Abstract
In B cell acute lymphoblastic leukemia (B-ALL), activation of Notch signaling leads to cell-cycle arrest and apoptosis. We aimed to harness knowledge acquired by understanding a mechanism of Notch-induced cell death to elucidate a therapeutically viable target in B-ALL. To this end, we identified that Notch activation suppresses Polo-like kinase 1 (PLK1) in a B-ALL specific manner. We identified that PLK1 is expressed in all subsets of B-ALL, and is highest in Philadelphia-like (Ph-like) ALL, a high-risk subtype of disease. We biochemically delineated a mechanism of Notch-induced PLK1 downregulation that elucidated stark regulation of p53 in this setting. Our findings identified a novel post-translational cascade initiated by Notch in which CHFR was activated via PARP1-mediated PARylation resulting in ubiquitination and degradation of PLK1. This led to hypophosphorylation of MDM2Ser260, culminating in p53 stabilization and upregulation of BAX. shRNA knockdown or pharmacologic inhibition of PLK1 using BI2536 or BI6727 (volasertib) in B-ALL cell lines and patient samples led to p53 stabilization and cell death. These effects were seen in primary human B-ALL samples in vitro and in patient-derived xenograft models in vivo. These results highlight PLK1 as a viable therapeutic target in B-ALL. Efficacy of clinically relevant PLK1 inhibitors in B-ALL PDX mouse models suggests that use of these agents may be tailored as an additional therapeutic strategy in future clinical studies.
Keywords: Notch, B-cell acute lymphoblastic leukemia, PLK1, CHFR, PLK1 inhibitor, volasertib
Introduction
B-cell acute lymphoblastic leukemia (B-ALL) is the most common single cancer in children, and patients of any age with relapsed B-ALL have poor outcomes (1-3). Treatment strategies and outcomes for B-ALL are partially based on the presence of high-risk genomic aberrations (2, 4). These include presence of the BCR-ABL oncogene, or the so-called Philadelphia chromosome (Ph+) (1, 2). Another high-risk subgroup in both pediatric and adult populations lacks BCR-ABL, but has gene expression profiles similar to Ph+ cases, and is thus referred to as “Ph-like” (5-7). Currently, standard treatment for B-ALL invariably includes cytotoxic chemotherapies that have significant long-term side effects (8). Therefore, treatment strategies that minimize the need for chemotherapy or utilize other targeted anti-leukemia agents are highly desirable. Thus, the need for effective and targeted therapeutic approaches is critical for both adult and pediatric patients with B-ALL. Despite recent advances in chimeric antigen receptor T cells, CD19-CD3 bi-specifics, and CD22 antibody drug conjugates, there remains a significant unmet medical need (9-11). We sought to use our knowledge of the apoptotic effects of Notch signaling on B-ALL cells (12, 13) to find a novel therapeutic approach that could be rapidly tested in the clinic.
Normal development of lymphoid cells is dependent on Notch signaling (14). When Notch receptors (Notch1-4) on the surface of a cell are bound by ligand, two successive proteolytic cleavages occur, resulting in the release of intracellular Notch (ICN1-4), which subsequently translocates to the nucleus (15). Once in the nucleus, ICN interacts with a number of co-factors to influence transcription of a variety of downstream target genes, including HES1, HEY1, and DTX1 (16). Constitutive activation of Notch signaling leads to T-ALL (17, 18). Conversely, potent activation of Notch signaling exerts pro-apoptotic effects in B-ALL cells (12, 13).
Inhibitors of the Notch pathway have demonstrated preclinical success and are in clinical trials for malignancies with overactive Notch signaling, such as breast, colorectal, gliomas, and T-cell malignancies (19). In contrast, options for translatable Notch agonists are extremely limited, due largely in part to lack of specificity. Therefore, we aimed to identify clinically actionable targets downstream of Notch that could mimic its pro-apoptotic effects in B-ALL. In this study, we identified Polo-like kinase 1 (PLK1) as a B-ALL specific pathway that contributes to the tumor suppressive effect of Notch activation.
PLK1 is a serine/threonine kinase and negative regulator of p53 that mediates mitotic entry, spindle formation, and chromosome segregation (20, 21). Its expression is elevated in solid tumors arising from several anatomic locations, including bladder, melanoma, colorectal, esophageal, and lung (22). In a variety of malignancies, PLK1 knockdown stabilizes p53, resulting in apoptosis (23). In this study, we describe a mechanism by which Notch activation downregulates PLK1, allowing for p53-mediated cell death. Using a clinically relevant PLK1 inhibitor, we demonstrate that inhibition of PLK1 in cell lines and patient-derived xenograft (PDX) models of B-ALL mimics Notch activation, resulting in cell-cycle arrest and apoptosis. Thus, our work supports the use of PLK1 inhibitors in B-ALL.
Materials and Methods
Cell culture
Pertinent cell line details are summarized in Table 1. All cells were maintained in RPMI-1640 medium (GIBCO, Gaithersburg, MD) containing 10% heat-inactivated fetal calf serum (Hyclone, Logan, UT) and 1 mM HEPES, 1 mM glutamine, 1 mM sodium pyruvate, and 1× non-essential amino acids (all, GIBCO). Cell samples from patients with T-ALL or B-ALL were acquired from the Leukemia Tissue Bank at The University of Texas MD Anderson Cancer Center, with approval from the MD Anderson Institutional Review Board.
Table 1.
Leukemic cell lines used in this study
| Human leukemia cell line |
P53 response to irradiation |
P53 status | BCR-ABL status |
Source ATCC |
|---|---|---|---|---|
| JM1 (B-ALL) | Yes | Wild type | None | CRL-10423 |
| SB (B-ALL) | Yes | Wild type | None | CCL-120 |
| MUTZ5 | Yes | Wild type | Ph-like | |
| MHH-CALL4 | Yes | Wild type | Ph-like | |
| Nalm6 (B-ALL) | Yes | Wild type | Ph+ | CRL-3273 |
| Nalm1(B-ALL) | NA | Wild type | Ph+ | CRL-1567 |
| Sup B15(B-ALL) | NA | Wild type | Ph+ | CRL-1929 |
| CEM(T-ALL) | No | Multiple mutation | NA | CCL-119 |
| SupT1(T-ALL) | Yes | Wild type | NA | CRL-1942 |
| Molt4(T-ALL) | Yes | Wild type | NA | CRL-1582 |
| Jurkat(T-ALL) | Weaker | Multiple mutation | NA | TIB-152 |
| HL60 (AML) | No | Null | NA | CCL-240 |
| Reh | Yes | Wild type | NA | CRL-8286 |
| HS5 | NA | Bone marrow fibroblast | NA | CRL-11882 |
Note: Finger printed positive cell lines with mycoplasma negative status were used within 20 passages from thawing. P53 protein expression in response to gamma-irradiation (800 rads) was confirmed by using cytometry (L.S. Golfman; unpublished observation). Wild-type p53 status was verified by sequencing or querying in the COSMIC database and/or searching the literature. NA: not applicable for irradiation/BCR-ABL studies performed.
Notch activation via co-culture on HS5 feeder system or with plate-bound ligands
HS5 cells were transduced with green fluorescent protein (GFP)-expressing MSCV-based retroviral plasmid MigR1 (as control) or full length human DLL1, sorted for 100% GFP positivity and plated. B-ALL cell lines, T-ALL CCRF-CEM cells, and secondarily engrafted B-ALL cells from patients were counted and plated (100,000 cells/well) onto the GFP or DLL1-expressing HS5 feeder system. Alternatively, Notch was activated by the plate-bound DLL1 ligand in protein-G–coated 6-well plates. Plates were treated with 1% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO), washed with phosphate-buffered saline solution (PBS), and incubated with 1 μg of human ligands fused to IgG Fc domains, i.e., DLL1-Fc (ALX-201-765-0025; Enzo Life Sciences, Farmingdale, NY), DLL3-Fc, DLL4-Fc (Adipogen), Jagged-1Fc or −2Fc (1277-JG-050 and 1726-JG-050, R &D Systems, Minneapolis, MN) or control IgG-Fc (Jackson Laboratory, Bar Harbor, ME) in 0.1% BSA independently, with rocking, overnight at 4°C.
