SUMMARY
Aggregation of damaged or misfolded proteins is a protective mechanism against proteotoxic stress, abnormalities of which underlie many aging-related diseases. Here, we show that in asymmetrically dividing yeast cells, aggregation of cytosolic mis-folded proteins does not occur spontaneously but requires new polypeptide synthesis and is restricted to the surface of ER, which harbors the majority of active translation sites. Protein aggregates formed on ER are frequently also associated with or are later captured by mitochondria, greatly constraining aggregate mobility. During mitosis, aggregates are tethered to well-anchored maternal mitochondria, whereas mitochondria acquired by the bud are largely free of aggregates. Disruption of aggregate-mitochondria association resulted in increased mobility and leakage of mother-accumulated aggregates into the bud. Cells with advanced replicative age exhibit gradual decline of aggregates-mitochondria association, likely contributing to their diminished ability to rejuvenate through asymmetric cell division.
INTRODUCTION
Formation and intercellular spreading of protein aggregates underlie several aging-related and amyloid diseases (Chiti and Dobson, 2006). Recent data have led to the general view that protein aggregation is a protective mechanism against disrupted protein homeostasis in the presence of acute or chronic stress (Arrasate et al., 2004; Tyedmers et al., 2010). Because misfolded proteins could interfere with normal biochemical processes or promote denaturation of otherwise properly folded proteins (Olzscha et al., 2011), aggregation of midfolded proteins and their sequestration into well-confined structures, such as the aggresome, potentially limit the harmful intracellular effect and prevent further spreading through cell division (Rujano et al., 2006; Tyedmers et al., 2010). In a crowed cytosolic environment, proteins with limited structural stability, especially newly synthesized polypeptides or mutated proteins, may be in a dynamic equilibrium between the folded and misfolded states (Hartl et al., 2011). Misfolded proteins could oligomerize spontaneously once reaching a concentration threshold due to stress (e.g., heat, oxidizing conditions) or impaired proteasome activity, which could grow into visible aggregates (Hartl et al., 2011). On the other hand, the protective role of protein aggregation and the recently revealed involvement of specific factors raise the possibility that aggregate formation may be a highly regulated process that requires energy input and occurs preferentially at specific cellular location (Escusa-Toret et al., 2013; Kawaguchi et al., 2003; Malinovska et al., 2012; Specht et al., 2011).
In the budding yeast Saccharomyces cerevisiae, accumulation of damaged proteins and formation of aggregates naturally accompany the process of replicative aging, during which protein aggregates are distributed asymmetrically between the mother cell and the daughter cell (bud) of each division (Liu et al., 2010; Steinkraus et al., 2008; Zhou et al., 2011). Aggregate retention by the mother cell frees the bud of the accumulated damaged products and may contribute to the latter’s fully renewed replicative potential (Steinkraus et al., 2008). Protein aggregation can also be induced acutely under a variety of stress conditions or by expression of poorly folded mutant or nonnative proteins. When proteasome activity is inhibited, aggregated proteins were shown to be deposited into two compartments: the juxtanuclear quality-control compartment (JUNQ) that recruits ubiquitinated-proteins and insoluble protein deposit (IPOD) (Kaganovich et al., 2008). A recent study using the model aggregate-forming protein Ubc9ts reported that JUNQ may be the fusion product of small inclusions termed Q bodies located at the surface of the ER during proteasome inhibition (Escusa-Toret et al., 2013). However, it remains unclear whether these structures represent sites of protein aggregation or later compartmentalization of spontaneously formed aggregates.
Experimentally induced protein aggregates in yeast also segregate asymmetrically and exhibit dynamic properties similar to natural aggregates accumulated during aging (Liu et al., 2010; Spokoini et al., 2012; Zhou et al., 2011). An earlier study of heat-induced aggregates led to a model of retrograde transport along actin cables that actively clears aggregates in the bud into the mother side (Liu et al., 2010). However, a later study found that the motion of aggregates does not represent active transport but is best characterized as random diffusion with confinement (Zhou et al., 2011). The constrained aggregate diffusion, together with the geometry of the dividing yeast cell, was predicted to be sufficient for asymmetric retention of damaged proteins. In this study, by tracking the fate of damaged proteins, we show that these proteins rely on new polypeptide synthesis to initiate aggregation. Sites of protein aggregation are predominantly located on the surface of ER, enriched in regions in close contact with or later captured by mitochondria. Such organelle association underlies a mechanism of quality control, ensuring asymmetric segregation of proteome damage.
RESULTS
Unstable Cytosolic Proteins Do Not Aggregate Spontaneously in the Cytosol during Stress
In order to gain general insights into the process of protein aggregation, we employed several different methods to induce protein aggregation in yeast, including heat shock (HS, at 42°C), treatment of cells with oxidizing agent H2O2, or pulse-feeding cells with azetidine-2-carboxylic acid (A2C), a proline analog that interferes with protein folding after incorporation into newly translated polypeptides (Liu et al., 2013). These conditions produce proteome-wide aggregation of damaged or misfolded proteins, visualized with green fluorescent protein (GFP)-tagged Hsp104, which binds protein aggregates nonspecifically (Glover and Lindquist, 1998). Hsp104-GFP, produced from the genomic locus possesses wild-type chaperone activity (Zhou et al., 2011) (Figure S1A available online). We also induced substrate-specific aggregates by expressing mCh-Ubc9ts and GFP-Ubc9ts, respectively, representing mCherry- and GFP-tagged Ubc9ts, or GFP-tagged luciferase, a thermally labile protein that aggregate at 42°C (Kaganovich et al., 2008; Winkler et al., 2012).
We first asked whether thermal labile proteins aggregate spontaneously in the cytosol or at certain predefined sites by performing a sequential aggregate induction experiment (Figure 1A). Expression of mCh-Ubc9ts was induced with Gal1 promoter prior to aggregate induction by growing cells in galatose-containing media for 2 hr, followed by promoter shutoff. Newly translated polypeptides are particularly prone to aggregation (Hartl et al., 2011; Medicherla and Goldberg, 2008), which can be exacerbated by treating cells transiently (10 min) with A2C (Liu et al., 2013). Numerous aggregates labeled with Hsp104-GFP emerged upon A2C treatment. After A2C washout, we induced aggregation of cytosolic mCh-Ubc9ts by HS and found that aggregates of mCh-Ubc9ts colocalized 100% with Hsp104-GFP-labeled aggregates induced by A2C (Figure 1A). A similar result was observed when H2O2 was used to induce mCh-Ubc9ts aggregates after A2C treatment (Figure S1B). Time-lapse movies showed that during HS, mCh-Ubc9ts only accumulated to preformed aggregates induced with A2C, without forming new aggregates in the cytosol, and concomitantly cytosolic mCh-Ubc9ts fluorescence decreased (Figure 1B; Movie S1). To confirm that the aggregated mCh-Ubc9ts came from the pre-existing cytosolic pool, mCherry fluorescence was photobeached prior to HS, and no visible mCh-Ubc9ts aggregates formed (Figure 1C). In addition, when mCh-Ubc9ts aggregates were induced by HS only (without the prior A2C treatment), mCh-Ubc9ts aggregation, which appeared at around 6 min of the HS at 42°C, also resided in Hsp104-GFP-labeled aggregates that emerged before accumulating red fluorescence (Figure 1D).
