Abstract
Somatic frameshift mutations in the calreticulin (CALR) gene are key drivers of cellular transformation in myeloproliferative neoplasms (MPN). All patients carrying these mutations (CALR+ MPN) share an identical sequence in the C-terminus of the mutated CALR protein (mut-CALR), with the potential for utility as a shared neoantigen. Here we demonstrate that while a subset of CALR+ MPN patients develops specific T cell responses against mut-CALR C-terminus, programmed cell death protein 1 (PD-1) or cytotoxic T lymphocyte antigen 4 (CTLA-4) expression abrogates the full complement of responses. Significantly, blockade, ex vivo by monoclonal antibodies and in vivo by pembrolizumab administration, restores mut-CALR-specific T cell immunity in some CALR+ MPN patients. Moreover, mut-CALR elicits antigen-specific responses from both CD4+ and CD8+ T cells, confirming its broad applicability as an immunogen. Collectively, these results establish mut-CALR as a shared, MPN-specific neoantigen and inform the design of novel immunotherapies targeting mut-CALR.
Keywords: Myeloproliferative neoplasms, Calreticulin, Neoantigens, Immune checkpoint blockade, Immunotherapy
Introduction
Immune-based therapies have revolutionized the treatment of cancer resulting in unprecedented response rates and even complete remission (1,2). Inhibition of immune checkpoint receptors, such as programmed cell death protein 1 (PD-1) and cytotoxic T lymphocyte antigen 4 (CTLA-4), in particular, have proved successful in the treatment of several different tumor types, and their combination with other modalities is also gaining approval. Blockade of PD-1 and CTLA-4 signaling, either alone or in combination, has been reported to reinvigorate tumor antigen-specific T cells, thereby ameliorating antitumor activity (3,4). The clinical efficacy of these immunotherapies is correlated in part with mutation load and is likely based on restoring T cell recognition of neoantigens, non-self antigens that arise from somatic mutations in tumors, in the context of major histocompatibility complex (MHC) molecules (3,5–8). Neoantigen-specific T cells are not subject to immune tolerance, and hence, they have the potential to exhibit strong effector responses specifically against malignant cells. However, due to intertumoral heterogeneity of mutations, neoantigen-based immunotherapies for the most part remain personalized and limited to individual patients (9,10).
BCR-ABL1-negative myeloproliferative neoplasms (MPN), which include polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (MF), are chronic hematological malignancies that are characterized by hyperproliferation of blood cells. The initiation and progression of MPN are associated with recurrent somatic driver mutations in Janus kinase 2 (JAK2), the thrombopoietin receptor gene MPL and calreticulin (CALR). 67% of ET and 88% of MF patients, who lack JAK2 and MPL mutations, carry mutations in the exon 9 of CALR gene (11,12). Mut-CALR mediates transformation by activating JAK-STAT signaling through its binding to MPL (13,14). Unlike JAK2 and MPL mutations, CALR mutations are frameshift mutations. To date, over 50 types of insertions or deletions in the exon 9 of CALR gene have been reported (15). All of these mutations lead to a +1 shift in the open reading frame and result in the formation of an altered C-terminus with an identical 36-amino acid (aa) sequence that is shared by all CALR+ MPN patients (11). The two most frequent mutation types, L367fs*46 (Type 1) and K385fs*47 (Type 2), found in 80% of CALR+ MPN patients, share a 44-aa sequence (16).
Except for hematopoietic stem cell transplantation, curative treatments for MPN patients are not available (17). Therefore, it is imperative to identify more effective treatment options for these patients. We hypothesized that the uniformity of mut-CALR marks it as an attractive MPN-specific, shared neoantigen candidate that could be targeted for the development of immunotherapy regimens for CALR+ patients. The shared mut-CALR C-terminus is at least 36-aa long and exhibits limited similarity to wild type (WT) CALR (16). Hence, mut-CALR could incorporate multiple epitopes, which if presented on MHC could elicit anti-tumor T cell responses with minimal cross-reactivity to WT protein expressed on nonmalignant cells. We therefore evaluated the immunogenicity of mut-CALR and found that a subset of CALR+ MPN patients indeed develops specific T cell responses against mut-CALR that can be detected in vitro.
We also considered that an exhausted state, driven by chronic antigen exposure, might blunt the detection of mut-CALR responses in some patients. Therefore, we determined whether the expression of checkpoint molecules, namely PD-1 and CTLA-4, regulated mut-CALR-specific T cell immunity. We report here that blockade of PD-1 and CTLA-4 signaling in CALR+ MPN patients led to both in vivo and ex vivo clonal expansion of T cells recognizing mut-CALR. Together, our results support the development of immunotherapy approaches targeting mut-CALR, either in the form of neoantigen-specific vaccines or adoptive T cell therapies for elimination of malignant clones and also provide a rationale for testing immune-checkpoint blockade in CALR+ MPN patients.
Results
T cells from MPN patients recognize shared neopeptides originating from somatic frameshift mutations in calreticulin
Patients carrying frameshift mutations in the CALR gene share an identical 36 aa sequence in the C-terminus of the mut-CALR protein (11). This novel sequence exhibits limited similarity to the WT CALR (16), is not expressed elsewhere and hence not subject to immune tolerance. Therefore, we hypothesized that mut-CALR as a neoantigen would elicit specific T cell responses that exhibit minimal cross-reactivity to the WT CALR. To assess whether this neoantigen could potentially elicit a cellular immune response, we investigated T cell responses against mut-CALR neopeptide in peripheral blood mononuclear cells (PBMCs) from a cohort of MPN patients carrying CALR mutations. 18 MPN patients with CALR+ ET (n=7), MF arising from ET (ET-MF) (n=7) or primary MF (n=4) (table S1) were assessed for underlying mut-CALR-specific immunity. To this end, PBMCs from CALR+ MPN patients were stimulated in vitro with pooled overlapping long peptides (OLPs; 14-15 aa) spanning the mutated (fig. S1A) or WT (fig. S1B) CALR C-terminus. After expansion, the cells were re-stimulated with the OLP pools and IFN-γ production was measured by ELISPOT (Fig. 1A) (18). A significant increase in IFN-γ production was observed when the cells were stimulated with mut-CALR OLPs as compared to WT OLPs that spanned the C-terminus tail of the protein (Fig. 1B–C). Of note, mut-CALR-induced IFN-γ production was observed with a greater frequency in ET, but not in primary MF patients (fig. S2A–C). Next, we characterized the mut-CALR-specific T cell subsets in MPN patients by intracellular staining. Due to limitations in cell numbers, we were able to analyze 11 of the 18 patients in our cohort. Mut-CALR-induced IFN-γ production, identified by comparing stimulation with mut-CALR OLPs and control peptides derived from myelin oligodendrocyte glycoprotein (MOG), was observed primarily in CD4+ T cells (Fig. 1D–E). These results demonstrated that T cells from CALR+ MPN patients could react to mut-CALR. Yet, as T cells in these assays were stimulated with mut-CALR OLPs prior to expansion and immunogenicity evaluation, it remains possible that T cells from CALR+ MPN patients were primed in vitro. In order to assess directly whether in vivo T cell priming has occurred in CALR+ MPN patients, we performed ex vivo T cell ELISPOT assays using PBMCs from a total of 19 JAK2V617F+ and 22 CALR+ MPN patients. PBMCs from MPN patients were stimulated with mut-CALR OLPs or control peptides and mut-CALR-specific T cell responses were monitored after 48 hours. No mut-CALR-specific T cell responses were detected ex vivo (fig. S2D), suggesting either a lack of spontaneous mut-CALR-specific T cell immunity in CALR+ MPN patients or limitations in the detection of responses in non-expanded cells due to a low frequency of mut-CALR-specific T cells.
Fig. 1.
T cell immunity against mut-CALR in MPN patients. (A) Overview of the T cell immunogenicity assay used to evaluate antigen-specific T cell responses. PBMCs from CALR+ MPN patients were expanded in vitro following stimulation with WT or mut-CALR OLPs. Stimulation with CEFT pool was used as control. Expanded T cells were re-stimulated with either the peptide pool they were expanded with or the control peptide pool MOG. Representative ELISPOT images (B) and summary of ELISPOT results (C) generated in PBMCs from 18 CALR+ MPN patients. Each data point represents one MPN patient. Statistical significance was evaluated by Wilcoxon signed-rank test: *, p=0.0327. Representative flow cytometry plots (D) and summary of intracellular staining analysis for IFN-γ in CD4 and CD8 T cell subsets of 11 CALR+ MPN patients (E). Statistical significance for MOG vs mut-CALR OLPs was evaluated by Wilcoxon signed-rank test. P values were 0.0113 and 0.3223 for CD4+ and CD8+ T cells, respectively. The spot numbers and % IFN-γ values were calculated by subtracting the values obtained after MOG stimulation from the values after OLP pool stimulation and negative values were set to zero. Horizontal lines indicate the mean.
