Abstract
The majority of people with prediabetes transition to type 2 diabetes. Weight gain is a known predictor of increasing the risk of diabetes, but another reason may be a focus on immediate rewards and discounting of the future. Delay discounting (DD: devaluation of future consequences) is related to obesity and poor glycemic control in persons with type 2 diabetes. This study was designed to assess whether changes in DD are associated with HbA1c change beyond BMI change in individuals with prediabetes. Hierarchical regression showed changes in BMI (p=0.008) and the $1000 DD task (p=0.04) were associated with HbA1c change beyond demographic characteristics, with the full model accounting for 25.8% of the variance. Those with greater BMI increases and greater increases in discounting of the future showed the greatest increases in HbA1c. DD represents a novel target to prevent progression from prediabetes to type 2 diabetes.
Keywords: Delay discounting, prediabetes, glycemic control
The development of type 2 diabetes proceeds from normoglycemia to prediabetes to type 2 diabetes (Nichols, Hillier, & Brown, 2007; Tabak, Herder, Rathmann, Brunner, & Kivimaki, 2012) and the prevalence of type 2 diabetes is increasing (Tabak et al., 2012). Weight gain is a significant risk factor for type 2 diabetes (Colditz, Willett, Rotnitzky, & Manson, 1995), and conversely, the transition from prediabetes to type 2 diabetes is preventable by weight loss and lifestyle change (Knowler et al., 2002). The rational approach for those at risk for diabetes is to implement lifestyle changes to avoid the transition to serious chronic disease. However, research suggests that individuals with type 2 diabetes discount the future (Lebeau et al., 2016; Reach et al., 2011), which could lead to a failure to engage in current behaviors to prevent this transition to type 2 diabetes.
Discounting the future is an important aspect of self-regulation, which has been shown to be related to a variety of biomedical and societal problems (Bradford, 2010; Reach, 2010; Story, Vlaev, Seymour, Darzi, & Dolan, 2014). Lifestyle changes to prevent type 2 diabetes involves changing diet, as well as becoming more active (Knowler et al., 2002), but this can be challenging for people since both food (Epstein, Leddy, Temple, & Faith, 2007) and a sedentary lifestyle (Epstein & Saelens, 2000) can be strong, and immediately available, reinforcers. The focus on immediate rewards may lead people to overvalue present rewards and discount future rewards.
One way to assess whether people discount the future is by studying delay discounting. Delay discounting (DD) measures the extent to which people prefer small immediate rewards over larger, delayed rewards (Bickel & Marsch, 2001). DD is relevant to understanding why people do not adopt behaviors beneficial to their long term health (Bradford, 2010). A wealth of data shows that DD is a predictor of poor follow through on many preventive health behaviors (Bradford, 2010). Excessive discounting of the future may make it challenging for obese persons to not consume highly reinforcing, highly valued, high energy dense foods and not engage in healthier behaviors that have a delayed benefit on health. Likewise, individuals with type 2 diabetes in poor metabolic control (Lebeau et al., 2016; Reach et al., 2011) excessively discount the future, as they may make decisions that focus on their immediate pleasure at the expense of better long-term health. Since commitment to preventive health behaviors requires a person to alter current behaviors with the goal of preventing a future outcome, discounting the future is a risk factor for not engaging in preventive health behaviors.
The conceptual basis for studying how changes in delay discounting may be related to changes in glycemic control is the experimental medicine approach (Bernard, 1927). This approach, which serves as the platform for the NIH Science of Behavior Change Initiative (Riddle & Science of Behavior Change Working Group, 2015), seeks to identify a behavioral target that is related to an important health outcome, including both cross-sectional and prospective observational studies, identify methods to manipulate the target, and finally demonstrate that manipulation of the target leads to a health outcome improvement. While research shows that DD is cross-sectionally related to HbA1c control (Lebeau et al., 2016; Reach et al., 2011), no studies have demonstrated that changes in DD would be related to changes in HbA1c. This finding is an important piece of the experimental medicine approach, which provides support for the next intervention step.
We hypothesize that excessive discounting of the future for those with prediabetes is a risk factor for worsening glycemic control, and that excessive discounting of the future incrementally is associated with increases in HbA1c values beyond contributions of weight gain (Colditz et al., 1995), controlling for years of education, which also is related to changes in glycemic control over time (Agardh, Allebeck, Hallqvist, Moradi, & Sidorchuk, 2011).
Methods
Participants and Procedures.
Participants with prediabetes, no prior history of diabetes, and a diagnosis of hypertension and/or dyslipidemia who were being treated with medications were recruited from the Buffalo, New York and Roanoke, Virginia communities via physician networks using flyers, media ads, referrals, and direct mailings. Participants were at least 18 years of age, were not pregnant, and did not have any health factors that influenced their blood glucose.
