Abstract
Introduction
Chronic kidney disease (CKD) risk staging is based on estimated glomerular filtration rate (eGFR) and albumin-creatinine ratio (ACR). However, the relationship between all-cause hospitalization risk and the current CKD staging system has not been well-studied among older adults, despite a high prevalence of CKD and a high risk of hospitalization in older age.
Methods
Among 4,766 participants of the Atherosclerosis Risk in Communities study, CKD was staged according to Kidney Disease Improving Global Outcomes (KDIGO) criteria, using creatinine-based eGFR (eGFRcr) and ACR. Incidence rates of all-cause hospitalization associated with each CKD risk group were analyzed using negative binomial regression. Additionally, cause-specific hospitalization risks for cardiovascular, infectious, kidney, and other diseases were estimated. The impacts of using cystatin C-based eGFR (eGFRcys) to estimate prevalence of CKD and risks of hospitalization were also quantified.
Results
Participants experienced 5,548 hospitalizations and 29% had CKD. Hospitalization rates per 1,000 person-years according to KDIGO risk categories were: 208–223 (“low risk”), 288–376 (“moderately increased risk”), 363–548 (“high risk”), and 499–1083 (“very high risk”). The increased risk associated with low eGFR and high ACR persisted in adjusted analyses, examinations of cause-specific hospitalizations, and when CKD was staged by eGFRcys or eGFRcr-cys, a combined equation based on both creatinine and cystatin C. In comparison to eGFRcr, staging by eGFRcys increased the prevalence of CKD to 50%, but hospitalization risks remained similarly high.
Discussion/Conclusion
In older adults, decreased eGFR, increased ACR, and KDIGO risk stages based on a combination of these measures, were strong risk factors for hospitalization. These relationships held, regardless of the marker used to estimate GFR, but the use of cystatin C resulted in a substantially higher prevalence of CKD than the use of creatinine. Older adults in the population with very high risk stages of CKD have hospitalization rates exceeding 500 per 1,000 person-years.
Keywords: Chronic kidney disease, hospitalization, aging, estimated, glomerular, filtration rate, albuminuria
Introduction
As individuals age, physiological changes, such as vascular stiffening and fibrosis, gradually lead to decreased kidney function over time [1]. However, numerous studies have shown that worsened estimated glomerular filtration rate (eGFR) and albumin-creatinine ratio (ACR), the markers currently used to stage CKD [2], are drivers of increased risks for cardiovascular mortality, overall mortality, and end-stage kidney disease (ESKD), even among older adults [3–6]. Thus, refuting the notion that decreased eGFR and increased ACR are consequences of normal ageing that require no intervention.
Hospitalizations detract from quality of life and are an important marker of morbidity, with particularly detrimental effects in old age. In those with CKD, older individuals are at greatest risk of hospitalization, with rates among those aged 85 and older as high as 752.2 admissions per 1,000 patient-years [7]. Furthermore, CKD patients account for 20% of all Medicare expenditures in those 65 years and older, and hospitalizations are a major source of these costs [7, 8]. However, hospitalizations have not been well-studied in relation to the current Kidney Disease Improving Global Outcomes (KDIGO) staging system. Previously, risks of hospitalization among adults in Washington State were found to increase by CKD stage [9]. However, CKD was defined based on International Classification of Disease (ICD9-CM) codes which are known to have low sensitivity and variable validity depending on disease severity [10]. A cohort of older adults in the UK saw increased risks of hospitalization associated with decreased eGFR and evidence of proteinuria, but the latter measure was based solely on dipstick proteinuria testing [11]. Hospitalization risk has been analyzed by cross-classifications of eGFR and quantitatively measured albuminuria, but only with respects to certain causes, such as cardiovascular [12] and infection-related hospitalization [13].
Therefore, our primary objective was to characterize the risk of all-cause hospitalization among older individuals as related to key markers and stages of CKD, in a population sample of older adults. We hypothesized that more severe CKD, as indicated by decreased eGFR and elevated ACR, would be positively associated with all-cause hospitalization risk. We also aimed to identify the most common causes of hospitalizations in this population and to estimate risks of cause-specific hospitalizations.
Our secondary objective was to examine how cystatin C-based eGFR (eGFRcys) or a combined equation (eGFRcr-cys) would compare to creatinine-based eGFR (eGFRcr) in estimating hospitalization risk in an older population. eGFRcr is dependent on muscle mass [14] and consequently, categorizing risk by eGFRcr among older adults with above average muscle loss, tends to overestimate GFR. In contrast, serum cystatin C is unaffected by muscle mass [14] and eGFRcys or eGFRcr-cys may be better indicators of kidney function among older individuals [5, 15]. We hypothesized that eGFRcys would have stronger, more linear associations with hospitalization risk than eGFRcr.
Methods
Study Population
The Atherosclerosis Risk in Communities (ARIC) study was established in 1987 as a community-based cohort of 15,792 individuals aged 45 to 65 years [16]. Participants were recruited from four US communities: Forsyth County, NC; Jackson, MS ; suburban Minneapolis, MN; and Washington County, MD. Demographic information was collected and physical examinations were performed at visit 1 (1987–1989). Follow-up visits were conducted during: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), and visit 5 (2011–2013). Details of the ARIC study have been published elsewhere [16].