Figure 1. Coaggregation of Cytosolic Misfolded Proteins with Newly Synthesized Polypeptides.
(A) Sequential protein aggregation experiment in which cells expressing Hsp104-GFP from its endogenous promoter and mCh-Ubc9ts (briefly induced with Gal1 promoter) were treated for 10 min with A2C. After A2C washout, HS or H2O2 treatment was applied to induce aggregation of mCh-Ubc9ts. Top: experimental scheme and two possible outcomes; bottom: representative cell images from HS experiment.
(B) Left: selected frames from a representative movie showing recruitment of cytosolic mCh-Ubc9ts to pre-existing A2C-induced aggregates (labeled with Hsp104-GFP) during HS. Right: quantification showing gradual depletion of cytosolic mCh-Ubc9ts, while aggregate-associated mCh-Ubc9ts signal increased.
(C) Selected frames from the fluorescence recovery after photobleaching experiment in which A2C-treated cells with mCh-Ubc9ts were photobleached with a 561nm laser before being subjected to HS. Top: experimental scheme with two possible outcomes. Bottom: frames from a representative movie showing no accumulation of mCh-Ubc9ts fluorescence into A2C-induced Hsp104-GFP-containing aggregates upon HS.
(D) Delayed mCh-Ubc9ts aggregation during heat shock. The culture of same strain as in (A) was heat shocked for indicated time at 42°C, and representative cells are shown.
(E) Two possible pathways leading to recruitment of mCh-Ubc9ts to pre-existing aggregates. Possibility 1: misfolded GFP-Ubc9ts monomers directly add to existing aggregates. Possibility 2: misfolded GFP-Ubc9ts monomers first spontaneously form oligomers and then merge with existing aggregates.
(F) Molecular brightness of diffusing GFP-Ubc9ts or GFP-luciferase species as a function of time during 42°C HS, as determined by FCS. Plots shown mean and SEM from >20 FCS runs from at least nine cells per time point for GFP-Ubc9ts or GFP-luciferase and >9 FCS runs from >3 cells for each time point for 1x and 2xGFP, used as controls for monomer or dimer brightness. Scale bar for all panels, 4 μm. Time stamps in (B) and (C) refer to time after temperature shift. See also Figure S1 and Movie S1.
To distinguish the possibility that misfolded Ubc9ts first formed invisible soluble oligomers before their incorporation into visible aggregates from that misfolded Ubc9ts monomers are directly incorporated into pre-existing aggregates (Figure 1E), we used live-cell fluorescence correlation spectroscopy (FCS) (Slaughter and Li, 2010) to measure the molecular brightness and thus the oligomeric state of diffusing GFP-Ubc9ts species during HS. This analysis showed that the diffusing GFP-Ubc9ts species remained monomeric throughout HS, whereas visible aggregates grew (Figures 1F and S1C), supporting the scenario that misfolded Ubc9ts monomers do not accumulate as oligomers prior to partitioning into existing aggregates. The FCS experiment was also performed on the other model substrate, GFP-luciferase, or in the experiment with A2C pretreatment as in Figure 1B, yielding the same results (Figures 1F, S1D, and S1E).
Active Translation Is Required for Protein Aggregation
Next, we performed an experiment in which the order of aggregate inductions was reversed from that in Figure 1A (Figure 2A). Surprisingly, A2C treatment not only increased the intensity of pre-existing aggregates but also induced many new aggregates outside the preformed aggregates (Figure 2A; Movie S2). This raised a possibility that newly synthesized polypeptides initiate aggregate formation. To test this further, we treated cells with cycloheximide (CHX), a translation inhibitor, before induction of protein aggregation. CHX treatment inhibited aggregate formation under all conditions examined (Figures 2B, 2C, and S1F). CHX did not affect aggregate formation in a CHX-resistant strain (Kawai et al., 1992) (Figure 2B). Anisomycin, a translation inhibitor structurally different from CHX (Pestka et al., 1972), prt1ts, a temperature-sensitive mutation disrupting translation initiation (Li et al., 2011), and glucose depletion, which is known to arrest translation (Ashe et al., 2000), also inhibited heat-induced protein aggregation (Figure S1G). These experiments, however, did not rule out that conditions that inhibit translation (e.g., CHX treatment) may also help “free up” the capacity of chaperone and proteasome systems to prevent the aggregation of native proteins under stress. We therefore blocked chaperone activity by depleting ATP during CHX treatment and HS. The combined treatment with 2-deoxy-d-glucose (2-DG) and antimycin cause over 95% (Serrano, 1977) reduction of cellular ATP by blocking both glycolysis and mitochondrial respiration (Figure S1I). However, CHX still fully prevented HS-induced aggregation in ATP-depleted cells (Figure S1H), suggesting that CHX suppression of protein aggregation was unlikely to be due to elevated chaperone activity but more likely to be due to a requirement for newly synthesized polypeptides in aggregate initiation.
Figure 2. Active Translation Is Required for Stress-Induced Protein Aggregation.
(A) Sequential protein aggregation experiment in which cells of the same strain as in Figure 1A were first heat shocked at 42°C to induce mCh-Ubc9ts aggregates, followed by A2C treatment. Left: schematic and two possible outcomes. Right: selected frames from a time-lapse movie of A2C-induced aggregation. Arrowheads follow new Hsp104-GFP-labeled aggregates outside of existing aggregates.
(B) Cycloheximide blocks protein aggregation induced by HS. Left: examples of cells with different treatment as indicated. Right: quantification of aggregate amounts from different treatments. WT, wild-type; CHXr, cycloheximide-resistant strain (Δrpl42a rpl42bP56Q); CHX, cycloheximide; DMSO, solvent control. Histogram shows the average (3–4 experiments, >80 cells per experiment) amount of aggregates per cell normalized to the DMSO control (set at 100%). Error bars show SEM. ***p < 0.001, **p < 0.01, *p < 0.05.
(C) Aggregates induced by indicated stress conditions in the presence or absence of CHX. Left, representative images of cells. Right, quantification of aggregate amount as in (B). All scale bars, 2 μm.
