Abstract
Assembly of cell-surface receptors into specific oligomeric states and/or clusters before and after ligand binding is an important feature governing their biological function. Receptor oligomerization can be mediated by specific domains of the receptor, ligand binding, configurational changes or other interacting molecules. In this review we summarize our understanding of the oligomeric state of discoidin domain receptors (DDR1 and DDR2), which belong to the receptor tyrosine kinase family (RTK). DDRs form an interesting system from an oligomerization perspective as their ligand collagen(s) can also undergo supramolecular assembly to form fibrils. Even though DDR1 and DDR2 differ in the domains responsible to form ligand-free dimers they share similarities in binding to soluble, monomeric collagen. However, only DDR1b forms globular clusters in response to monomeric collagen and not DDR2. Interestingly, both DDR1 and DDR2 are assembled into linear clusters by the collagen fibril. Formation of these clusters is important for receptor phosphorylation and is mediated in part by other membrane components. We summarize how the oligomeric status of DDRs shares similarities with other members of the RTK family and with collagen receptors. Unraveling the multiple macro-molecular configurations adopted by this receptor-ligand pair can provide novel insights into the intricacies of cell-matrix interactions.
Keywords: discoidin, dimer, cluster, oligomer, collagen, receptor tyrosine kinases
1. Introduction
Discoidin domain receptors (DDRs) are widely expressed cell-surface receptors belonging to two major families of receptors. The first among these is the receptor tyrosine kinase (RTK) family. RTKs are cell-surface receptors that are characterized by a ligand-binding extracellular domain (ECD), a single transmembrane domain (TMD) and an intracellular domain (ICD) consisting of the kinase domain (KD)[1]. In addition an unstructured intra- or extracellular juxtamembrane (IJXM or EJXM) region or a cytoplasmic tail (CT) is present in certain RTKs. There are 58 known RTKs, which can be categorized into 20 sub-families based on the homology of their ECDs, with DDR1 and DDR2 belonging to their own sub-family called DDRs.
Binding of ligand to the ECD of RTKs induces tyrosine phosphorylation and activation of their KD leading to downstream cell-signaling. Triple helical collagen(s) serve as the ligand for DDRs. Because of their ligand, DDRs also belong to the family of collagen receptors[2]. There are four different sub-families of collagen receptors, namely DDRs, select integrins (α1β1, α2β1, α10β1 and α11β1), few immunoglobulin (IgG)-like receptors (GPVI, LAIR1, OSCAR), and mannose receptors. Unlike RTKs, the various sub-families of collagen receptors do not share a similar domain organization and are different in their structures. However, like RTKs, binding of the ligand (collagen) to collagen receptors leads to receptor activation and downstream cell-signaling.
The assembly of both RTKs and collagen receptors into various oligomeric states is important for receptor function. The oligomeric state can range from monomers, dimers, higher-order ‘oligomers’ of well-defined stoichiometry or ‘clusters’, which are higher-order structures heterogeneous in size. The inherent oligomeric state of receptors on the cell-surface can dictate their ligand-binding abilities and modulation of an auto-inhibitory state[3][4]. Transitions in the oligomeric state of receptors upon ligand binding can be important for receptor activation, receptor endocytosis, recruitment of adapter proteins or formation of a signaling node[5].
The oligomeric status of DDRs has been of importance ever since their discovery as RTKs. The ECD of DDRs consists of a discoidin (DS) domain which bears homology to the discoidin I protein found in the slime mold Dictyostelium discoideum[6]. Discoidin I is a lectin which is known to oligomerize[7][8] and modulates cell aggregation. In search for the DDR ligand(s), recombinant ECDs of DDRs were expressed as Fc-tagged homodimers and observed to bind to collagen[9]. Since then several studies have investigated the oligomeric status of DDRs pre and post-ligand binding and how it modulates ligand binding as well as ensuing receptor phosphorylation, receptor activation and downstream signalling. DDR oligomerization and clustering is a complex process and is modulated in part by various receptor domains, collagen binding as well as other cell-based factors. This review summarizes our current understanding of the oligomeric status of DDRs and their impact on receptor function.
2. Oligomeric status of DDRs pre-ligand binding
2.1. Homodimers of DDR1
The DDR sub-family consists of two members: DDR1 and DDR2. Among these, DDR1 is known to exist in five different isoforms (DDR1a to DDR1e) obtained through alternative splicing (Figure 1a). Two of these isoforms, DDR1d and DDR1e lack a functional kinase domain[10], whereas DDR1b and DDR1c consist of an extra 37 amino acid insert in their IJXM. Isoforms DDR1a and DDR1b are most widely expressed and have been most studied. Besides these splice variants, DDR1 can also undergo shedding, resulting in a soluble ECD protein and a membrane tethered C-terminal fragment (DDR1-ΔECD)[11].
Figure 1.
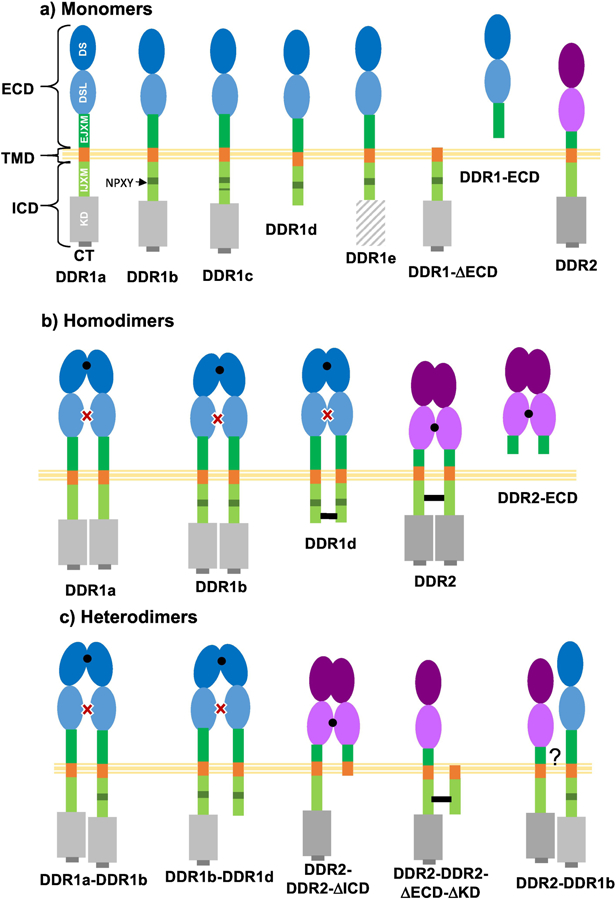
Members of the DDR family known to exist as (a) monomers, (b) homodimers and (c) heterodimers before ligand stimulation. (a) Besides naturally occurring isoforms DDR1a to DDR1e, DDR1 is also known to shed its ECD resulting in a membrane tethered C-terminal fragment lacking the DDR1 ECD (DDR1-ΔECD) and a soluble DDR1 ECD. Only one naturally occurring isoform of DDR2 has been reported. (b) Homodimers and (c) heterodimers reported to exist for recombinantly expressed full-length or truncated receptors. Other possible combinations of homo- and heterodimers may exist but have not been reported. Black dots and dashes represent regions mediating dimer contacts. Red cross indicates glycosylation in DS-like domain which inhibits dimer formation in DDR1. DS: discoidin; DSL: discoidin like; EJXM: extracellular juxtamembrane; TMD: transmembrane domain; IJXM: intracellular juxtamembrane; KD: kinase domain; CT: C-terminal.
