Abstract
Unique functions of specialised cells such as those of the immune and haemostasis systems, skin, blood vessels, lung, and bone require specialised compartments, collectively referred to as lysosome-related organelles (LROs), that share features of endosomes and lysosomes. LROs harbour unique morphological features and cell type-specific contents, and most if not all undergo regulated secretion for diverse functions. Ongoing research, largely driven by analyses of inherited diseases and their model systems, is unravelling the mechanisms involved in LRO generation, maturation, transport and secretion. A molecular understanding of these features will provide targets and markers that can be exploited for diagnosis and therapy of a myriad of diseases.
Keywords: endosomes, lysosome-related organelles, melanosomes, Hermansky-Pudlak syndrome, exosomes, Weibel-Palade bodies, lytic granules
Introduction
The endolysosomal system in eukaryotic cells is a dynamic network of organelles with essential roles in nutrient uptake, metabolic control, macromolecule degradation and signalling [1]. Several cell types have adapted their endolysosomal system to fulfil specific physiological needs by generating a group of specialised, functionally and morphologically diverse secretory compartments known as lysosome-related organelles (LROs [2]). The unique properties of each LRO, which include pigment cell melanosomes, platelet dense and alpha granules, endothelial cell Weibel-Palade bodies (WPBs), and cytotoxic T cell (CTL) lytic granules among others (Table 1), are conferred by cell type-specific cargoes that localize specifically to those organelles. Accordingly, most LROs share common components such as the tetraspanin CD63, and effectors such as the small GTPases RAB27A or RAB27B [1,3]. Intriguingly, these features are also shared by classical multivesicular endosomes (MVEs) that release their intraluminal vesicles (ILVs) into the extracellular space upon fusion with the surface of some cell types (Figure 1). The released ILVs, called exosomes, play key roles in cell-to-cell communication in health and disease [4]. Accordingly, we propose that secretory MVEs should be considered as members of the LRO family.
Table 1.
ELROs in vertebrates and invertebrates
| Vertebrates | ||||
| ELRO | Cell Type | RAB27/CD63 | Human ELRO Disease/Disease Model | Cellular, Physiological Function |
| Melanosomes | Melanocytes or melanophores in skin, retinal pigment epithelia and choroid | +/+ | HPS, CHS, GS | Synthesis, storage and/or release of melanin for skin and eye pigmentation, visual acuity, and photoprotection |
| Weibel Palade Bodies | Endothelial cells | +/+ | HPS | Storage and release of vWF and other proteins for platelet recruitment |
| Cytolytic granules | Cytotoxic T Cells, Natural Killer Cells | +/+ | HPS, CHS, GS, FHL | Storage and release of lytic hydrolases for lysis of target cells |
| Dense granules | Platelets, Megakaryocytes | +/+ | HPS, FHL | Storage and release of small active molecules for platelet adhesion and activation |
| Basophilic secretory granules | Mast cells, Basophils | +/+ | CHS | Storage and release of specific molecules for vasodilation at sites of damage |
| Lamellar bodies | Alveolar type II cells | +/+ | HPS | Synthesis, storage and release of lung surfactant phospholipids and proteins for pulmonary gas exchange |
| Phagosomes | Macrophages, Neutrophils, Dendritic cells | +/+ (depending on the ingested particle) | HPS | Storage and degradation of large particles (e.g. microorganisms, apoptotic cells) and initiation of signal transduction cascades |
| MHC class II compartments | Dendritic Cells, B lymphocytes, Macrophages, Langerhans cells | ?/+ | CHS | Processing of antigenic peptides for antigen presentation and stimulation of CD4+ T cells |
| Alpha granules | Platelets, Megakaryocytes | −/+ | GPS, ARC | Storage and release of components for blood clotting, platelet adhesion, haemostasis, inflammation, vascularization |
| Azurophil (primary) granules | Neutrophils, Eosinophils | +/? | HPS, CHS | Storage and release of lysosomal enzymes and anti-microbial peptides into phagosomes for degradation |
| NOX2+ inhibitory lysosomes | Dendritic cells | +/? | GS | Store the NADPH oxidase N0X2, fuse with phagosomes to limit acidification |
| Acrosomes | Sperm | +/? | GS | Penetration of the egg prior to fertilization |
| Large dense-core vesicles | Specialized secretory cells (e.g. adrenal chromaffin cells) | +/? | HPS, FHL | Storage and release of hormones and neuropeptides |
| IRF7 signalling lysosomes | Plasmacytoid dendritic cells | ?/? | HPS | Accumulate IRF7 and TRAF3, initiate TLR signalling cascade for type 1 interferon production |
| Notochord vacuoles | Notochord inner cell | ?/? | HPS | Required for body axis elongation and spine morphogenesis |
| ELRO (putative) | Cell Type | RAB27/CD63 | Human ELRO Disease/Disease Model | Cellular, Physiological Function |
| Melanocore-containing organelle | Epidermal Keratinocytes | ?/+ | ? | Melanin storage for skin pigmentation and photoprotection |
| Secretory MVEs | Most cell types, Model organisms | + (not all MVEs)l+ | ? | Store ILVs that upon secretion are called exosomes and serve multiple functions in target cell differentiation, immunity, cancer progression, pigmentation, bone regeneration, communication in the CNS, pathogen dissemination, and other systems. |
| Fusiform vesicle | Urothelium | +/? | ? | Storage of crystalline uroplakin arrays forming, upon secretion, urothelial plaques for bladder expansion |
| Osteoclast secretory lysosome | Osteoclast | +/? | ? | Secretory lysosomes involved in bone resorption and remodelling |
| Specific (secondary) granules | Neutrophils | +/? | ? | Secretory granules containing cytotoxic proteins involved in the initiation of the inflammatory response and collagen degradation |
| Gelatinase (tertiary) granules | Neutrophils | +/? | ? | Secretory granules containing gelatinase and adhesion molecules (among others) for neutrophils adhesion to and penetration through the endothelium |
| Lamellar bodies Lamellar granules | Epidermal Keratinocytes | ?/? | ARC | Storage and release of lipids for skin permeability |
| Surfactant production and storage organelles | Teleost swimbladder epithelium | ?/? | HPS | Necessary for the proper formation and storage of surfactant; similar to lamellar bodies in mammals |
| Presynaptic vesicles | Neuron synaptic cleft | +/? | ? | Secretory vesicles containing neurotransmitters that are released at the synapse upon stimulation |
| Pathogen-containing phagosomes or vacuoles | Various host cells | ?/? | HPS? | Modification of host phagosome dynamics by pathogen effectors to promote survival |
| Non-acidic late endosomes | Neurons (axons, dendrites) | ?/+ | ? | LAMP1- and/or CD63-positive organelles lacking acid hydrolases for further degradation |
| Invertebrates | ||||
| Evolutionary related ELRO | Organism | ELRO Disease Model | Cellular, Physiological Function | |
| Pigment granules | Drosophila melanogaster retinal cells | HPS | Contain pigments necessary for light insulation throughout the eye | |
| Zinc storage granules | Drosophila melanogaster Malpighian tubule epithelial cells | HPS | Storage compartment for the major total body pool of chelatable zinc | |
| Gut granules | Caenorhabditis elegans intestinal cells | HPS | Storage compartment containing zinc, anthranilic acid and lipofuscin | |
| Post-lysosomes | Dictyostelium discoideum | CHS | Poorly acidic compartments that secrete undigested material into the extracellular space | |
| Mucocysts | Tetrahymena thermophila | HPS | Secretory granules containing dense crystalline cores that, upon stimulated exocytosis, function as a cellular defence | |
| Riboflavin granules | Bombyx mori Malpighian tubules | HPS | Needle-shaped yellow granules that store riboflavin | |
| Integument urate granules | Bombyx mori epidermal cells | HPS | Uric acid storage and secretory compartment for photoprotection of the larvae | |
Figure 1. Examples of ELRO ultrastructure.