Given the focus on older age, this study included all individuals who attended visit 5 (n=6,538). Participants who were missing measures of eGFRcr, eGFRcys, eGFRcr-cys, or ACR (n=821), and those missing adjustment covariates (n=901, most frequently due to unknown smoking status in 399 participants) were excluded. Individuals who had no follow-up beyond visit 5 (n=7) were also excluded. Participants who self-reported race as neither black nor white, whites in Jackson, blacks in Washington County, and blacks in Minneapolis (n=43) were excluded due to small numbers. The final analytic population consisted of 4,766 participants. The ARIC study has been approved by the Johns Hopkins University Institutional Review Board (IRB No. N.34.99.07.02.A1) and informed consent was provided by all participants.
Assessments of Kidney Measures
The primary measures were eGFRcr, eGFRcys, eGFRcr-cys, and ACR. eGFRcr was estimated using the 2009 CKD Epidemiology Collaboration (CKD-EPI) creatinine equation [17] whereas eGFRcys was estimated using the 2012 CKD-EPI cystatin C equation [18, 19] The 2012 CKD-EPI creatinine-cystatin C equation was used to estimate eGFRcr-cys [19]. Serum creatinine was measured on a Roche Modular P Chemistry Analyzer (Roche Diagnostics Corporation) using a creatinase enzymatic method (Roche Diagnostics, Indianapolis, IN 46250) and standardized to isotope-dilution mass spectroscopy (IDMS). Serum cystatin C was measured using a turbidometric method (Gentian AS, PO Box 733, N-1509, Moss, Norway) and standardized to International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) reference [20]. Urinary albumin was measured using an immunoturbidometric method on the ProSpec nephelometric analyzer (Dade Behring GMBH. Marburg, Germany D-35041) and urinary creatinine was measured using a modified kinetic Jaffe method to calculate ACR.
Ascertainment of Hospitalizations
All hospitalizations occurring through December 31st, 2016 were captured through ARIC study surveillance. Participants’ self-reported events were ascertained during annual telephone interviews and in-person study visits. In addition, data was abstracted from discharge records of ARIC community hospitals. The primary outcome was all-cause hospitalizations. Using the Clinical Classifications Software (CCS) produced by the Agency for Healthcare Research and Quality [21], the primary ICD code of each hospitalization was used to classify events by cause, focusing on: cardiovascular, infection, kidney, or other causes. Before October 1st, 2015, hospitalizations were classified using ICD-9 codes, while hospitalizations afterwards, were classified using ICD-10 codes. During analysis, this change was accounted for by converting ICD-10 to ICD-9 codes.
Assessments of Covariates
At visit 5, demographic information, medical history, and health behavior data were collected using both participant self-report and standardized examinations. All assessments were performed by trained staff. Age, sex, race, and cigarette consumption were ascertained through questionnaires. Smoking status was classified as current, former, or never. Body mass index (BMI) was calculated by dividing weight (in kilograms) by squared height (in meters). Diabetes was defined as having a self-reported history of physician-diagnosed diabetes, having used anti-diabetic medication over the past two weeks, having a fasting blood glucose of ≥126 mg/dL, or having a casual blood glucose of ≥200 mg/dL. Hypertension was defined as having a systolic blood pressure of ≥140 mmHg, having a diastolic blood pressure of ≥90 mmHg, or having used antihypertensive medication over the past four weeks. A history of cancer or cardiovascular disease including heart failure, coronary heart disease (CHD), and stroke was identified through self-report at visit 1 or captured by active surveillance between visit 1 and visit 5.
Statistical Analysis
Baseline characteristics of participants were compared using analysis of variance and Pearson’s χ2 tests across five categories of eGFR [≥90 (G1), 60–89 (G2, Reference), 45–59 (G3a), 30–44 (G3b), <30 (G4+) mL/min/1.73 m2]. G2 was selected as the referent because it was the most prevalent category in the cohort. All individuals with eGFR <30 mL/min/1.73 m2 were pooled into one group to produce more stable estimates. Likelihood ratio tests revealed that Poisson regression would be an inappropriate method for analysis due to an overdispersion of study values. Consequently, crude incidence rates, incidence rate ratios of hospitalization, and their associated 95% confidence intervals (95% CI) were estimated using negative binomial regression.
Numbers of hospitalizations, crude incidence rates per 1,000 person-years, and adjusted incidence rate ratios were calculated for cross-classifications of eGFRcr and ACR to best represent the current staging system of CKD, thus creating a 5 × 3 gradient (heat map) of risk. These estimates are presented in a color-coded format, in accordance with KDIGO CKD risk groups and designations of risk: green (“low risk”), yellow (“moderately increased risk”), orange (“high risk”), red (“very high risk”). Models were adjusted for age, sex, BMI, race-center, smoking status, diabetes, hypertension and history of heart failure, CHD, stroke, or cancer. These values were similarly calculated for eGFRcys and eGFRcr-cys.