ER and Mitochondria Are Main Organelle Hosts for Stress-Induced Protein Aggregation
Previous fractionation studies in mammalian cell lines revealed that a large fraction of mRNAs are translated at ER-bound ribosomes (Reid and Nicchitta, 2012). If this is also true in budding yeast, ER could be the main organelle host for protein aggregation under stress. We found, by adapting a puromycin-based assay (David et al., 2012) (Figures S2A and S2B), that ~75% of active translation sites are associated with ER surface (Figure S2C). By using both confocal microscopy and structured illumination microscopy (SIM) with 3D reconstruction, we observed that the vast majority of HS-induced protein aggregates were associated with ER (Figures 3A and S2D; Movie S3C). Under electron microscopy (EM), aggregates formed from HS appeared as electron-dense and amorphous bodies (Figures S2E–S2G), and the association with ER membrane can be observed (Figures 3B and S2G). 3D time-lapse movies showed that ~90% of aggregates formed on the surface of ER (Figure 3C; Movie S3A). This association remained at ~90%, much higher than 42% expected for random association simulated using measured aggregate numbers and ER surface area in SIM images, even though aggregates underwent considerable movement with or along ER (Movie S3B). The association of protein aggregation sites with ER was also observed for A2C or H2O2-induced ones (Figure S2D).
Figure 3. ER and Mitochondria Are Main Organelle Hosts for Protein Aggregation.
(A) Representative SIM images showing association of protein aggregates (Hsp104-GFP) with ER (labeled with mCherry-tagged transmembrane domain of the ER protein Scs2) induced with 6 min HS at 42°C.
(B) Thin-sectioning EM showing association of protein aggregates (magenta) with ER (blue) and mitochondria (yellow).
(C) Fast confocal time-lapse imaging of HS-induced aggregate emergence (Hsp104-GFP) on the surface of ER (DsRed-HDEL). Arrows follow emerging aggregates and the time stamps indicate the time since start of the movie.
(D) Representative pseudocolored three-channel confocal images showing simultaneous association of HS-induced aggregates (Hsp104-GFP) with ER (DsRed-HDEL) and mitochondria (mito-BFP). Images were taken after 6 min HS at 42°C, followed by 10 min recovery at 30°C.
(E) Percentage of aggregates with illustrated spatial relationship with ER and mitochondria quantified from tricolor confocal microscopy after 6 min of HS and 10 min recovery. Histogram shows mean and SEM from four experiments of >100 aggregates examined per experiment.
(F) Left: thin-sectioning EM showing association of protein aggregates (magenta) with ER (blue) and mitochondria (yellow) near organelle contact sites. Right: a plane from of 3D reconstruction of serial sections. Scale bars for (B) and (F), 100 nm. Scale bars for all other images, 4 μm.
Interestingly, protein aggregates formed under all stress conditions examined also demonstrated extensive association with mitochondria (Figures 3B, 4A, and 4B; Figures S3A and S3F; Movie S4C). Time-lapse movies revealed that 60%–70% of aggregates initially formed on the surface of and remained continuously associated with mitochondria (Figure 4C; Movie S4A). Furthermore, many aggregates that were not initially associated were later captured by mitochondria once stress was removed, such that eventually >85% aggregates were attached to mitochondria (Figure S3G; Movies S4B and S5A). Aggregates did not show prominent association with the rest of the organelles examined (Figures S3B–S3E). Importantly, Ubc9ts aggregates formed under the previously described conditions for IPOD and JUNQ were also found to be associated with ER and mitochondria (Figure S3I).
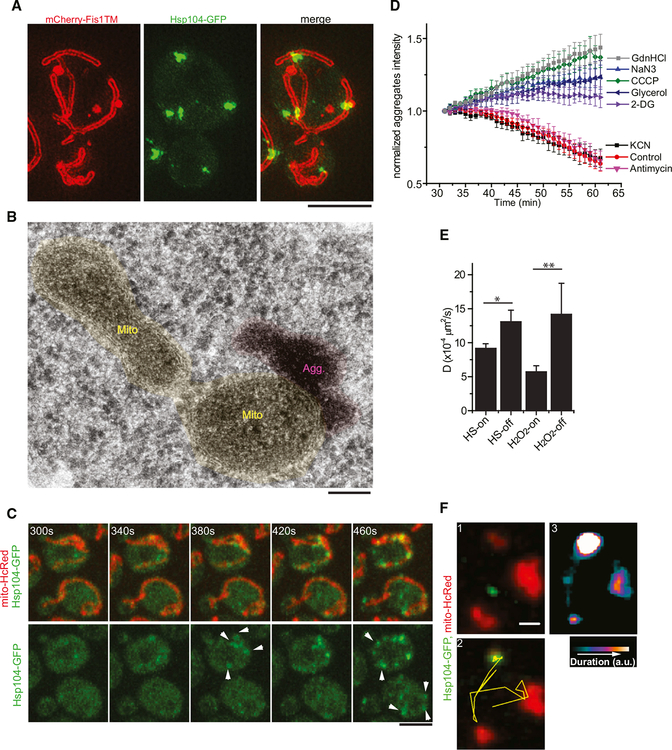
Figure 4. Mitochondria Play Key Roles in the Dissolution and Dynamics of Aggregates.
(A) Representative SIM images showing association of HS aggregates (Hsp104-GFP) with mitochondria (labeled with the mitochondria outer membrane marker mCh-Fis1TM). Scale bar, 4 μm.
(B) Thin-sectioning EM images showing association of protein aggregates (magenta) with mitochondria (yellow). Scale bar, 100 nm.
(C) Fast confocal time-lapse images of HS-induced aggregates emerging from the surface of mitochondria. Arrowheads follow newly formed aggregates, and the time stamps indicate the time since the start of the movie. Scale bar, 4 μm.
(D) Quantification of aggregate dissolution kinetics after HS under indicated conditions. Plots are normalized Hsp104-GFP-labeled aggregate intensity as a function of time from >3 movies of a field of cells (50–100 cells per field) starting from the 30 min frame when aggregates in the control no longer grew in brightness. GdnHCl, guanidine hydrochloride; NaN3, sodium azide; CCCP, carbonyl cyanide m-chlorophenyl hydrazine; 2-DG, 2-deoxy-d-glucose; KCN, potassium cyanide.
(E) Diffusion coefficients of aggregates between two different pools of aggregates induced by heat shock or H2O2. HS-on or H2O2-on, mitochondria-associated aggregates; HS-off or H2O2-off, aggregates not associated with mitochondria. Aggregates (>500) from two movies/strain were tracked.
(F) Two frames near the beginning (1) and end (2) of a time-lapse movie tracking the motion of a H2O2-induced aggregate (green) relative to mitochondria (red).(2) has the aggregate trajectory (yellow line) superimposed. Heatmap in (3) shows duration of aggregate staying at a given location. Scale bar, 1 μm.