During recruitment, participants were told that the study required four sessions with each lasting about 90–120 minutes, and that they may also be asked to participate in a fMRI during one of their sessions. The study procedures included height and weight measurements, blood sampling for HbA1c and cholesterol values, blood pressure measurement, diary completion, and computer tasks. The focus of that study was the relationship between delay discounting and medication adherence in people with prediabetes. Participants who completed all four sessions earned $225. Baseline data collection occurred from 3/28/2016 to 9/16/2016. A one-year follow-up appointment was added that included measurement of height and weight, delay discounting, and HbA1c. The follow-up measures were collected 12 ± 2 months from baseline, from 1/31/2017 to 11/15/2017. Participants were paid $55 for follow-up data collection. All procedures were conducted in accordance with guidelines for the ethical conduct of human research outlined by the National Institute of Health and with approval of the University at Buffalo Institutional Review Board and by the Virginia Tech Institutional Review Board.
The original sample included 81 participants, 73 of whom were studied at 12-month follow-up (90%). Over the follow-up, 7 of persons transitioned to type 2 diabetes (9.6%), based on whether their HbA1c values were greater than or equal to 6.5% (48 mmol/mol) or they had been diagnosed with type 2 diabetes by their doctor and placed on diabetes medications (N = 4). People on diabetes medications were not included as this could influence the relationship between BMI or DD change and HbA1c change. Of the 69 persons who were followed and not on medication, 4 did not have complete data, resulting in a sample of 65 individuals.
Measures
Race/ethnicity, educational level and demographics were assessed. Education is a predictor of DD, as those at lower education are more likely to discount the future (Jaroni, Wright, Lerman, & Epstein, 2004). Weight was measured using a Tanita (Hong Kong, China) digital scale. Height was measured using a SECA (Chino, California) stadiometer. HbA1c was measured using the validated (Bode, Irvin, Pierce, Allen, & Clark, 2007) A1CNow+® system (PTS Diagnostics, Sunnyvale, CA).
Delay Discounting.
DD was assessed using a computerized adjusting amount task (Johnson & Bickel, 2002). The task was used to calculate DD for two delayed monetary rewards ($100 and $1000). In the task participants were asked if they wanted either a fraction of the total delayed amount of money now, or the full amount ($100 or $1000, depending on the monetary amount used for the task) at different delays. The delays included seven different time periods (1 day, 1 week, 1 month, 3 months, 1 year, 5 years, and 25 years). The order of the time periods was randomized across subjects. Depending on the participant’s response, the immediate available amount was adjusted up (if delayed choice selected) or down (if immediate choice selected). Each time delay included six trials with the amount adjusting half that of the previous adjustment (Du, Green, & Myerson, 2002). For example, a participant might be asked whether they preferred to receive $50 now or $100 in one month. If they chose $50, then the amounts would be changed to $25 now versus $100 in one month. Indifference points, or discounted value (V), were calculated for each delay based on the seven delay intervals. Indifference points represent values for which the subject is indifferent to, or does not prefer the immediate or delayed rewards. The value of V reflects the amount of discounting of the large delayed reward amount. For example, for a $100 delayed reward, V = $95 would reflect only 5% discounting of the reward, as opposed to a steeper discounting V = $50, reflects 50% discounting.
Instructions to the participants for the task were as follows:
“In the following tasks, you will be asked to choose between receiving different amounts of money at different points in time. For these tasks, you’ll be using these pedals to submit your answer choices. To choose the option on the left side of the screen, press the left pedal, and to choose the option on the right side of the screen, press the right pedal. These are hypothetical choices, but please choose the answers as if they were real. There are no right or wrong answers in this task. Please take your time and answer thoughtfully. We will be monitoring your answers to make sure you are paying attention and you may be discontinued if you are not. While you are completing this task, I’ll be in the next room. The rooms are connected by a microphone. If you have any questions, please ask out loud and I will be able to hear and answer you. Do you have any questions?”
Delay discounting data were subjected to a preliminary analysis to detect patterns of responding that were not systematically affected by delay. Such data may be the product of inattention to the task or failure to understand task instructions; in this case, nonsystematic data would not reflect the construct of interest (delay discounting) and, therefore, may need to be excluded. We adapted standardized diagnostic criteria (Johnson & Bickel, 2008) commonly used in studies of delay discounting (Hendrickson & Rasmussen, 2013; Lansing, Stanger, Crochiere, Carracher, & Budney, 2017; Peters & Buchel, 2010), in which systematic data are assumed to meet two, minimally restrictive criteria (Johnson, Johnson, Herrmann, & Sweeney, 2015): 1) evidence of consistent effects of contiguous delays on discounted value, in which no indifference point at any delay exceed 20% of the indifference point at the previous delay; and 2) evidence of an overall reduction in discounted value as a function of delay, in which the indifference point at the last delay (in this case, 25 years) must be at least 10% lower than the indifference point at the first delay (in this case, 1 day). This algorithm identified no participants out of the 73 who completed follow-up who violated these rules.
