Abstract
A moderate reduction of body temperature can induce a remarkable lifespan extension. Here we examine the link between cold temperature, germ line fitness and organismal longevity. We show that low temperature reduces age-associated exhaustion of germ stem cells (GSCs) in Caenorhabditis elegans, a process modulated by thermosensory neurons. Notably, robust self-renewal of adult GSCs delays reproductive aging and is required for extended lifespan at cold temperatures. These cells release prostaglandin E2 (PGE2) to induce cbs-1 expression in the intestine, increasing somatic production of hydrogen sulfide (H2S), a gaseous signaling molecule that prolongs lifespan. Whereas loss of adult GSCs reduces intestinal cbs-1 expression and cold-induced longevity, application of exogenous PGE2 rescues these phenotypes. Importantly, tissue-specific intestinal overexpression of cbs-1 mimics cold-temperature conditions and extends longevity even at warm temperatures. Thus, our results indicate that GSCs communicate with somatic tissues to coordinate extended reproductive capacity with longevity.
Introduction
The aging process is modulated by environmental and genetic factors1,2. Although extreme cold is detrimental3, a moderate reduction of body temperature induces a remarkable lifespan extension4. As such, lowering body temperature is one of the most effective interventions to extend organismal lifespan4. This phenomenon was first observed in poikilotherms, including Caenorhabditis elegans 5–7, the fruit fly Drosophila melanogaster 8, and distinct fish species9,10. For instance, C. elegans lives shorter when shifted from the standard culturing temperature (20°C) to higher temperatures, whereas lower temperature (e.g., 15°C) extends lifespan7,11,12. In mouse models, a 0.5-0.6°C reduction of core body temperature induces a 20% lifespan extension13. Although the longevity effects of lowering core body temperature were originally reported over a century ago14, little is known about the mechanisms underlying this process. Since temperature essentially influences every chemical and biological process, it was conventionally considered that longevity ensues from a passive thermodynamic process. In this view, cold temperature reduces the rate of chemical reactions and metabolism, resulting in slower molecular entropy, energy expenditure and pace of living. However, recent work in C. elegans reported that the cold-sensitive TRPA-1 channel detects low temperature during adulthood, leading to a calcium ion influx that promotes lifespan extension12,15. Conversely, loss of TRPA-1 channel shortens lifespan at cold temperature, while it does not affect lifespan at warm temperature12. Further studies identified that TRPA-1 acts in IL1 neurons, inducing neuroendocrine signaling circuits to extend longevity at cold temperature16. In addition, the co-chaperone daf-41/p23 is also necessary for lifespan extension at low temperature17. Thus, cold-induced longevity is a regulated process that cannot be simply explained by passive changes in chemical reactions.
As temperature, fecundity is also negatively correlated with lifespan18. Among invertebrates, birds and mammals, interventions that limit investment in the germ line and reproduction induce lifespan extension18. Since tissue homeostasis and physiological integrity are constantly challenged by ever-changing metabolic, pathological and environmental conditions, evolutionary pressure has been theorized to force a re-allocation of energetic resources to prevent and repair damage to the germ line19. By this compromised distribution, the organism will ensure its reproduction, generating a healthy and fit progeny. In these lines, extensive evidence in C. elegans indicates that the germ line promotes somatic aging under standard and stress conditions20–22. As such, germline-lacking worms can live up to 60% longer and exhibit increased resistance to a variety of environmental stressors, including high temperatures20–22. Remarkably, these effects are not simply a result of sterility20,22. Under standard (20°C) and warm temperatures, the germ line is responsible for the generation of signals that promote progressive deterioration of the soma and organismal aging20,21. Conversely, removal of the germ line activates pro-longevity transcriptional factors in somatic tissues, resulting in lifespan extension20–24. Eventually, these transcription factors modulate downstream processes within somatic cells, such as increased autophagy or proteome stability, that contribute more directly to the longevity phenotype22,24–26. Thus, these findings establish proliferating germ cells as active inhibitors of pro-longevity pathways in somatic tissues, reducing organismal lifespan.
While the impact of the germ line on lifespan at standard and warm temperatures has been extensively studied, here we examine the link between low temperature, germ line fitness and organismal longevity. Remarkably, we find that cold temperature does not extend lifespan of germline-lacking worms. Since germline-lacking worms live significantly shorter at low temperatures when compared with wild-type worms, our results indicate that the germ line is required for cold-induced longevity. Prompted by these results, we assess whether low temperature impacts on germline aging. Indeed, low temperature ameliorates exhaustion of the germ stem cell (GSC) pool during adulthood, resulting in a delay of reproductive aging. This process is regulated by thermosensory neurons, which detect low temperatures to maintain GSC self-renewal with age. Notably, cold-induce longevity is blocked by chemical and genetic interventions that specifically diminish adult GSC proliferation. On the contrary, these interventions do not affect lifespan at higher temperatures. Robust proliferation of adult GSCs induces the expression of CBS-1 in somatic tissues such as the intestine, increasing the production of hydrogen sulfide (H2S), a gaseous signaling molecule that extends lifespan27,28. Importantly, overexpression of cbs-1 in the intestine extends lifespan even at warm temperatures. In addition, we find that low temperature increases the production and release of prostaglandin E2 (PGE2) by GSCs, a hormone that modulates somatic tissues and promotes cold-induced longevity. Whereas loss of adult GSCs reduces somatic cbs-1 expression and cold-induced longevity, application of exogenous PGE2 rescues these phenotypes. Taken together, our results indicate that adult GSCs communicate with somatic tissues via prostaglandin signals to extend longevity at cold temperature. This process coordinates extended reproductive capacity and long lifespan, without compromising on either the germ line or the soma.
Results
The germ line is essential for cold-induced longevity during adulthood
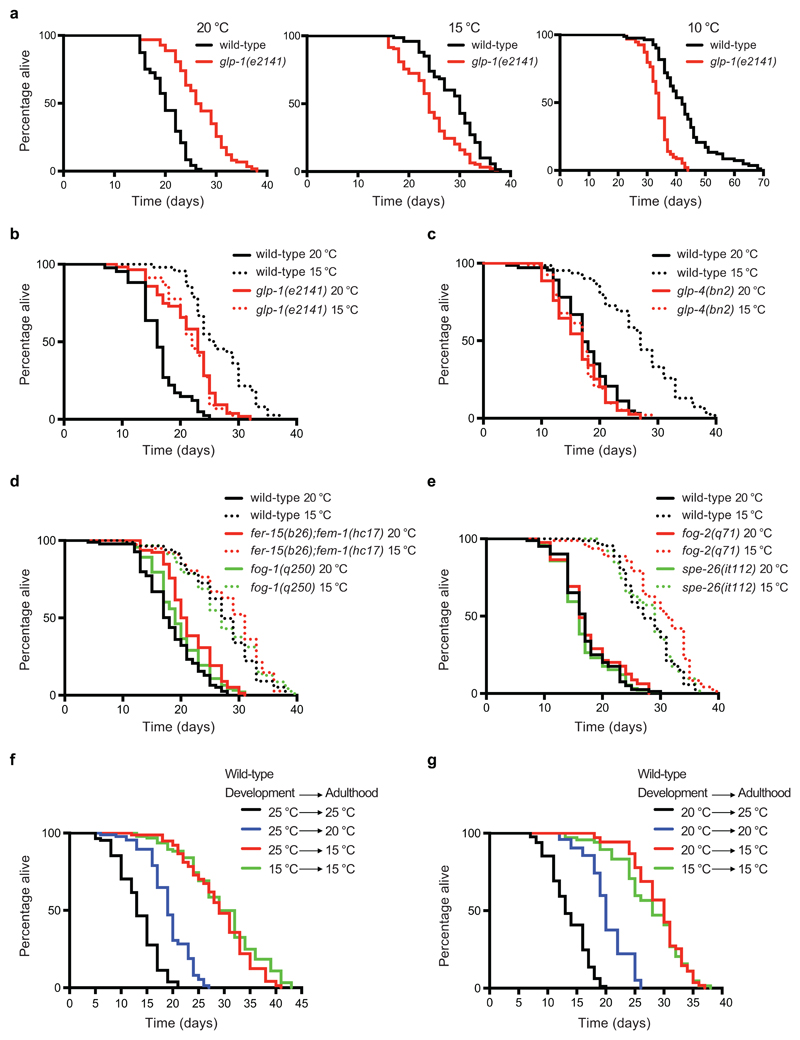
With the strong lifespan extension induced by lowering body temperature, we asked what role, if any, do germ cells have in this phenotype. For this purpose, we first examined glp-1(e2141) mutant worms, which develop into adults lacking a germ line due to a failure in germ-cell proliferation during development at the restrictive temperature (25°C)29. Thus, we raised glp-1(e2141) mutant larvae at 25°C to obtain germline-lacking adults and shifted them to distinct temperatures. As extensively reported20,23, glp-1 germline-lacking worms were long-lived at 20°C when compared with wild-type animals under the same conditions (Fig. 1a). On the contrary, we found that glp-1 mutants lived significantly shorter in comparison with wild-type worms at lower temperatures (i.e., 15°C, 10°C) (Fig. 1a).
Figure 1. The germ line is essential for cold-induced longevity during adulthood.
a, Germline-lacking worms (glp-1(e2141)) are long lived compared to wild-type (N2) strain at 20°C (N2 20°C mean ± s.e.m: 20.15 ± 0.40, glp-1 20°C: 26.90 ± 0.58, P<0.0001). In contrast, germline-lacking worms are short lived in comparison with wild-type worms at cold temperatures (N2 15°C: 29.01 ± 0.58, glp-1 15°C: 24.39 ± 0.56, P<0.0001; N2 10°C: 42.94 ± 1.05, glp-1 10°C: 33.95 ± 0.46, P<0.0001). b, Temperature reduction (15°C) extends lifespan of wild-type worms (N2 20°C mean ± s.e.m: 16.40 ± 0.60, N2 15°C: 26.73 ± 0.77, P<0.0001) but not glp-1 germline-lacking worms (glp-1 20°C: 21.59 ± 0.66, glp-1 15°C: 21.78 ± 0.60, P= 0.6396). Wild-type worms are long lived compared with glp-1 mutant worms at cold temperature (N2 15°C versus glp-1 15°C, P<0.0001). c, Temperature reduction extends lifespan of wild-type worms (N2 20°C mean ± s.e.m: 17.83 ± 0.58, N2 15°C: 26.81 ± 0.90, P<0.0001) but not glp-4(bn2) germline-lacking worms (glp-4 20°C: 16.44 ± 0.49, glp-4 15°C: 16.71 ± 0.45, P= 0.7379). Wild-type worms are long lived compared with glp-4 mutant worms at cold temperature (N2 15°C versus glp-4 15°C, P<0.0001). d, Temperature reduction extends lifespan of sterile fer-15(b26);fem-1(hc17) and fog-1(q250) mutant worms with a proliferating germ line. N2 20°C mean ± s.e.m: 18.17 ± 0.53 versus N2 15°C: 27.20 ± 0.67, P<0.0001; fer-15;fem-1 20°C: 21.42 ± 0.50 versus fer-15;fem-1 15°C: 28.46 ± 0.74, P<0.0001; fog-1 20°C: 19.62 ± 0.49 versus fog-1 15°C: 27.22 ± 0.70, P<0.0001. e, Temperature reduction (15°C) extends lifespan of sterile fog-2(q71) and spe-26(it112) mutant worms with a proliferating germ line. N2 20°C mean ± s.e.m: 17.01 ± 0.48 versus N2 15°C: 27.79 ± 0.60, P<0.0001; fog-2 20°C: 16.55 ± 0.46 versus fog-2 15°C: 27.94 ± 0.62, P<0.0001; spe-26 20°C: 17.51 ± 0.54 versus spe-26 15°C: 29.96 ± 0.67, P<0.0001. f, Wild-type larvae were raised at 25°C and then adult worms were shifted to the indicated temperatures. Exposure to low temperature (15°C) during adulthood is sufficient to extend longevity (P<0.0001). 25°C (development and adulthood) mean ± s.e.m: 13.38 ± 0.43, shifted to 20°C after development: 19.28 ± 0.44, shifted to 15°C after development: 29.07 ± 0.72, 15°C (development and adulthood): 29.89 ± 0.76. g, After development at 20°C, adult wild-type worms were shifted to the indicated temperatures. Exposure to low temperature during adulthood extends longevity (P<0.0001). 20°C (development and adulthood) mean ± s.e.m: 20.25 ± 0.44, shifted to 25°C after development: 13.66 ± 0.37, shifted to 15°C after development: 29.11 ± 0.53, 15°C (development and adulthood): 26.65 ± 0.70. P-values: two-sidedlog-rank test, n= 96 worms/condition. See Supplementary Data 3 for statistical analysis and replicate data of lifespan experiments.
Although our results indicate that the pro-longevity effects of low temperature are diminished in glp-1 germline-lacking mutants (Fig. 1b), the fact that these worms are long-lived at 20°C makes our results difficult to interpret. To further assess the impact of germline depletion in cold-induced longevity, we used glp-4(bn2) mutants, a distinct germline-lacking strain which has no lifespan increase relative to wild-type at 20°C30,31 (Fig. 1c). Notably, low temperature (15°C) did not extend lifespan of glp-4 germline-lacking worms, as we observed in glp-1 mutants (Fig. 1b, c). To determine whether this is a consequence of sterility per se, we assessed fer-15(b26);fem-1(hc17) worms that are also sterile but which still contain a proliferating germ line when raised at the restrictive temperature (25°C) during development (Supplementary Fig. 1). We found that low temperature during adulthood increased lifespan of the control sterile strain whereas it did not extend lifespan of glp-1 germline-lacking mutants (Supplementary Fig. 2). As a result, sterile control worms lived longer in comparison with glp-1 mutants at low temperature (Supplementary Fig. 2). Similar to fer-15;fem-1 worms, low temperature also extended the lifespan of distinct sterile mutants with spermatogenesis defects but which contain a proliferating germ line and generate oocytes, such as fog-1, fog-2 and spe-26 mutant worms32–36 (Fig. 1d, e). Thus, our results indicate that loss of cold-induced longevity is not due to sterility, but specific to depletion of the germ line. In all the above experiments, we raised both mutant and wild-type worms at the same temperature until they reached adulthood and then shifted them to the distinct temperatures to assess adult lifespan. Thus, our results suggest that the germ line is particularly important during adulthood for the cold-induced longevity phenotype. In support of this hypothesis, exposure of wild-type C. elegans to low temperature only during adulthood was sufficient to prolong lifespan after development at either 25°C or 20°C (Fig. 1f, g)15.
Cold temperature delays exhaustion of GSCs and reproductive aging
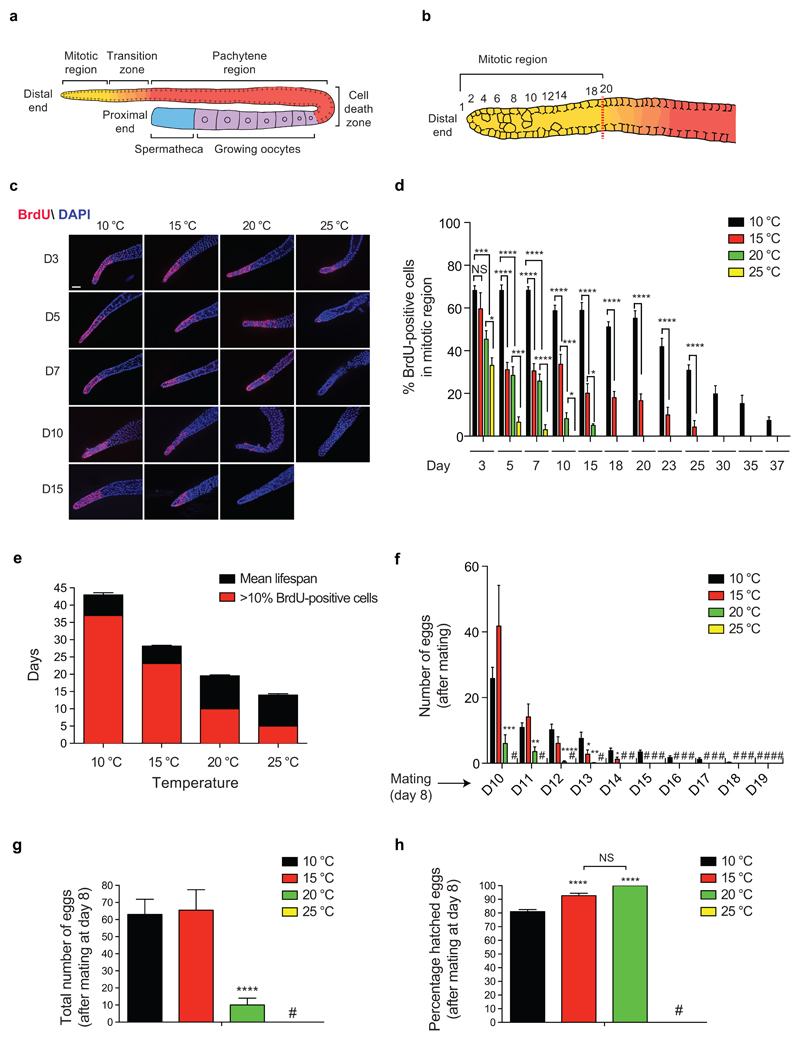
Prompted by our results in germline-lacking worms, we hypothesized that temperature-associated changes in the adult germ line determine cold-induced longevity. In adult C. elegans, the germ line is composed by a mitotic zone at the distal end followed by regions containing cells from early meiotic stages to maturing gametes, extending proximally37 (Fig. 2a, b). Whereas adult germ cells are continuously lost during gametogenesis and cell death events, proliferating germ stem cells (GSCs) of the mitotic region self-renew and provide new pools of cells which enter meiosis to replenish the germ line and generate gametes38. To determine the impact of temperature and age in adult germline proliferation, we raised wild-type larvae at 20°C until adulthood and then shifted them to different temperatures. Notably, the total number of germ cells within the mitotic region did not change with age or temperature (Fig. 2c and Supplementary Fig. 3). On the contrary, low temperature delayed the age-associated decline in the percentage of mitotic cells, which were labeled with bromodeoxyuridine (BrdU) (Fig. 2c, d). In addition, we observed active proliferation of germ cells for an extended fraction of the animal’s mean lifespan at cold temperatures (Fig. 2e). At 25°C, the percentage of proliferating germ cells was strongly reduced (i.e., less than 10% BrdU-positive cells) at day 5 whereas the organismal mean lifespan was 14 days (Fig. 2d, e). At 20°C, worms had a mean lifespan of 19.5 days with a strong decline in proliferating germ cells at day 10. However, adult worms at 15°C delayed the acute decline in proliferating germ cells until day 23, closer to their mean lifespan of 28 days. Likewise, worms at 10°C maintained a high percentage of proliferating germ cells until day 37, while their mean lifespan was 43 days (Fig. 2d, e). Thus, these results indicate that low temperature extends the ability of GSCs to self-renew during aging, sustaining the pool of proliferating cells within the mitotic region.