Crude and adjusted continuous associations between baseline eGFR and risk of all-cause hospitalizations were plotted using linear splines with knots at 60 and 90 mL/min/1.73 m2, and the reference value at 75 mL/min/1.73 m2. These models were adjusted to the mean levels of all covariates, including albuminuria. Similarly, continuous associations were plotted for risks of cause-specific hospitalizations due to cardiovascular, infection, kidney, and other causes. The ICD-9 codes used to classify hospitalizations are listed in Supplementary Table 3. For further comparison, risks for cross-classifications of eGFRcr and eGFRcys were also estimated. All statistical analyses were performed using Stata, version 14.2 (StataCorp).
Results
Baseline Characteristics
The mean age of the cohort at visit 5 was 75.7 years (range: 60–90). Overall, 56.0% of the 4,766 participants were female and 21.6% were black. As classified by eGFRcr, 29.3% of the cohort had Stage G3 CKD or worse (eGFR <60 mL/min/1.73 m2) (Table 1). Those with low eGFRcr tended to be older, female, and white. Notably, more than half (53.7%) of those with eGFRcr ≥90 mL/min/1.73m2 were black, while the majority of those with severely decreased eGFRcr (<30 mL/min/1.73 m2) were white (66.3%). Those with reduced eGFRcr had slightly lower BMI and were less likely to self-report current smoking. Severely decreased eGFRcr was associated with albuminuria (ACR ≥30 mg/g; 71%), hypertension (94.2%), diabetes (43.0%), and history of cancer (8.1%), heart failure (26.7%), CHD (31.4%), and stroke (8.1%).
Table 1.
Study Population Characteristics by eGFRcr
| eGFRcr≥90 | 60≤eGFRcr<90 | 45≤eGFRcr<60 | 30≤eGFRcr<45 | eGFRcr<30 | p-value | ||
|---|---|---|---|---|---|---|---|
| N=4766 (Row %) | 410 (8.6%) | 2962 (62.1%) | 953 (20.0%) | 355 (7.5%) | 86 (1.8%) | ||
| Age, y | 72.7 (4.1) | 75.3 (4.9) | 77.1 (5.3) | 78.0 (5.5) | 78.0 (5.7) | <0.001 | |
| Sex | Male | 152 (37.1%) | 1328 (44.8%) | 424 (44.5%) | 150 (42.3%) | 44 (51.2%) | 0.03 |
| Female | 258 (62.9%) | 1634 (55.2%) | 529 (55.5%) | 205 (57.7%) | 42 (48.8%) | ||
| Race | White | 190 (46.3%) | 2441 (82.4%) | 772 (81.0%) | 276 (77.7%) | 57 (66.3%) | <0.001 |
| Black | 220 (53.7%) | 521 (17.6%) | 181 (19.0%) | 79 (22.3%) | 29 (33.7%) | ||
| Center | Forsyth | 61 (14.9%) | 661 (22.3%) | 219 (23.0%) | 76 (21.4%) | 18 (20.9%) | <0.001 |
| Jackson | 205 (50.0%) | 492 (16.6%) | 168 (17.6%) | 73 (20.6%) | 28 (32.6%) | ||
| Minneapolis | 78 (19.0%) | 991 (33.5%) | 283 (29.7%) | 96 (27.0%) | 19 (22.1%) | ||
| Washington | 66 (16.1%) | 818 (27.6%) | 283 (29.7%) | 110 (31.0%) | 21 (24.4%) | ||
| BMI, kg/m2 | 29.9 (7.2) | 28.5 (5.4) | 28.9 (5.5) | 29.9 (6.0) | 28.8 (6.4) | <0.001 | |
| Smoking status | Current | 44 (10.7%) | 185 (6.2%) | 45 (4.7%) | 17 (4.8%) | 5 (5.8%) | 0.004 |
| Former | 218 (53.2%) | 1551 (52.4%) | 500 (52.5%) | 198 (55.8%) | 42 (48.8%) | ||
| Never | 148 (36.1%) | 1226 (41.4%) | 408 (42.8%) | 140 (39.4%) | 39 (45.3%) | ||
| Albuminuria | ACR<30 | 347 (84.6%) | 2497 (84.3%) | 734 (77.0%) | 209 (58.9%) | 25 (29.1%) | |
| 30<ACR<300 | 59 (14.4%) | 422 (14.2%) | 194 (20.4%) | 119 (33.5%) | 33 (38.4%) | <0.001 | |
| ACR>300 | 4 (1.0%) | 43 (1.5%) | 25 (2.6%) | 27 (7.6%) | 28 (32.6%) | ||
| Medical History | |||||||
| Hypertension | 302 (73.7%) | 2119 (71.5%) | 772 (81.0%) | 319 (89.9%) | 81 (94.2%) | <0.001 | |
| Diabetes | 174 (42.4%) | 894 (30.2%) | 323 (33.9%) | 164 (46.2%) | 37 (43.0%) | <0.001 | |
| Cancer | 7 (1.7%) | 89 (3.0%) | 30 (3.1%) | 15 (4.2%) | 7 (8.1%) | 0.02 | |
| Heart failure | 10 (2.4%) | 106 (3.6%) | 74 (7.8%) | 53 (14.9%) | 23 (26.7%) | <0.001 | |
| Coronary heart disease | 34 (8.3%) | 404 (13.6%) | 177 (18.6%) | 98 (27.6%) | 27 (31.4%) | <0.001 | |
| Stroke | 10 (2.4%) | 96 (3.2%) | 42 (4.4%) | 30 (8.5%) | 7 (8.1%) | <0.001 | |
| Number of Hospitalizations, Mean (SD) | 1.1 (1.9) | 1.0 (1.7) | 1.3 (1.8) | 1.9 (2.7) | 3.0 (2.0) | <0.001 | |
As classified by eGFRcys, 49.8% of participants had CKD (Supplementary Table 1). Characteristics associated with reduced eGFRcys were similar to those found in association with reduced eGFRcr, with the exception of high BMI and current smoking, which were more common among those with low eGFRcys. By eGFRcr-cys, 38.2% of participants had CKD (eGFRcr-cys <60 mL/min/1.73 m2) (Supplementary Table 2). Similar factors to those associated with decreased eGFRcr were associated with decreased eGFRcr-cys, except for higher BMI. A considerable proportion of the study population (23%) was re-classified to a lower level of kidney function by eGFRcys in comparison to eGFRcr (Supplementary Figure 1). Classification to a worsened eGFR category by cystatin C was associated with increased hospitalization risk (IRR, 1.4 [95% CI, 1.2–1.5]).