Area under the curve (AUC) was used to calculate discount rates (Myerson, Green, & Warusawitharana, 2001). AUC is the total area under the curve that connects the indifference points where the x-axis represents the delays and the y-axis represents the indifference points. AUC provides an easy to understand metric, with high values representing low discounting of the future and low values representing high discounting of the future. The range of AUC is from 0 (very impulsive) to 1 (not impulsive).
Analytic plan
The analytic plan assessed if demographic variables, baseline and change in BMI and DD predicted HbA1c at baseline or 12-month HbA1c change. All change scores were calculated by post-pre, so that a positive number means an increase, and a negative number a decrease. First zero-order predictors of HbA1c change were assessed. Significant predictors were included in the hierarchical regression model, in addition to demographic variables of age, income and sex. The hierarchical regression model included three steps. Step 1 included years of education, age, income and sex, followed by BMI change (Step 2) and DD change (Step 3).
Based on significant relationships between DD change and HbA1c change, and BMI and HbA1c change, a mediational model was used to test whether the relationship between DD and HbA1c change was mediated by the change in BMI. The mediational model used 10,000 bootstrapping simulations to determine the indirect effects of DD change on HbA1c change mediated by changes in BMI, controlling for level of education (Hayes, 2013; Preacher & Hayes, 2004).
Results
Characteristics of the sample and variables tested for their association with baseline HbA1c and HbA1c change are shown in Tables 1 and 2, respectively. The sample is predominantly female, obese, with a high school education or some college. The only variable that was related to baseline HbA1c was sex, as women had higher HbA1c values than men (r = 0.24, p = 0.042). Baseline $1000 DD values were marginally related (r = −0.22, p = 0.058), as those who discounted more had high HbA1 values. Changes in HbA1c were related to change in $1000 DD (r = −0.33, p = 0.008), years of education (r = −0.25, p = 0.043), and BMI change (r = 0.32, p = 0.009). Those who showed increases in delay discounting were less educated and had higher BMI changes and greater HbA1c changes.
Table 1.
Relationship between baseline characteristics and baseline HbA1c (N = 73)
| Characteristic | r | p | |
|---|---|---|---|
| Sex (male/female) | 26/47 | 0.24 | 0.042 |
| Minority (minority/non-minority) | 19/54 | 0.23 | 0.055 |
| Black/African American | 17 | ||
| Native American | 1 | ||
| White | 54 | ||
| Multirace | 1 | ||
| Site (UB/ VT) | 49/24 | −0.05 | 0.699 |
| Age | 55.21 ± 11.58 | 0.21 | 0.070 |
| Height (cm) | 167.44 ± 8.65 | −0.21 | 0.070 |
| Weight (kg) | 90.28 ± 27.07 | 0.05 | 0.675 |
| Body Mass Index (BMI) | 32.04 ± 8.76 | 0.15 | 0.222 |
| HbAlc | 5.98 + 0.20 | ------- | ------- |
| Annual Household Income ($) | 63,151 ± 48,157 | −0.13 | 0.276 |
| < $30,000 | 24 | ||
| $30,000 – $70,000 | 16 | ||
| $70,000–$110,000 | 25 | ||
| $110,000–$140,000 | 3 | ||
| > $140,000 | 5 | ||
| Years of Education | 14.62 ± 2.12 | −0.07 | 0.573 |
| High School Graduate or less | 16 | ||
| Some college/vocational school | 28 | ||
| College graduate | 15 | ||
| Graduate degree | 11 | ||
| Refused to answer | 3 | ||
| Adjusting Amount $100 AUC | 0.25 ± 0.21 | −0.15 | 0.207 |
| Adjusting Amount $1000 AUC | 0.28 ± 0.24 | −0.22 | 0.058 |
Note – significant (p < 0.05) correlations are in bold.
Table 2.