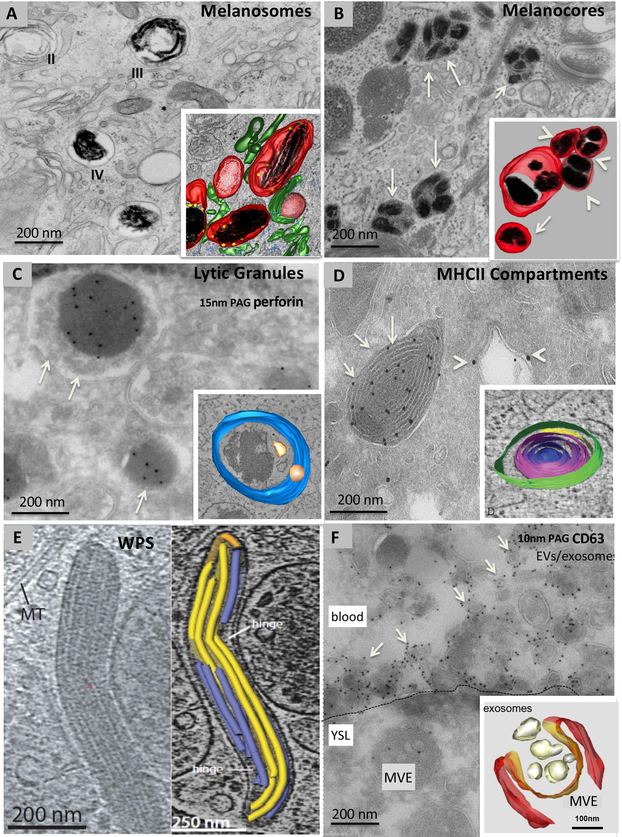
Conventional electron microscopy (A, B), immunogold labelling on ultrathin cryosections (C, D, F), cryomicroscopy (E) and three-dimensional (3D) reconstructions of electron tomograms of model ELROs and their derivatives (A, B, C, D, E, F). A, stage II immature and stage III and IV mature melanosomes in an MNT-1 human melanoma cell fixed by high pressure freezing and embedded in plastic by freeze substitution. Note the striated appearance in stages II and III. Inset: 3D reconstruction showing dark melanin on fibrillar structures (black) in stage III/ IV melanosomes. Unpigmented fibrillar structures present in stage II melanosomes are shown in white. Melanosomes are surrounded by tubular membranes (green), corresponding to endosomal transport carriers.Ribosomes are in blue B, melanocore containing organelles (arrows) in a keratinocyte in human skin biopsies. Inset: 3D reconstruction. Note several melanocores (black) enclosed by a single membrane (red). These organelles appear isolated (arrow) or in a network of clusters (arrowheads). C, lytic granules (arrows) of a human cytotoxic T cell depicting the characteristic dense core containing perforin, immunolabelled with 15 nm protein A gold particles (PAG), surrounded by small membrane vesicles. Inset: 3D reconstruction showing the limiting membrane (blue) and ILVs (yellow). The dense core is not pseudocoloured. D, an ultrathin cryosection of a mouse dendritic cell showing MHC class II (MHCII) compartments (arrows) immunolabeled for MHCII molecules with 10 nm PAG and endosomes (arrowheads) immunolabeled for the endosomal protein EEA1 with 15 nm PAG. Inset: 3D reconstruction showing the multiple concentric membrane layers of an MHCII compartment. E, Cigar-shaped WPBs in a thick section of a human umbilical vein endothelial cell visualized by cryomicroscopy (left panel). The right panel shows a 3D reconstruction of the vWF tubules (blue and yellow) contained within the WPB. F, ultrathin cryosection of a zebrafish embryo expressing CD63-pHluorin in the yolk syncytial layer and labelled for GFP with 10 nm PAG. The plasma membrane of the yolk sac layer is indicated by the dashed line. Note the MVE in the yolk cell and numerous membrane vesicles labelled for CD63 (arrows) in the blood. Inset: 3D reconstruction of an MVE in a HeLa cell in the process of fusing with the plasma membrane. Magnifications are indicated in the panels.
LROs require specific endosomal effectors and molecular mechanisms for their biogenesis, maturation, transport and release. These effectors and mechanisms are being elucidated largely through analyses of genetic disorders and of their associated animal models in which LRO subsets are functionally disrupted [3,5]. Here we summarize recent advances in understanding LRO biology propose additional new members of the LRO family.
LROs defined.
LROs are traditionally defined as cell type-specific organelles that derive a large component of their membranes and contents from the early and/ or late endosomal system [2,3]. However, this definition is complicated by the diversity not only of the morphology (Figure 1) and content of individual family members, but also of the (i) origin of their membranes from endosomal and/ or secretory sources and (ii) the machineries required for their formation, maturation and secretion. For example, whereas some LROs appear to derive predominantly from endosomes (e.g. melanosomes, lung lamellar bodies and secretory MVEs), other well-accepted LROs (e.g. WPBs and neuronal dense core granules) originate from the secretory pathway while acquiring additional contents from the endolysosomal system. Moreover, the term “lysosome-related” – as well as “secretory lysosomes”, which some apply to LROs [6] – belies the coexistence of most LROs with conventional lysosomes, which can also be secreted under certain conditions [7]. Because all LROs have features associated with early/ late endosomes (and not necessarily lysosomes), we propose endo-lysosome-related organelles (ELROs) as a more appropriate designation. In addition, we propose that ELROs encompass all organelles with at least some endolysosomal contents that are affected by genetic disorders such as the Hermansky-Pudlak (HPS), Chediak-Higashi (CHS) and Griscelli syndromes (GS) and familial haemophagocytic lymphohistiocytosis (FHL) and their animal models [3]; these diseases result from loss of function of effectors of ELRO generation or secretion as detailed below. By this definition, dense core granules [8,9], secretory MVEs, and several invertebrate secretory organelles may be considered ELROs (Table 1). Also, some additional newly characterized organelles might join the list (discussed below, Table 1).
ELRO biogenesis from the endocytic and exocytic pathways.