Overall Risk of Hospitalizations by eGFR and ACR
During 21,120 person-years of follow-up (median follow-up, 4.6 years), 5,548 hospitalizations occurred among 4,766 participants; 2,389 (50.1%) individuals did not experience any hospitalizations, but among those with at least one event, the mean number of hospitalizations was 2.3.
Lower eGFRcr and higher albuminuria were independently associated with greater incidence of hospitalizations (Figure 1, colors indicating KDIGO risk categories as defined a priori). The crude hospitalization rate (Incidence rate per 1,000 person-years [95% CI]) in the reference group (G2, A1) was 208 [195–222]. The crude hospitalization rate was higher in individuals with similar eGFRcr but elevated albuminuria (548 [356–844] for G2, A3) and also higher progressively higher in individuals with similar levels of albuminuria but lower eGFRcr (A1, G1; G2; G3a; G3b; G4) 223, 208, 288, 363 and 870. Hospitalization risk was consistent with pre-defined KDIGO risk categories and was greater than 500 per 1,000 person-years in “very high risk” CKD (red). Participants with the lowest eGFRcr and highest ACR (G4+, A3), experienced more than three times the adjusted incidence rate of hospitalizations (IRR, 3.3 [95% CI, 2.0–5.5]) compared to the reference group (G2, A1).
Figure 1. Risk of Hospitalizations by Estimated Glomerular Filtration Rate and Albuminuria.
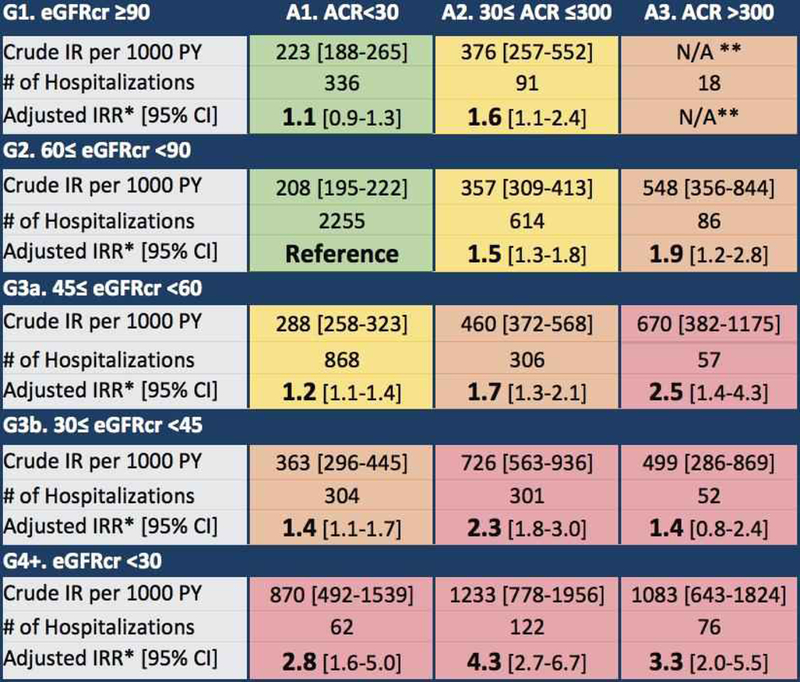
Estimates for eGFRcr are shown. Colors indicate risk groups (green: low risk; yellow: moderately increased risk; orange: high risk; red: very high risk) as defined by KDIGO 2012 criteria. *Incidence rate ratios adjusted for age, sex, race-center, diabetes, smoking status, hypertension, and history of cancer, heart failure, coronary heart disease, and stroke. **Omitted due to underpowered estimation.