Relationship between baseline and change characteristics and HbA1c changes (N = 65)
| Characteristic | r | p | |
|---|---|---|---|
| Sex (male/female) | 25/40 | −0.11 | 0.389 |
| Minority (minority/non-minority) | 16/49 | 0.11 | 0.401 |
| Black/African American | 15 | ||
| Native American | 0 | ||
| White | 49 | ||
| Multirace | 1 | ||
| Site (UB/ VT) | 43/22 | 0.10 | 0.424 |
| Age | 54.92 ± 11.81 | −.12 | 0.351 |
| Height (cm) | 167.58 ± 8.81 | 0.07 | 0.566 |
| Weight (kg) | 89.11 ± 25.51 | 0.05 | 0.675 |
| Body Mass Index (BMI) | 31.52 ± 7.79 | 0.02 | 0.874 |
| Body Mass Index change | 0.01 ± 2.59 | 0.32 | 0.009 |
| HbA1c | −0.27 ± 0.35 | −0.14 | 0.283 |
| Annual Household Income ($) | 65,000 ± 49,347 | −0.14 | 0.28325 |
| < $30,000 | 20 | ||
| $30,000 – $70,000 | 15 | ||
| $70,000–$110,000 | 22 | ||
| $110,000–$140,000 | 3 | ||
| > $140,000 | 5 | ||
| Years of Education | 14.62 ± 2.12 | −0.25 | 0.043 |
| High School Graduate or less | 16 | ||
| Some college/vocational school | 26 | ||
| College graduate | 153 | ||
| Graduate degree | 10 | ||
| Adjusting amount $100 AUC | 0.25 ± 0.21 | −0.03 | 0.831 |
| Adjusting amount $100 AUC change | −0.03 ± 0.16 | −0.06 | 0.651 |
| Adjusting amount $1000 AUC | 0.28 ± 0.24 | −0.19 | 0.127 |
| Adjusting amount $1000 AUC change | −0.001 ± 0.22 | −0.33 | 0.008 |
Note – significant (p < 0.05) correlations are in bold.
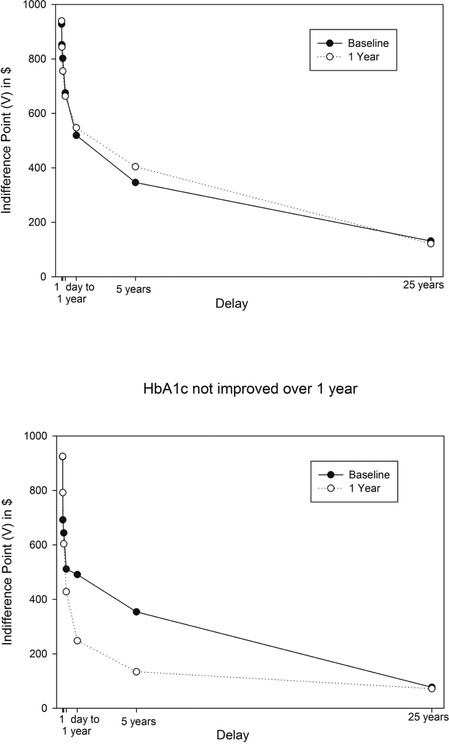
The relationship between DD change and HbA1c change is shown in Figure 1, which compares plots of indifference points and thus area under the curve at baseline and 12 months for people who improved HbA1c over 12 months (top graph) versus those who did not improve in their HbA1c values (bottom graph). As shown by the 12-month curves being higher than baseline, those who improved HbA1c showed less discounting (increased area under the curve). Conversely, there was a large increase in discounting of the future (decreased area under the curve) for those people who showed an increase in their HbA1c values.
Figure 1.
Plots of indifference points at baseline and after one year for people who showed a reduction in HbA1c values versus did not improve their HbA1c values.
The hierarchical regression showed that years of education, age, income and sex in the first step accounted for 9.7% of the variance, which increased to 20.1% when BMI change was added to the model (Finc(1,58) = 7.55, p = 0.008). Introducing DD change increased the variance accounted for to 25.8% (Finc(1,57) = 4.38, p = 0.041). Thus, DD predicted worsening of glycemic control as measured by HbA1c beyond education and other demographics or increases in BMI.
The mediational model (Table 3) showed that the indirect effect of DD on BMI change accounted for only 7 percent of the total effect of DD attributable to HbA1c change, a non-significant effect (95% CI −0.135 to +0.367). The direct effect of DD on HbA1c change accounted for 93% of the total effect (95% CI −.8124 to −.0507) confirming the independence of DD and BMI changes as predictors of HbA1c change.
Table 3.