Figure 2. Cold temperature delays exhaustion of GSCs and reproductive aging.
a, Schematic representation of one gonad of the young adult hermaphrodite germ line. Germ cells are derived from proliferating GSCs located at the mitotic region. Proliferating cells enter meiosis and move proximally from the mitotic region to the meiotic zones (transition zone, pachytene). Germ cell apoptosis occurs in the germline loop region (death cell zone). GSCs generate sperm during larval stages, then switch to oocyte production during adulthood. b, Representation of the mitotic region. Red dashed line corresponds to the point at which multiple germ cells exhibit an early meiotic phenotype, considered the boundary between the mitotic region and the transition zone (row 20 of cells from the most distal cell). c, Bromodeoxyuridine (BrdU) staining of proliferating germ cells. Wild-type C. elegans were shifted to the distinct temperatures during adulthood and the germ lines were extruded after 2 h BrdU treatment at the indicated day (D) of age. Cell nuclei were stained with DAPI. Scale bar represents 20 µm. The images are representative of 4 independent experiments. d, Graph represents the percentage of BrdU-positive cells/total nuclei within the mitotic region (mean ± s.e.m., 10°C D3-37, 15°C D3-23 (n=20 germ lines), 15°C D23, 20°C D3-15, 25°C D3-10 (n= 15), germ lines were scored from 3 independent experiments). We did not examine the germ line after D15 at 25°C, D18 at 20°C, D25 at 15°C and D37 at 10°C as the percentage of BrdU-positive cells was below 10% at these ages/temperatures. NS= not significant (P=0.3322). e, Graph represents the average ± s.e.m. of the mean lifespan at 10°C (n= 15 independent experiments), 15°C (n= 56), 20°C (n= 29) and 25°C (n= 22). The graph also indicates until what day of the respective mean lifespan is germ cell proliferation maintained at each temperature (>10% BrdU-positive cells in the mitotic region), as inferred from the data presented in the previous panel. f, Adult worms were cultured at the indicated temperature during adulthood. After the self-reproductive period, worms were mated at day 8 of adulthood with young males raised at 20°C and kept at the respective temperatures. Graph represents the number of eggs laid per worm every 24 h after mating (mean ± s.e.m., n= 24 worms scored per condition from 3 independent experiments). (#) no eggs were laid. g, Total number of eggs laid per worm at different temperatures after mating at day 8 of adulthood (mean ± s.e.m., n= 24 worms from 3 independent experiments). (#) no eggs were laid by mated worms at 25°C. h, Percentage of hatched eggs at different temperatures (mean ± s.e.m., n= 24 worms from 3 independent experiments). The percentage of viable eggs after mating is similar at 15°C and 20°C (NS= not significant, P=0.0715). Although egg hatching decreases at 10°C, most of the eggs (81%) were still viable. (#) no eggs were laid. Statistical comparisons were made by two-tailed Student’s t-test for unpaired samples. P-value: *(P<0.05), **(P<0.01), ***(P<0.001), ****(P<0.0001).
Besides their self-renewal ability, GSCs also produce differentiating gametes. Since more than half of the developing oocytes undergo apoptosis whereas the remaining cells progress to terminal differentiation and fertilization, GSCs are critical to replenish the pool of oocytes39,40. During development, the first germ cells in hermaphrodites differentiate into spermatocytes, resulting in a limited number of 240-320 sperm, whereas subsequent germ cells generate oocytes during adulthood which can be then self-fertilized and ovulated32,34 (Fig. 2a). At standard temperature, C. elegans hermaphrodites reproduce during the first 4-5 days of adulthood and quickly exhaust their supply of self-sperm41. Likewise, worms at 15°C also self-reproduced until day 5 (Supplementary Fig. 4a). Although worms extended their self-reproductive period until day 8 of adulthood at 10°C (Supplementary Fig. 4a), the total number of eggs laid by hermaphrodites was not increased at cold temperatures (Supplementary Fig. 4b). Moreover, most of the laid eggs hatched in all the temperatures tested (Supplementary Fig. 4c). However, this period is not sufficient to assess reproductive capacity because self-progeny brood sizes are determined by the number of self-sperm41. To circumvent this limitation, 8-day adult hermaphrodites at the distinct temperatures were mated with young males for 48 h to replenish the pool of sperm. At 10°C and 15°C, worms were able to lay eggs during the following 9 and 5 days after mating, respectively (Fig. 2f). In contrast, worms at 20°C laid eggs essentially within the first 2 days after mating whereas we did not observe eggs at higher temperature. Notably, the total number of eggs laid at low temperatures was markedly higher when compared with 20°C (Fig. 2g). In addition, most of these eggs were hatched into viable larvae (Fig. 2h). Taken together, our results indicate that cold temperature sustains the GSC pool during adulthood, resulting in a higher maintenance of the proliferating germline region and reproductive capacity with age.
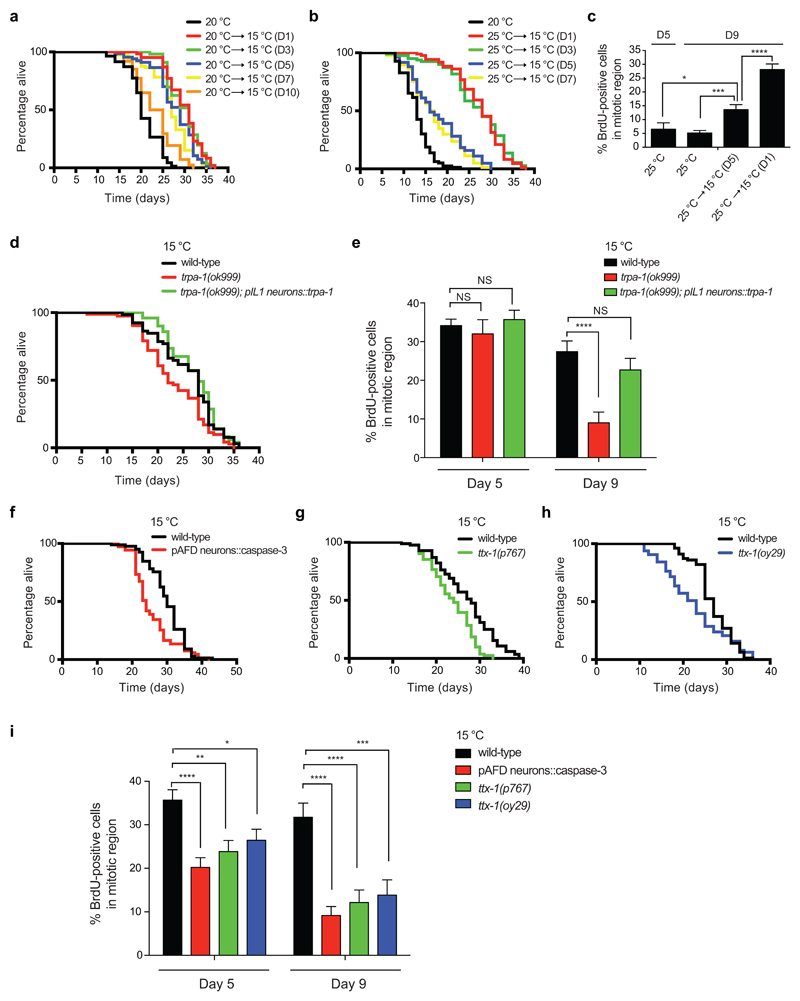
To assess whether maintenance of GSCs correlates with the capacity to induce cold-associated longevity, we shifted worms from higher temperature to lower temperature at different adult stages. Remarkably, cold-induced longevity was dramatically reduced when worms at 20°C were shifted to lower temperature at day 10 of adulthood (Fig. 3a), correlating with the acute decline in germline proliferation at 20°C (i.e., <10% BrdU-positive cells). Similarly, worms displayed a diminished ability to live longer under cold temperature once they reached day 5 of adulthood at 25°C (Fig. 3b), correlating with the demise of germline proliferation at this temperature (Fig. 2d). Despite the reduction in the length of cold-induced longevity, worms with low percentage of proliferating germ cells still exhibited a partial lifespan extension when shifted to cold temperature (Fig. 3a, b). In these lines, we found that shifting the worms to cold temperature after an acute decline in germline proliferation could also partially recover this phenotype (Fig. 3c).
Figure 3. Thermosensory neurons regulate cold-induced longevity and GSC proliferation.
a, Lifespan of worms at 20°C shifted to lower temperature (15°C) at the indicated days (D). 20°C mean ± s.e.m: 20.72 ± 0.43, shifted to 15°C at D1: 29.85 ± 0.52 (P<0.0001), shifted to 15°C at D3: 29.86 ± 0.49 (P<0.0001), shifted to 15°C at D5: 27.75 ± 0.58 (P<0.0001), shifted to 15°C at D7: 26.92 ± 0.53 (P<0.0001), shifted to 15°C at D10: 23.26 ± 0.48 (P<0.0001). Cold-induced longevity decreases when worms are shifted to lower temperature at day 10 (D10 versus D1-7, P<0.0001). b, Lifespan of worms at 25°C shifted to 15°C at different days. 25°C mean ± s.e.m: 13.09 ± 0.35, shifted to 15°C at D1: 27.43 ± 0.60 (P<0.0001), shifted to 15°C at D3: 26.90 ± 0.80 (P<0.0001), shifted to 15°C at D5: 17.74 ± 0.68 (P<0.0001), shifted to 15°C at D7: 16.98 ± 0.62 (P<0.0001). Cold-induced longevity decreases when worms are shifted to lower temperature at day 5 (D5 versus D1-3, P<0.0001). Worms shifted to 15°C at either day 5 or day 7 exhibit a similar lifespan (P<0.2661). c, Percentage of BrdU-positive cells/total nuclei within the mitotic region (mean ± s.e.m., 25°C D5 and D9 (n=16 germ lines), shifted to 15°C at D1 or D5 (n=20), germ lines from 2 independent experiments). Worms were maintained at 25°C until BrdU analysis (D5 and D9) or shifted to 15°C at the indicated days before BrdU analysis at D9 of adulthood. NS= not significant (D5: WT versus trpa-1 (P=0.6091); WT vs trpa-1;pIL1::trpa-1 (P=0.5948). D9: WT vs trpa-1;pIL1::trpa-1 (P=0.2502). d, Transgenic expression of trpa-1 in IL1 neurons driven by aqp-6 promoter rescues the short-lived phenotype of trpa-1 mutants at 15°C. Wild-type mean ± s.e.m: 25.97 ± 0.73, trpa-1(ok999): 23.34 ± 0.72 (P=0.0127), trpa-1(ok999);pIL1::trpa-1: 27.70 ± 0.70 (P=0.4163). e, Expression of trpa-1 in IL1 neurons rescues low germline proliferation in trpa-1 mutant worms at 15°C. Graph represents the percentage of BrdU-positive cells/total nuclei in the indicated strains at day 5 and 9 of adulthood (mean ± s.e.m., 15 germ lines from 2 independent experiments). f, Specific AFD ablation by driving reconstituted caspase-3 under gcy-8 promoter reduces cold-induced longevity at 15°C (N2 wild-type mean ± s.e.m: 29.88 ± 0.58, pAFD::caspase-3: 25.61 ± 0.69, P= 0.0002). g, ttx-1(p767) mutant worms are short-lived at 15°C (N2 mean ± s.e.m: 27.05 ± 0.69, ttx-1(p767): 23.64 ± 0.54, P< 0.0001). h, ttx-1(oy29) mutant worms are short-lived at 15°C (N2 mean ± s.e.m: 26.81 ± 0.50, ttx-1(oy29): 22.49 ± 0.91, P= 0.0267). i, Percentage of BrdU-positive cells/total nuclei in the indicated strains at day 5 and 9 of adulthood (mean ± s.e.m., D5 (n=30 germ lines), D9 (n=15), germ lines from 3 independent experiments). In lifespan experiments, P-values: two-sided log-rank test, n= 96 worms/condition (see Supplementary Data 3 for statistical analysis and replicate data). In Figures 3c, 3e and 3i, statistical comparisons were made by two-tailed Student’s t-test for unpaired samples. P-value: *(P<0.05), **(P<0.01), ***(P<0.001), ****(P<0.0001).
Since somatic tissues such as thermosensory neurons detect changes in temperature12,16,42,43, we asked whether these cells modulate germline maintenance at cold temperature. The cold-sensitive channel TRPA-1 senses temperature decrease in the intestine and neurons to extend lifespan12,15. Concomitantly, loss of function of trpa-1 reduces the length of cold-induced longevity12,15 (Fig. 3d). At early adulthood stages, lack of trpa-1 did not affect germline proliferation under cold temperature (Fig. 3e and Supplementary Fig. 5). However, germline proliferation dramatically decreased in trpa-1-lacking mutants when compared with wild-type worms at older age, whereas the total number of germ cells within the mitotic region remained similar (Fig. 3e and Supplementary Fig. 5). Cold-sensitive IL1 neurons express trpa-1 44 and are required for the long-lived phenotype induced by low temperature16. Because transgenic expression of trpa-1 in IL1 neurons alone is sufficient to rescue the short lifespan of trpa-1-lacking mutants at lower temperatures16 (Fig. 3d), we examined whether it also ameliorates the decline in germline proliferation. Indeed, specific transgenic expression of trpa-1 in IL1 neurons rescued the defects in germline proliferation of trpa-1 mutants at cold temperature (Fig. 3e and Supplementary Fig. 5). Since loss of trpa-1 does not completely abolish cold-induced longevity at 15°C12, we hypothesized that other thermosensory neurons could also contribute to this phenotype. A pair of thermoreceptor neurons, called AFD, are required for temperature-related behaviors42 but do not express TRPA-1 channel44. To assess whether AFD neurons modulate cold-induced longevity, we used worms expressing reconstituted caspase-3 under AFD-specific promoter to kill these pair of neurons43. As we performed in our previous experiments with trpa-1 mutants, we raised AFD-ablated worms at 20°C during development and then shifted them to distinct temperatures to assess adult lifespan. Notably, we found that AFD-ablated transgenic animals lived significantly shorter at cold temperature when compared with wild-type worms (Fig. 3f and Supplementary Fig. 6a). On the contrary, loss of AFD neurons did not affect lifespan at 20°C and 25°C (Supplementary Fig. 6b-c). To further assess the role of these neurons in cold-induced longevity, we examined two distinct strains carrying mutations in the ttx-1 gene that specifically disrupt AFD structure and function45,46. Both ttx-1 mutants were short-lived when cultured at 10°C or 15°C during adulthood (Fig. 3g-h and Supplementary Fig. 7a-b). By contrast, ttx-1 mutants had no significant lifespan change relative to wild-type at either 20°C or 25°C (Supplementary Fig. 7c-f). In both AFD-ablated and ttx-1 mutant worms, we observed a decrease in germline proliferation under cold temperature, particularly at older age (Fig. 3i and Supplementary Fig. 8). Collectively, our results indicate that detection of low temperature by thermosensory circuits such as IL1 and AFD neurons delays GSC exhaustion.
