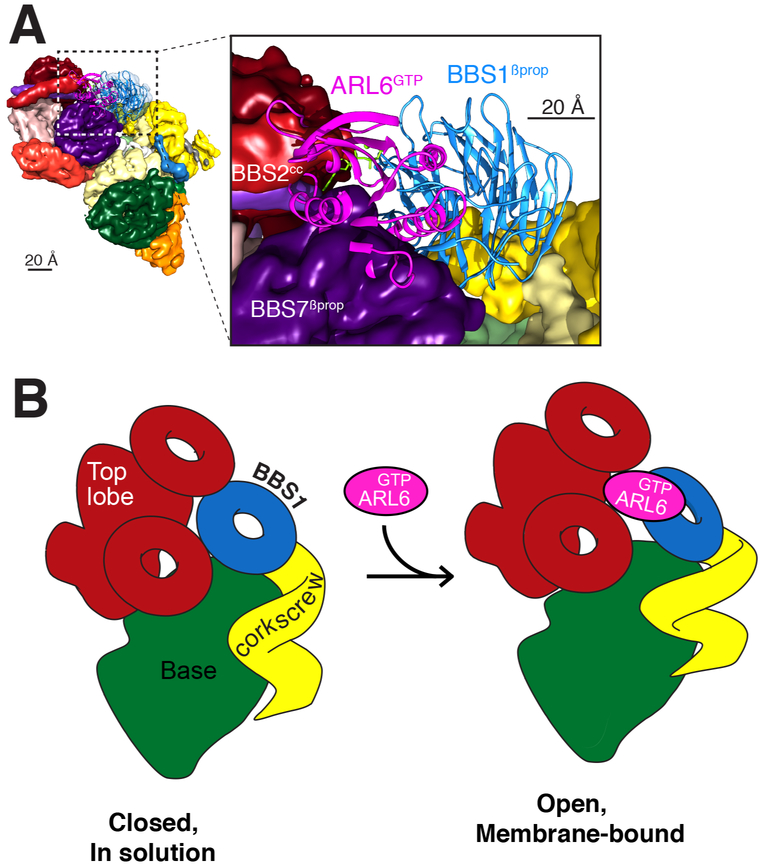
Figure 5. BBSome recruitment to membranes is coupled to a conformational change.
A. Placement of the crystal structure of the BBS1βprop, which was determined in complex with ARL6GTP (PDB id: 4V0M; Stenson et al., 2017), into the corresponding density of the 4.9-Å cryo-EM map results in a major steric clash between ARL6GTP and BBS7 βprop. The crystal structures are shown in ribbon representation and the cryo-EM map as a colored segmented map. Left, overview. Right, close-up view. B. Diagram of the predicted conformational change in the BBSome induced by ARL6GTP binding. Left panel: In solution, the BBSome exists predominantly in a closed conformation, in which BBS1βprop is too close to the top lobe to allow binding of ARL6GTP. Right panel: Binding of ARL6GTP locks the membrane-bound BBSome in an open, active conformation. This conformation would allow the BBSome to interact with cargoes and/or IFT and to cross the TZ.