Abstract
Proline-rich antimicrobial peptides (PrAMPs) kill bacteria via a non-lytic mechanism in which they permeate through the outer membrane, utilize protein-mediated transport across the inner membrane, and target the ribosome to inhibit protein synthesis. We previously reported that substitutions of oncocin (VDKPPYLPRPRPPRRIYNR-NH2) with a pair of cationic residues improved the antimicrobial activity. In this work, we applied the design protocol to three other PrAMPs: apidaecin-1b, pyrrhocoricin and bactenecin 7(1-16) and found that the substitutions (R4K and I8K/R) for apidaecin-1b improve the activity by 2 fold (p<0.05) against non-pathogenic E. coli. Moreover, the substitutions (L7K/R and R14K) for pyrrhocoricin improves the activity by 2-10 fold (p<0.05) against some strains of E. coli and S. Typhimurium. We also performed activity tests against inner membrane protein (SbmA or YgdD) knockout strains. The result is consistent with previous studies that SbmA is the major transporter for apidaecin-1b and pyrrhocoricin derivatives. However, bactenecin 7(1-16) functions independently of these transporters. In addition, several apidaecin-1b derivatives exhibit enhanced activity relative to wild-type only in the absence of SbmA, which is consistent with mutations that enhance transport across the inner membrane. An HPLC-based kinetic assay for cellular association and internalization demonstrates that the selected cationic mutations can improve cellular association in minimal media, but this enhanced association is not required for increased activity, which suggests the importance of inner membrane transport. These functional studies on cationic mutants of PrAMPs advance understanding of potency and mechanism and advance the ability to engineer improved antimicrobials as evidenced by the identification of the pyrrhocoricin mutant (L7R, R14K) with 10-fold elevated potency against pathogenic E. coli.
Keywords: Proline-rich antimicrobial peptides, apidaecin-1b, pyrrhocoricin, bactenecin, SbmA receptor
1. INTRODUCTION
Proline-rich antimicrobial peptides (PrAMPs) belong to a subclass of non-membrane-lytic peptides that exert their antimicrobial activities through binding and inhibition of intracellular targets(Scocchi, Tossi, & Gennaro, 2011; Li et al., 2014; Krizsan et al., 2014; Roy, Lomakin, Gagnon, & Steitz, 2015; Seefeldt et al., 2015; Gagnon et al., 2016; Seefeldt et al., 2016; Graf et al., 2017). These PrAMPs are an important element of natural defense mechanisms(Konovalova, Zubareva, Lutsenko, & Svirshchevskaya, 2018; Zasloff et al., 2002) and are promising alternatives to traditional antibiotics, which suffer from emerging resistance(S. Wang, Zeng, Yang, & Qiao, 2016; Joerger, 2003; Baltzer & Brown, 2011). Advanced knowledge of the relationship between sequence and function would improve our understanding of these natural systems and enhance our ability to engineer therapeutic variants.
Previously, we evaluated a systematic mutagenesis library of oncocin, which revealed enhanced activity against E. coli upon mutagenic introduction of particular cationic residues(Lai, Geldart, Ritter, Kaznessis, & Hackel, 2018). Specifically, the combination of P4K with either L7K or L7R increased activity against multiple E. coli strains. Cellular association assays, in vitro translation inhibition experiments, and measurement of transporter expression revealed that the enhanced activity resulted from improved peptide uptake rate. In this work, we evaluate the generality of the impact of cationic mutations on other PrAMPs: apidaecin-1b, pyrrhocoricin, and bactenecin. Apidaecin-1b is one of three natural forms of a PrAMP isolated from the hemolymph of honeybees(Casteels, Ampe, Jacobs, Vaeck, & Tempst, 1989). Pyrrhocoricin was isolated from firebug(Cociancich et al., 1994). Bactenecin 7 (Bac7) is a 60-residue mammalian PrAMP from cows(Gennaro, Skerlavaj, & Romeo, 1989). A C-terminal truncated form Bac7(1-16) was shown to have antimicrobial activities comparable to the full-length version(Benincasa et al., 2004).
PrAMPs pass through cell membranes without causing cell lysis at bacteriostatic and bactericidal concentrations. The non-lytic mechanism is consistent with the presence of membrane transporters to facilitate cellular uptake into bacteria(Graf et al., 2017). The inner membrane protein SbmA was shown to play an important role in the transportation of insect and mammalian PrAMPs(Salomón & Farías, 1995; Mattiuzzo et al., 2007). However, it was found that bacteria without SbmA did not fully confer resistance towards PrAMPs as other inner membrane proteins were also discovered to regulate PrAMPs transportation. The inner membrane protein MdtM was reported as a transporter protein for some PrAMPs(Krizsan, Knappe, & Hoffmann, 2015). In addition, YgdD protein was found to be essential for the complete susceptibility of E. coli for arasin 1(1-25)(Paulsen etal., 2016).
The ribosome is considered as the intracellular target of PrAMPs(Krizsan et al., 2014; Krizsan, Prahl, Goldbach, Knappe, & Hoffmann, 2015; Seefeldt et al., 2015; Gagnon et al., 2016). However, the inhibition mechanisms of action might vary for different PrAMPs(Krizsan, Prahl, et al., 2015). The oncocin-type peptides inhibit protein translation in E. coli by binding to the exit tunnel of the 70S ribosome whereas the apidaecin-type peptides block the assembly of the large (50S) subunit of the ribosome. The crystal structures of pyrrhocoricin and Bac7(1-16) bound on the ribosome reveals that pyrrhocoricin and Bac7(1-16) overlap with the binding site of oncocin derivatives which is consistent with inhibition of protein translation(Seefeldt et al., 2015; Roy et al., 2015; Seefeldt et al., 2016; Gagnon et al., 2016). An alternative mechanism for apidaecin and its derivative is that they bind to the ribosome and trap release factors RF1 or RF2 which causes the majority of ribosomes to stall at stop codons and interfere with polypeptide release(Matsumoto et al., 2017; Florin et al., 2017).
There have been several studies aiming to improve the functions of these PrAMPs. Mutation of the first three residues of apidaecin to RVR (from GNN) markedly enhanced antimicrobial activity(Taguchi, Mita, Ichinohe, & Hashimoto, 2009). In addition, an analog of apidaecin-1b, Api137 (gu-ONNRPVYIPRPRPPHPRL-OH, gu = N,N,N’,N’-tetramethylguanidino, O = ornithine), was engineered to increase the serum stability (half-life: 345 min) compared to the apidaecin-1b with C-terminal amide (12 min) and with free acid (161 min)(Berthold et al., 2013). A cyclic pyrrhocoricin derivative was designed to link the N- and C-termini by a linker of nine amino acids. The macrocyclic derivative does not adversely affect the antimicrobial potency but leads to a broader spectrum of activity(Rosengren, Göransson, Otvos, & Craik, 2004). Chemical modifications of pyrrhocoricin with cyclohexane-1-carboxylic acid and monoacetyl-diamino-propionic acid reduce toxicity and enhance serum stability(Cudic, Bulet, Kragol, & Otvos, 2001). PEGylation of the peptide Bac7(1-35) reduces renal clearance without affecting antibacterial activity and bacterial cell penetration capacity(Benincasa et al., 2015).
In our previous work, we designed a peptide library with monosubstitution of the first 11 residues of oncocin and found that particular lysine and arginine substitutions resuited in more active mutants. Several multisubstituted variants were synthesized based on the monosubstitution results. The optimized multisubstituted mutants further increased the antimicrobial activities. Following this design strategy, in this work, we performed systematic mutagenesis of apidaecin-1b, pyrrhocoricin and Bac7(1-16) with lysine and arginine substitutions at each position. The active variants were combined to design multisubstitution analogs to further increase antimicrobial activities. The multisubstituted mutants were tested against some strains of E. coli and S. Typhimurium. In addition, activity testing against inner membrane protein (SbmA or YgdD) knockout E. coli strains was performed to elucidate the impact of these transporters in context of cationic mutants. Moreover, an HPLC-based binding and internalization assay was conducted to give insights into the binding and transportation mechanisms of the improved mutants under various growth media conditions and against different bacterial strains.