Robust proliferation of adult GSCs determines cold-induced longevity
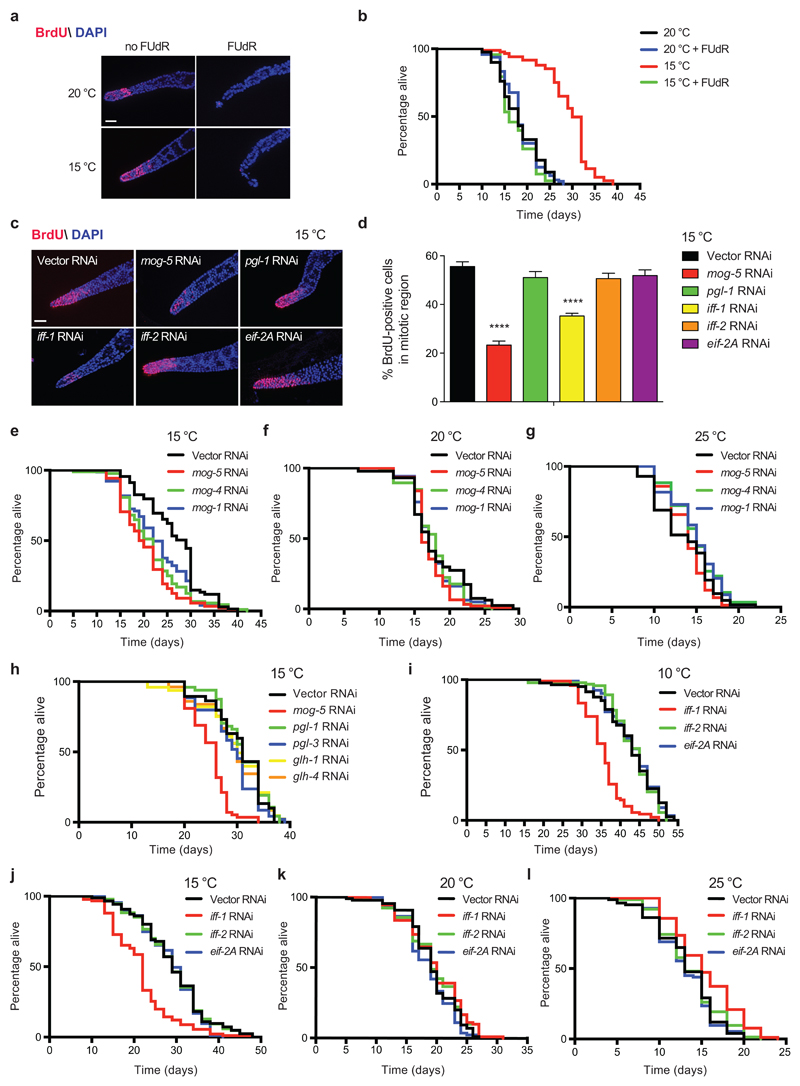
Our experiments with germline-lacking worms indicated that the germ line is required for cold-induced longevity. In addition, we found that low temperature maintains robust GSC proliferation during adulthood. Intrigued by these results, we asked whether there is a direct and active role of adult GSCs in cold-induced longevity. To address this hypothesis, we let worms to develop at 20°C into adults with a normal germ line and then inhibited adult GSC function by different means. In adult worms, the germ cells within the mitotic region constitute the only pool of proliferating cells in the organism47. Thus, the treatment with 5-fluoro-2′deoxyuridine (FUdR), an inhibitor of DNA synthesis, could particularly affect these cells in adult worms. Indeed, FUdR treatment initiated during adulthood dramatically reduced GSC proliferation at either 20°C or 15°C (Fig. 4a). Whereas FUdR treatment did not affect adult lifespan at 20°C, it blocked the lifespan extension induced by cold temperature (Fig. 4b).
Figure 4. Adult GSC proliferation determines cold-induced longevity.
a, BrdU staining of germ lines from wild-type worms treated with 100 μg ml-1 FUdR during adulthood at the indicated temperatures after development at 20°C. Worms were examined at day 5 of adulthood. Nuclei were stained with DAPI. Scale bar represents 20 µm. The images are representative of 3 independent experiments. b, FUdR during adulthood does not affect lifespan at 20°C (20°C mean ± s.e.m: 18.23 ± 0.49, 20°C + FUdR: 18.28 ± 0.40, P= 0.8617), but reduces cold-induced longevity (15°C: 29.08 ± 0.60, 15°C + FUdR: 17.49 ± 0.40, P<0.0001). c, BrdU staining of germ lines from wild-type worms examined at day 5 of adulthood. Scale bar represents 20 µm. The images are representative of 3 independent experiments. d, Percentage of BrdU-positive cells/total nuclei (mean ± s.e.m., Vector/mog-5/iff-1 RNAi (n= 33 germ lines), pgl-1/iff-2/eif-2A RNAi (n= 36), germ lines from 3 independent experiments). Analysis by two-tailed Student’s t-test for unpaired samples (**** (P<0.0001)). e, Knockdown of mog genes shortens cold-induced longevity (15°C) in wild-type worms. Vector RNAi mean ± s.e.m: 26.60 ± 0.74, mog-5: 20.52 ± 0.63 (P<0.0001); mog-4: 21.83 ± 0.69 (P<0.0001); mog-1: 22.87 ± 0.76 (P= 0.0020). f, mog RNAi does not reduce lifespan of wild-type worms at 20°C. Vector RNAi mean ± s.e.m: 17.96 ± 0.46, mog-5: 17.13 ± 0.28 (P= 0.0653), mog-4: 17.68 ± 0.34 (P= 0.3608), mog-1: 17.58 ± 0.34 (P= 0.2567). g, mog RNAi does not shorten lifespan of wild-type worms at 25°C. Vector RNAi mean ± s.e.m: 13.50 ± 0.42, mog-5: 13.93 ± 0.27 (P= 0.7344), mog-4: 14.88 ± 0.27 (P= 0.0420), mog-1: 14.76 ± 0.36 (P= 0.0685). h, Knockdown of pgl and glh genes does not suppress cold-induced longevity in wild-type worms. Vector RNAi mean ± s.e.m: 30.35 ± 0.85, mog-5: 24.92 ± 0.44 (P<0.0001), pgl-1: 30.96 ± 0.65 (P= 0.6424), pgl-3: 28.95 ± 0.72 (P= 0.2590), glh-1: 29.64 ± 0.94 (P= 0.9853), glh-4: 29.57 ± 0.81 (P= 0.7474). i, Loss of iff-1 shortens cold-induced longevity in wild-type worms at 10°C. Vector RNAi mean ± s.e.m: 42.45 ± 0.84, iff-1: 35.52 ± 0.54 (P<0.0001), iff-2: 43.00 ± 0.66 (P= 0.6186), eif-2A: 43.11 ± 0.68 (P= 0.8526). j, iff-1 RNAi reduces cold-induced longevity in wild-type worms at 15°C. Vector RNAi mean ± s.e.m: 29.19 ± 0.86, iff-1: 21.66 ± 0.76 (P<0.0001), iff-2: 29.24 ± 0.85 (P= 0.8227), eif-2A: 28.53 ± 0.74 (P= 0.3853). k, iff-1 RNAi does not reduce lifespan at 20°C. Vector RNAi: mean ± s.e.m: 19.33 ± 0.45, iff-1: 19.79 ± 0.51 (P= 0.1317), iff-2: 19.61 ± 0.50 (P= 0.2137), eif-2A: 18.65 ± 0.44 (P= 0.2061). l, iff-1 RNAi induces a moderate lifespan extension at 25°C. Vector RNAi mean ± s.e.m: 13.23 ± 0.40, iff-1: 15.36 ± 0.39 (P<0.0001), iff-2: 13.69 ± 0.42 (P= 0.4603), eif-2A: 13.19 ± 0.33 (P= 0.6935). In lifespan experiments, P-values: two-sided log-rank test, n= 96 worms/condition. RNAi initiated at day 1 of adulthood in all the experiments. Supplementary Data 3 contains replicate data of lifespan experiments.
To further assess the requirement for adult proliferating germ cells in cold-induced longevity, we silenced distinct regulators of GSC proliferation. First, we knocked down mog-1, mog-4 and mog-5 genes, which encode DEAH-box RNA helicases required for robust proliferation of germ cells during development48–50. When we initiated RNAi treatment in young adults after normal development of the germ line, we also observed reduced proliferation within the mitotic region (Fig. 4c, d and Supplementary Fig. 9-10). Notably, downregulation of mog genes during adulthood diminished the lifespan extension induced by low temperature (Fig. 4e). On the contrary, loss of mog expression during adulthood did not decrease lifespan at 20°C and 25°C (Fig. 4f, g). Besides mog genes, other RNA-binding proteins are also involved in maintenance of germline proliferation such as PGL-1, PGL-3 and GLH-1, which are all components of P-granules, a germline-specific RNA/protein complex51–53. However, the germline proliferation requirement for these specific P-granule components is sensitive to temperature. As such, their individual loss can diminish germ-cell proliferation at high temperature (25°C), but not at lower temperatures51–53. Interestingly, knockdown of pgl genes slightly extends lifespan of wild-type worms at high temperature30. In contrast to downregulation of mog genes, we found that knockdown of pgl-1 alone during adulthood did not reduce germline proliferation (Fig. 4c-d) and cold-induced longevity at 15°C (Fig. 4h). Likewise, downregulation of glh genes did not alter the long lifespan phenotype induced by low temperature (Fig. 4h).
Although these results indicate a link between germline proliferation and cold-induced longevity during adulthood, it is important to note that mog genes are also expressed in somatic tissues. To circumvent potential direct effects on somatic tissues, we knocked-down the orthologues of the translational initiation factor EIF5A (i.e., iff-1, iff-2), which is duplicated in C. elegans. Whereas iff-2 is only expressed in somatic tissues54, iff-1 is specifically expressed in the germ line and, particularly, abundant in the distal gonad where mitotic divisions occur54. In fact, iff-1 is essential for germline proliferation at either larvae or adult stages54,55. As such, iff-1 RNAi initiated during adulthood was sufficient to decrease the proliferation of germ cells in the distal gonad (Fig. 4c, d and Supplementary Fig. 9). Notably, knockdown of iff-1 during adulthood diminished lifespan extension induced by low temperatures (Fig. 4i, j), but did not shorten lifespan at higher temperatures (i.e., 20°C and 25°C) (Fig. 4k, l). On the contrary, loss of iff-2 during adulthood did not impair germline proliferation (Fig. 4c, d) and cold-induced longevity (Fig. 4i, j). Upon knockdown of eif-2A, a different translational initiation factor, worms also exhibited a normal proliferating germ line (Fig. 4c, d) and extended lifespan when compared with control worms at cold temperature (Fig. 4k, l). Altogether, our data suggest that maintenance of adult GSC proliferation determines cold-induced longevity.
Prompted by these results, we assessed how temperature impinges on iff-1 mutants, which develop into young adult worms with very few germ cells (~40 germ nuclei) when compared with controls (~2000 germ nuclei)54, resembling glp-1 mutants. As glp-1 worms (Fig. 1b), iff-1 germline-less mutants were also long-lived at 20°C while they lived shorter than the wild-type strain at 15°C (Supplementary Fig. 11a, b). Although ablation of the germ line from development in either iff-1 or glp-1 mutants cannot be directly equated to the impairment of GSC proliferation induced by iff-1 RNAi at the adult stage, these results further support a role of the germ line in cold-induced longevity. In these lines, iff-1 knockdown during adulthood did not further decrease the lifespan of distinct germline-lacking strains at cold temperature (Supplementary Fig. 12a, b). As a more formal test of the specific role of adult GSCs, we knock-downed iff-1 in adult sterile worms with a proliferating germline and found a decrease in cold-induced longevity (Supplementary Fig. 12c). These results not only strengthened the link between adult GSCs and cold-induce longevity, but also indicate that sterility does not affect the long lifespan phenotype. Indeed, knockdown of iff-1 during adulthood reduces GSC proliferation and cold-induced longevity (Fig. 4d, j), but did not strongly affect the number of eggs laid by self-fertilizing hermaphrodites (Supplementary Fig. 13a, b). Because maintenance of germline proliferation is required for cold-induced longevity, we asked whether an over-proliferative germ line could further extend lifespan at low temperature. Mutations in gld-1, a tumor-suppressor factor, promote that germ cells reenter the mitotic cycle and overproliferate56. Likewise, knockdown of gld-1 also induces over-proliferation of the germ line57. Eventually, these over-proliferating germ cells break out of the gonad and fill the animal’s body, killing the worms at early adulthood stages. Concomitantly, these worms exhibit a short lifespan at 20°C58 (Supplementary Fig. 14a). Similarly, both tumorous gld-1 mutations and gld-1 knockdown from either development or adulthood also killed C. elegans early in life at lower temperature (Supplementary Fig. 14b). Collectively, our data indicate that cold-induced longevity is determined by an extended maintenance of the physiological pool of proliferating GSCs, while abnormal over-proliferation of these cells is deleterious for the animal.
Adult proliferating germ cells induce cbs-1 in the intestine to extend lifespan
Since our results suggest a direct regulation of cold-induced longevity by adult GSCs, we asked whether these cells regulate somatic tissues to prolong lifespan. Whereas low temperature slightly increased motility, we did not observe defects in germline-lacking mutant worms when compared with either wild-type or fer-15;fem-1 control sterile worms (Supplementary Fig. 15a). Likewise, inhibition of adult germline proliferation upon iff-1 or mog-5 RNAi treatment did not affect motility at cold temperature (Supplementary Fig. 15b). We also examined body size and found that neither germline ablation nor inhibition of adult germline proliferation have a notable effect on the animal’s size (Supplementary Fig. 15c-f). In C. elegans, metabolic rates increase with temperature and are often negatively correlated with longevity59. As indicated by lower oxygen consumption, long lifespan of wild-type worms was accompanied by reduced metabolic rates at 15°C (Supplementary Fig. 16a). However, metabolic rates increased in fer-15;fem-1 sterile worms at cold temperature (Supplementary Fig. 16a), despite they exhibited a cold-induced longevity phenotype (Fig. 1d). In glp-1 germline-lacking mutants, we did not find significant changes in metabolic rates when they were shifted to 15°C (Supplementary Fig. 16a). More importantly, the short lifespan of iff-1 RNAi-treated worms did not correlate with increased metabolic rates at 15°C (Supplementary Fig. 16b). In fact, iff-1 RNAi-treated worms had a decrease in metabolic rates compared with control worms, resembling iff-2 RNAi treatment which does not affect cold-induced longevity (Supplementary Fig. 16b). Thus, inhibition of adult GSC proliferation did not have a strong effect on features such as motility, body size or metabolic rates, indicating that these cells regulate cold-induced longevity by distinct mechanisms.
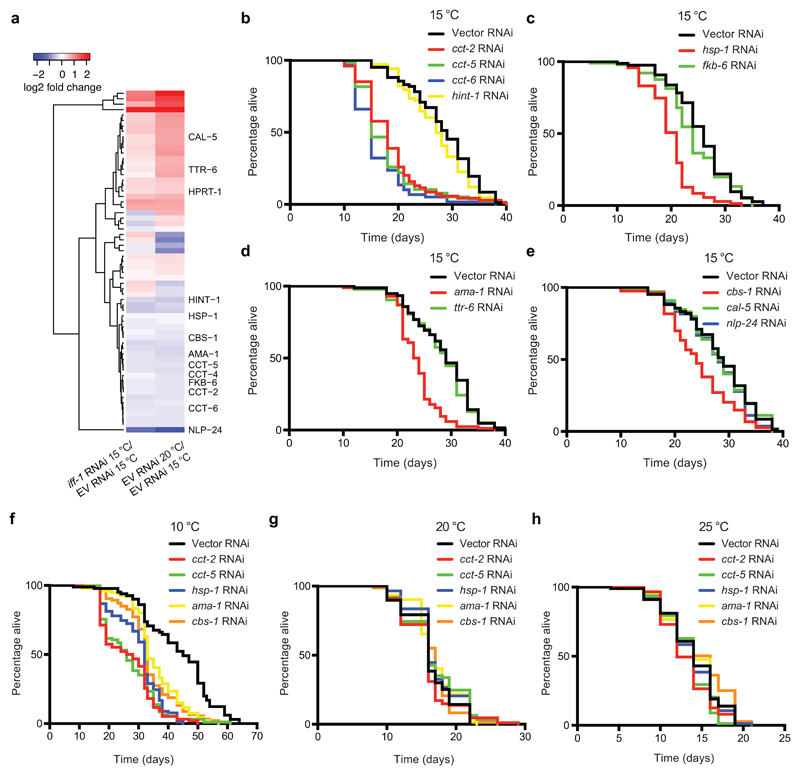
To gain further insights into somatic changes induced by GSCs at low temperature, we used a quantitative proteomics approach. We identified 225 up-regulated and 233 down-regulated proteins at 20°C when compared with C. elegans cultured at 15°C (Supplementary Data 1). We hypothesized that inhibition of germ-cell proliferation during adulthood at 15°C triggers similar changes in lifespan regulators to those induced by temperature increase. Thus, we examined the proteome of iff-1 RNAi-treated worms at cold temperature (Supplementary Data 1). Besides IFF-1 levels, quantitative proteomics analysis revealed that other 152 proteins are significantly changed upon iff-1 knockdown during adulthood at 15°C (Supplementary Data 1). Among them, 27 proteins were also up-regulated at 20°C (Fig. 5a, Supplementary Fig. 17 and Supplementary Data 1). Likewise, 27 proteins were downregulated in both iff-1 RNAi and 20°C conditions (Fig. 5a, Supplementary Fig. 17 and Supplementary Data 1). To examine whether these proteins modulate longevity at cold temperatures, we performed a RNAi screen (Supplementary Table 1). Notably, knockdown of several genes reduced cold-induced longevity as we further validated in independent experiments (Fig. 5b-e). In particular, genes encoding proteins which are increased at cold-temperature and down-regulated by loss of iff-1 (Fig. 5a). The strongest effect was induced by knockdown of distinct subunits (cct-2, cct-5, cct-6) of the chaperonin TRiC/CCT complex (Fig. 5b). Since loss of a single subunit is sufficient to impair the assembly of the complex25, our results suggest that increased TRiC/CCT assembly is required for cold-induced longevity. Indeed, knockdown of a different cct subunit (cct-8) also shortened lifespan at 15°C (Supplementary Fig. 18). Besides cct subunits, loss of either the hsp70 chaperone hsp-1 or the RNA polymerase II ama-1 reduced lifespan extension at cold temperature (Fig. 5c, d). Likewise, up-regulated levels of CBS-1, the orthologue of human cystathionine β-synthase (CBS)27,60, were required for the full extent of the longevity phenotype at 15°C (Fig. 5e). At lower temperature (10°C), knockdown of cct subunits, hsp-1, ama-1 and cbs-1 also induced a decrease in the longevity phenotype (Fig. 5f). On the contrary, knockdown of these genes did not shorten lifespan at higher temperatures (20°C and 25°C) (Fig. 5g, h). Whereas these factors regulated cold-induced longevity in wild-type worms with a proliferating germ line, their knockdown did not further decrease the short lifespan of germline-lacking worms at 15°C (Supplementary Fig. 19). Taken together, our results indicate a requirement for high expression of cct subunits, hsp-1, ama-1, and cbs-1 in the cold-induced longevity mediated by proliferating germ cells.