ER and mitochondria are known to have extensive areas of contact. Quantification with tricolor time-lapse confocal microscopy showed that 58% ± 3% (mean ± SEM, three experiments, >20 cells imaged per experiment) of aggregates emerged initially from regions in which ER and mitochondria were juxtaposed, and this fraction increased to nearly 80% of all aggregates because of capture by mitochondria (Figures 3D and 3E). The association of aggregates at or near these organelle contact sites was verified with SIM (Figure S2H), as well as with serial sectioning EM with 3D reconstruction (Figure 3F; Movie S3D). However, only a small portion of aggregates showed colocalization with Mdm34 (Figure S2I), a component of the well-studied ER-mitochondria encounter structures (ERMES) (Kornmann et al., 2009), possibly because a considerable portion of ER-mitochondrial contact regions are not marked by ERMES components (Kornmann, 2013; Nguyen et al., 2012; Youngman et al., 2004).
Influence of Mitochondria on Aggregate Dynamics
Mitochondria might provide the ATP for the dissolution of protein aggregates. However, electron transport chain poisons, such as cyanide and antimycin, did not affect the kinetics of aggregate dissolution (Figure 4D). By contrast, like GdnHCl, which inhibits Hsp104 (Kummer et al., 2013), 2-DG or replacing glucose with glycerol after HS, which prevents cytosolic ATP production, significantly delayed aggregate dissolution, suggesting that glycolysis is the main source of ATP for aggregate dissolution. Interestingly, agents that disrupt mitochondrial membrane potential, such as azide or carbonyl cyanide m-chlorophenyl hydrazine (CCCP), did not by themselves induce protein aggregation (data not shown) but strongly blocked aggregate dissolution (Figure 4D).
A second effect of mitochondria on aggregates is constraining the latter’s mobility. As shown in previous work, aggregates were visibly motile after a short period of recovery (Liu et al., 2010; Zhou et al., 2011). Those aggregates not initially associated with mitochondria exhibited dampened mobility immediately upon capture by mitochondria (Movie S5A). The average diffusion coefficient was significantly lower for aggregates attached to mitochondria compared to those that were not (Figure 4E). Once attached, the aggregates’ motion resembled mostly that of their associated mitochondria. This is most readily observed in H2O2-treated cells, where mitochondria became mostly immobile. A typical example is shown in Figure 4F (Movie S5B), where an aggregate exhibited rapid movement until it eventually formed stable attachment to mitochondria and became nearly immobile.
Mitochondria Control Aggregate Asymmetric Segregation
Previous studies showed that aggregates are largely retained by the mother during budding and cell division (Liu et al., 2010; Zhou et al., 2011), but it was also known that mitochondria are trafficked into the bud via actin cables and myosin-V (Altmann et al., 2008; Boldogh et al., 2004). If mitochondrial movement accounts for the motility of associated aggregates, why do aggregates not passively follow mitochondria into the bud? To address this question, we performed two-color time-lapse imaging of mitochondria and HS aggregates in cells undergoing budding and mitochondria inheritance. Remarkably, the majority of aggregates present in the mother did not follow the mitochondria that entered the bud, but they either underwent dissolution/degradation or remained associated with the mitochondria that were kept in the mother cell and only exhibited confined local mobility (Figure 5A; Movies S6 and S8). As such, the bud-inherited mitochondria were largely devoid of aggregates. Aggregates that occasionally entered the bud were usually already at the bud neck region and followed the movement of bud-bound mitochondria (Movie S10; Figure 6G). This pattern of motility was also observed for foci of Dmn1 and Mdm34 (Figures 5B and 5C; Movies S7A and S7B), consistent with previously observed stability of ERMES complex (Nguyen et al., 2012). A similar inheritance pattern was also observed for mitochondrial nucleoids (Figure 5D; Movie S7C). Note that nucleoids in the mother were overall less anchored than Dmn1 and Mdm34 foci, but the ones entering the bud also often originated from the bud neck region. These observations suggest that some structural assemblies of mitochondria, including the associated protein aggregates, are segregated conservatively during organelle inheritance during yeast asymmetric cell division.
Figure 5. Mitochondria Confine Protein Aggregates during Asymmetric Segregation.
(A) Left: montage of a representative movie showing that mitochondria inheritance does not result in movement of mitochondria-associated aggregates into the bud. Right: trajectories of aggregates (all colors, except red lines) and mitochondrial tips (red lines) from the same movie. Blue and red dots associated with the more extended tracks represent the beginning and end of these tracks, respectively. Time stamps indicate the time since the start of the movie. This representation applies also to (B)–(D). See also Movie S6.
(B) Left: montage of a representative movie showing that Dnm1-GFP foci in the mother remain largely stationary as mitochondria extended into the bud. Right: trajectories of Dnm1-GFP foci (black and blue lines) and mitochondrial tips (red lines) from the same movie. Blue arrowhead follows an aggregate moving with mitochondria into the bud; white arrowhead points to one staying in the mother. See also Movie S7A.
(C) Left: montage of a representative movie showing the extension of mitochondria into bud (blue arrowhead) and the relative immobility of Mdm34-GFP foci in mother cell. Right: trajectories of Mdm34 foci and mitochondrial tip from the same movie. See also Movie S7B.
(D) Left: montage of a representative movie showing the restrained motion of mitochondrial nucleoids labeled with Abf2-GFP within mother, contrasting extension of mitochondrial tip into bud. The two white arrowheads follow the front most (near bud neck) maternal nucleoids. Blue arrow-heads follow a part of Abf2 foci that split and move into bud with mitochondria. Right: trajectories of nucleoids (all colors, except red lines) and mito-chondrial tip (red lines) from the same movie. See also Movie S7C. All scale bars, 4 μm.
Figure 6. Association with Mitochondria Contributes to Mother Retention of Aggregates.
(A) Representative images and quantification of aggregates-mitochondria colocalization in Δfis1, Δdnm1, and Δmdv1 cells. Δfis1SRD and WTSRD: colocalization from random distribution (SRD) simulated using parameters measured from respective cells. Histogram shows mean and SEM from 3–4 experiments with >150 aggregates per experiment. Scale bars, 2 mm. Statistical representation as in Figure 2.
(B) Average diffusion coefficients of all aggregates in WT and Δfis1 mutant. Histogram shows mean and SEM of diffusion coefficient calculated from >100 aggregate trajectories per strain.
(C) Diffusion coefficients of aggregates associated (Δfis1-on) or not associated with mitochondria (Δfis1-off) in Δfis1 cells using same analysis as in Figure 4E. Histogram shows mean and SEM from >3 movies with >50 budding cells per movie examined. See also Movie S9.
(D) Percentage of cells showing at least one MTB aggregate leakage among all budding cells observed in time-lapse movies. Histograms show mean and SEM from at least three movies with >50 budding cells per movie.