CALR protein is critical for the folding and assembly of MHC class I molecules and CALR-deficient cells are reported to have reduced levels of cell surface MHC class I expression as well as reduced efficiency in antigen presentation (19,20). Therefore, we examined whether the presence of CALR mutations in MPN patients was associated with a decrease in cell surface expression of MHC molecules. Both MHC class I and II molecules were expressed by patient PBMCs (fig. S3A–B), and the expression of MHC class I molecules was significantly greater in CALR+ MPN PBMCs than in healthy donor (HD) PBMCs (fig. S3A). The pro-inflammatory milieu that characterizes the MPNs (21), as well as certain treatment modalities, such as IFN-α, among MPN patients (22,23) may contribute to the increased MHC class I expression. Since some of the patients in our cohort have been treated with IFN-α, we assessed the role of IFN-α treatment in modulating surface MHC class I expression levels. To this end, we treated cells from a CALR+ MPN patient-derived cell line (24), with IFN-α and observed a dose dependent increase in MHC class I expression (fig. S3C). Importantly, CD34+ cells of MPN patients, which may harbor CALR mutations (25), also expressed MHC molecules (fig. S3D–F). These observations suggest that the paucity of anti-mut-CALR responses in CALR+ MPN patients was not due to a reduction of MHC class I expression by PBMCs or CD34+ cells. Altogether, our results indicate that a subset of CALR+ MPN patients develops T cell responses that are specific to mut-CALR, which are primarily of the CD4+ T cell phenotype.
Blockade of checkpoint receptors in vitro can restore mut-CALR-specific immune responses
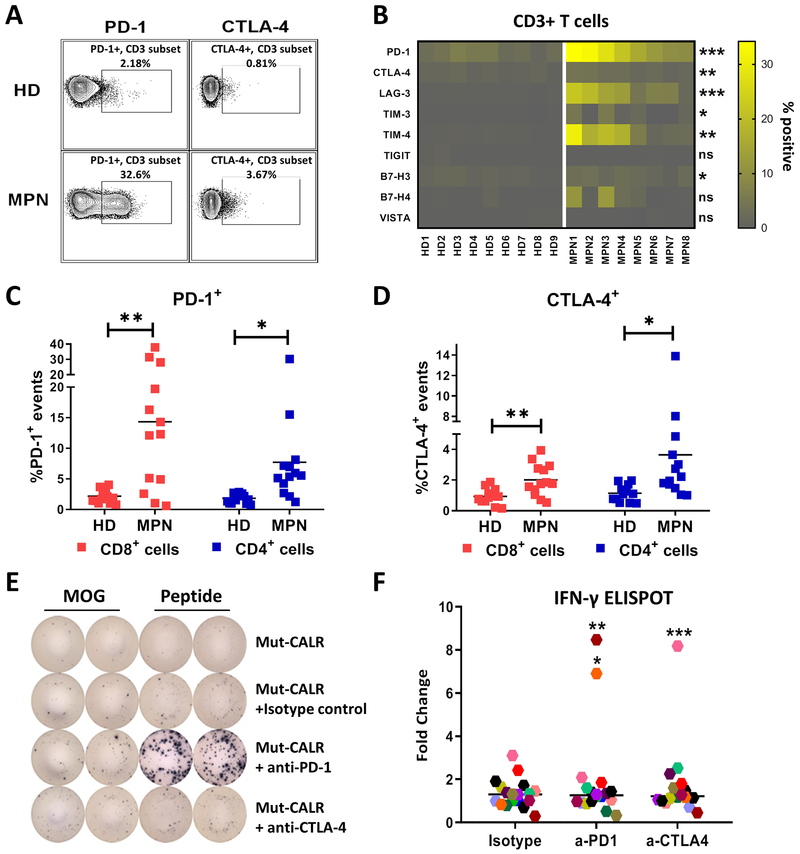
Although we observed mut-CALR-specific T cell responses in CALR+ MPN patients, the responses were found only in a subset of study subjects and were of low frequency in peripheral blood. Therefore, we considered the possibility that antigen-specific T cells were undergoing exhaustion due to chronic antigen exposure. We, therefore, evaluated the surface expression levels of a number of immune checkpoint receptors (PD-1, CTLA-4, LAG-3, TIM-3, TIM-4, TIGIT, B7-H3, B7-H4 and VISTA) on PBMCs of CALR+ MPN patients. We found that MPN T cells exhibited greater degrees of expression of multiple cell surface inhibitory molecules compared to HD T cells (Fig. 2A–B). Among these checkpoint receptors, PD-1 displayed the highest proportional increase (19.2±10% in MPN vs. 3.62±1.69% in HD), with expression detected in both CD8+ and CD4+ T cell subsets (Fig. 2C). Similarly, increased CTLA-4 expression was also detected in both T cell subsets (Fig. 2D). Based on this, we hypothesized that the elevated PD-1 and CTLA-4 expression could play a role in suppressing mut-CALR-specific T cell responses. Accordingly, we re-examined mut-CALR-specific T cell responses in CALR+ MPN PBMCs in the context of PD-1 or CTLA-4 blockade. PD-1 or CTLA-4 signaling on T cells was inhibited by monoclonal blocking antibodies and mut-CALR-specific T cell immunity was evaluated by ELISPOT, as described in Fig. 1A–C. T cell responses against mut-CALR OLPs were recovered in 3 CALR+ MPN. These cells now produced IFN-γ when PD-1 or CTLA-4 signaling was blocked (Fig. 2E–F). In addition to IFN-γ, cells produced multiple other cytokines including TNF, suggesting that mut-CALR-specific T cells are polyfunctional (fig. S4). When pre and post-checkpoint inhibition responses were combined, 11 of the 18 patients in the MPN cohort exhibited mut-CALR-specific T cell immunity.
Fig. 2.
T cells from MPN patients are exhausted and blockade of checkpoint receptors restore mut-CALR-specific T cell immunity in vitro. (A) Representative flow cytometric analyses showing PD-1 and CTLA-4 expression in peripheral blood T cells from CALR+ MPN patients and HD. (B) Summary of flow data for cell surface expression of checkpoint receptors, listed on the left, in HD and MPN T cells (n=9 and 8, respectively). Each cell corresponds to one HD or MPN patient. The color intensity indicates the % expression for each checkpoint receptor as gated under live, CD3+ cells. Statistical significance of MPN vs HD for each checkpoint receptor was evaluated by t test. PD-1: *** p=0.0002, CTLA-4: ** p=0.0037, LAG-3: *** p=0.0004, TIM-3: * p=0.0217, TIM-4: ** p=0.0051, TIGIT: ns p=0.2433, B7-H3: * p=0.0101, B7-H4: ns p=0.0615, VISTA: ns p=0.99. Quantification of (C) PD-1 and (D) CTLA-4 expressing cells within CD8+ and CD4+ T cell subsets (n=13 for HD and MPN). Each square represents one subject. Data were pooled from 3 independent experiments. Statistical significance was evaluated by t test; HD vs MPN: CD8+PD-1+ **p=0.0014, CD4+PD-1+ *p=0.0106, CD8+CTLA-4+ **p=0.0024, CD4+CTLA-4+ *p=0.0207. PBMCs from CALR+ MPN patients were stimulated in vitro with pooled mut-CALR in the absence or presence of monoclonal antibodies blocking PD-1 or CTLA-4 (10 μg/mL). Representative IFN-γ ELISPOT images (E) and summary of ELISPOT results (F) generated in PBMCs from 18 CALR+ MPN patients. Each data point represents one MPN patient. The change in spot numbers were displayed as fold change by dividing the number of spots formed after OLP pool stimulation to the number of spots formed after MOG stimulation. Horizontal lines indicate the median. Statistical significance for changes at population level was evaluated by Wilcoxon signed rank test. Isotype vs a-PD-1: p=0.3465, isotype vs CTLA-4: 0.4171. Additionally, statistical significance was evaluated for each subject by t test by comparing isotype vs checkpoint blockade. 3 subjects that showed significant response to checkpoint blockade were denoted. *p=0.0121, **p=0.0045, ***p=0.0005.
Apart from the increased checkpoint receptor expression, suppression of T cells in cancer patients can be attributed to the expansion of other immunoregulatory cell populations (26). Therefore, we investigated the presence of myeloid-derived suppressor cells (MDSCs) and regulatory T cells (Tregs) in MPN PBMCs from the same cohort. We found that MDSCs, defined as Lin-CD33+CD11b+HLA-DR-CD14- cells (27), were significantly expanded in CALR+ MPN patients when compared to HD (fig. S5A–B). However, the frequencies of Treg populations, CD4+CD25+FoxP3+, were comparable between CALR+ MPN patients and HD (fig. S5C–D). We also found that the average percentage of CD3+ T cells in the peripheral blood of MPN patients was lower than healthy controls (fig. S5E–G). These observations may account for why blockade of PD-1 and CTLA-4 failed to rescue mut-CALR-specific T cell responses in some patients. Together, these data indicate that peripheral blood T cells in CALR+ MPN patients exhibit an exhausted phenotype and checkpoint blockade might be an effective strategy to restore mut-CALR-specific T cell responses in a subset of patients.
