Abstract
Many patients who meet core/root criteria for Primary Progressive Aphasia (PPA) are not classifiable as a recognized variant and are often excluded from neuroimaging studies. Here, we detail neurological, neuropsychological, speech and language assessments, and anatomic and molecular neuroimaging (MRI, PiB-PET, and FDG-PET) for fifteen (8 female) unclassifiable PPA patients. Median age of onset was 64 years old with median 3 years disease duration at exam. Three patients were amyloid positive on PiB-PET. 14/15 patients had abnormal FDG-PETs with left predominant hypometabolism, affecting frontal, temporal, parietal, and even occipital lobes. Patients had mild to severe clinical presentations. Visualization of the FDG-PETs principal component analysis revealed patterns of hypometabolism similar to those seen in the PPA variants and suggests the brain regions affected in unclassifiable PPA patients are no different from those who are more easily classifiable. These findings may inform future modifications to the diagnostic criteria to improve diagnostic classification.
Keywords: primary progressive aphasia, frontotemporal dementia, amyloid imaging, PET imaging, hypometabolism
Introduction
Primary progressive aphasia (PPA) captures a group of neurodegenerative syndromes in which progressive language impairment is the initial and predominant deficit (Mesulam, 1982, 2001). The current diagnostic consensus criteria (Gorno-Tempini et al., 2011) reflect the broad diagnosis of PPA and three clinical variants, including 1) a nonfluent/agrammatic variant (agPPA), characterized by grammatical errors in speech and writing, often accompanied by apraxia of speech, 2) a semantic variant (svPPA) characterized by loss of knowledge about the meaning of words, and 3) a logopenic variant (lvPPA) characterized by word retrieval problems, poor sentence repetition, and phonological errors. Research has demonstrated that 10–41% of patients who meet the root/ core criteria for PPA are not classifiable as one of the aforementioned variants (Gil-Navarro et al., 2013; Harris et al., 2013; Matias-Guiu et al., 2014; Mesulam, Wieneke, Thompson, Rogalski, & Weintraub, 2012; Sajjadi, Patterson, Arnold, Watson, & Nestor, 2012; Wicklund et al., 2014). This cohort of individuals is collectively referred to as PPA-Unclassifiable (PPA-U). The source of classification difficulty is not well established, although it is possibly influenced by the severity of impairments or the simultaneous presence of features of more than one of the aforementioned variants, such as in (Mesulam et al., 2008; Spinelli et al., 2017).
Patients who do not reliably meet criteria for a variant of PPA are often excluded from neuroimaging and clinical studies of PPA. Here, we detail the neurologic, neuropsychological, speech, and language profiles, along with neuroimaging characteristics of a cohort of 15 PPA-U patients. We also looked for patterns in [18F] fluorodeoxyglucose (FDG) PET scans, utilizing principal components analysis (PCA), to better describe the underlying heterogeneity of PPA-U and determine if the source of classification difficulty may inform future modifications to diagnostic criteria.
Materials and methods
Between September 2011 and January 2018, 118 patients with PPA were recruited into NIH funded studies, 15 of whom (12.7%) met root criteria for PPA but did not meet criteria for any one of the currently recognized three PPA variants (Gorno-Tempini et al., 2011) and hence were classified as PPA-U (detailed in Supplementary Tables). For tests utilized to assess each diagnostic feature, see (Wicklund et al., 2014). Two of the 15 PPA-U patients were previously published as case reports (Flanagan et al., 2016; Utianski et al., Under Review), indicated in each table, and others were included in previous group analyses (Botha et al., 2015; Wicklund et al., 2014). Given the novelty of the current analyses, the comprehensive nature of the clinical descriptions, and the inclusion of FDG-PET, all 15 patients were included here. All patients underwent a standard protocol of neurological, neuropsychological, speech, and language assessments and neuroimaging, described below in further detail. If necessary, features of PPA were assessed qualitatively by history and clinical neurology evaluations. Two expert speech-language pathologists reviewed all clinical data and came to a consensus agreement regarding diagnostic classification. The study was approved by the Institutional Review Board at Mayo Clinic. All participants provided written consent for enrollment into the study.
Clinical Data
The neurological evaluations included: the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005) to index general cognition; the short version of the Neuropsychiatric Inventory Questionnaire (NPI-Q) (Kaufer et al., 2000) and the Frontal Behavioral Inventory (FBI) (Kertesz, Davidson, & Fox, 1997) to assess behavioral and psychiatric features; the Frontal Assessment Battery (FAB) (Dubois, Slachevsky, Litvan, & Pillon, 2000) to evaluate executive dysfunction; and the Movement Disorder Society Sponsored revision of the Unified Parkinson’s disease Rating Scale, motor section (MDS-UPDRS III) (Goetz et al., 2008) to assess the presence, nature, and severity of parkinsonism.
The neuropsychological test battery included: the Auditory Verbal Learning Test (Rey, 1964) to assess verbal episodic memory; Trail Making Test (TMT) A and B (Spreen & Strauss, 1998) to evaluate processing speed and mental flexibility, respectively; and the Rey-Osterrieth Complex Figure test (Rey-O) to evaluate visual-constructional ability (Osterrieth, 1944; Rey, 1964).
The speech and language assessments were performed by a certified speech-language pathologist and included: the Western Aphasia Battery revised (WAB) (Kertesz, 2006) to index global language ability; supplementary tasks from Part 2 of the WAB to assess reading and writing; the Token Test, Part V (De Renzi & Vignolo, 1962) to assess comprehension of complex grammar; the Pyramids and Palm Trees Test (PPTT) to index semantic access (Howard & Patterson, 1992); and the 15-item Boston Naming Test (Lansing, Ivnik, Cullum, & Randolph, 1999) to evaluate confrontation naming ability. Grammatic integrity was further assessed in spoken and written picture descriptions (e.g. omission of articles/function words or syntax errors). A comprehensive evaluation of oral structure and function was completed, as was auditory-perceptual assessment of speech production to characterize the presence and nature of possible motor speech disorders.
Neuroimaging Data
MRI Acquisition
For all patients, a 3T magnetization prepared rapid gradient echo (MPRAGE) T1-weighted MRI sequence was acquired. Each patient’s MRI scan was segmented and corrected for intensity inhomogeneity with the unified segmentation algorithm (Ashburner & Friston, 2005) implemented in SPM12 using tissue priors and settings from the Mayo Clinic Adult Lifespan Template (MCALT) (Schwarz et al., 2017), a publicly-available population-matched brain template (https://www.nitrc.org/projects/mcalt/). The Advanced Normalization Tools (ANTs) symmetric normalization (SyN) algorithm was used with the MCALT template image to nonlinearly transform the MCALT atlas to the voxel space of each patient’s MRI.
PiB PET Acquisition and Analysis
Each patient was injected with Pittsburgh Compound B (PiB) and after a 40 minute uptake period, a 20 minute PiB PET scan was obtained consisting of four 5-minute dynamic frames following a low dose CT transmission scan. Standard corrections were applied. Emission data were reconstructed into a 256 × 256 matrix with a 30-cm FOV. A global PiB standard uptake value ratio (SUVR) was generated; a cut-point of 1.42 was used to establish Aβ positivity, as previously described (Jack et al., 2008).
