Antibodies to the M protein and SpeC resolve streptococcal toxic shock syndrome in HLA-humanized mice.
Abstract
Invasive streptococcal disease (ISD) and toxic shock syndrome (STSS) result in over 160,000 deaths each year. We modelled these in HLA-transgenic mice infected with a clinically lethal isolate expressing Streptococcal pyrogenic exotoxin (Spe) C and demonstrate that both SpeC and streptococcal M protein, acting cooperatively, are required for disease. Vaccination with a conserved M protein peptide, J8, protects against STSS by causing a dramatic reduction in bacterial burden associated with the absence of SpeC and inflammatory cytokines in the blood. Furthermore, passive immunotherapy with antibodies to J8 quickly resolves established disease by clearing the infection and ablating the inflammatory activity of the M protein, which is further enhanced by addition of SpeC antibodies. Analysis of 77 recent isolates of Streptococcus pyogenes causing ISD, demonstrated that anti-J8 antibodies theoretically recognize at least 73, providing strong support for using antibodies to J8, with or without antibodies to SpeC, as a therapeutic approach.
INTRODUCTION
Seemingly mild streptococcal infections can rapidly escalate to serious invasive infections with a high mortality rate. The overall incidence for invasive group A streptococcal disease (ISD) was reported to vary between 2 and 4 per 100,000 people in developed countries. Most of these data were garnered from multiple surveys conducted between 1996 and 2007 (1). However, more recent data point to an alarming increase in rates (2–4). In Alberta, Canada, for example, the rate has steadily risen from 4.2 per 100,000 in 2003 to 10.2 per 100,000 in 2017 (4). In developing countries, very high rates are reported among the young and the elderly (up to 75 per 100,000) (1). The true current global incidence rates are unknown, but available data point to the true rates being substantially higher than many recently reported.
In approximately 20% of cases, ISD is accompanied by a streptococcal toxic shock syndrome (STSS) with multiorgan failure and case fatality rates approaching 50% even in the best-equipped facilities (5). It can occur after any streptococcal infection but most commonly occurs after infections of the skin and is usually associated with necrotizing fasciitis, myositis, or deep bruising. Pregnancy and the puerperium are periods of excessive risk, especially in developing countries (6).
Streptococcal “superantigens” (SAgs) are thought to play the key role in the pathogenesis of STSS (7). Eight of the 13 streptococcal SAg genes are located within bacteriophages (8). The phage-encoded streptococcal pyrogenic exotoxin A (SpeA) and SpeC are commonly found in STSS. In a Swedish study of 297 invasive isolates (of varying emm types), 73% were shown to carry spec, with spea being almost entirely restricted to emm1 isolates (9). SAgs have profound immunological potency that is derived from their binding to human major histocompatibility complex [human leukocyte antigen (HLA)] class II molecules (outside the peptide binding groove) and conserved regions of the T cell receptor chains, resulting in polyclonal T cell activation often with >25% of CD4+ T cells being activated. The resulting T helper 1 (TH1) cytokine storm is the proposed causal link responsible for the hypotension and multiorgan failure that define STSS. This has led to toxoids of SAgs being proposed as vaccine candidates (10). However, the pathogenesis of STSS is not fully understood. Other streptococcal virulence factors, including Streptolysin O (SLO), peptidoglycan, lipoteichoic acid, and the M protein (11–13), have been shown to be potent inducers of inflammatory cytokines in vitro, and these or other factors may play important roles in STSS and may be key to the development of successful vaccines and immunotherapies.
“J8” is a vaccine candidate based on the C-3 repeat region of the M protein. J8 vaccination can protect mice from skin, mucosal, and intraperitoneal streptococcal infection via antibody-mediated neutrophil opsonophagocytosis (14, 15) and is effective against organisms bearing J8 or the closely related allelic sequence, J8.1 (14, 16–18). When conjugated to diphtheria toxoid (DT), J8 is immunogenic in nonhuman primates (19) and in humans (20) and is currently undergoing further clinical trials to study immunogenicity and efficacy.
In this study, we used HLA DR3 DQ2-transgenic B6 mice to model STSS. We asked whether vaccination with J8 could prevent STSS-like disease and whether passive immunotherapy with J8- and SpeC-specific antibodies could treat established STSS. Our data demonstrate critical roles for both the M protein and SpeC in pathogenesis. We further show that vaccination can prevent disease and that passive immunotherapy can rapidly ablate the potent mitogenic and inflammatory activity of Streptococcus pyogenes, negate the clinical signs of disease, and clear the infection caused by an organism isolated from a patient who succumbed to ISD and STSS.
RESULTS
HLA-transgenic mice are a suitable model for studying STSS
SN1 is an emm89 strain of S. pyogenes isolated in 2013 from the blood of a patient in Brisbane, Australia, who succumbed to ISD and STSS. At the time, there was a cluster of four patients who experienced ISD due to emm89 S. pyogenes. Genomic analysis revealed that of 10 streptococcal SAg genes examined, SN1 expressed the phage-encoded spec gene and the chromosomally encoded smez and speg genes (fig. S1). The organism was negative for spea. Organisms from the other three patients had the same SAg profile. A group C streptococcal (GCS) organism, NS33 (isolated from a patient with a foot ulcer), did not contain any SAg genes and was used as a control for the experiments described below.
BALB/c mice were infected via the skin with SN1 or NS33. They developed a skin infection but only a very limited systemic infection (Fig. 1, A and B). However, blood samples collected from mice infected via the skin with SN1 were positive for SpeC as determined by Western blot, whereas mice infected with NS33 were not (Fig. 1, C to F). Sterile-filtered sera from mice infected with SN1, or recombinant SpeC (rSpeC), were added to B6 or HLA-transgenic B6 splenocyte cultures and caused significant proliferation of the HLA-transgenic spleen cells (but not the B6 cells) (Fig. 2A). Proliferation caused by SN1 serum was largely, but not completely, blocked by rSpeC antiserum, indicating that another molecule(s) present in SN1 serum, apart from SpeC, exerted some mitogenic activity (Fig. 2A). The proliferative responses were mirrored by the production of tumor necrosis factor (TNF) and interferon-γ (IFN-γ)—key cytokines implicated in STSS pathogenesis (Fig. 2, B and C). Serum from NS33-infected mice induced neither proliferation nor cytokine responses from splenocytes from either HLA-B6 or B6 mice. A significant mitogenic response was observed when serum from SN1 skin–infected BALB/c mice, but not serum from NS33-infected mice, was added to human peripheral blood mononuclear cells (PBMCs) (Fig. 2, D to F).
Fig. 1. BALB/c mice as a model for STSS.
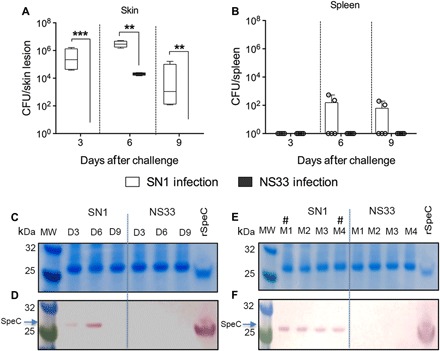
(A and B) Virulence of human isolates in murine skin infection model. Cohorts of BALB/c mice (n = 5 per group) were infected with SN1 or NS33 strain via the skin route of infection. After day 3, 6, or 9 of challenge, the mice were culled and skin biopsy (A) and spleen (B) samples were harvested, processed, and plated to determine the bacterial burden. The results are shown as box and whisker plot, where the line in the box indicates the median, the box extremities indicate the upper and lower quartiles, and the whiskers show the minimum to maximum values. Statistical analysis was performed using nonparametric, unpaired Mann-Whitney U test to compare the two groups at each time point. **P < 0.01 and ***P < 0.001. (C and D) SDS–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blot profile of SpeC in serum samples collected at various time points after infection. Serum samples from SN1- or NS33-infected mice were collected on days 3, 6, and 9 after infection and run on 4 to 12% SDS-PAGE gels (C). Following protein transfer from the gel, the membrane was probed with rabbit anti-SpeC immunoglobulin G (IgG) primary antibody, followed by detection with sheep anti-rabbit IgG-AP (alkaline phosphatase), and developed using SIGMAFAST BCIP/NBT (bromochloroindolyl phosphate–nitro blue tetrazolium) substrate. rSpeC protein was also run as a positive control. Detection of SpeC is shown (D). MW, molecular weight. (E and F) SpeC detection in individual mouse serum samples from the day 6 collection. Serum samples from each individual mouse on day 6 following SN1/NS33 infection were also assessed for the presence of SpeC as described. A representative image of SDS-PAGE (E) and Western blot (F) is shown. The symbol “#” indicates the mice that had positive spleen culture.