Models including both eGFRcr and albuminuria as independent risk factors show that each effect increases risk of hospitalization in a statistically significant manner and that neither factor strongly confounds the other. Relative to albuminuria category A1, A2 was associated with an IRR of 1.5 [95% CI, 1.3–1.7] while A3 had an IRR of 1.7 [95% CI, 1.3–2.2]. In comparison to eGFR category G2, G3a and G3b were associated with IRRs of 1.2 [95% CI, 1.1–1.3] and 1.4 [95% CI, 1.2–1.6], respectively; the effects of these stages of eGFRcr were weaker than the associations observed with albuminuria. However, having G4+ eGFRcr conferred a greater relative risk of incident hospitalizations than even the worst category of ACR (IRR: 2.5 [95% CI, 1.9–3.4] vs 1.7 [95% CI, 1.3–2.2]).
In sensitivity analyses, using eGFRcys or eGFRcr-cys, and ACR to classify risk resulted in similar patterns of elevated hospitalization risk with increased CKD risk groups (Supplementary Figures 2A–B). Surprisingly, adjusted relative risks were not higher with cystatin C-based estimates than with creatinine-based estimates of GFR. The continuous associations between eGFR, by all three estimating equations, and risk of all-cause hospitalizations are plotted in Figure 2. Risk gradients were greatly attenuated after adjustment for albuminuria, demographics, health behavior, and medical history (dashed lines). Risk estimates by all three equations, both crude and adjusted, fell to their lowest points at eGFR 60–90 mL/min/1.73 m2. An uptick in risk was observed in the crude and adjusted incidence of hospitalization when eGFR exceeded 90 mL/min/1.73 m2, resulting in reverse J-shaped risk curves.
Figure 2. Incidence of All-Cause Hospitalizations by Baseline Estimated Glomerular Filtration Rate.
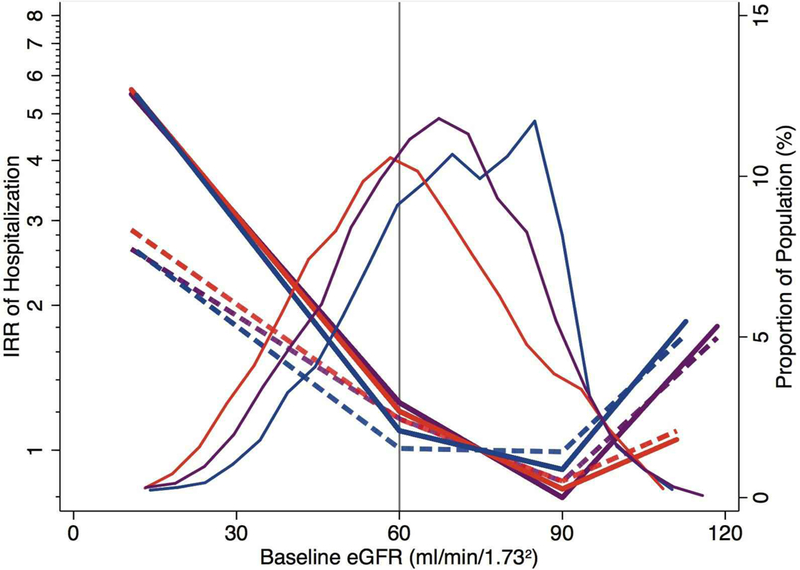
Density curves show the distributions of the three GFR estimates (eGFRcr in blue, eGFRcys in red, and eGFRcr-cys in purple). Risk curves show the crude (solid) and adjusted (dash) risk ratios of hospitalization by baseline level of eGFR, with the reference point at eGFR 75 mL/min/1.73 m2. Risk was adjusted to the mean level of all adjustment variables (76 years, female, white, recruited from Minneapolis, BMI of 29, non-diabetic, former smoker, hypertensive, ACR<30 mg/g, no history of heart failure, coronary heart disease, stroke, or cancer).
Cause-Specific Hospitalizations
Of the 5,548 hospitalizations that occurred, cardiovascular, infection, and kidney events accounted for 24.7%, 10.5%, and 2.0% of hospitalizations respectively (Table 2). The remaining 62.8% of events was related to a range of other causes, with musculoskeletal and iatrogenic illness/injury being the most common. Across various causes of hospitalization, risk relationships with eGFR were similar, despite marked differences in absolute risk (Supplementary Figures 3A–D). The slopes of adjusted incidence rates were much smaller than those of crude incidence rates, and at high levels of eGFR (>90 mL/min/1.73 m2), the adjusted risk ratios for most hospitalization types were more linear by eGFRcys than eGFRcr. Notably, upticks of cardiovascular hospitalization were observed at high levels of eGFRcr and eGFRcr-cys, but not eGFRcys (Supplementary Figure 3A). The incidence of kidney-related hospitalizations showed the steepest risk gradient; even after full adjustment, a more than 10-fold increase in risk was observed at low levels of eGFR, by all estimating equations (Supplementary Figure 3C).
Table 2.
