation study. Journal of Epidemiology and Community Health 1980;34(4):281‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kanas 2012
- Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, et al. Survival after liver resection in metastatic colorectal cancer: review and meta‐analysis of prognostic factors. Clinical Epidemiology 2012;4:283‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kievit 2000
- Kievit J. Colorectal cancer follow‐up: a reassessment of empirical evidence on effectiveness. European Journal of Surgical Oncology 2000;26(4):322‐8. [DOI] [PubMed] [Google Scholar]
Lee 2007
- Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, et al. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. International Journal of Colorectal Diseases 2007;22(6):699‐704. [DOI] [PubMed] [Google Scholar]
McArdle 2000
- McArdle C. ABC of colorectal cancer: effectiveness of follow‐up. BMJ 2000;321(7272):1332‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moertel 1993
- Moertel CG, Fleming TR, Macdonald JS, Haller DG, Laurie JA, Tangen C. An evaluation of the carcinoembryonic antigen (CEA) test for monitoring patients with resected colon cancer. JAMA 1993;270(8):943‐7. [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 6;7:e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mokhles 2016
- Mokhles S, Macbeth F, Farewell V, Fiorentino F, Williams NR, Younes RN, et al. Meta‐analysis of colorectal cancer follow‐up after potentially curative resection. British Journal of Surgery 2016;103(10):1259‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Muratore 2007
- Muratore A, Zorzi D, Bouzari H, Amisano M, Massucco P, Sperti E, et al. Asymptomatic colorectal cancer with un‐resectable liver metastases: immediate colorectal resection or up‐front systemic chemotherapy?. Annals of Surgical Oncology 2007;14(2):766‐70. [DOI] [PubMed] [Google Scholar]
Oellerich 2017
- Oellerich M, Schültz E, Beck J, Kanzow P, Plowman P, Weiss G, et al. Using circulating cell‐free DNA to monitor personalized cancer therapy. Critical Reviews in Clinical Laboratory Science 2017;54(3):205‐18. [DOI] [PubMed] [Google Scholar]
Ovaska 1989
- Ovaska JT, Järvinen HJ, Mecklin JP. The value of a follow‐up programme after radical surgery for colorectal carcinoma. Scandinavian Journal of Gastroenterology 1989;24(4):416‐22. [DOI] [PubMed] [Google Scholar]
Pawlik 2005
- Pawlik TM, Scoggins CR, Zorzi D, Abdalla EK, Andres A, Eng C, et al. Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Annals of Surgery 2005;241(5):715‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pfister 2004
- Pfister DG, Benson AB, Somerfield MR. Clinical practice. Surveillance strategies after curative treatment of colorectal cancer. New England Journal of Medicine 2004;350(23):2375‐82. [DOI] [PubMed] [Google Scholar]
Pita‐Fernández 2014
- Pita‐Fernández S, Alhayek‐Aí M, González‐Martin C, López‐Calviño B, Seoane‐Pillado T, Pértega‐Díaz S. Intensive follow‐up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta‐analysis. Annals of Oncology Advance Access 2014;00:1‐12. [DOI] [PubMed] [Google Scholar]
Rabenek 2008
- Rabeneck L, Paszat LF, Hilsden RJ, Saskin R, Leddin D, Grunfeld E, et al. Bleeding and perforation after outpatient colonoscopy and their risk factors in usual clinical practice. Gastroenterology 2008;135(6):1899‐1906. [DOI] [PubMed] [Google Scholar]
Renehan 2002
- Renehan A, Egger M, Saunders MP, O'Dwyer ST. Impact on survival of intensive follow‐up after curative resection for colorectal cancer: systematic review and meta‐analysis of randomised trials. BMJ 2002;324:813. [DOI] [PMC free article] [PubMed] [Google Scholar]
Renehan 2004
- Renehan AG, O'Dwyer ST, Whynes DK. Cost effectiveness analysis of intensive versus conventional follow up after curative resection for colorectal cancer. BMJ 2004;328(7431):81‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rojas 2005
- Rojas MP, Telaro E, Russo A, Fossati R, Palli D, Rosselli Del Turco M, et al. Follow‐up strategies for women treated for early breast cancer. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD001768.pub2] [DOI] [PubMed] [Google Scholar]
Ryuk 2014
- Ryuk JP, Choi GS, Park JS, Kim HJ, Park SY, Yoon GS, et al. Predictive factors and the prognosis of recurrence of colorectal cancer within 2 years after curative resection. Annals of Surgical Treatment and Research 2014;86(3):143‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sadahiro 2003