2. MATERIALS AND METHODS
2.1. SPOT synthesis
The SPOT-synthesized peptides of apidaecin-1b, pyrrhocoricin and Bac7(1-16) with monosubstituion of lysine or arginine at each position were purchased from Kinexus (Vancouver, Canada). The peptides have an additional glycine at the C-terminus to increase the yield during immobilized synthesis(Kramer & Schneider-Mergener, 1998). Each peptide, delivered as a paper disc form in a plastic tube, was dissolved in 160 μL Milli-Q water. A subset of peptides were randomly picked for quality control using HPLC and matrix-associated laser-desorption time-of-flight mass spectrometry. The purity for monosubstituted apidaecin-1b was 38 ± 2 %, for pyrrhocoricin was 53 ± 18 % and for Bac7(1-16) was 49 ± 8 %, respectively (Table S1).
2.2. Purified peptides
The purified peptides of apidaecin-1b, pyrrhocoricin and Bac7(1-16) derivatives were synthesized from Synpeptide (Shanghai, China). The purities of all peptides were > 95% (Fig. S1). The purified oncocin derivatives were obtained from our previous work(Lai et al., 2018).
2.3. Microdilution broth assay
The antimicrobial activity of each peptide was measured by microdilution broth assay. The bacteria strains (Table 1) were cultivated on lysogeny broth (LB, Fisher Scientific BP1427-2) with agar overnight at 37 °C. A single colony was added to 2 mL nutrient broth (RPI N15100) and incubated overnight at 37 °C to make liquid culture. 150 μL overnight liquid culture was added to 3 mL of growth media and shaken at 37 °C for 6 h to reach exponential growth phase. The bacterial culture was diluted with growth media to reach a suspension of 5 × 105 colony-forming unit (cfu)/mL. The growth media were 12.5% Mueller Hinton Broth (MHB, Sigma-Aldrich 70192) or 5% and 20% minimal M9 broth (Cat. No. M8000, TEKNOVA). The peptide solution was twofold diluted in Milli-Q water and 10 μL of the peptide solution was added to 90 μL of the diluted bacterial culture in a 96-well plate. The culture was incubated at 37 °C for 18 ± 1 h and the cell density was measured by optical density at 600 nm (OD600). The minimum inhibitory concentration (MIC) was defined as the lowest peptide concentration with no observable bacterial growth (OD600 < 0.08). The experiment was conducted in triplicate on different days to ensure the results are reproducible.
Table 1:
Bacteria and mammalian cells used in this study
| Bacterial strain | Description | Source |
|---|---|---|
| E. coli BW25113 | Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Keio Collection, GenoBase |
| E. coli JW0013 | ΔdnaK734::kan, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Keio Collection, GenoBase |
| E. coli JW0368 | ΔsbmA742::kan, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Keio Collection, GenoBase |
| E. coli JW2778 | ΔygdD735::kan, Δ(araD-araB)567, ΔlacZ4787(::rrnB-3), λ-, rph-1, Δ(rhaD-rhaB)568, hsdR514 | Keio Collection, GenoBase |
| E. coli MC1061 F’ | Plasmid-free, recA+, non-amber suppressor strain | Lucigen |
| E. coli J2210 | Common pathogenic species | University of Minnesota |
| E. coli JJ2487 | Common pathogenic species | Minneapolis VA hospital |
| E. coli FVEC 638 | Commensal human isolate from fecal sample | Minneapolis VA hospital |
| E. coli FVEC 964 | Commensal human isolate from fecal sample | Minneapolis VA hospital |
| E. coli PUTI 379 | Commensal human isolate from fecal sample | Minneapolis VA hospital |
| Salmonella enterica serovar Enteritidis MH91989 | Common pathogenic species | University of Minnesota |
| Salmonella enterica serovar Typhimurium ATCC SL1344 | Common pathogenic species | University of Minnesota |
| Salmonella enterica serovar Tennessee | Common pathogenic species | University of Minnesota |
| Salmonella enterica serovar Newport | Common pathogenic species | University of Minnesota |
| Salmonella Infantis #129 B | Common pathogenic species | University of Minnesota |
| Mammalian Cell Types | ||
| A431 | human epidermoid carcinoma | University of Minnesota |
| MDA-MB-231 | human breast adenocarcinoma | University of Minnesota |
2.4. Statistical analysis of MIC values
The MIC value is defined as the lowest concentration without detectable cell growth. However, the actual MIC value is between the measured MIC value and the next lower concentration in the dilution series. We have previously proposed a statistical analysis method for these censored data(Lai et al., 2018). The actual MIC value is assumed normally distributed with mean μ, and standard deviation σ. The random variable x is the MIC value from experiment. The piecewise probability between two values a and b is defined as
| (1) |
| (2) |
where b is the measured MIC value and a is the next lower peptide concentration. In order to obtain the marginal distribution of μ contingent on the observed data, the probability P is integrated over σ and normalized:
| (3) |
Each combination of experimental measurements has a distribution of μ. The actual MIC value is defined as the mode of μ. The uncertainty of μ is defined as the average difference between the mode and the limits of the 68% confidence interval. A MATLAB script for MIC value calculation is available from our previous work(Lai et al., 2018).
2.5. Cellular binding and internalization assay
Previously, we have designed an HPLC-based kinetic assay to measure the binding and internalization rate(Lai et al., 2018). The rate was obtained by calculating the concentration of peptides left in the cell-free supernatant over time by HPLC. Briefly, 0.2 mL of two peptide solutions were added to 4 mL of 5% or 20% M9 minimal growth media at 0 min. The concentrations of the peptides were 50 μmol/L. 0.2 mL overnight bacterial culture (~ 2 × 108 cells) was centrifuged, and the pellet was resuspended in the diluted peptide solutions. The bacterial culture was then incubated at 37 °C. At 0, 30 and 60 min, 1 mL of the culture was centrifuged at 3000 × g for 3 min and the supernatant was sterile filtered (0.2 μm) and analyzed by reverse-phase HPLC (Dionex UltiMate 3000 UHPLC, Sunnyvale, CA, USA) with an XBridge Peptide BEH C18 column (Waters Corp., Milford, MA, USA) using a linear gradient from 5.5 to 35.5% aqueous acetonitrile for 17 min in the presence of 0.1% trifluoroacetic acid as ion pair reagent. The absorbance was measured at 280 nm.
2.6. Cytotoxicity assay
A431 human epidermoid carcinoma cells and MDA-MB-231 human breast adenocarcinoma cells were grown at 37°C in a humidified atmosphere with 5% CO2 in DMEM with 10% fetal bovine serum and 1% penicillin streptomycin. Cells were washed with sterile PBS and 10000 cells were inoculated into 200 μL media in sterile, clear 96-well plates and grown overnight. After overnight growth, cells were washed with PBS and 180 μL fresh media was added. Subsequently peptide solutions diluted in PBS were added (20 μL per well, 10 μmol/L final well concentration) and incubated for 24 h at growth conditions. Cell viability was determined using the Vybrant MTT Cell Viability Assay (ThermoFisher Scientific V13154). Cell media was removed and replaced with 100 μL of fresh media. 10 μL of 12mM 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) stock solution prepared in PBS was added and incubated at growth conditions for 4 h. 100 μL of sodium dodecyl sulfate (SDS)-HCl solution (10 mL of 0.01M HCl, 1 g SDS) was then added and incubated for 4 h at growth conditions. The samples were then mixed using a pipette and absorbance was measured at 570 nm. Positive and negative controls were conducted by substituting the peptide solution with the same volumes of dimethyl sulfoxide (DMSO) and PBS, respectively. All experimental conditions were conducted in duplicate.