Figure 5. cbs-1 and other factors modulate the long lifespan induced by adult GSCs at low temperatures.
a, Heatmap depicting the log2-fold change of the differentially expressed proteins in both iff-1 RNAi-treated worms at 15°C and empty vector (EV) RNAi-treated worms at 20°C when compared with EV RNAi-treated worms at 15°C. fer-15(b26);fem-1(hc17) control strain was raised at the restrictive temperature (25°C) during development to obtain sterile worms with a proliferating germline, which were then shifted to the indicated temperatures and RNAi treatment until day 6 of adulthood. Statistical comparisons were made by two-tailed Student’s t-test (n= 3, p-value <0.05 was considered significant). Lifespan experiments upon knockdown of the indicated proteins are presented in the following panels. Supplementary Fig. 17 presents the heatmap with the full list of significantly changed proteins. For lifespan data of the other differentially expressed proteins, please see Supplementary Table 1. b, CCT subunits are required for cold-induced longevity (15°C) in wild-type worms (Empty vector RNAi mean ± s.e.m: 28.26 ± 0.69; cct-2 RNAi: 18.58 ± 0.71, P<0.0001; cct-5 RNAi: 17.83 ± 0.68, P<0.0001; cct-6 RNAi: 16.15 ± 0.66, P<0.0001; hint-1 RNAi: 27.15 ± 0.69, P=0.1722). c, The hsp-1 chaperone is necessary for cold-induced longevity (15°C) (Empty vector RNAi mean ± s.e.m: 25.49 ± 0.62; hsp-1 RNAi: 19.84 ± 0.49, P<0.0001; fkb-6 RNAi: 24.10 ± 0.73, P=0.2535). d, The RNA polymerase II ama-1 regulates longevity of wild-type worms at 15°C (Empty vector RNAi mean ± s.e.m: 28.54 ± 0.68; ama-1 RNAi: 23.67 ± 0.42, P<0.0001; ttr-6 RNAi: 27.95 ± 0.65 P=0.4734). e, Loss of cbs-1 decreases longevity of wild-type worms at 15°C (Empty vector RNAi mean ± s.e.m: 28.26 ± 0.69; cbs-1 RNAi: 24.54 ± 0.69, P=0.0005; cal-5 RNAi: 28.09 ± 0.78, P=0.8688; nlp-24 RNAi: 27.16 ± 0.78, P=0.0664). f, Knockdown of cct subunits, hsp-1, ama-1 or cbs-1 decreases the long lifespan phenotype induced by 10°C in wild-type worms (P<0.0001). Empty vector RNAi mean ± s.e.m: 43.31 ± 1.39; cct-2 RNAi: 26.36 ± 0.02; cct-5 RNAi: 26.76 ± 0.95; hsp-1 RNAi: 30.17 ± 0.82; ama-1 RNAi: 35.37 ± 0.87; cbs-1 RNAi: 33.57 ± 0.93. g, Knockdown of cct subunits, hsp-1, ama-1, or cbs-1 does not shorten lifespan at 20°C. Empty vector RNAi mean ± s.e.m: 16.34 ± 0.37; cct-2 RNAi: median= 15.97 ± 0.41, P=0.5386; cct-5 RNAi: 17.01 ± 0.48, P=0.1106; hsp-1 RNAi: 17.10 ± 0.39, P=0.1571; ama-1 RNAi: 17.02 ± 0.35, P=0.4403; cbs-1 RNAi: 16.26 ± 0.43, P=0.9592. h, Knockdown of cct subunits, hsp-1, ama-1, or cbs-1 does not shorten lifespan at 25°C. RNAi was initiated at day 1 of adulthood. Empty vector RNAi mean ± s.e.m: 13.95 ± 0.37; cct-2 RNAi: 13.20 ± 0.31, P=0.0695; cct-5 RNAi: 13.43 ± 0.29, P=0.0472; hsp-1 RNAi: 13.83 ± 0.36, P=0.7389; ama-1 RNAi: 14.07 ± 0.36, P=0.8221; cbs-1 RNAi: 14.53 ± 0.44, P=0.1069. In all the lifespan experiments, RNAi was started at day 1 of adulthood (P-values: two-sided log-rank test, n= 96 worms/condition). See Supplementary Data 3 for statistical analysis and replicate data of lifespan experiments.
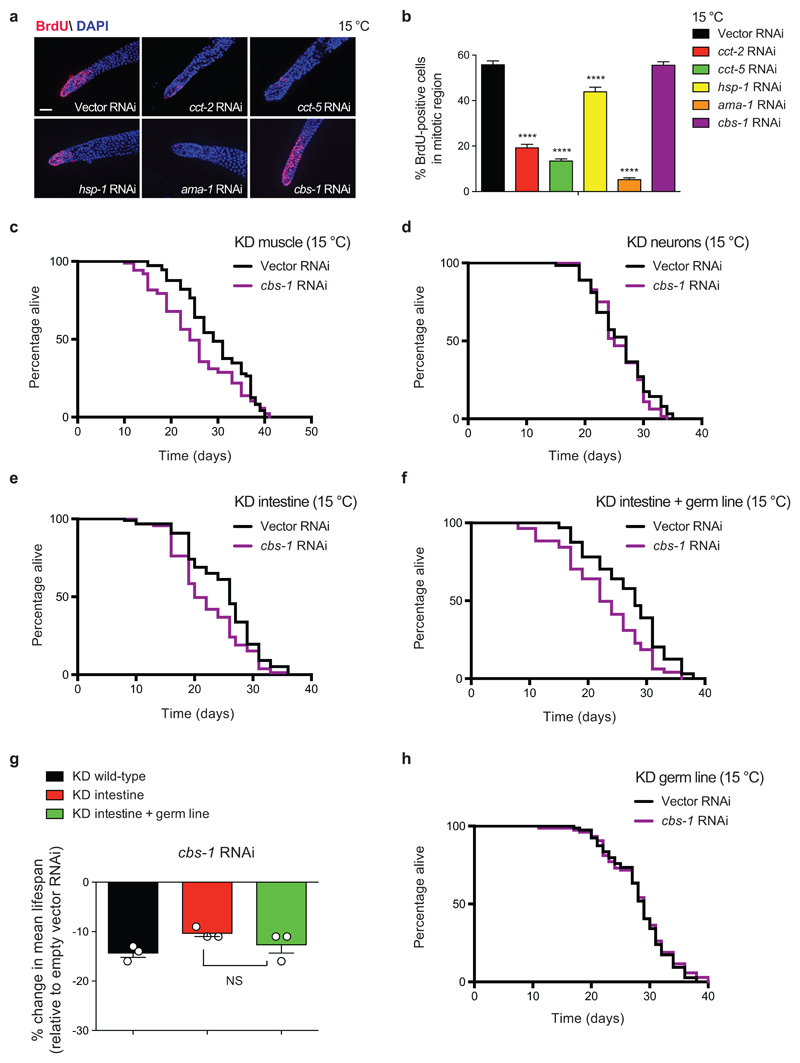
Prompted by these results, we asked how these factors impinge upon cold-induced longevity. For instance, they could modulate longevity by directly affecting germ-cell proliferation. Another intriguing possibility is that proliferating germ cells induce specific factors in somatic tissues, resulting in lifespan extension. To define the factors that fall into each category, we first assessed their effects on germline proliferation (Fig. 6a-b and Supplementary Fig. 20). Loss of cct subunits dramatically decreased the number of proliferating germ cells in wild-type worms (Fig. 6a, b). In these lines, tissue-specific knockdown in the germ line alone reduced cold-induced longevity (Supplementary Fig. 21a). Remarkably, tissue-specific knockdown in either neurons or intestine also partially diminished the longevity phenotype (Supplementary Fig. 21b-e). Thus, cct subunits could act in both the germ line and the soma to prolong lifespan at low temperature. Besides cct subunits, loss of ama-1 also resulted in a strong decrease of germline proliferation (Fig. 6a, b). Whereas knockdown of ama-1 in the intestine alone did not affect lifespan, we found a reduction of cold-induced longevity when the RNAi was also efficient in the germ line (Supplementary Fig. 22). In addition, tissue-specific knockdown of ama-1 in neurons also slightly decreased cold-induced longevity (Supplementary Fig. 22). In comparison with cct subunits or ama-1, loss of hsp-1 slightly decreased germline proliferation (Fig. 6a, b), indicating an important role in somatic tissues. Indeed, neuron-specific knockdown of hsp-1 dramatically shortened longevity at cold temperature, whereas its loss in either muscle or intestine did not significantly affect lifespan (Supplementary Fig. 23a-c). However, knockdown of hsp-1 in the germ line alone also decreased cold-induced longevity (Supplementary Fig. 23d, e). Thus, although cct subunits and hsp-1 could act in somatic tissues to extend lifespan, their direct effects in germ-cell proliferation also modulate organismal longevity. For this reason, we focused on cbs-1, the only regulator of cold-induced longevity that did not affect the number of proliferating germ cells (Fig. 6a, b). Whereas specific knockdown of cbs-1 in neurons did not change lifespan, its downregulation in either the muscle or intestine significantly shortened cold-induce longevity (Fig. 6c-e). Notably, longevity was not further reduced when the germ line also responded to RNAi treatment (Fig. 6f, g). In addition, we found that knockdown of cbs-1 in the germ line alone does not impair cold-induced longevity (Fig. 6h).
Figure 6. Tissue-specific knockdown of cbs-1 in the intestine or muscle reduces cold-induced longevity.
a, BrdU staining of germ line from wild-type C. elegans fed with the indicated RNAi from day 1 of adulthood. Worms were examined at day 5 of adulthood. Cell nuclei were stained with DAPI. Scale bar represents 20 µm. The images are representative of 3 independent experiments. b, Graph represents the percentage of BrdU-positive cells/total nuclei (mean ± s.e.m., Vector, cbs-1 RNAi (n= 46 germ lines), cct-2, cct-5, hsp-1, ama-1 RNAi (n= 40), germ lines scored per each condition from 3 independent experiments). Statistical comparisons were made by two-tailed Student’s t-test for unpaired samples. P-value: **** (P<0.0001). c, Muscle-specific knockdown (KD) of cbs-1 decreases lifespan extension induced by cold temperature (15°C). RNAi rescued in the muscle of RNAi-deficient worms (rde-1(ne300); myo-3p::rde-1 strain). Empty vector RNAi mean ± s.e.m: 29.49 ± 0.81; cbs-1 RNAi: 25.33 ± 0.90, P=0.0048. d, Neuronal-specific KD of cbs-1 does not affect longevity at 15°C. RNAi rescued in the neurons of RNAi-deficient worms (sid-1(pk3321); unc-119p::sid-1 strain). Empty vector RNAi mean ± s.e.m: 26.09 ± 0.60; cbs-1 RNAi: 25.78 ± 0.51, P=0.3367. e, Intestinal-specific KD of cbs-1 decreases longevity at 15°C. RNAi rescued in the intestine of RNAi-deficient worms (rde-1(ne219); nhx-2p::rde-1 strain). Empty vector RNAi mean ± s.e.m: 25.02 ± 0.66; cbs-1 RNAi: 22.25 ± 0.65, P<0.0001. f, RNAi rescued in both the intestine and germ line of RNAi-deficient worms (rde-1(ne219); mex-5p::rde-1 strain). Empty vector RNAi mean ± s.e.m: 26.97 ± 0.79; cbs-1 RNAi: 22.72 ± 1.00, P<0.0019. g, Percentage of mean lifespan change in different strains upon knockdown of cbs-1 (relative to the respective empty vector RNAi control). Graph represents the mean ± s.e.m. from 3 independent lifespan experiments for each strain. Longevity was not further reduced when both the intestine and the germ line responded to RNAi treatment when compared to knockdown in the intestine alone (KD intestine versus KD intestine + germ line, P= 0.2634). Statistical comparisons were made by two-tailed Student’s t-test for unpaired samples. NS= not significant. h, Knockdown of cbs-1 in the germ line alone does not decrease cold-induced longevity at 15°C. RNAi rescued in the germ line of RNAi-deficient worms (rde-1(mkc36); sun-1p::rde-1::sun-1 3'UTR strain). Empty vector RNAi mean ± s.e.m: 28.32 ± 0.55; cbs-1 RNAi: 28.40 ± 0.64, P=0.5733. In all the lifespan experiments, P-values: two-sided log-rank test, n= 96 worms/condition. RNAi was started at day 1 of adulthood. See Supplementary Data 3 for statistical analysis and replicate data of lifespan experiments.
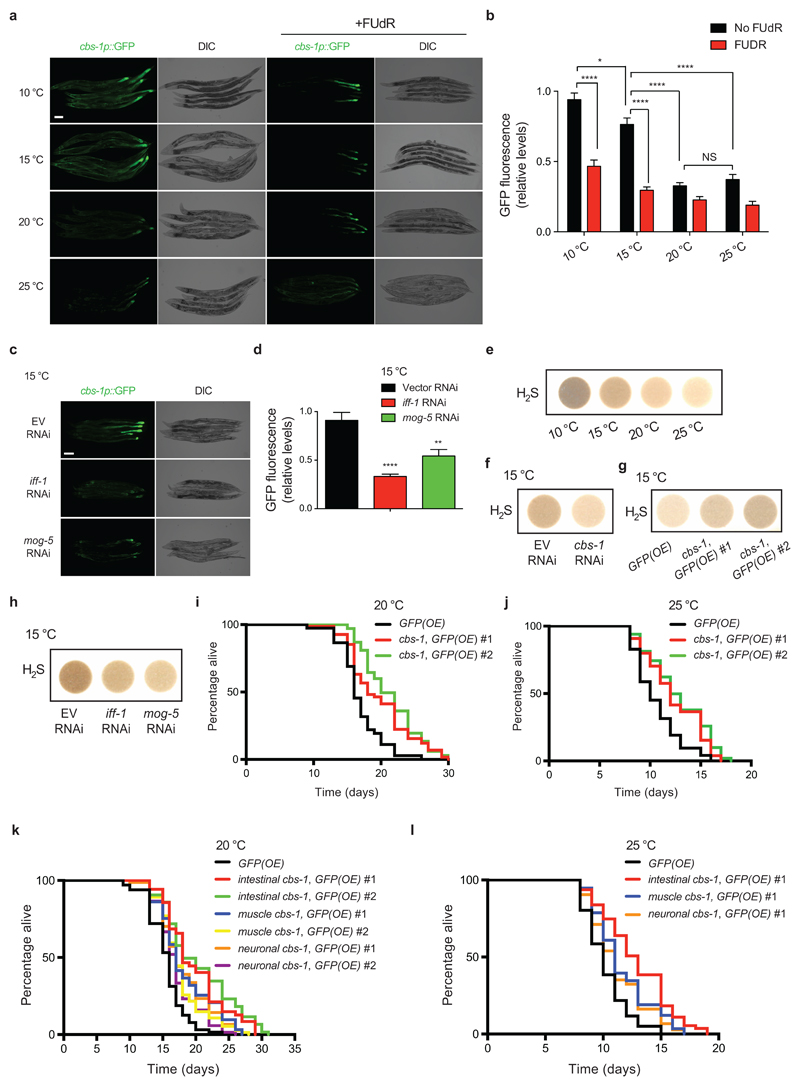
Altogether, our results suggest that proliferating germ cells induce cbs-1 in specific somatic tissues to extend longevity at cold temperature. To assess this hypothesis, we generated a transcriptional reporter construct. At 20°C and 25°C, cbs-1 was mostly expressed in the posterior intestine (Fig. 7a). At cold temperatures, cbs-1 expression increased particularly in the intestine and also the body muscle (Fig. 7a, b). Notably, FUdR treatment reduced the high expression of cbs-1 in somatic tissues induced by cold temperature (Fig. 7a, b). Likewise, knockdown of either iff-1 or mog-5 decreased somatic expression of cbs-1 at cold temperature (Fig. 7c, d). Importantly, we found that somatic expression of cbs-1 was also downregulated when we specifically knocked-down these factors in the germ line alone (Supplementary Fig. 24a, b). Thus, our results indicate that proliferating germ cells regulate cold-associated induction of cbs-1 in somatic tissues such as the intestine.
Figure 7. GSCs induce cbs-1 expression in somatic tissues at cold temperature.
a, GFP expressed under cbs-1 promoter in day 5 adult-worms grown at the indicated temperatures during adulthood. At 20°C and 25°C, cbs-1 is mostly expressed in the posterior intestine. At cold temperatures, cbs-1 expression increases in the intestine and muscle. FUdR reduces cold-induced expression of cbs-1 in muscle and intestine. DIC, differential interference contrast. Scale bar, 100 μm. Images are representative of four independent experiments. b, Quantification of cbs-1p::GFP (mean ± s.e.m. relative to 10°C with no FUdR, 40 worms/condition from 4 independent experiments). Analysis by two-tailed Student’s t-test for unpaired samples: *(P<0.05), ****(P<0.0001). NS= not significant (P=0.2683). c, cbs-1p::GFP in day 7 adult-worms at 15°C. Knockdown of either iff-1 or mog-5 during adulthood decreases cbs-1 expression when compared to empty vector (EV) RNAi. Scale bar, 50 μm. Images are representative of two independent experiments. d, Quantification of cbs-1p::GFP (mean ± s.e.m. relative to EV RNAi, 20 worms/condition from 2 independent experiments). Analysis by two-tailed Student’s t-test for unpaired samples: **(P<0.01), ****(P<0.0001). e, H2S gas production in control sterile strain at day 6 of adulthood (fer-15(b26);fem-1(hc17)). Worms were raised at the restrictive temperature (25°C) and shifted to the indicated temperatures during adulthood. The images are representative of four independent experiments. f, H2S production in day 6-adult control sterile worms upon knockdown of cbs-1 during adulthood. The images are representative of four independent experiments. g, Somatic overexpression (OE) of cbs-1 increases H2S production. Two independent cbs-1(OE) lines were tested. The images are representative of two independent experiments. h, Loss of either iff-1 or mog-5 during adulthood decreases H2S production. Control sterile worms at day 6 of adulthood were examined. The images are representative of three independent experiments. i, Ubiquitous somatic overexpression of cbs-1 under sur-5 promoter extends lifespan at 20°C (P<0.0001). GFP(OE) mean ± s.e.m: 16.83 ± 0.36; cbs-1,GFP(OE) #1: 19.65 ± 0.59; cbs-1,GFP(OE) #2: 21.28 ± 0.49. j, Ubiquitous somatic cbs-1(OE) extends lifespan at 25°C (P<0.0001). GFP(OE): 10.61 ± 0.26; cbs-1,GFP(OE) #1: 12.33 ± 0.35; cbs-1,GFP(OE) #2: 12.75 ± 0.38. k, Tissue-specific overexpression of cbs-1 in the intestine (gly-19p), muscle (myo-3p) or neurons (rgef-1p) extends lifespan at 20°C (P<0.0001). Intestinal-specific OE induced longer longevity compared with muscle or neuronal overexpression (P<0.01). GFP(OE): 15.56 ± 0.34; intestinal cbs-1,GFP(OE) #1: 19.82 ± 0.59; intestinal cbs-1,GFP(OE) #2: 20.47 ± 0.66; muscle cbs-1,GFP(OE) #1: 18.31 ± 0.51; muscle cbs-1,GFP(OE) #2: 17.73 ± 0.40; neuronal cbs-1,GFP(OE) #1: 18.11 ± 0.44; neuronal cbs-1,GFP(OE) #2: 17.17 ± 0.36. Two independent tissue-specific cbs-1(OE) lines were tested for each tissue. l, Tissue-specific overexpression of cbs-1 in the intestine (P<0.0001), muscle(P=0.0002) or neurons (P=0.0034) extends lifespan at 25°C. Intestinal-specific OE induced longer lifespan compared with muscle (P=0.0142) or neuronal (P=0.0008) overexpression. GFP(OE) mean ± s.e.m: 10.24 ± 0.24; intestinal cbs-1,GFP(OE) #1: 12.82 ± 0.36; muscle cbs-1,GFP(OE) #1: 11.62 ± 0.28; neuronal cbs-1,GFP(OE) #1: 11.25 ± 0.28. In lifespan experiments, comparisons were made by two-sided log-rank test, n= 96 worms/condition. Supplementary Data 3 contains replicate data of lifespan experiments.