Fig. 2. Mitogenic and inflammatory activity of SN1-infected sera in vitro.
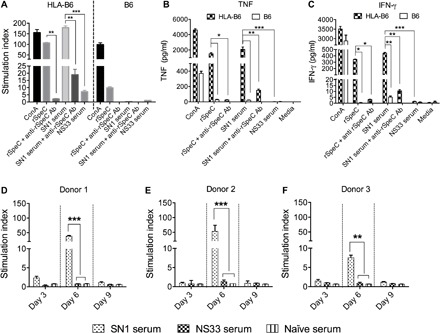
(A) Splenocyte proliferation in response to serum from SN1-infected mice and rSpeC. Splenocytes from HLA-B6 and B6 mice were stimulated in vitro either with 20 μl of sterile-filtered serum from SN1-infected BALB/c mice or with rSpeC. As controls, sterile-filtered serum from mice infected with a SAg-negative GCS isolate (NS33) and Concanavalin A (ConA) were also included. Proliferation of splenocytes was assessed after 72 hours by measuring incorporation of tritiated [3H]thymidine, and data are represented as stimulation indices (see below). The specificity of the response was confirmed by addition of 20 μl of anti-rSpeC serum. Ab, antibody. (B and C) Cytokine profiles following splenocyte proliferation. Cytokine responses of splenocytes from HLA-B6 and B6 mice were measured at 72 hours after incubation with various stimulants. Concentrations of TNF (B) and IFN-γ (C) in the culture supernatants were measured using a TH1/TH2/TH17 cytometric bead array (CBA) kit (BD Biosciences). The specificity of the responses was confirmed by addition of rSpeC antiserum. (D to F) Proliferation of human PBMCs in response to stimulation with serum from SN1- or NS33-infected mice. PBMCs from three different individuals were cultured in the presence of serum collected at various time points following infection with SN1 or NS33. An optimized amount of serum (20 μl) was used for PBMC stimulation. Proliferation was measured by [3H]thymidine uptake after 72 hours. Data are mean ± SEM of three replicates in each experiment, with experiments repeated twice. Stimulation index was defined as counts per minute in the presence of antigen/counts per minute in the absence of antigen. One-way analysis of variance (ANOVA) with Tukey’s post hoc method was used to calculate significance between various groups. *P < 0.05, **P < 0.01, and ***P < 0.001.
We then assessed the clinical outcome of SN1 infection in HLA-transgenic B6 mice. HLA-B6 and B6 mice were infected intraperitoneally with 106 SN1. HLA-B6 mice developed systemic infection with splenic and blood bacterial burden of 2 × 103 and 4 × 104 colony-forming units (CFU)/ml, respectively. In comparison, the bacterial burden in B6 mice was substantially lower (10 and 12 CFU/ml in blood and spleen, respectively). SpeC was detected in sera from both mice, but TNF, IFN-γ, and interleukin-2 (IL-2) were detected only in the serum of HLA-B6 mice. SpeC and the cytokines were evident by 24 hours after infection (Fig. 3, A to D). Similarly, we observed that skin infection also caused STSS-like pathology in HLA-B6 mice. At day 6 after skin infection with 106 CFU of SN1, mice demonstrated bacteremia and high bacterial burden in the skin, which was >3 logs higher in the HLA-B6 mice in comparison to B6 mice (Fig. 3, E and F). SpeC was detected in sera from both HLA-B6 and B6 mice (Fig. 3G), but again, inflammatory cytokines were only detected in the blood of HLA-B6 mice (Fig. 3, H to J). The levels were lower than observed following intraperitoneal infection (e.g., TNF was 1110 pg/ml following intraperitoneal infection and 175 pg/ml following skin infection; IFN-γ was 2184 pg/ml versus 1615 pg/ml). Serum from NS33-infected mice did not contain SpeC.
Fig. 3. Infectivity of S. pyogenes SN1 in HLA-B6 mice following intraperitoneal and skin infection.
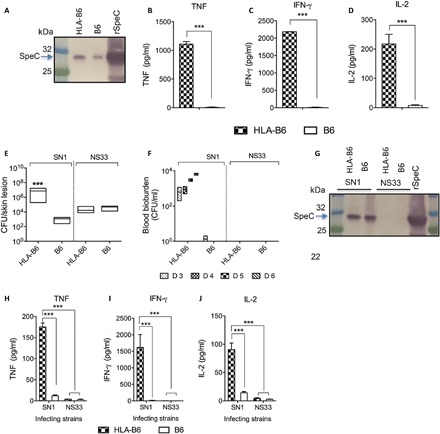
(A) Western blot analysis of serum from SN1-infected HLA-B6 and B6 mice following intraperitoneal infection. Naïve HLA-B6 and B6 mice (n = 10 per group) were infected with 106 SN1 via the intraperitoneal route of infection. Serum samples were collected at 24 hours after infection and analyzed to detect the toxin. The samples were run on 4 to 12% SDS-PAGE gel. Following protein transfer from the gel, the membrane was probed with rabbit anti-SpeC IgG primary antibody, followed by detection with sheep anti-rabbit IgG-AP, and developed using SIGMAFAST BCIP/NBT substrate. rSpeC protein was also run as a positive control. (B to D) Serum cytokine profile of HLA-B6 and B6 mice following intraperitoneal infection with SN1. The mice infected with SN1 were culled at 24 hours after infection. Blood cytokine levels were measured from the cohort that received 106 SN1 using a CBA kit. TNF, IFN-γ, and IL-2 responses are shown. Statistical analysis was performed using nonparametric, unpaired Mann-Whitney U test to compare the two cohorts. ***P < 0.001. (E and F) Bacterial burden in HLA-B6 mice after skin infection with SN1. Naïve HLA-B6 and B6 mice (n = 10 per group) were infected with SN1 or NS33 via skin. On day 6 after infection, mice were culled and skin bacterial burdens were assessed (E). Mann-Whitney U test used to compare the two cohorts. ***P < 0.001. The presence of systemic infection was assessed by plating blood samples at days 3 to 6 after infection (F). The results are shown as box and whisker plot, where the line in the box indicates the median, the box extremities indicate the upper and lower quartiles, and the whiskers show the minimum to maximum values. (G) Western blot analysis of serum from SN1- or NS33-infected mice. Serum samples collected from SN1- or NS33-infected HLA-B6 and B6 mice were analyzed to detect the presence of SpeC in their serum. The samples were run on 4 to 12% SDS-PAGE gel. Following protein transfer from the gel, the membrane was probed with rabbit anti-SpeC IgG primary antibody, followed by detection with sheep anti-rabbit IgG-AP, and developed using SIGMAFAST BCIP/NBT substrate. The band at 26 kDa in the serum sample from SN1-infected mice corresponds to rSpeC in the positive control sample. (H to J) Cytokine responses in the serum of HLA-B6 and B6 mice following skin infection. Cytokine responses in the serum of HLA-B6 and B6 mice were measured at day 6 after infection with SN1 or NS33. Concentrations of TNF (H), IFN-γ (I), and IL-2 (J) were measured using a CBA kit. One-way ANOVA with Tukey’s post hoc method was used to calculate significance between various groups. ***P < 0.001.