- Sadahiro S, Suzuki T, Ishikawa K, Nakamura T, Tanaka Y, Masuda T, et al. Recurrence patterns after curative resection of colorectal cancer in patients followed for a minimum of ten years. Hepatogastroenterology 2003;50(53):1362‐6. [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Spinhoven 1997
- Spinhoven P, Ormel J, Sloekers PP, Kempen GI, Speckens E, Hemert M, et al. A validation study of the Hospital Anxiety and Depression Scale (HADS) in different groups of Dutch subjects. Psychological Medicine 1997;27(2):363‐70. [DOI] [PubMed] [Google Scholar]
Sprangers 1993
- Sprangers MA, Cull A, Bjordal K, Groenvold M, Aaronson NK. The European Organization for Research and Treatment of Cancer. Approach to quality of life assessment: guidelines for developing questionnaire modules. EORTC Study Group on Quality of Life. Quality of Life Research 1993;2(4):287‐95. [DOI] [PubMed] [Google Scholar]
Stiggelbout 1997
- Stiggelbout AM, Haes JC, Vree R, Velde CJ, Bruijninckx CM, Groningen K, et al. Follow‐up of colorectal cancer patients: quality of life and attitudes towards follow‐up. British Journal of Cancer 1997;75(6):914‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sugarbaker 1987
- Sugarbaker PH, Gianola FJ, Dwyer A, Neuman NR. A simplified plan for follow‐up of patients with colon and rectal cancer supported by prospective studies of laboratory and radiologic test results. Surgery 1987;102(1):79‐87. [PubMed] [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007; Vol. 8:16. [DOI] [PMC free article] [PubMed]
Tjandra 2007
- Tjandra JJ, Chan MK. Follow‐up after curative resection of colorectal cancer: a meta‐analysis. Diseases of the Colon and Rectum 2007;50(11):1783‐99. [DOI] [PubMed] [Google Scholar]
Van Cutsem 2006
- Cutsem EM, Nordlinger B, Adam R, Köhne CH, Pozzo C, Poston G, et al. European Colorectal Metastases Treatment Group. Towards a pan‐European consensus on the treatment of patients with colorectal liver metastases. European Journal of Cancer 2006;42(14):2212‐21. [DOI] [PubMed] [Google Scholar]
Vernava 1994
- Vernava AM, Longo WE, Virgo KS, Coplin MA, Wade TP, Johnson FE. Current follow‐up strategies after resection of colon cancer. Results of a survey of members of the American Society of Colon and Rectal Surgeons. Diseases of the Colon and Rectum 1994;37(6):573‐83. [DOI] [PubMed] [Google Scholar]
Virgo 1995
- Virgo KS, Vernava AM, Longo WE, McKirgan LW, Johnson FE. Cost of patient follow‐up after potentially curative colorectal cancer treatment. JAMA 1995;273(23):1837‐41. [PubMed] [Google Scholar]
Walker 1988
- Walker AM, Martin‐Moreno JM, Artalejo FR. Odd man out: a graphical approach to meta‐analysis. American Journal of Public Health 1988;78(8):961‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ware 1993
- Ware J, Snoww KK, Kosinski MA, Gandek BG. SF36 Health Survey: Manual and Interpretation Guide. 165‐6. Vol. 30, Lincoln: Quality Metric, Inc, 1993. [Google Scholar]
Ware 1995
- Ware J, Kosinski M, Keller S. SF‐12: How to Score the SF‐12 Physical and Mental Health Summary Scales. 2nd Edition. Boston Massachusetts: The Health Institute, New England Medical Centre, 1995. [Google Scholar]
Whistance 2009
- Whistance RN, Conroy T, Chie W, Costantini A, Sezer O, Koller M, et al. European Organisation for the Research and Treatment of Cancer Quality of Life Group. Clinical and psychometric validation of the EORTC QLQ‐CR29 questionnaire module to assess health‐related quality of life in patients with colorectal cancer. European Journal of Cancer 2009;45(17):3017‐26. [DOI: 10.1016/j.ejca.2009.08.014.] [DOI] [PubMed] [Google Scholar]
Yoo 2006
- Yoo PS, Lopez‐Soler RI, Longo WE, Cha CH. Liver resection for metastatic colorectal cancer in the age of neoadjuvant chemotherapy and bevacizumab. Clinical Colorectal Cancer 2006;6(3):202‐7. [DOI] [PubMed] [Google Scholar]
Zabora 2001
- Zabora J, BrintzenhofeSzoc K, Jacobsen P, Curbow B, Piantadosi S, Hooker C, et al. A new psychosocial screening instrument for use with cancer patients. Psychosomatics 2001;42:241‐6. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jeffery 2007
- Jeffery M, Hickey BE, Hider PN. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD002200.pub2] [DOI] [PubMed] [Google Scholar]
Jeffery 2016
- Jeffery M, Hickey BE, Hider PN, See AM. Follow‐up strategies for patients treated for non‐metastatic colorectal cancer. Cochrane Database of Systematic Reviews 2016, Issue 11. [DOI: 10.1002/14651858.CD002200.pub3] [DOI] [PubMed] [Google Scholar]