With the strong connection between germ line, cold-induced longevity and up-regulation of somatic expression of cbs-1, we assessed whether intestinal overexpression of cbs-1 can rescue the short lifespan of germline-lacking worms at cold temperature. Whereas tissue-specific overexpression of cbs-1 in the intestine did not further increase cold-induced longevity of wild-type worms, it extended the short lifespan of glp-1 germline-lacking mutants (Supplementary Fig. 25a). In addition, intestinal overexpression of cbs-1 also partially rescued the short lifespan of worms with defects in adult germline proliferation induced by FUdR treatment at low temperature (Supplementary Fig. 25b). Collectively, our data suggest that intestinal up-regulation of cbs-1 modulated by GSC proliferation contributes to cold-induced longevity.
CBS is a key enzyme of the transsulfuration pathway that mediates the interconversion of cysteine and homocysteine. CBS activity produces cystathionine and hydrogen sulfide (H2S) 27,60, which acts as a gaseous signaling molecule that reduces blood pressure61 and prevents neurodegeneration in mammals62. In addition, dietary restriction induces high levels of H2S that contribute to lifespan extension phenotype in yeast, worm, fruit fly and rodents27. Importantly, exogenous addition of H2S is sufficient to extend lifespan of C. elegans 28. Since up-regulated levels of cbs-1 contributed to cold-induced longevity, we examined H2S amounts. Notably, low temperatures increased H2S gas levels (Fig. 7e). Knockdown of cbs-1 diminished the production of H2S at cold temperature whereas its overexpression increased H2S levels (Fig. 7f, g). Moreover, inhibition of germline proliferation by knockdown of either iff-1 or mog-5 was sufficient to decrease H2S production at 15°C, providing a link between H2S levels and the cold-induced longevity modulated by adult GSCs (Fig. 7h).
As shown above, tissue-specific overexpression of cbs-1 in the intestine alone did not further extend longevity of worms with endogenous high levels of this protein at cold temperature (Supplementary Fig. 25, 26). Likewise, ubiquitous somatic overexpression of cbs-1 did not significantly increase cold-induced longevity (Supplementary Fig. 26). Thus, we asked how somatic overexpression of cbs-1 affects lifespan at higher temperatures when worms have lower endogenous levels of cbs-1. Ubiquitous somatic overexpression of cbs-1 extends lifespan at 20°C27 (Fig. 7i). Notably, we found that ubiquitous somatic up-regulation of cbs-1 induced longevity even at warm temperature (25°C) (Fig. 7j). Although tissue-specific overexpression in the muscle or neurons also extended longevity, we observed the strongest effects upon intestinal overexpression of cbs-1 at both 20°C and 25°C (Fig. 7k, l). Thus, up-regulation of cbs-1 in the intestine could mimic low temperature conditions and prolong lifespan. Altogether, our results suggest that adult proliferating germ cells induce cbs-1 expression in somatic tissues such as the intestine, a process that contributes to cold-induced longevity.
Release of prostaglandin E2 by GSCs promotes longevity at cold temperatures
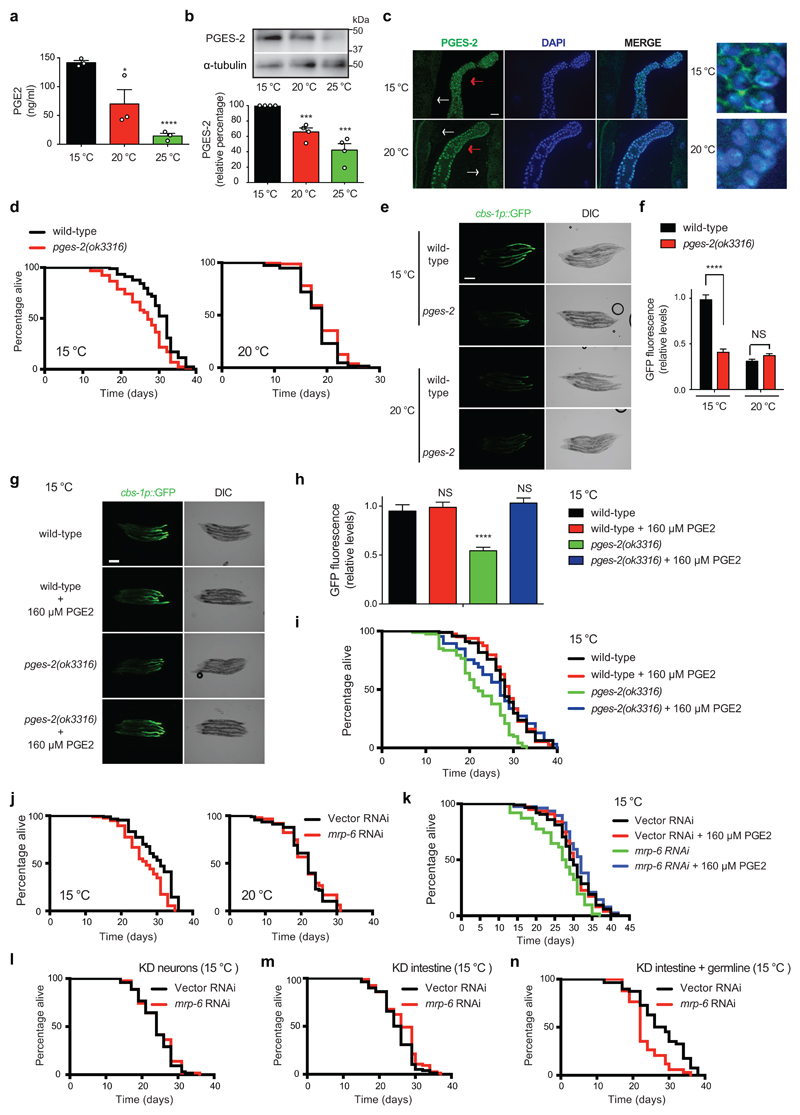
To assess the mechanism by which adult germ cells induce cbs-1 expression in the intestine at cold temperature, we performed transcriptome analysis of extruded germ lines from wild-type worms upon iff-1 knockdown or temperature increase (Supplementary Data 2). When compared with the germ line of nematodes cultured at 15°C, we found 248 up-regulated transcripts and 433 down-regulated transcripts in the germ line of both iff-1 RNAi-treated worms and 20°C-cultured worms (Supplementary Fig. 27a and Supplementary Data 2). Among these common down-regulated transcripts, we did not observe decreased expression of signaling peptides (i.e., nlp, flp or insulin-like peptides) or genes involved in the synthesis of sex steroid hormones (e.g., pregnenolone) (Supplementary Data 2). However, the expression of pges-2, the worm orthologue of human PTGES2, was down-regulated in the germ line by either iff-1 knockdown or temperature increase (Supplementary Fig. 27b and Supplementary Data 2). PTGES2 encodes a membrane-associated prostaglandin E synthase, which catalyzes the conversion of prostaglandin H2 into the more stable prostaglandin E2 (PGE2)63. Notably, we found that low temperature increases the levels of PGE2 (Fig. 8a). By western blot, we confirmed that worms exhibit higher protein levels of PGES-2 at cold temperature (Fig. 8b). Immunohistochemistry experiments indicated that PGES-2 is highly expressed in the germ line compared to the intestine (Fig. 8c). Strikingly, low temperature promoted the enrichment of PGES-2 in the plasma membrane of germ cells whereas this enzyme was essentially concentrated in the nucleus at higher temperature (Fig. 8c). Prompted by these results, we examined a potential link between pges-2 and cold-induced longevity. Notably, pges-2 mutant animals lived significantly shorter when compared with wild-type worms at cold temperatures (Fig. 8d and Supplementary Fig. 28). However, they did not exhibit decreased lifespan at 20°C or 25°C (Fig. 8d and Supplementary Fig. 28). Moreover, we found that pges-2 mutant worms exhibit a significant decline in somatic expression of cbs-1 at cold temperature when compared with wild-type worms (Fig. 8e, f). In contrast, pges-2 mutation did not further decrease the low cbs-1 expression of worms at 20°C (Fig. 8e, f). To further assess whether the prostaglandin E synthase activity of pges-2 is necessary for cold-induced longevity, we added exogenous PGE2 hormone. Indeed, PGE2 rescued the low somatic expression of cbs-1 in pges-2 mutants and extended their short lifespan at cold temperature (Fig. 8g-i). Recently, a study reported that the co-chaperone daf-41/p23 shortens lifespan at warm temperature, whereas it is necessary for the long lifespan induced by cold temperature17. Importantly, epistasis experiments showed that the co-chaperone activity of daf-41/p23 is involved in lifespan regulation at warm temperature, but not cold temperature17. Thus, daf-41/p23 may modulate cold-induced longevity through a different pathway, although the detailed mechanism is not entirely understood. Besides its co-chaperone activity, p23 also exhibits prostaglandin E2 synthase activity in vitro 64. Although experiments in mouse models failed to support a function of p23 in PGE2 biosynthesis65, we asked whether the potential PGE2 synthase activity of daf-41/p23 modulates the effects of the germ line in cold-induced longevity. We found that ubiquitous and germline-specific knockdown of daf-41/p23 did not diminish the somatic up-regulation of cbs-1 induced by cold temperature (Supplementary Fig. 29a-d). In addition, knockdown of daf-41/p23 in the germ line alone did not shorten longevity at cold temperature (Supplementary Fig. 29e). Taken together, our results support an important role of pges-2 in the germ line to modulate cold-induced longevity, whereas daf-41/p23 is dispensable in this tissue for the long lifespan phenotype.
Figure 8. Release of PGE2 by GSCs promotes cold-induced longevity.
a, PGE2 levels in fer-15;fem-1 control sterile worms at day 6 of adulthood (8.4 mg worms/ml, mean ± s.e.m. from 3 independent experiments). Two-tailed Student’s t-test for unpaired samples: * (P<0.05), ****(P<0.0001), NS= not significant (P=0.0519). b, Western blot of day 6-adult control sterile worms with antibodies to PGES-2 and α-tubulin loading control. The graph represents PGES-2 relative percentage values (corrected for α-tubulin) to 15°C (mean ± s.e.m., 4 independent experiments). Two-tailed Student’s t-test for unpaired samples: ***(P<0.001). c, Immunostaining with PGES-2 antibody. White and red arrows indicate intestine and germ line of day 6-adult wild-type worms, respectively. On the right, higher magnification of germ cells. Nuclei stained with DAPI. Scale bar, 20 μm. Images representative of three independent experiments. d, pges-2(ok3316) are short lived at cold temperature compared with wild-type animals (pges-2 15°C mean ± s.e.m: 30.10 ± 0.52, wild-type 15°C: 26.09 ± 0.66, P<0.0001), but do not live shorter at 20°C (pges-2 20°C: 18.13 ± 0.41, wild-type 20°C 19.11 ± 0.37, P=0.1303). e, cbs-1p::GFP in adult wild-type and pges-2(ok3316) mutant worms (day 5). DIC, differential interference contrast. Scale bar, 1000 μm. Images representative of three independent experiments. f, Quantification of cbs-1p::GFP in day 5-adult animals (mean ± s.e.m. relative to 15°C wild-type worms, 30 worms/condition from 3 independent experiments). Student’s t-test for unpaired samples: ****(P<0.0001), NS (P=0.0519). g, cbs-1p::GFP in day 8 adult-worms at 15°C. Exogenous PGE2 rescues low expression of cbs-1 in pges-2(ok3316) mutants. Scale bar, 200 μm. Images representative of two independent experiments. h, Quantification of cbs-1p::GFP in day 8-adult animals at 15°C (mean ± s.e.m. relative to non-treated wild-type worms. Wild-type (n=12) and pges-2 (n=15) from 2 independent experiments). Two-tailed Student’s t-test for unpaired samples: ****(P<0.0001), NS= wild-type versus wild-type + PGE2 (P=0.6614), wild-type versus pges-2 + PGE2 (P=0.4073). i, Exogenous PGE2 extends the short lifespan of pges-2(ok3316) mutants at 15°C (pges-2 versus pges-2 +PGE2, P<0.0001). Wild-type mean ± s.e.m: 28.07 ± 0.59; wild-type + PGE2: 28.60 ± 0.58; pges-2: 22.43 ± 0.73; pges-2 + PGE2: 26.54 ± 0.95. j, mrp-6 RNAi decreases lifespan of wild-type worms at 15°C (Vector RNAi mean ± s.e.m: 29.64 ± 0.59; mrp-6: 26.66 ± 0.61, P<0.0001), but not at 20°C (Vector: 21.23 ± 0.58; mrp-6: 21.58 ± 0.64, P=0.3707). RNAi was initiated during adulthood. k, Exogenous PGE2 does not prolong cold-induced longevity of control worms (Vector RNAi mean ± s.e.m: 29.85 ± 0.61 versus Vector + PGE2: 29.98 ± 0.59, P=0.9106), but extends the short lifespan of mrp-6 RNAi-treated worms (mrp-6: 26.37 ± 0.82 versus mrp-6 + PGE2: 31.43 ± 0.60, P<0.0001). l, Neuronal-specific mrp-6 RNAi (Vector RNAi: 23.87 ± 0.56, mrp-6: 24.18 ± 0.61, P=0.5119). m, Intestinal-specific mrp-6 RNAi (Vector RNAi: 24.86 ± 0.51, mrp-6: 26.16 ± 0.57, P=0.0531). n, When the germ line is sensitive to RNAi, mrp-6 RNAi reduces cold-induced longevity (Vector RNAi: 27.77 ± 1.03; mrp-6: 23.32 ± 0.78, P=0.0007). Lifespan experiments were analyzed by two-sided log-rank test, n= 96 worms/condition. Supplementary Data 3 contains replicate lifespan experiments.