J8 vaccination prevents STSS
HLA-B6 mice were vaccinated (three times, intramuscularly) with J8-DT/alum or phosphate-buffered saline (PBS)/alum as a control. Two weeks after vaccination, mice were challenged via the skin with SN1 and we observed a 1000- to 1,000,000-fold reduction in bacterial burden in skin, blood, and spleen (P < 0.05, P < 0.001, and P < 0.01, respectively) (Fig. 4A). SpeC was evident in the serum of control mice but not in vaccinated mice (Fig. 4B). IFN-γ and TNF (TH1 cytokines) were detected in the sera of control mice but were absent in the sera of vaccinated mice, whereas IL-4 and IL-10 (TH2 cytokines) were found in the sera of vaccinated mice (Fig. 4, C and D).
Fig. 4. Abolition of mitogenic and inflammatory effects after J8-DT vaccination.
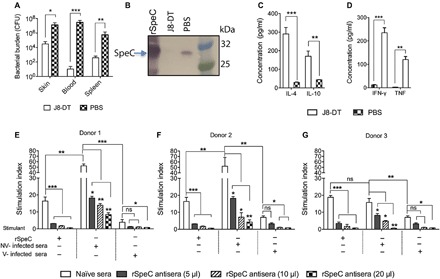
(A) Protective efficacy of J8-DT vaccine against S. pyogenes SN1 infection. HLA-B6 mice were vaccinated with 30 μg of J8-DT/alum or PBS/alum on days 0, 21, and 28. Two weeks after immunization, mice were infected with SN1 via the skin. On day 6 after infection, mice were culled and bacterial burden in skin (CFU/lesion), blood (CFU/ml), and spleen (CFU/spleen) is shown. Statistical analysis was performed using nonparametric, unpaired Mann-Whitney U test to compare the two cohorts. *P < 0.05, **P < 0.01, and ***P < 0.001. (B) Western blot analysis to detect SpeC in infected mice serum. Pooled serum samples from vaccinated and control cohorts collected at day 6 after SN1 infection were run on 4 to 12% SDS-PAGE gels. Following protein transfer from the gel, the membrane was probed with rabbit anti-SpeC IgG primary antibody, followed by detection with sheep anti-rabbit IgG-AP, and developed using SIGMAFAST BCIP/NBT substrate. The band at 26 kDa in the serum sample from PBS-treated (control) mice corresponds to rSpeC in the positive control sample. (C and D) Cytokine responses in the serum of HLA-B6 mice following skin infection. Cytokine responses in the serum of vaccinated and control HLA-B6 mice were measured at day 6 after infection with SN1. Concentration of IL-4 and IL-10 (C) and TNF and IFN-γ (D) in serum was measured using a CBA kit. One-way ANOVA with Tukey’s post hoc method was used to calculate significance between various groups. ***P < 0.001. (E to G) Assessment of PBMC proliferation induced by serum from vaccinated/control-infected mice. PBMCs from three different individuals were stimulated with pre-optimized concentration of serum from J8-DT–vaccinated SN1-infected (V-infected sera) or nonvaccinated SN1-infected (NV-infected sera) mice. rSpeC was used as controls for stimulation. The specificity of response was assessed by addition of various amounts of rSpeC antisera. PBMCs in the presence of naïve sera were used as a control for specificity of neutralization. Proliferation was measured by [3H]thymidine uptake after 72 hours. Data are mean ± SEM of three replicates in each experiment, with experiments repeated twice. Representative data from two individuals are shown. One-way ANOVA with Tukey’s post hoc method was used to calculate significance. *P < 0.05, **P < 0.01, and ***P < 0.001. ns, not significant.
Sera from J8-vaccinated/SN1-challenged and control (PBS-vaccinated/SN1-challenged) mice were added to cultures of human PBMCs from healthy individuals. Serum from control mice caused robust proliferation of PBMCs from all individuals (and this was reduced by 80 to 90% by addition of rSpeC antiserum) (Fig. 4, E to G), whereas serum from J8-vaccinated/challenged mice caused significantly less proliferation (up to 95% reduction) and this was further reduced to background levels (stimulation index, ~1; P < 0.05 to 0.01) by addition of rSpeC antiserum (Fig. 4, E to G). Thus, there were very low levels of residual SpeC present in the serum of vaccinated/challenged mice responsible for the minimal proliferation of lymphocytes, although SpeC was not evident from inspection of the Western blot (Fig. 4B). Induction of inflammatory cytokines (IFN-γ, TNF, IL-2, IL-6, and IL-17) by human PBMCs stimulated with serum from J8-DT–vaccinated/challenged mice was almost nonexistent, whereas the cytokines were abundantly produced by addition of sera from control mice (fig. S2). J8 vaccination could thus prevent all of the inflammatory cytokine responses observed in vivo (Fig. 4D) and in vitro (fig. S2). The vaccine effect was likely due in large part to the observed reduction in bacterial load (Fig. 4A) with reduced production of mitogenic/inflammatory factors.
Immunotherapy for STSS
HLA-B6 mice were infected intraperitoneally with 106 SN1 and developed bacteremia and became ill within 18 hours (Fig. 5, A to C). Clinical scoring throughout this experiment was performed with the observer blinded to the groups. We asked whether these mice could be rescued by passive immunotherapy. Eighteen hours after infection, they were given 200 μl of J8 antisera, 200 μl of rSpeC antisera, 100 μl of each antiserum, or 200 μl of naïve serum intravenously (Fig. 5A). All mice that received J8 and/or rSpeC antisera recovered within 24 hours with significant reduction in clinical scores (P < 0.01 to P < 0.001; Fig. 5B); however, it was only in those mice that received J8 antiserum (either alone or in combination with rSpeC antiserum) that we observed bacterial clearance from blood and spleen (P < 0.01; Fig. 5C) and we only observed clearance of SpeC in the blood in those mice that received rSpeC antiserum (either alone or in combination with J8 antiserum) (Fig. 5, D to G). Concentrations of inflammatory cytokines (IFN-γ, TNF, and IL-2) in the serum from mice treated with either J8 antisera or rSpeC antisera were significantly reduced (P < 0.01) and were absent entirely from mice treated with both antisera (P < 0.001) (Fig. 5, H to J). The reduced, but not absent, cytokine response in J8-DT antisera–treated mice (with no bacteria in their blood; Fig. 5C) indicated that factors not directly related to J8 or the M protein were contributing to the cytokine response. Addition of rSpeC antisera completely eliminated this cytokine response (Fig. 5, H to J). On the other hand, treatment with rSpeC antisera alone (with no effect on bacterial burden; Fig. 5C) resulted in a reduced cytokine response, suggesting that another factor(s) apart from SpeC, present in the blood of infected mice, contributed to the cytokine response. It is possible that the chromosomally encoded SpeG or SmeZ or other factors present in SN1 may have contributed to this.
Fig. 5. Therapeutic potential of combination immunotherapy.