FDG-PET Acquisition and Analysis
FDG-PET scans were acquired using a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin) as previously described (Josephs et al., 2012). Individual patterns of hypometabolism were assessed using the clinical tool of 3-dimensional stereotactic surface projections (SSP) (Minoshima, Frey, Koeppe, Foster, & Kuhl, 1995) as implemented in the CortexID software package (GE Healthcare, Waukesha, Wisconsin). Activity was normalized to the pons and compared with an age-stratified normative database, yielding a 3-dimensional SSP z score image. Regional FDG-PET metabolism in both the gray and white matter was calculated with the MCALT (Schwarz et al., 2017). For this study, regions-of-interest (ROIs) were selected a priori based on regions that are typically affected in the three PPA variants (Bouwman et al., 2018; Guo et al., 2016; Josephs, Duffy, Fossett, & et al., 2010; Matias-Guiu, Cabrera-Martín, Matías-Guiu, & Carreras, 2017; Nestor et al., 2018; Sonty et al., 2003) and included the following regions, keeping the left and right hemispheres separate: inferior frontal (combined opercularis, triangularis, and orbitalis), medial temporal, lateral temporal, lateral parietal, sensorimotor region (combined supplementary motor area and precentral), and cerebellum (combined cortical cerebellar regions).
Principal Component Analysis
To characterize the patterns of neurodegeneration visible on imaging, we performed a PCA on log-transformed FDG-PET SUVR data from the 15 patients across the above listed ROIs. PCA is a useful data reduction tool that maps large datasets onto orthogonal axes where each axis can be thought of as an independent, one number summary of the sources of variation in the data, with the axes ranked based on the proportion of variation explained. PCA was performed using R (version 3.4.2; R Foundation for Statistical Computing, Vienna, Austria). The loadings of the principal components are projected using Surf Ice (2017). To ascertain the relationship between imaging patterns and clinical presentations, correlations between component loadings and clinical variables were calculated.
Results
Clinical Data
Review of individual diagnostic classification criteria are presented in Supplementary Tables 1 and 2. Briefly, all patients demonstrated 1) initial and 2) prominent language difficulties that 3) were the cause of impaired activities of daily living. Ten patients did not meet core criteria for any one variant; one of these patients had aphasia (in the presence of apraxia of speech), but was not agrammatic. Three patients nearly met criteria for more than one variant, but did not fully meet criteria for a single variant. One patient met criteria for both nonfluent/agrammatic and logopenic variants and one met criteria for both semantic and logopenic variants.
Patient demographics and neurological data are detailed in Table 1. The median age at exam was 68 years old, with median of 3 years disease duration. Median education was 14 years; all patients had at least a high school education. Overall, two patients were normal [Patient IDs (PIDs): 1, 2], while others were mild-moderately (PIDs: 5–11) or severely (PIDs: 12, 13, 14, 15) impaired, on the reported tests of neurologic function. The median MoCA was 24/30, with only two patients scoring in the range of normal performance (score > 26). Overall, there were little to no neuropsychiatric symptoms for any patient, per the NPI-Q. Median FAB was 15, consistent with relatively preserved frontal lobe function; however 10/15 patients demonstrated some evidence of frontal lobe dysfunction (scores ≤ 15). Median FBI was 9.5, consistent with minimal behavioral dysfunction, but 7/14 tested patients demonstrated at least mild impairment. Median MDS-UPDRS III was 2, suggesting absent parkinsonism; only 4/15 patients (PIDs: 5, 6, 7, 15) demonstrated evidence of parkinsonism. The neurologic data indicated that no patient met criteria for another neurologic disease (e.g. corticobasal syndrome, progressive supranuclear palsy, Alzheimer’s disease dementia).
Table 1.
Demographic information and neurological data for each patient. A portion of these data for patients 7 and 11 were previously published in case studies (Flanagan et al., 2016; Utianski et al., Under Review). Notes: * indicates left-handed; disease duration = years since symptom onset; F = Female; M = Male; MoCA = Montreal Cognitive Assessment; NPI-Q = Neuropsychiatric Inventory Questionnaire; FAB = Frontal Assessment Battery; FBI = Frontal Behavioural Inventory; MDS-UPDRS III = the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale motor subsection; PiB SUVR = global Pittsburgh Compound B (PiB) standard uptake value ratio (SUVr); DNT = did not test; where appropriate, maximum scores are noted in column headers.
| ID | Age at Onset (years) | Age at Exam (years) | Disease Duration (years) | Sex | Education (years) | MoCA (/30) | NPI-Q (/36) | FAB (/18) | FBI (/72) | MDS - UPD RS III (/120) | Global PiB SUVR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | 54 | 2 | F | 14 | 27 | 1 | 17 | 6 | 0 | 1.19 |
| 2 | 59 | 62 | 3 | F | 18 | 28 | 1 | 18 | 9 | 0 | 2.19 |
| 3 | 69 | 71 | 1.5 | M | 20 | 24 | 4 | 15 | 4 | 8 | 1.24 |
| 4 | 64 | 68 | 4 | M | 16 | 25 | 2 | 16 | 4 | 0 | 1.25 |
| 5 * | 63 | 67 | 4 | M | 17 | 24 | 0 | 13 | 12 | 25 | 1.3 |
| 6 | 53 | 57 | 4 | M | 14 | 23 | 2 | 14 | 30 | 21 | 1.19 |
| 7 | 77 | 78 | 1 | F | 14 | 24 | 7 | 15 | 8 | 24 | 1.21 |
| 8 | 68 | 69 | 1 | F | 13 | 23 | 7 | 13 | 27 | 1 | 1.27 |
| 9 | 67 | 69 | 2 | F | 14 | 16 | 1 | 16 | 0 | 4 | 2.37 |
| 10 | 69 | 72 | M | 13 | 24 | 0 | 15 | 7 | 2 | 1.28 | |
| 11 | 60 | 65 | 5 | F | 12 | 24 | 1 | 18 | 15 | 0 | 1.21 |
| 12 | 60 | 64 | 4 | F | 14 | 11 | 7 | 8 | DNT | 0 | DNT |
| 13 | 59 | 64 | 6 | M | 12 | DNT | 9 | 3 | 34 | 6 | 1.13 |
| 14 | 74.5 | 76 | 1.5 | M | 16 | 12 | 1 | 13 | 10 | 2 | 1.66 |
| 15 | 68 | 70 | 2 | F | 16 | 10 | 1 | 9 | 32 | 24 | 1.35 |
Neuropsychological data are detailed in Table 2. Data included Mayo’s Older Americans Normative Studies (MOANS) scores, which are age and education adjusted standard scores with a mean of 10 and standard deviation of 3. MOANS scores ≤ 6 are generally considered abnormal. Overall, some patients were normal on all (PIDs: 1, 3, 4, 11) or most (PIDs: 5, 7) neuropsychological tests, while others were mildly (PIDs: 2, 6, 8, 15) or severely (PIDs: 9, 10, 12, 13, 14) impaired on most tests. Median MOANS score on the AVLT (Steinberg, Bieliauskas, Smith, Ivnik, & Malec, 2005) was 6 for short-term retention (mildly impaired); 5/13 patients demonstrated impairment. Median MOANS score on the AVLT was 8 (average) for long-term retention; 5/13 patients scored one standard deviation below the mean. Median MOANS score was 7 for TMT A (Steinberg, Bieliauskas, Smith, & Ivnik, 2005), consistent with average motor speed but 6/15 patients’ scores indicated impairment. Median MOANS was 6.5 for TMT B (Steinberg, Bieliauskas, Smith, & Ivnik, 2005), consistent with reduced cognitive flexibility. This may be an under-estimate as the test was discontinued for 5/15 patients; of the remaining 10 testable patients, half scored in the impaired range. Median PPTT was 47.5, indicating mildly reduced semantic access; 7 patients scored in the impaired range. Median MOANS on the Rey-O (Machulda et al., 2007) was 9, suggesting overall intact visual constructional skills; however, the assessment indicated impairment in 6/15 patients. A test of recognition of famous faces suggested intact facial recognition for all patients.
Table 2.