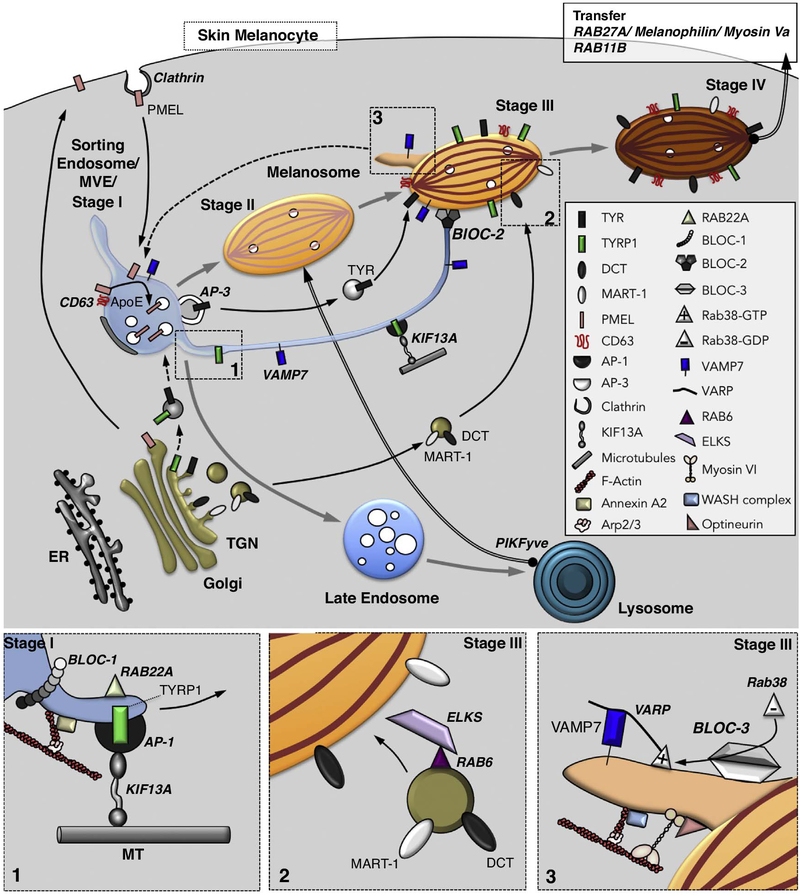
Melanosomes in skin melanocytes are well characterized ELROs that derive primarily from the endosomal system (Figure 2). Melanosomes mature from sorting endosomes/ MVEs, within which physiological amyloid fibrils assemble from fragments of the pigment cell-specific PMEL protein and serve as a matrix upon which melanin pigments ultimately deposit [10]. The fibrils nucleate on the forming ILVs in a process requiring CD63 and apolipoprotein E (ApoE), a component of secreted cholesterolcarrying lipoprotein particles [11]. Optimal fibril formation also requires transient fusion of the immature melanosome precursors with degradative lysosomes, a step that is facilitated by the phosphatidylinositol(3)phosphate 5-kinase PIKFyve [12]. The PMEL amyloid fibrils ultimately assemble into sheets [10], concomitant with the segregation of the non-pigmented melanosome precursors from the endocytic pathway [13]. Once segregated, these precursors mature to pigmented late stage melanosomes following the import of melanogenic enzymes and transporters via transport carriers originating either from early/ recycling endosomes or from the TGN (Figure 2; see next section). Carrier formation from the TGN requires the small GTPase RAB6 and their docking to melanosomes relies on its effector ELKS (Figure 2, box2; [14]). Thus, melanosomes emerge from sorting endosomes but require interactions at multiple stages with lysosomes, recycling endosomes and TGN-derived membranes. Similarly, MVEs are endocytic intermediates that also receive components such as LAMP1 from the secretory pathway [15].
Figure 2.
Model of membrane dynamics during melanosome biogenesis. Shown are pathways of membrane transport from the Golgi and early endosomes to generate different stages of melanosomes. Melanosomes mature from stage I (equivalent to vacuolar domain of a sorting early endosome) to stage IV by progressive acquisition of protein cargoes, mirrored by morphological changes. Following clathrin-dependent endocytosis from the plasma membrane, PMEL is targeted to stage I melanosomes within which it forms fibrils, with the assistance of CD63 and ApoE, on intraluminal vesicles (white circles). The fibrils then mature to fully assembled sheets in stage II in a process requiring collisions with lysosomes mediated by PIKFyve (thick arrow). Melanin synthesis begins upon acquisition of Tyrosinase (TYR), additional enzymes (e.g. TYRP1 and DCT), and transporters that neutralize the luminal pH. The newly generated melanin accumulates on the sheets in stage III and throughout the organelle in stage IV. Cargo transport from endosomes occurs by an AP-3-dependent vesicular pathway taken by TYR and a tubular pathway taken by TYRP1 and other cargoes that requires BLOC-1, RAB22A, AP-1 and KIF13A for membrane tubule formation along microtubules (Box 1), BLOC-2 for targeting to maturing stage III melanosomes, and the vSNARE VAMP7 for fusion with melanosomes. An additional pathway to target DCT and MART-1 to melanosomes from the Golgi requires RAB6 for vesicle formation and its effector ELKS for docking to melanosomes (Box 2). VAMP7 is recycled from melanosomes in tubules that require RAB38, its GEF BLOC-3, and the scaffold protein VARP for formation and/ or VAMP7 incorporation, and that require Myosin VI, optineurin, the WASH complex, Arp2/3 and actin for tubule constriction, scission and release (Box 3); the target organelle for these tubules remains speculative (dashed arrow). Mature stage IV melanosomes in skin melanocytes are captured in the periphery by the RAB27A/ Melanophilin/ Myosin Va complex, and may fuse with the plasma membrane in a RAB11B-dependent manner to release melanocores for uptake by neighbouring keratinocytes.
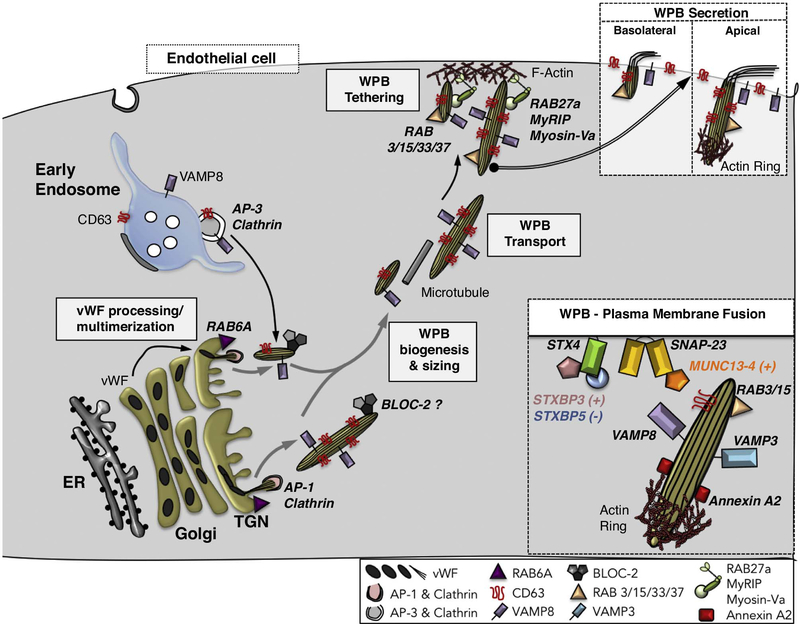
By contrast to melanosomes, WPBs exemplify ERLOs derived primarily from the TGN (Figure 3). Narrow tubule-like polymers of von Willebrand Factor (vWF) form in the Golgi complex, their lengths confined by individual Golgi stacks and by the Golgi ribbon [16]. Efficient platelet recruitment upon vWF release at sites of vascular damage requires a critical length [16,17], such that only ultra-long vWF polymers are ultimately secreted apically into the blood-stream [18]. Once released from the TGN, vWFcontaining WPBs mature by the AP-3-dependent input of endosomal membranes and proteins such as CD63 and the exocytic SNARE VAMP8 [19,20]. A superficially similar process likely underlies the maturation of chromaffin dense core granules and some other secretory granules [8,9].
Figure 3.
Model of membrane dynamics during Weibel-Palade body (WPB) biogenesis. WPB biogenesis begins with the assembly of tubular multimers of von Willebrand factor (vWF) in the Golgi. The quantal length of the multimers is limited by the length of the Golgi cisterna within an individual stack, the size of which is controlled by RAB6A; vWF multimer quanta are then assembled into longer tubules depending on the integrity of the Golgi ribbon. Immature WPBs encasing vWF tubules bud from the Golgi in a process requiring AP-1 and clathrin, and then mature by fusion with vesicles bearing CD63 and the vSNARE, VAMP8, originating from early endosomes in an AP-3-dependent process. BLOC-2 also contributes to WPB maturation in as yet unknown ways. Small WPBs are secreted basolaterally. By contrast, maturing large WPBs are transported to the apical plasma membrane of endothelial cells along microtubules and tethered to the plasma membrane through the action of the RAB27A/ MyRIP/ MyosinVa complex. Mature WPBs are decorated by several RABs (e.g. RAB3, 15, 33 and 37), some of which (e.g. RAB3 and RAB15) might positively regulate the release step. Apical secretion of large WPBs (Box) requires actin polymerization at the basal tip mediated by Annexin A2, calcium-dependent tethering mediated by MUNC13–4, and fusion mediated by the Syntaxin 4/ SNAP-23 tSNARE and either VAMP3 or VAMP8 as the vSNARE. The latter step is facilitated by STXBP3 (Munc18c) and antagonized by STXBP5.