PD-1 blockade in vivo augments T cell responses
To gain further insights into checkpoint receptor-mediated T cell suppression in CALR+ MPN patients, we took advantage of an ongoing phase 1/2 clinical trial (), in which patients with advanced MPN are undergoing treatment with pembrolizumab (Fig. 3A). One of the patients enrolled in the study expressed Type 1 CALR mutation (c1092_1143del52) (PT#15 in table S1). Using longitudinal blood samples available from this patient, we monitored the changes in the frequencies of T cells before and after pembrolizumab treatment by both flow cytometry and TCR sequencing. Prior to therapy, only 4% of patient’s PBMCs were T cells and of these 32% expressed PD-1 (fig. S6A). Importantly, the proportion of T cells in peripheral blood greatly increased after pembrolizumab treatment, reaching up to 26% and 18% of PBMCs after 2 (T1) and 6 (T2) cycles of treatment, respectively (Fig. 3B–D). Pembrolizumab treatment was accompanied by increases in both peripheral blood CD4+ and CD8+ T cells (fig. S6A). After pembrolizumab administration, PD-1 expressing T cells in peripheral blood were undetectable by commercial anti-PD-1 antibodies (fig. S6A), probably due to the persistent binding of pembrolizumab to PD-1 on T cells in vivo, as described previously (28,29). In addition, TCR Vβ sequencing was undertaken to evaluate the extent of clonal expansion. TCR Vβ chains of peripheral blood T cells before and after pembrolizumab treatment were sequenced and the diversity of TCR repertoire was calculated by Pielou’s evenness metric. The results indicated that T cells not only expanded but also showed greater clonality after pembrolizumab treatment (Fig. 3E). After the initial treatment cycles, 8 T cell clones were significantly expanded compared to baseline (pre-treatment) (Fig. 3F). Each of these clones remained significantly expanded after additional cycles of treatment and there were 15 more T cell clones that were also significantly expanded compared to baseline (Fig. 3G). These observations suggested that in vivo PD-1 blockade may help reinvigorate T cells in MPN patients.
Fig. 3.
Pembrolizumab administration rescues mut-CALR-specific immunity. (A) Schema of a phase 1/2 clinical trial to assess the efficacy, safety and tolerability of pembrolizumab in patients with chronic phase myelofibrosis. The FDA-approved dose of 200 mg pembrolizumab is administered via intravenous (IV) infusion every 3 weeks. 9 patients will be enrolled in the first stage of the Simon-two stage design, and 15 in the second stage. A treatment cycle is 3 weeks and the core study period is 6 cycles. PBMCs were collected from a CALR+ MPN patient receiving pembrolizumab, before (baseline) and after 2 and 6 cycles of treatment, T1 and T2, respectively. The frequency of T cells was analyzed by (B-C) flow cytometry (CD3+ cells) or by (D) TCRseq (number of cells expressing TCR/number of total nucleated cells). (E) Clonality of T cells was calculated as 1-Pielou’s evenness. Changes in the abundance of unique TCR Vβ sequences were analyzed using ImmunoSeq platform: (F) T1 vs baseline and (G) T2 vs baseline. Only clones with a cumulative abundance of 10 or above were included in the analysis. Binomial method was used to calculate p-values. False discovery rates were controlled by Benjamini Hochberg method. Differential abundance of clones was considered significant (red and blue circles) when p-value was equal or less than 0.01. (H) PBMCs collected at T1 were stimulated in vitro with either WT or mut-CALR OLPs, alone or in the presence of PD-1 blocking antibodies. IFN-γ production by expanded T cells was measured by ELISPOT. Statistical significance was evaluated by t test, comparing MOG and peptide stimulation for each group: a-PD-1 *p=0.0168, CEFT at baseline ****p<0.0001, at T1 **p=0.0016, at T2 ****p<0.0001. (I) 2 clones that were significantly expanded in peripheral blood upon pembrolizumab treatment were also expanded in in vitro cultures upon stimulation with mut-CALR OLPs when PD-1 was blocked. TCR Vβ rearrangements for each clone are indicated next to frequency graphs.
To address this possibility, we evaluated changes in antigen-specific, namely, mut-CALR-specific T cell responses in this CALR+ MPN patient prior to and during pembrolizumab administration. At baseline, peripheral blood T cells from this patient did not have detectable mut-CALR-specific responses, even after in vitro inhibition of PD-1 with antibody blockade. After pembrolizumab treatment, however, mut-CALR-specific T cell responses were evident, as shown by increased IFN-γ production (Fig. 3H). Interestingly, in vitro addition of PD-1 blocking antibody was necessary to observe such responses. We speculate that this was due to the upregulation of PD-1 expression on the T cells during the in vitro expansion (fig. S6B). Hence the blockade of PD-1 was required in order to uncover mut-CALR-specific responses. Next, we investigated the changes in clonal T cell populations in these in vitro cultures in response to stimulation with mut-CALR OLPs. Peripheral blood T cells, collected after the initial treatment (T1), were stimulated and expanded in vitro with WT or mutant CALR peptides, in the absence or presence of PD-1 blocking antibodies. Subsequently, TCR Vβ chains of the expanded T cells were sequenced. Multiple T cell clones uniquely expanded in response to stimulation with mut-CALR OLPs in the presence of PD-1 blocking antibodies (fig. S7A–D). Among these, 2 clones that also significantly expanded in peripheral blood after pembrolizumab treatment were identified (Fig. 3I). Interestingly, the frequency of 1 of these 2 clones reached above 30% in all of the in vitro groups. The cells utilized in these in vitro cultures were collected after pembrolizumab administration and clonal expansion of T cells was evident at this point compared to baseline. We speculate that this specific clone of T cells was invigorated in vivo after pembrolizumab treatment and had a proliferative advantage, thereby populating the in vitro cultures. Although these cells might recognize mut-CALR epitopes, it remains possible that these are bystander T cells recognizing epitopes unrelated to mut-CALR (30). Together, these observations, while limited to one patient, indicate that PD-1 blockade in CALR+ MPN patients may lead to expansions of clonal T cell populations that could potentially recognize neoantigens derived from mut-CALR.
Mut-CALR reactive T cells can be elicited from healthy donor T cells
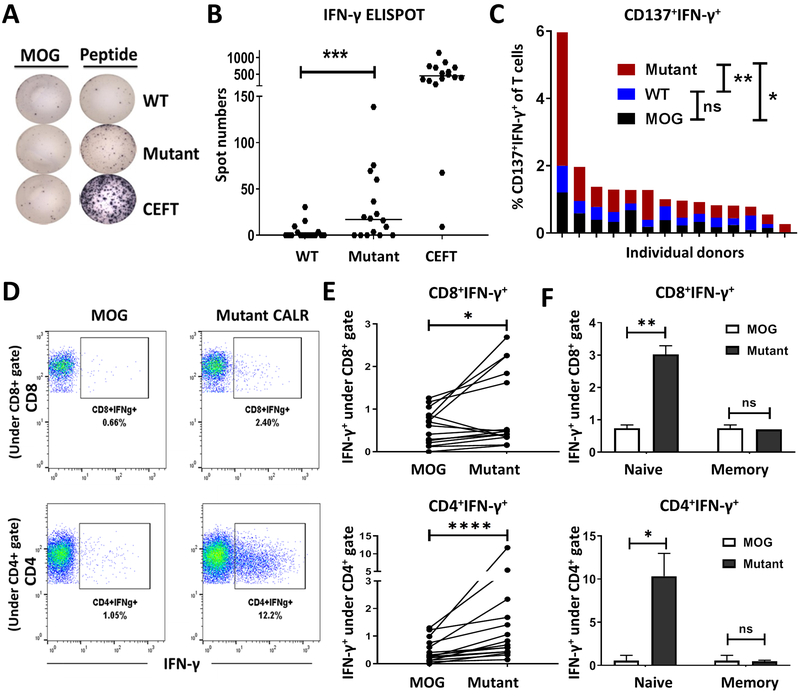
Our data suggest that systemic T cell exhaustion may limit the ability to observe mut-CALR-specific T cell responses in MPN patients. Schumacher et al. have previously demonstrated that neoantigen-specific T cells can be primed from T cells in HD blood (31). Similarly, we hypothesized that mut-CALR-specific T cells could be readily primed from HD blood. To test this hypothesis PBMCs from 16 HD were stimulated with OLPs derived from mut-CALR and the responses were measured by ELISPOT and ICS. Responses to mut-CALR were observed in both CD4+ and CD8+ T cells and were characterized by the production of effector cytokines, such as IFN-γ, as well as an increase in the surface expression of the activation marker CD137 (4-1BB) (Fig. 4A–E). Importantly, the observed T cell responses were specific to mut-CALR, as responses to stimulation with WT CALR OLPs were not higher than the background (Fig. 4A–C). Consistent with the hypothesis that T cell exhaustion limits mut-CALR-specific T cell responses in MPN patients, we observed mut-CALR-specific T cell responses in a greater fraction of HD PBMCs than MPN PBMCs (p=0.001 for HD [Fig. 4B] and p=0.037 for MPN [Fig. 1C]). Additionally, T cell responses observed in HD PBMCs were of a higher magnitude than those observed in MPN PBMCs.
Fig. 4.