Neuropsychological data for each patient. A portion of these data for patients 7 and 11 were previously published in case studies (Flanagan et al., 2016; Utianski et al., Under Review). Notes: MOANS = The Mayo Older Americans Normative Studies (Machulda et al., 2007; Steinberg, Bieliauskas, Smith, & Ivnik, 2005; Steinberg, Bieliauskas, Smith, Ivnik, & Malec, 2005); AVLT = Auditory Verbal Learning Test; ST = Short-term retention; LT = Long-term retention; TMT = Trail Making Test; Rey-O = Rey-Osterrieth Complex Figure test; Famous Faces = Recognition test; CND = could not determine, although test was attempted (discontinued); DNT = did not test; where appropriate, maximum score is noted in column headers.
| ID | AVLT ST MOANS | AVLT LT MOANS | TMTA MOANS | TMTB MOANS | Rey-O MOANS | Famous Faces (/10) |
|---|---|---|---|---|---|---|
| 1 | 12 | 13 | 7 | 7 | 10 | 10 |
| 2 | 7 | 8 | 5 | 6 | 2 | 10 |
| 3 | 11 | 12 | 7 | 6 | 11 | 10 |
| 4 | 14 | 14 | 10 | 9 | 9 | 10 |
| 5 | 10 | 14 | 8 | 5 | 10 | 10 |
| 6 | 5 | 6 | 3 | 2 | 10 | 10 |
| 7 | 2 | 6 | 13 | 9 | 12 | 9 |
| 8 | 6 | 8 | 7 | CND | 7 | 10 |
| 9 | 2 | 3 | 4 | CND | 10 | |
| 10 | 2 | 4 | 8 | 7 | 11 | 10 |
| 11 | 10 | 11 | 9 | 7 | 10 | 10 |
| 12 | DNT | DNT | 11 | CND | 6 | 10 |
| 13 | DNT | DNT | 2 | DNT | 10 | |
| 14 | 2 | 4 | 5 | CND | 2 | 10 |
| 15 | 6 | 8 | 4 | 2 | 2 | 9 |
Language data are detailed in Table 3. Overall, four patients (PIDs: 1–4) were nearly normal on all formal tests, despite presenting with language complaints that were apparent conversationally. Three additional patients had moderate impairment on most language tests (PIDs: 5–7). Four additional patients (PIDs: 8–11) had evidence of agrammatism in speaking or writing and impaired auditory comprehension (per the Token Test). Four patients (PIDs: 12–15) were severely impaired, or untestable on most or all tests; they were too severely impaired to confidently classify, although all presented with language complaints. Median BNT was 13/15; 6/13 tested patients demonstrated reduced confrontation naming ability (a score < 12). Median TT was 13/22; 9/13 tested patients demonstrated reduced comprehension (a score < 19). Median WAB-AQ was 92.35/100, consistent with mild aphasia; 9 patients scored below the cutoff for normal of 93.8. Median scores on reading commands (20/20) and comprehension (40/40) reflect intact reading comprehension. Median accuracy was 10/10 reading irregular words and 6.5/10 nonwords. Two patients scored in the impaired range reading regular words (i.e. ≤ 7) while 8 demonstrated difficulty reading nonwords. Median accuracy was 6.5/10 writing irregular words and 5/10 nonwords. Ten patients scored in the impaired range writing regular words (i.e. ≤ 7) and 12 demonstrated difficulty writing nonwords. Median writing score, derived from the WAB picture description, was 32.5/34, and reflected paraphasic, grammatical, or spelling errors; only 4/14 patients had error-free writing samples. Two patients were judged to have evidence of agrammatism (i.e. telegraphic, missing function words or articles, disrupted syntax) in both spoken language and written language; another 6 patients had agrammatism in only written language. No patient had agrammatism in spoken language only. Three patients demonstrated dysarthria (two hypokinetic; one unilateral upper motor neuron). One patient had apraxia of speech.
Table 3.
Speech and language data for each patient. A portion of these data for patients 7 and 11 were previously published in case studies (Flanagan et al., 2016; Utianski et al., Under Review). Notes: MSD = Motor Speech disorder; Hypo = Hypokinetic dysarthria; UUMN = Unilateral upper motor neuron dysarthria; AOS = Apraxia of Speech; BNT = Boston Naming Test, short form; PPTT =The Pyramids and Palm Trees Test; TT = Token Test; AQ = Aphasia Quotient; Western Aphasia Battery Part II Subtests; Comp. = Comprehension; Irr. = Irregular; CND = Could not determine, given missing information; DNT = did not test; maximum score noted in each column header.
| Western Aphasia Battery | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reading | Writing | Agrammatism? | ||||||||||||
| ID | MS D? | BNT (/I5) | PPTT (/52) | TT (/22) | AQ (/100) | Commands (/20) | Comp. (/40) | Irr. Words (/10) | Non Words (/10) | Irr. Words (/10) | Non Words (/10) | Picture (/34) | Speaking | Writing |
| 1 | None | 15 | 51 | 20 | 93.3 | 20 | 40 | 10 | 10 | 10 | 9 | 34 | No | No |
| 2 | None | 14 | 46 | 19 | 95.8 | 19 | 40 | 10 | 7 | 7 | 7 | 33 | No | No |
| 3 | None | 15 | 47 | 17 | 94.4 | 20 | 40 | 10 | 10 | 10 | 9 | 33.5 | No | No |
| 4 | None | 13 | 49 | 19 | 96.6 | 20 | 40 | 10 | 8 | 9 | 7 | 34 | No | No |
| 5 | Hypo. | 14 | 47 | 13 | 95 | 20 | 40 | 9 | 4 | 5 | 33 | No | No | |
| 6 | None UU | 10 | 49 | 9 | 84. 9 | 17 | 40 | 9 | 2 | 2 | 3 | 17 | No | No |
| 7 | MN and AOS | 10 | 50 | 19 | 94.8 | 20 | 40 | 10 | 10 | 6 | 5 | 32 | No | No |
| 8 | None | 13 | 52 | 10 | 92.2 | 20 | 32 | 10 | 5 | 7 | 6 | 32 | No | Yes |
| 9 | None | 3 | 8 | 8 | 92.5 | 20 | 32 | 8 | 9 | 5 | 5 | 17 | No | Yes |
| 10 | None | 14 | 48 | 15 | 91.2 | 20 | 40 | 10 | 6 | 6 | 0 | 9 | No | Yes |
| 11 | None | 11 | 51 | 4 | 72. 4 | 15 | 40 | 4 | 6 | 7 | 2 | 34 | Yes | Yes |
| 12 | None | DNT | 45 | DNT | 60.8 | 16 | 40 | 7 | 6 | 0 | 0 | 34 | Yes | Yes |
| 13 | None | DNT | DNT | DNT | CND | DNT | 2 | DNT | DNT | DNT | DNT | DNT | CND | CND |
| 14 | None | 2 | 35 | 8 | 78.1 | 18 | 32 | 10 | 10 | 10 | 6 | 29 | No | Yes |
| 15 | Hypo. | 6 | 43 | 6 | 55.3 | 16 | 40 | 10 | 5 | 1 | 0 | 0 | No | No |
PiB PET
Individual global PiB SUVRs for 14/15 patients are included in Table 1. Median global PiB SUVR was 1.27. Three patients were identified as amyloid positive (SUVR > 1.42) (Jack et al., 2008), implying some degree of Alzheimer’s pathologic change.