Homo-dimerization of DDR1b has been extensively examined using biochemical analysis of cell-lysates as well as live cell-microscopy. It is now well-established that before collagen stimulation DDR1b exists as a sub-population of monomers and non-covalent homodimers on the cell-surface[12][13][14][15] (Figure 1b). Homodimer formation has also been reported for the kinase dead isoform DDR1d[16]. Various regions in DDR1b have been examined for dimer formation by using deletion mutants in cell-surface cross-linking assays[12][13][17][18]. DDR1b dimer formation is primarily mediated by the DS-domain[12], as the DS-like and EJXM domains in the DDR1 ECD have been shown to be dispensable for dimerization[13][12]. Enforced covalent dimerization of almost the entire DDR1b population could be achieved when individual residues in the EJXM or TMD were mutated to Cys[17], but similar mutations in constructs lacking the ECD yielded a sub-population of monomers[18], consistent with the importance of DS domain in dimer formation. The TMD in DDRs has been shown to exhibit a high propensity to dimerize in TOXCAT assays[19] (an assay used to measure transmembrane helix–helix association in a biological membranes[20]). However, deletion of the putative dimerization motifs, namely GXXXG and the leucine zipper motif in the TMD did not affect the dimeric state of DDR1 on the cell surface[13].
The role of cysteines in mediating disulphide-linked dimers has also been explored using non-reducing gels. An earlier study showed some evidence of disulphide-linked dimer of wild-type DDR1 and postulated that Cys303 and Cys348 in the DS-like domain mediated inter-molecular dimer formation[16]. However, recent structural studies of DDR1 ECD (consisting of its DS and DS-like domains but lacking the EJXM) have revealed that these Cys residues are buried too deep into the DS-like domain to mediate inter-molecular interactions[21]. A similar feature has been reported for other proteins consisting of homologous discoidin domain(s) e.g. retinoschisin (RS1) where the cysteines within the discoidin domain are known to create intramolecular links, whereas the inter-molecular dimer and oligomer formation is only mediated by cysteines flanking the discoidin domain in RS1[22]. Recent reports have confirmed that wild-type DDR1 does not form disulphide-linked dimers before ligand stimulation[17]. Interestingly, the kinase dead DDR1d has been shown to have a significant percentage of disulphide-linked dimers[16]. The unstructured DDR1b IJXM consists of one cysteine (Cys499), which is also present in DDR1d. It is possible that this cysteine may be constrained in the full-length receptor but relieved upon deletion of the KD in DDR1d to form disulphide-linked dimers.
Receptor glycosylation and binding of sugars is another factor which can modulate dimerization of DDR1. The DS-like domain in DDR1 ECD has a conserved N-glycosylation site at Asn211, Asn260 and a Ca-ion binding site at Asp233. Glycosylation of DDR1 ECD is likely modulated by the Ca-ion binding site as shown by mutating the analogous Asp234 in DDR2 [23]. Mutation of Asn211 resulted in constitutively active dimers of DDR1b, thus revealing that glycans in this region inhibit dimerization[15]. It is thus likely that the location and extent of DDR1 ECD glycosylation may modulate homodimer formation. It is interesting to note that glycosylation of DDR1 ECD can be isoform specific, with DDR1a being more heavily glycosylated than DDR1b[24]. Besides intrinsic glycosylation, it is possible that binding of extrinsic mono- or disaccharides to the DS domain of DDR1 also mediate dimer formation, in a manner analogous to the discoidin I protein[8]. Further studies are required to understand the role of sugars in modulating DDR oligomerization pre and post-ligand binding.
Several lines of evidence indicate that DDR1 monomers by themselves cannot self-interact to form ligand-independent dimers. Purified recombinant His-tagged DDR1 ECD has been shown to exist as a monomer using gel-filtration analysis[25]. SDS-PAGE under reducing conditions confirmed that both N-terminal[25] and C-terminal[26] His-tagged DDR1 ECD expressed as ~ 64 kDa protein, consistent with the predicted weight of the monomer and absence of covalent dimer. Even at high concentrations (6.8 mg/ml) recombinant DDR1 ECD failed to show dimer formation in analytical centrifugation (unpublished observations)[21]. In another study, DDR1b-myc and DDR1b-flag could be successfully co-immunoprecipitated only when co-expressed in cells but not when lysates of cells expressing either DDR1b-myc or DDR1b-flag were mixed together[13]. Thus cell-mediated processes appear to modulate the assembly of DDR1 into dimers, driven primarily by its DS domain. It is tempting to speculate that a small molecule or sugar may bind to the DDR1 DS domain and mediate its dimer formation.
2.2. Homodimers of DDR2
DDR2 is known to exist in only one isoform(Figure 1a). The oligomeric status of DDR2, appears to be similar to DDR1, with a fraction of DDR2 being in dimeric state on the cell surface[13][27][28]. However, in contrast to DDR1 ECD, recombinant N- or C-terminal His-tagged human DDR2 ECD has been shown to exist as a non-covalent dimer via size-exclusion chromatography and chemical cross-linking analysis with a small fraction ~ 20% in higher-order oligomeric form[25][27]. Dimer analysis via use of reducing and non-reducing gels confirmed that a His-tagged mouse DDR2 ECD lacked disulphide-links[29]. The propensity of DDR2 ECD to dimerize can also be witnessed in atomic force microscopy (AFM) studies where anti-Fc antibody mediated oligomers of recombinant DDR2-Fc were observed to be a single-lobed globular protein[30] whereas in contrast DDR1-Fc dimers and oligomers were resolved to be bi-lobed or multi-lobed[31] consistent with their inability to self-interact.
The DDR2 dimer formation appears to be mediated by its DS-like domain as His-tagged DDR2 ECD lacking this domain was reported to be a monomer[25]. A double mutant DDR2 ECD protein, consisting of F96A and T98A at the DS and DS-like interface, did not affect the non-covalent dimer but disrupted oligomer formation[27]. Thus the DS-like domain and its interface appear to be important for the dimeric and oligomeric status of DDR2. Like DDR1, the TMD of DDR2 has a high propensity to dimerize in TOXCAT assays[19] but evidence of TMD directly modulating DDR2 dimerization is currently lacking. Truncated segments of DDR2 consisting only of TMD and portions of its IJXM formed dimers when expressed in cells and a segment of the IJXM was found to be necessary for DDR2 dimer formation[28]. Thus unlike DDR1, more than one domain modulates dimer formation in DDR2 (Figure 1b).