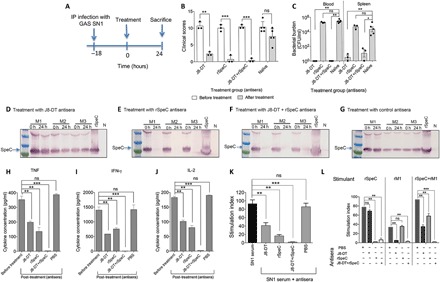
(A) Timeline of infection and treatment protocol. (B) Clinical scores for SN1-infected mice before and after antisera treatment. Four cohorts of HLA-B6 mice (n = 3 to 5 per group) were infected intraperitoneally (IP) with 106 S. pyogenes SN1. Eighteen hours after infection (0 hours), mice were scored for clinical symptoms and intravenously administered 200 μl of either anti–J8-DT, anti-rSpeC, a combination of anti–J8-DT and anti-rSpeC, or PBS-treated (control) sera. All mice were again scored for clinical symptoms after treatment to assess disease severity. The scoring system included appearance, level of consciousness, activity, response to stimulus, eyes, respiration rate, and respiration quality. The mice were scored from 0 to 4. The clinical scores for all cohorts before (0 hours) and after (24 hours) antisera treatment are shown. At 24 hours after treatment (42 hours after infection), mice were culled. (C) Bacterial burden in SN1-infected mice before and after antisera treatment. Blood and spleen samples were harvested, processed, and plated for quantification of bacteria. The bacterial burdens in blood and spleen of mice are shown. Mann-Whitney U test was performed to compare each group with the PBS-treated (control) group. *P < 0.05; **P < 0.01; ***P < 0.001; and ns, P > 0.05. (D to G) Assessment of SpeC in serum samples from mice before and after treatments. To assess SpeC neutralization in vivo, sera samples from all cohorts were collected before (0 hours) and then at 24 hours after antisera administration. Pooled serum samples from treated and untreated cohorts were run on 4 to 12% SDS-PAGE gels. Following protein transfer from the gel, the membrane was probed with rabbit anti-SpeC IgG primary antibody, followed by detection with sheep anti-rabbit IgG-AP, and developed using SIGMAFAST BCIP/NBT substrate. The presence of SpeC in HLA-B6 mice treated with J8-DT antiserum (D), rSpeC antiserum (E), J8-DT + rSpeC antiserum (F), or control serum (G) sera before and after treatment is shown. “N” represents pooled serum from naive mice. (H to J) Cytokine profile in SN1-infected mice before and after treatment. Following treatment with various antisera, at 24 hours, mice were culled and cytokine responses in the pre- and post-treatment serum were measured using a CBA kit. Concentration of TNF (H), IFN-γ (I), and IL-2 (J) in serum is shown. Statistical analysis was carried out using one-way ANOVA with Tukey’s post hoc method to calculate significance between various groups, with color of asterisk (*) denoting the groups being compared. *P < 0.05, **P < 0.01; ***P < 0.001; and ns, P > 0.05. (K and L) Splenocyte proliferation and inhibition in response to S. pyogenes antigens and various antisera. (K) Splenocytes from HLA-B6 mice (n = 3) were stimulated with 20 μl of SN1-infected serum. Proliferation was measured in the presence or absence of J8-DT, rSpeC, J8-DT + rSpeC, or PBS-treated (control) sera by [3H]thymidine uptake after 72 hours. Statistical analysis was performed using nonparametric, unpaired Mann-Whitney U test to compare the two groups. ns, P > 0.05; **P < 0.01; and ***P < 0.001. (L) Splenocytes from HLA-B6 mice were stimulated with pre-optimized concentration (5 μg/ml) of rSpeC, rM1, or rSpeC + rM1. To assess inhibition by various sera, 20 μl of J8-DT, rSpeC, or J8-DT + rSpeC antisera was added to each well. Nonimmune sera were used as control. Proliferation of splenocytes in the presence or absence of various antisera was assessed after 72 hours by [3H]thymidine uptake. Data are represented as stimulation indices, which were defined by comparing the proliferation in the presence or absence of antisera. One-way ANOVA with Tukey’s post hoc method was used to calculate significance between various groups. **P < 0.01; ***P < 0.001; and ns, not significant.
M protein from SN1 exerts a mitogenic effect and contributes to the pro-inflammatory response
We investigated the mechanism by which J8 and SpeC antisera could resolve STSS-like pathology. We observed that the inflammatory and mitogenic effects of sterile-filtered serum from SN1-infected mice were partially blocked by either rSpeC or J8 antisera but were completely blocked by the combination of both antisera (Fig. 5K), suggesting that SN1 sera contained only two mitogenic/inflammatory factors: SpeC and M protein. The data collectively suggested that J8-specific antibodies have a dual role in treating STSS in this model: They clear bacteria, thus indirectly reducing production of mitogenic/inflammatory factors, but they also directly block the mitogenic/inflammatory effect of the serum, presumably by neutralizing the M protein. This has a synergistic effect with the anti-rSpeC antibodies. To ask whether J8-specific antibodies might block the mitogenic effect of M proteins more generally, we tested whether they would block the activity of a recombinant emm1 M protein, (rM1). Figure 5L again shows that rSpeC has profound mitogenic activity but shows that rM1 also has significant activity and that the effect of both is synergistic. However, the mitogenic activity of rM1 is completely blocked by J8 antisera. Similarly, rSpeC antiserum completely blocks the mitogenic activity of rSpeC and a combination of J8 and SpeC antisera completely blocks the combined mitogenic activities of rM1 + rSpeC. The mitogenic effect of rM1 was paralleled in cytokine responses (IFN-γ, TNF, and IL-2), where addition of J8-DT antisera also ablated the activity (fig. S3). These data collectively show that anti-J8 antibodies can block the mitogenic activities of two distinct M proteins, M89 and M1. The data do not suggest that the J8 epitope itself has mitogenic activity but that antibodies to J8 can neutralize the M protein. Vaccination of HLA-B6 transgenic mice with J8-DT is not associated with any side effects, which would have been expected if J8 were mitogenic. Furthermore, others have shown that the mitogenic determinant on the M protein is located in the semiconserved B repeat region of the protein (12, 21), whereas the J8 epitope is located in the C-terminal C-3 repeat region.
J8 immunotherapy would provide theoretical coverage for most ISD cases
Given the recent epidemic of ISD in Canada (4), we asked whether passive immunotherapy with J8 and SpeC antibodies could have theoretically provided treatment for the infections. We examined 77 S. pyogenes strains from 2017–2018 (11 different emm types) isolated from patients in Alberta who presented with ISD, of whom 8 had a diagnosis of STSS. We performed genomic analysis to determine the various J8 alleles present in that collection. Seventy-three isolates contained either J8 or J8.1 in their emm gene, and each of the 8 isolates taken from patients with STSS expressed either J8 or J8.1. This is in agreement with a previous observation in 2005 that, within a collection of 37 clinical isolates from India, all expressed either J8 or J8.1 (22) and with a study from Fiji in 2009 showing that 94% of 521 isolates expressed J8 or J8.1 (23). We tested immunological cross-reactivity between these two sequences and observed complete cross-reactivity by enzyme-linked immunosorbent assay (ELISA) (fig. S4). More notably, we have shown that vaccination with J8 can protect against intraperitoneal and skin infections with organisms expressing eight distinct emm types and bearing either the J8 or J8.1 sequence (14–16). We have yet to find an organism against which J8 does not protect. Thus, to the best of our knowledge, and to the limit of the STSS model, J8 vaccination or J8-specific immunotherapy is likely to provide significant protection/treatment for the vast majority, if not all, cases of ISD and STSS.
We also determined the presence of the spec gene in the 77 ISD isolates. Twenty-four (31%) of the isolates had spec, with very high degree of conservation (nucleotide sequence identity, 99.5 to 100%). The data demonstrated a widespread distribution of the spec gene among a range of emm types (7 of 11 types harbored the spec gene), whereas distribution of the spea gene was found to be limited to emm1 (14% of total) (table S3). Four of the eight isolates from patients with STSS expressed spec, with none of these expressing spea.
DISCUSSION
The data presented here show that in an HLA-humanized mouse, it is possible to prevent STSS-like disease by vaccination with a highly conserved M protein peptide, J8, and to rapidly treat established disease by specific immunotherapy containing antibodies to J8 and another highly conserved antigen, SpeC. Antibodies to J8 have a dual effect: They not only eliminate the bacteria but also directly block the mitogenic effect of the M protein, while antibodies to SpeC block the activity of that protein. Together, the effect is synergistic and can completely resolve STSS-like disease.