FDG-PET
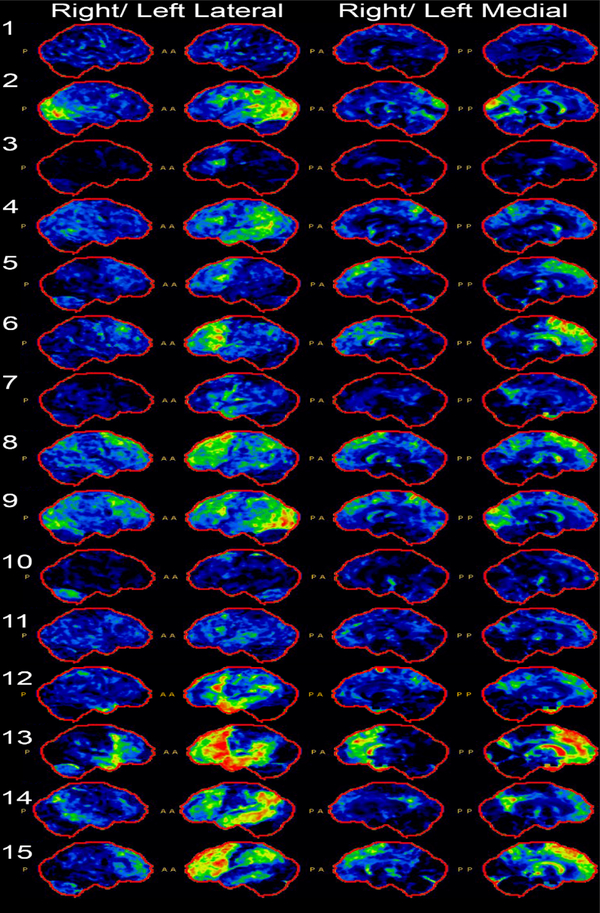
Individual FDGs are included in Figure 1 and demonstrate several patterns of hypometabolism. All patients had left greater than right involvement, consistent with their language predominant presentation. Six patients (PIDs: 3, 5, 6, 8, 10, 15) demonstrated predominantly frontal hypometabolism. Two patients (PIDs: 4, 14) demonstrated predominantly parietal and temporal hypometabolism. Two patients (PIDs: 2, 9) demonstrated predominantly occipital hypometabolism. Two patients demonstrated equal frontal and temporal hypometabolism (PIDs: 12, 13). One patient (PID: 1) demonstrated essentially normal metabolism. Additionally, five patients had involvement of the caudate (PIDs: 1, 5, 8, 10, 15). Five patients (PIDs: 5, 9, 10, 13, 15) had cerebellar hypometabolism.
Figure 1.
Cortex-ID analysis of fluorodeoxyglucose-F18 PET (FDG-PET) for each patient, showing right and left lateral and medial views. Notes: P = Posterior; A = Anterior.
Principal Component Analysis (PCA)
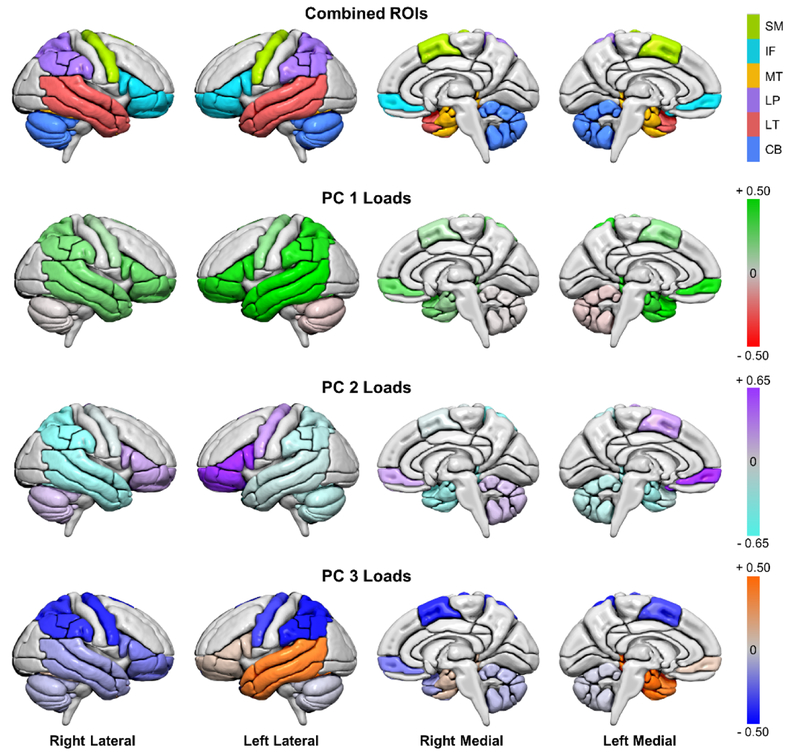
The first, second, and third components explain 56%, 19%, and 14% of the variation in the data, respectively. Loadings of the first three principal components (PC) on log-transformed FDGPET SUVRs for regions of interest are reported in Table 4. PC 1, PC 2, and PC 3 loadings are shown in Figure 2, outlining ROIs. Finally, to quantify the gestalt associations of the patterns of hypometabolism and clinical presentations, correlations were computed between each patients’ factor loadings and clinical variables. Correlations are reported in Table 5; no PC correlated with age or disease duration. Detailed results for the above analyses for each PC are discussed below.
Table 4.
Loadings of the first three principal components (PC) on log-transformed FDG-PET SUVRs for regions of interest. The factor loadings of the regions with the 6 highest magnitudes in each component are bolded.
| Region of Interest | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| Left Cerebellum | −0.043 | −0.153 | −0.030 |
| Right Cerebellum | −0.010 | 0.113 | −0.070 |
| Left Inferior Frontal | 0.441 | 0.689 | 0.071 |
| Right Inferior Frontal | 0.238 | 0.193 | −0.191 |
| Left Lateral Parietal | 0.427 | −0.164 | −0.430 |
| Right Lateral Parietal | 0.202 | −0.364 | −0.368 |
| Left Lateral Temporal | 0.533 | −0.100 | 0.380 |
| Right Lateral Temporal | 0.214 | −0.283 | −0.112 |
| Left Medial Temporal | 0.373 | −0.266 | 0.463 |
| Right Medial Temporal | 0.129 | −0.270 | 0.087 |
| Left Sensorimotor | 0.166 | 0.239 | −0.320 |
| Right Sensorimotor | 0.102 | −0.042 | −0.389 |
Figure 2.
Lateral and medial views of regions of interest (ROIs) from the AAL atlas shown in MCALT space; colors note larger regions of interested often affected in PPA. Loadings are shown for each principal component (PC) in those ROIs. Note: SM = Supplementary Motor. IF = Inferior frontal; MT = medial temporal; LP = lateral parietal; LT = lateral temporal; CB = cerebellum. For clarity, the range for PC 2 loadings was increased because of the similarity in high loadings across multiple regions. Renders created using Surf Ice (2017).
Table 5.
Spearman’s nonparametric correlations for clinical variables and PC loadings. Notes: Text in bold- italics indicates statistically significant relationship; PC = principal component; MoCA = Montreal Cognitive Assessment; Rey-O = Rey-Osterrieth Complex Figure test; BNT = Boston Naming Test, short form; FAB = Frontal Assessment Battery; FBI = Frontal Behavioural Inventory; MDS-UPDRS III = the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale, motor subsection; TMT B = Trail Making Test B; TT = Token Test. PiB = global Pittsburgh Compound B (PiB) ratio; WAB AQ = Western Aphasia Battery Aphasia Quotient; PPTT = The Pyramids and Palm Trees Test; NPI-Q = Neuropsychiatric Inventory Questionnaire; TMT A= Trail Making Test; AVLT = Auditory Verbal Learning Test; ST = Short-term retention; LT = Long-term retention.