HES1 induction and PLK1 knockdown
Human cDNA for Notch target gene HES1 was cloned into the MigR1 retroviral vector (12, 24). PLK1 short-hairpin (sh) RNA from a TRIPZ-Inducible Transfection Starter Kit (RHS4741-EG5347; Dharmacon, Lafayette, CO) was co-transfected with second-generation packaging vectors psPAX2 and pMD2 (ratio 1:1:0.5, respectively), using jetPEI transfection reagents according to the manufacturer’s protocol, for 72 h into 293T cells (provided by Dr. Faye Johnson, MD Anderson). Cells were transduced using the following method: Cells (0.1-2×106) were plated with 250-500 μL of a viral supernatant and 4-8 μg/mL of polybrene (Sigma-Aldrich). After centrifugation at 1000g for 90 min, cells were incubated at 37ºC in 5% CO2 for 3-6 h before addition of fresh complete culture medium. Upon recovery, cells transduced with doxycycline-inducible PLK1 shRNA or non-targeted control were selected against puromycin. To induce PLK1 knockdown, B-ALL cells were exposed to doxycycline (2 ug/mL) for 2 days. Doxycycline-induced red fluorescent protein expression was confirmed by flow cytometry analysis.
siRNA transfection
Oligonucleotide small-interfering RNA (siRNA) targeting human PLK1 (sc36277) or CHFR (sc37567) and siFITC-nontargeting control scramble (sc36869) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Each siRNA (100 nm) was transfected into cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) along with control siFITC transfection reagent according to the manufacturer’s protocol. After 12 h of siRNA transfection, FITC-positive leukemic cells were sorted for co-culture.
RNA extraction and RT-PCR
Total RNA was isolated from the cells and reverse-transcribed using the RNeasy Mini Kit (Qiagen, Germantown, MD). Prepared RNA was primed with random hexamers to synthesize cDNA using AMV reverse-transcriptase (Amersham, Little Chalfont, UK) according to the manufacturer’s instructions. Quantitative reverse-transcriptase PCR (qRT-PCR) was performed using human-specific Taqman probes for HES1 (Hs00172878_m1), Deltex1 (Hs00614837), HEY1 (Hs03005884), PLK1 (Hs00153444_m1), and GAPDH (4333764F); (Applied Biosystems, Foster City, CA) per the manufacturer’s instructions. CHFR cDNA was amplified with the following primers (forward/reverse, 5′-3′): CAGCAGTCCAGGATTACGTGTG and AGCAGTCAGGACGGGATGTTAC (25). The qPCR reactions were performed on an iCycler (Bio-Rad, Hercules, CA). All values were normalized to the expression of the control GAPDH housekeeping gene using the 2delta-delta Ct method.
Western blotting
Co-cultured cells were collected and whole-cell lysates were prepared according to established methods utilizing Triton X-100 or RIPA lysis buffer and 1 mM PMSF supplemented with protease inhibitor cocktail (Calbiochem, San Diego, CA) and phosphatase inhibitor cocktails I and II (Sigma-Aldrich). BCA Protein Assay Kit (ThermoFisher Scientific, Waltham, MA) was used to determine protein concentrations. HRP–linked secondary antibodies against rabbit, mouse, and goat IgGs were purchased from Amersham-GE Life Sciences (Pittsburgh, PA). 50μg of protein were loaded for western analyses as previously described(26).
Immunoprecipitation
For immunoprecipitation of PLK1, CHFR, MDM2, pMDM2, and p53, cells were subjected to lysis with 400 μL of HEPES lysis buffer (pH 7.5) containing 50 mM HEPES, 150 mM Nacl, 1 mM EGTA, 10 mM Na pyrophosphate, 10 mM NaF, 10% glycerol, 1% Triton X-100, and 1.5 mM MgCl2 at 4°C for 30 min. Whole-cell lysates were subjected to centrifugation at 15,000g for 20 min at 4°C and diluted in RIPA immunoprecipitation buffer (500 μL) containing 50 mm Tris-HCl (pH 7.5), 150 mm NaCl, 0.5% Nonidet P-40, 0.1% sodium dodecyl sulfate (SDS), and 0.1% sodium deoxycholate with 1× protease inhibitor mixture (Roche Applied Science, Indianapolis, IN) and were precleared with 30 μL of a 50% slurry of protein A-Sepharose (Amersham). Supernatants were incubated with normal mouse serum and appropriate antibodies at 4°C for 3 h. Immunocomplexes were incubated with protein G–coated magnetic beads (53014; Active Motif, Carlsbad, CA) for 45 min at 4°C. Immunoprecipitation and wash steps were performed by pelleting the magnetic beads on the side of the tube using a magnetic bar for the removal of supernatant. The precipitates were washed three times with the lysis buffer at 4°C, resuspended in 30 μL of the SDS sample buffer, and heated at 100°C for 5 min. Proteins were then resolved by 10% SDS–polyacrylamide gel electrophoresis and transferred onto Immobilon-P membranes for Western blot analysis using the specified antibodies.
Antibodies and pharmacologic inhibitors
Antibodies beta-actin (A2066; Sigma-Aldrich), cleaved Notch2 (C651.6DbHN; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA), HES1 (ab71559; AbCam, Cambridge, MA; TA500014, Origene, Rockville, MD), Notch-1, −3, and-4(ab52627, ab23426, and ab184742, Abcam), Jagged1 (sc11376; Santa Cruz Biotechnology), DLL1 (sc73899; Santa Cruz Biotechnology), p53 (DO1; Santa Cruz Biotechnology), FITC anti-p53 antibody (645803; Biolegend, San Diego, CA), PLK1 (50-171-861; Millipore, Billerica, MA), CHFR (H00055743-M01; Abnova, Taipei, Taiwan), PAR (4335-MC-100-AC; Trevigen, Gaithersburg, MD), MDM2 (OP115; Millipore), pMDM2(ser260) (orb129684; Biorbyt LLC, San Francisco, CA) (Bioworld Technology, Inc., St Louis Park, MN), phycoerythrin-labeled donkey anti-rabbit IgG antibody (406421; Biolegend), and anti-ubiquitin (U5379; Sigma-Aldrich) were used. PARP1 inhibitor 3ABA (300 μM; Sigma-Aldrich) (27), dimethyl sulfoxide (DMSO), Notch inhibitor N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT; Sigma-Aldrich), and PLK1 inhibitors BI6727 (volasertib) (28) and BI2536 (29) (Selleck Chemicals, Houston, TX) were used.
Ph-like B-ALL engraftment and therapy
B-ALL patient cells were injected into the tail vein of 5- to 6-week-old NSG-SGM3 mice (500,000 per mouse) after sub-lethal total body irradiation (800 rads). Peripheral blood was drawn from the tail vein at relevant time points and monitored for engraftment via flow cytometry for human CD45 and CD19 expression. When circulating leukemic cells were observed (3 weeks from initial injection), engrafted mice were treated twice weekly for 2 weeks with DMSO or PLK1 inhibitor BI2536 or BI6727. Peripheral blood leukemia burden was monitored beginning with the first treatment, weekly for 4 weeks. At the end of the 4-week monitoring period, mice were euthanized and peripheral blood, femurs, and tibias harvested. Animals were maintained and all animal experiments were performed under Institutional Animal Care and Use Committee with prior approval from the MD Anderson in compliance with the US Department of Health and Human Services guidelines.
Flow cytometry
Cells were stained with antibodies against human PLK1, total p53 and pMDM2 were quantified by flow cytometry (30). For intracellular staining, cells were fixed and permeabilized with BD Cytofix/Cytoperm (554714), stained with antibody for fourty minutes at room temeperature with gentle shacking, and run on a FACSCalibur. Annexin V staining was performed using standard methods (550475; BD Biosciences, San Jose, CA). FlowJo software (FlowJo LLC, Ashland, OR) was used for analysis.
CyTOF mass cytometry
Metal-tagged antibodies were provided by the MD Anderson Flow Cytometry Core, and samples were stained as described previously (31, 32), and run on a DVS Sciences CyTOF mass cytometer (Fluidigm, South San Francisco, CA). Samples were analyzed using Spanning Tree Progression of Density Normalized Events (SPADE) algorithm as described elsewhere (33).
Expression of relevant genes in ALL patient samples
mRNA expression levels in samples from 206 children with ALL (34 T-ALL, 172 B-ALL) were measured on Affymetrix U133A arrays. To identify significant differences, we used LIMMA (Linear Models for Microarray Analysis), the empirical Bayes t-test implemented in Bioconductor, and the Benjamini-Hochberg method of false discovery rate estimation. Data were deposited at the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession no. GSE26366.
Statistical analysis
Differences between treatment groups were analyzed by the Student t-test or multiple measures ANOVA to assess statistical significance. All in vitro experiments were performed in triplicate unless otherwise noted.