The role of SAgs in superficial and invasive streptococcal infections has been well demonstrated (24, 25). Among the bacteriophage-encoded SAgs, SpeA and SpeC are believed to be responsible for most of the STSS cases. More recently, a critical role for SpeA in the establishment of nasopharyngeal infection has been described (26), and the utility of SpeA toxoid antibodies in protection against nasopharyngeal infection was demonstrated (24). The study provided evidence that humoral immunity to specific SAg can protect against streptococcal nasopharyngeal infection. The evidence that streptococcal SAgs have the potential to suppress antibody production via Fas-FasL–dependent apoptosis of B cells and the reduced antibody response to SAgs is associated with STSS (27) and recurrent tonsillitis (28) further highlight the critical role of SAg-specific antibodies in the prevention and/or treatment of SAg-mediated diseases.
Efforts to develop vaccines to prevent STSS are limited. One group has developed toxoids to SpeA and SpeC and shown that vaccination of rabbits induces antibodies that neutralize the toxin and protect rabbits from native toxin administered via a mini-osmotic pump. The rabbits were not exposed to a streptococcal infection (10, 29). While this vaccine approach is promising, it suffers from the need to vaccinate with multiple toxoids to protect against only one aspect of streptococcal disease. Our data suggest that this approach would not reduce bacterial sepsis. HLA-transgenic mice have been used to show that certain HLA types are more prone to STSS (30) but not to model vaccine or immunotherapy development.
We previously developed J8 as a candidate S. pyogenes vaccine [reviewed in (31)]. J8 has a sequence that contains 12 amino acids of the C3 repeat of the M protein. It is highly conserved, with only a single major C-3 variant (J8.1) reported—both previously (22, 23) and in this study. Vaccination with J8 induces antibodies that opsonize S. pyogenes in vitro and protect mice from multiple strains that infect the peritoneum, skin, and upper respiratory mucosa, irrespective of whether the organism expresses J8 or J8.1 (14–16, 18). Here, we show that J8 vaccination can also protect against emm89 (which contains J8.1) and can neutralize both M89 and M1 proteins. We also show that of the 77 recent ISD cases in Alberta, Canada, anti-J8 antibodies theoretically recognize at least 73. On the basis of these data, we believe that J8-specific antibodies will likely recognize most, if not all, M proteins and will prove highly effective in treatment.
Because J8 vaccination can prevent S. pyogenes infections, it was assumed that vaccine-mediated protection would extend to protection against STSS, although this had not been tested in HLA-humanized mice. However, it was not assumed that passive immunotherapy with anti-J8 antibodies would resolve established disease, even if there was some reduction in bacterial burden because SAgs are believed to play the central role in the disease and there was no suggestion that antibodies to J8 would affect the levels of serum SAgs. We were surprised that J8 antiserum (with or without rSpeC antiserum) could quickly eliminate virtually the entire bacterial burden in the blood and spleen as well as resolve the clinical scores. While the clinical signs that we observe in mice (32) differ from the clinical signs that define STSS in humans, there is no doubt that infection can make HLA-humanized mice ill with high clinical scores. Furthermore, the chemical pathology is very similar between mice and humans, viz., the presence of SpeC in the blood as well as high levels of inflammatory cytokines. It is reasonable to assume that antibodies to J8 might also “indirectly block” the chemical and cytokine imbalance in patients with STSS and contribute to an enhanced clinical outcome. Our data also emphasize the important role for SpeC in the pathogenesis of STSS, particularly because anti-rSpeC antibodies can also rapidly resolve clinical signs and correct the chemical profile, although they do not clear the infection.
Our data show that the effects of the M protein and SpeC are cooperative, and we argue, based on the model in HLA-humanized mice, that the pathogenesis of STSS requires both immune stimulants and, conversely, that blocking either will block disease; however, optimal immunotherapy will require antibodies to both the M protein and SpeC. J8 is an obvious choice for antibody therapy to block the M protein because of its very high degree of immunological conservation between different M proteins. However, a major issue, not resolved by this study, is whether other M proteins also demonstrate mitogenic activity that contributes to STSS pathology. Our study shows that M89 has this activity and that rM1 has a similar mitogenic effect, as has been reported previously (33). Tomai et al. (34) have also shown that an extract of M5 has mitogenic properties. It has been suggested that the partially conserved B repeat region of the M protein is the source of its mitogenic activity (12), and hence, it is likely that many M proteins will have mitogenic activity. If other M proteins do contribute to the pathogenesis of STSS in humans, then a combination of antibodies to J8 and SpeC should provide optimal immunotherapy for STSS caused by spec+ organisms. SpeC and SpeA are the SAgs most commonly associated with STSS, with very few case descriptions of the other SAgs playing a role (35). spea has been mostly associated with emm1 strains [(36) and this study]. We have not studied the pathogenesis of STSS following infection with S. pyogenes containing spea; however, we would suggest that in cases where SpeA or other SAgs have contributed to pathology, a combination of antibodies to the SAg and J8 would be highly effective. Nevertheless, we would also argue that J8-specific antibodies alone would exert a potent beneficial effect. In support of the need for J8-specific antibodies, others have shown that the disruption of the spea gene does not prevent sepsis in an HLA-transgenic model (37). Other bacterial factors including SLO, peptidoglycan, and lipoteichoic acid have been shown to promote inflammation. Our data do not deny that they may play an auxiliary role in pathogenesis, but we note that these molecules are found on all streptococcal isolates (including SN1) and alone do not cause disease.
Intravenous immunoglobulin (IVIG) (pooled from multiple donors) in conjunction with antibiotics has been shown to reduce the case fatality rate of STSS (38). Its mechanism of action is unknown; however, it is thought that anti-streptococcal antibodies in IVIG are responsible for its effect. The levels of type-specific M protein antibodies in IVIG will be variable and dependent on recent previous exposure of the donors to various strains. The use of IVIG treatment in experimental STSS, although providing therapeutic activity when used at the time of infection, did not confer additional therapeutic benefit when used in conjunction with antibiotic therapy in a delayed treatment setting (39). Together, the high costs of IVIG, batch-to-batch variation (40), and difficulties in supply underscore the need for alternative defined adjunctive therapies, in particular the use of monoclonal antibodies against the M protein and the SAgs. The work presented here provides proof of concept for this approach in STSS.
In conclusion, we show that J8 vaccination or a combination immunotherapy results in dual toxin neutralization as well as clearance of S. pyogenes from the blood, which is associated with a rapid improvement in the clinical and pathological features of STSS-like disease in HLA-humanized mice. Clearance of S. pyogenes will reduce the need for continuous antitoxin treatment. We demonstrate that S. pyogenes SAgs are not the sole determinant of the pathophysiology of STSS-like disease and that the M protein plays a critical role. We show that SpeC and the M protein work synergistically and contribute to the clinical disease. In vivo attack on the M protein by J8 antisera removes the organism from the circulation and also blocks the mitogenic activity of its M protein (at least for M89 and M1) and likely prevents its interaction with fibrinogen and subsequent recognition via β2 integrins on neutrophils (41). As an end result, there is greatly diminished production of inflammatory cytokines and no activation and release of mediators of vascular leakage, which are a key occurrence in STSS. While the combination of J8 and SpeC antibodies holds great promise for the treatment of STSS caused by spec-bearing organisms, the data show that J8 antibodies alone are likely to have a significant beneficial effect in STSS caused by organisms that contain spec or any other SAgs.
MATERIALS AND METHODS
Ethics statement
All animal procedures were conducted according to the Guide for the Care and Use of Laboratory Animals (National Health and Medical Research Council, Australia). Procedures using BALB/c, C57/BL6, and HLA-B6 mice were approved by the Griffith University Animal Ethics Committee. PBMCs were obtained from healthy volunteers, and the study was performed in accordance with an approved Human Research Ethics Committee protocol.