ELRO maturation and HPS-associated protein complexes.
Analyses of isoforms and corresponding animal models of HPS - a group of rare diseases characterized by oculocutaneous albinism, excessive bleeding, and often lung disease and/ or immune dysfunction – have provided insight into the mechanisms underlying ELRO maturation. HPS disease reflects malfunction and incomplete maturation of pigment cell melanosomes, platelet dense granules, lung lamellar bodies, and/ or ELROs in several immune cell types, and is caused by inactivating mutations in any of the genes encoding subunits of four obligate multisubunit protein complexes: AP-3 and biogenesis of LRO complexes (BLOC)-1, −2 and −3. Similar ELRO defects are observed in animal models with defects in homologues of these complexes, the homotypic fusion and vacuole protein sorting (HOPS) and the related class c core vacuole/ endosome tethering (CORVET) complexes, or the RAB38 GTPase [5]. The composition of these complexes and their requirement for ELRO biogenesis have been well reviewed [21,22]. Here we highlight recent findings on the cellular functions of BLOCs.
In mammalian melanocytes, BLOC-1 and BLOC-2 effect the delivery of integral membrane melanogenic enzymes and transporters to maturing melanosomes in a pathway mediated by membrane tubules with characteristics of recycling endosomes [23–27]. BLOC-1 is necessary for recycling tubule formation from early endosomes in several ways (Figure 2, box 1). Firstly, BLOC-1 interacts with the kinesin-3 heavy chain, KIF13A, which drives tubule elongation along microtubule tracks [23,25]. Secondly, BLOC-1 cooperates with Annexin A2 to nucleate Arp2/3-dependent branched actin filaments, which support the elongation of the tubules [24,26,27]. In neurons and HEK293 cells, BLOC-1 cooperates with a distinct effector, the WASH complex, to nucleate Arp2/3-dependent actin filaments on endosomes [28] (although WASH does not appear to impact BLOC-1-dependent trafficking to melanosomes in melanocytes [23,29]), and both Arp2/3 and Annexin A2 are depleted from neurons or neuronal cell lines lacking BLOC-1 [23,30]. These observations suggest that BLOC-1 is a primary cellular activator of Arp2/3 by distinct mechanisms in multiple cell types. BLOC-1 might be stabilized on endosomal membranes in melanocytes by interacting with UVRAG (UV radiation resistance associated gene), a regulator of autophagosome formation and maturation [31]. Intriguingly, UVRAG activities in pigmentation and autophagy appear to be separable, suggesting that some autophagy regulators might be subverted for novel roles in ELRO biogenesis. In non-neuronal cells that lack ELROs, BLOC-1 effects cargo recycling to the plasma membrane [23] and the delivery of specific components to the primary cilium [32], documenting cell-type plasticity of the endocytic pathway.
In melanocytes, the three-subunit BLOC-2 is necessary to direct BLOC-1-dependent tubules towards maturing melanosomes [27]. How BLOC-2 effects this function is not clear; it might either tether tubules to the melanosome membrane [27] or facilitate kinesin-3 function, perhaps by interacting with KIF13A and the small GTPase RAB22A [33]. BLOC-2 might function independently of BLOC-1 in endothelial cells, since WPB maturation and von Willebrand factor secretion in these cells is impaired by depletion of BLOC-2, but not of BLOC-1 [34,35].
BLOC-3 is a guanine nucleotide exchange factor (GEF) for RAB38 and RAB32 [36], two cell type-restricted Rab GTPases implicated in the biogenesis of melanosomes and other ELROs [3]. However, in melanocytes, BLOC-3 and RAB32/ 38 might primarily function from maturing melanosomes to effect retrograde trafficking of the fusion protein VAMP7/ TI-VAMP [26]. VAMP7 is an R-SNARE that mediates fusion of BLOC-1/ BLOC-2-dependent tubular transport intermediates with melanosomes (Figure 2; [26,37]; VAMP7 is also a cargo of the BLOC-1 pathway in neurons [28,38]). Following fusion, VAMP7 is sorted into distinct tubular carriers that emerge from melanosomes [26] and that are likely destined for endosomes after being released following membrane constriction mediated by branched actin filaments, Myosin VI and its scaffold, optineurin (Figure 2, box 3; [29]). The requirement for VAMP7 in anterograde transport to melanosomes and other LROs might explain why RAB32 and RAB38 are needed for forward cargo transport in some pigment cells [39]. While BLOC-3 subunits are not conserved in Drosophila and C. elegans, they are distantly related to the Mon1/ Ccz1 subunits of the RAB7 GEF [36]. In C. elegans, the Ccz1 orthologue pairs with either of two Mon1 orthologues, SAND1 or GLO3, to effect activation of RAB7 (for lysosome biogenesis) or the RAB32/ 38 orthologue, GLO1 (for biogenesis of gut granule LROs) [40].
ELRO secretion and FHL.
As with biogenesis, substantial insight into the molecular mechanisms that govern ELRO secretion have emerged from genetic diseases and their animal models, particularly variants of FHL - a hyperinflammatory disease caused primarily by a failure of CTLs and NK cells to kill their targets [41]. FHL types 3, 4 and 5 are due to mutations in genes encoding MUNC13–4, syntaxin (STX) 11 (STX11), and STX binding protein 2 (STXBP2, a.k.a. MUNC18–2 or MUNC18b) [42]. Their protein products function in fusion at the plasma membrane of cytolytic granules, platelet alpha and dense granules, and other ELROs. Consistent with earlier cellular studies, analyses from in vitro reconstitution systems show that STX11 and the Qbc SNARE protein SNAP23 serve as the plasma membrane tSNARE for docking and fusion of cytolytic granules bearing VAMP8 as the primary vSNARE [43]. VAMP8 might also facilitate the plasma membrane delivery of STX11 by mediating fusion of STX11-bearing recycling endosomes with the cell surface [44] – perhaps by forming a SNARE complex with the plasma membrane associated STX4 [45]. STXBP2, like other members of the Sec1/ MUNC18 (SM) family of SNARE chaperones, facilitates fusion by sequentially engaging with (i) the N-terminal domain of STX11 alone and (ii) the STX11/ SNAP23/ VAMP8 trans-SNARE complex [45,46]. Data from FHL patients with specific mutations that either block or stabilize STX11 binding indicate that both types of STXBP2 interactions are critical for its function [47,48]. Interestingly, STX11 is an unusual SNARE in that it is linked to the membrane by a glycolipid anchor; by facilitating full zipping of the STX11-containing SNARE complex, STXBP2 supports complete membrane fusion mediated by STX11 in vitro [43]. Another SM family member, STXBP5, plays an as yet unspecified role in promoting platelet granule secretion, but appears to antagonize WPB secretion from endothelial cells [49,50] (Figure 3, box).
MUNC13–4 is a calcium-binding effector of RAB27A that is thought to function in granule secretion as a tether [51,52], to directly regulate SNARE-dependent fusion [53], or both [54], most likely at the plasma membrane. GS patients with mutations in RAB27A that disrupt its interaction with MUNC13–4 exhibit FHL-like symptoms with defective CTL degranulation, indicating that this interaction is critical for lytic granule release [55,56]. MUNC13–4 interactions with RAB11 and STX7 also appear to regulate proper lytic granule release [57,58], but how these interactions are coordinated is not well understood. A distinct set of MUNC13–4 interactions appears to regulate WPB release in endothelial cells [59] (Figure 3, box), suggesting cell type specificity in MUNC13–4 functions.