(E and F) Quantification of aggregate number per cell (E) and size (average intensity of aggregates) (F) in Δfis1 and WT. Histograms show mean and SEM from at least three movies with >50 budding cells per movie.
(G) Montage showing how majority of aggregate leakage occurred in WT and Δfis1 cells. In WT cells (top), aggregates that leaked into the bud were usually associated with bud-bound mitochondrial extension; in Δfis1cells, the majority of leaked aggregates were not associated with bud-bound mitochondrial extension. Arrowheads point to example MTB aggregates. Scale bars, 2 μm. See also Movie S10.
(H) Percentage of MTB-leaking aggregates that were not associated with bud-bound mitochondrial extension among all MTB-leaking aggregates in wild-type and Δfis1. Histogram shows mean and SEM from at least three movies with ~30 MTB leakage events per movie observed.
To further test the idea that mitochondria tethering plays an important role in the asymmetric segregation of protein aggregates, we sought for conditions that could disrupt this association. As mitochondria are known to align with actin cables (Fehrenbacher et al., 2004), we first tested whether treatment with latrunculin A (LatA), an actin inhibitor, would disrupt mitochondria-aggregate association. Although LatA treatment grossly disrupted mitochondrial distribution and morphology, protein aggregates remained bound at a high frequency (Figure S3H). We next screened a subset (72) of the yeast nonessential open reading frame deletion library, encoding mitochondrial outer membrane proteins, and found several mutants showing reduced aggregate-mitochondria association (Figure S4A). The strongest defect was displayed by Δfis1, in which only 57% aggregates colocalized with mitochondria, compared to over 85% for the wild-type (WT) and 45% for simulated random distribution (Figure 6A). The association of aggregates with ER was not affected in Δfis1 (Figure S4B). Fis1 is an evolutionarily conserved mitochondrial protein known for its role in yeast mitochondria fission by forming a complex with Mdv1 and Dnm1 (Detmer and Chan, 2007). Both Δmdv1 and Δdnm1 showed a less severe, though statistically significant, defect in aggregate-mitochondria association (Figure 6A). However, colocalization was rarely observed between protein aggregates and Dnm1 foci or sites of mitochondrial fission (Figures S4C and S4D; Movie S8). Thus, Fis1 may have a function in aggregate-mitochondria association distinct from its role in mitochondrial fission.
HS-induced protein aggregates in Δfis1 cells were overall more mobile than those in WT cells (Figure 6B). As in WT cells, the mitochondria-free aggregates moved significantly faster than those associated with mitochondria (Figures 4E and 6C; Movie S9). Accompanying the increased mobility of aggregates, more Δfis1 cells exhibited mother-to-bud (MTB) leakage of at least one aggregate during bud formation, and this defect was not associated with increased aggregate number or size in the mutant (Figures 6D–6F). We further classified the MTB-leaking aggregates according to their mitochondrial association in cells with dual labels. In WT cells, the majority of the mitochondrial-associated aggregates stayed in the mother cell, while a small fraction of them (usually near the bud neck) entered the bud with mitochondria (Movie S10) and represented 57% of MTB events (Figure 6H). Aggregates not associated with mitochondria (~15% of total aggregates; Figure 6A) accounted for 43% of MTB leakage, suggesting that free aggregates are much more likely to leak into the bud than were mitochondria-associated ones. In Δfis1 cells, free aggregates represented the majority (73%) of MTB events (Figure 6H; Movie S10), correlating with the defect in mitochondria-aggregates association.
Gradual Loss of Mitochondria-Aggregate Attachments during Replicative Aging
Because mitochondrial association is required for efficient retention of aggregates in young cells, we examined how this quality control mechanism is affected in old cells with replicative age of 7–10 or 15–17 generations sorted as described (Lindstrom and Gottschling, 2009). Hsp104-GFP-bound aggregates that occurred naturally during the aging process were rare in young cells but could be observed in aged cells. The percentage of aggregates associated with mitochondria showed progressive reduction with increased replicative age (Figures 7A and 7B). This reduced mitochondrial association was not predicted by simulations of random distribution in aged cells. In the 15–17 generation population examined, the observed frequency of aggregate-mitochondria association was reduced to a level close to simulated random distribution (Figure 7B). The association of heat-induced aggregates with mitochondria showed similar age-dependent decline (Figures 7B and S4E).
Figure 7. Aggregate-Mitochondria Interaction in Aging Cells and Summary Model.
(A) Example images of natural (left panels) or HS-induced (right panels) aggregates, labeled with Hsp104-GFP, in relationship to mitochondria in cells of 7–10 or 15–17 generations old. Scale bar, 4 μm.
(B) Quantification showing a gradual decline of mitochondrial association of naturally occurring Hsp104-containing aggregates in cells with increasing replicative age. Histograms shows mean and SEM from at least three experiments with >100 aggregates examined per experiment. SRD, aggregate-mitochondria colocalization from simulated random distribution using parameters measured from the aged cells. See also Figure S4.
(C) Model for organelle-based formation and segregation of protein aggregates.
(1) Active polysomes, which are enriched on ER, provide the newly translated peptides that either fold into native proteins or misfold and aggregate on ER surface, a process exaggerated under stress.
(2) Native proteins denatured under stress aggregate to sites—initiated by newly translated peptides on ER—frequently close to mitochondria.
(3) Aggregates formed away from mitochondria move along ER and are eventually captured by mitochondria.
(4) Association of aggregates at site of ER-mitochondria juxtaposition constrains the mobility of aggregates.
(5) During bud formation, stable anchorage of mother mitochondria is also reflected by constrained motion of many mitochondrial components, such as nucleoids.
(6) Mitochondria that rapidly extend into the bud are likely to acquire these components from either the bud neck region or new assembly. The combination of (5) and (6) explains retention of protein aggregates in the mother during mitochondria inheritance.
(7) Disruption of aggregate-mitochondria association by certain mutations or during aging promotes spreading of aggregates into the bud.
DISCUSSION
The results described above establish a framework for protein aggregation under acute stress, from aggregates’ initial formation to distribution during asymmetric cell division (summarized in Figure 7C). Although our findings are consistent with the notion that misfolded proteins of different nature tend to aggregate together, a surprising observation was the requirement for active translation. Our data suggest that nascent translation products play a key role in initiating the sites for protein aggregation. It is known that newly translated polypeptides suffer a prolonged period of sensitivity to stress and environmental changes (Medicherla and Goldberg, 2008), and their high local concentration near sites of protein synthesis could favor aggregate initiation. In contrast to newly synthesized polypeptides, cytosolic misfolded proteins (Ubc9ts or luciferase at elevated temperature) did not appear to have the ability to initiate aggregation but can be substrates added to existing aggregates.