3. RESULTS AND DISCUSSION
3.1. Activity of monosubstituted PrAMPs analogs
Thirty-four apidaecin-1b, 37 pyrrhocoricin and 25 Bac7(1-16) variants – representing lysine and arginine amino acid variants at each site – were produced via SPOT synthesis. The antimicrobial activity was evaluated against E. coli strain JW0013 in 12.5% MHB. This strain was chosen because it lacks the secondary target of PrAMPs, heat shock protein DnaK, which allows the analysis to focus on the primary target, the ribosome, as well as PrAMP transport. It is noted that the antimicrobial activity is media dependent as shown in our previous work(Lai et al., 2018). The peptides tend to have higher activity in diluted media when the bacteria are in a disadvantaged condition. Some studies reported the MIC values in rich media; however, our work follows the protocol from Knappe et al.(Knappe, Ruden, et al., 2016) that used diluted 12.5% MHB medium for peptide screening.
The greatest decrease in MIC relative to wild-type apidaecin-1b (2-fold to 0.30 ± 0.07 μmol/L, p < 0.05) was achieved via mutation of arginine at site 4 to lysine or mutation of isoleucine at position 8 to lysine or arginine and mutation of histidine at position 15 to arginine (Fig. 1). For pyrrhocoricin, the greatest improvement relative to wild-type (2-fold to 0.30 ± 0.07 μmol/L, p < 0.05) was observed for mutation of leucine at position 7 to lysine and arginine and mutation of arginine at position 14 to lysine. Pyrrhocoricin has a very similar sequence to oncocin, which has been studied in our previous work (Table 2). Both peptides showed increased activity for L7K/R mutants. For Bac7(1-16), 14 out 24 wild-type variants showed improvement in activity, and the most enhanced analogs were from lysine mutation of arginine at position 4 or proline at position 5 and arginine at position 16 (2.5-fold to 0.24 ± 0.06 μmol/L, p < 0.05).
Figure 1:
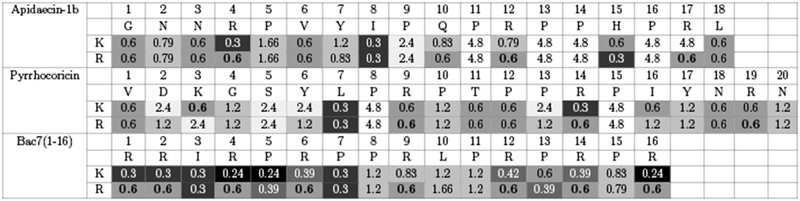
E. coli inhibition from lysine and arginine substitutions of apidaecin-1b, pyrrhocoricin and Bac7(1-16). Each column represents the minimum inhibitory concentration (MIC, in μmol/L) of an amino acid replacement at each position. The values are the mean MIC (n = 3) evaluated by dilution test against E. coli strain JW0013. Boxes are color coded: darker colors indicate lower MIC values and higher activity, and white color represents no activity at the highest concentration. The wild-type residues are emboldened.
Table 2:
Sequence of PrAMPs studied in this work. The sequence identity is calculated by EMBOSS Needle protein sequence alignment compared to oncocin
| Oncocin | V | D | K | P | P | Y | L | P | R | P | R | P | P | R | R | I | Y | N | R | -NH2 | Sequence Identity (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Apidaecin-1b | G | N | N | R | P | V | Y | I | P | Q | P | R | P | P | H | P | R | L | -OH | 38 | ||
| Pyrrhocoricin | V | D | K | G | S | Y | L | P | R | P | T | P | P | R | P | I | Y | N | R | N | -OH | 75 |
| Bactenecin 7(1-16) | R | R | I | R | P | R | P | P | R | L | P | R | P | R | P | R | -OH | 36 |
Beyond specific beneficial mutations, much can be learned from the relative tolerance of each site to basic amino acid mutations. All six proline residues for apidaecin-1b were not tolerable to Lys and Arg mutations. The MIC values increased from 2.8 to 8-fold. The MIC for R17K mutant also increased by 8-fold. For pyrrhocoricin, the activity of 12 out of 20 positions were decreased from mutations (with MIC increased at least 2-fold). The proline at 8 and 15 positions showed significant loss in potency (8-fold decrease). Bac7(1-16) was generally tolerant of mutation with only three of the 16 sites (P8, L10, P11) showed 2 to 2.8-fold increase in MIC values.
In general, the cationic state and relatively similar size of lysine and arginine result in comparable activity for the two options at most sites (Fig. 2). Only three sites exhibit more than 2-fold different MIC between the cationic pair: apidaecin-1b R17, for which wild-type arginine exhibits 8-fold lower MIC than lysine; pyrrhocoricin K3, which prefers wild-type lysine 4-fold; and Bac7(1-16) R4, for which mutant lysine outperforms wild-type arginine 2.5-fold. The benefit of apidaecin-1b R17 is consistent with previous literature demonstrating beneficial interactions with the ribosome(Krizsan et al., 2014) and release factors(Florin et al., 2017).
Figure 2:
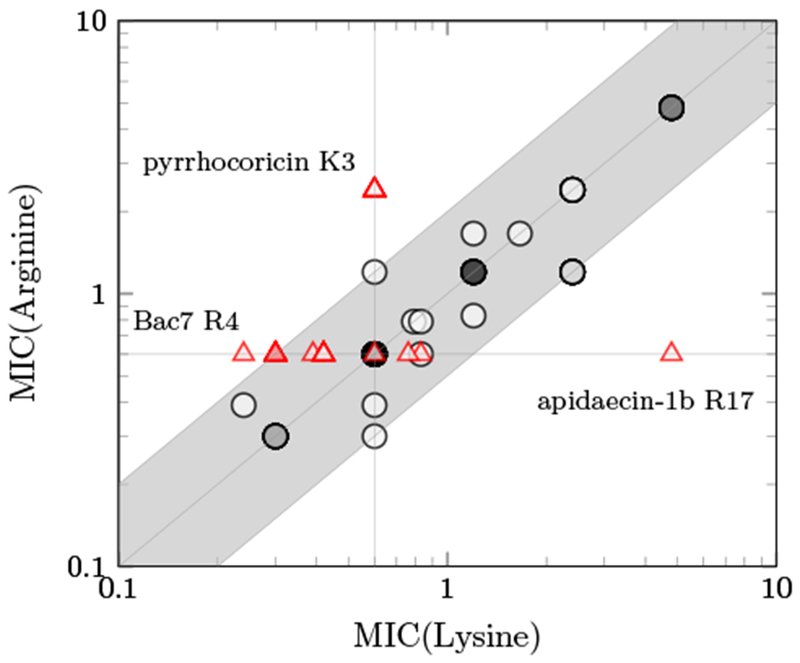
The MIC values of apidaecin-1b, pyrrhocoricin and Bac7(1-16) derivatives with lysine versus arginine substitutions against E. coli strain JW0013. The triangle indicates the wild-type residue is either lysine or arginine, and the circle indicates other residues. The darkness of each data point is proportional to the number of overlapping peptides at that MIC combination. The shaded area shows the MIC values within 2-fold.
3.2. Activity of multisubstituted PrAMPs analogs
From the results of the monosubstituted mutants, we combined several favorable substitutions to design multisubstitution variants for further evaluation (Table 3). We only selected variants that had at least two mutations to keep combinatorial diversity constrained for experimental efficiency. The peptides were synthesized on resin and purified (>95%) for antimicrobial activity measurement and mechanistic studies.