Novel ELRO family members - new organelles to discover and/ or old ones to revisit?
The ELRO family can be expanded not only by organelles newly identified to be affected by HPS, FHL or related diseases or disease models (Table 1), but also by organelles with features that differ from conventional endolysosomes. One example is the pigment storage organelle in keratinocytes. In the skin, melanosomes produced by melanocytes are transferred to neighbouring keratinocytes, within which they form a cap around the nucleus to protect the DNA from ultraviolet radiation. Different modes of melanosome transfer might operate [60], but the primary mechanism in human skin appears to initiate by fusion of melanosomes with the melanocyte cell surface, leading to the secretion of “melanocores” devoid of membrane. Melanocores are then phagocytosed by neighbouring keratinocytes [61–63]. While melanocore release requires RAB11B [62], melanocore uptake requires the G-protein coupled receptor PAR-2 [61]. Within keratinocytes, the pigment is stored within an organelle that is neither acidic nor degradative and that is marked by LAMP1 and CD63, but not by the autophagic marker LC3 [61,63]. This organelle may thus be considered as an ELRO. Interestingly, the melanin pigment itself might dictate the non-degradative capacity of the organelle in which it is enclosed, as suggested by the fate of melanized fungi. Airborne fungal human pathogens are internalized and ultimately killed by pulmonary phagocytes via LC3-associated phagocytosis (LAP) [64]. However, LAP activation and host defence to Aspergillus fumigatus is circumvented by melanin in the cell wall, which inhibits phagosome-lysosome fusion and pathogen destruction [65]. Whether melanin in keratinocytes might similarly “self-protect” its ELRO from degradation to ensure skin photoprotection remains to be determined.
Many hematopoietic lineage cells generate ELROs that support host defence against pathogens (Table 1). In macrophages and monocytes, defence against bacterial infection requires ELRO-associated effectors like RAB32 and its GEF, BLOC-3. The BLOC-3-dependent recruitment of RAB32 to vacuoles harbouring Salmonella Typhimurium [66] or Listeria monocytogenes [67] supports bacterial killing, likely by facilitating phagosome-lysosome fusion [68]. Not surprisingly, S. Typhimurium has developed strategies to attack this host defence by secreting a protease (GtgE) and a GAP (SopD2) specific for RAB32 [66]. This example illustrates how host cells restrict bacterial infection by reshaping bacterial vacuoles into an ELRO-like compartment, and how intracellular bacteria avert this pathway to create a replication niche.
Highly specialized neuronal cells harbour a subpopulation of non-acidic, LAMP1- and/ or CD63-positive organelles that lack acid hydrolases [69,70]. These populations are primarily restricted to axons and dendrites, and comprise amphisomes, MVEs and multilamellar lysosomes prior to fusion with somatic lysosomes and subsequent content degradation [69,70]. These organelles function as intermediates during degradation and display molecular and morphological features of ELROs. The peripheral positioning of these organelles and of presynaptic components bound for nascent synapses depends on BORC [71,72], an 8-subunit complex that shares three subunits with BLOC-1 and that links these organelles to kinesin-1 or kinesin-3 via its effector ARL8 and the kinesin adaptor SKIP [73,74]. Consistently, a partial loss of function of the BORCS7 subunit in mice leads to perinuclear accumulation of LAMP1-positive structures in neurons and to motor defects [75]. It is possible that cells derived from the neural crest (e.g. neurons, melanocytes and osteoclasts) more generally adapt endolysosomal trafficking pathways to generate functionally distinct ELRO subsets.
Perspectives: ELROs as mediators of intercellular communication.
The primary function of most metazoan ELROs is the stimulus-dependent secretion of their contents as a means of intercellular communication. Many ELRO contents are soluble and have well-characterized physiological effects on target cells and tissues (e.g. induction of target cell death by perforin and granzymes released from CTL lytic granules, recruitment of platelets to damaged endothelia by vWF released from WPBs, or stimulation of vascular constriction by release of histamine and other effectors from mast cell granules). Recent observations suggest that additional or alternative modes of intercellular communication are mediated by components released from ELROs. For example, melanocore release by melanocytes may be accompanied by signals – perhaps carried by contents of accompanying exosomes [76] – that might modify the fate of the phagocytosed melanocore within the keratinocyte, depending on skin phototype and exposure to stressors such as ultraviolet exposure or inflammation [61,63]. Similarly, exosomes derived from MVE exocytosis from many cell types harbour receptor ligands, genetic material including miRNAs, and second messengers that can transmit signals to target cells for numerous physiological and pathological functions, including development, tissue regeneration, innate and adaptive immunity, and metastatic progression. As such, exosome contents have a great potential to provide biomarkers for disease diagnosis, prognosis and therapy, such as in cancer and anti-tumour vaccine efficacy [4]. While some controversy exists regarding the mechanisms of formation, secretion and targeting of exosomes due to their similarity to other secreted membranes, the field is advancing. For example, because MVEs and their ILVs bear CD63, MVE fusion with the plasma membrane and exosome release were visualized by live total internal reflection fluorescence microscopy using CD63 fused to the pH-sensitive fluorescent protein, pHluorin (Figure 1F; [77]). A similar approach was then employed in zebrafish embryos to study the biogenesis, composition, transfer, uptake and fate of exosomes produced by yolk syncytial cells in vivo (Figure 1F; [78]) and to track the fate of extracellular vesicles derived from melanoma [79]. The latter studies showed that tumour extracellular vesicles are rapidly taken up by endothelial cells and blood-patrolling macrophages, leading to their activation and promoting metastatic outgrowth. These studies demonstrate the potential for extending cell biological analyses to whole animals to define how exosomes and other ELRO releasates function in physiological and/ or pathological contexts.
Acknowledgements
The authors are grateful to Dr. P. Rosenthal and colleagues, Dr M. Kleijmeer and colleagues and Drs. G. van Niel and F. Verweij for copyright permission (Figures 1D, E and F, respectively), Dr I. Hurbain for Figures A and B, Dr. M. Romao for Figure C. We thank members of our teams for fruitful insights and stimulating discussions, Dr. L. Ripoll for the original draft of Figure 2, and Dr. L. King for helpful comments on the manuscript. We acknowledge funding from the National Institutes of Health/ National Eye Institute (2R01 EY015625 to M.S.M. and G. R.) and National Heart, Lung and Blood Institute (2R01 HL121323 to M.S.M.), the Fondation pour la Recherche Médicale (FRM team label DEQ20140329491 to G.R.), the Fondation ARC pour la Recherche sur le Cancer (PJA20161204965 to C.D.), ANR “Myoactions” (ANR-17-CE11–0029-03 to C.D.), L’Oréal Research and Development, Clarins Research and Development, Institut Curie, INSERM and the Centre National de la Recherche Scientifique (CNRS).