2.3. Hetero-dimerization of DDRs
Hetero-dimerization between members of the DDR sub-family would imply dimers formed from two different isoforms of DDR1 or with DDR2. Much less is known about hetero-dimerization of DDRs, although there is indication that they may exist. During the discovery of DDR1a, it was reported that both endogenously expressed as well as transiently transfected DDR1a and DDR1b could be co-immunoprecipitated using isoform-specific antibodies, thus indicating hetero-dimer formation even before ligand binding[32]. In another study, co-transfection of full-length DDR1b with increasing amounts of a truncated, kinase-dead receptor diminished DDR1b phosphorylation in response to collagen[33]. Along similar lines, retroviral infection with a truncated DDR2 (lacking its ICD) construct inhibited cell proliferation and migration due to endogenous DDR2[34]. While these observations indicate that the truncated receptors may independently compete for ligand binding with the full length receptor, one could also hypothesize that the truncated receptors dimerize with full length DDRs in a manner which inhibits their phosphorylation. Supporting the hypothesis that various isoforms may form hetero-dimers, a truncated DDR2 receptor lacking the ECD and KD was reported to localize with endogenous DDR2 on the plasma membrane[28]. Thus several combinations of DDR hetero-dimers can putatively exist, linked by similar domains as present in homodimers (Figure 1c).
Very little is understood regarding hetero-oligomer formation between DDR1 and DDR2. Using immuno-fluorescence and co-immunoprecipitation it has been shown that DDR1 and DDR2 physically interact and co-localize both before and after interacting with the collagen fibril[35]. In our study DDR1b-YFP was found to co-localize with endogenous DDR2 in the filamentous structures formed after collagen stimulation[29]. Further, co-expression with DDR2 as the donor kinase led to phosphorylation of the signaling-incompetent DDR1b receiver mutants[18]. Thus it is likely that DDR1 and DDR2 when co-expressed in cells preserve the capacity to form hetero-oligomers. However it should be taken into consideration that formation of DDR hetero-oligomers has primarily been reported using recombinant proteins. It remains to be examined if hetero-oligomer formation of DDRs is a naturally occurring phenomenon. Further, the relative abundance, stoichiometry and receptor contacts of hetero-oligomers before and after interaction with collagen remain to be characterized.
3. Role of oligomeric status in ligand binding
There are 29 different types of collagen, several of which serve as ligands for DDRs[36][37]. Collagen serves as a complex ligand as it can exist as soluble monomers and also undergoes fibrillogenesis to form fibrils of various morphologies and composition[36]. The ligand binding ability of DDRs have mostly been studied using collagen I (henceforth referred to as collagen) or collagen II or III mimetic toolkit peptides (henceforth referred to as col II or col III). Regarding the role of oligomeric status of DDRs in ligand binding, it is has been well established that the DS domain in the DDR ECD is necessary for its binding to collagen but is it sufficient for high affinity binding to the ligand? Early studies reported that the monomeric GST-tagged DS domain of both DDR1 and DDR2 could bind to immobilized collagen in solid-phase binding assays[25][38]. It has subsequently been shown using NMR [39] and X-ray crystallography[40] that the monomeric DS domain of DDR2 can bind to collagen fibrils and to the coll-II mimetic triple helical peptide. However, studies have also shown that the presence of the entire ECD enhanced the binding of both DDR1 and DDR2 to immobilized collagen as compared to their DS-domain only[25][38]. In case of DDR2, one can postulate two reasons behind this enhanced affinity. Firstly, the presence of DS-like domain could exert an allosteric control over its DS-domain in collagen binding as shown by mutating F96A and T98A at the DS and DS-like interface[27]. Another possibility could be that the DS-like domain results in a dimeric state of DDR2 ECD which enhances its ligand binding. In the case of DDR1, the enhanced binding observed could be explained in part due to clustering of DDR1 ECD upon collagen binding (Section 4.1).
Several lines of evidence indicate that the oligomeric state of DDR ECDs plays a major role in their binding to collagen. Covalent dimers of DDR ECDs enforced by means of an Fc-tag at their C-terminus exhibited an enhanced binding to collagen as compared to their untagged forms. The DDR2 DS-domain alone when expressed as an Fc-tagged dimer showed enhanced binding to collagen in solid-phase binding assays as compared to the monomeric DS-domain, which showed limited binding[25]. Similarly, DDR1-Fc and DDR2-Fc (consisting of the entire ECDs) exhibited enhanced binding to collagen as compared to monomeric DDR1 ECD[14], or even non-covalently dimerized DDR2 ECD[29]. The Fc-tagged proteins have also been utilized to create higher-order oligomers by using an anti-Fc antibody. These enforced higher order oligomers further enhanced their binding to collagen as compared to Fc-tagged dimers for both DDR1[31][41] and DDR2[30][42][29] as revealed by their dissociation constant (using surface plasmon resonance) or half maximal effective concentration values (in solid-phase binding assays). Consistent with these observations, a non-covalent oligomeric form of His-tagged DDR2 ECD also exhibited enhanced binding than the corresponding dimeric fraction[27]. Thus increasing the oligomeric state of DDR ECD enhances their binding to collagen in in-vitro assays. This is also manifested in enhanced inhibition of collagen fibrillogenesis by DDR2 oligomers[29].
What could be the reasons behind this enhanced binding by dimers and oligomers of DDR ECDs? One possible explanation is that two (or more) DS domains in the DDR2 dimer (or oligomer) bind cooperatively to a high-affinity (e.g. GVMGFO motif) and an adjacent low affinity site (e.g. consisting of upstream Arg) on the collagen triple helix[43][40], thus reducing the number of dissociation events (Figure 2a). Another possibility, at least for DDR2, could be that the monomeric form only binds to one site on the collagen triple helix whereas the dimeric (and oligomeric) DDR2 ECD binds to additional sites (Figure 2b). Our earlier AFM studies had showed the existence of three possible binding sites for DDR2-Fc oligomers on the collagen I triple helix[30]. Consistent with multiple binding sites for DDR2, the dimeric DS2-Fc protein bound to both periods D1 and D2 of the col II triple helix[44]. Recent studies using col II and col III toolkit peptides have identified that besides the primary GVMGFO site, four additional DDR2 binding sites exist on the collagen triple helix[43,45]. It is interesting to note that in the collagen toolkit studies, the various DDR2 ECD variants showed different relative affinities to these additional binding sites[43,45]. For instance, while the covalent DDR2-Fc dimer recognized the toolkit peptide II-5[45], this site was not recognized by the non-covalent dimer, DDR2-His[43]. Thus, it is likely that binding of dimeric (and oligomeric) DDR2 ECD to additional sites on the collagen triple-helix could account for their enhanced binding as compared to the monomeric form. In case of DDR1 ECD, only one binding site has been identified on the collagen triple helix using toolkit peptides[45]. However, like DDR2, it is possible that additional binding sites exist for DDR1, which may not have been identified using toolkit peptides. For instance DDR1 also binds to collagen type IV[45] and its ligand-binding is affected by collagen glycosylation[46], features which are not present on the toolkit peptides.