| Clinical Variable | PC 1 | PC 2 | PC 3 |
|---|---|---|---|
| MoCA | 0.5986, p = 0.0237 | 0.3623, p = 0.2030 | −0.5086, p = 0.0633 |
| Rey-O | 0.7030, p = 0.0035 | −0.2047, p = .4642 | −0.0670, p = 0.8124 |
| BNT | 0.8239, p = 0.0005 | −0.1387, p = 0.6513 | −0.2663, p = 0.3791 |
| FAB | 0.4324, p = 0.1074 | 0.6955, p = 0.0040 | −0.1081, p = 0.7013 |
| FBI | −0.3454, p = 0.2264 | −0.5963, p = 0.0244 | 0.2222, p = 0.4451 |
| MDS-UPDRS III | −0.0528, p = 0.8517 | −0.6632, p = 0.0070 | 0.2569, p = 0.3554 |
| TMTB | −0.0186, p = 0.9594 | 0.6441, p = 0.044 | −0.6751, p = 0.0322 |
| TT | 0.4509, p = 0.1220 | 0.2490, p = 0.4121 | −0.7414, p = 0.0037 |
| Age | −0.2536, p = 0.3618 | −0.1107, p = 0.6945 | 0.1571, p = 0.5760 |
| Disease Duration | 0.1451, p = 0.6059 | −0.1523, p = 0.5878 | −0.1850, p = 0.5093 |
| PiB | −0.3106, p = 0.2798 | 0.3678, p = 0.1957 | 0.3987, p = 0.1579 |
| WAB AQ | 0.2879, p = 0.3182 | 0.3363, p = 0.2398 | −0.4462, p = 0.1098 |
| PPTT | 0.3925, p = 0.1651 | −0.0772, p = 0.7931 | −0.0353, p = 0.9047 |
| NPI-Q | −0.3376, p = 0.2185 | −0.1595, p = 0.5701 | −0.4415, p = 0.0995 |
| TMTA | 0.4009, p = 0.1554 | 0.2740, p = 0.3432 | −0.1314, p = 0.6543 |
| AVLT ST | 0.5476, p = 0.0527 | −0.0196, p = 0.9494 | −0.1481, p = 0.6293 |
| AVLT LT | 0.5298, p = 0.0626 | −0.1914, p = 0.5310 | −0.0832, p = 0.7869 |
Component One (PC 1)
The first component reflects lateralization in the data, with left hemisphere loadings greater in magnitude than right hemisphere loadings in each region, consistent with the fact that the patients presented with language predominant deficits. The cerebellum is not involved in this primary dimension. The t-map of PC 1 revealed a pattern similar to that seen in lvPPA, with diffuse, bilateral hypometabolism. Alternatively, PC 1 may reflect the overall severity of impairment. PC 1 loadings were significantly correlated with the MoCA, Rey-O, and BNT.
Component Two (PC 2)
In the second component, the bilateral inferior frontal lobes, left sensorimotor region, and right cerebellum had positive loadings, in contrast to negative loadings in other regions. The t-map of PC 2 reflects a pattern of involvement that is similar to what is seen in patients with agPPA. PC 2 significantly correlated with the FAB, FBI, MDS-UPDRS III, and TMT B.
Component Three (PC 3)
The third component loadings reflect asymmetry in the temporal regions, with positive loadings in the left lateral and medial temporal lobes with minimal if any contribution from the right hemisphere. There were equivalent negative loadings in the sensorimotor and lateral parietal regions and low loadings on the inferior frontal and cerebellar ROIs. PC 3, visualized in the tmap, demonstrates primary involvement of the left temporal lobe, similar to what is seen in the semantic variant of PPA. PC 3 significantly correlated with TMT B and the TT.
Discussion
Unclassifiable PPA, which accounts for up to one-third of patients with PPA (Gil-Navarro et al., 2013; Harris et al., 2013; Mesulam et al., 2012; Wicklund et al., 2014), and 12.7% of the research cohort from which the patients in this study were drawn, is a heterogeneous entity. All 15 patients described here met the root/core criteria for PPA (Gorno-Tempini et al., 2011), as they presented with language deficits as the initial and prominent symptoms that led to difficulties with activities of daily living. However, they did not clearly fit criteria for a clinical diagnosis of a single variant of PPA: logopenic, nonfluent/agrammatic, or semantic. The reason for difficulty of classification of patients varied: some were very mildly impaired, others were severely impaired, and others reflected a true combination of deficits associated with the aforementioned variants, meeting either none or more than one of the diagnostic criteria. We use standard cut-off scores for most language tests and age-adjusted norms for neuropsychological testing. Utilizing the skills and clinical judgments of expert speech-language pathologists and neuropsychologists, clinical judgment complemented interpretation of scores to ensure their validity. It is possible that differing definitions and thresholds of “impairment” account for differences in estimated proportions of unclassifiable PPA patients.
Overall, it appears the group-level clinical descriptions, or median tests scores, do not adequately reflect the individual patients; this is one reason we additionally described the proportion of patients who performed abnormally on a given assessment. When the clinical data are examined together, there are mixed profiles of language deficits and accompanying motor, cognitive, and memory symptoms deficits. Four patients (PIDs: 1–4) were nearly normal on all neurologic, neuropsychological, and language tests, but presented with language complaints that were otherwise evident outside of standard assessments (i.e. conversationally). Three additional patients had parkinsonism (PIDs: 5–7), with mild-moderate cognitive deficits and moderate aphasia. Four additional patients had agrammatism, as it occurs in the agrammatic variant, and notably impaired auditory comprehension (beyond single word comprehension), as it most often occurs in the logopenic variant (PIDs: 8–11); of these patients, two had significantly impaired cognition (PIDs: 9,10), whereas the other two were relatively spared cognitively (PIDs: 8, 11). The combination of agrammatism and comprehension deficits is akin to patients described as “Mixed” in a previous study (Mesulam et al., 2008). The final four patients (PIDs: 12–15) were severely impaired or untestable on most, if not all, tests. While acknowledging missing data, at least two of these severely impaired patients had evidence for agrammatism (PIDs: 12, 14) and one for parkinsonism (PID: 15). This descriptively stratifies the heterogeneity of the patients with otherwise unclassifiable PPA.
It is the case that many of the patients had impaired scores in non-language domains; however, most of the scores (e.g. MoCA) can be influenced by language impairments. For the few who had impaired scores not accounted for by language deficits, the disease duration was longer than 3 years. This does not preclude a diagnosis of PPA; as the disease progresses, we expect impairments to emerge in non-language domains.
While not explicitly mentioned in the diagnostic criteria, data for reading and writing abilities were interesting and informative in this cohort. Overall reading comprehension was intact for most patients. The data suggest that patients had more difficulty with reading and writing nonwords over irregular words, and more difficulty with writing than reading both nonwords and irregular words. The source of poor performance could include reduced working memory or impaired phonological processing, among others aspects of language or cognitive functioning. Identifying the mechanism may be helpful in anticipating other functional deficits and should be the topic of future studies. Additionally, reading and writing were the only clearly abnormal tests for some patients; this is currently not included in the diagnostic consensus criteria and consideration of its inclusion is worthwhile for future iterations.
A previous report that investigated a subset of unclassifiable PPA patients (Wicklund et al., 2014) suggested that the clinical presentations of their cohort reflected patterns seen in lvPPA and svPPA that were perhaps too mild to be captured by standardized testing. This may be the case in a subset of the current patients (PIDs: 1–4) in the context of their premorbid abilities (reflected in their levels of education). Despite performing in the “normal” range, it may be below what is expected for their prior level of functioning. Additionally, all but one of these patients had abnormal FDG-PETs. When abnormal, FDG-PETs for all patients demonstrated left greater than right involvement. No individual patient had a clinical or imaging profile suggestive of svPPA.