REFERENCES
Recommended reads:
• of special interest
•• of outstanding interest
- 1.Klumperman J, Raposo G: The complex ultrastructure of the endolysosomal system. Cold Spring Harb. Perspect. Biol 2014, 6:a016857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dell’Angelica EC, Mullins C, Caplan S, Bonifacino JS: Lysosome-related organelles. FASEB J. 2000, 14:1265–1278. [DOI] [PubMed] [Google Scholar]
- 3.Marks MS, Heijnen HFG, Raposo G: Lysosome-related organelles: unusual compartments become mainstream. Curr. Opin. Cell Biol 2013, 25:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Niel G, D’Angelo G, Raposo G: Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol 2018, 19:213–228. [DOI] [PubMed] [Google Scholar]
- 5.Raposo G, Marks MS, Cutler DF: Lysosome-related organelles: driving post-golgi compartments into specialisation. Curr. Opin. Cell Biol 2007, 19:394–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffiths GM: Secretion from myeloid cells: secretory lysosomes. Microbiol Spectr. 2016, 4:10.1128/microbiolspec.MCHD-0030–2016. [DOI] [PubMed] [Google Scholar]
- 7.Andrews NW, Almeida PE, Corrotte M: Damage control: cellular mechanisms of plasma membrane repair. Trends Cell Biol. 2014, 24:734–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao Z, Wei L, Feng Y, Chen X, Du W, Ma J, Zhou Z, Chen L, Li W: Impaired maturation of large dense-core vesicles in muted-deficient adrenal chromaffin cells. J. Cell Sci. 2015, 128:1365–1374. [DOI] [PubMed] [Google Scholar]
- 9.Sirkis DW, Edwards RH, Asensio CS: Widespread dysregulation of peptide hormone release in mice lacking adaptor protein AP-3. PLoS Genet. 2013, 9:e1003812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watt B, van Niel G, Raposo G, Marks MS: PMEL: A pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 2013, 26:300–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van Niel G, Bergam P, Di Cicco A, Hurbain I, Lo Cicero A, Dingli F, Palmulli R, Fort C, Potier MC, Schurgers LJ, et al. : Apolipoprotein E regulates amyloid formation within endosomes of pigment cells. Cell Rep. 2015, 13:43–51. [DOI] [PubMed] [Google Scholar]
- 12.Bissig C, Croisé P, Heiligenstein X, Hurbain I, Lenk GM, Kaufman E, Sannerud R, Annaert W, Meisler MH, Weisman LS, et al. : The PIKfyve complex regulates the early melanosome homeostasis required for physiological amyloid formation. J. Cell Sci 2019, 132:jcs.229500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raposo G, Tenza D, Murphy DM, Berson JF, Marks MS: Distinct protein sorting and localization to premelanosomes, melanosomes, and lysosomes in pigmented melanocytic cells. J. Cell Biol 2001, 152:809–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patwardhan A, Bardin S, Miserey-Lenkei S, Larue L, Goud B, Raposo G, Delevoye C: Routing of the RAB6 secretory pathway towards the lysosome related organelle of melanocytes. Nat. Commun 2017, 8:15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pols MS, van Meel E, Oorschot V, Ten Brink C, Fukuda M, Swetha MG, Mayor S, Klumperman J: hVps41 and VAMP7 function in direct TGN to late endosome transport of lysosomal membrane proteins. Nat. Commun 2013, 4:1361. [DOI] [PubMed] [Google Scholar]
- •16.Ferraro F, Kriston-Vizi J, Metcalf DJ, Martin-Martin B, Freeman J, Burden JJ, Westmoreland D, Dyer CE, Knight AE, Ketteler R, et al. : A two-tier Golgi-based control of organelle size underpins the functional plasticity of endothelial cells. Dev. Cell 2014, 29:292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that regulation of platelet aggregation at the vascular wall relies on changes in WPB size that consequently influences the adhesive activity of its vWF cargo.
- 17.Ferraro F, Mafalda Lopes da S, Grimes W, Lee HK, Ketteler R, Kriston-Vizi J, Cutler DF: Weibel-Palade body size modulates the adhesive activity of its von Willebrand Factor cargo in cultured endothelial cells. Sci. Rep 2016, 6:32473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopes da Silva M, Cutler DF: von Willebrand factor multimerization and the polarity of secretory pathways in endothelial cells. Blood 2016, 128:277–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harrison-Lavoie KJ, Michaux G, Hewlett L, Kaur J, Hannah MJ, Lui-Roberts WW, Norman KE, Cutler DF: P-selectin and CD63 use different mechanisms for delivery to Weibel-Palade bodies. Traffic 2006, 7:647–662. [DOI] [PubMed] [Google Scholar]
- 20.Karampini E, Schillemans M, Hofman M, van Alphen F, de Boer M, Kuijpers TW, van den Biggelaar M, Voorberg J, Bierings R: Defective AP-3-dependent VAMP8 trafficking impairs Weibel-Palade body exocytosis in Hermansky-Pudlak Syndrome type 2 blood outgrowth endothelial cells. Haematologica 2019, In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei A-H, Li W: Hermansky-Pudlak syndrome: Pigmentary and non-pigmentary defects and their pathogenesis. Pigment Cell Melanoma Res. 2013, 26:176–192. [DOI] [PubMed] [Google Scholar]
- 22.Bowman SL, Bi-Karchin J, Le L, Marks MS: The road to LROs: insights into lysosome-related organelles from Hermansky-Pudlak syndrome and other rare diseases. Traffic 2019, 20:404–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••23.Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L, Gokhale A, Morel E, Faundez V, et al. : BLOC-1 brings together the actin and microtubule cytoskeletons to generate recycling endosomes. Curr. Biol 2016, 26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that the BLOC-1 complex functions to initiate the formation of recycling endosomal membrane tubules from vacuolar endosomes in conjunction with a microtubule motor and local actin polymerization.
- 24.Delevoye C, Hurbain I, Tenza D, Sibarita J-B, Uzan-Gafsou S, Ohno H, Geerts WJC, Verkleij AJ, Salamero J, Marks MS, et al. : AP-1 and KIF13A coordinate endosomal sorting and positioning during melanosome biogenesis. J. Cell Biol 2009, 187:247–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Delevoye C, Miserey-Lenkei S, Montagnac G, Gilles-Marsens F, Paul-Gilloteaux P, Giordano F, Waharte F, Marks MS, Goud B, Raposo G: Recycling endosome tubule morphogenesis from sorting endosomes requires the kinesin motor KIF13A. Cell Rep. 2014, 6:445–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••26.Dennis MK, Delevoye C, Acosta-Ruiz A, Hurbain I, Romao M, Hesketh GG, Goff PS, Sviderskaya EV, Bennett DC, Luzio JP, et al. : BLOC-1 and BLOC-3 regulate VAMP7 cycling to and from melanosomes via distinct tubular transport carriers. J. Cell Biol 2016, 214:293–308. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that a vSNARE required for anterograde traffic to melanosomes is removed from melanosomes by membrane tubules, the formation of which requires BLOC-3.