Results
All four Notch receptor homologs are expressed across B-ALL subtypes
Previous work from our laboratory (12, 13) demonstrated that Notch signaling results in growth arrest and apoptosis in B-cell malignancies. To better understand the expression of Notch receptors on B-ALL, we evaluated surface expression of Notch1-4 in patient samples with various Ph status by flow cytometry. Expression of Notch2 was consistently highest, while the other receptors were present at lower and variable levels (Fig 1a). Importantly, Notch receptors were present in both Ph-negative and Ph-like B-ALL samples (Fig 1a).
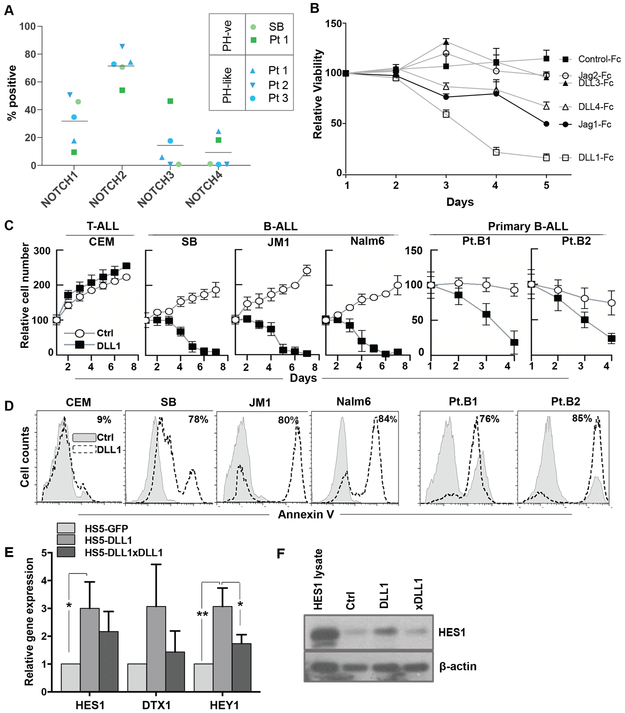
Figure 1. Notch receptors are expressed in B-ALL and Notch activation results in apoptosis.
(A) Percentage of Notch 1-4 receptor-positive cells on B-ALL patient samples and a representative cell line was determined by flow cytometry as the fraction of cells exceeding the intensity of the corresponding isotype control. Patients are represented by the same symbol for each receptor with PH-ve and PH-like subtypes discriminated by green and blue, respectively. Mean percentage for each receptor is represented by the horizontal bar. (B) B-ALL patient samples (n=3) were cultured on plate-bound human DLL1-Fc, DLL3-Fc, DLL4-Fc, Jagged1-Fc, Jagged2-Fc, and an IgG-Fc control. Viability was determined every 24 hours as the percentage of cells PI positive by flow cytometry. (C) Cell lines representing T-ALL (CEM) and B-ALL (SB, JM1, Nalm6) and samples from Ph-like B-ALL patients (Pt.B1, Pt.B2) were co-cultured on human bone marrow stromal HS5 cells expressing GFP (control) or DLL1. Viability was determined every 24 hours by trypan blue (n=3). (D) Representative histograms of Annexin V positivity in the same cells co-cultured with HS5 cells expressing DLL1 or control at 96 hours. (E) RNA expression of Notch downstream genes HES1, DTX1, and HEY1 in B-ALL patient samples (n=3) co-cultured for 24 hours on HS5 cells expressing GFP or DLL1; with and without a DLL1 blocking antibody, was assessed by qRT-PCR. (F) A representative western blot from the same experiment depicting HES1(abcam ab) at the protein level.
Activation of Notch signaling has anti-leukemic effects in B-ALL
Given the presence of all Notch receptors and the variety in receptor distribution, we next sought to determine whether cell death would be preferentially induced by individual Notch ligands. Plate-bound DLL1-Fc, Jagged1-Fc, or DLL4-Fc were all able to decrease viability in B-ALL patient samples (Fig 1b). In contrast, culture with DLL3-Fc or Jagged2-Fc had limited impact on cell viability. Notably, no ligand induced B-ALL cell growth (Fig 1b). Although several Notch ligands could induce B-ALL cell death, we selected the most potent ligand, DLL1, as our model ligand. Annexin V positivity was observed in a dose-dependent manner when patient samples were cultured with increasing concentrations of DLL1-Fc (Fig S1a). Similarly, B-ALL cell lines and patient samples co-cultured with human stromal cells overexpressing DLL1 (HS5-DLL1) exhibited decreased growth (Fig 1c) and increased apoptosis (Fig 1d) compared to those cultured with an HS5-GFP control. In line with previous work, this effect was not seen in a T-ALL cell line (Fig 1c,d). In support of active Notch signaling, B-ALL cells cultured on HS5-DLL1 demonstrated a consistent increase in Notch downstream target genes HES1, HEY1, and DTX1 mRNA (Fig 1e) and in HES1 protein (Fig 1f) when compared to control co-cultures. The addition of a DLL1-blocking antibody (xDLL1) to co-culture mitigated the induction of HES1 protein expression (Fig 1f), suggesting that the additional HES1 expression was due to the additional DLL1 ligand on these HS5 cells (HS5-DLL1). Induction of HES1 expression was seen across B-ALL cell lines and patient samples (Fig S1b). Together, these results illustrate that ligand-mediated Notch activation is capable of suppressing growth and survival of B-ALL.
Notch activation results in decreased PLK1 expression in a B-ALL-specific manner
Previous work showed that B-ALL cell death was induced by expression of the intracellular domain of any of the four Notch receptors (12). Here, we demonstrate that at least three recombinant Notch ligands induced significant B-ALL cell death (Fig 1b). These observations suggest that B-ALL cells apoptose via a common Notch-mediated mechanism, which is potentially shared to different degrees by many Notch ligand-receptor pairs. Given the challenge of translating Notch-activating therapeutics, we sought to target the downstream mechanism of Notch activation in B-ALL cells. PLK1, a putatively oncogenic kinase, is stabilized by a negative regulator of Notch in melanoma (34). Hematologic malignancies, including ALL, reportedly express high levels of PLK1 (35, 36). We thus chose to examine whether the inverse was true—namely, if Notch activation was a negative regulator of PLK1 expression in B-ALL cells, ultimately leading to apoptosis.
Flow cytometry demonstrated that our B-ALL cell lines and patient samples did express PLK1 (Fig 2a,c). While expression was observed across B-ALL samples of various Ph-status, PLK1 was highest in Ph-like B-ALLs (Fig 2b,d). Upon co-culture with HS5-DLL1 cells, B-ALL cell lines and patient samples demonstrated marked reduction in the expression of PLK1 protein in the context of activation of Notch signaling, supported by the appearance of cleaved intracellular Notch2 (ICN2) and the common Notch target gene HES1 (Fig 2e,g). Notably, Ph-like samples had the greatest reduction in PLK1 in response to DLL1-Notch activation (Fig 2d). Expression of the intracellular domain of any of the four Notch receptors was capable of decreasing PLK1 expression (Fig 2f), although ICN2 and ICN1 elicited the greatest reductions, and ICN4 only had a marginal decrease. These results are consistent with prior work demonstrating that expression of ICN1-4 could induce B-ALL cell death (12), and the activating potencies of Notch receptors, i.e., ICN1=ICN2>ICN3>ICN4.
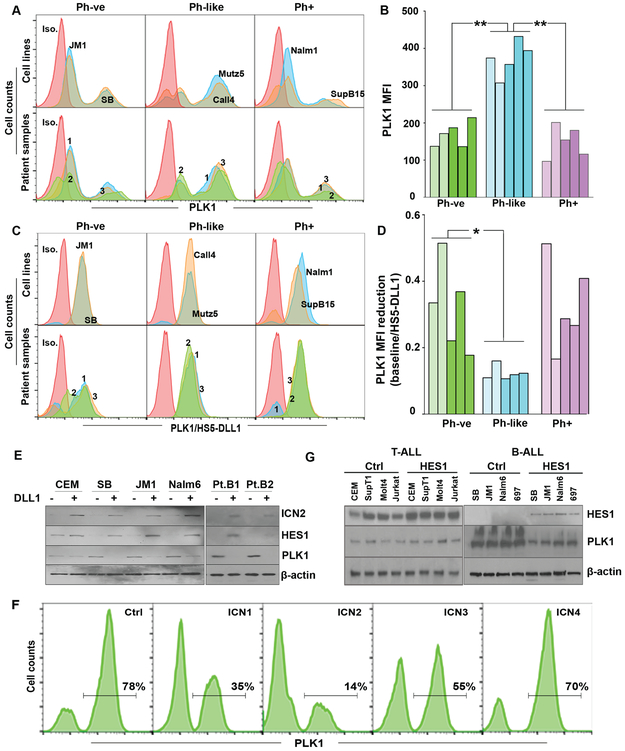
Figure 2. PLK1 is expressed in B-ALL and is suppressed by Notch activation.