Figure 2.
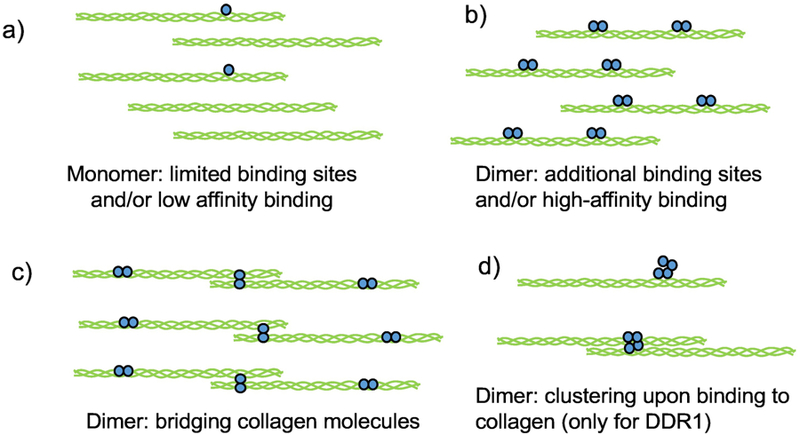
Possible mechanisms explaining enhanced binding of dimeric (or oligomeric DDR ECDs (shown in blue) to collagen (shown in green). As compared to monomers (a) dimers of DDR ECD (b) may bind to a high-affinity binding site as well as an adjacent low-affinity site thus enhancing the overall affinity. This could lead to reduced dissociation of the bound DDR ECD thus increasing the number of binding events on collagen molecules present. In addition dimers may bind to additional sites on the collagen triple helix (b) or to an end-to-end overlap of collagen molecules (c), which may not happen with DDR monomers. Clustering of DDR1 ECD can also increase the amount of bound protein present on collagen (d). While dimeric DDR1 ECD has been shown to cluster, clustering of monomeric DDR1 is not known.
Another explanation for enhanced binding by dimers and/or oligomers could be that the two DS domains each bind to a site on two adjacent collagen molecules brought together during the collagen assembly process[42] (Figure 2c). Consistent with this hypothesis we had shown that DDR1 ECD binds to collagen monomers which assemble into a dimer via an end-to end overlap of collagen molecules, in a manner reminiscent of early stages of collagen fibrillogenesis[31]. Along similar lines, a multi-D2 domain collagen II molecule resulted in increased phosphorylation of DDR2 expressing cells as compared to native collagen II[44], suggesting a possible scenario where a DDR2 dimer could bind to the D2 domains in two adjacent multi-D2 collagen II molecules. Finally, the clustering of DDR ECD upon binding to collagen could also exhibit an enhanced binding signal (Figure 2d). While the dimeric DDR1-ECD (DDR1-Fc) has been shown to form globular clusters upon binding to soluble collagen[41], it remains to be examined if monomeric DDR1 ECD participates in such cluster formation. However, neither monomeric nor dimeric DDR2 ECD cluster upon collagen binding[29]. Thus, while further studies can help elucidate the underlying mechanisms, it is clear that dimers and oligomers of DDRs exhibit enhanced binding to collagen as compared to monomers.
4. Ligand induced changes in the oligomeric status of DDRs
4.1. Effect of monomeric collagen
DDR-collagen interaction results in multi-faceted macro-molecular assemblies as their ligand collagen(s) can also undergo supramolecular assembly to form fibrils. Both monomeric and fibrillar form of collagen I has been used to evaluate the oligomeric state of DDRs post-ligand binding. Using live-cell FRET microscopy we had elucidated that stimulation with soluble, monomeric collagen led to rapid assembly of YFP and CFP-tagged DDR1b into higher-order globular clusters even beyond the dimeric state[14] (Figure 3a). Our FRET analysis also indicated that high-affinity DDR1b dimers preferentially interact with collagen (as compared to DDR1b monomers) to form these clusters. The DDR1b clusters thus formed are rapidly internalized into early endosomes[14], consistent with the presence of NPXY internalization motif present in the IJXM of DDR1b[37]. Immunofluorescence microscopy by others has also confirmed clustering of full length DDR1b upon stimulation with soluble collagen[47][18]. The DDR1b clusters thus formed are known to persist for several hours after stimulation with collagen [47][29]. Very little is known about clustering of DDR1 upon stimulation with other collagen types. One study has reported formation of DDR1 clusters with collagen IV, but the clusters were short-lived as compared to those formed by collagen I[47]. DDR2 on the other hand does not cluster upon binding to monomeric collagen and maintains a uniform distribution on the cell surface[29][48]. Binding of DDR2 to monomeric collagen, especially via its high-affinity dimers likely accounts for its inhibition of collagen fibrillogenesis on the cell surface as shown in our earlier studies[49][42][50] (Figure 3b).
Figure 3.
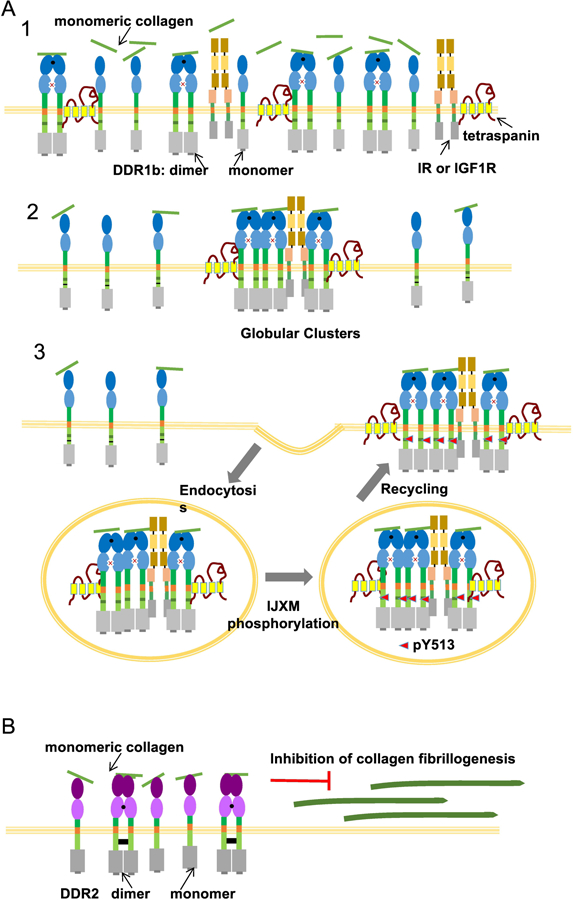
(a): Clustering of DDR1b in response to monomeric collagen. Upon exposure to soluble, monomeric collagen, high-affinity DDR1b dimers bind to collagen (as in 1) and rapidly undergo clustering (as in 2). Thus, while clustering of DDR1b sequesters the dimer population, it remains to be examined if monomers are also incorporated in clusters. DDR1b clusters also recruit other membrane components like the insulin receptor (IR), insulin-like growth factor receptor (IGF1R) and tetraspanins. The DDR1b clusters thus formed are endocytosed to early endosomes, likely via a clathrin mediated endocytosis pathway. The clusters recycle back to the cell membrane after several hours and exhibit DDR1b phosphorylation at Y513 in its IJXM region (as in 3). Since tyrosine phosphorylation in KD does not occur in these clusters the ensuing signaling due to DDR1b clusters is termed non-canonical signaling. While this process is likely to be present in DDR1c due to NPXY motif in its IJXM as in DDR1b, it remains to be investigated if DDR1a also undergoes clustering and endocytosis. (b) Unlike DDR1b, DDR2 does not undergo clustering or endocytosis in response to monomeric collagen. Binding of high-affinity dimers to monomeric collagen inhibits collagen fibril formation on the cell surface.