In vitro biochemical studies have shown that, in addition to intraprotein interactions, agents that discriminately bind to proteins in their folded states (such as proteins’ physiological ligands) could also prevent misfolding and aggregation by increasing the energetic barrier between the native and misfolded states (Carpenter et al., 1999). The opposite effect is expected with agents (such as surfactant) having higher affinity to misfolded states. In the cytosol, each protein interacts with a multitude of functional partners, helping to stabilize protein’s native states and possibly explain the low likelihood for aggregation, whereas in protein aggregates, the misfolded state is favored and stabilized through interaction with other misfolded proteins. Such opposite stabilizing effects on native versus mis-folded conformers could drive protein aggregation to proceed like phase separation seeded by newly translated polypeptides enriched near translation sites. It will be interesting to determine if specific types of newly synthesized polypeptides may be particularly potent in aggregate initiating and whether certain components of ER facilitate this process.
Another unexpected finding is the coinvolvement of ER and mitochondria in the formation and management of protein aggregates. This may be a consequence of the extensive interaction between these organelles but may also reflect a propensity of aggregates to bind both ER and mitochondria surface components. ER-mitochondria contact sites have been a subject of exciting recent research because of their role in mediating lipid transfer between the organelles, formation of autophagosomes, and mitochondrial fission (Böckler and Westermann, 2014; Kornmann, 2013). Several interacting proteins that form the ERMES complex were found to be required for ER-mitochondrial interaction, but ERMES themselves only represent a portion of ER-mitochondria contact sites (Kornmann, 2013). Protein aggregates do not show extensive overlap with ERMES or sites of mitochondrial fission and thus represent another type of interaction bridging these two organelles.
Mitochondria membrane potential, but not mitochondria-dependent ATP production, is required for the dissolution of aggregates. Mitochondrial membrane potential regulates processes such as mitochondrial protein import and calcium homeostasis (Duchen, 2000; Neupert and Herrmann, 2007). A previous study showed that stress conditions can induce hyper-polarization of mitochondrial membrane and that this is required for the induction of chaperone expression (Rikhvanov et al., 2005); however, this is unlikely to explain our observed inhibition of aggregate dissolution by uncouplers because Hsp104 induction after HS did not appear to be defective (Figure 4D; note the brightness increase of Hsp104-GFP). It is possible that mitochondrial membrane potential contributes to chaperone or pro-teosome efficiency or affects aggregate dissolution indirectly through other essential mitochondria-mediated processes.
Asymmetric inheritance, such that aggregated proteins are distributed to only one of the two progeny cells of mitosis, is another clearance mechanism that limits the spreading of proteome damage in a growing population (Bufalino et al., 2013; Rujano et al., 2006; Steinkraus et al., 2008). Our previous study with mathematic modeling proposed that confined slow mobility of aggregates was one of the key reasons for the high-probability of aggregate retention by the mother cell (Unruh et al., 2013; Zhou et al., 2011). While tight association of aggregates with mitochondria, as frequently observed under EM, could explain the observed confinement if mitochondria are relatively immobile, mitochondria in fact are known to undergo rapid and directed extension into the growing bud. What resolves this paradox is the apparent conservative segregation of at least a subset of mitochondrial components. This was evidenced by the retention of not only aggregates but also that of physiological components of mother mitochondria, such as foci of Dnm1 or Mdm34, or nucleoids that harbor mitochondrial genome, when daughter-bound mitochondria extend rapidly from the bud neck toward the bud tip. This observation may be consistent with recent studies showing that the volume of mitochondria in the mother cell remains roughly constant, while the daughter mitochondrial volume continuously grows throughout the cell cycle, and that daughter cells mitochondria are overall “fitter” than mother mitochondria (McFaline-Figueroa et al., 2011; Rafelski et al., 2012).
The increased leakage of mother-born aggregates into the bud in Δfis1 mutant corroborates the importance of mitochondria tethering as a quality control mechanism for limiting the spreading of damage products to newborn cells. Attaching to mitochondria may also allow irreversible aggregates to be cleared through mitophagy, a catabolic process for removing damaged mitochondria and thought to be antiaging (Kim et al., 2007). However, the association between aggregates and mitochondria appears to decline gradually in aging cells, and this correlates with increasingly abnormal mitochondrial morphology (Figure 7A) (Hughes and Gottschling, 2012). In humans, proteins that form aggregates in several degenerative diseases have also been associated with the mitochondrial membrane (Camilleri et al., 2013; Hashimoto et al., 2003; Pasinelli et al., 2004). Whereas this association could potentially help limit the spreading of disease proteins, it is also known to contribute to mitochondria dysfunction. In cultured mammalian cells, mitochondria were found to be enriched in the aggresome, which is partitioned unequally during mitosis (García-Mata et al., 1999; Johnston et al., 1998). Asymmetric partitioning of protein aggregates was also reported recently in asymmetrically dividing Drosophila stem cells (Bufalino et al., 2013). It is thus reasonable to speculate that the organelle-based aggregate formation and retention revealed in yeast represents a mechanism of proteome quality control that may also be important during development and aging in metazoans.
EXPERIMENTAL PROCEDURES
See the Extended Experimental Procedures for additional details.
Yeast Strains and Culture
Yeast strains used in this study are based on the BY4741 strain background as listed in Table S1. Gene deletion or GFP tagging were performed with PCR-mediated homologous recombination, and correct integrations were confirmed by PCR. Yeast cells were grown in synthetic complete (SC) or dropout media containing 2% dextrose, or 2% raffinose plus 2% galactose for Gal promoter induction. Cells expressing “mother enrichment program” with advanced replicative age were obtained by magnetic sorting as described previously (Lindstrom and Gottschling, 2009).
Light Microscopy
Live-cell confocal images were acquired using a Yokagawa CSU-10 spinning disc on the side port of a Carl Zeiss 200 m inverted microscope, PerkinElmer Ultraview VoX system, or a Carl Zeiss LSM-510 Confocor 3 system. Structured illumination microscopy (SIM) images were acquired on an Applied Precision OMX Blaze microscope equipped with PCO Edge sCMOS cameras. All image processing was performed in the ImageJ software (NIH). 3D reconstruction of SIM images was done with Imaris. FRET measurement was performed by using the acceptor photobleaching method. FCS measurements were done as described previously (Slaughter and Li, 2010).
Electron Microscopy
Yeast cells grown in yeast extract, peptone, and dextrose media (YPD) with or without heat shock (30 min at 42°C) were harvested, vacuum filtered, and freezing-fixed in a high-pressure freezer (EM PACT I; Leica). After freeze substitution and embedding, serial ultrathin sections of 50–70 nm thick were cut on an ultramicrotome (Leica), and serial images were collected on a TEM (FEI Tecnai). 3D reconstruction was done with ImageJ. For immunogold labeling of aggregates, an anti-GFP antibody (Invitrogen) was used followed by 10 nm gold-conjugated antibody labeling.