(A) Intracellular PLK1 expression in cell lines (top panels) and patient samples (bottom panels) representing the 3 B-ALL subsets; PH-ve, PH-like, and PH+, was assessed by flow cytometry and (B) median fluorescence intensity (MFI) was summarized. Pale and dark shades represent cell lines and patient samples, respectively, within each subset. PH-like cells had significantly higher PLK1 MFI when compared to both PH-ve and PH+ cells (p<0.01) using a one-way ANOVA with post-hoc Tukey’s test. (C) Intracellular PLK1 expression in the same cells was measured after 48 hours of co-culture with HS5 cells expressing DLL1. (D) Summary of the relative reduction of PLK1 MFI after co-culture with HS5-DLL1 compared to baseline. PLK1 MFI reduction was significantly greater in PH-like when compared to PH-ve cells (p<0.05) using a one-way ANOVA with post-hoc Tukey’s test. (E) T-ALL (CEM; a HES1-positive control) and B-ALL cell lines along with two representative Ph-like B-ALL patient samples (Pt.B1, Pt.B2) were assessed by Western blot for cleaved intra-cellular Notch 2 (ICN2), HES1(abcam ab), PLK1, and β-actin after co-culture on HS5 cells expressing either GFP or DLL1 for 48 hours. (F) Representative flow plots of PLK1 expression from the B-ALL cell line, SB, expressing intra-cellular Notch domains 1-4 (ICN1-4) or a GFP control 48 hours post induction. (G) T-ALL (CEM, SupT1, Molt4, Jurkat) and B-ALL (SB, JM1, Nalm6, 697) cells were transduced with GFP control or HES1. HES1(Origene ab) and PLK1 protein expression in transduced cells was determined by Western blot.
Importantly, overexpression of HES1, the most commonly reported Notch downstream target gene, was sufficient to decrease PLK1 protein expression in B-ALL cell lines, but not in T-ALL cell lines (Fig 2g), suggesting that the Notch/HES-mediated downregulation of PLK1 occurs in a B-ALL specific manner.
PLK1 is a survival kinase in B-ALL that mediates p53 suppression
Given these observations that Notch activation downregulates PLK1 expression, we sought to determine whether direct downregulation of PLK1 expression was sufficient to inhibit growth and survival in patient B-ALL cells. B-ALL with shPLK1 exhibited a significant decrease in viability, but this effect was not seen in T-ALL (Fig 3a,b). This reduction in cell viability with partial PLK1 downregulation suggests the importance of PLK1 in the survival of B-ALL cells.
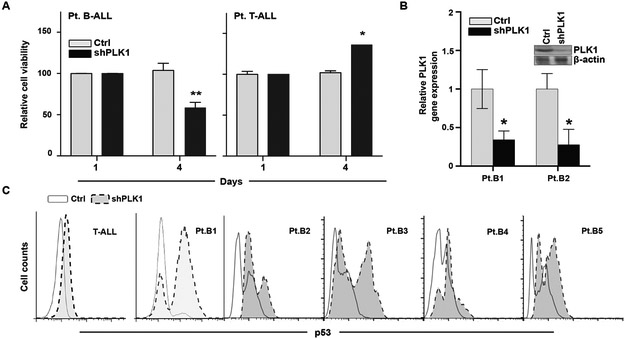
Figure 3. PLK1 inhibition promotes B-ALL specific cell death and is associated p53 accumulation.
(A) Primary cells from B-ALL (left) and T-ALL (right) patients (each n=3) were transduced with doxycycline- inducible PLK1 or scrambled shRNAs and cultured in low-dose doxycycline (2 μg/mL). Cell viability was determined on days 1 and 4 by trypan blue staining. (B) The PLK1-shRNA knockdown was confirmed both at the RNA and protein level by qRT-PCR and Western blot, respectively. PLK1 RNA was significantly reduced in both B-ALL patient samples (p<0.05, n=3) by two sample t-tests. (C) Representative flow cytometry data summarizing the differences in intracellular p53 expression from a T-ALL and 5 B-ALL patient samples transduced with the PLK1 or scrambled shRNA after 48 hours.
In other malignancies, PLK1 is proposed to exert oncogenic functions by suppressing the pro-apoptotic function of p53 (20, 37). In contrast, p53 can repress transcription of PLK1 in response to DNA damage (38). To explore this inverse relationship between PLK1 and p53, we evaluated p53 expression in B-ALL samples following PLK1 knockdown. p53 expression was significantly increased in shPLK1 cells compared to control, and this effect was not seen in T-ALL cells (Fig 3c). Together, this demonstrates that PLK1 is required for survival in B-ALL, and may exert its effects in part through suppression of p53.
Notch-induced loss of PLK1 stabilizes p53 through MDM2 hypophosphorylation
PLK1 has been shown to modulate p53 stability and function via phosphorylation of MDM2 at Ser260, resulting in p53 degradation (21). To evaluate whether these PLK1-p53 relationships are maintained in B-ALL, we tested the effect of activated Notch signaling on pMDM2Ser260 and p53 levels in B-ALL cell lines and B-ALL patient samples. Notch ligand-mediated activation dramatically decreased pMDM2Ser260 without altering total MDM2 levels, and markedly increased p53 protein levels (Fig 4a). Importantly, inhibition of Notch receptor cleavage with DAPT, a gamma-secretase inhibitor that acts as a non-specific inhibitor of Notch activation, could abrogate the majority of the p53 accumulation (Fig 4b) and attenuate HES1 induction in the context of Notch ligand exposure (Fig 4c), supporting the hypothesis that this mechanism is driven by canonical Notch signaling.
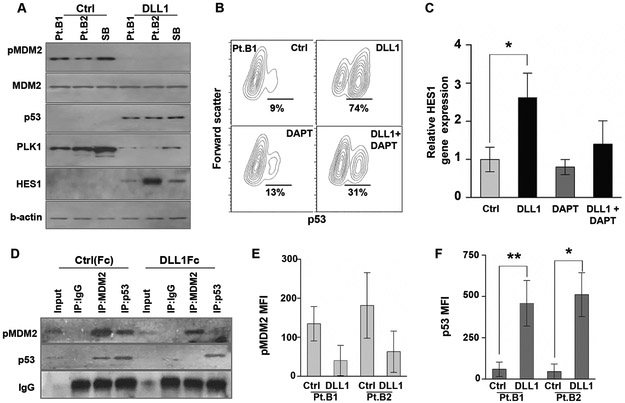
Figure 4. PLK1 inhibition prevents MDM2 phosphorylation and increases p53 accumulation in B-ALL.
(A) Ph-like B-ALL patient samples (Pt.B1, Pt.B2) and B-ALL cell line, SB, were co-cultured on HS5 cells expressing either the GFP-control or DLL1. MDM2 (phospho and total), p53, PLK1, HES1, and β-actin protein expression were quantified by Western blot. (B) Intracellular p53 was measured in primary B-ALL patient cells (Pt.B1) treated with soluble DLL1, γ-secretase (Notch) inhibitor DAPT (200 nM), or both for 48 h by flow cytometry. (C) Similarly, HES1 was quantified by qRT-PCR in the same primary B-ALL cells treated with DLL1, DAPT, or both, relative to untreated controls. Only the DLL1 treated cells had a significant increase in HES1 RNA expression (p<0.05) by one-way ANOVA with Dunnett’s post-hoc test. (D) A representative Ph-like B-ALL patient sample was cultured on either control (Fc) or DLL1 (DLL1Fc) plate-bound ligand for 48 hours. Cell lysates were immunoprecipitated with IgG control, MDM2, or p53 and the membrane was probed with pMDM2 and p53. (E,F) Similarly, intracellular pMDM2 and p53 was measured by flow cytometry for 2 B-ALL patient samples after 48 hours of culture on control (Fc) or DLL1 (DLL1Fc) plate-bound ligand. Intracellular p53 expression was significantly increased in both patient samples (p<0.05 and p<0.01) cultured on DLL1Fc when compared to their corresponding control cultures.