The formation of globular clusters of DDR1b upon stimulation with monomeric collagen appears to be governed by its ECD. Using AFM studies, we have shown how recombinant dimeric DDR1 ECD (DDR1-Fc) clusters upon binding to collagen[41]. Consistent with this observation, a function-blocking DDR1 antibody, which binds to an extracellular epitope (but not the collagen binding site) was shown to inhibit collagen induced DDR1 clustering[18]. It is possible that the DDR1b TMD and IJXM also modulate receptor clustering[18] although further studies are required to confirm their role on cluster formation. It is not known if DDR1a which lacks the NPXY motif also undergo ligand-induced clustering and endocytosis. Inhibition of the DDR1 kinase activity with the type II inhibitors, namely imatinib and ponatinib did not interfere with DDR1 clustering in response to soluble collagen, indicating that activation of the kinase domain does not modulate receptor clustering[47].
4.2. Effect of collagen fibrils
Fibrils of collagen I have a strikingly different effect on the oligomeric status of DDRs as compared to monomeric collagen. In our recent study using live cell microscopy we have shown that upon prolonged (4 hr) stimulation with soluble collagen I both DDR1b-YFP and DDR2-GFP assemble into linear clusters resembling filamentous structures which associate with collagen fibrils[29] (Figure 4). These linear clusters arise due to a spatial redistribution of the receptor molecules and exhibit a fluorescence intensity higher than their surrounding regions, characteristic of higher-order oligomeric states. Evidence for formation of such linear clusters of DDR1 and DDR2 can also be gathered by re-visiting other studies. For instance full length and kinase dead isoforms of DDR1 were stated to undergo ‘cluster’ formation when cells were cultured on collagen fibrils[51]. A close examination of the morphology of the clusters formed in this study indicates that these structures resemble linear clusters instead of small punctuate globular clusters of DDR1b. A similar observation can be extracted from two additional studies where DDR1 and DDR2 assembled along collagen fibrils when examined via immunostaining approaches[48][35]. A recent study has reported that in cells treated with soluble collagen I, a large fraction of DDR1 was insoluble in multiple detergents[47], which can be partly explained due to their assembly and association with insoluble fibrils formed upon collagen fibrillogenesis. Thus the morphological state of collagen can induce two different oligomeric states of DDRs: globular clusters or linear clusters. Both these oligomeric states once formed persist for several hours indicating stable structures[47][29].
Figure 4.
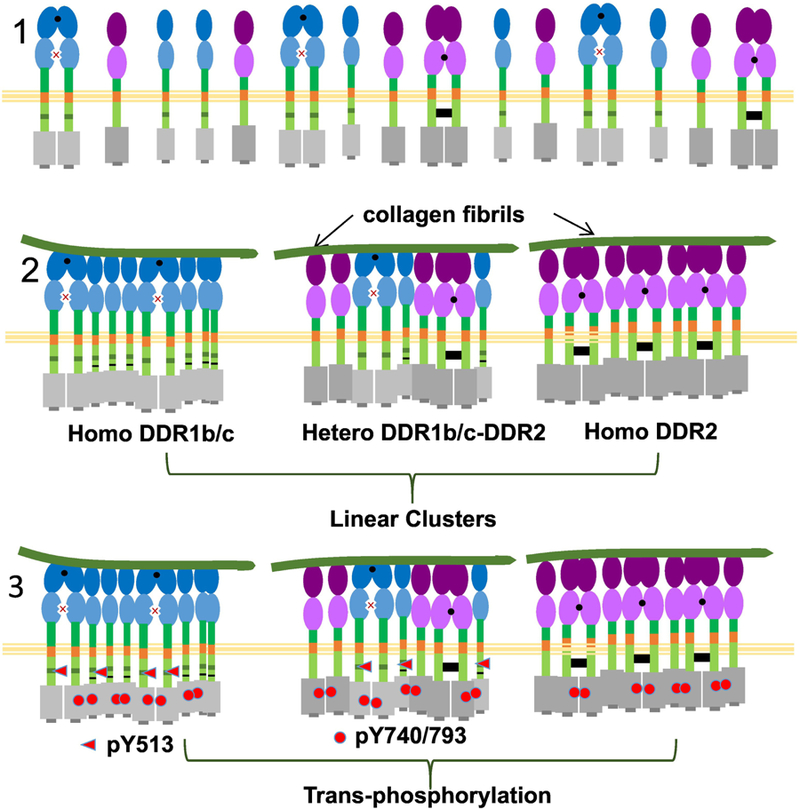
Formation of linear clusters of DDR1b and DDR2 in response to collagen fibrils. The collagen fibrils serves as a multivalent ligand and likely recruits both monomers and dimers of DDRs. The DDRs thus assemble together along the fibril length to form filamentous structures or linear clusters. These assemblies can be homo-oligomers or hetero-oligomers. Receptor phosphorylation (at Y513) in IJXM for DDR1b and (at Y793 for DDR1 and Y740 for DDR2) in KD occurs upon formation of linear clusters due to trans-phosphorylation leading to canonical DDR signaling. Formation of these linear clusters is likely mediated by several domains including the ECD, TMD, IJXM and KD. All DDR1 isoforms as well as DDR1-ΔECD could putatively participate in formation of linear clusters.
Not much is understood about the stoichiometry and molecular mechanisms behind formation of linear clusters. The receptor domains responsible for formation of linear clusters are likely to be those that mediate lateral association of DDR monomers upon ligand binding. In structural studies using x-ray crystallography, a conserved patch (containing Arg32 and Leu152) in the DS-domain of one DDR1 molecule is postulated to interact with the DS-like domain of the neighboring molecule, and mediate lateral association between monomers[11]. In another study, treatment with the type II kinase inhibitor, nilotinib suppressed DDR1 spatial redistribution in cells cultured on fibrillar collagen[51], suggesting that lateral associations between the kinase domain may also be important for these linear clusters.