Hierarchical model predicting HbA1c change at 1 year
| B | SE | P | r2 | Finc | df | p | % Model Δ | ES | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Step 1 | Variable | 0.097 | ||||||||
| Education | −0.04 | 0.02 | 0.09 | |||||||
| Age | 0.00 | 0.00 | 0.68 | |||||||
| Income | 0.00 | 0.00 | 0.86 | |||||||
| Sex | −0.05 | 0.09 | 0.56 | |||||||
| BL HbA1c | −0.24 | 0.24 | 0.32 | |||||||
| Step 2 | 0.201 | 7.55 | 1,58 | 0.008 | 10.40 | 0.13 | ||||
| BMI change | 0.05 | 0.02 | 0.01 | |||||||
| Step 3 | 0.258 | 4.38 | 1,57 | 0.041 | 5.70 | 0.075 | ||||
| $1000 AUC Δ | −0.42 | 0.20 | 0.04 |
Note – Finc = incremental F, Model Δ = model improvement, ES = effect size (f2)
Discussion
The results show that 12-month changes in DD are associated with HbA1c change, with the contribution beyond the influence of both weight change and education on HbA1c change, two other variables that were associated with HbA1c change in this sample. The observation of a relationship between education and weight gain replicates previous research. Previous research has shown that people with lower education discount the future more than those with higher education (Jaroni et al., 2004) but to our knowledge this is the first study to show that lower education is also related to increases in DD over time. Previous research has shown years of education predicts the transition from prediabetes to type 2 diabetes (Agardh et al., 2011). Weight gain and obesity are known to be risk factors for type 2 diabetes (Colditz et al., 1995). Importantly changes in DD are associated with HbA1c changes even after the contributions of weight gain to HbA1c change are accounted for in this sample of individuals with prediabetes.
These results provide the first evidence that discounting of the future is uniquely associated with changes in glycemic control in individuals with prediabetes. Many people with prediabetes will transition to type 2 diabetes (Tabak et al., 2012). Our findings suggest that people who discount the future to gain immediate gratification may not accomplish the long term goal of preventing diabetes. Our results support that we can predict that people who know they have prediabetes, and do not change their behavior to reduce the risk of transitioning to type 2 diabetes, discount the future more than those who successfully change their behaviors. Discounting of the larger amount of money ($1000) but not the smaller amount ($100) was the stronger predictor of HbA1c values. A reliable difference was observed in discounting of smaller versus larger amount of money, as people discount larger amounts of money less than smaller amounts of money (Green & Myerson, 2004). Perhaps, if a reward is too small, most people discount it, limiting the ability to predict other variables. In previous research we have found significant differences in DD between substance users and controls were evident at high and intermediate reward magnitudes, but not low magnitudes (Mellis, Woodford, Stein, & Bickel, 2017). The strategy of including multiple magnitudes of delayed rewards provides the best opportunity to predict important behavioral outcomes.
These results suggest that DD represents a target for behavioral interventions that could improve adherence to behaviors that reduce the likelihood of worsening dysglycemia and progression to type 2 diabetes. The experimental medicine approach suggests that the next step after identifying a cross-sectional relationship between DD and HbA1c (Lebeau et al., 2016; Reach et al., 2011) is to show that changes in DD are related to changes in HbA1c. In the present study, baseline DD was marginally related to baseline HbA1c (p = 0.058), however, the correlation was similar in this study (r = 0.22) to a larger study (n = 93) that did show this relationship showed a similar relationship (r = 0.24). The next step is to demonstrate that DD can be manipulated, and a consistent body of research has shown that episodic future thinking, an intervention that promotes choice of larger delayed rewards over smaller, immediate rewards by having people vividly imagine positive future events, can reduce discounting of the future (Schacter, Benoit, & Szpunar, 2017) (Daniel, Sawyer, Dong, Bickel, & Epstein, 2016; Daniel, Stanton, & Epstein, 2013a, 2013b; Stein et al., 2016; Sze, Daniel, Kilanowski, Collins, & Epstein, 2015), energy intake in obese persons (Daniel et al., 2013b) improve food purchasing behavior (Hollis-Hansen, O’Donnell, & Epstein, In press; O’Neill, Daniel, & Epstein, 2016) and improve success with weight loss (Sze et al., 2015). Given that DD was an independent predictor of HbA1c change beyond weight change, and the influence of DD on HbA1c was not explained by changes in BMI, our data suggest that improvements in DD may influence multiple health behaviors beyond weight change. Losing weight may not be necessary to improve glycemic control, as blood glucose control can be improved by physical activity with minimal changes in body weight (Hainer, Toplak, & Stich, 2009), and by improving the quality of foods consumed by reducing sugar intake and foods with a high glycemic index (Ludwig, 2002).