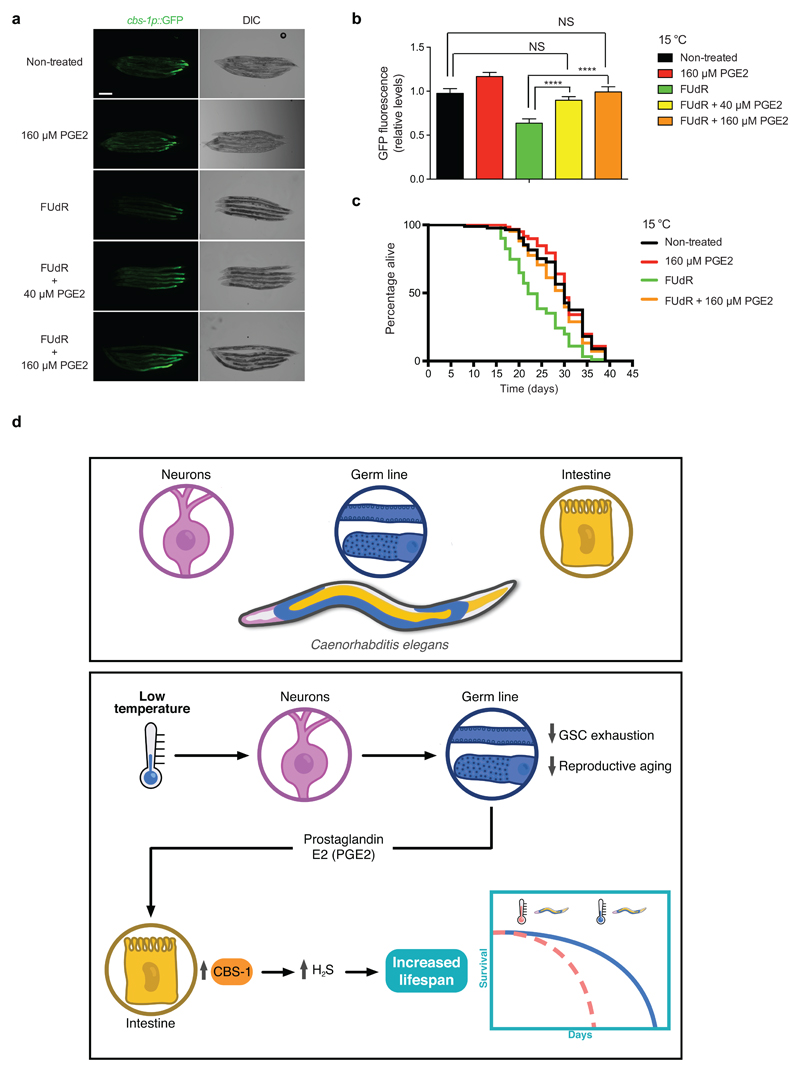
Since pges-2 mutants had normal numbers of proliferating germ cells (Supplementary Fig. 30), our results indicate that pges-2 activity in the germ line modulates cold-induced longevity by influencing somatic tissues rather than directly affecting germ cell proliferation. The release of prostaglandin from cells is mediated by a specific transporter, ABCC4 (ATP binding cassette subfamily C member 4)66. Knockdown of mrp-6, the worm orthologue of ABCC4, diminished cold-induced longevity (Fig. 8j). In contrast, loss of mrp-6 did not shorten lifespan of worms at 20°C (Fig. 8j). Whereas exogenous PGE2 hormone did not further extend cold-induced longevity of control worms, it rescued the short lifespan phenotype of mrp-6 RNAi-treated worms at cold temperature (Fig. 8k). Tissue-specific knockdown of mrp-6 in either the intestine or neurons did not affect longevity at cold temperature (Fig. 8l, m). Importantly, we found a decline in cold-induced longevity when the RNAi was efficient in the germ line (Fig. 8n and Supplementary Fig. 31). Thus, these results suggest that the release of PGE2 by the germ line regulates cold-induced longevity. To test this hypothesis, we inhibited adult GSC proliferation by FUdR treatment and examined intestinal cbs-1 expression upon application of exogenous PGE2 hormone. Remarkably, exogenous PGE2 was sufficient to rescue the low expression of intestinal cbs-1 induced by FUdR treatment (Fig. 9a, b). Likewise, PGE2 rescued the decline in cold-induced longevity induced by loss of GSC proliferation (Fig. 9c). In addition, exogenous PGE2 extended the short lifespan phenotype of glp-1 germline-lacking worms at cold temperature (Supplementary Fig. 32). Altogether, our data indicate that the germ line communicates with somatic tissues via PGE2 signals to extend longevity at cold temperature (Fig. 9d).
Figure 9. Application of exogenous PGE2 rescues cbs-1 expression and cold-induced longevity in GSC-impaired worms.
a, Representative images of GFP expressed under control of the cbs-1 promoter in day 8 adult-worms at 15°C. Application of exogenous PGE2 rescues low expression of cbs-1 caused by FUdR treatment (100 μg ml-1). PGE2 was added from day 5 of adulthood. DIC, differential interference contrast. Scale bar represents 200 μm. Images are representative of three independent experiments. b, Quantification of cbs-1p::GFP expression in day 8-adult animals at 15°C. Graph represents the mean ± s.e.m. relative to non-treated worms (Non-treated, PGE2, FUdR (n=50), FUdR + PGE2 (n=65), worms were analyzed per each condition from three independent experiments). Statistical comparisons were made by Student’s t-test for unpaired samples. P-value: ****(P<0.0001), NS (not significant): Non-treated versus FUdR+40 μM PGE2 (P=0.2436), Non-treated vs FUdR+160 μM PGE2 (P=0.8706). c, FUdR treatment reduces lifespan of wild-type worms at cold temperature (Non-treated versus FUdR, P<0.0001), a phenotype rescued by application of exogenous PGE2 (PGE2 + FUdR versus FUdR, P<0.0001). Non-treated mean ± s.e.m: 29.30 ± 0.69; PGE2: 30.39 ± 0.70, FUdR: 22.98 ± 0.63, FUdR + PGE2: 28.38 ± 0.64. P-values: two-sided log-rank test, n= 96 worms/condition. See Supplementary Data 3 for statistical analysis and replicate data of lifespan experiments. d, Model of cell-non-autonomous communication between somatic tissues and germ line at cold temperature. Somatic tissues such as IL1 and AFD neurons detect low temperature and ameliorate adult GSC exhaustion, which in turns delays reproductive aging. Adult GSCs release PGE2 hormone to induce cbs-1 expression in the intestine, resulting in higher somatic production of pro-longevity H2S gas. The rewiring of somatic tissues by GSCs extends organismal lifespan. This process coordinates extended reproductive capacity with long lifespan at cold temperature.
Discussion
The aging process is regulated by a complex network of interconnected cell non-autonomous events, as distinct tissues can influence the aging of distal organs67. In this intricate system, the germ line has a central role in the regulation of somatic aging68. Particularly, previous studies found that the germ line can actively promote the deterioration of the soma during the aging process20,68. Since food sources can be largely unpredictable and insufficient in nature, and the integrity of tissues is constantly challenged by environmental or metabolic conditions, this system may facilitate a compromised distribution of limited resources to protect the germ line ensuring the fitness of the progeny in line with the disposable soma theory of aging. However, here we find an unexpected role of the germ line in the long lifespan phenotype induced by cold temperature. Notably, low temperature does not extend lifespan in germline-lacking mutants, providing a first indication that this tissue is required for cold-induced longevity. Ablation of the germ line from development could induce numerous changes, but our results indicate that this tissue is particularly required during adulthood for cold-induced longevity. Intrigued by these results, we examined whether temperature-associated changes determine the fitness of the adult germ line and how these changes impinge on cold-induced longevity. Besides its effects on lifespan, we find that low temperature also slows down reproductive aging. This process ensues from a delay in the age-associated exhaustion of adult GSCs, resulting in extended reproductive capacity. Notably, maintenance of GSC function is determined by the TRAP-1 channel, which acts in IL1 neurons to detect low temperature. Besides IL1 neurons, we find that dysregulation of thermosensory AFD neurons also shortens lifespan and germline proliferation at cold temperatures, but it does not reduce lifespan at standard or warm temperatures. Since AFD neurons do not express TRPA-1 channel44, it will be fascinating to examine how these cells sensor and process low temperature to communicate with the germ line. We speculate that AFD neurons constitutively release a pro-longevity signal when they are inactive or at a low activity state under cold temperature conditions. Conversely, AFD activation by temperature increase may prevent the release of this pro-longevity factor. Besides their effects at cold temperature, AFD neurons also induce heat shock response under heat stress conditions (>30°C)69. Although we did not observe effects of AFD neurons in lifespan at moderately warm temperature (25°C), other studies reported that ablation of these neurons can reduce survival at 25°C11. In our experiments, worms were maintained and raised at 20°C during development before shifting them to distinct temperatures to assess adult lifespan. In contrast, worms were cultured in the aforementioned study at a given experimental temperature for at least two generations before adult lifespan analysis at this specific temperature. Moreover, they performed lifespan experiments on FUdR to inhibit progeny, while we avoided this treatment given its effects on germline proliferation. Although it is difficult to directly compare both studies because the divergent experimental conditions, these differences raise the intriguing possibility that adaptation to temperature and other conditions can also influence how AFD neurons process temperature signals to modulate lifespan.
Whereas our data indicate that somatic tissues such as IL1 and AFD neurons detect and process temperature changes to delay GSC exhaustion, we find that these cells, in turn, promote somatic fitness of distal tissues and organismal longevity at low temperatures. To assess the requirement for adult GSCs in cold-induced longevity, we first treated adult worms with FUdR, which is extensively used to inhibit progeny at standard temperatures because it does not affect longevity of wild-type worms under these conditions5,70–72. We find that FUdR inhibits GSC proliferation at both 20°C and 15°C, but this demise of adult GSCs only shortens lifespan at cold temperature. However, caution is needed to interpret these results. Besides its effects on germline proliferation, FUdR treatment also improves proteostasis73. In addition, FUdR affects bacterial ribonucleotide metabolism and has different effects depending on the E. coli strain used to feed the worms, modulating host-microbiome co-metabolism in C. elegans 74. For this reason, we validated our results by RNAi of modulators of GSC proliferation during adulthood. For instance, we knocked down the expression of iff-1, a regulator of germline proliferation which is only expressed in this tissue54. Loss of iff-1 during adulthood blocks cold-induced longevity, without reducing lifespan at warmer temperatures. Importantly, knockdown of iff-1 does not affect fertility in early adult stages while it has a strong effect in GSC proliferation at later stages, supporting a specific signaling role of adult GSCs in cold-induced longevity.
We then identify that robust proliferation of GSCs up-regulates proteostasis components (e.g., cct subunits and hsp-1) as well as pro-longevity factors such as H2S. This signaling gaseous molecule is produced by cbs-1, which expression is induced in somatic tissues such as the intestine by adult GSCs at low temperatures. Notably, intestinal cbs-1 overexpression is sufficient to extend longevity even at warm temperatures, mimicking cold-temperature conditions. It is important to note that intestinal cbs-1 overexpression does not completely rescue the pronounced short-lived phenotype of worms with defects in adult germline proliferation. This partial rescue upon intestinal cbs-1 overexpression could be explained by different possibilities. Besides its effects in the intestine, cold temperature also triggers cbs-1 expression in the muscle. Conversely, muscle-specific knockdown of cbs-1 decreases cold-induced longevity. Therefore, up-regulation of cbs-1 in the muscle could contribute to cold-induced longevity. In addition, our results indicate that cbs-1 is not the only factor involved in cold-induced longevity and up-regulation of other factors can also contribute to this phenotype. For instance, loss of cct subunits in the intestine and neurons as well as knockdown of hsp-1 in neurons diminish cold-induced longevity.
Interestingly, it has been reported that glp-1 germline-lacking mutant worms also exhibit increased levels of cct subunits and H2S production in somatic tissues at standard and warm temperatures, contributing to lifespan extension under these conditions25,75. Thus, a fascinating possibility is that germ cells can either promote or inhibit the same downstream pathways in somatic tissues depending on the environmental and physiological conditions. In addition, depletion of the germ line from development could induced additional physiological changes when compared to inhibition of GSC proliferation after the adult germ line is formed. Our experiments in germline-lacking models were a first indication of a potential role of the germ line in cold-induced longevity, as we further validated by rescue experiments overexpressing cbs-1 in the intestine of these worms. However, absence of the germ line cannot be directly equated with reduced adult GSC proliferation. In these lines, we observe that both iff-1 or glp-1 germline-lacking mutants are long-lived at 20°C, whereas iff-1 RNAi in adult wild-type worms does not extend lifespan at this temperature.
Little is known about the specific signaling molecules by which the germ line communicates with somatic tissues. At cold temperature, our findings establish a role of PGE2 release by adult GSCs in extended longevity. Thus, this hormone could be an important determinant of long lifespan under specific conditions such as low temperature. Prostaglandins are lipid compounds derived from arachidonic acid. Interestingly, administration of arachidonic acid increases resistance of C. elegans to starvation and extends life span in conditions of food abundance76. It is important to note that alterations in the synthesis or release of PGE2 do not completely block cold-induced longevity. Therefore, other signaling pathways could also contribute to modulation of somatic tissues by germ cells at cold temperature.
Taken together, our results suggest a cell non-autonomous mechanism (i.e., neurons-germline-intestine axis) that coordinates extended reproductive capacity with long lifespan under cold temperature, without the need of sacrificing either the germ line or the soma. Since it is generally considered that the germ line and fertility induce somatic aging, this unexpected pro-longevity role of adult GSCs have important implications for the understanding of the aging process. Indeed, our results raise the intriguing possibility that germ cells activate distinct signals to differentially modulate somatic tissues depending on the physiological and environmental conditions. This could be of particular interest for our ever-aging society, because several studies indicate that women who have later menopause tend to live longer as well as reduced risk of cardiovascular disease and less loss of bone density77–79. An intriguing question is why the germ line promotes longevity at cold temperature. In contrast to high temperatures, low temperature reduces the rate of chemical reactions and molecular entropy, a process that may decrease the damage and deterioration of distinct tissues triggered by cellular metabolism. Concomitantly, this process could ameliorate the pressure to prevent and repair damage to tissues. We speculate that these conditions facilitate the distribution of resources, reducing the need to compromise on either the soma or the germ line. Cold temperatures are detected by thermosensory neurons that delay exhaustion of GSCs, resulting in the ability to reproduce at older ages. Since this process may also require a healthy soma, we speculate that GSCs actively communicate with other tissues such as the intestine to ensure the fitness of the organism and the ability to reproduce for extended periods of time.
Methods
C. elegans strains and maintenance
C. elegans strains were grown and maintained on standard Nematode Growth Medium (NGM) seeded with E. coli (OP50)80. Wild-type (N2), CB4037 (glp-1(e2141)III), SS104 (glp-4(bn2)I), CF512 (fer-15(b26)II;fem-1(hc17)IV) (fer-15 is also named rrf-3), TQ233 (trpa-1(ok999)IV), GN112 (pgIs2[gcy-8p::TU#813 + gcy-8p::TU#814 + unc-122p::GFP + gcy-8p::mCherry + gcy-8p::GFP + ttx-3p::GFP]), JN215 (iff-1(tm483)III/hT2 [bli-4(e937) let-?(q782) qIs48](I;III)), PR767 (ttx-1(p767)V), PY1283 (ttx-1(oy29) oyIs17 [gcy-8p::GFP + lin-15(+)]V), BA837 (spe-26(it112)IV), CB4108 (fog-2(q71)V), JK560 (fog-1(q250)I) and JK1466 (gld-1(q485)/dpy-5(e61) unc-13(e51)I) were provided by the Caenorhabditis Genetics Center (CGC) (University of Minnesota), which is supported by the NIH Office of Research Infrastructure Programs (P40 OD010440). CF2424 (PR767 (ttx-1(p767)V) outcrossed 4 times to wild-type N2) was provided by S.J. Lee (Korea Advanced Institute of Science and Technology, South Korea). AA1724 (pges-2(ok3316)IV) was provided by A. Antebi (Max Planck Institute for Biology of Ageing, Germany) and was generated by outcrossing the RB2421 strain (pges-2(ok3316)IV) 4 times to wild-type N2. RB2421 was made by the C. elegans Gene Knockout Project at the Oklahoma Medical Research Foundation. TQ6891 (xuEx1670[aqp-6p::trpa-1::sl2::yfp]; trpa-1(ok999)IV) was a kind gift from X.Z. Shawn Xu (University of Michigan, USA).
For tissue-specific RNAi experiments, we used either rde-1 or sid-1 mutant animals in which wild-type rde-1 or sid-1 genes have been rescued using tissue-specific promoters, respectively. Tissue-specific knockdown was confirmed in the original publications. In WM118 strain (rde-1(ne300)V; neIs9[myo-3p::HA::RDE-1 + rol-6(su1006)], RNAi treatment is only effective in muscle81. In VP303 strain (rde-1(ne219)V; kbIs7[nhx-2p::rde-1 + rol-6(su1006)], RNAi treatment is only effective in intestine82. In AMJ345 strain (jamSi2[mex-5p::rde-1(+)]II; rde-1(ne219)V), transgene rescues effective RNAi treatment in both intestine and germ line83. TU3401 strain (sid-1(pk3321)V; uIs69[pCFJ90(myo-2p::mCherry) + unc-119p::sid-1]) was used for neuron-specific knockdown84. All the aforementioned tissue-specific RNAi-sensitive strains were provided by the CGC. For tissue-specific RNAi experiments in the germ line alone, we used DCL569 strain (mkcSi13[sun-1p::rde-1::sun-1 3'UTR + unc-119(+)]II; rde-1(mkc36)V)85. DCL569 was a kind gift from Di Chen (Nanjing University, China).
For the generation of worm strains DVG146–DVG147 (N2, ocbEx130[sur-5p::ZC373.1, myo-3p::GFP] and N2, ocbEx131[sur-5p::ZC373.1, myo-3p::GFP]), a DNA plasmid mixture containing 70 ng μl–1 of the plasmid sur5-p::cbs-1 and 20 ng μl–1 pPD93_97 (myo3-p::GFP) was injected into the gonads of adult N2 hermaphrodite animals, using standard methods86. GFP-positive F1 progeny were selected. Individual F2 worms were isolated to establish independent lines. Following this method we also generated the worm strains DVG162 (N2, ocbEx145[rgef-1p::ZC373.1, myo3-p::GFP]), DVG163 (N2, ocbEx146[rgef-1p::ZC373.1, myo3-p::GFP]), DVG166 (N2, ocbEx149[gly-19p::ZC373.1, myo-3p::GFP]), DVG167 (N2, ocbEx150[gly-19p::ZC373.1, myo-3p::GFP]), DVG173 (N2, ocbEx156[myo-3p::ZC373.1, myo-3p::GFP]) and DVG174 (N2, ocbEx157[myo-3p::ZC373.1, myo-3p::GFP]). Control worms DVG9 (N2, ocbEx9[myo3p::GFP]) were generated by microinjecting N2 worms with 20 ng μl–1 pPD93_9725. DVG186 (glp-1(e2141)III; ocbEx150[gly-19p::ZC373.1, myo-3p::GFP]) was generated by crossing DVG167 to CB4037.
For the generation of cbs-1 transcriptional reporter line DVG156 (N2, ocbEx140[ZC373.1p::GFP, pRF4(rol-6)]), a DNA plasmid mixture containing 50 ng μl–1 of the plasmid pDV184 and 30 ng μl–1 pAD126 (pRF4(rol-6)) was injected into the gonads of adult N2 hermaphrodite animals. Roller-phenotype-positive F1 progeny were selected. Individual F2 worms were isolated to establish independent lines. Similarly, we generated the cbs-1 transcriptional reporter line DVG185 by injecting a mixture of the plasmid pDV184 and pAD126 into the gonads of DCL569 germline-specific RNAi strain.