There is some evidence that the formation of linear clusters may alter the relative population of DDR monomers versus dimers. Two initial studies had reported that stimulation with soluble collagen for 90 minutes did not change the dimeric fraction of DDR1b in transfected cells[12][13]. However, in later reports the dimeric fraction of DDR1 was enhanced in a breast cancer cell line upon stimulation with soluble rat-tail collagen[15] for 2 hrs (a time at which this collagen forms fibrils[29]), and in cells cultured on collagen fibrils[51]. While some discrepancies can be attributed to differences in cell-type and/or protocols employed, it is tempting to surmise that assembly of DDRs into linear clusters upon binding to collagen fibrils, recruits both monomers and dimers and drives the receptor population towards a dimeric state.
5. Oligomeric status and receptor phosphorylation
5.1. Dimerization is not enough
Before ligand binding monomeric as well as dimeric DDRs present on the cell surface are primarily in an inactive auto-inhibitory state[13][15]. Several lines of evidence indicate that DDRs do not follow the mechanism of ligand induced conformational change across monomers or dimers leading to receptor phosphorylation. Studies using chimeric receptors (ECD-TMD-IJXM-KD) have shown that the combination (PDGFR-DDR1-DDR1-TrkA) resulted in maximal activity, whereas those with (PDGFR-DDR1-DDR1-DDR1) or (PDGFR-TrkA-TrkA-DDR1) exhibited reduced phosphorylation when stimulated by the ligand PDGF[52]. It is interesting to note that these chimeric receptors were dimeric before ligand stimulation due to the presence of the dimeric ECD of PDGFR. In another study monomeric chimeric receptors with (TrkA-DDR1-DDR1b-DDR1) did show cognate ligand (nerve growth factor) induced dimerization but failed to show phosphorylation[53]. Thus the dimer formation alone is not sufficient to induce ligand-induced phosphorylation in the DDR1 KD. This lack of correlation between induced dimerization and DDR phosphorylation is consistent with the DS domain structural studies that do not show any significant conformational differences between the ligand bound and unbound states[40][39]. Similarly, additions and deletions of segments in the DDR1 EJXM did not affect receptor phosphorylation[17][11], thus indicating lack of conformational coupling through its highly flexible EJXM.
On the other hand, constraining the structure of the DDR dimer by site-directed mutagenesis has shown to interfere with the normal collagen-induced phosphorylation of the receptor. Cysteine scanning mutagenesis has revealed that enforced disulphide-linked covalent dimerization at two positions in the DS-like domain (V220C and L247C mutants) or a P387C mutation in the EJXM proximal to the DS-like domain displayed low levels of constitutive phosphorylation with strongly attenuated collagen-induced tyrosine phosphorylation[17]. Along similar lines, mutation of the glycosylation site Asn211 in the DS-like domain resulted in constitutively active dimers of DDR1, with little response to collagen-induced phosphorylation[15]. Enforced dimerization of DDR2 via a chimeric receptor (Fc-DDR2) in which the entire DDR2 ECD was replaced by the Fc portion of immunoglobulin also resulted in constitutively active receptors with little response to collagen[34]. Thus normal functioning of DDRs, which can undergo collagen induced phosphorylation, requires the existence of receptor monomers and/or unconstrained, non-covalent dimers which can respond to collagen.
5.2. Globular clusters of DDR1b and a non-canonical pathway
As first proposed by Gao et al [47] and based on our own findings[29], DDR1 appears to follow two pathways for receptor phosphorylation (a) a non-canonical pathway via formation of DDR1b clusters[47][29] (Figure 3a) and (b) the canonical pathway via formation of linear clusters[29] (Figure 4). Differences in downstream cell-signaling resulting due to phosphorylation of DDR1a versus DDR1b isoforms [54][55] could in part be explained by the formation of these two distinct higher order oligomers aligned with the two pathways. In the case of DDR2, only the latter pathway appears to be true.
In the non-canonical pathway, soluble collagen induces clustering of DDR1b, internalization of receptor clusters and phosphorylation of tyrosines in the IJXM (i.e. Y513) but not in the receptor KD[29][47]. Even though receptor clusters are formed within minutes of exposure to collagen, phosphorylation of Y513 was detected only after a few hours and was primarily localized to receptor clusters[29]. Further, not all DDR1b clusters were phosphorylated suggesting that clustering precedes receptor phosphorylation. At this point it is not clear if phosphorylation of Y513 occurs upon receptor internalization in the early endosomes or upon their recycling to the cell surface. It is also possible that other binding partners such as insulin receptors (Section 6) and tetraspanins (Section 7) can modulate DDR1b cluster formation and receptor phosphorylation (Figure 3a). Alongwith Gao et al, we ascribe this to be a non-canonical pathway as it does not lead to phosphorylation of key tyrosines in the KD required for activation of the RTK[56]. This non-canonical signaling pathway of DDR1 would be isoform specific and would be only present in clusters comprising of DDR1b/c which possess Y513.
5.3. Linear clusters of DDR1 and DDR2 and the canonical pathway
Kinase activation in RTKs is typically initiated at the activation loop, which occludes the catalytic site until it is phosphorylated[1]. The canonical pathway of RTKs involves tyrosine phosphorylation in the activation loop, leading to RTK activation. This activation event phosphorylates other tyrosines in the JM or C-terminal domain and leads to recruitment of SH2 domain-containing proteins and downstream signal transduction pathway[1]. In case of DDRs, the assembly of DDR1 and DDR2 into linear clusters along the collagen fibrils was observed to be a prerequisite for phosphorylation of their tyrosines Y793 and Y740 respectively[29] (Figure 4). These tyrosines are located in the activation loop of DDR1 and DDR2 and are critical for receptor activation[56][57]. The formation of linear clusters also led to phosphorylation of Y513 (found in the IJXM of DDR1b/c). We thus ascribe the formation of linear clusters to initiate the canonical pathway for DDR signaling. It should be noted that the requirement of fibrillar collagen for DDR2 phosphorylation has also been elucidated in earlier studies where cells were plated on immobilized collagen substrates[46][58] instead of being stimulated with soluble collagen.
So how do these higher-order oligomeric states promote receptor phosphorylation? A comprehensive study on biochemical analysis of receptor phosphorylation concluded that DDR1 phosphorylates by lateral dimer association and trans-phosphorylation between dimers[38]. In this study even ECD deleted or kinase inactive receiver mutants could be phosphorylated in trans by kinase-active donors[38]. In addition, the leucine zipper motif in the DDR1 TMD has previously been shown to be important for collagen mediated receptor phosphorylation[4] and may be mediating lateral dimer association. It is interesting to note that DDR2 has also been shown to trans-phosphorylate DDR1, whereas the converse is still not known[38]. On the other hand, phosphorylation of DDR2 kinase domain has been shown to occur via an intramolecular, cis-phosphorylation mechanism mediated in part by phosphorylation of Y740 in the DDR2 activation loop by Src kinase[59]. Tyr740Cys mutation found in fibroblasts from patients with Warburg-Cinotti Syndrome resulted in enhanced receptor phosphorylation, postulating auto-phosphorylation of the wild-type protein partner in a DDR2 dimer in heterozygous cells[60]. Further, as described earlier (Section 2.2) the DS and DS-like interface[27] and the IJXM[28] regions of DDR2 may be exerting an allosteric control over the receptor upon collagen binding and may contribute to release of its cis auto-inhibition.