Table 3:
Amino acid substitutions of the three PrAMPs in the multisubstitution library based on improved MIC values against E. coli strain JW0013 as monosubstitutions
| Api_WT | G | N | N | R | P | V | Y | I | P | Q | P | R | P | P | H | P | R | L | ||
| Api_R4K/I8K | G | N | N | K | P | V | Y | K | P | Q | P | R | P | P | H | P | R | L | ||
| Api_R4K/I8R | G | N | N | K | P | V | Y | R | P | Q | P | R | P | P | H | P | R | L | ||
| Api_R4K/H15R | G | N | N | K | P | V | Y | I | P | Q | P | R | P | P | R | P | R | L | ||
| Api_I8K/H15R | G | N | N | R | P | V | Y | K | P | Q | P | R | P | P | R | P | R | L | ||
| Api_I8R/H15R | G | N | N | R | P | V | Y | R | P | Q | P | R | P | P | R | P | R | L | ||
| Api_R4K/I8K/H15R | G | N | N | K | P | V | Y | K | P | Q | P | R | P | P | R | P | R | L | ||
| Api_R4K/I8R/H15R | G | N | N | K | P | V | Y | R | P | Q | P | R | P | P | R | P | R | L | ||
| Pyr_WT | V | D | K | G | S | Y | L | P | R | P | T | P | P | R | P | I | Y | N | R | N |
| Pyr_L7K/R14K | V | D | K | G | S | Y | K | P | R | P | T | P | P | K | P | I | Y | N | R | N |
| Pyr_L7R/R14K | V | D | K | G | S | Y | R | P | R | P | T | P | P | K | P | I | Y | N | R | N |
| Bac_WT | R | R | I | R | P | R | P | P | R | L | P | R | P | R | P | R | ||||
| Bac_R4K/P5K | R | R | I | K | K | R | P | P | R | L | P | R | P | R | P | R | ||||
| Bac_R4K/R16K | R | R | I | K | P | R | P | P | R | L | P | R | P | R | P | K | ||||
| Bac_P5K/R16K | R | R | I | R | K | R | P | P | R | L | P | R | P | R | P | K | ||||
| Bac_R4K/P5K/R16K | R | R | I | K | K | R | P | P | R | L | P | R | P | R | P | K |
Peptide activity was evaluated, across multiple E. coli and Salmonella species, for the multisubstituted mutants in a more purified form (Table 4). It is noted that the resin-synthesized peptides have no additional glycine at the C-terminus compared to the SPOT synthesized peptides. For apidaecin-1b, the Api_R4K/I8K and Api_R4K/I8R mutants showed 2-fold improvement relative to wild-type (p < 0.05) for nonpathogenic E. coli strains JW0013 and MC1041, but only showed nominal increase in potency against pathogenic E. coli and Salmonella from clinical isolates. Moreover, all the H15R-containing mutants were less active compared to the wild-type apidaecin-1b. One possible reason for the lack of efficacy for H15R is that the C-terminal region is essential for the antimicrobial activity of apidaecin, and the additional glycine from the SPOT synthesis might affect the mutagenesis scanning. For instance, the substitution of the penultimate arginine to alanine in hornet apidaecin (GKPRPQQVPPRPPHPRL) resulted in an 2500-fold increase in MIC(Castle, Nazarian, Tempst, et al., 1999).
Table 4:
Antimicrobial activity of multisubstituted PrAMPs derivatives against some strains of the Gram-negative bacteria E. coli and S. Typhimurium in 12.5% MHB. The values indicate the mode ± the uncertainty. The MIC values that are at least 2 times smaller than the wild-type are shown in bold. The MIC values are in μmol/L.
| Nonpathogenic E. coli | Pathogenic E. coli | Salmonella | ||||
|---|---|---|---|---|---|---|
| Peptides | JW0013 | MC1041 | J2210 | JJ2489 | MH91989 | SL1344 |
| Api_WT | 0.024 ± 0.006 | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.031 ± 0.014 | 0.048 ± 0.011 | 0.048 ± 0.011 |
| Api_R4K/I8K | 0.012 ± 0.003* | 0.024 ± 0.006* | 0.034 ± 0.017 | 0.031 ± 0.014 | 0.031 ± 0.014 | 0.034 ± 0.017 |
| Api_R4K/I8R | 0.012 ± 0.003* | 0.024 ± 0.006* | 0.034 ± 0.017 | 0.034 ± 0.017 | 0.031 ± 0.014 | 0.034 ± 0.017 |
| Api_R4K/H15R | 0.048 ± 0.011 | 0.096 ± 0.023 | 0.133 ± 0.061 | 0.067 ± 0.033 | 0.067 ± 0.033 | 0.126 ± 0.052 |
| Api_I8K/H15R | 0.062 ± 0.027 | 0.133 ± 0.061 | 0.252 ± 0.087 | 0.133 ± 0.061 | 0.096 ± 0.023 | 0.192 ± 0.044 |
| Api_I8R/H15R | 0.048 ± 0.011 | 0.096 ± 0.023 | 0.126 ± 0.052 | 0.126 ± 0.052 | 0.067 ± 0.033 | 0.126 ± 0.052 |
| Api_R4K/I8K/H15R | 0.096 ± 0.023 | 0.192 ± 0.044 | 0.267 ± 0.097 | 0.252 ± 0.087 | 0.133 ± 0.061 | 0.192 ± 0.044 |
| Api_R4K/I8R/H15R | 0.096 ± 0.023 | 0.126 ± 0.052 | 0.252 ± 0.087 | 0.133 ± 0.061 | 0.096 ± 0.023 | 0.192 ± 0.044 |
| Pyr_WT | 0.150 ± 0.034 | 0.416 ± 0.205 | 0.786 ± 0.322 | 0.600 ± 0.140 | 1.200 ± 0.278 | 1.200 ± 0.278 |
| Pyr_L7K/R14K | 0.075 ± 0.018* | 0.150 ± 0.034* | 0.208 ± 0.106* | 0.104 ± 0.053* | 0.600 ± 0.140* | 0.150 ± 0.034* |
| Pyr_L7R/R14K | 0.037 ± 0.008* | 0.075 ± 0.018* | 0.075 ± 0.018* | 0.078 ± 0.064* | 0.150 ± 0.034* | 0.075 ± 0.018* |
| Bac_WT | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.120 ± 0.028 | 0.120 ± 0.028 | 0.120 ± 0.028 | 0.060 ± 0.014 |
| Bac_R4K/P5K | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.157 ± 0.054 | 0.120 ± 0.028 | 0.120 ± 0.028 | 0.120 ± 0.028 |
| Bac_R4K/R16K | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.120 ± 0.028 | 0.120 ± 0.028 | 0.157 ± 0.054 | 0.120 ± 0.028 |
| Bac_P5K/R16K | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.157 ± 0.054 | 0.120 ± 0.028 | 0.120 ± 0.028 | 0.120 ± 0.028 |
| Bac_R4K/P5K/R16K | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.167 ± 0.061 | 0.120 ± 0.028 | 0.167 ± 0.061 | 0.120 ± 0.028 |
denotes p<0.05
In pyrrhocoricin, the swap of cation (R14K) and introduction of a new cation (L7K or L7R) enhanced activity up to 10.5-fold against some strains of the Gram-negative bacteria E. coli and S. Typhimurium, with greater potency for L7R. In our previous study, the L7K/R mutants for oncocin also showed improved activity. Since oncocin and pyrrhocoricin have high sequence identity (75%, Table 2), it could be compelling to evaluate the substitution of leucine to lysine or arginine residues at this position for potential improvement of antimicrobial activity for other oncocin-like PrAMPs such as riptocin (VDKGGYLPRPTPPRPVYRS)(J. K. Kim et al., 2015).
The MIC values for Bac7(1-16) derivatives did not show any improvement compared to Bac_WT against all species. The enhanced potency from SPOT synthesis might be due to differences in synthesis preparations, the additional glycine on the C-terminus, or negative synergy.
To expand the breadth of assessment for the most potent mutants, we tested activity on additional E. coli and Salmonella strains (Table 5). The pyrrhocoricin mutants Pyr_L7K/R14K and Pyr_L7R/R14K were more active than wild-type against all six additional strains. In particular, Pyr_L7R/R14K was at least 3- to 4-fold and 6- to 11-fold more potent than wild-type against E. coli and Salmonella strains, respectively. The apidaecin-1b mutants Api_R4K/I8K and Api_R4K/I8R were more potent than wild-type against the Salmonella strains but did not show improvement for three commensal E. coli strains.