To generate DVG177 strain (ocbEx160[cbs-1p::GFP, pRF4(rol-6)]; pges-2(ok3316)IV), DVG156 was crossed to AA1724. Roller-phenotype-positive F1 progeny were selected and transferred to individual plates. Screening of pges-2(ok3316) homozygote worms was done in F2 progeny by PCR using the following primers: TGAACGCTGAGCATCCATAG and CGCCCGTTTTCTTTTAATG.
RNAi constructs
RNAi-treated strains were fed E. coli (HT115) containing an empty control vector (L4440) or expressing double-stranded RNAi. pgl-1, pgl-3, glh-1, mog-4, mog-5, iff-1, iff-2, ifg-1, ife-2, rps-15, rsks-1, cct-2, cct-6, cct-8, hint-1, hsp-1, fkb-6, ttr-6, cal-5 and gld-1 RNAi constructs were obtained from the Vidal RNAi library. glh-4, mog-1, eif-2A, cct-5, ama-1, cbs-1, nlp-24, daf-41 and mrp-6 RNAi constructs were obtained from the Ahringer RNAi library. All constructs were sequence verified. For each RNAi, we assessed the knockdown efficiency at the distinct temperatures the RNAi was used (Supplementary Fig. 33).
Lifespan studies
In most of the lifespan experiments, synchronized larvae were raised and fed OP50 E. coli at 20 °C until day 1 of adulthood. If wild-type larvae were raised at 25 °C, this was noted in the figure (e.g., Fig. 1f-g and Fig. 3b). In experiments containing glp-1, glp-4, fer-15;fem-1, fog-1, fog-2 or spe-26 mutants, larvae were raised at the restrictive temperature (25 °C) to obtain germline-lacking and sterile worms. Once worms reached adulthood, they were shifted to a given temperature (i.e., 10 °C, 15 °C, 20 °C or 25 °C) on plates with HT115 E. coli carrying empty vector or RNAi clones. 96 animals were used per condition and scored every day or every other day25. From the initial worm population, the worms that are lost or burrow into the medium as well as those that exhibit ‘protruding vulva’ or undergo bagging were censored. n= total number of uncensored animals/total number (uncensored+censored) of animals observed in each experiment. Lifespans were conducted at the temperatures indicated in the corresponding figure. For non-integrated lines DVG9, DVG146, DVG147, DV162, DV163, DVG166, DVG167, DVG173 and DVG174, GFP-positive worms were selected for lifespan studies. PRISM 6 software was used to determine median lifespan and generate lifespan graphs. OASIS software was used for statistical analysis to determine mean lifespan87. P values were calculated using the log-rank (Mantel–Cox) method. The P values refer to experimental and control animals in a single experiment. In the main text, each graph shows a representative experiment. See Supplementary Data 3 for statistical analysis and replicate data.
Construction of cbs-1 C. elegans expression plasmids
To construct the C. elegans plasmid (pDV163) for ubiquitous somatic overexpression of cbs-1, pPD95.77 from the Fire Lab kit was digested with SphI and XmaI to insert 3.6KB of the sur-5 promoter. The resultant vector was then digested with KpnI and EcoRI to excise GFP and insert a multi-cloning site. ZC373.1a (cbs-1) was PCR amplified from cDNA to include 5′ NheI and 3′ NotI restriction sites and then cloned into the aforementioned vector. To generate neuronal-specific overexpression cbs-1 plasmid (pDV188), pMS1 plasmid (rgef-1p::rpn-6.1::unc-54 3′UTR) with an inserted multi-cloning site was digested with 5′ NheI and 3′ NotI restriction sites. Then, cbs-1 was PCR amplified from cDNA to include 5′ NheI and 3′ NotI restriction sites and cloned into pMS1. Following the same strategy, cbs-1 was cloned into pMS2 (gly-19p::rpn-6.1::unc-54 3′UTR) to construct intestinal-specific expression plasmid (pDV189). To generate muscle-specific expression plasmid (pDV190), pMS3 plasmid (myo-3p::rpn-6.1::unc-54 3′UTR) was digested with 5′ XbaI and 3′ AscI restriction sites. All constructs were sequence verified. pMS1-3 were a gift from A. Dillin.
Construction of cbs-1 transcriptional reporter construct
To construct pDV184, pPD95.77 from the Fire Lab kit was digested with SalI and BamHI. The promoter region and part of exon 1 of ZC373.1a (cbs-1) was PCR amplified from N2 gDNA to include −1428 to 187 and then cloned into the aforementioned vector using the same enzymes.
cbs-1 transcriptional reporter experiments and imaging
DVG156 worms were grown at 20 °C until day 1 of adulthood and then transferred onto plates with E. coli (HT115) expressing empty vector or double-stranded RNAi. Worms were then cultured until day 7 at the indicated temperatures in the respective figure. For FUdR treatment experiments, day 1 adults were transferred onto plates with E. coli (HT115) expressing empty vector covered with 100 μg ml-1 FUdR. Adult worms were then grown until day 5 at the indicated temperatures under FUdR treatment. In rescue experiments, worms were transferred at day 5 adulthood onto plates with PGE2 (Tocris, #363-24-6) spotted on top of the bacterial lawn. For imaging, adult worms were immobilized using 0.1% Azide in M9 buffer and covered with cover slip. Images of whole worms were acquired with Zeiss Axio Zoom.V16 fluorescence microscope. To quantify GFP fluorescence, worms were outlined and quantified using ImageJ software.
Body size imaging and quantification
Images of worms were acquired with Zeiss Axio Zoom.V16 microscope. To quantify body size, the whole worm was outlined and quantified using ImageJ software.
Bromodeoxyuridine (BrdU) proliferation assays
Worms were incubated with 33mM solution of BrdU (Sigma-Aldrich) in S medium for 2h at 20 °C. Worms were washed with EBT buffer (1X egg buffer, 0.2 % Tween 20, 20 mM Sodium Azide). Worms were then decapitated to extract germ line on a coverslip and covered with a poly-lysine-coated microscope slide. The coverslip was removed and the slide was fast frozen on dry ice. Then, the slide was fixed in methanol for 2 min at -20 °C and washed with PBST (1X PBS and 0.1% Tween 20). DNA was denatured in 2M HCl for 45 min at room temperature followed by washing with PBST. After blocking for 30 min in PBST 10% donkey serum, anti-BrdU antibody (Abcam, ab6326, RRID:AB_305426) was added (1:250) followed by overnight incubation in a humid chamber. Anti-Rat IgG secondary antibody (Life Technologies, A11081, RRID:AB_141738) was added (1:500) for 2 h at room temperature. Finally, slides were mounted with Precision coverslip (Roth) using DAPI fluoromount-G (Southern Biotech 0100-20).
Egg counting
Synchronized animals were raised and fed OP50 E. coli at 20 °C or 25 °C until late L4 stage. Number of eggs during the self-reproductive period was measured by singly plating late L4 worms and cultured them at the distinct temperatures indicated in the corresponding figures. Each adult worm was then transferred to a new plate every 24 h and the previous plate was kept at the respective temperature for another 24 h when the number of alive progeny, visible as L1 larvae was scored to assess percentage of viable eggs. This procedure was repeated until no alive progenies were counted.
For egg counting after the self-reproductive period, worms were grown at the distinct temperatures indicated in the corresponding figures for 8 days during adulthood. Then, worms were singly plated and mated for 48 h at the indicated temperatures with young males which were raised at 20°C. After mating, females were transferred to a new plate every 24 h and the previous plate was kept at the respective temperature for another 24 h when the number of alive progeny was scored. This procedure was repeated until no alive progenies were counted.
H2S detection
H2S production capacity was measured following the lead sulfide method27. First, worms were homogenated in lysis buffer (PBS supplemented with 10 mM L-cysteine and 1 mM pyridoxal 5’-phosphate hydrate (PLP)) using a Precellys 24 homogenizer (Bertin technologies). 300 μg of the lysate was transferred onto a well of a 96-well plate. Then, lead acetate paper was placed directly over the well for detection of H2S at 37°C. H2S production was detected by the black precipitate, lead sulfide, resulting from the specific reaction between H2S and lead acetate.
Western blot
Worms were lysed in protein lysis buffer (50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 0.25% sodium deoxycholate, 1 mM EDTA and protease inhibitor (Roche)) using a Precellys 24 homogenizer. Worm lysates were centrifuged at 10,000 rpm for 10 min at 4 °C and the supernatant was collected. Protein concentrations were determined with standard BCA protein assay (Thermoscientific). 30 μg of total protein was separated by SDS-PAGE, transferred to nitrocellulose membranes (Millipore) and subjected to immunoblotting. Western blot analysis was performed with anti-PGES2 antibody (Bioss, bs-2639R, 1:500; RRID:AB_10860215) and α-tubulin (Sigma, T6199, 1:5,000; RRID:AB_477583).
C. elegans germline and gut immunostaining
Worms were washed with EBT buffer (1X egg buffer, 0.2 % Tween 20, 20 mM Sodium Azid), decapitated to extract germ line and intestine on a coverslip and covered with a poly-lysine-treated microscope slide. The slide was fast frozen on dry ice, fixed in methanol for 2 min at -20 °C and washed with PBST (1X PBS and 0.1 % Tween 20). Immunostaining was processed by blocking with 10 % donkey serum in PBST for 30 min followed by overnight incubation with anti-PGES2 antibody (Bioss, bs-2639R, 1:100; RRID:AB_10860215) at room temperature. Alexa Fluor 488 goat anti-rabbit IgG (Life Technologies, A11008, RRID: AB_143165, 1:500) was added for 2 h. Slides were mounted with DAPI fluoromount-G (Southern Biotech 0100-20).
PGE2 measurements
We quantified PGE2 levels using a PGE2 ELISA Kit (amsbio, AMS.E-EL-0034), following manufacturer’s instructions. fer-15;fem-1 control sterile worms were lysed in protein lysis buffer (50 mM Tris-HCl, pH 7.8, 150 mM NaCl, 0.25% sodium deoxycholate, 1 mM EDTA and protease inhibitor (Roche)) using a Precellys 24 homogenizer. Worm lysates were centrifuged at 10,000 rpm for 10 min at 4°C and supernatant was collected. Protein concentrations were determined with standard BCA protein asaay (Thermoscientific). 3 mg of total protein were used for measurement.
Oxygen consumption
Oxygen consumption rates were measured using an Oroboros Oxygraph 2k (Oroboros Instruments GmbH). 300 adult worms were used for each measurement. Data was analyzed using DatLab7 software (version 7 3.0.3).
Motility assay
At day 6 of adulthood, worms were transferred to a drop of M9 buffer and after 30 s of adaptation the number of body bends was counted for 30 s. A body bend is defined as change in direction of the bend at the mid-body.
Sample preparation for label-free quantitative proteomics and analysis
fer-15;fem-1 control sterile worms were lysed in urea buffer (8 M urea, 2 M Thiourea, 10 mM HEPES, pH 7.6) by sonication and cleared using centrifugation (13,000 rpm, 10 min). Supernatants were reduced (1 mM DTT, 30 min), alkylated (5 mM iodoacetamide (IAA), 45 min) and digested with trypsin at a 1:100 w/w ratio after diluting urea concentration to 2 M. One day after, samples were cleared (16,000g, 20 min) and supernatant was acidified. Peptides were cleaned up using stage tip extraction. The liquid chromatography tandem mass spectrometry (LC-MS/MS) equipment consisted out of an EASY nLC 1000 coupled to the quadrupole based QExactive instrument (Thermo Scientific) via a nano-spray electroionization source. Peptides were separated on an in-house packed 50 cm column (1.9 µm C18 beads, Dr. Maisch) using a binary buffer system: A) 0.1% formic acid and B) 0.1% formic acid in acetonitrile. The content of buffer B was raised from 7% to 23% within 120 min and followed by an increase to 45% within 10 min. Then, within 5 min buffer B fraction was raised to 80% and held for further 5 min after which it was decreased to 5% within 2 min and held there for further 3 min before the next sample was loaded on the column. Eluting peptides were ionized by an applied voltage of 2.2 kV. The capillary temperature was 275°C and the S-lens RF level was set to 60. MS1 spectra were acquired using a resolution of 70,000 (at 200 m/z), an Automatic Gain Control (AGC) target of 3e6 and a maximum injection time of 20 ms in a scan range of 300-1750 Th. In a data dependent mode, the 10 most intense peaks were selected for isolation and fragmentation in the HCD cell using a normalized collision energy of 25 at an isolation window of 2.1 Th. Dynamic exclusion was enabled and set to 20 s. The MS/MS scan properties were: 17.500 resolution at 200 m/z, an AGC target of 5e5 and a maximum injection time of 60 ms. All label-free proteomics data sets were analyzed with the MaxQuant software (version 1.5.3.8). We employed the LFQ mode and used MaxQuant default settings for protein identification and LFQ quantification88. All downstream analyses were carried out on LFQ values with Perseus (v. 1.5.2.4).
RNA isolation and sequencing
RNA from extruded germ lines of day 6-adult N2 worms was extracted using RNAbee (Tel-Test Inc.). Libraries were prepared using the TruSeq Stranded mRNA Library Prep Kit. Library preparation started with 1 µg total RNA. After selection (using poly-T oligo-attached magnetic beads), mRNA was purified and fragmented using divalent cations under elevated temperature. The RNA fragments underwent reverse transcription using random primers followed by second strand cDNA synthesis with DNA Polymerase I and RNase H. After end repair and A-tailing, indexing adapters were ligated. The products were then purified and amplified (20 µl template, 14 PCR cycles) to create the final cDNA libraries. After library validation and quantification (Agilent 2100 Bioanalyzer), equimolar amounts of library were pooled. The pool was quantified by using the Peqlab KAPA Library Quantification Kit and the Applied Biosystems 7900HT Sequence Detection System. The pool was sequenced on an Illumina HiSeq 4000 sequencer with a paired-end (2x75bp) protocol.
RNA-seq data were analysed using a QuickNGS pipeline89. This workflow system provided basic read quality check using FastQC (version 0.10.1) and read statistics using SAMtools (version 0.1.19). The basic data processing of the QuickNGS pipeline consists of a splicing-aware alignment using Tophat2 (version 2.0.10) followed by reference-guided transcriptome reassembly with Cufflinks2 (version 2.1.1). The QuickNGS pipeline calculated read count means, fold change and P-values with DEseq2 (version 1.4.5) and gene expression for the individual samples with Cufflinks2 (version 2.1.1) as FPKMs, using in both cases genomic annotation from the Ensembl database version 87.
Quantitative RT–PCR
Total RNA was isolated from ~2,000 synchronized day 5-adult worms using RNAbee (Tel-Test Inc.). cDNA was generated using qScript Flex cDNA synthesis kit (Quantabio). SybrGreen real-time qPCR experiments were performed with a 1:20 dilution of cDNA using a CFC384 Real-Time System (Bio-Rad). Data were analysed with the comparative 2ΔΔC t method using the geometric mean of cdc-42 and pmp-3 as housekeeping genes90. See Supplementary Table 2 for details about the primers used for this assay.
Supplementary Material
Acknowledgements
This work was supported by the European Research Council (ERC Starting Grant-677427 StemProteostasis) and the Deutsche Forschungsgemeinschaft (DFG) (VI742/1-2 and CECAD). We are grateful to E. Llamas for the graphical model and the germline scheme. We thank U. Pham and Niels Fritsma for their help in BrdU and lifespan experiments, respectively. We thank J.P. Derks for data analysis and preparation of heat map figures with R stats package of proteomics experiments. We also thank J. Horák for generation of tissue-specific cbs-1 expression plasmids. We thank T. Hoppe for critical comments on the manuscript. We are grateful to the CECAD Proteomics and Imaging Facilities for their advice and contribution to proteomics and imaging experiments, respectively. We would also like to thank P. Wagle from the CECAD Bionformatics Facility for data analysis of RNA-sequencing experiments.
Footnotes
Reporting Summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Author Contributions
H.J.L., A.N. and D.V. performed most of the experiments, data analysis and interpretation. S.K. assessed knockdown levels and contributed to lifespan assays and other experiments. G.C. performed some of the BrdU assays and helped with other experiments. M.S.S. generated the plasmids for tissue-specific overexpression that were used to clone cbs-1. M.H. performed oxygen consumption experiments. A.T. contributed with her expertise on metabolic rates and provided critical advice on the project. The manuscript was written by D.V. All the authors discussed the results and commented on the manuscript.
Competing interests
The authors declare no competing interests.
Data availability
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary files. Transcriptome data have been deposited in Gene Expression Omnibus (GEO) under the accession code GSE123054. All the other data are also available from the corresponding author upon request.