5.4. Multivalency of ligand in DDR oligomerization and phosphorylation
The formation of higher order oligomers upon ligand binding can be either ligand mediated or receptor mediated or a combination of both[1]. Collagen in particular can serve as a multivalent ligand as it has been shown to have multiple binding sites for collagen receptors (including DDRs) along its triple helix[61]. Secondly collagen can assemble into supramolecular fibrils, which can bring together binding sites located on individual collagen monomers in close proximity[62]. Three independent studies indicate that simply binding to one specific site the collagen triple helix appears to be insufficient to induce DDR phosphorylation. (i) In-vitro AFM studies have shown that both DDR1[41] and DDR2[30] ECD bind to the collagen I triple helix, but a fibrillar form of collagen is required to induce receptor phosphorylation in the kinase domain[29]. (ii) Solid phase binding assays have identified multiple toolkit peptides which can bind DDR1[45] and DDR2[43]. However, not all of these peptides can induce receptor phosphorylation. Structural studies using X-ray crystallography have elucidated that the DDR2 DS domain binds to the GVMGFO motif[40] but additional triplets and a concentration nearly 10 times that required for the natural ligand collagen is required to induce receptor phosphorylation. It is possible that the toolkit peptides self-assemble into supramolecular structures at higher concentrations as reported for other collagen mimetic peptides[63]. (iii) Along similar lines, recombinant bacterial collagen-like constructs have been shown to bind to both DDR1 and DDR2 but could not induce receptor phosphorylation in cells[64]. Thus assembly of collagen into a supramolecular structure, enabling multi-valency of ligand appears to be a prerequisite for formation of linear clusters and ensuing DDR phosphorylation via the canonical pathway.
6. Other partners in oligomeric states of DDRs
DDRs are not known to associate with other collagen receptors (like with β1 integrin[33]), but there is evidence on association of DDRs with certain members of the RTK family. DDR1 has been characterized to co-localize and functionally interact with members of the insulin receptor (IR) and its homologous insulin growth factor receptor (IGF-R)[65]. In human breast cancer cells and in fibroblasts, DDR1a rapidly co-internalized with the IR after stimulation of either insulin or IGF-2 even in the absence of exogenous collagen[66]. Moreover, IGF-1 induced rapid DDR1 phosphorylation even in the absence of collagen. Another study elucidated how DDR1a and DDR1b associated with IGF-IR at the cell membrane, and within minutes of IGF-I stimulation, IGF-IR and DDR1 co-localized in early endosomes and at the perinuclear regions[67]. IGF-1R depletion also severely impaired collagen-induced DDR1 phosphorylation[67]. It should be noted that the tyrosine residues in the auto-phosphorylation sites (Y793, Y797, and Y798) of DDR1 are conserved within the catalytic domains of the insulin receptor[6] and it is likely the two receptors may trans-phosphorylate each other in non-canonical DDR1b signaling (Figure 3a). In contrast to insulin, stimulation with EGF did not affect DDR1 phosphorylation in the presence or absence of collagen[33] indicating that members of the EGFR family do not co-cluster with DDR1. DDR2 also appears to interact with members of the RTK family because cognate ligands such as insulin[68] and platelet-derived growth factor (PDGF)[69] have been shown to enhance collagen-induced DDR2 tyrosine phosphorylation. Besides RTKs, the Notch1 receptor is also reported to associate with DDR1 and collagen-mediated activation of DDR1 activated canonical Notch1 signaling [70]. Thus hybrid-oligomerization of DDRs with members of other receptor families could modulate cell signaling in a manner beyond the confines of a single receptor.
Metallo-proteases like membrane anchored MT-MMPs[11] and ADAM10 have been identified to cleave DDR1 at its EJXM region even before ligand binding and are postulated to form a complex with DDR1 on the cell surface[71]. In addition the rhomboid RHBDL2, an intramembrane serine protease which localizes to the plasma membrane, has been reported to cleave DDR1 at A411 in the EJXM region, proximal to the TMD[72]. Both DDR1a[11] and DDR1b[73] have been reported to undergo shedding of their ECD. It remains to be investigated if these proteases associate with and act on a specific isoform or oligomeric state of DDR1. Besides proteases, DDR1 is also reported to form a complex with other proteins like the cell-adhesion molecule, E-cadherin in epithelial cells[74]. In addition, Wnt5a has been shown to enhance DDR1-collagen binding[75]. Further studies are required to understand if these proteins modulate the oligomeric state of DDR1 pre or post-ligand binding.
7. Role of lipid environment
Spatial distribution of the RTKs into specific lipid environments can have a multi-faceted impact on receptor clustering. Specific lipid components as well as microdomains in the plasma membrane such as caveolae, clathrin-coated structures, lipid rafts and invadosomes[76] have been shown to control receptor density and oligomeric state, modulate interaction of receptors with their ligand and with other biomolecules, clustering, endocytosis, receptor phosphorylation and signaling outcomes[77]. It should be noted however, that the role of lipid environment in regulating spatial distribution and clustering of receptors can be dependent on the cell-type, receptor and/or ligand density and the time course examined.
Lipids solvate the membrane proteins and in many cases regulate their activity through direct, specific contacts[78][79]. Only recently, however, has there been direct investigation that RTK structure and function is regulated by specific lipids. The anionic phospholipids, namely phosphatidylinositol-4,5-bisphosphate (PIP2) and phosphatidylserine (PS)[80] present in the inner leaflet of the cell membrane can directly interact with the IJXM regions of RTKs, leading to clustering of these lipids around the IJXM. Coarse-grained molecular dynamics simulations have revealed that the first few residues of the DDR1 and DDR2 IJXM show a high propensity to interact with PIP2 and to a lesser extent with PS[80].
Ubiquitously expressed small proteins like tetraspanins present in the cell membrane can also interact with RTKs, modulate clustering and/or recruit cytoplasmic signaling proteins. DDR1 is reported to associate with the tetraspanin TM4SF1, via its ECD, even before ligand binding[47] (Figure 3a). Silencing of TM4SF1 suppressed collagen-induced clustering of DDR1, indicating that TM4SF1 is required for this process. Interaction of DDR1 with TM4SF1 was also found to enhance the formation of invadopodia in pancreatic cancer cell lines[81]. Another tetraspanin, namely CD9 was found to endogenously associate with DDR1 in MDA-MB-231 cells. Stimulation of these cells with native type IV collagen induced a transient increase of CD9-cell surface levels[82] primarily in clathrin-coated pits. Thus DDR1 clustering appears to be mediated in part by multiple members of the tetraspanisn family. No such reports exist on interaction of DDR2 with tetraspanins.