Table 5:
Antimicrobial activity of the most potent PrAMPs derivatives against some strains of the Gram-negative bacteria E. coli and S. Typhimurium in 12.5% MHB. The values indicate the mode ± the uncertainty. The MIC values that are at least 2 times smaller than the wild-type are shown in bold. The MIC values are in μmol/L.
| Commensal E. coli | Salmonella | |||||
|---|---|---|---|---|---|---|
| Peptides | FVEC 638 | FVEC 964 | PUTI 379 | Tennessee | Newport | Infantis #129 B |
| Api_WT | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.031 ± 0.014 | 0.048 ± 0.011 |
| Api_R4K/I8K | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.024 ± 0.006* | 0.024 ± 0.006 | 0.024 ± 0.006* |
| Api_R4K/I8R | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.048 ± 0.011 | 0.024 ± 0.006* | 0.017 ± 0.009 | 0.024 ± 0.006* |
| Pyr_WT | >0.416 | >0.600 | >0.600 | >0.600 | >0.416 | >0.600 |
| Pyr_L7K/R14K | 0.197 ± 0.088* | 0.300 ± 0.070* | 0.300 ± 0.070* | 0.300 ± 0.070* | 0.075 ± 0.018* | 0.300 ± 0.070* |
| Pyr_L7R/R14K | 0.150 ± 0.034* | 0.150 ± 0.034* | 0.208 ± 0.106* | 0.075 ± 0.018* | 0.037 ± 0.008* | 0.098 ± 0.045* |
denotes p<0.05
3.3. Activity of SbmA/YgdD knockout strains
The transportation of PrAMPs into cells requires inner membrane proteins(Graf et al., 2017). The inner membrane protein SbmA is considered as the major transporter for most PrAMPs(Mattiuzzo et al., 2007). Recently, other inner membrane proteins were found to facilitate transportation as well(Krizsan, Knappe, & Hoffmann, 2015; Paulsen et al., 2016). In particular, the YgdD protein was reported to be essential for the complete susceptibility of E. coli for arasin 1(1-25)(Paulsen et al., 2016). It is informative to test the role of SbmA and YgdD proteins to the antimicrobial activities for apidaecin-1b, pyrrhocoricin and Bac7(1-16) derivatives.
To assess the impact of the SbmA and YgdD transporters, we compared antimicrobial potency on parental BW25113 E. coli and individual knockouts of each transporter: ΔSbmA (JW0368) and ΔYgdD (JW2778) (Table 6). The MIC values of Api_WT increased by 16-fold and 2-fold for the ΔSbmA and ΔYgdD strains compared to the BW25113 strain (from 0.024 to 0.384 and 0.048 μmol/L), respectively. This is consistent with SbmA as the primary transporter and YgdD as the secondary transporter for apidaecin-1b. The role of SbmA receptor as the major transporter agrees with previous work(Narayanan et al., 2014; Krizsan, Knappe, & Hoffmann, 2015). Consistent with previous results, Api_R4K/I8K and Api_R4K/I8R mutants displayed improvement for non-pathogenic BW25113 strain. These mutants similarly enhanced potency against ΔYgdD, which implies their improvement does not stem from improved interaction with YgdD. Interestingly, all apidaecin-1b mutants except Api_R4K/H15R showed higher activity than wild-type against the SbmA knockout strain. This is consistent with the mutations enhancing transport across the inner membrane, which is not impactful in the presence of SbmA but limiting in the transporter’s absence.
Table 6:
Antimicrobial activity of multisubstituted PrAMPs derivatives against parental and inner membrane protein knockout E. coli strains in 12.5% MHB. The values indicate the mode ± the uncertainty. The MIC values that are at least 2 times smaller than the wild-type are shown in bold. The MIC values are in μmol/L.
| Parental E. coli | ΔSbmA | ΔYgdD | |
|---|---|---|---|
| Peptides | BW25113 | JW0368 | JW2778 |
| Api_WT | 0.024 ± 0.006 | 0.384 ± 0.080 | 0.048 ± 0.011 |
| Api_R4K/I8K | 0.017 ± 0.009 | 0.096 ± 0.023* | 0.024 ± 0.006* |
| Api_R4K/I8R | 0.012 ± 0.003* | 0.096 ± 0.023* | 0.024 ± 0.006* |
| Api_R4K/H15R | 0.048 ± 0.011 | 0.384 ± 0.080 | 0.096 ± 0.023 |
| Api_I8K/H15R | 0.067 ± 0.033 | 0.096 ± 0.023* | 0.133 ± 0.061 |
| Api_I8R/H15R | 0.062 ± 0.027 | 0.096 ± 0.023* | 0.126 ± 0.052 |
| Api_R4K/I8K/H15R | 0.096 ± 0.023 | 0.192 ± 0.044* | 0.192 ± 0.044 |
| Api_R4K/I8R/H15R | 0.096 ± 0.023 | 0.133 ± 0.061* | 0.192 ± 0.044 |
| Pyr_WT | 0.197 ± 0.088 | 2.400 ± 0.499 | 0.600 ± 0.140 |
| Pyr_L7K/R14K | 0.075 ± 0.018* | 0.834 ± 0.382* | 0.208 ± 0.106* |
| Pyr_L7R/R14K | 0.037 ± 0.008* | 0.300 ± 0.070* | 0.104 ± 0.053* |
| Bac_WT | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.083 ± 0.038 |
| Bac_R4K/P5K | 0.078 ± 0.032 | 0.060 ± 0.014 | 0.120 ± 0.028 |
| Bac_R4K/R16K | 0.060 ± 0.014 | 0.060 ± 0.014 | 0.120 ± 0.038 |
| Bac_P5K/R16K | 0.078 ± 0.032 | 0.060 ± 0.014 | 0.120 ± 0.038 |
| Bac_R4K/P5K/R16K | 0.078 ± 0.032 | 0.060 ± 0.014 | 0.120 ± 0.038 |
denotes p<0.05
Similarly to apidaecin-1b, the MIC values of Pyr_WT increased for the ΔSbmA and ΔYgdD strains compared to the BW25113 strain (by 12-fold and 3-fold, respectively, from 0.197 to 2.400 and 0.600 μmol/L). This indicated that SbmA is also the major transporter for pyrrhocoricin. Conversely to apidaecin-1b variants, the pyrrhocoricin variants did not exhibit further preferential enhancement in the ΔSbmA strain: The Pyr_L7K/R14K and Pyr_L7R/R14K mutants were 2.6 and 5.3-fold more active than Pyr_WT for the BW25113 strain (p<0.05) and 2.9 and 8-fold more potent than the wild-type for ΔSbmA (p<0.05).
The MIC values for Bac7(1-16) and the mutants were similar for the BW25113, JW0368 and JW2778 strains, which indicates that Bac7(1-16) could permeate through the inner membranes without any active transporter. This result is consistent with the previous study for the full length Bac7 in 100% MHB and Bac7(1-35) in 33% tryptic soy broth (TSB) medium(Krizsan, Knappe, & Hoffmann, 2015). In contrast, it has also been reported that the activity of Bac7(1-35) and Bac7(1-16) reduced against SbmA knockout strain in 100% MHB(Mattiuzzo et al., 2007; Krizsan, Knappe, & Hoffmann, 2015). From this work, Bac7(1-16) seems to lose SbmA dependency in diluted MHB medium. Because of the high arginine content, 8 out of 16 residues, Bac7(1-16) might possess similar transportation mechanisms as the arginine-rich cell-penetrating peptides via the formation of bidentate bonds (Lai & Kaznessis, 2018; Tang, Waring, & Hong, 2007; Pantos, Tsogas, & Paleos, 2008; Su, Li, & Hong, 2013) or perhaps gain cell entry via a different mechanism.
3.4. Internalization rate of the optimized peptides
We next aimed to assess if the enhanced antimicrobial activity of Api_R4K/I8R and/or Pyr_L7R/R14K result from improvements in cellular binding and internalization. Evaluation of the Api_R4K/I8R and Pyr_L7R/R14K revealed no appreciable increase in cellular association and internalization (Fig. 3 AC). Oncocin and its P4K/L7K variant were evaluated against the strain under current study, BW25113, to assess any strain-dependent differences versus the previous work with the similar, yet unique, strain JW0013. The oncocin P4K/L7K variant demonstrated enhanced binding/internalization relative to its wild-type (Fig. S2). The difference between oncocin and pyrrhocoricin is striking given their high sequence identity (Table 3). In earlier work (Lai et al., 2018), we developed an assay to measure cellular association/internalization of peptides, which revealed that particular potent cationic variants of oncocin exhibited elevated binding/internalization relative to wild-type. The transportation rate difference between oncocin and pyrrhocoricin, despite their high sequence identity, might be due to the presence of C-terminal amidation for oncocin and the absence for pyrrhocoricin (Table 3). Kim et al. showed that C-terminal amidation of antimicrobial peptide PMAP-23 increased the positive charge and stabilized the helical structure to facilitate membrane transportation(J.-Y. Kim, Park, Yoon, Hahm, & Park, 2011). It will be informative to investigate the effect of C-terminal amidation for PrAMPs on membrane transportation in future work. In addition, comparing the binding and internalization rate, oncocin shows a higher transportation rate in the presence of DnaK, which is in line with the speculation of a transportation function of DnaK for PrAMPs in previous study(Knappe, Goldbach, et al., 2016).