Mice
The mice BALB/c and C57/BL6 (B6) were sourced from Animal Resource Centre, Western Australia, Perth. HLA-expressing humanized mice (HLA-DR3-DQ2) have been previously described (42). These mice were bred in the animal facility at Griffith University, Gold Coast, and were routinely genotyped for the appropriate transgene(s).
Bacteria
A total of four S. pyogenes isolates (SN1 to SN4) were recovered from patients during an STSS outbreak in one hospital in Brisbane, Australia. The isolation site for these isolates and the clinical description of patients are summarized in table S1. The molecular typing of S. pyogenes was performed by emm typing as reported elsewhere (43). For in vivo studies, SN1 and a SAg-negative group C streptococcal (GCS) isolate, NS33, were grown in Todd Hewitt Broth (THB) supplemented with 1% (w/v) neopeptone.
SAg gene profiling
Genes for SAgs including spea, spec, speg, speh, spei, spej, spek, spel, spez, and ssa were detected by polymerase chain reaction with primers as shown in table S2. Amplification for all genes was performed with an initial 5-min denaturation step at 95°C followed by 30 cycles of denaturation at 95°C for 30 s, 30 s of annealing at the appropriate temperature for each gene, as specified in table S2, followed by extension at 72°C for 30 s, with a final extension step of 72°C for 7 min.
Whole-genome sequencing of Alberta isolates
Alberta S. pyogenes isolates were sequenced on an Illumina MiSeq using a V3 600-cycle kit after library preparation with Nextera XT. Genomes were assembled with SPAdes 3.12.1 (PMID: 22506599) using Shovill 1.0.1 as a wrapper (github.com/tseemann/shovill), annotated with Prokka (PMID: 24642063) filtering any contigs that had a depth less than 3 or was <500 base pairs in length. Protein BLAST (PMID: 9254694) was used to look for alignments within the assembled S. pyogenes genomes and the J8 or SpyCEP epitopes, excluding any amino acid alignments that were shorter than the epitope length.
Peptides and proteins
The J8, J14, and J14.1 peptides were synthesized and conjugated to DT as described (16). rSpeC was purchased from Toxin Technology (USA) and has been previously described (44). To produce the rM1 protein, the gene encoding M1 protein (amino acids 13 to 455) was cloned into pGEX-2T (GE Healthcare Life Sciences), incorporating a C-terminal 6× His-tag as previously described (45).
Vaccination and generation of immune sera
For protection studies, mice were immunized subcutaneously on days 0, 21, and 28 with 30 μg of J8-DT/alum. The same protocol was used to generate immune serum where, following three immunizations, mice were bled periodically via submandibular veins to collect immune serum. To generate rSpeC-specific antibodies, mice were immunized with 10 μg of rSpeC formulated in alum and serum was collected after three immunizations. At defined time points, during and after immunizations, serum samples were collected, and antigen-specific immunoglobulin G (IgG) titers were determined by ELISA as described elsewhere.
Murine infection models
The superficial skin infection model has previously been described (15). Briefly, mice were infected with a preoptimized dose of SN1 or NS33. Mice were sacrificed at the noted time points, and the skin biopsy sample, spleen, and blood were collected. Tissues were homogenized, serially diluted, and plated on blood agar plate for enumeration of bacteria. Blood samples were also used for detection of toxin using Western blots assays. To model acute onset of STSS, the HLA-B6 and B6 mice were also challenged intraperitoneally as previously described (14). Briefly, mice were administered S. pyogenes (at varying doses) intraperitoneally in a 400-μl volume. To assess bacterial burden, mice were culled at defined time points; spleen and blood samples were harvested, serially diluted, and plated. For immunotherapy experiments following skin infection, mice were intraperitoneally administered 200 μl of designated antisera. For immunotherapy following intraperitoneal infection, mice intravenously received 200 μl of various antisera.
Clinical scoring
Following superficial skin or intraperitoneal infection and/or serum treatment, mice were monitored closely for the development of STSS-related pathology. Clinical scoring was performed blindly as per the scoring system defined by Shrum et al. (32). The scoring system included a number of variables to assess disease severity including appearance, level of consciousness, activity, response to stimulus, eyes, respiration rate, and respiration quality. The mice were scored from 0 to 4, where 0 represented the baseline and 4 was the highest score for each category.
Ex vivo experiments
For splenocyte activation experiments, cells were harvested from BALB/c, B6, or HLA-B6 mouse spleens, treated with ammonium chloride–potassium (ACK) lysis buffer (15 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA), and suspended (2 × 105 cells per well) in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum (Invitrogen), 0.1 mM minimal essential medium, nonessential amino acids, 2 nM l-glutamine, 1 nM sodium pyruvate, penicillin (100 U ml−1), streptomycin (100 μg ml−1) (all from Gibco Life Technologies), and 50 μM β-mercaptoethanol (Sigma). Optimized amounts of SAg-containing serum or recombinant proteins were added to wells, and 72 hours after stimulation, culture supernatants were collected for assessment of ex vivo cytokine production. Cytokines were measured using a TH1/TH2/TH17 cytometric bead array (CBA) kit (BD Biosciences) according to the manufacturer’s instructions. Samples were analyzed using a CyAn ADP flow cytometer, and data analysis was performed using BD FCAP array software. Proliferation was measured by the addition of [3H]thymidine (1 μCi per well) after 72 hours. After 18 hours, cells were harvested onto glass fiber mats (PerkinElmer, Australia) using a FilterMate cell harvester and thymidine incorporation was assessed using a β-scintillation microplate counter (PerkinElmer). To assess the SAg blocking effect of antibodies/antisera, the SAg-containing serum and test antiserum were premixed before addition to the wells.
PBMC stimulation assays
PBMCs were thawed and washed three times in complete medium [RPMI 1640 containing 10% heat-inactivated human serum, 2 nM l-glutamine, penicillin (100 U ml−1), and streptomycin (100 μg ml−1)], resuspended in complete medium, and counted using trypan blue (Sigma). For T cell proliferation assay, 2 × 105 cells in 100 μl were added per well. Subsequently, 100 μl of antigen or antigen + antisera (at a pre-optimized concentration) was added as a stimulant. As controls, wells with 1% phytohemagglutinin (Gibco) or media alone were used. PBMCs were cultured for 72 hours, following which proliferation was measured as described above. Following 72 hours of culture, supernatants were collected for measurement of cytokine responses using a TH1/TH2/TH17 CBA kit for humans (BD Biosciences).
SDS–polyacrylamide gel electrophoresis and Western blots
Standards, controls, and test samples including serum samples collected from SN1-infected and control mice were run in duplicate on precast 4 to 12% gradient bis-tris SDS–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Invitrogen) under reducing conditions at a constant current of 200 V per gel for approximately 25 min using a bolt electrophoresis mini cell apparatus (Invitrogen). One gel was stained with Coomassie brilliant blue (Coomassie Brilliant Blue R-250, Bio-Rad), and the other was transferred to nitrocellulose membrane (Novex) at 10-V constant for 60 min. After transfer, the membrane was blocked with blocking buffer (5% skim milk in PBS) for 60 min, followed by incubation for 1 hour at room temperature with specific monoclonal antibodies (anti-rabbit SpeC IgG, Toxin Technology). After excessive washing in PBS with 0.5% Tween 20, the membrane was incubated with sheep anti-rabbit IgG alkaline phosphatase antibody (Sigma-Aldrich) for 1 hour at room temperature. Following further washes, the blots were developed using SIGMAFAST BCIP/NBT (5-bromo-4-chloro-3-indolyl phosphate–nitro blue tetrazolium) (Sigma-Aldrich) according to the manufacturer’s instructions. The reaction was stopped when additional bands cease to develop.