- 27.Dennis MK, Mantegazza AR, Snir OL, Tenza D, Acosta-Ruiz A, Délevoye C, Zorger R, Sitaram A, de Jesus-Rojas W, Ravichandran K, et al. : BLOC-2 targets recycling endosomal tubules to melanosomes for cargo delivery. J. Cell Biol 2015, 209:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ryder PV, Vistein R, Gokhale A, Seaman MN, Puthenveedu M, Faundez V: The WASH complex, and endosomal Arp2/3 activator, interacts with the Hermansky-Pudlak syndrome complex BLOC-1 and its cargo phosphatidylinositol-4 kinase type II alpha. Mol. Biol. Cell 2013, 24:2269–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ripoll L, Heiligenstein X, Hurbain I, Domingues L, Figon F, Petersen KJ, Dennis MK, Houdusse A, Marks MS, Raposo G, et al. : Myosin VI and branched actin filaments mediate membrane constriction and fission of melanosomal tubule carriers. J. Cell Biol 2018, 217:2709–2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gokhale A, Hartwig C, Freeman AH, Das R, Zlatic SA, Vistein R, Burch A, Carrot G, Lewis AF, Nelms S, et al. : The proteome of BLOC-1 genetic defects identifies the Arp2/3 actin polymerization complex to function downstream of the schizophrenia susceptibility factor Dysbindin at the synapse. J. Neurosci 2016, 36:12393–12411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Jang GB, Yang X, Wang Q, He S, Li S, Quach C, Zhao S, Li F, Yuan Z, et al. : Central role of autophagic UVRAG in melanogenesis and the suntan response. Proc. Natl. Acad. Sci. U.S.A 2018, 115:E7728–E7737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monis WJ, Faundez V, Pazour GJ: BLOC-1 is required for selective membrane protein trafficking from endosomes to primary cilia. J Cell Biol 2017, 216:2131–2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shakya S, Sharma P, Bhatt AM, Jani RA, Delevoye C, Setty SR: Rab22A recruits BLOC-1 and BLOC-2 to promote the biogenesis of recycling endosomes. EMBO Rep. 2018, 19:e45918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ma J, Zhang Z, Yang L, Kriston-Vizi J, Cutler DF, Li W: BLOC-2 subunit HPS6 deficiency affects the tubulation and secretion of von Willebrand factor from mouse endothelial cells. J. Genet. Genomics 2016, 43:686–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharda A, Kim SH, Jasuja R, Gopal S, Flaumenhaft R, Furie BC, Furie B: Defective PDI release from platelets and endothelial cells impairs thrombus formation in Hermansky-Pudlak syndrome. Blood 2015, 125:1633–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerondopoulos A, Langemeyer L, Liang J-R, Linford A, Barr FA: BLOC-3 mutated in Hermansky-Pudlak syndrome is a Rab32/38 guanine nucleotide exchange factor. Curr. Biol 2012, 22:2135–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamura K, Ohbayashi N, Ishibashi K, Fukuda M: Structure-function analysis of VPS9-ankyrin-repeat protein (Varp) in the trafficking of tyrosinase-related protein 1 in melanocytes. J. Biol. Chem 2011, 286:7507–7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salazar G, Craige B, Styers ML, Newell-Litwa KA, Doucette MM, Wainer BH, Falcon-Perez JM, Dell’Angelica EC, Peden AA, Werner E E, et al. : BLOC-1 complex deficiency alters the targeting of Adaptor Protein complex-3 cargoes. Mol. Biol. Cell 2006, 17:4014–4026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ohishi Y, Kinoshita R, Marubashi S, Ishida M, Fukuda M: The BLOC-3 subunit HPS4 is required for activation of Rab32/38 GTPases in melanogenesis, but its Rab9 activity is dispensable for melanogenesis. J. Biol. Chem 2019, 294:6912–6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •40.Morris C, Foster OK, Handa S, Peloza K, Voss L, Somhegyi H, Jian Y, Vo MV, Harp M, Rambo FM, et al. : Function and regulation of the C. elegans Rab32 family member GLO-1 in lysosome-related organelle biogenesis. PLoS Genet. 2018, 14:e1007772. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that Glo-1, the C. elegans orthologue of RAB32/ 38, functions in biogenesis of ELROs but not conventional lysosomes, and that the Ccz-1 subunit of its GEF associates with either of two distinct partner subunits to mediate exchange on either GLO-1 or RAB7.
- 41.Sieni E, Cetica V, Hackmann Y, Coniglio ML, Da Ros M, Ciambotti B, Pende D, Griffiths G, Arico M: Familial hemophagocytic lymphohistiocytosis: when rare diseases shed light on immune system functioning. Front. Immunol 2014, 5:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tang BL: A unique SNARE machinery for exocytosis of cytotoxic granules and platelets granules. Mol. Membr. Biol 2015, 32:120–126. [DOI] [PubMed] [Google Scholar]
- •43.Spessott WA, Sanmillan ML, McCormick ME, Kulkarni VV, Giraudo CG: SM protein Munc18–2 facilitates transition of Syntaxin 11-mediated lipid mixing to complete fusion for T-lymphocyte cytotoxicity. Proc Natl Acad Sci U S A 2017, 114:E2176–E2185. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper employs in vitro reconstitution assays to show that STXBP2 is required to ensure full fusion mediated by the lipid-anchored tSNARE, STX11, and SNAP-23 with its cognate SNARE VAMP8 for CTL lytic granule secretion.
- 44.Marshall MR, Pattu V, Halimani M, Maier-Peuschel M, Muller ML, Becherer U, Hong W, Hoth M, Tschernig T, Bryceson YT, et al. : VAMP8-dependent fusion of recycling endosomes with the plasma membrane facilitates T lymphocyte cytotoxicity. J Cell Biol 2015, 210:135–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spessott WA, Sanmillan ML, Kulkarni VV, McCormick ME, Giraudo CG: Syntaxin 4 mediates endosome recycling for lytic granule exocytosis in cytotoxic Tlymphocytes. Traffic 2017, 18:442–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dieckmann NM, Hackmann Y, Arico M, Griffiths GM: Munc18–2 is required for Syntaxin 11 Localization on the Plasma Membrane in Cytotoxic T-Lymphocytes. Traffic 2015, 16:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller ML, Chiang SC, Meeths M, Tesi B, Entesarian M, Nilsson D, Wood SM, Nordenskjold M, Henter JI, Naqvi A, et al. : An N-Terminal Missense Mutation in STX11 Causative of FHL4 Abrogates Syntaxin-11 Binding to Munc18–2. Front Immunol 2014, 4:515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spessott WA, Sanmillan ML, McCormick ME, Patel N, Villanueva J, Zhang K, Nichols KE, Giraudo CG: Hemophagocytic lymphohistiocytosis caused by dominant negative mutations in STXBP2 that inhibit SNARE-mediated membrane fusion. Blood 2015, 125:1566–1577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ye S, Huang Y, Joshi S, Zhang J, Yang F, Zhang G, Smyth SS, Li Z, Takai Y, Whiteheart SW: Platelet secretion and hemostasis require syntaxin-binding protein STXBP5. J. Clin. Invest 2014, 124:4517–4528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhu Q, Yamakuchi M, Ture S, de la Luz Garcia-Hernandez M, Ko KA, Modjeski KL, LoMonaco MB, Johnson AD, O’Donnell CJ, Takai Y, et al. : Syntaxin-binding protein STXBP5 inhibits endothelial exocytosis and promotes platelet secretion. J. Clin. Invest 2014, 124:4503–4516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Elstak ED, Neeft M, Nehme NT, Voortman J, ., Cheung M, Goodarzifard M, Gerritsen HC, van Bergen En Henegouwen PM, Callebaut I, de Saint Basile G, et al. : The munc13–4-rab27 complex is specifically required for tethering secretory lysosomes at the plasma membrane. Blood 2011, 118:1570–1578. [DOI] [PubMed] [Google Scholar]
- 52.Chicka MC, Ren Q, Richards D, Hellman LM, Zhang J, Fried MG, Whiteheart SW: Role of Munc13–4 as a Ca2+-dependent tether during platelet secretion. Biochem. J 2016, 473:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •53.Boswell KL, James DJ, Esquibel JM, Bruinsma S, Shirakawa R, Horiuchi H, Martin TF: Munc13–4 reconstitutes calcium-dependent SNARE-mediated membrane fusion. J. Cell Biol. 2012, 197:301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]; Using a mast cell line and in vitro assays, this paper shows that MUNC13–4 responds to calcium to both tether ELRO-like compartments to recycling endosomes prior to secretion and to directly facilitate SNARE-dependent membrane fusion.