Figure 3:
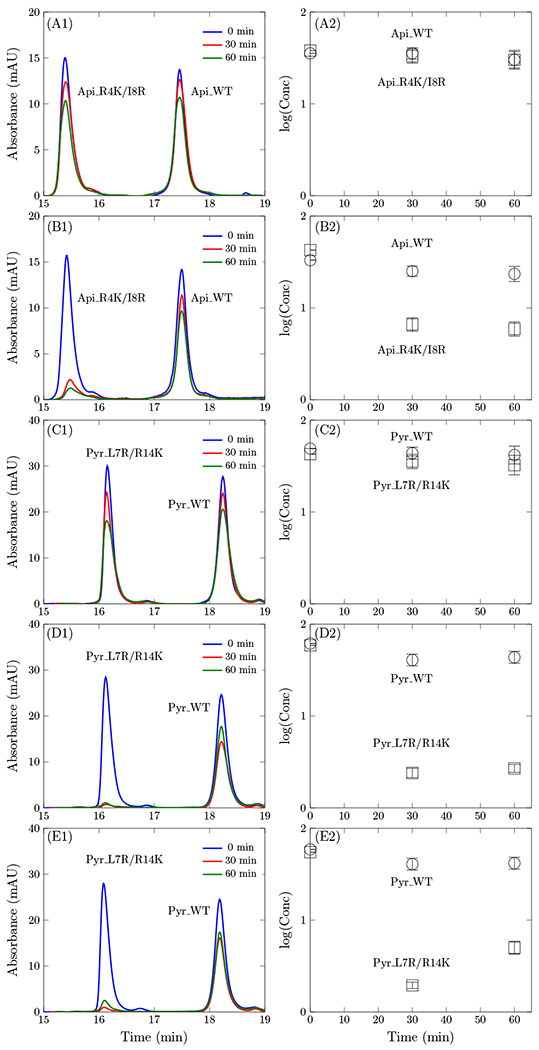
Cellular binding and internalization. Peptides (50 μmol/L) were incubated with BW25113 (A-D) or ΔSbmA (E) cells in 20% (A,C) or 5% (B,D,E) M9 growth medium. Cell-free supernatant was collected at 0, 30, or 60 min and analyzed via HPLC. HPLC traces at 280 nm are provided in column 1, and quantified peptide concentrations are provided in column 2.
Inspired by the previously observed increased cellular binding and internalization for oncocin variants in less-rich M9 broth, we evaluated the impact of reduced growth medium on Api_R4K/I8R and Pyr_L7R/R14K. Indeed, reduction of the growth medium to 5% M9 revealed substantially elevated binding/internalization of the cationic variants of apidaecin-1b and pyrrhocoricin whereas the wild-type peptides remained largely unassociated with cells (Fig. 3 BD). The HPLC data for Api_WT and Api_R4K/I8R in shorter time are shown in Fig. S3.
The kinetic assay was also conducted for the SbmA knockout strain and compared with the parental strain for Pyr_WT and Pyr_L7R/R14K (Fig. 3 DE). The peptides showed comparable association and internalization rate for both strains. This indicates that the HPLC assay measures the kinetic rate of cell binding and peptide permeation through the outer membrane. However, the timescale for peptide transportation through the inner membrane receptor might be longer than outer membrane permeation. It would be informative to measure the kinetic rate over a longer timescale as future work although experimental consideration of cell death will be required.
It is worth noting that other factors such as peptide degradation by protease or binding on the filter may attribute to the peptide loss. No degraded product is present in the HPLC analysis (Figs. S4 and S5) for apidaecin-1b and pyrrhocoricin mutants within 60 min. This is consistent with our previous work on homologous peptides in which we conducted a control experiment to evaluate the peptides in supernatant after cell growth for 2 h, and no peptide degradation was observed(Lai et al., 2018). In addition, the HPLC quantification at the first time point was performed to ensure the two peptides have equivalent initial concentrations, and no significant peptide loss was due to filtration. We should also stress that this technique measures peptide binding and/or internalization, which are essential steps for transportation.
3.5. Growth media dependence of the antimicrobial activity
Antimicrobial activity is growth medium dependent(G. Wang, 2017). It is valuable to evaluate if apidaecin-1b and pyrrhocoricin and their derivatives remain potent in the minimal M9 broth, which was used in the internalization and kinetic assay (the minimal medium enables definitive identification of the peptides via HPLC whereas the initial MIC assays were performed in the richer MHB medium). Table 7 illustrates the MIC values of Api_WT, Api_R4K/I8R, Pyr_WT and Pyr_L7R/R14K in 5% and 20% of the minimal M9 broth. The peptides were less active in 20% media relative to that in 5% media. The wild-type peptides were not potent in 20% M9 broth at the highest concentration. The MIC values for Api_R4K/I8R and Pyr_L7R/R14K increased by 2-fold in 20% media (from 0.192 to 0.384 and 0.150 to 0.300 μmol/L, respectively. p < 0.05).
Table 7:
Antimicrobial activity of multisubstituted PrAMPs derivatives against strain BW25113 in 5% M9 and 20% M9 growth media. The values indicate the mode ± the uncertainty. The MIC values are in μmol/L. The MIC values that are at least 2 times smaller than the wild-type are shown in bold.
| E. coli BW25113 | ||
|---|---|---|
| Peptides | 5% M9 | 20% M9 |
| Api_WT | 0.384 ± 0.080 | >0.384 |
| Api_R4K/I8R | 0.192 ± 0.044* | 0.384 ± 0.080* |
| Pyr_WT | 0.300 ± 0.070 | >0.600 |
| Pyr_L7R/R14K | 0.150 ± 0.034* | 0.300 ± 0.070* |
denotes p<0.05
3.6. Cytotoxicity assay
We evaluated the cytotoxicity of Api_WT, Api_R4K/I8R, Pyr_WT, and Pyr_L7R/R14K against MDA-MB-231 human breast adenocarcinoma cells and A431 epidermoid carcinoma cells to determine if their improved antimicrobial activity resulted in increased toxicity to mammalian cells. None of these PrAMPs exhibited any toxicity towards MDA-MB-231 (Fig. 4). While Pyr_WT and Pyr_L7R/R14K also showed no toxicity to A431 cells, Api_WT and Api_R4K/I8R showed 40% cytotoxicity. Mild apidaecin-1b toxicity has been previously observed at 600 μg/mL on HeLa cells (Hansen, Schafer, Knappe, Seibel, & Hoffmann, 2012) and at 2000 μg/mL on HEK293 and HepG2 cells (Berthold et al., 2013). A possible explanation for the increased cytotoxicity observed here is the use of different cells (A431 epidermoid cancer cells) with a relatively fast growth rate. Moreover, while fresh medium was provided for the 24 h peptide incubation, nutrient limitations could feasibly drive peptide uptake despite the lack of the traditional receptor system. While the precise mechanism of A431 toxicity requires further study, the existence of toxicity warrants careful consideration for applied use. Importantly, both apidaecin-1b and pyrrhocoricin mutants show comparable toxicity to their wild-type variants, showing that these mutations enable increased antibacterial activity without increased cytotoxicity.