Supplementary Material
Acknowledgments
We thank S. Yanow for critically reviewing the manuscript. Funding: This work was supported by grants from the National Health and Medical Research Council, Australia (grant nos. APP1037304 and APP1083548); National Foundation of Medical Research and Innovation (NFMRI, Australia); and an Australian Tropical Medicine Commercialization grant. Author contributions: M.P. and M.F.G. conceptualized and designed the experiments, analyzed the data, and wrote the manuscript. M.P., A.C., V.O., J.P., E.L., and J.-L.M. carried out in vitro and in vivo animal experiments. F.E.-C.J. provided intellectual and technical support for the toxin characterization work. Z.C. and J.M. provided the HLA-humanized mice, protocols for their breeding and genotyping, and also intellectual input. M.C. and G.J.T. performed genome analysis and provided data from Canadian isolates. J.R. provided clinical isolates and also intellectual input during the studies. All authors reviewed the manuscript. Competing interests: M.P. and M.F.G. are inventors on a Patent Cooperation Treaty (PCT) application related to this work filed by Griffith University [PCT application no. PCT/AU2019/050469, claiming priority of AU2018901709 (16 May 2018) and AU2018904377 (16 November 2018), filed on 16 May 2019]. The authors declare no other competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. The immune sera generated during the study and the HLA-humanized mice may be provided by Griffith University pending scientific review and a completed material transfer agreement. Requests for these materials should be submitted to M.P. and/or M.F.G. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax3013/DC1
Fig. S1. SAg profile of streptococcal isolates.
Fig. S2. Cytokine response of PBMCs following stimulation with vaccinated and control sera.
Fig. S3. Cytokine profile in HLA-B6 mice after stimulation with rM1.
Fig. S4. Immunological cross-reactivity between J14 and J14.1.
Table S1. Clinical isolates and medical history of patients.
Table S2. Primers used for SAg gene profiling.
Table S3. Distribution of spec and spea genes in ISD isolates from Canada.
Reference (46)
REFERENCES AND NOTES
- 1.Steer A. C., Lamagni T., Curtis N., Carapetis J. R., Invasive group a streptococcal disease: Epidemiology, pathogenesis and management. Drugs 72, 1213–1227 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention, ABCs report: Group A Streptococcus, 2016; www.cdc.gov/abcs/reports-findings/survreports/gas16.html.
- 3.Group A streptococcal diseases: For health professionals; www.canada.ca/en/public-health/services/diseases/group-a-streptococcal-diseases/health-professionals.html#a5.
- 4.Tyrrell G. J., Fathima S., Kakulphimp J., Bell C., Increasing rates of invasive group a streptococcal disease in Alberta, Canada; 2003–2017. Open Forum Infect. Dis. 5, ofy177 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lamagni T. L., Neal S., Keshishian C., Alhaddad N., George R., Duckworth G., Vuopio-Varkila J., Efstratiou A., Severe Streptococcus pyogenes infections, United Kingdom, 2003–2004. Emerg. Infect. Dis. 14, 202–209 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.A. Efstratiou, T. Lamagni, Epidemiology of Streptococcus pyogenes, in Streptococcus Pyogenes: Basic Biology to Clinical Manifestations, J. J. Ferretti, D. L. Stevens, V. A. Fischetti, Eds. (University of Oklahoma Health Sciences Center, 2016). [PubMed] [Google Scholar]
- 7.T. Proft, J. D. Fraser, Streptococcal superantigens: Biological properties and potential role in disease, in Streptococcus pyogenes: Basic Biology to Clinical Manifestations, J. J. Ferretti, D. L. Stevens, V. A. Fischetti, Eds. (University of Oklahoma Health Sciences Center, 2016). [PubMed] [Google Scholar]
- 8.Reglinski M., Sriskandan S., Turner C. E., Identification of two new core chromosome-encoded superantigens in Streptococcus pyogenes; speQ and speR. J. Inf. Secur. 78, 358–363 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Darenberg J., Luca-Harari B., Jasir A., Sandgren A., Pettersson H., Schalen C., Norgren M., Romanus V., Norrby-Teglund A., Normark B. H., Molecular and clinical characteristics of invasive group A streptococcal infection in Sweden. Clin. Infect. Dis. 45, 450–458 (2007). [DOI] [PubMed] [Google Scholar]
- 10.McCormick J. K., Tripp T. J., Olmsted S. B., Matsuka Y. V., Gahr P. J., Ohlendorf D. H., Schlievert P. M., Development of streptococcal pyrogenic exotoxin C vaccine toxoids that are protective in the rabbit model of toxic shock syndrome. J. Immunol. 165, 2306–2312 (2000). [DOI] [PubMed] [Google Scholar]
- 11.Kotb M., Ohnishi H., Majumdar G., Hackett S., Bryant A., Higgins G., Stevens D., Temporal relationship of cytokine release by peripheral blood mononuclear cells stimulated by the streptococcal superantigen pep M5. Infect. Immun. 61, 1194–1201 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B., Schlievert P. M., Gaber A. O., Kotb M., Localization of an immunologically functional region of the streptococcal superantigen pepsin-extracted fragment of type 5 M protein. J. Immunol. 151, 1419–1429 (1993). [PubMed] [Google Scholar]
- 13.Pahlman L. I., Olin A. I., Darenberg J., Mörgelin M., Kotb M., Herwald H., Norrby-Teglund A., Soluble M1 protein of Streptococcus pyogenes triggers potent T cell activation. Cell. Microbiol. 10, 404–414 (2008). [DOI] [PubMed] [Google Scholar]
- 14.Batzloff M. R., Hayman W. A., Davies M. R., Zeng M., Pruksakorn S., Brandt E. R., Good M. F., Protection against group A streptococcus by immunization with J8-diphtheria toxoid: Contribution of J8- and diphtheria toxoid-specific antibodies to protection. J. Infect. Dis. 187, 1598–1608 (2003). [DOI] [PubMed] [Google Scholar]
- 15.Pandey M., Langshaw E., Hartas J., Lam A., Batzloff M. R., Good M. F., A synthetic M protein peptide synergizes with a CXC chemokine protease to induce vaccine-mediated protection against virulent streptococcal pyoderma and bacteremia. J. Immunol. 194, 5915–5925 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Pandey M., Mortensen R., Calcutt A., Powell J., Batzloff M. R., Dietrich J., Good M. F., Combinatorial synthetic peptide vaccine strategy protects against hypervirulent CovR/S mutant streptococci. J. Immunol. 196, 3364–3374 (2016). [DOI] [PubMed] [Google Scholar]
- 17.Pandey M., Ozberk V., Langshaw E. L., Calcutt A., Powell J., Batzloff M. R., Rivera-Hernandez T., Good M. F., Skin infection boosts memory B-cells specific for a cryptic vaccine epitope of group A streptococcus and broadens the immune response to enhance vaccine efficacy. NPJ Vaccines 3, 15 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaman M., Ozberk V., Langshaw E. L., McPhun V., Powell J. L., Phillips Z. N., Ho M. F., Calcutt A., Batzloff M. R., Toth I., Hill G. R., Pandey M., Good M. F., Novel platform technology for modular mucosal vaccine that protects against streptococcus. Sci. Rep. 6, 39274 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Caro-Aguilar I., Ottinger E., Hepler R. W., Nahas D. D., Wu C., Good M. F., Batzloff M., Joyce J. G., Heinrichs J. H., Skinner J. M., Immunogenicity in mice and non-human primates of the group A streptococcal J8 peptide vaccine candidate conjugated to CRM197. Hum. Vaccin. Immunother. 9, 488–496 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sekuloski S., Batzloff M. R., Griffin P., Parsonage W., Elliott S., Hartas J., O’Rourke P., Marquart L., Pandey M., Rubin F. A., Carapetis J., McCarthy J., Good M. F., Evaluation of safety and immunogenicity of a group A streptococcus vaccine candidate (MJ8VAX) in a randomized clinical trial. PLOS ONE 13, e0198658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh P., The nonideal coiled coil of M protein and its multifarious functions in pathogenesis. Adv. Exp. Med. Biol. 715, 197–211 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vohra H., Dey N., Gupta S., Sharma A. K., Kumar R., McMillan D., Good M. F., M protein conserved region antibodies opsonise multiple strains of Streptococcus pyogenes with sequence variations in C-repeats. Res. Microbiol. 156, 575–582 (2005). [DOI] [PubMed] [Google Scholar]