Frequency of Hospitalizations by Cause
| Cause | Hospitalizations | |
|---|---|---|
| N | % | |
| Cardiovascular Disease | 1,372 | 24.7 |
| Infection | 584 | 10.5 |
| Musculoskeletal | 579 | 10.4 |
| Iatrogenic/Injury | 424 | 7.6 |
| Gastrointestinal | 409 | 7.4 |
| Neurologic | 376 | 6.8 |
| Respiratory | 306 | 5.5 |
| Genitourinary | 294 | 5.3 |
| Cancer | 270 | 4.9 |
| Endocrine | 158 | 2.9 |
| Other | 776 | 14.0 |
| Total | 5,548 | 100.0 |
Discussion/Conclusion
Among 4,766 older individuals, both decreased eGFR and increased ACR were robust risk factors for all-cause hospitalization. These findings indicate that the current KDIGO staging system, which was largely developed using risk estimates of mortality and ESKD, is also applicable to hospitalization risk in older adults. The hospitalization risk gradient in this population was dramatic, with crude incidence rates per 1000 person-years ranging from 208–223 among individuals with no CKD (“low risk”) to 499–1083 among individuals with “very high risk” CKD. Risk relationships were consistent across the three equations for eGFR based on serum creatinine, cystatin C, or both. However, in a direct comparison of eGFRcr and eGFRcys, the cystatin C-based equation re-classified a large proportion of participants to a lower eGFR, with approximately twice as many individuals categorized as having CKD by eGFRcys. Despite this increased prevalence, the absolute and relative risk of hospitalizations associated with CKD remained high when using cystatin C-based estimates.
Other studies have examined hospitalization risk in relation to KDIGO crossclassifications but have focused on specific causes, including cardiovascular disease [12] and infection [13]. Nichols et al. studied first cardiovascular hospitalizations among individuals with diabetes in Kaiser Permanente Northwest [12]. While their findings of increased hospitalization risk at low eGFR and high albuminuria were comparable to ours, we found that overall hospitalization risk was exceedingly high in old age (>500 per 1,000 person-years with very high risk CKD) compared to their finding of 23–31% incidence of first cardiovascular hospitalizations among similarly staged middle-aged adults [12]. Ishigami et al. found that ARIC participants with very high risk CKD experienced a more than five-fold increased risk of infection-related hospitalization [13]. Similarly, we showed that individuals with the lowest levels of eGFR had more than eight times the risk of infection-related hospitalization than participants with eGFR 75 mL/min/1.73 m2.
Our findings expand on previous research which has focused on eGFR [22–28] by showing the added risk that is detectable with measured albuminuria, reinforcing KDIGO recommendations for measuring albuminuria to fully stage CKD. Although the United States Preventive Services Task Force (USPSTF) has found insufficient evidence to recommend screening for CKD in asymptomatic individuals [29] in many clinical settings, eGFR is known to be reduced and albuminuria measurement is recommended but not necessarily implemented [30–32]. The strong relationship between these markers and hospitalization risk suggests an opportunity for early intervention to reduce further hospitalizations and associated costs among older adults with suboptimal measures of eGFR and ACR. For example, medical nutrition therapy is currently covered by Medicare for individuals with CKD and would also avoid the medication side effects which have been cited as potential harms of screening for CKD by USPSTF [29, 33]. Likewise, it has been shown in ARIC that attention to vaccination and other measures to reduce infection are warranted to reduce risks of hospitalization in older adults with CKD [13]. Diagnoses of other specific comorbidities, such as heart failure, should guide additional strategies for physiologic monitoring and hospitalization risk reduction in this population.
Furthermore, our results extend the findings of studies that have noted the increased sensitivity of detecting CKD by eGFRcys [5, 15, 34]. A previous meta-analysis of mortality and ESKD in over 90,000 older individuals found similar distributions of eGFRcr, eGFRcys, and eGFRcr-cys to our study [35]. In agreement with the stronger relationships we observed between eGFRcys and hospitalization risk, Shlipak et al. found that eGFRcys was more strongly associated with hazard of death than eGFRcr [35]. We demonstrated an increased adjusted risk of hospitalization among individuals with eGFR >90 mL/min/1.73 m2, a reverse J-shaped curve which has been reported in other studies of eGFRcr [35–37]. High eGFRcr may result from low muscle mass, leading to falsely elevated estimates of GFR among those at higher risk due to muscle wasting.
This study has several strengths. Our use of objective measurements of both eGFR and ACR to stage CKD according to KDIGO criteria presents as an improvement over previous methods that have used either marker in isolation, binary disease classifications, or ICD codes to define CKD and describe its relationship with hospitalization risk [9, 27, 38]. As the ARIC cohort is comprised of older individuals, sampled from communities across the US, our results are likely to be broadly generalizable to older adults. We were also able to describe the risks of a wider range of hospitalization types than previous studies of CKD and hospitalization. Furthermore, estimates of GFR based on creatinine, cystatin C, and both, were all examined in relation to albuminuria, consistent with current guidelines. To the best of our knowledge, this is the first time that hospitalization risk has been compared across all three CKD-EPI estimating equations for eGFR. Importantly, our results shed light on the relationship between markers of CKD and hospitalizations of various causes in old age, when hospitalization risk is already high.