Figure 4:
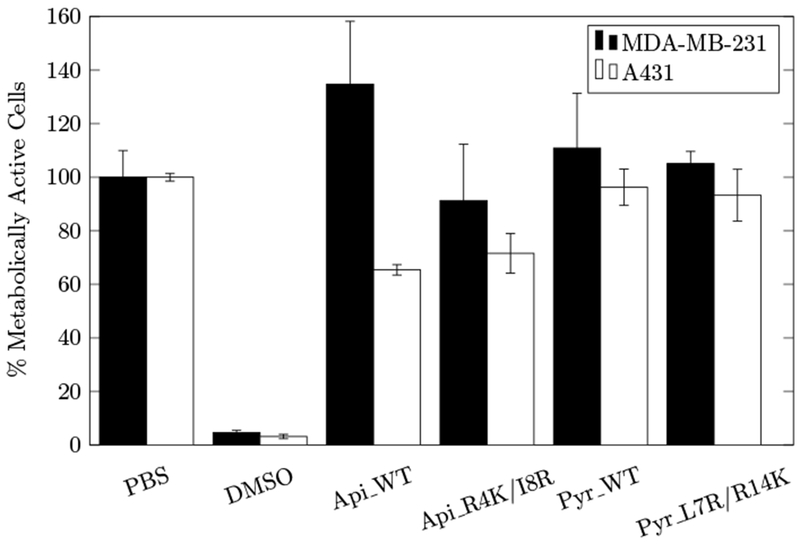
Impact of peptides or controls on human cell viability. MDA-MB-231 (black) and A431 (white) human cells were treated with the 10 μmol/L peptide (or control) for 24 h, and metabolic activity was assessed via MTT assay. Bars and error bars represent mean and standard deviation of duplicate samples normalized to PBS as negative control.
4. CONCLUSIONS
In this work, we performed systematic lysine and arginine mutagenesis of three proline-rich antimicrobial peptides: apidaecin-1b, pyrrhocoricin and bactenecin 7(1-16). Bac7(1-16) is widely tolerant of mutation. Aside from wild-type proline sites, apidaecin-1b is generally tolerant of mutations. Conversely, pyrrohocoricin was much less tolerant with five strongly non-tolerated mutations and nine weakly tolerated mutations. Lysine and arginine provide comparable activity at most locations, with one noted exception in each peptide. Selected single mutations enhanced activity 2- to 2.5-fold. Combination of two mutations in apidaecin-1b enhanced potency twofold against non-pathogenic E. coli but not pathogenic E. coli or Salmonella. Combination of two mutations in pyrrhocoricin substantially (2-fold to 10.5-fold) increased potency across all of these strains. Multimutants of Bac7(1-16) did not impact activity. The benefit of pyrrhocoricin mutation was maintained in the absence of SbmA or YgdD transporters suggesting either mutational benefit on interaction with both transporters or a non-transporter mode of enhanced activity. Conversely, the apidaecin-1b mutants were substantially improved in their relative activity enhancement in the absence of SbmA transporter, which is consistent with a mode of action that is obviated by SbmA transport. This work builds upon previous systematic mutation of the proline-rich oncocin peptide and reveals the scientific and technological merit of select cationic mutations within PrAMPs.
Supplementary Material
5. ACKNOWLEDGMENTS
This work was supported by a grant from the National Institutes of Health (R01GM121777).
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- Baltzer SA, & Brown MH (2011). Antimicrobial peptides–promising alternatives to conventional antibiotics. J. Mol. Microbiol. Biotechnol, 20,228–235. doi: 10.1159/000331009 [DOI] [PubMed] [Google Scholar]
- Benincasa M, Scocchi M, Podda E, Skerlavaj B, Dolzani L, & Gennaro R (2004). Antimicrobial activity of Bac7 fragments against drug-resistant clinical isolates. Peptides, 25,2055–2061. doi: 10.1016/j.peptides.2004.08.004 [DOI] [PubMed] [Google Scholar]
- Benincasa M, Zahariev S, Pelillo C, Milan A, Gennaro R, & Scocchi M (2015). PEGylation of the peptide Bac7(1–35) reduces renal clearance while retaining antibacterial activity and bacterial cell penetration capacity. Eur. J. Med. Chem, 95, 210–219. doi: 10.1016/j.ejmech.2015.03.028 [DOI] [PubMed] [Google Scholar]
- Berthold N, Czihal P, Fritsche S, Sauer U, Schiffer G, Knappe D, Alber G, & Hoffmann R (2013). Novel apidaecin 1b analogs with superior serum stabilities for treatment of infections by Gram-Negative pathogens. Antimicrob. Agents Chemother, 57,402–409. doi: 10.1128/aac.01923-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels P, Ampe C, Jacobs F, Vaeck M, & Tempst P (1989). Apidaecins: antibacterial peptides from honeybees. EMBO J., 8, 2387–2391. doi: 10.1002/j.1460-2075.1989.tb08368.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle M, Nazarian A, Tempst P, et al. (1999). Lethal effects of apidaecin on escherichia coliinvolve sequential molecular interactions with diverse targets. J. Biol. Chem, 274, 32555–32564. doi: 10.1074/jbc.274.46.32555 [DOI] [PubMed] [Google Scholar]
- Cociancich S, Dupont A, Hegy G, Lanot R, Holder F, Hetru C, Hoffmann JA, & Bulet P (1994). Novel inducible antibacterial peptides from a hemipteran insect, the sap-sucking bug pyrrhocoris apterus. J. Insect Physiol., 300 (Pt 2), 567–575. doi: 10.1042/bj3000567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudic M, Bulet P, Kragol G, & Otvos L (2001). Antibacterial activity spectrum of designed pyrrhocoricin analogs. In (p. 493–494). Springer. doi: 10.1007/978-94-010-0464-0_228 [DOI] [Google Scholar]
- Florin T, Maracci C, Graf M, Karki P, Klepacki D, Berninghausen O, Beckmann R, Vdzquez-Laslop N, Wilson DN, Rodnina MV, & Mankin AS (2017). An antimicrobial peptide that inhibits translation by trapping release factors on the ribosome. Nat. Struct. Mol. Biol, 24, 752–757. doi: 10.1038/nsmb.3439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon MG, Roy RN, Lomakin IB, Florin T, Mankin AS, & Steitz TA (2016). Structures of proline-rich peptides bound to the ribosome reveal a common mechanism of protein synthesis inhibition. Nucleic Acids Res., 44, 2439–2450. doi: 10.1093/nar/gkw018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennaro R, Skerlavaj B, & Romeo D (1989). Purification, composition, and activity of two bactenecins, antibacterial peptides of bovine neutrophils. Infect. Immun, 57, 3142–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M, Mardirossian M, Nguyen F, Seefeldt AC, Guichard G, Scocchi M, Innis CA, & Wilson DN (2017). Proline-rich antimicrobial peptides targeting protein synthesis. Nat. Prod. Rep, 34, 702–711. doi: 10.1039/C7NP00020K [DOI] [PubMed] [Google Scholar]
- Hansen A, Schafer I, Knappe D, Seibel P, & Hoffmann R (2012). Intracellular toxicity of proline-rich antimicrobial peptides shuttled into mammalian cells by the cell-penetrating peptide penetratin. Antimicrob. Agents Chemother., 56, 5194–5201. doi: 10.1128/aac.00585-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joerger RD (2003). Alternatives to antibiotics: bacteriocins, antimicrobial peptides and bacteriophages. Poultr. Sci, 82, 640–647. doi: 10.1093/ps/82.4.640 [DOI] [PubMed] [Google Scholar]
- Kim JK, Son DW, Kim C-H, Cho JH, Marchetti R, Silipo A, Sturiale L, Park HY, Huh YR, Nakayama H, et al. (2015). Insect gut symbiont’s susceptibility to host antimicrobial peptides caused by alteration of bacterial cell envelope. J. Biol. Chem, jbc–M115. doi: 10.1074/jbc.M115.651158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-Y, Park S-C, Yoon M-Y, Hahm K-S, & Park Y (2011). C-terminal amidation of pmap-23: translocation to the inner membrane of gram-negative bacteria. Amino acids, 40, 183–195. doi: 10.1007/s00726-010-0632-1 [DOI] [PubMed] [Google Scholar]
- Knappe D, Goldbach T, Hatfield MPD, Palermo NY, Weinert S, Strater N, Hoffmann R, & Lovas S (2016). Proline-rich antimicrobial peptides optimized for binding to escherichia coli chaperone dnak. Protein Pept. Lett., 23, 1061–1071. [DOI] [PubMed] [Google Scholar]