Adding further to this mechanism, co-immunoprecipitation assays in a B-ALL patient sample demonstrated loss of endogenous MDM2-p53 interaction following Notch activation (Fig 4d). Likewise, flow cytometry revealed a decrease in pMDM2Ser260 and a corresponding increase in p53 staining intensity in these B-ALL patient samples (Fig 4e,f). Together, these results demonstrate that Notch-mediated PLK1 downregulation leads to a significant reduction of pMDM2Ser260 and subsequent p53 stabilization. This regulation of the MDM2:p53 interaction through PLK1-mediated phosphorylation of MDM2 at Ser260 is a novel mechanism in B-ALL and provides insight into Notch-mediated effects in B-ALL while also highlighting PLK1 as a potential therapeutic target in B-ALL.
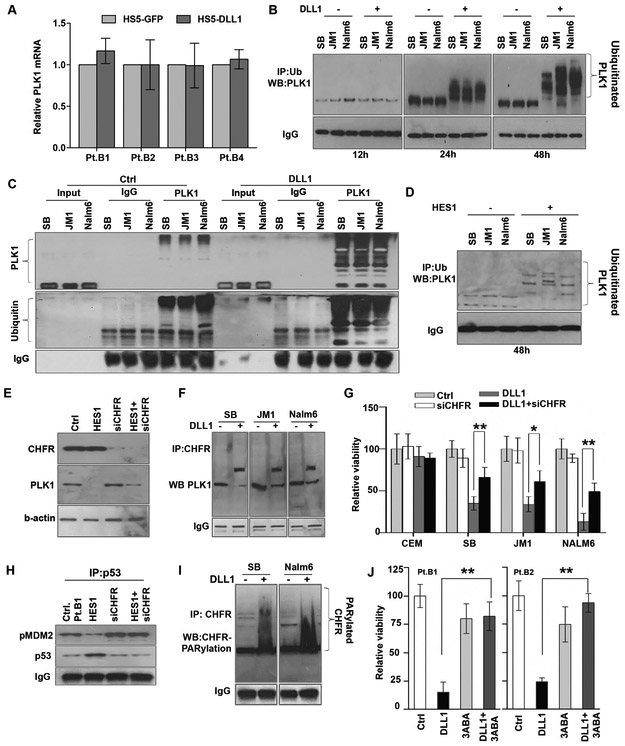
Notch-HES1 signaling promotes CHFR-mediated ubiquitination of PLK1
To further understand the mechanism of Notch/HES-mediated downregulation of PLK1, we examined PLK1 mRNA expression following exposure to Notch ligand and found no significant differences (Fig 5a), suggesting that Notch-mediated regulation of PLK1 protein levels occurs post-translationally. Since PLK1 is known to be ubiquitinated and subsequently degraded by the proteasome (39, 40), we tested whether PLK1 protein was ubiquitinated following Notch activation. Following Notch activation, PLK1 was ubiquitinated in B-ALL cells, with ubiquitination seen at 24 and 48 hours (Fig 5 b,c). PLK1 was ubiquitinated in the same cells following HES1 overexpression (Fig 5d), confirming the role for Notch signaling in the ubiquitination of PLK1.
Figure 5. Notch-mediated PLK1 ubiquitination involves PARylation of CHFR1 (checkpoint ubiquitin E3 ligase) in B-ALL.
(A) PLK1 mRNA expression was determined by qRT-PCR after co-culture with HS5-DLL1 or HS5-GFP for 48 hours (n=3). (B) B-ALL cell lines were treated with MG132 4 hours prior to various time points (12, 24, or 48 h) of co-culture with HS5-GFP or HS5-DLL1. Cell lysates were subjected to immunoprecipitation with ubiquitin and probed for PLK1. (C) Similary, after 48 hours of co-culture cells were treated with MG132 for 4 hours and harvested. Cell lysates were subjected to immunoprecipitation with PLK1 and blots were probed with PLK1 and ubiquitin. (D) The same panel of B-ALL cells was transduced with retrovirus-mediated HES1 for 48 hours to induce ectopic expression of the gene. The cells were treated with MG132 and the cell lysates subjected to immunoprecipitation with ubiquitin and probed for PLK1. (E) Primary B-ALL cells (Pt. B1) were either transduced with HES1 retrovirus, transfected with CHFR siRNA (siCHFR), or both for 24 hours; these cells and untreated controls were probed for CHFR and PLK1 expression. (F) Cell lysates from B-ALL cell lines co-culture with HS5-GFP or HS5-DLL1 were immunoprecipitated with CHFR and probed for PLK1. (G) T-ALL cells (CEM) and B-ALL cells (SB, JM1, Nalm6) were either co-cultured on control or DLL1-expressing HS5 cells and subjected to CHFR depletion via siRNA for 4 days. Viability was determined by trypan blue. (H) Cell lysates from (E) were immunoprecipitated with p53, and blots were probed with pMDM2(ser260) and p53. (I) B-ALL cells were co-cultured on control or DLL1-expressing HS5 cells for 48 hours, and cell lysates were immunoprecipitated with CHFR and resolved on 4-15% non-denaturing gradient gels; the membrane was probed for polyADP ribosylation (PAR). (J) Cells from patients with Ph-like B-ALL (Pt.B1, Pt.B2) were co-cultured on control or DLL1-expressing HS5 cells and treated for 48 hours with the PARP inhibitor, 3ABA. Viability was determined by trypan blue. *p<0.05; **p<0.01.
Because the E3 ligase CHFR has been reported to target PLK1 for ubiquitination (40), we sought to investigate whether CHFR was important for PLK1 ubiquitination in B-ALL. Indeed knockdown of CHFR impaired HES1-mediated loss of PLK1 expression (Fig 5e), linking HES1 to CHFR and PLK1. We then confirmed an interaction between PLK1 and CHFR via immunoprecipitation of endogenous proteins in B-ALL cell lines (Fig 5f). To determine whether CHFR was necessary for the Notch-mediated growth inhibition in B-ALL, we partially knocked down CHFR via siRNA and observed partial rescue of Notch ligand-mediated reductions in B-ALL cell counts, but not in a T-ALL cell line (Fig 5g), suggesting an important role for CHFR in this mechanism. In line with the notion that this cell death is due, at least in part, to p53 stabilization, we sought to understand the role of CHFR in p53 stabilization in B-ALL. Indeed, CHFR knockdown prevented the loss of pMDM2Ser260 and the stabilization of p53 in B-ALL patient cells (Fig 5h).
Notch-mediated PLK1 degradation involves PARylation of CHFR
Our previous work demonstrated that HES1 activates PARP1, resulting in global poly-ADP-ribosylation (PARylation) in B-ALL (13). Following exposure to DLL1, CHFR was found to be PARylated in B-ALL cells (Fig 5i). Addition of a PARP1 inhibitor (3ABA) rescued the majority of Notch ligand-mediated growth inhibition in B-ALL samples (Fig 5j), underscoring the importance of CHFR and PARP activity in this mechanism of Notch-mediated growth inhibition.
The experiments described above reveal a novel mechanism of Notch/HES-mediated PARP/CHFR-mediated PLK1 loss and subsequent dysregulation of MDM2/p53 leading to growth arrest and apoptosis in B-ALL cells. However, as Notch ligands/agonists are a challenge to translate into a clinical therapeutic, the remainder of the manuscript focuses on the use of PLK1 inhibitors as a clinically available method to mimic Notch-mediated apoptosis in B-ALL.