One interpretation of this data is that increases in discounting of the future contributes to increases in HbA1c by not engaging in preventive health behaviors. Previous research has shown that changes in cognitive and executive functions occur with prediabetes (Dybjer, Nilsson, Engstrom, Helmer, & Nagga, 2018; Vanhanen et al., 1997) which may result in increased DD, as valuation of future rewards requires executive function resources (Diamond, 2013). The duration and degree of blood glucose control is related to cognitive impairment (Dybjer et al., 2018; Geijselaers et al., 2017), but longstanding prediabetes may have altered executive function, influenced decision making and increased discounting of the future if DD is impacted by the length of time that person has been exposed to higher glycemic levels.
Limitations of the present study that may impact interpretation of the results should be acknowledged. The sample size is relatively small, and primarily female, and the majority had high school, vocational training or some college. Including more males, and people with more education might have changed the observed result. The impact of the sample size on interpretation of the results can be seen by a marginally significant relationship between baseline DD and HbA1c in this study, while a study with a slightly larger sample and a similar effect size showed a significant relationship (Lebeau et al., 2016). In addition, the study used a monetary discounting task that asked people to decide whether the preferred a small immediate amount of money versus a larger delayed amount. While this task provides an individual difference variable that indexes discounting of the future, it is not analogous to the decision that a person with prediabetes might make when deciding not eat a palatable, high energy dense, high glycemic food for immediate gratification or maintain a lower blood glucose value and prevent later transition to type 2 diabetes. Possibly, discounting tasks analogous to the decisions that people with prediabetes have to make might be more sensitive and show bigger relationships with glycemic control.
In summary, this study provides new evidence for the relationship between changes in DD and changes in HbA1c. High DD is associated with obesity (Amlung, Petker, Jackson, Balodis, & MacKillop, 2016), a significant risk factor for type 2 diabetes (Colditz et al., 1995). Results in this study suggest that the trajectory of DD predicts worsening metabolic control. Preventing people from further discounting the future may lead to more stable metabolic control over time that may not be mediated by changes in BMI. DD may provide a unique window into temporal decisions that people make as they decide about satisfying an immediate craving to eat a highly palatable, high energy dense, high glycemic food, or bypass the food for a lower glycemic index alternative that will not cause spikes in their blood glucose to attain a delayed future goal of not transitioning to type 2 diabetes. People who discount the future make impulsive choices to maximize their current pleasure (such as eating high energy/low nutrient dense, high glycemic index foods or not being physically active) rather than choosing to forego these experiences for the future greater good of not becoming diabetic. Reducing discounting of the future may have important benefits for glycemic control, helping those with those with prediabetes also make other behaviors that can improve their health. Understanding and treating processes related to discounting the future may provide a new platform for the prevention of type 2 diabetes.
Acknowledgments
This research was funded in part by the National Institutes of Health (NIH) Science of Behavior Change Common Fund Program through an award administered by the National Institute of Diabetes and Digestive and Kidney Diseases (1UH2DK109543), awarded to Drs. Epstein and Bickel.
Footnotes
Conflict of Interest: Dr. Epstein was a consultant and had equity in Daltri when the study was implemented. Dr. Bickel is a consultant or has equity in HealthSim LLC, NotifiUs LLC, Sober Grid Inc., DxRx, Prophase LLC, Teva Branded Pharmaceuticals, General Genetic Corporation. The other authors do not declare any conflict of interest with respect to the authorship or publication of this article.
Ethical approval: All procedures performed were in accordance with the ethical standards of the institution and with the 1964 Helsinki declaration and its later amendment of comparable ethical standards.
Informed consent: Informed consent was obtained from all participants included in the study.