However, we also recognize the limitations of our study. Our analysis excluded the few participants who were neither white nor black and consequently, our findings may not be applicable to other minorities. Also, the primary ICD-9 codes used to classify hospitalizations may not have accurately attributed events to their underlying causes. Lastly, survival bias may have affected our results as participants must have been able to attend visit 5 of the study. Retention in the ARIC cohort for nearly 25 years may represent selection, but this could also be similar to aging in the general population.
In conclusion, the markers of kidney function currently used to stage CKD, eGFR and ACR, were both strong, independent risk factors for hospitalization among older adults. In older age, very high risk CKD was associated with a hospitalization rate of over 500 per 1,000 person-years, and a greater than 3-fold adjusted relative risk of hospitalization compared to those classified as low risk. These results extend evidence to support the current guidelines for staging CKD by measuring both eGFR and albuminuria. Our findings further demonstrate that staging CKD with both markers allows for hospitalization risk stratification and thus, provides an opportunity for early interventions to prevent hospitalizations among older adults with CKD.
Supplementary Material
Acknowledgements
The authors thank the staff and participants of the ARIC study for their important contributions.
Funding Sources
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract nos. (HHSN268201700001I, HHSN268201700002I,HHSN268201700003 I,HHSN268201700005I,HHSN268201700004I). Dr. Ishigami is supported by a National Heart, Lung, and Blood Institute grant (T32HL007024). Dr. Rebholz is supported by a mentored research scientist development award from the National Institute of Diabetes and Digestive and Kidney Diseases (K01 DK107782) and a grant from the National Heart, Lung, and Blood Institute (R21 HL143089)
Statement of Ethics
The ARIC study has been approved by the Johns Hopkins University Institutional Review Board (IRB No. N.34.99.07.02.A1) and informed consent was provided by all participants.
Footnotes
Disclosure Statement
The authors have no conflicts of interest to declare. Abstracts of interim findings were presented at the American Heart Association’s EPI Lifestyle Scientific Sessions 2018 and the Society of Epidemiologic Research 2018 Annual Meeting.
References
- 1.O’Sullivan ED, Hughes J, Ferenbach DA. Renal Aging: Causes and Consequences. J Am Soc Nephrol. 2017;28(2):407–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney International Supplements. 2013;3(1):1–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hallan SI, Matsushita K, Sang Y, Mahmoodi BK, Black C, Ishani A, et al. Age and association of kidney measures with mortality and end-stage renal disease. JAMA. 2012;308(22):2349–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, et al. Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int. 2003;63(3):1121–9. [DOI] [PubMed] [Google Scholar]
- 5.Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, et al. Cystatin C and the risk of death and cardiovascular events among elderly persons. The New England journal of medicine. 2005;352(20):2049–60. [DOI] [PubMed] [Google Scholar]
- 6.Muntner P, Bowling CB, Gao L, Rizk D, Judd S, Tanner RM, et al. Age-specific association of reduced estimated glomerular filtration rate and albuminuria with all-cause mortality. Clin J Am Soc Nephrol. 2011;6(9):2200–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saran R, Robinson B, Abbott KC, Agodoa LYC, Bhave N, Bragg-Gresham J, et al. US Renal Data System 2017 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am J Kidney Dis. 2018;71(3S1):A7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St Peter WL, Khan SS, Ebben JP, Pereira BJ, Collins AJ. Chronic kidney disease: the distribution of health care dollars. Kidney Int. 2004;66(1):313–21. [DOI] [PubMed] [Google Scholar]
- 9.Daratha KB, Short RA, Corbett CF, Ring ME, Alicic R, Choka R, et al. Risks of subsequent hospitalization and death in patients with kidney disease. Clin J Am Soc Nephrol. 2012;7(3):409–16. [DOI] [PubMed] [Google Scholar]
- 10.Grams ME, Plantinga LC, Hedgeman E, Saran R, Myers GL, Williams DE, et al. Validation of CKD and related conditions in existing data sets: A systematic review. Am J Kidney Dis. 2011;57(1):44–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nitsch D, Nonyane BA, Smeeth L, Bulpitt CJ, Roderick PJ, Fletcher A. CKD and hospitalization in the elderly: a community-based cohort study in the United Kingdom. Am J Kidney Dis. 2011;57(5):664–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nichols GA, Deruaz-Luyet A, Hauske SJ, Brodovicz KG. The association between estimated glomerular filtration rate, albuminuria, and risk of cardiovascular hospitalizations and all-cause mortality among patients with type 2 diabetes. J Diabetes Complications. 2018;32(3):291–7. [DOI] [PubMed] [Google Scholar]
- 13.Ishigami J, Grams ME, Chang AR, Carrero JJ, Coresh J, Matsushita K. CKD and Risk for Hospitalization With Infection: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2017;69(6):752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baxmann AC, Ahmed MS, Marques NC, Menon VB, Pereira AB, Kirsztajn GM, et al. Influence of muscle mass and physical activity on serum and urinary creatinine and serum cystatin C. Clin J Am Soc Nephrol. 2008;3(2):348–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballew SH, Chen Y, Daya NR, Godino JG, Windham BG, McAdams-DeMarco M, et al. Frailty, Kidney Function, and Polypharmacy: The Atherosclerosis Risk in Communities (ARIC) Study. Am J Kidney Dis. 2017;69(2):228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.The ARIC investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 17.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, , Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inker LA, Eckfeldt J, Levey AS, Leiendecker-Foster C, Rynders G, Manzi J, et al. Expressing the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) cystatin C equations for estimating GFR with standardized serum cystatin C values. Am J Kidney Dis. 2011;58(4):682–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. Estimating glomerular filtration rate from serum creatinine and cystatin C. The New England journal of medicine. 2012;367(1):20–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grubb A, Blirup-Jensen S, Lindstrom V, Schmidt C, Althaus H, Zegers I, et al. First certified reference material for cystatin C in human serum ERM-DA471/IFCC. Clin Chem Lab Med. 2010;48(11):1619–21. [DOI] [PubMed] [Google Scholar]
- 21.Clinical A Classifications Software for ICD-10 Data Rockville, MD2012 [updated December 2012. Available from: https://www.ahrq.gov/research/data/hcup/icd10usrgd.html.