Six patients (PIDs: 3, 5, 6, 8, 10, 15), representing a range of severity, demonstrated predominant frontal hypometabolism. This is consistent with patterns of hypometabolism seen in agPPA (Bouwman et al., 2018; Josephs et al., 2010; Matias-Guiu et al., 2017; Nestor et al., 2018). Two patients (PIDs: 4, 14), representing the continuum of clinical impairment, demonstrated predominant parietal and temporal hypometabolism, not unlike documented patterns of hypometabolism in lvPPA (Bouwman et al., 2018; Josephs et al., 2010; Matias-Guiu et al., 2017; Nestor et al., 2018). Two patients (PIDs: 2, 9) demonstrated predominantly occipital hypometabolism. These patients represent two of the PiB positive cases; it is possible they are cases of language onset posterior cortical atrophy, a form of atypical Alzheimer’s disease, similar to what we and others previously reported in the literature (Magnin et al., 2013; Tabuchi, Horigome, Konishi, & Mimura, 2017; Wicklund et al., 2013). Two patients (PIDs: 12,13) had equivalent frontal and temporal hypometabolism, possibly suggesting mixed phenotypes, including elements of agPPA (Bouwman et al., 2018; Josephs et al., 2010; Matias-Guiu et al., 2017; Nestor et al., 2018) or possibly behavioral variant of frontotemporal dementia (Cerami et al., 2016); this could reflect comorbid underlying pathologies of tau and the TAR DNA binding protein of 43 kDa (TDP-43). A third of patients had hypometabolism in the cerebellum, not previously discussed in PPA; however, several recent studies have shown cerebellar atrophy in PPA (Guo et al., 2016; Sonty et al., 2003) and associations with it and cognitive deficits in PPA and other frontotemporal dementias (Chen et al.). Overall, it appears the patterns of hypometabolism are not a direct reflection of the clinical impairments, in either severity or character, suggesting the need to identify other complementary indices of individual impairment.
In another study of clinically mixed, or unclassifiable, patients (Sajjadi, Patterson, & Nestor, 2014), the atrophy patterns resembled that of lvPPA. In the current study, the t-map of PC 1 revealed a pattern similar to that seen in lvPPA, with diffuse, bilateral hypometabolism in regions associated with Alzheimer’s disease. While only nearing statistical significance, correlations between PC 1 factor loadings and tests of short- and long-term retention were modest in magnitude (0.55 and 0.53, respectively). It may be clinically signficant that nearly half of the patients performed in the impaired range on these tests. However, the correlation with global PiB ratio, which affords the use of amyloid status as a continuous variable rather than binary distinction, was not significant. Overall, at this time, the data do not establish that Alzheimer’s disease pathology is solely responsible for the clinical presentations of the current cohort. The notion that Alzheimer’s disease is not solely responsible is compatible with a past study that utilized a data-driven approach to classifying PPA (Hoffman, Sajjadi, Patterson, & Nestor, 2017).
An alternative interpretation of PC 1 as representing an lvPPA pattern is that it reflects the overall severity of language impairment. It is apparent from the individual FDG-PETs, as well as the component loadings, that a larger source of variation in this cohort is accounted for by the left hemisphere compared to the right. This again provides support for the clinical diagnosis of aphasia, rather than broader cognitive dysfunction. Further support for this interpretation lies in the significant correlation between the PC loadings and the MoCA, which, even though it is a test of general cognition, is heavily influenced by language abilities.
Given the nearly 20% increase in variance accounted for by PC 2, it offers a meaningful contribution to understanding the data. In PC 2, the bilateral inferior frontal lobes, left sensorimotor region, and right cerebellum have positive loadings, contrasted by the negative loadings in other regions. In fact, this relates to patterns seen in a recent study of classifiable cases of nonfluent/agrammatic PPA utilizing tau imaging (Josephs et al., 2018). The involvement of the medial cerebellum is consistent with areas involved in tauopathies (e.g. progressive supranuclear palsy or corticobasal degeneration), a possible underlying pathology. Further support is provided by the correlation between PC 2 loadings and scores on the FAB, FBI, MDSUPDRS III, and TMT B, measures that are also affected in patients with agPPA (Butts et al., 2015; Modirrousta, Price, & Dickerson, 2013).
PC 3 included positive loadings in the left lateral and medial temporal lobes, contrasted by bilateral negative loadings in the sensorimotor and lateral parietal lobes. The primary involvement of the left medial and lateral temporal lobe in PC 3, visualized in the t-map, is akin to patterns seen in past research of autopsy-proven hippocampal sclerosis (Botha et al., 2018) and overlaps partially with that seen in the semantic variant of PPA, both associated with TDP-43 pathology. This might suggest underlying, either primary or co-occurring, TDP-43 pathology. Of unclear significance, PC 3 significantly correlated with TMT B and the TT. It is essential to remember this is the lowest contributing PC and caution is therefore warranted in interpreting the clinical correlations.
The question of whether patients who meet criteria for PPA, but are not classifiable into a specific variant, should be excluded from studies is controversial. This study provides support for the conclusions of past studies that the current diagnostic criteria fall short of capturing all cases of PPA (Mesulam et al., 2014; Sajjadi et al., 2014). However, we have demonstrated through the data-driven PCA that the 3 PPA variants are in fact represented in this cohort of unclassifiable patients, both in clinical and imaging profiles. It is possible the three variants outlined in the current consensus criteria are adequate to classify many cases of PPA, but that any given patient may fall into more than one of the variants, with a person-specific variant indicated by either language testing or imaging results. Perhaps the diagnostic criteria capture the clinical syndrome of the patients, but the available clinical tests or severity of impairment are the limiting factor, at least in some cases. Additionally, a patient may not meet criteria because they have progressive aphasia but are not otherwise captured (e.g. pure word deafness). Future diagnostic criteria should work to capture these patients. As unclassifiable cases account for up to 30% of PPA (twice that of the current sample), a great deal of potentially important clinical and biological information may be lost if they are excluded. Studying unclassifiable PPA in future studies of PPA may result in refinements of clinical instruments and biomarkers that more fully describe these complex patients. This will help us better understand the ways in which they may be less heterogeneous than previously thought and inform how we can better account for subtle, mixed, or not-otherwise specified presentations in future iterations of diagnostic criteria. As we learn more about the relationship between clinical presentation and underlying pathology in the currently defined variants, we may come to better identify any such relationships in mixed, novel, or atypical patterns of PPA.
Future studies should focus on the sensitivity and specificity of tests of language functioning as it relates to patient complaints, clinical judgments, and abnormal neuroimaging rather than simply using these to categorize patients into one of a handful of syndromic diagnoses. This is especially important given differing definitions and thresholds for defining “impairment” across different areas of language functioning across different clinical practices and research institutions. Whether patterns on imaging should be a primary element of diagnostic criteria, as previously suggested (Bouwman et al., 2018), should also be considered, as hypometabolism on FDG-PET may precede deficits detectable on standardized testing; however, it may also over-classify some variants (Matias-Guiu et al., 2014). The balance of maximal sensitivity and specificity of clinical and imaging tests must be identified. As more biomarkers become available and accessible, clinical subtyping may not be necessary to predict underlying pathology and prognosis. However, it is certain that a patient’s individual deficits should dictate treatment planning. Characterizing a patient’s strengths and deficits facilitate planning for behavioral and other non-pharmacological treatments. This can also help affected individuals and their families anticipate future difficulties and put in place strategies that may help manage them when needed.