References
- Agardh E, Allebeck P, Hallqvist J, Moradi T, & Sidorchuk A (2011). Type 2 diabetes incidence and socio-economic position: a systematic review and meta-analysis. International Journal of Epidemiology, 40(3), 804–818. doi: 10.1093/ije/dyr029 [DOI] [PubMed] [Google Scholar]
- Amlung M, Petker T, Jackson J, Balodis I, & MacKillop J (2016). Steep discounting of delayed monetary and food rewards in obesity: a meta-analysis. Psychological Medicine, 46(11), 2423–2434. doi: 10.1017/S0033291716000866 [DOI] [PubMed] [Google Scholar]
- Bernard C (1927). An introduction to the study of experimental medicine. North Chelmsford, MA: Courier Corporation. [Google Scholar]
- Bickel WK, & Marsch LA (2001). Toward a behavioral economic understanding of drug dependence: delay discounting processes. Addiction, 96, 73–86. [DOI] [PubMed] [Google Scholar]
- Bode BW, Irvin BR, Pierce JA, Allen M, & Clark AL (2007). Advances in hemoglobin A1C point of care technology. Journal of Diabetes Science and Technology, 1, 405–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford WD (2010). The association between individual time preferences and health maintenance habits. Medical Decision Making, 30(1), 99–112. doi:0272989X09342276[pii]10.1177/0272989X09342276 [pii] [DOI] [PubMed] [Google Scholar]
- Colditz GA, Willett WC, Rotnitzky A, & Manson JE (1995). Weight-gain as a risk factor for clinical diabetes-mellitus in women. Annals of Internal Medicine, 122(7), 481–486. doi: 10.7326/0003-4819-122-7-199504010-00001 [DOI] [PubMed] [Google Scholar]
- Daniel TO, Sawyer A, Dong YL, Bickel WK, & Epstein LH (2016). Remembering versus imagining: When does episodic retrospection and episodic prospection aid decision making? Journal of Applied Research in Memory and Cognition, 5(3), 352–358. [Google Scholar]
- Daniel TO, Stanton CM, & Epstein LH (2013a). The future is now: comparing the effect of episodic future thinking on impulsivity in lean and obese individuals. Appetite, 71, 120–125. doi: 10.1016/j.appet.2013.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel TO, Stanton CM, & Epstein LH (2013b). The future is now: Reducing impulsivity and energy intake using episodic future thinking. Psychological Science, 24(11), 2339–2342. doi: 10.1177/0956797613488780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A (2013). Executive functions. Annual Review of Psychology, 64, 135–168. doi: 10.1146/annurev-psych-113011-143750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du WJ, Green L, & Myerson J (2002). Cross-cultural comparisons of discounting delayed and probabilistic rewards. Psychological Record, 52(4), 479–492. [Google Scholar]
- Dybjer E, Nilsson PM, Engstrom G, Helmer C, & Nagga K (2018). Pre-diabetes and diabetes are independently associated with adverse cognitive test results: a cross-sectional, population-based study. Bmc Endocrine Disorders, 18 doi:ARTN9110.1186/s12902-018-0318-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Leddy JJ, Temple JL, & Faith MS (2007). Food reinforcement and eating: A multilevel analysis. Psychological Bulletin, 133(5), 884–906. doi: 10.1037/0033-2909.133.5.884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, & Saelens BE (2000). Behavioral economics of obesity: Food intake and energy expenditure In Bickel WK & Vuchinich RE (Eds.), Reframing health behavior change with behavioral economics (pp. 293–311). Mahwah, N.J: Lawrence Erlbaum. [Google Scholar]
- Geijselaers SLC, Sep SJS, Claessens D, Schram MT, van Boxtel MPJ, Henry RMA, … Stehouwer CDA (2017). The role of hyperglycemia, insulin resistance, and blood pressure in diabetes-associated differences in dognitive performance-The Maastricht Study. Diabetes Care, 40(11), 1537–1547. doi: 10.2337/dc17-0330 [DOI] [PubMed] [Google Scholar]
- Green L, & Myerson J (2004). A discounting framework for choice with delayed and probabilistic rewards. Psychological Bulletin, 130(5), 769–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hainer V, Toplak H, & Stich V (2009). Fat or Fit: What Is More Important? Diabetes Care, 32, S392–S397. doi: 10.2337/dc09-S346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF (2013). An introduction to mediation, moderation, and conditional process modeling: A regression-based approach. New York, New York: Guilford Press. [Google Scholar]
- Hendrickson KL, & Rasmussen EB (2013). Effects of mindful eating training on delay and probability discounting for food and money in obese and healthy-weight individuals. Behaviour Research and Therapy, 51(7), 399–409. doi: 10.1016/j.brat.2013.04.002 [DOI] [PubMed] [Google Scholar]
- Hollis-Hansen K, O’Donnell S, & Epstein LH (In press). Episodic future thinking and grocery shopping online. Appetite. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaroni JL, Wright SM, Lerman C, & Epstein LH (2004). Relationship between education and delay discounting in smokers. Addictive Behaviors, 29(6), 1171–1175. [DOI] [PubMed] [Google Scholar]