Healthy donors provide mut-CALR reactive T cells. PBMCs collected from healthy donors were stimulated in vitro with pooled WT or mut-CALR OLPs. Stimulation with CEFT pool was used as control. Representative IFN-γ ELISPOT images (A) and summary of ELISPOT results (B) generated in PBMCs from 16 healthy donors. The spot numbers were calculated by subtracting the number of spots formed after MOG stimulation from the number of spots formed after OLP pool stimulation and negative values were set to zero. P value was calculated by Wilcoxon signed-rank test. ***p=0.001. Horizontal lines indicate the median. (C) Percentage of CD137+IFN-γ+ T cells, assessed by flow cytometry. Each bar represents an individual donor. P values were calculated by Wilcoxon signed-rank test. MOG vs. WT: *p=0.0144, WT vs. Mutant: **p=0.007. (D) Representative flow plots showing IFN-γ production by CD8+ and CD4+ T cells upon priming with MOG or mut-CALR OLPs. (E) Quantitative summary of frequencies of T cell subsets producing IFN-γ (n=15). P values were calculated by Wilcoxon signed-rank test. *p=0.0413, ****p<0.0001. Each data point represents one healthy donor. (F) Naïve (CD45RO−CD45RA+CCR7+) and memory (CD45RO+ CD45RA−) T cells and APCs (CD3−) were isolated by fluorescence-activated cell sorting (FACS) from HD PBMCs (n=2). APCs pulsed with MOG or mut-CALR OLPs were co-cultured with naïve or memory T cells. IFN-γ production by each T cell population was measured by flow cytometry. P values were calculated by t test. **p=0.078, *p=0.0365.
Healthy blood donors presumably have had no prior exposure to mut-CALR. Therefore, any observed mut-CALR-specific T cell response should result from effective priming of naïve mut-CALR-specific T cell precursors. To test this hypothesis naïve (CD3+CD45RO−CD45RA+CCR7+) and memory (CD3+CD45RO+) T cells, and non T-cell (CD3−) antigen presenting cells (APC) were sorted from PBMCs of reactive HD. APCs, pulsed with mut-CALR OLPs, were then separately co-cultured with either naïve or memory T cells. Consistent with the hypothesis that mut-CALR-induced responses were derived from de novo T cell priming, we observed mut-CALR-specific T cells in co-cultures with sorted naïve T cells, but not with memory T cells (Fig. 4F). To confirm these findings, umbilical cord blood mononuclear cells, which are unlikely to have memory T cells (32), were stimulated with mut-CALR OLPs. The induction of mut-CALR-specific T cell responses was also evident in these cultures (fig. S8). These data confirm the immunogenicity of the mut-CALR neoantigen and demonstrate the feasibility of priming antigen-specific T cell responses from naïve T cells.
T cells recognize multiple epitopes in mut-CALR C-terminus that are endogenously processed and presented
The mut-CALR C-terminus is >36 aa long (16) and may give rise to multiple immunogenic epitopes. Additionally, our tested cohorts of MPN patients and HD will exhibit diverse human leukocyte antigens (HLA) haplotypes. An individual’s HLA alleles will determine whether they are able to present mut-CALR epitopes, which will depend upon the relative binding affinities. To this end, we interrogated the binding affinities of epitopes derived from the altered C-terminus using in silico peptide binding prediction algorithms through the immune epitope database (IEDB). For maximal population coverage, we used IEDB’s HLA reference set that covers >97% and >99% of the population for class I and class II alleles, respectively. Our analysis showed that multiple epitopes could theoretically bind to several HLA class I and II alleles (fig. S9A–B and tables S2–3). Furthermore, HLA-I alleles with predicted high binding affinities for mut-CALR-derived epitopes (table S2) were represented in approximately 60% of the population of the USA, according to recorded allele frequencies of 2.9 million typed donors covering 16 races (33,34). A significant proportion of MPN patients with the CALR frameshift mutation, hence, may have the potential to develop antigen-specific T cells.
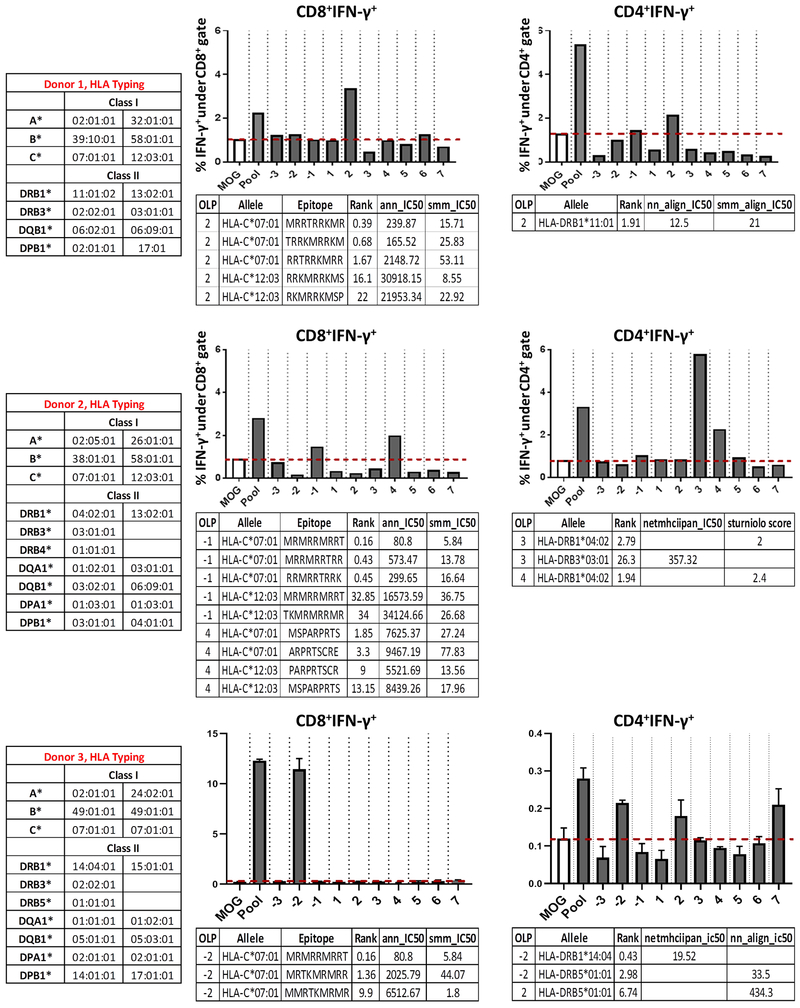
Next, we aimed to identify which, if any, of the predicted HLA-I/II-restricted epitopes could induce T cell responses. We selected 3 HDs that displayed both CD8+ and CD4+ T cell effector responses upon stimulation with mut-CALR OLP pool. We deconvoluted the mut-CALR OLP pool by priming HD precursor T cells with individual OLPs within the pool (fig. S1A). We observed CD8+ and CD4+ T cell responses against multiple individual OLP (Fig. 5). We also performed sequence-based HLA-I/II genotyping (SBT) for these subjects and determined the predicted HLA-I/II binding affinities of the epitopes that induced IFN-γ production by CD8+ or CD4+ T cells using the IEDB’s recommended algorithm. Each stimulating OLP yielded epitopes that were predicted to bind strongly to the HLA alleles of the donors tested (Fig. 5), suggesting that the predicted epitope may have been responsible for the observed mut-CALR-specific T cell response. Additionally, we evaluated whether the mut-CALR epitopes predicted to be strong binders to donor HLA (percentile rank < 2) were enriched within the OLPs that induced CD8+ T cell responses. Although such enrichment was observed for each donor tested, the correlation was not significant as there were a few OLPs that did not induce T cell responses, yet were enriched for predicted epitopes (fig. S9C). This observation is expected since many studies have reported that the majority of predicted epitopes fail to elicit T cell responses (35) and that high HLA-peptide binding affinity does not equate to immunogenicity. Additionally, when we perform T cell immunogenicity assays, we start with a limited number of PBMCs, typically around 1 million. Therefore, it is possible that we may not capture all reactive T cells, especially the ones that are low in precursor frequency.
Fig. 5.
T cells recognize multiple epitopes in mut-CALR. PBMCs from 3 HD, donors 1, 2 and 3 were stimulated in vitro with individual OLPs and IFN-γ production by CD8+ and CD4+ T cells was measured by flow cytometry. HLA alleles of individuals were identified by sequence-based genotyping. Binding affinities of donors’ alleles to epitopes within the OLPs that induced IFN-γ production were predicted by IEDB’s recommended algorithm. ANN: artificial neural network (NetMHC 4.0), SMM: stabilized matrix method, NN-align: NetMHCII 2.2, SMM-align: NetMHCII 1.1.