There are limitations to the current study. A larger sample size would allow us to do more thorough, voxel-wise imaging analyses whilst retaining sufficient statistical power. It would also allow for PCA on cohorts of different severities to assess the degree to which our findings apply across the severity continuum. Following these patients longitudinally, including to autopsy, will also allow us to better understand the underlying pathology and relate it to previous studies of typical and atypical PPA (Spinelli et al., 2017). Finally, we assessed neuroimaging patterns in FDG-PET only. Hypometabolism is only one element of neurodegeneration and exploring these relationships in multiple imaging modalities may offer further insight into correlations with clinical variables and the interaction of multiple disease processes. There may be added variable in comparing the structural and metabolic changes to one another and to other functional imaging methodologies. However, the goal of the current study was to address the similarities and patterns among this cohort. The strength of this study is its comprehensive detail about the clinical language, neuropsychological, and neurological presentations of a cohort of diagnostically challenging patients with PPA. We utilized data-driven statistical methods to understand the FDG-PET patterns, which were then directly related to the clinical presentations. Overall, this study expands our understanding of an “unclassifiable” PPA group, at least a portion of who may be better characterized as “mixed” presentations of PPA.
Conclusions
This study has provided comprehensive detail about the clinical presentations of a cohort of diagnostically challenging patients. The findings have also described the similarity and differences among patients with clinically unclassifiable PPA, an overall heterogeneous cohort. The data suggest that the severity continuum is not the only reason for an unclassifiable diagnosis. Mixed presentations of PPA should be studied to better define diagnostic categories and predict underlying pathology. Overall, the presented findings may guide refinements of clinical assessment tools to capture mild language deficits and inform future modifications to the current diagnostic criteria to account for mixed, novel, or atypical manifestations of PPA.
Supplementary Material
Statement of Significance to the Neurobiology of Language
Demographic, clinical, and imaging profiles of patients with Primary Progressive Aphasia who do not meet criteria for a specific sub-variant (PPA-Unclassifiable), are detailed. Visualization of the results of principal component analysis of FDG-PETs suggested brain regions affected in PPA-Unclassifiable patients are no different from those who are more easily classifiable.
Highlights
Many patients with Primary Progressive Aphasia are unclassifiable.
Unclassifiable PPA (PPA-U) patients not well studied.
Brain regions affected in PPA-U are no different from classifiable patients.
Severity is not the only reason for an unclassifiable categorization.
Acknowledgments
We thank the patients and their families for their time and participation in our research program. We express gratitude to Sarah Boland, psychometrist and study coordinator, for her assistance in data collection and coordinating patient visits.
Funding
This study was funded by the National Institutes of Health (NIH), National Institute on Deafness and Other Communication Disorders [grants R01 DC010367 (PI: Josephs), R01 DC014942 (PI: Josephs), and R01 DC012519 (PI: Whitwell)], National Institute of Neurological Disorders and Stroke (NINDS) [grant R21 NS094684 (PI: Josephs)], and National Institute on Aging [grant R37 AG11378 (PI: Jack)].
Declaration of interest: All authors receive research support from the National Institutes of Health. Dr. Jack consults for Lily and serves on an independent data monitoring board for Roche but he receives no personal compensation from any commercial entity; he receives research support from the NIH and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Clinic. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma, Piramal Life Sciences and receives research support from GE Healthcare, Siemens Molecular Imaging, AVID Radiopharmaceuticals and the NIH (NIA, NCI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashburner J, & Friston KJ (2005). Unified segmentation. Neuroimage, 26(3),839–851. [DOI] [PubMed] [Google Scholar]
- Botha H, Duffy JR, Whitwell JL, Strand EA, Machulda MM, Schwarz CG, … Josephs KA (2015). Classification and clinicoradiologic features of primary progressive aphasia (PPA) and apraxia of speech. Cortex, 69, 220–236. doi: 10.1016/j.cortex.2015.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha H, Mantyh WG, Murray ME, Knopman DS, Przybelski SA, Wiste HJ, … Jones DT (2018). FDG-PET in tau-negative amnestic dementia resembles that of autopsy-proven hippocampal sclerosis. Brain, 141(4), 1201–1217. doi: 10.1093/brain/awy049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwman F, Orini S, Gandolfo F, Altomare D, Festari C, Agosta F, … Boccardi M (2018). Diagnostic utility of FDG-PET in the differential diagnosis between different forms of primary progressive aphasia. European Journal of Nuclear Medicine and Molecular Imaging, 45(9), 1526–1533. doi: 10.1007/s00259-018-4034-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butts AM, Machulda MM, Duffy JR, Strand EA, Whitwell JL, & Josephs KA (2015). Neuropsychological Profiles Differ among the Three Variants of Primary Progressive Aphasia. J Int Neuropsychol Soc, 21(6), 429–435. doi: 10.1017/s1355617715000399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerami C, Dodich A, Lettieri G, Iannaccone S, Magnani G, Marcone A, … Perani D (2016). Different FDG-PET metabolic patterns at single-subject level in the behavioral variant of fronto-temporal dementia. Cortex, 83, 101–112. doi: 10.1016/j.cortex.2016.07.008 [DOI] [PubMed] [Google Scholar]
- Chen Y, Kumfor F, Landin-Romero R, Irish M, Hodges JR, & Piguet O (2018). Cerebellar atrophy and its contribution to cognition in frontotemporal dementias. Annals of Neurology, 84(1), 98–109. doi:doi: 10.1002/ana.25271 [DOI] [PubMed] [Google Scholar]
- De Renzi E, & Vignolo LA (1962). The token test: A sensitive test to detect receptive disturbances in aphasics. Brain, 85, 665–678. [DOI] [PubMed] [Google Scholar]
- Dubois B, Slachevsky A, Litvan I, & Pillon B (2000). The FAB: a Frontal Assessment Battery at bedside. Neurology, 55(11), 1621–1626. [DOI] [PubMed] [Google Scholar]
- Flanagan EP, Duffy JR, Whitwell JL, Vemuri P, Dickson DW, & Josephs KA (2016). Mixed tau and TDP-43 pathology in a patient with unclassifiable primary progressive aphasia. Neurocase, 22(1), 55–59. doi: 10.1080/13554794.2015.1041534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gil-Navarro S, Lladó A, Rami L, Castellví M, Bosch B, Bargalló N, … Sánchez-Valle R (2013). Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dementia and geriatric cognitive disorders, 35(1–2), 106–117. doi: 10.1159/000346289 [DOI] [PubMed] [Google Scholar]
- Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, … LaPelle N (2008). Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord, 23(15), 2129–2170. doi: 10.1002/mds.22340 [DOI] [PubMed] [Google Scholar]
- Gorno-Tempini M, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SE, & Manes F (2011). Classification of primary progressive aphasia and its variants. Neurology, 76 (11), 1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo CC, Tan R, Hodges JR, Hu X, Sami S, & Hornberger M (2016). Network-selective vulnerability of the human cerebellum to Alzheimer’s disease and frontotemporal dementia. Brain, 139(5), 1527–1538. doi: 10.1093/brain/aww003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JM, Gall C, Thompson JC, Richardson AMT, Neary D, du Plessis D, … Jones M (2013). Classification and pathology of primary progressive aphasia. Neurology, 81(21), 1832–1839. doi: 10.1212/01.wnl.0000436070.28137.7b [DOI] [PubMed] [Google Scholar]