- Johnson MW, & Bickel WK (2002). Within-subject comparison of real and hypothetical money rewards in delay discounting. Journal of the Experimental Analysis of Behavior, 77(2), 129–146. doi: 10.1901/jeab.2002.77-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, & Bickel WK (2008). An algorithm for identifying nonsystematic delay-discounting data. Experimental and Clinical Psychopharmacology, 16(3), 264–274. doi:Doi 10.1037/1064-1297.16.3.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson MW, Johnson PS, Herrmann ES, & Sweeney MM (2015). Delay and probability discounting of sexual and monetary outcomes in individuals with cocaine use disorders and matched controls. PLoS One, 10(5). doi:ARTNe012864110.1371/journal.pone.0128641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, & Nathan DM (2002). Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. New England Journal of Medicine, 346, 393–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansing AH, Stanger C, Crochiere R, Carracher A, & Budney A (2017). Delay discounting and parental monitoring in adolescents with poorly controlled type 1 diabetes. Journal of Behavioral Medicine, 40(6), 864–874. doi: 10.1007/s10865-017-9856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeau G, Consoli SM, Le Bouc R, Sola-Gazagnes A, Hartemann A, Simon D, … Lemogne C (2016). Delay discounting of gains and losses, glycemic control and therapeutic adherence in type 2 diabetes. Behavioural Processes, 132, 42–48. doi: 10.1016/j.beproc.2016.09.006 [DOI] [PubMed] [Google Scholar]
- Ludwig DS (2002). The glycemic index - Physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. Journal of the American Medical Association, 287(18), 2414–2423. doi:DOI 10.1001/jama.287.18.2414 [DOI] [PubMed] [Google Scholar]
- Mellis AM, Woodford AE, Stein JS, & Bickel WK (2017). A second type of magnitude effect: Reinforcer magnitude differentiates delay discounting between substance users and controls. Journal of the Experimental Analysis of Behavior, 107(1), 151–160. doi: 10.1002/jeab.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerson J, Green L, & Warusawitharana M (2001). Area under the curve as a measure of discounting. Journal of the Experimental Analysis of Behavior, 76(2), 235–243. doi:DOI 10.1901/jeab.2001.76-235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols GA, Hillier TA, & Brown JB (2007). Progression from newly acquired impaired fasting glusose to type 2 diabetes. Diabetes Care, 30(2), 228–233. doi: 10.2337/dc06-1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill J, Daniel TO, & Epstein LH (2016). Episodic future thinking reduces eating in a food court. Eating Behaviors, 20, 9–13. doi: 10.1016/j.eatbeh.2015.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters J, & Buchel C (2010). Episodic future thinking reduces reward delay discounting through an enhancement of prefrontal-mediotemporal interactions. Neuron, 66(1), 138–148. doi:DOI 10.1016/j.neuron.2010.03.026 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, & Hayes AF (2004). SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behavior Research Methods, Instruments, & Computers, 36(4), 717–731. [DOI] [PubMed] [Google Scholar]
- Reach G (2010). Is there an impatience genotype leading to non-adherence to long-term therapies? Diabetologia, 53(8), 1562–1567. doi:DOI 10.1007/s00125-010-1755-3 [DOI] [PubMed] [Google Scholar]
- Reach G, Michault A, Bihan H, Paulino C, Cohen R, & Le Clesiau H (2011). Patients’ impatience is an independent determinant of poor diabetes control. Diabetes & Metabolism, 37(6), 497–504. doi:DOI 10.1016/j.diabet.2011.03.004 [DOI] [PubMed] [Google Scholar]
- Riddle M, & Science of Behavior Change Working Group. (2015). News from the NIH: using an experimental medicine approach to facilitate translational research. Translational Behavioral Medicine, 5(4), 486–488. doi: 10.1007/s13142-015-0333-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Benoit RG, & Szpunar KK (2017). Episodic future thinking: mechanisms and functions. Current Opinion in Behavioral Sciences, 17, 41–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JS, Wilson AG, Koffarnus MN, Daniel TO, Epstein LH, & Bickel WK (2016). Unstuck in time: episodic future thinking reduces delay discounting and cigarette smoking. Psychopharmacology, 233(21–22), 3771–3778. doi: 10.1007/s00213-016-4410-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Story GW, Vlaev I, Seymour B, Darzi A, & Dolan RJ (2014). Does temporal discounting explain unhealthy behavior? A systematic review and reinforcement learning perspective. Frontiers in Behavioral Neuroscience, 8 doi:Artn 76 Doi 10.3389/Fnbeh.2014.00076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sze YY, Daniel TO, Kilanowski CK, Collins RL, & Epstein LH (2015). Web-based and mobile delivery of an Episodic Future Thinking intervention for overweight and obese families: a feasibility study. JMIR mHealth uHealth, 16, e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabak AG, Herder C, Rathmann W, Brunner EJ, & Kivimaki M (2012). Prediabetes: a high-risk state for diabetes development. Lancet, 379(9833), 2279–2290. doi: 10.1016/S0140-6736(12)60283-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhanen M, Koivisto K, Karjalainen L, Helkala EL, Laakso M, Soininen H, & Riekkinen P (1997). Risk for non-insulin-dependent diabetes in the normoglycaemic elderly is associated with impaired cognitive function. Neuroreport, 8(6), 1527–1530. doi:Doi 10.1097/00001756-199704140-00041 [DOI] [PubMed] [Google Scholar]