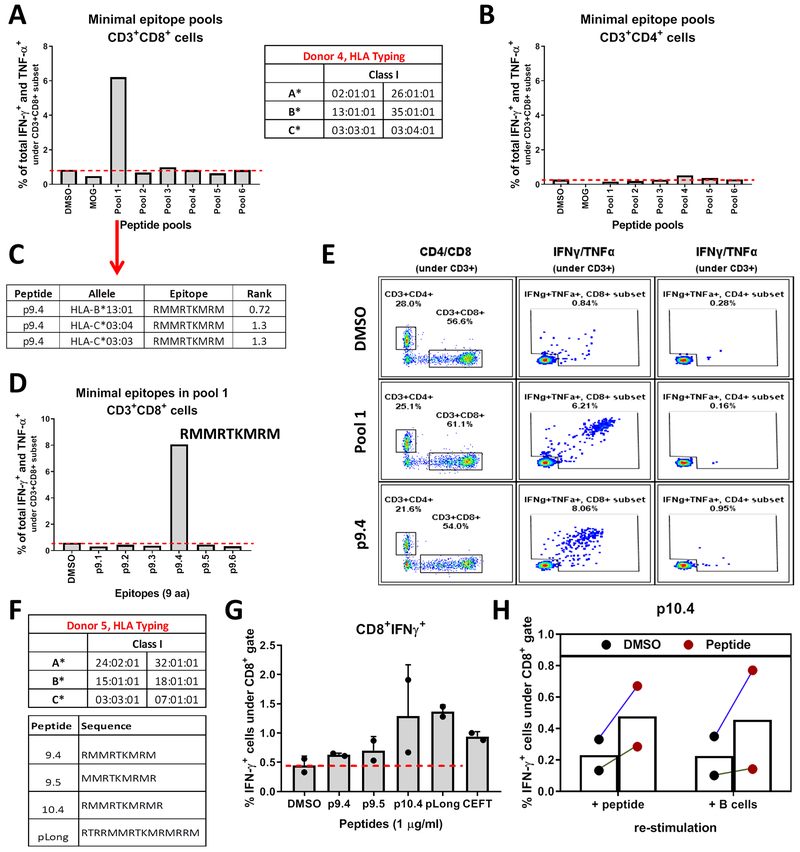
Since mut-CALR OLPs induced CD8+ T cell responses, we aimed to identify the minimal epitopes that were recognized by CD8+ T cells. To perform an unbiased screening of all potential epitopes, a complete library of peptides with 9 aa length was synthesized. The 9mers overlapped with an offset of 1 aa and spanned the last 46 aa of the C-terminus of mut-CALR, formed by the 52-bp deletion. PBMCs from 3 additional HD were stimulated with pools of these overlapping 9mers (6-7 peptide/pool) to screen for CD8 responses. CD8 responses were detected in one of the donors tested (Fig. 6A). As expected, short peptides did not induce CD4 responses (Fig. 6B). Next, we aimed to identify which epitope(s) within the stimulating pool (pool 1) was inducing the response. First, we investigated the predicted binding affinities of each epitope to the donor’s HLA alleles. Out of the 6 epitopes constituting the pool, only “RMMRTKMRM” was predicted to have a percentile rank <2 by NetMHCpan 3.0 (Fig. 6C and fig. S10A). We then re-stimulated cells expanded with pool 1 with individual epitopes constituting the pool. In accordance with the predictions, only one epitope, “RMMRTKMRM”, was recognized by CD8+ T cells (Fig. 6D–E). To further confirm the immunogenicity of this epitope, we predicted the binding affinities of “RMMRTKMRM” and other related epitopes (fig. S10B) to common HLA class I alleles (fig. S10C). Next, we identified HDs with at least one HLA allele that was predicted to bind to the epitopes tested (fig. S10D). As an example, we observed CD8+ T cell responses in donor 5 (Fig. 6F–G), further demonstrating the immunogenicity of these epitopes. Notably, this donor carried 3 of the predicted alleles (fig. S10D). CD8+ T cells from donor 5 produced effector cytokines both when stimulated with minimal epitopes and with a long peptide containing these epitopes (Fig. 6G). These data suggest that the identified epitopes can be processed and loaded onto MHC molecules within the cells. To further test this hypothesis, we expanded T cells from donor 5 by stimulating with the minimal epitope p10.4, and co-cultured the expanded T cells with autologous APC that were pulsed with a long peptide containing p10.4 sequence. We observed that long peptide loaded APC could induce p10.4-specific CD8+ T cells (Fig. 6H), suggesting this epitope is endogenously processed and presented to T cells by APC.
Fig. 6.
T cells recognize mut-CALR epitopes that are endogenously processed and presented. PBMCs from donor 4 were stimulated in vitro with pools of short peptides (9 aa and 6-7 peptides/pool) and frequencies of IFN-γ and TNF-α producing CD8+ (A) and CD4+ (B) T cells were measured by flow cytometry. HLA alleles of individuals were identified by sequence-based genotyping. (C) Binding affinities of donor 4’s alleles to the 6 epitopes within pool 1 were predicted by NetMHCPan 3.0. Epitopes with a predicted percentile rank <2 are listed. (D) Donor 4 PBMCs were stimulated with individual short peptides constituting pool 1. Frequencies of IFN-γ and TNF-α producing CD8+ T cells were measured by flow cytometry. (E) Flow plots showing IFN-γ and TNF-α production by CD8+ and CD4+ T cells upon stimulation with p9.4. (F) PBMCs from donor 5 were stimulated in vitro with peptides listed. (G) Frequencies of IFN-γ producing CD8+ T cells were measured by flow cytometry. Data were pooled from 2 independent experiments. (H) Donor 5 PBMCs expanded with p10.4 were re-stimulated either by the short peptide they were expanded with or by co-culturing autologous B cells that were pulsed with pLong. DMSO or DMSO-pulsed B cells were used as background controls, respectively. Data were pooled from 2 independent experiments.
Together, these data show that mut-CALR can induce T cell responses, even in HD without prior exposure to mut-CALR. Moreover, it provides evidence that healthy donor T cells can be utilized as an alternative approach to evaluate the breadth and specificity of mut-CALR-specific T cell responses and their HLA restriction.
Discussion
In this study we demonstrate that mut-CALR is a shared MPN-specific neoantigen that can induce T cell responses. Our results reveal that mut-CALR elicits specific T cell responses in CALR+ MPN patients, delineate the immunogenic properties of mut-CALR and identify a role for immune checkpoint blockade in enhancing mut-CALR-specific T cell immunity in CALR+ MPN patients.
Recent studies from Holmstörm et al., and Tubb et al. also evaluated mut-CALR-specific T cell immunity (36–38). Holmstörm et al. reported that CALR mutations could be recognized in vitro by T cells from CALR+ MPN patients. However, the responses were restricted to CD4+ T cells (36,37). Tubb et al. focused on identifying mut-CALR epitopes recognized by CD8+ T cells based on predicted epitope-MHC information. However, mut-CALR reactive CD8+ T cells were elicited only in healthy donors but not in MPN patients (38). In either case, the majority of predicted peptides failed to elicit T cell responses and the observed mut-CALR-specific T cell responses were weak. In the present study, we systematically evaluated the mut-CALR C-terminus for peptides binding to MHC molecules by using in silico MHC-I and MHC-II peptide binding prediction algorithms. We identified 103 peptides predicted to bind to representative MHC-I and MHC-II molecules with high affinity (Table S1–2). To account for MHC-restricted peptides that may have been missed or otherwise incorrectly assigned by in silico predictors (35), we utilized an unbiased screening approach based on overlapping peptides spanning the full length of the shared mut-CALR C-terminus. Screening was performed in parallel in MPN patients and healthy donors to control for factors associated with disease burden that may inhibit mut-CALR-specific T cells. We demonstrated that several MHC-I and MHC-II restricted peptides from mut-CALR were recognized by CD8+ and CD4+ T cells.
Significantly, mut-CALR-specific T cell responses were lower in magnitude and less prevalent in MPN patients when compared to healthy donors. Several putative explanations for these observations were explored. We stratified the patients in our cohort according to their type of MPN and explored associations with the mut-CALR-specific T cell responses. We observed that the majority of patients with mut-CALR-specific T cell responses had ET. Although mut-CALR-specific T cell responses were occasionally observed in MF patients that had a prior history of ET, none of the patients with primary MF had detectable T cell responses. ET can evolve to MF, which is associated with greater symptom burden, inferior prognosis and higher number of somatic mutations (39). Consistent with Holmstörm et al.’s report (37), these observations suggest that mut-CALR-specific T cell responses may occur more frequently in CALR+ MPN patients with low symptom burden. Additionally, some of the MF patients in our cohort were being treated with ruxolitinib at the time their samples were used in this study. Ruxolitinib has been previously shown to inhibit T cell responses (40). Therefore, it is possible that ruxolitinib treatment impaired the reactivity of mut-CALR-specific T cells in some patients in our cohort, a possibility being examined in separate experiments.