Drug Treatments
All drugs used were purchased from Sigma. Final concentrations were Cycloheximide, 100 μg/ml, MG132, 80 μM, Anisomycin, 200 μg/ml, A2C, 1 mg/ml, hydrogen peroxide, 0.7 mM, and CCCP, 25 μM was used to treat cells after heat shock for 15 min before time-lapse imaging. Also used were Antimycin A, 10 μM, 2-DG, 20 mM, KCN, 1 mM, NaN3, 0.2 mM, and GdnHCl, 3 mM.
Aggregate Analyses
Aggregate dissolution assays were done as described previously (Zhou et al., 2011). The same method was also used to find the total intensity of aggregate-bound and free cytosolic Hsp104-GFP. Tracking and extrapolation of diffusion coefficient were done as described previously (Zhou et al., 2011).
Aggregate Colocalization Simulations
Organelle segmentation was performed in ImageJ. For ER simulations, single slices were taken from structured illumination images; for all other organelles, the simulations were run on maximum projections of the z stack. All possible aggregate centers were surveyed, and positions less than three pixels from the nearest organelle pixel were considered “colocalized.”
Supplementary Material
ACKNOWLEDGMENTS
The authors thank L. Parnell and B. Rubinstein for assistance with data analysis, J. Lange for assistance in microscopy, D. Bradford and S. McCroskey for assistance in strain construction, R. Parker, Y. Ohsumi, K. Weiss, J. Nunnari, D. Kaganovich, D. Gottschling, S. Jaspersen, and L. Pon for providing plasmids and strains, and R. Halfmann for comments on the manuscript. This work was supported by an American Heart Association predoctoral fellowship (13PRE16750001) (to C.Z.) and an NIH grant (RO1-GM057063) (to R.L.).
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Extended Experimental Procedures, four figures, one table, and ten movies and can be found with this article online at http://dx.doi.org/10.1016/j.cell.2014.09.026.
REFERENCES
- Altmann K, Frank M, Neumann D, Jakobs S, and Westermann B (2008). The class V myosin motor protein, Myo2, plays a major role in mitochondrial motility in Saccharomyces cerevisiae. J. Cell Biol. 181, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrasate M, Mitra S, Schweitzer ES, Segal MR, and Finkbeiner S (2004). Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 431, 805–810. [DOI] [PubMed] [Google Scholar]
- Ashe MP, De Long SK, and Sachs AB (2000). Glucose depletion rapidly inhibits translation initiation in yeast. Mol. Biol. Cell 11, 833–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Böckler S, and Westermann B (2014). Mitochondrial ER contacts are crucial for mitophagy in yeast. Dev. Cell 28, 450–458. [DOI] [PubMed] [Google Scholar]
- Boldogh IR, Ramcharan SL, Yang HC, and Pon LA (2004). A type V myosin (Myo2p) and a Rab-like G-protein (Ypt11p) are required for retention of newly inherited mitochondria in yeast cells during cell division. Mol. Biol. Cell 15, 3994–4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bufalino MR, DeVeale B, and van der Kooy D (2013). The asymmetric segregation of damaged proteins is stem cell-type dependent. J. Cell Biol. 201, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri A, Zarb C, Caruana M, Ostermeier U, Ghio S, Högen T, Schmidt F, Giese A, and Vassallo N (2013). Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim. Biophys. Acta 1828, 2532–2543. [DOI] [PubMed] [Google Scholar]
- Carpenter JF, Kendrick BS, Chang BS, Manning MC, and Randolph TW (1999). Inhibition of stress-induced aggregation of protein therapeutics. Methods Enzymol. 309, 236–255. [DOI] [PubMed] [Google Scholar]
- Chiti F, and Dobson CM (2006). Protein misfolding, functional amyloid, and human disease. Annu. Rev. Biochem 75, 333–366. [DOI] [PubMed] [Google Scholar]
- David A, Dolan BP, Hickman HD, Knowlton JJ, Clavarino G, Pierre P, Bennink JR, and Yewdell JW (2012). Nuclear translation visualized by ribosome-bound nascent chain puromycylation. J. Cell Biol. 197, 45–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Detmer SA, and Chan DC (2007). Functions and dysfunctions of mitochondrial dynamics. Nat. Rev. Mol. Cell Biol. 8, 870–879. [DOI] [PubMed] [Google Scholar]
- Duchen MR (2000). Mitochondria and calcium: from cell signalling to cell death. J. Physiol 529, 57–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escusa-Toret S, Vonk WI, and Frydman J (2013). Spatial sequestration of misfolded proteins by a dynamic chaperone pathway enhances cellular fitness during stress. Nat. Cell Biol. 15, 1231–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbacher KL, Yang HC, Gay AC, Huckaba TM, and Pon LA (2004). Live cell imaging of mitochondrial movement along actin cables in budding yeast. Curr. Biol 14, 1996–2004. [DOI] [PubMed] [Google Scholar]
- García-Mata R, Bebök Z, Sorscher EJ, and Sztul ES (1999). Characterization and dynamics of aggresome formation by a cytosolic GFP-chimera. J. Cell Biol. 146, 1239–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover JR, and Lindquist S (1998). Hsp104, Hsp70, and Hsp40: a novel chaperone system that rescues previously aggregated proteins. Cell 94, 73–82. [DOI] [PubMed] [Google Scholar]
- Hartl FU, Bracher A, and Hayer-Hartl M (2011). Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332. [DOI] [PubMed] [Google Scholar]
- Hashimoto M, Rockenstein E, Crews L, and Masliah E (2003). Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 4, 21–36. [DOI] [PubMed] [Google Scholar]
- Hughes AL, and Gottschling DE (2012). An early age increase in vacuolar pH limits mitochondrial function and lifespan in yeast. Nature 492, 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, and Kopito RR (1998). Aggresomes: a cellular response to misfolded proteins. J. Cell Biol. 143, 1883–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaganovich D, Kopito R, and Frydman J (2008). Misfolded proteins partition between two distinct quality control compartments. Nature 454, 1088–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kovacs JJ, McLaurin A, Vance JM, Ito A, and Yao TP (2003). The deacetylase HDAC6 regulates aggresome formation and cell viability in response to misfolded protein stress. Cell 115, 727–738. [DOI] [PubMed] [Google Scholar]