- 22.Nishikawa K, Takahashi K, Yamada R, Kinaga T, Masato M, Yamamoto M. Influence of chronic kidney disease on hospitalization, chronic dialysis, and mortality in Japanese men: a longitudinal analysis. Clin Exp Nephrol. 2017;21(2):316–23. [DOI] [PubMed] [Google Scholar]
- 23.Tellez-Plaza M, Orozco-Beltran D, Gil-Guillen V, Pita-Fernandez S, Navarro-Perez J, Pallares V, et al. Renal function and attributable risk of death and cardiovascular hospitalization in patients with cardiovascular risk factors from a registry-based cohort: the Estudio Cardiovascular Valencia-risk study. J Hypertens. 2016;34(11):2266–73. [DOI] [PubMed] [Google Scholar]
- 24.Dalrymple LS, Katz R, Kestenbaum B, de Boer IH, Fried L, Sarnak MJ, et al. The risk of infection-related hospitalization with decreased kidney function. Am J Kidney Dis. 2012;59(3):356–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ronksley PE, Hemmelgarn BR, Manns BJ, Wick J, James MT, Ravani P, et al. Potentially Preventable Hospitalization among Patients with CKD and High Inpatient Use. Clin J Am Soc Nephrol. 2016;11(11):2022–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James MT, Quan H, Tonelli M, Manns BJ, Faris P, Laupland KB, et al. CKD and risk of hospitalization and death with pneumonia. Am J Kidney Dis. 2009;54(1):24–32. [DOI] [PubMed] [Google Scholar]
- 27.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. The New England journal of medicine. 2004;351(13):1296–305. [DOI] [PubMed] [Google Scholar]
- 28.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, et al. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: the Anemia in Chronic Heart Failure: Outcomes and Resource Utilization (ANCHOR) Study. Circulation. 2006;113(23):2713–23. [DOI] [PubMed] [Google Scholar]
- 29.Moyer VA, Force USPST. Screening for chronic kidney disease: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(8):567–70. [DOI] [PubMed] [Google Scholar]
- 30.Perkins RM, Chang AR, Wood KE, Coresh J, Matsushita K, Grams M. Incident chronic kidney disease: trends in management and outcomes. Clin Kidney J. 2016;9(3):432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevens PE, O’Donoghue DJ, de Lusignan S, Van Vlymen J, Klebe B, Middleton R, et al. Chronic kidney disease management in the United Kingdom: NEOERICA project results. Kidney Int. 2007;72(1):92–9. [DOI] [PubMed] [Google Scholar]
- 32.Leddy J, Green JA, Yule C, Molecavage J, Coresh J, Chang AR. Improving proteinuria screening with mailed smartphone urinalysis testing in previously unscreened patients with hypertension: a randomized controlled trial. BMC Nephrol. 2019;20(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer H, Jimenez EY, Brommage D, Vassalotti J, Montgomery E, Steiber A, et al. Medical Nutrition Therapy for Patients with Non-Dialysis-Dependent Chronic Kidney Disease: Barriers and Solutions. J Acad Nutr Diet. 2018;118(10):1958–65. [DOI] [PubMed] [Google Scholar]
- 34.Shlipak MG, Katz R, Kestenbaum B, Fried LF, Newman AB, Siscovick DS, et al. Rate of kidney function decline in older adults: a comparison using creatinine and cystatin C. American journal of nephrology. 2009;30(3):171–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shlipak MG, Matsushita K, Arnlov J, Inker LA, Katz R, Polkinghorne KR, et al. Cystatin C versus creatinine in determining risk based on kidney function. The New England journal of medicine. 2013;369(10):932–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Astor BC, Levey AS, Stevens LA, Van Lente F, Selvin E, Coresh J. Method of glomerular filtration rate estimation affects prediction of mortality risk. J Am Soc Nephrol. 2009;20(10):2214–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, et al. Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int. 2011;79(12):1341–52. [DOI] [PubMed] [Google Scholar]
- 38.Iwagami M, Caplin B, Smeeth L, Tomlinson LA, Nitsch D. Chronic kidney disease and cause-specific hospitalisation: a matched cohort study using primary and secondary care patient data. Br J Gen Pract. 2018;68(673):e512–e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