Systemic T cell dysfunction is frequently observed in cancer patients. The severity of T cell exhaustion is associated with disease progression due to factors such as prolonged exposure to tumor antigens, which results in elevated expression of checkpoint receptors that directly inhibit T cell function (41). Based on the observed association with disease burden, we hypothesized that systemic T cell exhaustion may limit the ability to observe spontaneous mut-CALR-specific T cell responses in some CALR+ MPN patients. In support of this hypothesis, we observed that T cells from CALR+ MPN patients exhibited elevated expression of immune checkpoint receptors PD-1 and CTLA-4, which have been associated with T cell exhaustion. Second, we found that, in MPN patients, antibody-mediated blockade of PD-1 or CTLA-4 signaling ex vivo rescued mut-CALR-specific T cell responses. Third, we observed that mut-CALR reactive T cells can be produced from naïve T cells from healthy donors in greater frequencies, again suggesting a role for suppression of mut-CALR-specific T cells in MPN patients. Finally, administration of pembrolizumab was shown to rescue mut-CALR-specific T cell responses in a CALR+ MPN patient. Collectively, these data show that spontaneous mut-CALR-specific T cell responses that occur in CALR+ MPN patients may be actively suppressed by immune checkpoint receptor signaling and that blockade of said inhibitors augments the mut-CALR-specific T cell responses in vitro and in vivo.
However, mut-CALR-specific T cell responses were restored upon blockade of PD-1 and CTLA-4 signaling only in a subset of patients. Several mechanisms might underlie these observations. The patients in our MPN cohort were not selected based on their HLA type and exhibit diverse HLA alleles. Hence, the lack of mut-CALR-specific T cell responses could be the absence of the HLA alleles binding to mut-CALR epitopes with high affinity. Also, checkpoint receptors other than PD-1 and CTLA-4 might contribute to suppression of mut-CALR-specific T cell responses. Phenotypic analysis showed that several checkpoint receptors, including LAG-3 and TIM-4, were also highly expressed in peripheral blood T cells of CALR+ MPN patients. Therefore, inhibition of such molecules could be beneficial in restoring mut-CALR-specific T cell responses. Chronic antigen exposure can lead to terminal exhaustion with limited reinvigoration potential in later stages of disease (41), thus earlier intervention would be a more favorable approach.
A key biomarker of response to PD-1 blockade is tumor mutational burden (TMB) (3,7). TMB for MPN has previously been reported to be significantly low, 1 to 32 mutations per patient (12). Hence, it can be argued that the activity of anti-PD1 treatment in MPN patients would be low. However, even tumors with low TMB have been shown to provide high quality neoantigens that elicit antitumor T cell responses (42). Recent work by Cristescu et al. corroborate such observations by demonstrating that factors, such as a T cell-inflamed microenvironment, can independently predict response to PD-1 blockade even when the TMB is low (43). We observed that pembrolizumab treatment led to an overall increase in the proportion of peripheral blood T cells and significant expansion of several T cell clones. It is possible that some of the expanded clones target high quality neoantigens, mut-CALR and potentially others. Although limited to one patient, these observations suggest that in vivo PD-1 blockade in CALR+ MPN patients may reinvigorate neoantigen-specific T cells.
While we demonstrated that mut-CALR epitopes could be recognized by both CD8+ and CD4+ T cells from healthy donors, patient-derived mut-CALR-specific T cells were primarily CD4+. We hypothesized that the lack of CD8+ mut-CALR-specific T cells in CALR+ MPN patients was the result of inefficient antigen presentation by MHC class I. CALR is a chaperone protein critical for the folding and assembly of MHC class I molecules (20). CALR-deficient cells are reported to have reduced levels of cell surface MHC class I expression as well as reduced efficiency in antigen presentation to cytotoxic T lymphocytes (19). Even a heterozygous expression of the mut-CALR protein, as is the case with majority of CALR+ MPN patients, has been shown to lead to a significant, albeit small, reduction in surface expression of MHC class I molecules (44). However, we did not observe a reduction in the cell surface expression of MHC class I molecules in either PBMCs or CD34+ cells from CALR+ MPN patients as compared to HD. Similarly, MHC class II expression was also intact in CALR+ MPN patients. Hence the paucity of anti-mut-CALR responses in CALR+ MPN patients cannot be explained by a reduction of MHC expression. Yet it remains possible that the presentation of mut-CALR on MHC class I may be compromised, which warrants further investigation to evaluate the impact of mut-CALR on peptide loading and presentation.
Among the current standard treatment options for MPN patients, only hematopoietic stem cell transplantation (HSCT) is potentially curative (17). However, the application of HSCT is limited due to lack of appropriate donor options, the advanced age of patients, comorbidities and poor functional status (45). Other treatment modalities, though they improve symptom burden, show only minimal effects in eliminating malignant clones and providing molecular remissions (21,46,47). Therefore, it is essential to identify new approaches that advance the treatment of MPN patients. We anticipate that our findings will form the basis for three avenues of therapy. A neoantigen vaccine targeting the C-terminal of mut-CALR can be administered, perhaps as a prevention of progression strategy, given that the disease is long standing in nature and impacts longevity considerably later. Studies by several groups demonstrated that administration of neoantigen vaccines could induce neoantigen-specific T cell priming and also boost spontaneous neoantigen-specific T cell immunity in melanoma patients (48–50). Alternatively, the vaccine may be given in combination with checkpoint inhibitors, as is being done in several clinical trials for solid tumors (). Finally, an adoptive T cell therapy for the treatment of MPN patients harboring mutated CALR may be feasible, in which the patients will receive autologous reinvigorated mut-CALR-specific T cells that are expanded ex vivo. Sequencing and cloning of patients’ TCRs could also be used to transduce healthy donor HLA-matched T cells for an alternative form of adoptive cell therapy, an approach we are pursuing. These immunotherapy regimens could potentially establish an effective mut-CALR-specific T cell immunity, which would target and eliminate CALR+ malignant cells, thereby leading to improved clinical outcomes in this patient population. Since CALR mutations are the second most common MPN driver mutation and mut-CALR neopeptide is shared among patients carrying each type of CALR mutation (11), strategies targeting mut-CALR are anticipated to be applicable to a significant number of MPN patients.
Materials and Methods
Detailed materials and methods are provided in the supplementary data.
Patient samples
The use of patient-derived specimens was approved by the Institutional Review Boards at Mount Sinai (HS#11-02054) and all patients provided written informed consent before the initiation of any study procedures. The specimens were provided by the Hematological Malignancies Tissue Bank (HMTB) of the Tisch Cancer Institute. Patient blood was collected by the clinical personnel and MNCs were isolated by HMTB personnel by Ficoll-Paque density gradient. All patients analyzed in this study were diagnosed with MPN and tested positive for a CALR or JAKV617F mutation. Due to low viability of patient cells after thawing, only freshly isolated patient PBMCs were used in immunogenicity assays, unless noted otherwise. Therefore, assays were performed once for each patient. Healthy donor specimens were procured from New York Blood Center as leukopak and MNCs were isolated by density gradient centrifugation using Ficoll-Paque™ Plus (GE Healthcare). PBMCs were cryopreserved in human serum containing 10% DMSO. HD PBMCs were used after thawing and assays were repeated at least twice.
Neoepitope predictions
Peptide predictions were performed using algorithms available at immune epitope database and analysis resource (www.iedb.org). Unless indicated otherwise, IEDB recommended method was selected to perform prediction analyses for both MHC-I and MHC-II binding. Epitopes that were assigned an IC50 value lower than 500 and/or percentile rank lower than 2 were considered high affinity binders.
Peptide synthesis
Custom peptide libraries for WT and mut-CALR peptides were chemically synthesized by JPT Peptide Technologies (Germany). Each peptide had >80% purity as determined by high performance liquid chromatography. MOG and CEFT peptide pools were commercially available at JPT Peptide Technologies (Germany). Each peptide was resuspended in DMSO and used at a final concentration of 1 μg/mL.
Induction of neoantigen reactive T cells
For the induction of antigen-specific T cells, a previously published method (18) was used with modifications. Briefly, unfractionated PBMCs were cultured in X-VIVO15 media (LONZA) with cytokines and were stimulated with peptide(s) (1 μg/mL) or equal volume of DMSO in the presence of adjuvants. Cells were expanded with IL-2 (R&D Systems, 10 IU/mL) and IL-7 (R&D Systems, 10 ng/mL) in RPMI media containing 10% human serum (Gibco) (R10 media). Cells were fed every 2-3 days. After 9-11 days of culture, cells were harvested and all wells were pooled within group. After washing with R10 media, cells were re-stimulated with either MOG to account for background signal or with test peptide(s) they were initially stimulated with (1 μg/mL) in the presence of anti-CD28 (1 μg/mL) and anti-CD49d (1 μg/mL) antibodies (BD Biosciences). As controls, some cells were stimulated with PMA (Sigma-Aldrich, 50 ng/mL) and ionomycin (Sigma-Aldrich, 1 μg/mL).
Same immunogenicity assay was utilized to induce antigen-specific T cell responses when checkpoint receptors were inhibited with the following modifications: on day 2, when PBMCs were stimulated with peptides and adjuvants, anti-PD-1 (clone EH12.1) or anti-CTLA-4 (clone BNI3) antibodies (no azide/low endotoxin mouse anti-human antibodies by BD Biosciences, both used at 10 μg/mL) or same concentration of isotype controls, mouse IgG1, κ or IgG2a, κ, respectively, were also added.