- 23.Steer A. C., Magor G., Jenney A. W. J., Kado J., Good M. F., McMillan D., Batzloff M., Carapetis J. R., emm and C-repeat region molecular typing of beta-hemolytic Streptococci in a tropical country: Implications for vaccine development. J. Clin. Microbiol. 47, 2502–2509 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeppa J. J., Kasper K. J., Mohorovic I., Mazzuca D. M., Mansour Haeryfar S. M., McCormick J. K., Nasopharyngeal infection by Streptococcus pyogenes requires superantigen-responsive Vβ-specific T cells. Proc. Natl. Acad. Sci. U.S.A. 114, 10226–10231 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCormick J. K., Yarwood J. M., Schlievert P. M., Toxic shock syndrome and bacterial superantigens: An update. Annu. Rev. Microbiol. 55, 77–104 (2001). [DOI] [PubMed] [Google Scholar]
- 26.Kasper K. J., Zeppa J. J., Wakabayashi A. T., Xu S. X., Mazzuca D. M., Welch I., Baroja M. L., Kotb M., Cairns E., Cleary P. P., Haeryfar S. M. M., McCormick J. K., Bacterial superantigens promote acute nasopharyngeal infection by Streptococcus pyogenes in a human MHC class II-dependent manner. PLOS Pathog. 10, e1004155 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Darenberg J., Ihendyane N., Sjölin J., Aufwerber E., Haidl S., Follin P., Andersson J., Norrby-Teglund A.; The Streptlg Study Group , Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: A European randomized, double-blind, placebo-controlled trial. Clin. Infect. Dis. 37, 333–340 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Dan J. M., Havenar-Daughton C., Kendric K., Al-Kolla R., Kaushik K., Rosales S. L., Anderson E. L., LaRock C. N., Vijayanand P., Seumois G., Layfield D., Cutress R. I., Ottensmeier C. H., Arlehamn C. S. L., Sette A., Nizet V., Bothwell M., Brigger M., Crotty S., Recurrent group A Streptococcus tonsillitis is an immunosusceptibility disease involving antibody deficiency and aberrant TFH cells. Sci. Transl. Med. 11, eaau3776 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roggiani M., Stoehr J. A., Olmsted S. B., Matsuka Y. V., Pillai S., Ohlendorf D. H., Schlievert P. M., Toxoids of streptococcal pyrogenic exotoxin A are protective in rabbit models of streptococcal toxic shock syndrome. Infect. Immun. 68, 5011–5017 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nooh M. M., El-Gengehi N., Kansal R., David C. S., Kotb M., HLA transgenic mice provide evidence for a direct and dominant role of HLA class II variation in modulating the severity of streptococcal sepsis. J. Immunol. 178, 3076–3083 (2007). [DOI] [PubMed] [Google Scholar]
- 31.Good M. F., Pandey M., Batzloff M. R., Tyrrell G. J., Strategic development of the conserved region of the M protein and other candidates as vaccines to prevent infection with group A streptococci. Expert Rev. Vaccines 14, 1459–1470 (2015). [DOI] [PubMed] [Google Scholar]
- 32.Shrum B., Anantha R. V., Xu S. X., Donnelly M., Haeryfar S. M., McCormick J. K., Mele T., A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 7, 233 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Påhlman L. I., Mörgelin M., Eckert J., Johansson L., Russell W., Riesbeck K., Soehnlein O., Lindbom L., Norrby-Teglund A., Schumann R. R., Björck L., Herwald H., Streptococcal M protein: A multipotent and powerful inducer of inflammation. J. Immunol. 177, 1221–1228 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Tomai M., Kotb M., Majumdar G., Beachey E. H., Superantigenicity of streptococcal M protein. J. Exp. Med. 172, 359–362 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Proft T., Sriskandan S., Yang L., Fraser J. D., Superantigens and streptococcal toxic shock syndrome. Emerg. Infect. Dis. 9, 1211–1218 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ekelund K., Skinhøj P., Madsen J., Konradsen H. B., Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: Results from a nationwide study. J. Clin. Microbiol. 43, 1789–1796 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sriskandan S., Unnikrishnan M., Krausz T., Dewchand H., Van Noorden S., Cohen J., Altmann D. M., Enhanced susceptibility to superantigen-associated streptococcal sepsis in human leukocyte antigen-DQ transgenic mice. J. Infect. Dis. 184, 166–173 (2001). [DOI] [PubMed] [Google Scholar]
- 38.Linnér A., Darenberg J., Sjölin J., Henriques-Normark B., Norrby-Teglund A., A. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: A comparative observational study. Clin. Infect. Dis. 59, 851–857 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Sriskandan S., Ferguson M., Elliot V., Faulkner L., Cohen J., Human intravenous immunoglobulin for experimental streptococcal toxic shock: Bacterial clearance and modulation of inflammation. J. Antimicrob. Chemother. 58, 117–124 (2006). [DOI] [PubMed] [Google Scholar]
- 40.Jolles S., Sewell W. A. C., Misbah S. A., Clinical uses of intravenous immunoglobulin. Clin. Exp. Immunol. 142, 1–11 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B., Yurecko R. S., Dedhar S., Cleary P. P., Integrin-linked kinase is an essential link between integrins and uptake of bacterial pathogens by epithelial cells. Cell. Microbiol. 8, 257–266 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Chen Z., Dudek N., Wijburg O., Strugnell R., Brown L., Deliyannis G., Jackson D., Koentgen F., Gordon T., McCluskey J., A 320-kilobase artificial chromosome encoding the human HLA DR3-DQ2 MHC haplotype confers HLA restriction in transgenic mice. J. Immunol. 168, 3050–3056 (2002). [DOI] [PubMed] [Google Scholar]
- 43.Centers for Disease Control and Prevention, Protocol for emm typing; www.cdc.gov/streplab/groupa-strep/emm-typing-protocol.html.
- 44.Goshorn S. C., Bohach G. A., Schlievert P. M., Cloning and characterization of the gene, speC, for pyrogenic exotoxin type C from Streptococcus pyogenes. Mol. Gen. Genet. 212, 66–70 (1988). [DOI] [PubMed] [Google Scholar]
- 45.Sanderson-Smith M., Batzloff M., Sriprakash K. S., Dowton M., Ranson M., Walker M. J., Divergence in the plasminogen-binding group a streptococcal M protein family: Functional conservation of binding site and potential role for immune selection of variants. J. Biol. Chem. 281, 3217–3226 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Berman H. F., Tartof S. Y., Reis J. N., Reis M. G., Riley L. W., Distribution of superantigens in group A streptococcal isolates from Salvador, Brazil. BMC Infect. Dis. 14, 294 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/9/eaax3013/DC1
Fig. S1. SAg profile of streptococcal isolates.
Fig. S2. Cytokine response of PBMCs following stimulation with vaccinated and control sera.
Fig. S3. Cytokine profile in HLA-B6 mice after stimulation with rM1.
Fig. S4. Immunological cross-reactivity between J14 and J14.1.
Table S1. Clinical isolates and medical history of patients.
Table S2. Primers used for SAg gene profiling.
Table S3. Distribution of spec and spea genes in ISD isolates from Canada.
Reference (46)