Pharmacologic PLK1 inhibition mimics Notch-induced effects in B-ALL
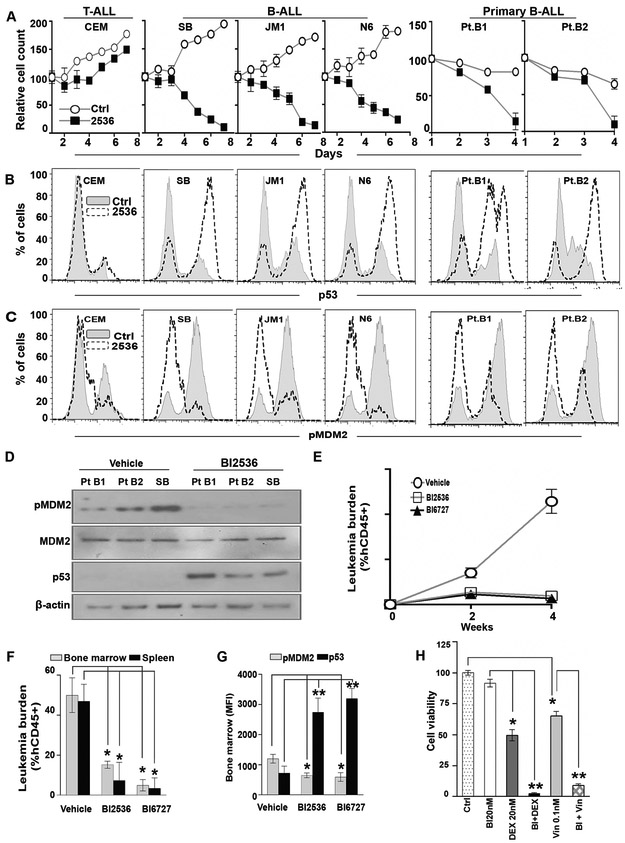
The PLK1 inhibitor BI2536 dramatically decreased cell growth of B-ALL cell lines and patient samples, but had little effect on a T-ALL cell line (Fig 6a). Consistent with the Notch/HES-mediated mechanism revealed above, exposure to BI2536 resulted in a decrease in pMDM2Ser260 and subsequent p53 stabilization in B-ALL, but not T-ALL (Fig 6b,c). These results were further corroborated by western blot (Fig 6d). These data confirm the importance of this PLK1/MDM2/p53 mechanism in B-ALL and demonstrate the therapeutic potential of PLK1 inhibition in this disease.
Figure 6. Pharmacologic inhibition of PLK1 shows anti-leukemia activity in Ph-like B-ALL.
(A) Cells representing T-ALL (CEM) or B-ALL (SB, JM1, Nalm6 [N6]) and primary samples from patients with Ph-like B-ALL were treated with a vehicle control or PLK1 inhibitor, BI2536 (100 nM), for 1 week. Cells were counted by trypan blue daily and the counts normalized the seed number. (B, C) Intracellular p53 (B) and pMDM2 (ser260) (C) for all cells in (A) were determined by flow cytometry at 48 hours. (D) Similarly, cell lysates were collected at the same time point and probed for pMDM2(ser260), MDM2, p53, and β-actin. (E) NSG-SGM3 mice engrafted with cells from a patient with Ph-like B-ALL (Pt.B1) were treated, beginning 3 weeks after initial tail vein injection, with vehicle (control), PLK1 inhibitor BI2536 (15 mg/kg), or BI6727 (volasertib; 15 mg/kg) by gavage twice per week for 2 weeks (n=10 mice per group) and continuously monitored for 2 additional weeks. The leukemia burden was measured weekly in the peripheral blood by hCD45 staining. (F) Mice were euthanized 4 weeks after beginning treatment and the leukemia burden was measured in the bone marrow and spleen by hCD45 staining. (G) Intracellular pMDM2 and p53 levels in the bone marrow of the treated mice were quantified by flow-based analysis. (H) SB B-ALL were treated with various combinations of dexamethasome (DEX) or vincristine (Vi) and PLK1 inhibitor BI2536 (BI) for 3 days (n=3 per treatment group). Cell viability was determined by alamar blue. *p<0.05; **p<0.01.
Therapeutic PLK1 inhibition demonstrates anti-leukemia activity in B-ALL PDX models
To evaluate whether PLK1 inhibition reduces leukemia burden in vivo, we treated a Ph-like B-ALL patient-derived xenograft (PDX) mouse model with the PLK1 inhibitors BI2536 or BI6727 (volasertib). Strikingly, mice that received either PLK1 inhibitor had leukemic burden reductions of 70% in blood (Fig 6e), 90% in bone marrow, and 95% in spleen compared to mice receiving vehicle (Fig 6f). Analysis of bone marrow cells isolated from mice receiving BI2636 or BI6727 revealed a decrease in pMDM2Ser260 with concomitant p53 stabilization compared to vehicle-treated mice (Fig 6g). In addition to increased p53, mice that received BI6727 also had increased expression of the pro-apoptotic protein BAX in leukemia blasts, suggesting a further downstream mechanism of apoptosis (S2a-f). These data support the existence of a PLK1-MDM2-p53 mechanism in B-ALL and demonstrate the potential therapeutic efficacy of direct PLK1 inhibition in B-ALL.
PLK1 inhibition enhances efficacy of standard chemotherapeutics in B-ALL
While PLK1 inhibition appears to have marked anti-leukemic effects in B-ALL, single-agent therapies are only rarely utilized clinically. Thus, we evaluated whether addition of a PLK1 inhibitor would confer any additional benefit to standardly used chemotherapeutic agents. When combined with dexamethasone or vincristine, a low dose of BI2536 (5-fold lower than previous in vitro experiments) significantly reduced cell viability compared to any of the three agents alone (Fig 6h). This suggests that the anti-leukemia effects of dexamethasone and vincristine can be potentiated by PLK1 inhibition.
Levels of PARP1 and MDM2 transcripts are higher in B-ALL patient samples than in T-ALL patient samples
Our data suggest an important role for Notch-mediated PLK1 inhibition, occurring via PARylation of CHFR, hypophosphorylation of MDM2Ser260, stabilization of p53, and upregulation of pro-apoptotic proteins such as BAX. To evaluate whether this mechanism could be reasonably generalized across a larger subset of B-ALL, we analyzed data from 172 bone marrow samples from B-ALL cases (representing all major biologic subtypes) and 34 T-ALL cases compared with 9 leukemia-free donors. Figure S3 displays a heat map of the relative mRNA expression of the genes involved in this mechanism. Of the Notch receptors, transcript levels of NOTCH1 and NOTCH2 were highest, consistent with their relatively abundant surface expression (Fig 1a). HES1 levels were elevated in T-ALL, but were uniformly low in B-ALL, consistent with the constitutive Notch activation seen in many T-ALL cases, with low HES1 protein (Fig 1 e,f) and with our prior findings of a lack of constitutive Notch signaling in B-ALL (12, 13). CHFR and PLK1 were expressed at similar levels in B-ALL and T-ALL, though these transcript expression array data do not reflect the post-translational regulation described here. Likewise, TP53 levels appear similar between B-ALL and T-ALL. Importantly, both PARP1 and MDM2 transcripts were significantly higher in B-ALL than T-ALL, potentially revealing why B-ALL is sensitive to the PLK1-MDM2-p53 mechanism described here.
Discussion
The present study demonstrates that Notch ligand-mediated Notch activation induces B-ALL-specific growth inhibition and apoptosis, in part through PLK1 reduction. Our data demonstrate that PLK1 is ubiquitinated by CHFR, which is PARylated by PARP1 in response to Notch activation via HES1 expression. The decrease in PLK1 results in MDM2 hypophosphorylation, p53 stabilization, and upregulation of pro-apoptotic proteins (BAX).
It appears that multiple Notch ligands and receptors may contribute to this mechanism. DLL1 was used as the model ligand in these studies, though multiple Notch ligands were able to induce apoptosis (Fig 1b), suggesting that this mechanism is not specific to a single ligand. While patient samples had variable distribution of Notch receptors (Fig 1a,S3), each sample tested exhibited PLK1 downregulation and ultimately cell death in response to Notch activation. Likewise, expression of ICN1-4 or HES1 phenocopied Notch-ligand mediated PLK1 downregulation (Fig 2f,g), suggesting that any Notch receptor can induce this mechanism through HES gene upregulation. Finally, DAPT abrogated the effects of Notch activation on PLK1 downregulation in B-ALL (Figs 4c,d) suggesting that this mechanism relies on cleavage of the Notch receptors and canonical Notch signaling. Together, these observations allude to a general Notch-HES pathway effect, that is not exclusive to one ligand or receptor. Deciphering relative contributions of each Notch receptor or ligand in different patient samples may be helpful in further understanding the physiologic effects of Notch signaling in B-ALL. However, by therapeutically targeting the downstream mechanism via direct PLK inhibition, the contribution of Notch pathway becomes less relevant for the clinic.
In our current study, PLK1 inhibitor dramatically potentiated the effects of both vincristine and dexamethasone (Fig 6h). This may suggest that addition of a PLK1-targeted agent may reduce the exposure to chemotherapy necessary to achieve clinical response. To this end, PLK1 inhibitors, including volasertib (BI6727), have shown acceptable toxicity profiles and have had modest clinical response in combination with other agents for advanced solid tumors and acute myeloid leukemia (41-43). While PLK1 inhibition has not been used clinically in B-ALL, accumulating evidence supports a role for PLK1 in B-cell oncogenesis. In B-cell lymphoma, overexpression of PLK1 has been described as an independent prognostic factor (44). A recent publication has demonstrated a pivotal role for PLK1 in double-hit diffuse large B-cell lymphoma, and proposed combination therapy of PLK1 inhibition and venetoclax, a BCL2 inhibitor, in this disease (45). This aligns nicely with our current study, in which PLK1 inhibition upregulated p53 and the pro-apoptotic BAX protein, ultimately resulting in B-ALL growth arrest and apoptosis. Future studies using these combinations in B-ALL would certainly be of use given the widespread use and clinical efficacy of venetoclax (46, 47).