Functional analysis of T cell responses
For ELISPOT analysis, plates with mixed cellular ester membrane (Millipore) were coated with anti-IFN-γ antibody (clone 1-D1k by Mabtech, used at 4 μg/mL) overnight at 4°C. Plates were washed 3x with plain RPMI and blocked by incubating with R10 media at 37°C for at least 1 h prior to addition of cells expanded in immunogenicity assay. Cells were seeded at either 5×104 or 105 per well in duplicates and re-stimulated as detailed above for 48 h and plates were processed for IFN-γ detection. Plates were first incubated with biotinylated anti-IFN-γ antibody (clone 7-B6-1 by Mabtech, used at 0.2 μg/mL) for 2 h at 37°C, then 1 h at room temperature with streptavidin-AP conjugate (Roche, used at 0.75 U/mL) and lastly with the SigmaFast BCIP/NBT substrate for 10 minutes at room temperature. Plates were washed 6x with PBS containing 0.05% Tween-20 and 3x with water in between each step. Plates were scanned and analyzed by ImmunoSpot software.
For flow cytometry, cells expanded in immunogenicity assay were seeded at 1-2x105 per well. 1 hour after re-stimulation, cells were added BD GolgiStop™, containing monensin and BD GolgiPlug™, containing brefeldin A, according to manufacturer’s suggestion. 12 h after the addition of protein transport inhibitors, cells were processed for flow cytometry. Cells were first stained for surface molecules with the following antibodies: anti-CD3 (OKT3), anti-CD4 (RPA-T4), anti-CD8 (RPA-T8) and anti-CD137 (4B4-1) and then processed for intracellular staining using BD Cytofix/Cytoperm™ reagents according to manufacturer’s protocol. Cells were then stained with anti-IFN-γ (B27) and anti-TNF-α (MAb11) antibodies. All antibodies were purchased BioLegend.
TCR sequencing
Longitudinal blood samples were available from a CALR+ MF patient (PT#15 in Table S1) undergoing pembrolizumab treatment. PBMCs from this patient were isolated before and after 2 and 6 cycles of pembrolizumab treatment. PBMCs isolated after 2 cycles were expanded in vitro in an immunogenicity assay as described above by stimulating with WT or mut-CALR OLP pools, with or without PD-1 blocking monoclonal antibodies. After expansion, cells were harvested. Genomic DNA was isolated from these 6 samples using DNeasy Blood and Tissue Kit (Qiagen) according to the manufacturer’s instructions. The quality and quantity of the extracted DNA samples were analyzed by NanoDrop. DNA samples were sent to Adaptive Biotechnologies for TCR Vβ sequencing. Briefly, TCRβ CDR3 regions for each sample were amplified by a multiplexed PCR method using a mix of forward and reverse primers specific to TCR Vβ and TCR Jβ, respectively. Amplified regions were sequenced using Illumina HiSeq System. Data analyses were performed using Adaptive Biotechnologies ImmunoSeq Analyzer 3.0. Clonality was calculated as (1 – normalized entropy). Normalized entropy was defined as (Shannon entropy / log2R), where R = the total number of rearrangements. The frequency of T cells was calculated as (number of cells expressing TCR / number of total nucleated cells). Total nucleated cell numbers were obtained by converting the input DNA amount in the assay based on the assumption that each diploid cell have about 6.4 pg genomic DNA. In differential abundance analyses of TCRβ chain sequencing, only clones with a cumulative abundance of 10 or above were included. P values were calculated using the binomial method and false discovery rates were controlled by Benjamini Hochberg method. P values ≤ 0.01 were considered statistically significant. The frequencies of VJ combinations for abundant clones were visualized using circular plots. To this end, the most abundant 50 clones at each time point were selected and the combination of these clones were mapped out. Circular plots were generated using circos software package (http://circos.ca/). Each arch represents a V or J allele. Joining ribbon indicates a unique VJ cassette combination. The arc length and the width of the ribbons indicate the frequency of V/J alleles and their cassette combination respectively.
Cell Lines
MARIMO cells were provided by N. Arshad and P. Cresswell (Yale School of Medicine). MARIMO cells were maintained at a density of 1-2x106 cells/mL in RPMI media (Gibco) containing 10% heat-inactivated fetal bovine serum (Gibco) and penicillin streptomycin (Gibco, used at 100 U/mL, 100 μg/mL, respectively). Cells were regularly tested for mycoplasma contamination by PCR. Latest testing was performed on November 2018 and cells were negative for mycoplasma.
Statistical analyses
Statistical analyses performed were detailed in the figure legends. Briefly, statistical differences were assessed by Wilcoxon matched-pairs signed rank test or t test for comparisons between two groups. P values < 0.05 were considered statistically significant. Statistical analyses were performed using GraphPad Prism software version 7 and 8. Differential abundance analyses of TCRβ chain sequencing data were performed using Adaptive Biotechnologies ImmunoSeq Analyzer 3.0. P values were calculated using the binomial method and false discovery rates were controlled by Benjamini Hochberg method. P values ≤ 0.01 were considered statistically significant.
Supplementary Material
Significance.
Current treatment modalities for MPN are not effective in eliminating malignant cells. Here we show that mutations in the CALR gene, which drive transformation in MPN, elicit T cell responses that can be further enhanced by checkpoint blockade, suggesting immunotherapies could be employed to eliminate CALR+ malignant cells in MPN.
Acknowledgments
We thank N. Arshad and P. Cresswell (Yale School of Medicine) for providing the MARIMO cells and C. McClain (Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai) for performing mycoplasma testing of the MARIMO cells. We also thank J. Arandela at HMTB (Tisch Cancer Institute at the Icahn School of Medicine at Mount Sinai) for assistance in specimen processing.
Funding: Research funds were provided by the Myeloproliferative Neoplasms Research Foundation and Leukemia & Lymphoma Society to C.I.R and N.B. and by the Parker Institute for Cancer Immunotherapy to N.B. Human specimens were provided by the HMTB of the Tisch Cancer Institute supported by the NCI P30 Cancer Center Support Grant P30 CA196521.
Conflict of interests: C.C.B, V.R, J.P.F. and C.I.R. declare that they have no competing financial interests. J.M. receives research support from Incyte, Novartis, CTI Biopharma, Janssen, Roche and is on the scientific advisory board for Celgene, Roche and Incyte. R.H. receives research support from Formation Biologics, Merus, Novartis, Roche/Genentech, Scholar Rock, Elstar and is on the scientific advisory board for Novartis. N.B. has received research funds from NIH (R01CA201189 , R01CA180913, R01AI081848), Cancer Research Institute, Merck, Regeneron, Novacure, Celldex, Ludwig Institute, Genentech, Oncovir, Melanoma Research Alliance, Leukemia & Lymphoma Society, NYSTEM and is on the advisory boards of Avidea, Check Point Diagnostics, Curevac, Prime-vax, Neon, Roche, Tempest Therapeutics, Novartis, Array BioPharm and is an extramural member researcher at Parker Institute for Cancer Immunotherapy.