- 54.Woo SS, James DJ, Martin TFJ: Munc13–4 functions as a Ca2+ sensor for homotypic secretory granule fusion to generate endosomal exocytic vacuoles. Mol. Biol. Cell 2017, 28:792–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cetica V, Hackmann Y, Grieve S, Sieni E, Ciambotti B, Coniglio ML, Pende D, Gilmour K, Romagnoli P, Griffiths GM, et al. : Patients with Griscelli syndrome and normal pigmentation identify RAB27A mutations that selectively disrupt MUNC13–4 binding. J. Allergy Clin. Immunol 2015, 135:1310–1318.e1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Netter P, Chan SK, Banerjee PP, Monaco-Shawver L, Noroski LM, Hanson IC, Forbes LR, Mace EM, Chinen J, Gaspar HB, et al. : A novel Rab27a mutation binds melanophilin, but not Munc13–4, causing immunodeficiency without albinism. J. Allergy Clin. Immunol 2016, 138:599–601 e593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.He J, Johnson JL, Monfregola J, Ramadass M, Pestonjamasp K, Napolitano G, Zhang J, Catz SD: Munc13–4 interacts with syntaxin 7, regulates late endosomal maturation, endosomal signaling and TLR9-initiated cellular responses. Mol. Biol. Cell 2016, 27:572–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnson JL, He J, Ramadass M, Pestonjamasp K, Kiosses WB, Zhang J, Catz SD: Munc13–4 Is a Rab11-binding protein that regulates Rab11-positive vesicle trafficking and docking at the plasma membrane. J. Biol. Chem 2016, 291:3423–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chehab T, Santos NC, Holthenrich A, Koerdt SN, Disse J, Schuberth C, Nazmi AR, Neeft M, Koch H, Man KNM, et al. : A novel Munc13–4/S100A10/annexin A2 complex promotes Weibel-Palade body exocytosis in endothelial cells. Mol. Biol. Cell 2017, 28:1688–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu X, Hammer JA: Melanosome transfer: it is best to give and receive. Curr. Opin. Cell Biol 2014, 29:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •61.Correia MS, Moreiras H, Pereira FJC, Neto MV, Festas TC, Tarafder AK, Ramalho JS, Seabra MC, Barral DC: Melanin transferred to keratinocytes resides in nondegradative endocytic compartments. J. Invest. Dermatol 2018, 138:637–646. [DOI] [PubMed] [Google Scholar]; This paper shows that keratinocyte uptake of melanocores, but not membrane-bound melanosomes, is PAR2-dependent as it is in vivo. As in (59) the results show that the internalized melanocores are stored in a non-acidic, non-degradative compartment marked by lysosomal membrane proteins.
- 62.Tarafder AK, Bolasco G, Correia MS, Pereira FJ, Iannone L, Hume AN, Kirkpatrick N, Picardo M, Torrisi MR, Rodrigues IP, et al. : Rab11b mediates melanin transfer between donor melanocytes and acceptor keratinocytes via coupled exo/endocytosis. J. Invest. Dermatol 2014, 134:1056–1066. [DOI] [PubMed] [Google Scholar]
- •63.Hurbain I, Romao M, Sextius P, Bourreau E, Marchal C, Bernerd F, Duval C, G. R: Melanosome distribution in keratinocytes in different skin types: melanosome clusters are not degradative organelles. J. Invest. Dermatol 2018, 138:647–656. [DOI] [PubMed] [Google Scholar]; This paper shows in skin biospsies that the fate of melanosomes in keratinocytes is dependent on the skin phototype and that in mildly and lightly pigmented skins melanocores are stored in a non-acidic, non-degradative compartment marked by lysosomal membrane proteins.
- 64.Kanayama M, Shinohara ML: Roles of autophagy and autophagy-related proteins in antifungal immunity. Front. Immunol 2016, 7:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Akoumianaki T, Kyrmizi I, Valsecchi I, Gresnigt MS, Samonis G, Drakos E, Boumpas D, Muszkieta L, Prevost MC, Kontoyiannis DP, et al. : Aspergillus Cell Wall Melanin Blocks LC3-Associated Phagocytosis to Promote Pathogenicity. Cell Host Microbe 2016, 19:79–90. [DOI] [PubMed] [Google Scholar]
- ••66.Spano S, Gao X, Hannemann S, Lara-Tejero M, Galan JE: A bacterial pathogen targets a host Rab-family GTPase defense pathway with a GAP. Cell Host Microbe 2016, 19:216–226. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that unlike wild-type mice, mice lacking RAB32 or BLOC-3 are permissive for infection by Salmonella Typhi, and that the related pathogen Salmonella Typhimurium secretes two effectors that degrade RAB32 or inhibit its activation.
- 67.Li Y, Wang Y, Zou L, Tang X, Yang Y, Ma L, Jia Q, Ni Q, Liu S, Tang L, et al. : Analysis of the Rab GTPase Interactome in Dendritic Cells Reveals Anti-microbial Functions of the Rab32 Complex in Bacterial Containment. Immunity 2016, 44:422–437. [DOI] [PubMed] [Google Scholar]
- 68.Seto S, Tsujimura K, Koide Y: Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes. Traffic 2011, 12:407–420. [DOI] [PubMed] [Google Scholar]
- 69.Cheng XT, Xie YX, Zhou B, Huang N, Farfel-Becker T, Sheng ZH: Characterization of LAMP1-labeled nondegradative lysosomal and endocytic compartments in neurons. J. Cell Biol 2018, 217:3127–3139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yap CC, Digilio L, McMahon LP, Garcia ADR, Winckler B: Degradation of dendritic cargos requires Rab7-dependent transport to somatic lysosomes. J. Cell Biol 2018, 217:3141–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Farías GG, Guardia CM, De Pace R, Britt DJ, Bonifacino JS: BORC/kinesin-1 ensemble drives polarized transport of lysosomes into the axon. Proc. Natl. Acad. Sci. U.S.A 2017, 114:E2955–E2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Niwa S, Tao L, Lu SY, Liew GM, Feng W, Nachury MV, Shen K: BORC regulates the axonal transport of synaptic vesicle precursors by activating ARL-8. Curr Biol 2017, 27:2569–2578 e2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pu J, Schindler C, Jia R, Jarnik M, Backlund P, Bonifacino JS: BORC, a multisubunit complex that regulates lysosome positioning. Dev. Cell 2015, 33:176–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••74.Guardia CM, Farías GG, Jia R, Pu J, Bonifacino JS: BORC functions upstream of Kinesins 1 and 3 to coordinate regional movement of lysosomes along different microtubule tracks. Cell Rep. 2016, 17:1950–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper shows that BORC and Arl8 recruit two different kinesins to lysosomes that mediate outward motility on distinct microtubule tracks in the pericentriolar area or the cell periphery.
- 75.Snouwaert JN, Church RJ, Jania L, Nguyen M, Wheeler ML, Saintsing A, Mieczkowski P, Manuel de Villena FP, Armao D, Moy SS, et al. : A mutation in the Borcs7 subunit of the lysosome regulatory BORC complex results in motor deficits and dystrophic axonopathy in mice. Cell Rep. 2018, 24:1254–1265. [DOI] [PubMed] [Google Scholar]
- 76.Lo Cicero A, Delevoye C, Gilles-Marsens F, Loew D, Dingli F, Guere C, Andre N, Vie K, van Niel G, Raposo G: Exosomes released by keratinocytes modulate melanocyte pigmentation. Nat. Commun 2015, 6:7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Messenger SW, Woo SS, Sun Z, Martin TFJ: A Ca(2+)-stimulated exosome release pathway in cancer cells is regulated by Munc13–4. J. Cell Biol 2018, 217:2877–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••78.Verweij FJ, Revenu C, Arras G, Dingli F, Loew D, Pegtel DM, Follain G, Allio G, Goetz JG, Zimmermann P, et al. : Live tracking of inter-organ communication by endogenous exosomes in vivo. Dev. Cell 2019, 48:573–589.e574. [DOI] [PubMed] [Google Scholar]; This paper employs a CD63-pHluorin fusion protein expressed in zebrafish embryos and high resolution microscopy techniques to characterize extracellular vesicle release, targeting, uptake and degradation within a living animal.
- 79.Hyenne V, Ghoroghi S, Collot M, Bons J, Follain G, Harlepp S, Mary B, Bauer J, Mercier L, Busnelli I, et al. : Studying the fate of tumor extracellular vesicles at high spatiotemporal resolution using the zebrafish embryo. Dev. Cell 2019, 48:554–572.e557. [DOI] [PubMed] [Google Scholar]