- Kawai S, Murao S, Mochizuki M, Shibuya I, Yano K, and Takagi M (1992). Drastic alteration of cycloheximide sensitivity by substitution of one amino acid in the L41 ribosomal protein of yeasts. J. Bacteriol 174, 254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim I, Rodriguez-Enriquez S, and Lemasters JJ (2007). Selective degradation of mitochondria by mitophagy. Arch. Biochem. Biophys 462, 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B (2013). The molecular hug between the ER and the mitochondria. Curr. Opin. Cell Biol. 25, 443–448. [DOI] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, and Walter P (2009). An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325, 477–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer E, Oguchi Y, Seyffer F, Bukau B, and Mogk A (2013). Mechanism of Hsp104/ClpB inhibition by prion curing Guanidinium hydrochloride. FEBS Lett. 587, 810–817. [DOI] [PubMed] [Google Scholar]
- Li Z, Vizeacoumar FJ, Bahr S, Li J, Warringer J, Vizeacoumar FS, Min R, Vandersluis B, Bellay J, Devit M, et al. (2011). Systematic exploration of essential yeast gene function with temperature-sensitive mutants. Nat. Biotechnol 29, 361–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom DL, and Gottschling DE (2009). The mother enrichment program: a genetic system for facile replicative life span analysis in Saccharomyces cerevisiae. Genetics 183, 413–422, 411SI-413SI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Han Y, and Qian SB (2013). Cotranslational response to proteotoxic stress by elongation pausing of ribosomes. Mol. Cell 49, 453–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Larsson L, Caballero A, Hao X, Oling D, Grantham J, and Nyström T (2010). The polarisome is required for segregation and retrograde transport of protein aggregates. Cell 140, 257–267. [DOI] [PubMed] [Google Scholar]
- Malinovska L, Kroschwald S, Munder MC, Richter D, and Alberti S (2012). Molecular chaperones and stress-inducible protein-sorting factors coordinate the spatiotemporal distribution of protein aggregates. Mol. Biol. Cell 23, 3041–3056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFaline-Figueroa JR, Vevea J, Swayne TC, Zhou C, Liu C, Leung G, Boldogh IR, and Pon LA (2011). Mitochondrial quality control during inheritance is associated with lifespan and mother-daughter age asymmetry in budding yeast. Aging Cell 10, 885–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medicherla B, and Goldberg AL (2008). Heat shock and oxygen radicals stimulate ubiquitin-dependent degradation mainly of newly synthesized proteins. J. Cell Biol. 182, 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neupert W, and Herrmann JM (2007). Translocation of proteins into mitochondria. Annu. Rev. Biochem 76, 723–749. [DOI] [PubMed] [Google Scholar]
- Nguyen TT, Lewandowska A, Choi JY, Markgraf DF, Junker M, Bilgin M, Ejsing CS, Voelker DR, Rapoport TA, and Shaw JM (2012). Gem1 and ERMES do not directly affect phosphatidylserine transport from ER to mitochondria or mitochondrial inheritance. Traffic 13, 880–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olzscha H, Schermann SM, Woerner AC, Pinkert S, Hecht MH, Tartaglia GG, Vendruscolo M, Hayer-Hartl M, Hartl FU, and Vabulas RM (2011). Amyloid-like aggregates sequester numerous metastable proteins with essential cellular functions. Cell 144, 67–78. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Belford ME, Lennon N, Bacskai BJ, Hyman BT, Trotti D, and Brown RH Jr. (2004). Amyotrophic lateral sclerosis-associated SOD1 mutant proteins bind and aggregate with Bcl-2 in spinal cord mitochondria. Neuron 43, 19–30. [DOI] [PubMed] [Google Scholar]
- Pestka S, Rosenfeld H, Harris R, and Hintikka H (1972). Studies on transfer ribonucleic acid-ribosome complexes. XXI. Effect of antibiotics on peptidyl-puromycin synthesis by mammalian polyribosomes. J. Biol. Chem 247, 6895–6900. [PubMed] [Google Scholar]
- Rafelski SM, Viana MP, Zhang Y, Chan YH, Thorn KS, Yam P, Fung JC, Li H, Costa Lda.F., and Marshall WF (2012). Mitochondrial network size scaling in budding yeast. Science 338, 822–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DW, and Nicchitta CV (2012). Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J. Biol. Chem 287, 5518–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikhvanov EG, Varakina NN, Rusaleva TM, Rachenko EI, Knorre DA, and Voinikov VK (2005). Do mitochondria regulate the heat-shock response in Saccharomyces cerevisiae? Curr. Genet 48, 44–59. [DOI] [PubMed] [Google Scholar]
- Rujano MA, Bosveld F, Salomons FA, Dijk F, van Waarde MA, van der Want JJ, de Vos RA, Brunt ER, Sibon OC, and Kampinga HH (2006). Polarised asymmetric inheritance of accumulated protein damage in higher eukaryotes. PLoS Biol. 4, e417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R (1977). Energy requirements for maltose transport in yeast. Eur. J. Biochem 80, 97–102. [DOI] [PubMed] [Google Scholar]
- Slaughter BD, and Li R (2010). Toward quantitative “in vivo biochemistry” with fluorescence fluctuation spectroscopy. Mol. Biol. Cell 21, 4306–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S, Miller SB, Mogk A, and Bukau B (2011). Hsp42 is required for sequestration of protein aggregates into deposition sites in Saccharomyces cerevisiae. J. Cell Biol. 195, 617–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spokoini R, Moldavski O, Nahmias Y, England JL, Schuldiner M, and Kaganovich D (2012). Confinement to organelle-associated inclusion structures mediates asymmetric inheritance of aggregated protein in budding yeast. Cell Reports 2, 738–747. [DOI] [PubMed] [Google Scholar]
- Steinkraus KA, Kaeberlein M, and Kennedy BK (2008). Replicative aging in yeast: the means to the end. Annu. Rev. Cell Dev. Biol 24, 29–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyedmers J, Mogk A, and Bukau B (2010). Cellular strategies for controlling protein aggregation. Nat. Rev. Mol. Cell Biol. 11, 777–788. [DOI] [PubMed] [Google Scholar]
- Unruh JR, Slaughter BD, and Li R (2013). Quality control: putting protein aggregates in a bind. Curr. Biol 23, R74–R76. [DOI] [PubMed] [Google Scholar]
- Winkler J, Tyedmers J, Bukau B, and Mogk A (2012). Hsp70 targets Hsp100 chaperones to substrates for protein disaggregation and prion fragmentation. J. Cell Biol. 198, 387–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman MJ, Hobbs AE, Burgess SM, Srinivasan M, and Jensen RE (2004). Mmm2p, a mitochondrial outer membrane protein required for yeast mitochondrial shape and maintenance of mtDNA nucleoids. J. Cell Biol. 164, 677–688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Slaughter BD, Unruh JR, Eldakak A, Rubinstein B, and Li R (2011). Motility and segregation of Hsp104-associated protein aggregates in budding yeast. Cell 147, 1186–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.