- Hoffman P, Sajjadi SA, Patterson K, & Nestor PJ (2017). Data-driven classification of patients with primary progressive aphasia. Brain Lang, 174, 86–93. doi: 10.1016/j.bandl.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard D, & Patterson K (1992). The pyramids and palm trees test: a test of semantic access from words and picture. Bury St Edmunds, UK: Thames Valley Test Company. [Google Scholar]
- Jack CR Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, … Petersen RC (2008). 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain, 131(Pt 3), 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Fossett TR, & et al. (2010). Fluorodeoxyglucose f18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Archives of Neurology, 67(5), 596–605. doi: 10.1001/archneurol.2010.78 [DOI] [PubMed] [Google Scholar]
- Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, … Whitwell JL (2012). Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain, 135(5), 1522–1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephs KA, R. MP, Hugo B,G, S. C. R, D. J. M, C. H., … L. WJ (2018). [18F]AV-1451 tau-PET and primary progressive aphasia. Annals of Neurology, 83(3), 599–611. doi:: 10.1002/ana.25183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, … DeKosky ST (2000). Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci, 12(2), 233–239. [DOI] [PubMed] [Google Scholar]
- Kertesz A (2006). Western Aphasia Battery (Revised). San Antonio, TX: PsychCorp. [Google Scholar]
- Kertesz A, Davidson W, & Fox H (1997). Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Canadian Journal of Neurological Sciences, 24 (1), 29–36. [DOI] [PubMed] [Google Scholar]
- Lansing AE, Ivnik RJ, Cullum CM, & Randolph C (1999). An empirically derived short form of the Boston naming test. Archives of clinical neuropsychology : the official journal of the National Academy of Neuropsychologists, 14(6), 481–487. [PubMed] [Google Scholar]
- Machulda MM, Ivnik RJ, Smith GE, Ferman TJ, Boeve BF, Knopman D, … Tangalos EG (2007). Mayo’s Older Americans Normative Studies: Visual Form Discrimination and copy trial of the Rey-Osterrieth Complex Figure. J Clin Exp Neuropsychol, 29(4), 377–384. doi: 10.1080/13803390600726803 [DOI] [PubMed] [Google Scholar]
- Magnin E, Sylvestre G, Lenoir F, Dariel E, Bonnet L, Chopard G, … Rumbach L (2013). Logopenic syndrome in posterior cortical atrophy. J Neurol, 260(2), 528–533. doi: 10.1007/s00415-012-6671-7 [DOI] [PubMed] [Google Scholar]
- Matias-Guiu JA, Cabrera-Martín MN, García-Ramos R, Moreno-Ramos T, Valles-Salgado M, Carreras JL, & Matias-Guiu J (2014). Evaluation of the New Consensus Criteria for the Diagnosis of Primary Progressive Aphasia Using Fluorodeoxyglucose Positron Emission Tomography. Dementia and geriatric cognitive disorders, 38(3–4), 147–152. doi: 10.1159/000358233 [DOI] [PubMed] [Google Scholar]
- Matias-Guiu JA, Cabrera-Martín MN, Matías-Guiu J, & Carreras JL (2017). FDG-PET/CT or MRI for the Diagnosis of Primary Progressive Aphasia? American Journal of Neuroradiology, 38(9), E63–E63. doi: 10.3174/ajnr.A5255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM (1982). Slowly progressive aphasia without generalized dementia. Ann Neurol, 11(6), 592–598. [DOI] [PubMed] [Google Scholar]
- Mesulam MM (2001). Primary progressive aphasia. Ann Neurol, 49(4), 425–432. [PubMed] [Google Scholar]
- Mesulam MM, Weintraub S, Rogalski EJ, Wieneke C, Geula C, & Bigio EH (2014). Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain, 137(Pt 4), 1176–1192. doi: 10.1093/brain/awu024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, … Bigio EH (2008). Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol, 63 (6), 709–719. doi: 10.1002/ana.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM, Wieneke C, Thompson C, Rogalski E, & Weintraub S (2012). Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain, 135(5), 1537–1553. doi: 10.1093/brain/aws080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Frey KA, Koeppe RA, Foster NL, & Kuhl DE (1995). A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med, 36(7), 1238–1248. [PubMed] [Google Scholar]
- Modirrousta M, Price BH, & Dickerson BC (2013). Neuropsychiatric symptoms in primary progressive aphasia: phenomenology, pathophysiology, and approach to assessment and treatment. Neurodegener Dis Manag, 3(2), 133–146. doi: 10.2217/nmt.13.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, … Chertkow H (2005). The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc, 53(4), 695–699. doi: 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nestor PJ, Altomare D, Festari C, Drzezga A, Rivolta J, Walker Z, … Frisoni GB (2018). Clinical utility of FDG-PET for the differential diagnosis among the main forms of dementia. European Journal of Nuclear Medicine and Molecular Imaging, 45 (9), 1509–1525. doi: 10.1007/s00259-018-4035-y [DOI] [PubMed] [Google Scholar]
- Osterrieth P (1944). Le test de copie d’une figure complex: Coontribution a l’etude de la perception et de la memoire. Archives de Psychologie, 30, 286–356. [Google Scholar]
- Rey A (1964). L’examen clinique en psychologie. Paris: Presses Universitaires de France. [Google Scholar]
- Sajjadi SA, Patterson K, Arnold RJ, Watson PC, & Nestor PJ (2012). Primary progressive aphasia: a tale of two syndromes and the rest. Neurology, 78(21), 1670–1677. doi: 10.1212/WNL.0b013e3182574f79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjadi SA, Patterson K, & Nestor PJ (2014). Logopenic, mixed, or Alzheimer-related aphasia? Neurology, 82(13), 1127–1131. doi: 10.1212/wnl.0000000000000271 [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Ward CP, Vemuri P, Senjem ML, Wiste HJ, … Jack CR (2017). The Mayo Clinic Adult Lifespan Template (MCALT): Better Quantification across the Lifespan. Paper presented at the Alzheimer’s Association International Conference. [Google Scholar]
- Sonty SP, Mesulam MM, Thompson CK, Johnson NA, Weintraub S, Parrish TB, & Gitelman DR (2003). Primary progressive aphasia: PPA and the language network. Annals of Neurology: Official Journal of the American Neurological Association and the Child Neurology Society, 53(1), 35–49. [DOI] [PubMed] [Google Scholar]
- Spinelli EG, Mandelli ML, Miller ZA, Santos-Santos MA, Wilson SM, Agosta F, … Gorno-Tempini ML (2017). Typical and atypical pathology in primary progressive aphasia variants. Ann Neurol, 81(3), 430–443. doi: 10.1002/ana.24885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreen O, & Strauss E (1998). Compendium of Neuropsychological tests, second edition: administration, norms and commentary. New York: Oxford University Press. [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, & Ivnik RJ (2005). Mayo’s Older Americans Normative Studies: Age- and IQ-Adjusted Norms for the Trail-Making Test, the Stroop Test, and MAE Controlled Oral Word Association Test. Clinical Neuropsychology, 19(3–4), 329–377. doi: 10.1080/13854040590945210 [DOI] [PubMed] [Google Scholar]
- Steinberg BA, Bieliauskas LA, Smith GE, Ivnik RJ, & Malec JF (2005). Mayo’s Older Americans Normative Studies: Age-and IQ-Adjusted Norms for the Auditory Verbal Learning Test and the Visual Spatial Learning Test. The Clinical Neuropsychologist, 19 Initial Symptom of Alzheimer’s disease: Case Report. (3–4), 464–523. [DOI] [PubMed] [Google Scholar]
- Tabuchi H, Horigome T, Konishi M, & Mimura M (2017). Agraphia as an Initial Symptom of Alzheimer’s disease:Case Report. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association, 13(7), P1518–P1519. doi: 10.1016/j.jalz.2017.07.641 [DOI] [Google Scholar]
- Utianski RL, Duffy JR, Clark HM, Machulda MM, Dickson DW, Whitwell JL, & Josephs KA (Under Review). Auditory Verbal Agnosia in Primary Progressive Aphasia: A Case Study. Cortex. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Machulda MM, Whitwell JL, & Josephs KA (2014). Quantitative Application of the PPA Consensus Criteria. Neurology, 82, 1119–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund MR, Duffy JR, Strand EA, Whitwell JL, Machulda MM, & Josephs KA (2013). Aphasia with left occipitotemporal hypometabolism: a novel presentation of posterior cortical atrophy? J Clin Neurosci, 20(9), 1237–1240. doi: 10.1016/j.jocn.2013.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.