In this study, we utilized samples with wildtype p53. The majority of pediatric B-ALL presents with wildtype p53, though relapse is associated with a higher incidence of TP53 mutations (48). In adult B-ALL, p53 is mutated in ~15% of newly diagnosed cases (49-51). More frequently, other mechanisms of p53 inactivation—such as deregulation of MDM2 or ARF—occur (52, 53). This is also evident in our analysis of patient samples, where MDM2 levels were elevated (Fig S3). Hypophosphorylation of MDM2 by Notch-HES1-mediated PLK1 reduction appears to act as a means to re-activate wildtype p53, which results in upregulation of pro-apoptotic proteins, such as BAX (Fig S2). Future studies evaluating whether this mechanism remains intact when p53 is mutated in B-ALL would be worth exploring.
Previously, we showed that HES1 interacts with and activates PARP1 in B-ALL, resulting in nuclear translocation of apoptosis inducing factor (AIF), caspase cleavage, and apoptosis of B-ALL cells (13). In this study, we expand on these findings by further relating PARP1 to PARylation and activation of CHFR, which is required for the ubiquitination and subsequent degradation of PLK1 in B-ALL. These mechanisms are likely to work in concert with one another, which may explain why knockdown of CHFR only partially rescues DLL1-mediated reductions in B-ALL cell counts (Fig 5g). Therefore, a therapeutic means to selectively activate Notch in B-ALL cells may ultimately be more beneficial than PLK1 inhibition in isolation. The anti-leukemic activity of Notch signaling in B-ALL resides in striking contrast to the oncogenic Notch activation seen in various malignancies, including T-ALL (54, 55). This reasonably causes hesitation regarding the notion of developing Notch-activating therapeutics to treat B-ALL. Should such a venture be evaluated, ensuring specificity to B-lineage cells via conjugation of Notch ligand to an antibody raised against an antigen such as CD19, CD20, or CD22 may be useful.
Notch-activation induced B-ALL apoptosis appears to be a phenomenon observed across B-ALL subtypes (Fig 2). Intriguingly, this effect is particularly pronounced in Ph-like samples, representing a high-risk phenotype (Fig 2d). Likewise, Ph-like cell lines and patient samples had the highest PLK1 expression (Fig 2c), which may brand this subtype as particularly amenable to PLK1 inhibition. These compelling findings require further investigation.
In summary, this study demonstrated a role for active Notch/HES signaling in downregulating PLK1 in a PARP1/CHFR-dependent manner. This led to hypophosphorylation of MDM2, stabilization of p53, and upregulation of the pro-apoptotic protein BAX, ultimately resulting in B-ALL cell death. PLK1 inhibitors had marked pro-apoptotic effects in vitro and in PDX models of B-ALL. Together, these data strongly suggest that Notch activators or PLK1 inhibitors are viable therapeutic approaches in B-ALL that warrant clinical investigation.
Supplementary Material
Key Points:
Investigation of Notch-mediated anti-leukemia effects in B-ALL reveals PLK1 as a targetable therapeutic opportunity
Notch signaling decreases PLK1 expression in B-ALL through CHFR-mediated ubiquitination and degradation
Decreased PLK1 in B-ALL leads to apoptosis via hypophosphorylation of MDM2Ser260, stabilization of p53, upregulation of BAX
Acknowledgements
This work was supported by the National Cancer Institute (R01CA138816 to P.A. Zweidler-McKay) and by a research grant from Alex’s Lemonade Stand (ALSF ID# 4497 to J. Chandra). Additional funding support was provided by a Cancer Prevention & Research Institute of Texas (CPRIT: RP150006 to M. Konopleva) and by a Laboratory Incentive Fund from the Division of Pediatrics (to S. Kannan) and additional resources from Richard Gorlick, MD. Thanks to Dr. Michael Roth (Division of Pediatrics) and the Department of Scientific Publications (MD Anderson Cancer Center) for reviewing the manuscript. The authors are grateful to Drs. Michelle Barton, Dean of The University of Texas Graduate School of Biomedical Sciences, and Faye M Johnson, Associate Professor of Thoracic/Head and Neck Medical Oncology at MD Anderson Cancer Center, for helpful discussion about the manuscript and providing MDM2 and p53 reagents. The authors also are thankful to the Cellular Imaging Core, Department of Leukemia for their valuable support in analyzing the CyTOF samples with the support of the NIH/NCI through the MD Anderson Cancer Center Support Grant (CCSG) under award number P30CA016672.
Financial Support:
This research was supported by grants from National Cancer Institute (R01CA138816), Alex’s Lemonade Stand (ALSF ID# 4497), Cancer Prevention & Research Institute of Texas (CPRIT: RP150006), and Laboratory Incentive Fund from the Division of Pediatrics (MDACC).
Footnotes
Disclosure of Conflicts of Interest:
The authors declare no conflict of interest.
References
- 1.Kato M, Manabe A. Treatment and biology of pediatric acute lymphoblastic leukemia. Pediatr Int. 2018;60(1):4–12. [DOI] [PubMed] [Google Scholar]
- 2.Jabbour E, O’Brien S, Konopleva M, Kantarjian H. New insights into the pathophysiology and therapy of adult acute lymphoblastic leukemia. Cancer. 2015;121(15):2517–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mody R, Li S, Dover DC, Sallan S, Leisenring W, Oeffinger KC, et al. Twenty-five-year follow-up among survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. Blood. 2008;111(12):5515–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vrooman LM, Silverman LB. Treatment of Childhood Acute Lymphoblastic Leukemia: Prognostic Factors and Clinical Advances. Current hematologic malignancy reports. 2016;11(5):385–94. [DOI] [PubMed] [Google Scholar]
- 5.Mullighan CG, Collins-Underwood JR, Phillips LA, Loudin MG, Liu W, Zhang J, et al. Rearrangement of CRLF2 in B-progenitor- and Down syndrome-associated acute lymphoblastic leukemia. Nat Genet. 2009;41(11):1243–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roberts KG, Morin RD, Zhang J, Hirst M, Zhao Y, Su X, et al. Genetic alterations activating kinase and cytokine receptor signaling in high-risk acute lymphoblastic leukemia. Cancer cell. 2012;22(2):153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain N, Roberts KG, Jabbour E, Patel K, Eterovic AK, Chen K, et al. Ph-like acute lymphoblastic leukemia: a high-risk subtype in adults. Blood. 2017;129(5):572–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness KK, Armenian SH, Kadan-Lottick N, Gurney JG. Adverse effects of treatment in childhood acute lymphoblastic leukemia: general overview and implications for long-term cardiac health. Expert review of hematology. 2011;4(2):185–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, et al. Tisagenlecleucel in Children and Young Adults with B-Cell Lymphoblastic Leukemia. N Engl J Med. 2018;378(5):439–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kantarjian H, Stein A, Gokbuget N, Fielding AK, Schuh AC, Ribera JM, et al. Blinatumomab versus Chemotherapy for Advanced Acute Lymphoblastic Leukemia. N Engl J Med. 2017;376(9):836–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, et al. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zweidler-McKay PA, He Y, Xu L, Rodriguez CG, Karnell FG, Carpenter AC, et al. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005;106(12):3898–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannan S, Fang W, Song G, Mullighan CG, Hammitt R, McMurray J, et al. Notch/HES1-mediated PARP1 activation: a cell type-specific mechanism for tumor suppression. Blood. 2011;117(10):2891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maillard I, Fang T, Pear WS. Regulation of lymphoid development, differentiation, and function by the Notch pathway. Annu Rev Immunol. 2005;23:945–74. [DOI] [PubMed] [Google Scholar]
- 15.Hori K, Sen A, Artavanis-Tsakonas S. Notch signaling at a glance. Journal of cell science. 2013;126(Pt 10):2135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9(3):179–88. [DOI] [PubMed] [Google Scholar]
- 17.Weng AP, Ferrando AA, Lee W, Morris JPt, Silverman LB, Sanchez-Irizarry C, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306(5694):269–71. [DOI] [PubMed] [Google Scholar]