- Knappe D, Ruden S, Langanke S, Tikkoo T, Ritzer J, Mikut R, Martin LL, Hoffmann R, & Hilpert K (2016). Optimization of oncocin for antibacterial activity using a spot synthesis approach: extending the pathogen spectrum to staphylococcus aureus. Amino acids, 48, 269–280. doi: 10.1007/s00726-015-2082-2 [DOI] [PubMed] [Google Scholar]
- Konovalova M, Zubareva A, Lutsenko G, & Svirshchevskaya E (2018). Antimicrobial peptides in health and disease. Appl. Biochem. Microbiol, 54, 238–244. doi: 10.1056/NEJMe020106 [DOI] [Google Scholar]
- Kramer A, & Schneider-Mergener J (1998). Synthesis and screening of peptide libraries on continuous cellulose membrane supports. Methods Mol. Biol., 87, 25–39. [DOI] [PubMed] [Google Scholar]
- Krizsan A, Knappe D, & Hoffmann R (2015). Influence of the yjiL-mdtM gene cluster on the antibacterial activity of proline-rich antimicrobial peptides overcoming Escherichia coli resistance induced by the missing SbmA transporter system. Antimicrob. Agents Chemother, 59, 5992–5998. doi: 10.1128/AAC.01307-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krizsan A, Prahl C, Goldbach T, Knappe D, & Hoffmann R (2015). Short proline-rich antimicrobial peptides inhibit either the bacterial 70S ribosome or the assembly of its large 50S subunit. ChemBioChem, 16, 2304–2308. doi: 10.1002/cbic.201500375 [DOI] [PubMed] [Google Scholar]
- Krizsan A, Volke D, Weinert S, Strater N, Knappe D, & Hoffmann R (2014). Insect-derived proline-rich antimicrobial peptides kill bacteria by inhibiting bacterial protein translation at the 70S ribosome. Angew. Chem. Int. Ed. Engl, 53, 12236–12239. doi: 10.1002/anie.201407145 [DOI] [PubMed] [Google Scholar]
- Lai P-K, Geldart K, Ritter S, Kaznessis YN, & Hackel BJ (2018). Systematic mutagenesis of oncocin reveals enhanced activity and insights into the mechanisms of antimicrobial activity. Mol. Syst. Des. Eng, 3,930. doi: 10.1039/C8ME00051D [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai P-K, & Kaznessis YN (2018). Insights into membrane translocation of protegrin antimicrobial peptides by multistep molecular dynamics simulations. ACS Omega, 3,6056–6065. doi: 10.1021/acsomega.8b00483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Tailhades J, O’Brien-Simpson NM, Separovic F, Otvos L, Hossain MA, & Wade JD (2014). Proline-rich antimicrobial peptides: potential therapeutics against antibiotic-resistant bacteria. Amino acids, 46,2287–2294. doi: 10.1007/s00726-014-1820-1 [DOI] [PubMed] [Google Scholar]
- Matsumoto K, Yamazaki K, Kawakami S, Miyoshi D, Ooi T, Hashimoto S, & Taguchi S (2017). In vivo target exploration of apidaecin based on acquired resistance induced by gene overexpression (argo assay). Sci. Rep, 7, 12136. doi: 10.1038/s41598-017-12039-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattiuzzo M, Bandiera A, Gennaro R, Benincasa M, Pacor S, Antcheva N, & Scocchi M (2007). Role of the Escherichia coli SbmA in the antimicrobial activity of proline-rich peptides. Mol. Microbiol, 66, 151–163. doi: 10.1111/j.1365-2958.2007.05903.x [DOI] [PubMed] [Google Scholar]
- Narayanan S, Modak JK, Ryan CS, Garcia-Bustos J, Davies JK, & Roujeinikova A (2014). Mechanism of escherichia coli resistance to pyrrhocoricin. Antimicrob. Agents Chemother., 58, 2754–2762. doi: 10.1128/AAC.02565-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantos A, Tsogas I, & Paleos CM (2008). Guanidinium group: a versatile moiety inducing transport and multicompartmentalization in complementary membranes. Biochim. Biophys. Acta. Biomembr, 1778, 811–823. doi: 10.1016/j.bbamem.2007.12.003 [DOI] [PubMed] [Google Scholar]
- Paulsen VS, Mardirossian M, Blencke H-M, Benincasa M, Runti G, Nepa M, Haug T, Stensvag K, & Scocchi M (2016). Inner membrane proteins YgdD and SbmA are required for the complete susceptibility of E. coli to the proline-rich antimicrobial peptide arasin 1(1-25). Microbiology, 162,601–609. doi: 10.1099/mic.0.000249 [DOI] [PubMed] [Google Scholar]
- Rosengren KJ, Goransson U, Otvos L, & Craik DJ (2004). Cyclization of pyrrhocoricin retains structural elements crucial for the antimicrobial activity of the native peptide. Biopolymers, 76,446–458. doi: 10.1002/bip.20159 [DOI] [PubMed] [Google Scholar]
- Roy RN, Lomakin IB, Gagnon MG, & Steitz TA (2015). The mechanism of inhibition of protein synthesis by the proline-rich peptide oncocin. Nat. Struct. Mol. Biol, 22,466. doi: 10.1038/nsmb.3031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomón RA, & Farías RN (1995). The peptide antibiotic microcin 25 is imported through the TonB pathway and the SbmA protein. J Bacteriol, 177, 3323–3325. doi: 10.1128/jb.177.11.3323-3325.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocchi M, Tossi A, & Gennaro R (2011). Proline-rich antimicrobial peptides: converging to a non-lytic mechanism of action. Cell. Mol. Life Sci., 68, 2317–2330. doi: 10.1007/s00018-011-0721-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeldt AC, Graf M, Pérébaskine N, Nguyen F, Arenz S, Mardirossian M, Scocchi M, Wilson DN, & Innis CA (2016). Structure of the mammalian antimicrobial peptide bac7(1-16) bound within the exit tunnel of a bacterial ribosome. Nucleic Acids Res., 44,2429–2438. doi: 10.1093/nar/gkv1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seefeldt AC, Nguyen F, Antunes S, Pdrdbaskine N, Graf M, Arenz S, Inampudi KK, Douat C, Guichard G, Wilson DN, & Innis CA (2015). The proline-rich antimicrobial peptide Onc112 inhibits translation by blocking and destabilizing the initiation complex. Nat. Struct. Mol. Biol, 22, 470–475. doi: 10.1038/nsmb.3034 [DOI] [PubMed] [Google Scholar]
- Su Y, Li S, & Hong M (2013). Cationic membrane peptides: atomic-level insight of structure-activity relationships from solid-state NMR. Amino Acids, 44, 821–833. doi: 10.1007/s00726-012-1421-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi S, Mita K, Ichinohe K, & Hashimoto S (2009). Targeted engineering of the antibacterial peptide apidaecin, based on an in vivo monitoring assay system. Appl. Environ. Microbiol, 75, 1460–1464. doi: 10.1128/aem.02096-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang M, Waring AJ, & Hong M (2007). Phosphate-mediated arginine insertion into lipid membranes and pore formation by a cationic membrane peptide from solid-state nmr. J. Am. Chem. Soc, 129, 11438–11446. doi: 10.1021/ja072511s [DOI] [PubMed] [Google Scholar]
- Wang G (2017). Antimicrobial peptides: Discovery, design and novel therapeutic strategies. CABI. doi: 10.1079/9781786390394.0000 [DOI] [Google Scholar]
- Wang S, Zeng X, Yang Q, & Qiao S (2016). Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int. J. Mol. Sci, 17. doi: 10.3390/ijms17050603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zasloff M, et al. (2002). Antimicrobial peptides in health and disease. N. Engl. J. Med, 347, 1199–1199. doi: 10.1007/978-3-319-53737-5_8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