References:
- 1.Rosenberg SA, Restifo NP. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 2015;348(6230):62–8 doi 10.1126/science.aaa4967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sharma P, Allison JP. The future of immune checkpoint therapy. Science 2015;348(6230):56–61 doi 10.1126/science.aaa8172. [DOI] [PubMed] [Google Scholar]
- 3.Hellmann MD, Ciuleanu TE, Pluzanski A, Lee JS, Otterson GA, Audigier-Valette C, et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N Engl J Med 2018;378(22):2093–104 doi 10.1056/NEJMoa1801946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 2015;373(1):23–34 doi 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engels B, Engelhard VH, Sidney J, Sette A, Binder DC, Liu RB, et al. Relapse or eradication of cancer is predicted by peptide-major histocompatibility complex affinity. Cancer Cell 2013;23(4):516–26 doi [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015;348(6230):124–8 doi 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yarchoan M, Hopkins A, Jaffee EM. Tumor Mutational Burden and Response Rate to PD-1 Inhibition. N Engl J Med 2017;377(25):2500–1 doi 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017;357(6349):409–13 doi 10.1126/science.aan6733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Capietto AH, Jhunjhunwala S, Delamarre L. Characterizing neoantigens for personalized cancer immunotherapy. Curr Opin Immunol 2017;46:58–65 doi 10.1016/j.coi.2017.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Schumacher TN, Schreiber RD. Neoantigens in cancer immunotherapy. Science 2015;348(6230):69–74 doi 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 11.Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013;369(25):2379–90 doi 10.1056/NEJMoa1311347. [DOI] [PubMed] [Google Scholar]
- 12.Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC, et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013;369(25):2391–405 doi 10.1056/NEJMoa1312542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Elf S, Abdelfattah NS, Baral AJ, Beeson D, Rivera JF, Ko A, et al. Defining the requirements for the pathogenic interaction between mutant calreticulin and MPL in MPN. Blood 2018;131(7):782–6 doi 10.1182/blood-2017-08-800896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Elf S, Abdelfattah NS, Chen E, Perales-Paton J, Rosen EA, Ko A, et al. Mutant Calreticulin Requires Both Its Mutant C-terminus and the Thrombopoietin Receptor for Oncogenic Transformation. Cancer Discov 2016;6(4):368–81 doi 10.1158/2159-8290.CD-15-1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luo W, Yu Z. Calreticulin (CALR) mutation in myeloproliferative neoplasms (MPNs). Stem Cell Investig 2015;2:16 doi 10.3978/j.issn.2306-9759.2015.08.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ha JS, Kim YK. Calreticulin exon 9 mutations in myeloproliferative neoplasms. Ann Lab Med 2015;35(1):22–7 doi 10.3343/alm.2015.35.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deeg HJ, Bredeson C, Farnia S, Ballen K, Gupta V, Mesa RA, et al. Hematopoietic Cell Transplantation as Curative Therapy for Patients with Myelofibrosis: Long-Term Success in all Age Groups. Biol Blood Marrow Transplant 2015;21(11):1883–7 doi 10.1016/j.bbmt.2015.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lissina A, Briceno O, Afonso G, Larsen M, Gostick E, Price DA, et al. Priming of Qualitatively Superior Human Effector CD8+ T Cells Using TLR8 Ligand Combined with FLT3 Ligand. J Immunol 2016;196(1):256–63 doi 10.4049/jimmunol.1501140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gao B, Adhikari R, Howarth M, Nakamura K, Gold MC, Hill AB, et al. Assembly and antigen-presenting function of MHC class I molecules in cells lacking the ER chaperone calreticulin. Immunity 2002;16(1):99–109. [DOI] [PubMed] [Google Scholar]
- 20.Raghavan M, Wijeyesakere SJ, Peters LR, Del Cid N. Calreticulin in the immune system: ins and outs. Trends Immunol 2013;34(1):13–21 doi 10.1016/j.it.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koschmieder S, Mughal TI, Hasselbalch HC, Barosi G, Valent P, Kiladjian JJ, et al. Myeloproliferative neoplasms and inflammation: whether to target the malignant clone or the inflammatory process or both. Leukemia 2016;30(5):1018–24 doi 10.1038/leu.2016.12. [DOI] [PubMed] [Google Scholar]
- 22.Bellucci S, Harousseau JL, Brice P, Tobelem G. Treatment of essential thrombocythaemia by alpha 2a interferon. Lancet 1988;2(8617):960–1. [DOI] [PubMed] [Google Scholar]
- 23.Silver RT, Vandris K, Goldman JJ. Recombinant interferon-alpha may retard progression of early primary myelofibrosis: a preliminary report. Blood 2011;117(24):6669–72 doi 10.1182/blood-2010-11-320069. [DOI] [PubMed] [Google Scholar]
- 24.Kollmann K, Nangalia J, Warsch W, Quentmeier H, Bench A, Boyd E, et al. MARIMO cells harbor a CALR mutation but are not dependent on JAK2/STAT5 signaling. Leukemia 2015;29(2):494–7 doi 10.1038/leu.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vannucchi AM, Rotunno G, Bartalucci N, Raugei G, Carrai V, Balliu M, et al. Calreticulin mutation-specific immunostaining in myeloproliferative neoplasms: pathogenetic insight and diagnostic value. Leukemia 2014;28(9):1811–8 doi 10.1038/leu.2014.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zarour HM. Reversing T-cell Dysfunction and Exhaustion in Cancer. Clin Cancer Res 2016;22(8):1856–64 doi 10.1158/1078-0432.CCR-15-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang JC, Kundra A, Andrei M, Baptiste S, Chen C, Wong C, et al. Myeloid-derived suppressor cells in patients with myeloproliferative neoplasm. Leuk Res 2016;43:39–43 doi 10.1016/j.leukres.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Huang AC, Postow MA, Orlowski RJ, Mick R, Bengsch B, Manne S, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017;545(7652):60–5 doi 10.1038/nature22079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci U S A 2017;114(19):4993–8 doi 10.1073/pnas.1705327114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simoni Y, Becht E, Fehlings M, Loh CY, Koo SL, Teng KWW, et al. Bystander CD8(+) T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018;557(7706):575–9 doi 10.1038/s41586-018-0130-2. [DOI] [PubMed] [Google Scholar]
- 31.Stronen E, Toebes M, Kelderman S, van Buuren MM, Yang W, van Rooij N, et al. Targeting of cancer neoantigens with donor-derived T cell receptor repertoires. Science 2016;352(6291):1337–41 doi 10.1126/science.aaf2288. [DOI] [PubMed] [Google Scholar]
- 32.Beck R, Lam-Po-Tang PR. Comparison of cord blood and adult blood lymphocyte normal ranges: a possible explanation for decreased severity of graft versus host disease after cord blood transplantation. Immunol Cell Biol 1994;72(5):440–4 doi 10.1038/icb.1994.65. [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez-Galarza FF, Takeshita LY, Santos EJ, Kempson F, Maia MH, da Silva AL, et al. Allele frequency net 2015 update: new features for HLA epitopes, KIR and disease and HLA adverse drug reaction associations. Nucleic Acids Res 2015;43(Database issue):D784–8 doi 10.1093/nar/gku1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gragert L, Madbouly A, Freeman J, Maiers M. Six-locus high resolution HLA haplotype frequencies derived from mixed-resolution DNA typing for the entire US donor registry. Hum Immunol 2013;74(10):1313–20 doi 10.1016/j.humimm.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 35.The problem with neoantigen prediction. Nat Biotechnol 2017;35(2):97 doi 10.1038/nbt.3800. [DOI] [PubMed] [Google Scholar]
- 36.Holmstrom MO, Martinenaite E, Ahmad SM, Met O, Friese C, Kjaer L, et al. The calreticulin (CALR) exon 9 mutations are promising targets for cancer immune therapy. Leukemia 2018;32(2):429–37 doi 10.1038/leu.2017.214. [DOI] [PubMed] [Google Scholar]
- 37.Holmstrom MO, Riley CH, Svane IM, Hasselbalch HC, Andersen MH. The CALR exon 9 mutations are shared neoantigens in patients with CALR mutant chronic myeloproliferative neoplasms. Leukemia 2016;30(12):2413–6 doi 10.1038/leu.2016.233. [DOI] [PubMed] [Google Scholar]
- 38.Tubb VM, Schrikkema DS, Croft NP, Purcell AW, Linnemann C, Freriks MR, et al. Isolation of T cell receptors targeting recurrent neoantigens in hematological malignancies. J Immunother Cancer 2018;6(1):70 doi 10.1186/s40425-018-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rumi E, Cazzola M. Diagnosis, risk stratification, and response evaluation in classical myeloproliferative neoplasms. Blood 2017;129(6):680–92 doi 10.1182/blood-2016-10-695957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Parampalli Yajnanarayana S, Stubig T, Cornez I, Alchalby H, Schonberg K, Rudolph J, et al. JAK1/2 inhibition impairs T cell function in vitro and in patients with myeloproliferative neoplasms. Br J Haematol 2015;169(6):824–33 doi 10.1111/bjh.13373. [DOI] [PubMed] [Google Scholar]
- 41.Wherry EJ. T cell exhaustion. Nat Immunol 2011;12(6):492–9. [DOI] [PubMed] [Google Scholar]
- 42.Balachandran VP, Luksza M, Zhao JN, Makarov V, Moral JA, Remark R, et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 2017;551(7681):512–6 doi 10.1038/nature24462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cristescu R, Mogg R, Ayers M, Albright A, Murphy E, Yearley J, et al. Pan-tumor genomic biomarkers for PD-1 checkpoint blockade-based immunotherapy. Science 2018;362(6411) doi 10.1126/science.aar3593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Arshad N, Cresswell P. Tumor-associated calreticulin variants functionally compromise the peptide loading complex and impair its recruitment of MHC-I. J Biol Chem 2018;293(25):9555–69 doi 10.1074/jbc.RA118.002836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kroger NM, Deeg JH, Olavarria E, Niederwieser D, Bacigalupo A, Barbui T, et al. Indication and management of allogeneic stem cell transplantation in primary myelofibrosis: a consensus process by an EBMT/ELN international working group. Leukemia 2015;29(11):2126–33 doi 10.1038/leu.2015.233. [DOI] [PubMed] [Google Scholar]
- 46.Devlin R, Gupta V. Myelofibrosis: to transplant or not to transplant? Hematology Am Soc Hematol Educ Program 2016;2016(1):543–51 doi 10.1182/asheducation-2016.1.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Harrison C, Kiladjian JJ, Al-Ali HK, Gisslinger H, Waltzman R, Stalbovskaya V, et al. JAK inhibition with ruxolitinib versus best available therapy for myelofibrosis. N Engl J Med 2012;366(9):787–98 doi 10.1056/NEJMoa1110556. [DOI] [PubMed] [Google Scholar]
- 48.Carreno BM, Magrini V, Becker-Hapak M, Kaabinejadian S, Hundal J, Petti AA, et al. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science 2015;348(6236):803–8 doi 10.1126/science.aaa3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ott PA, Hu Z, Keskin DB, Shukla SA, Sun J, Bozym DJ, et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017;547(7662):217–21 doi 10.1038/nature22991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahin U, Derhovanessian E, Miller M, Kloke BP, Simon P, Lower M, et al. Personalized RNA mutanome vaccines mobilize poly-specific therapeutic immunity against cancer. Nature 2017;547(7662):222–6 doi 10.1038/nature23003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.