- 18.Aster JC, Pear WS, Blacklow SC. Notch signaling in leukemia. Annu Rev Pathol. 2008;3:587–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamagnone L, Zacchigna S, Rehman M. Taming the Notch Transcriptional Regulator for Cancer Therapy. Molecules. 2018;23(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ando K, Ozaki T, Yamamoto H, Furuya K, Hosoda M, Hayashi S, et al. Polo-like kinase 1 (Plk1) inhibits p53 function by physical interaction and phosphorylation. The Journal of biological chemistry. 2004;279(24):25549–61. [DOI] [PubMed] [Google Scholar]
- 21.Dias SS, Hogan C, Ochocka AM, Meek DW. Polo-like kinase-1 phosphorylates MDM2 at Ser260 and stimulates MDM2-mediated p53 turnover. FEBS Lett. 2009;583(22):3543–8. [DOI] [PubMed] [Google Scholar]
- 22.Liu Z, Sun Q, Wang X. PLK1, A Potential Target for Cancer Therapy. Transl Oncol. 2017;10(1):22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu X, Erikson RL. Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(10):5789–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pui JC, Allman D, Xu L, DeRocco S, Karnell FG, Bakkour S, et al. Notch1 expression in early lymphopoiesis influences B versus T lineage determination. Immunity. 1999;11(3):299–308. [DOI] [PubMed] [Google Scholar]
- 25.Privette LM, Gonzalez ME, Ding L, Kleer CG, Petty EM. Altered expression of the early mitotic checkpoint protein, CHFR, in breast cancers: implications for tumor suppression. Cancer Res. 2007;67(13):6064–74. [DOI] [PubMed] [Google Scholar]
- 26.Kannan S, Sutphin RM, Hall MG, Golfman LS, Fang W, Nolo RM, et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J Exp Med. 2013;210(2):321–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Purnell MR, Whish WJ. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980;185(3):775–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rudolph D, Steegmaier M, Hoffmann M, Grauert M, Baum A, Quant J, et al. BI 6727, a Polo-like kinase inhibitor with improved pharmacokinetic profile and broad antitumor activity. Clin Cancer Res. 2009;15(9):3094–102. [DOI] [PubMed] [Google Scholar]
- 29.Steegmaier M, Hoffmann M, Baum A, Lenart P, Petronczki M, Krssak M, et al. BI 2536, a potent and selective inhibitor of polo-like kinase 1, inhibits tumor growth in vivo. Current biology : CB. 2007;17(4):316–22. [DOI] [PubMed] [Google Scholar]
- 30.Krutzik PO, Trejo A, Schulz KR, Nolan GP. Phospho flow cytometry methods for the analysis of kinase signaling in cell lines and primary human blood samples. Methods Mol Biol. 2011;699:179–202. [DOI] [PubMed] [Google Scholar]
- 31.Nolo R, Herbrich S, Rao A, Zweidler-McKay P, Kannan S, Gopalakrishnan V. Targeting P-selectin blocks neuroblastoma growth. Oncotarget. 2017;8(49):86657–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Herbrich SM, Kannan S, Nolo RM, Hornbaker M, Chandra J, Zweidler-McKay PA. Characterization of TRKA signaling in acute myeloid leukemia. Oncotarget. 2018;9(53):30092–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu P, Simonds EF, Bendall SC, Gibbs KD Jr., Bruggner RV, Linderman MD, et al. Extracting a cellular hierarchy from high-dimensional cytometry data with SPADE. Nat Biotechnol. 2011;29(10):886–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmit TL, Nihal M, Ndiaye M, Setaluri V, Spiegelman VS, Ahmad N. Numb regulates stability and localization of the mitotic kinase PLK1 and is required for transit through mitosis. Cancer Res. 2012;72(15):3864–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ikezoe T, Yang J, Nishioka C, Takezaki Y, Tasaka T, Togitani K, et al. A novel treatment strategy targeting polo-like kinase 1 in hematological malignancies. Leukemia. 2009;23(9):1564–76. [DOI] [PubMed] [Google Scholar]
- 36.Hartsink-Segers SA, Exalto C, Allen M, Williamson D, Clifford SC, Horstmann M, et al. Inhibiting Polo-like kinase 1 causes growth reduction and apoptosis in pediatric acute lymphoblastic leukemia cells. Haematologica. 2013;98(10):1539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bussey KJ, Bapat A, Linnehan C, Wandoloski M, Dastrup E, Rogers E, et al. Targeting polo-like kinase 1, a regulator of p53, in the treatment of adrenocortical carcinoma. Clin Transl Med. 2016;5(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McKenzie L, King S, Marcar L, Nicol S, Dias SS, Schumm K, et al. p53-dependent repression of polo-like kinase-1 (PLK1). Cell cycle. 2010;9(20):4200–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim JS, Park YY, Park SY, Cho H, Kang D, Cho H. The auto-ubiquitylation of E3 ubiquitin-protein ligase Chfr at G2 phase is required for accumulation of polo-like kinase 1 and mitotic entry in mammalian cells. The Journal of biological chemistry. 2011;286(35):30615–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. The Journal of cell biology. 2002;156(2):249–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schoffski P, Awada A, Dumez H, Gil T, Bartholomeus S, Wolter P, et al. A phase I, dose-escalation study of the novel Polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer. 2012;48(2):179–86. [DOI] [PubMed] [Google Scholar]
- 42.Gjertsen BT, Schoffski P. Discovery and development of the Polo-like kinase inhibitor volasertib in cancer therapy. Leukemia. 2015;29(1):11–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dohner H, Lubbert M, Fiedler W, Fouillard L, Haaland A, Brandwein JM, et al. Randomized, phase 2 trial of low-dose cytarabine with or without volasertib in AML patients not suitable for induction therapy. Blood. 2014;124(9):1426–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu L, Zhang M, Zou P. Expression of PLK1 and survivin in diffuse large B-cell lymphoma. Leuk Lymphoma. 2007;48(11):2179–83. [DOI] [PubMed] [Google Scholar]
- 45.Ren Y, Bi C, Zhao X, Lwin T, Wang C, Yuan J, et al. PLK1 stabilizes a MYC-dependent kinase network in aggressive B cell lymphomas. J Clin Invest. 2018;128(12):5517–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 with Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med. 2016;374(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, Busman T, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov. 2016;6(10):1106–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hof J, Krentz S, van Schewick C, Korner G, Shalapour S, Rhein P, et al. Mutations and deletions of the TP53 gene predict nonresponse to treatment and poor outcome in first relapse of childhood acute lymphoblastic leukemia. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(23):3185–93. [DOI] [PubMed] [Google Scholar]
- 49.Chiaretti S, Brugnoletti F, Tavolaro S, Bonina S, Paoloni F, Marinelli M, et al. TP53 mutations are frequent in adult acute lymphoblastic leukemia cases negative for recurrent fusion genes and correlate with poor response to induction therapy. Haematologica. 2013;98(5):e59–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stengel A, Schnittger S, Weissmann S, Kuznia S, Kern W, Kohlmann A, et al. TP53 mutations occur in 15.7% of ALL and are associated with MYC-rearrangement, low hypodiploidy, and a poor prognosis. Blood. 2014;124(2):251–8. [DOI] [PubMed] [Google Scholar]
- 51.Kanagal-Shamanna R, Jain P, Takahashi K, Short NJ, Tang G, Issa GC, et al. TP53 mutation does not confer a poor outcome in adult patients with acute lymphoblastic leukemia who are treated with frontline hyper-CVAD-based regimens. Cancer. 2017;123(19):3717–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mullighan CG, Phillips LA, Su X, Ma J, Miller CB, Shurtleff SA, et al. Genomic analysis of the clonal origins of relapsed acute lymphoblastic leukemia. Science. 2008;322(5906):1377–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Iacobucci I, Ferrari A, Lonetti A, Papayannidis C, Paoloni F, Trino S, et al. CDKN2A/B alterations impair prognosis in adult BCR-ABL1-positive acute lymphoblastic leukemia patients. Clin Cancer Res. 2011;17(23):7413–23. [DOI] [PubMed] [Google Scholar]
- 54.Ferrando AA, Herblot S, Palomero T, Hansen M, Hoang T, Fox EA, et al. Biallelic transcriptional activation of oncogenic transcription factors in T-cell acute lymphoblastic leukemia. Blood. 2004;103(5):1909–11. [DOI] [PubMed] [Google Scholar]
- 55.Demarest RM, Ratti F, Capobianco AJ. It’s T-ALL about Notch. Oncogene. 2008;27(38):5082–91. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.