References
- 1.Fontana L, Partridge L, Longo VD. Extending healthy life span--from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- 3.Robinson JD, Powell JR. Long-term recovery from acute cold shock in Caenorhabditis elegans. BMC Cell Biol. 2016;17:2. doi: 10.1186/s12860-015-0079-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conti B. Considerations on temperature, longevity and aging. Cell Mol Life Sci. 2008;65:1626–1630. doi: 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosono R, Mitsui Y, Sato Y, Aizawa S, Miwa J. Life span of the wild and mutant nematode Caenorhabditis elegans. Effects of sex, sterilization, and temperature. Exp Gerontol. 1982;17:163–172. doi: 10.1016/0531-5565(82)90052-3. [DOI] [PubMed] [Google Scholar]
- 6.Klass MR. Aging in the nematode Caenorhabditis elegans: major biological and environmental factors influencing life span. Mech Ageing Dev. 1977;6:413–429. doi: 10.1016/0047-6374(77)90043-4. [DOI] [PubMed] [Google Scholar]
- 7.Wu D, Rea SL, Cypser JR, Johnson TE. Mortality shifts in Caenorhabditis elegans: remembrance of conditions past. Aging Cell. 2009;8:666–675. doi: 10.1111/j.1474-9726.2009.00523.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lamb MJ. Temperature and lifespan in Drosophila. Nature. 1968;220:808–809. doi: 10.1038/220808a0. [DOI] [PubMed] [Google Scholar]
- 9.Liu RK, Walford RL. Increased Growth and Life-Span with Lowered Ambient Temperature in Annual Fish Cynolebias Adloffi. Nature. 1966;212:1277. &. [Google Scholar]
- 10.Valenzano DR, Terzibasi E, Cattaneo A, Domenici L, Cellerino A. Temperature affects longevity and age-related locomotor and cognitive decay in the short-lived fish Nothobranchius furzeri. Aging Cell. 2006;5:275–278. doi: 10.1111/j.1474-9726.2006.00212.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee SJ, Kenyon C. Regulation of the longevity response to temperature by thermosensory neurons in Caenorhabditis elegans. Curr Biol. 2009;19:715–722. doi: 10.1016/j.cub.2009.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao R, et al. A genetic program promotes C. elegans longevity at cold temperatures via a thermosensitive TRP channel. Cell. 2013;152:806–817. doi: 10.1016/j.cell.2013.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Conti B, et al. Transgenic mice with a reduced core body temperature have an increased life span. Science. 2006;314:825–828. doi: 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- 14.Loeb J, Northrop JH. Is There a Temperature Coefficient for the Duration of Life? Proc Natl Acad Sci U S A. 1916;2:456–457. doi: 10.1073/pnas.2.8.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang B, et al. Environmental Temperature Differentially Modulates C. elegans Longevity through a Thermosensitive TRP Channel. Cell Rep. 2015;11:1414–1424. doi: 10.1016/j.celrep.2015.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang B, et al. Brain-gut communications via distinct neuroendocrine signals bidirectionally regulate longevity in C. elegans. Genes Dev. 2018;32:258–270. doi: 10.1101/gad.309625.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horikawa M, Sural S, Hsu AL, Antebi A. Co-chaperone p23 regulates C. elegans Lifespan in Response to Temperature. PLoS Genet. 2015;11:e1005023. doi: 10.1371/journal.pgen.1005023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Partridge L, Gems D, Withers DJ. Sex and death: what is the connection? Cell. 2005;120:461–472. doi: 10.1016/j.cell.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 19.Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 20.Arantes-Oliveira N, Apfeld J, Dillin A, Kenyon C. Regulation of life-span by germ-line stem cells in Caenorhabditis elegans. Science. 2002;295:502–505. doi: 10.1126/science.1065768. [DOI] [PubMed] [Google Scholar]
- 21.Hsin H, Kenyon C. Signals from the reproductive system regulate the lifespan of C. elegans. Nature. 1999;399:362–366. doi: 10.1038/20694. [DOI] [PubMed] [Google Scholar]
- 22.Vilchez D, et al. RPN-6 determines C. elegans longevity under proteotoxic stress conditions. Nature. 2012;489:263–268. doi: 10.1038/nature11315. [DOI] [PubMed] [Google Scholar]
- 23.Berman JR, Kenyon C. Germ-cell loss extends C. elegans life span through regulation of DAF-16 by kri-1 and lipophilic-hormone signaling. Cell. 2006;124:1055–1068. doi: 10.1016/j.cell.2006.01.039. [DOI] [PubMed] [Google Scholar]
- 24.Lapierre LR, Gelino S, Melendez A, Hansen M. Autophagy and lipid metabolism coordinately modulate life span in germline-less C. elegans. Curr Biol. 2011;21:1507–1514. doi: 10.1016/j.cub.2011.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noormohammadi A, et al. Somatic increase of CCT8 mimics proteostasis of human pluripotent stem cells and extends C. elegans lifespan. Nat Commun. 2016;7:13649. doi: 10.1038/ncomms13649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shemesh N, Shai N, Ben-Zvi A. Germline stem cell arrest inhibits the collapse of somatic proteostasis early in Caenorhabditis elegans adulthood. Aging Cell. 2013 doi: 10.1111/acel.12110. [DOI] [PubMed] [Google Scholar]
- 27.Hine C, et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell. 2015;160:132–144. doi: 10.1016/j.cell.2014.11.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miller DL, Roth MB. Hydrogen sulfide increases thermotolerance and lifespan in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2007;104:20618–20622. doi: 10.1073/pnas.0710191104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Priess JR, Schnabel H, Schnabel R. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell. 1987;51:601–611. doi: 10.1016/0092-8674(87)90129-2. [DOI] [PubMed] [Google Scholar]
- 30.Curran SP, Wu X, Riedel CG, Ruvkun G. A soma-to-germline transformation in long-lived Caenorhabditis elegans mutants. Nature. 2009;459:1079–1084. doi: 10.1038/nature08106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Syntichaki P, Troulinaki K, Tavernarakis N. eIF4E function in somatic cells modulates ageing in Caenorhabditis elegans. Nature. 2007;445:922–926. doi: 10.1038/nature05603. [DOI] [PubMed] [Google Scholar]
- 32.Andux S, Ellis RE. Apoptosis maintains oocyte quality in aging Caenorhabditis elegans females. PLoS Genet. 2008;4:e1000295. doi: 10.1371/journal.pgen.1000295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barton MK, Kimble J. fog-1, a regulatory gene required for specification of spermatogenesis in the germ line of Caenorhabditis elegans. Genetics. 1990;125:29–39. doi: 10.1093/genetics/125.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.L'Hernault SW. Spermatogenesis. WormBook. 2006:1–14. doi: 10.1895/wormbook.1.85.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schedl T, Kimble J. fog-2, a germ-line-specific sex determination gene required for hermaphrodite spermatogenesis in Caenorhabditis elegans. Genetics. 1988;119:43–61. doi: 10.1093/genetics/119.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Varkey JP, Muhlrad PJ, Minniti AN, Do B, Ward S. The Caenorhabditis elegans spe-26 gene is necessary to form spermatids and encodes a protein similar to the actin-associated proteins kelch and scruin. Genes Dev. 1995;9:1074–1086. doi: 10.1101/gad.9.9.1074. [DOI] [PubMed] [Google Scholar]
- 37.Hubbard EJ, Greenstein D. Introduction to the germ line. WormBook. 2005:1–4. doi: 10.1895/wormbook.1.18.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crittenden SL, Leonhard KA, Byrd DT, Kimble J. Cellular analyses of the mitotic region in the Caenorhabditis elegans adult germ line. Mol Biol Cell. 2006;17:3051–3061. doi: 10.1091/mbc.E06-03-0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimble J, Seidel H. StemBook. 2008 [Google Scholar]
- 40.Morita Y, Tilly JL. Oocyte apoptosis: like sand through an hourglass. Dev Biol. 1999;213:1–17. doi: 10.1006/dbio.1999.9344. [DOI] [PubMed] [Google Scholar]
- 41.Hodgkin J, Barnes TM. More is not better: brood size and population growth in a self-fertilizing nematode. Proc Biol Sci. 1991;246:19–24. doi: 10.1098/rspb.1991.0119. [DOI] [PubMed] [Google Scholar]
- 42.Garrity PA, Goodman MB, Samuel AD, Sengupta P. Running hot and cold: behavioral strategies, neural circuits, and the molecular machinery for thermotaxis in C. elegans and Drosophila. Genes Dev. 2010;24:2365–2382. doi: 10.1101/gad.1953710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Glauser DA, et al. Heat Avoidance Is Regulated by Transient Receptor Potential (TRP) Channels and a Neuropeptide Signaling Pathway in Caenorhabditis elegans. Genetics. 2011;188:91–U150. doi: 10.1534/genetics.111.127100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kindt KS, et al. Caenorhabditis elegans TRPA-1 functions in mechanosensation. Nat Neurosci. 2007;10:568–577. doi: 10.1038/nn1886. [DOI] [PubMed] [Google Scholar]
- 45.Perkins LA, Hedgecock EM, Thomson JN, Culotti JG. Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol. 1986;117:456–487. doi: 10.1016/0012-1606(86)90314-3. [DOI] [PubMed] [Google Scholar]
- 46.Satterlee JS, et al. Specification of thermosensory neuron fate in C. elegans requires ttx-1, a homolog of otd/Otx. Neuron. 2001;31:943–956. doi: 10.1016/s0896-6273(01)00431-7. [DOI] [PubMed] [Google Scholar]
- 47.Joshi PM, Riddle MR, Djabrayan NJ, Rothman JH. Caenorhabditis elegans as a model for stem cell biology. Dev Dyn. 2010;239:1539–1554. doi: 10.1002/dvdy.22296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Graham PL, Schedl T, Kimble J. More mog genes that influence the switch from spermatogenesis to oogenesis in the hermaphrodite germ line of Caenorhabditis elegans. Dev Genet. 1993;14:471–484. doi: 10.1002/dvg.1020140608. [DOI] [PubMed] [Google Scholar]
- 49.Puoti A, Kimble J. The Caenorhabditis elegans sex determination gene mog-1 encodes a member of the DEAH-Box protein family. Mol Cell Biol. 1999;19:2189–2197. doi: 10.1128/mcb.19.3.2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Puoti A, Kimble J. The hermaphrodite sperm/oocyte switch requires the Caenorhabditis elegans homologs of PRP2 and PRP22. Proc Natl Acad Sci U S A. 2000;97:3276–3281. doi: 10.1073/pnas.97.7.3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kawasaki I, et al. The PGL family proteins associate with germ granules and function redundantly in Caenorhabditis elegans germline development. Genetics. 2004;167:645–661. doi: 10.1534/genetics.103.023093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawasaki I, et al. PGL-1, a predicted RNA-binding component of germ granules, is essential for fertility in C. elegans. Cell. 1998;94:635–645. doi: 10.1016/s0092-8674(00)81605-0. [DOI] [PubMed] [Google Scholar]
- 53.Kuznicki KA, et al. Combinatorial RNA interference indicates GLH-4 can compensate for GLH-1; these two P granule components are critical for fertility in C. elegans. Development. 2000;127:2907–2916. doi: 10.1242/dev.127.13.2907. [DOI] [PubMed] [Google Scholar]
- 54.Hanazawa M, et al. The Caenorhabditis elegans eukaryotic initiation factor 5A homologue, IFF-1, is required for germ cell proliferation, gametogenesis and localization of the P-granule component PGL-1. Mech Dev. 2004;121:213–224. doi: 10.1016/j.mod.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 55.Green RA, et al. A high-resolution C. elegans essential gene network based on phenotypic profiling of a complex tissue. Cell. 2011;145:470–482. doi: 10.1016/j.cell.2011.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Francis R, Barton MK, Kimble J, Schedl T. gld-1, a tumor suppressor gene required for oocyte development in Caenorhabditis elegans. Genetics. 1995;139:579–606. doi: 10.1093/genetics/139.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Marin VA, Evans TC. Translational repression of a C-elegans Notch mRNA by the STAR/KH domain protein GLD-1. Development. 2003;130:2623–2632. doi: 10.1242/dev.00486. [DOI] [PubMed] [Google Scholar]
- 58.Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C-elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- 59.Van Voorhies WA, Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. P Natl Acad Sci USA. 1999;96:11399–11403. doi: 10.1073/pnas.96.20.11399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vozdek R, Hnizda A, Krijt J, Kostrouchova M, Kozich V. Novel structural arrangement of nematode cystathionine beta-synthases: characterization of Caenorhabditis elegans CBS-1. Biochem J. 2012;443:535–547. doi: 10.1042/BJ20111478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang G, et al. H2S as a physiologic vasorelaxant: hypertension in mice with deletion of cystathionine gamma-lyase. Science. 2008;322:587–590. doi: 10.1126/science.1162667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Paul BD, Snyder SH. H2S signalling through protein sulfhydration and beyond. Nat Rev Mol Cell Bio. 2012;13:499–507. doi: 10.1038/nrm3391. [DOI] [PubMed] [Google Scholar]
- 63.Kudo I, Murakami M. Prostaglandin E synthase, a terminal enzyme for prostaglandin E2 biosynthesis. J Biochem Mol Biol. 2005;38:633–638. doi: 10.5483/bmbrep.2005.38.6.633. [DOI] [PubMed] [Google Scholar]
- 64.Tanioka T, et al. Regulation of cytosolic prostaglandin E2 synthase by 90-kDa heat shock protein. Biochem Biophys Res Commun. 2003;303:1018–1023. doi: 10.1016/s0006-291x(03)00470-4. [DOI] [PubMed] [Google Scholar]
- 65.Lovgren AK, Kovarova M, Koller BH. cPGES/p23 is required for glucocorticoid receptor function and embryonic growth but not prostaglandin E-2 synthesis. Molecular and Cellular Biology. 2007;27:4416–4430. doi: 10.1128/MCB.02314-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kochel TJ, Fulton AM. Multiple drug resistance-associated protein 4 (MRP4), prostaglandin transporter (PGT), and 15-hydroxyprostaglandin dehydrogenase (15-PGDH) as determinants of PGE2 levels in cancer. Prostaglandins Other Lipid Mediat. 2015;116–117:99–103. doi: 10.1016/j.prostaglandins.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor RC, Berendzen KM, Dillin A. Systemic stress signalling: understanding the cell non-autonomous control of proteostasis. Nat Rev Mol Cell Biol. 2014;15:211–217. doi: 10.1038/nrm3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Khodakarami A, Mels J, Saez I, Vilchez D. Mediation of organismal aging and somatic proteostasis by the germline. Frontiers in Molecular Biosciences. 2015;2 doi: 10.3389/fmolb.2015.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Prahlad V, Cornelius T, Morimoto RI. Regulation of the cellular heat shock response in Caenorhabditis elegans by thermosensory neurons. Science. 2008;320:811–814. doi: 10.1126/science.1156093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gandhi S, Santelli J, Mitchell DH, Stiles JW, Raosanadi D. A Simple Method for Maintaining Large, Aging Populations of Caenorhabditis-Elegans. Mechanisms of Ageing and Development. 1980;12:137–150. doi: 10.1016/0047-6374(80)90090-1. [DOI] [PubMed] [Google Scholar]
- 71.Mitchell DH, Stiles JW, Santelli J, Rao SD. Synchronous Growth and Aging of Caenorhabditis-Elegans in the Presence of Fluorodeoxyuridine. J Gerontol. 1979;34:28–36. doi: 10.1093/geronj/34.1.28. [DOI] [PubMed] [Google Scholar]
- 72.Van Raamsdonk JM, Hekimi S. FUdR causes a twofold increase in the lifespan of the mitochondrial mutant gas-1. Mech Ageing Dev. 2011;132:519–521. doi: 10.1016/j.mad.2011.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Feldman N, Kosolapov L, Ben-Zvi A. Fluorodeoxyuridine improves Caenorhabditis elegans proteostasis independent of reproduction onset. PLoS One. 2014;9:e85964. doi: 10.1371/journal.pone.0085964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scott TA, et al. Host-Microbe Co-metabolism Dictates Cancer Drug Efficacy in C. elegans. Cell. 2017;169:442–456 e418. doi: 10.1016/j.cell.2017.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wei Y, Kenyon C. Roles for ROS and hydrogen sulfide in the longevity response to germline loss in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2016;113:E2832–2841. doi: 10.1073/pnas.1524727113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.O'Rourke EJ, Kuballa P, Xavier R, Ruvkun G. omega-6 Polyunsaturated fatty acids extend life span through the activation of autophagy. Gene Dev. 2013;27:429–440. doi: 10.1101/gad.205294.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ossewaarde ME, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16:556–562. doi: 10.1097/01.ede.0000165392.35273.d4. [DOI] [PubMed] [Google Scholar]
- 79.Shadyab AH, et al. Ages at menarche and menopause and reproductive lifespan as predictors of exceptional longevity in women: the Women's Health Initiative. Menopause. 2017;24:35–44. doi: 10.1097/GME.0000000000000710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yigit E, et al. Analysis of the C. elegans Argonaute family reveals that distinct Argonautes act sequentially during RNAi. Cell. 2006;127:747–757. doi: 10.1016/j.cell.2006.09.033. [DOI] [PubMed] [Google Scholar]
- 82.Espelt MV, Estevez AY, Yin X, Strange K. Oscillatory Ca2+ signaling in the isolated Caenorhabditis elegans intestine: role of the inositol-1,4,5-trisphosphate receptor and phospholipases C beta and gamma. J Gen Physiol. 2005;126:379–392. doi: 10.1085/jgp.200509355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Marre J, Traver EC, Jose AM. Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans. Proc Natl Acad Sci U S A. 2016;113:12496–12501. doi: 10.1073/pnas.1608959113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Calixto A, Chelur D, Topalidou I, Chen X, Chalfie M. Enhanced neuronal RNAi in C. elegans using SID-1. Nat Methods. 2010;7:554–559. doi: 10.1038/nmeth.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou L, et al. Construction of a germline-specific RNAi tool in C. elegans. Sci Rep. 2019;9:2354. doi: 10.1038/s41598-019-38950-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mello CC, Kramer JM, Stinchcomb D, Ambros V. Efficient gene transfer in C.elegans: extrachromosomal maintenance and integration of transforming sequences. EMBO J. 1991;10:3959–3970. doi: 10.1002/j.1460-2075.1991.tb04966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yang JS, et al. OASIS: online application for the survival analysis of lifespan assays performed in aging research. PLoS One. 2011;6:e23525. doi: 10.1371/journal.pone.0023525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Cox J, et al. Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics. 2014;13:2513–2526. doi: 10.1074/mcp.M113.031591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wagle P, Nikolic M, Frommolt P. QuickNGS elevates Next-Generation Sequencing data analysis to a new level of automation. BMC Genomics. 2015;16:487. doi: 10.1186/s12864-015-1695-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hoogewijs D, Houthoofd K, Matthijssens F, Vandesompele J, Vanfleteren JR. Selection and validation of a set of reliable reference genes for quantitative sod gene expression analysis in C. elegans. BMC Mol Biol. 2008;9:9. doi: 10.1186/1471-2199-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the main data supporting the findings of this study are available within the article and its supplementary files. Transcriptome data have been deposited in Gene Expression Omnibus (GEO) under the accession code GSE123054. All the other data are also available from the corresponding author upon request.