Emerging evidence indicates that DDRs may also associate with various membrane microdomain modulating receptor endocytosis such as clathrin coated pits and flat-clathrin lattices[83]. We have demonstrated how use of a dynamin inhibitor could inhibit endocytosis of DDR1b clusters[41] suggesting a clathrin mediated pathway. Interaction of DDR1 with the tetrapanin CD9 is also dependent on clathrin[82]. Further studies are required to reveal if DDR1b and other isoforms associate with clathrin coated pits or flat-clathrin lattices. Sequence analysis shows that motifs which interact with another membrane microdomain, i.e. calveolin scaffolding domain[84] are also present in the DS-like domain of DDR1 (WpgydYvgW) and DDR2 (WpgydYvgW) and in the KD of DDR1 (WaFgvtlW) and DDR2 (WaFgvtlWetF). It has been shown for other RTKs like EphB1[85] and insulin receptors that the caveolin binding motif in the KD is important for their cell-surface expression and that the receptors localize with caveolin primarily after ligand stimulation. It remains to be examined if the oligomeric state of DDRs is dependent on caveolin and if so in which cell-type(s).
8. Comparison of DDR Oligomerization with other RTKs
DDRs exist as sub-populations of monomers and homodimers on the cell-surface, in a manner similar to that reported for other RTKs like fibroblast growth factor receptor (FGFR), Eph receptors and epidermal growth factor receptor (EGFR). Like DDRs, the fraction of these RTK dimers is dependent on parameters like cell type, receptor density, and receptor isoform. Members of the FGFR sub-family, form dimers in the absence of ligand, although the stability of these dimers varies quantitatively among its members[86]. Dimerization of FGFR is driven largely by the ECD as reported in early crystallographic studies. However, mutations in the TMD and JM domains also affect FGFR dimer stability in cell membranes. The Eph receptors (e.g. EphA1 and EphA2) also exist as dimers in the absence of ligand and their ECD is understood to drive dimerization and oligomerization[87][88]. EGFR (aka ErbB1 or Her1) is the most scrutinized RTK and has served as prototype for RTK structure and function studies. While EGFR activation was originally characterized as a ligand induced monomer to dimer transition[89], several biophysical studies have confirmed a non-negligible fraction of ligand independent dimers. Ligand-independent EGFR dimer formation is mediated by three non-covalent interfaces in the ECD, KD, and the TMD. Thus analogous to other RTKs, the DDR dimer formation is mediated primarily by its ECD with the likelihood that the TMD and JXM domains affect the dimer stability.
Similar to DDR dimers which show a high-affinity binding to collagen, there is evidence that ligand-free EGFR dimers are primed for ligand binding[90], and that differences in ligands can alter their conformation and stability[91]. Recently a quantitative FRET study combined with thermodynamic modelling concluded that VEGFR dimers bind ligand nearly fifty times more strongly than VEGFR monomers[92]. Hetero-dimerization also affects ligand binding in RTKs. For example, EGFR forms heterodimers within the ErbB sub-family and these heterodimeric interactions[93] play a variety of roles in receptor activation and ligand sensitivity. Depending on the heterodimer composition, ErbB receptors show differential sensitivity to ligand binding and downstream activation[94]. This paradigm needs to be explored for DDRs.
Ligand induced changes in the oligomeric status of DDRs also share similarities with that observed for other RTKs. DDR1b (originally called TrkE) exhibits many traits like TrkA namely ligand induced clustering, receptor endocytosis and recycling[95]. Similar to DDR1b clustering, liganded EGFR dimers can further assemble into multimeric clusters through recently reported interfaces [96][97][98] leading to assembly of their kinase domains in an asymmetric geometry that has quantitative, allosteric effect on trans-phosphorylation[99]. Ligand binding also drives Eph receptor clustering and increased tyrosine phosphorylation. Ephrin binds to the N-terminal ligand binding domain of Eph receptors and drives assembly of the ECDs into polymeric structures that are large enough to be visualized in optical micrographs[100][101][102]. These oligomers are formed through alternating dimer contacts in the ectodomain. Two structural motifs drive receptor clustering, a dimerization interface in the ligand binding domain and a clustering interface in the cysteine-rich or sushi domain membrane[103]. The macromolecular DDR assemblies, namely clusters and linear clusters thus share similarities with the RTK family.
Finally, as postulated for DDRs other RTKs have also been described to exhibit a canonical and a non-canonical signaling pathway. For instance the canonical VEGFR signaling is understood to occur via interaction of homodimeric VEGF ligands with homodimeric VEGFRs whereas non-canonical VEGFR signaling involves receptor activation through alternative ligands e.g. galectins[104]. In case of EGFR, the canonical pathway involves ligand-induced receptor clustering, endocytosis and kinase activation while the non-canonical pathway involves translocation of the receptor to the nucleus in response to certain DNA-damage pathways[105]. Another non-canonical pathway for several RTKs is proposed to be via nuclear translocation of proteolytic fragments released from the receptor intracellular domain[106]. Further studies are required to compare and contrast DDRs with other RTKs with respect to their oligomeric status and signaling pathways.
9. Comparison of DDR Oligomerization with other collagen receptors
The oligomeric status of DDRs appears to be different from that of collagen receptors. In contrast to fraction of DDRs existing as homodimers, a major fraction of the collagen binding integrins namely α1β1, α2β1, α10β1 and α11β1 exist as heterodimers on the cell surface. This hetero-dimerization of integrins occurs via the β-propeller surface of the α chain and the hybrid domain of the β chain in the cytoplasm[107]. Another collagen receptor GPVI exists as a hybrid dimer consisting of two GPVI units linked at the D2 domain[108]. The other three collagen receptors, namley LAIR-1, osteoclast associated receptor (OSCAR) and G-protein-coupled receptor 56 (GPR56, gene name ADGRG1) are understood to exist in a monomeric state [109].
Several parallels can be drawn between DDRs and members of the integrin family. Like DDRs, integrins recognize the supramolecular state of collagen. The α1β1 integrin effectively binds collagen I monomers while α2β1 integrin is a functional receptor for collagen fibrils[110]. Similar to enforced dimerization and oligomerization of DDR ECDs, clustering of integrin α2β1 by a phorbal ester increased the avidity of this integrin for collagen[111]. In response to collagen, integrin α2β1 was clustered to the focal contacts in fibroblasts adherent on monomeric collagen, but was arranged into long punctuate arrays in cells cultured on collagen fibrils[112]. Time-lapse imaging of endothelial cells has shown that β1 integrin clusters at focal contacts between 1 to 2 hours after plating the cells[113]. Integrin clustering is also driven by the interaction of vinculin with talin at the integrin intracellular domain, which in turn is promoted by contractile forces[114]. In contrast, none of the cytosolic proteins examined (vimentin, vinculin and actin) were found to associate with DDR assemblies when either soluble collagen[29] or collagen fibrils[51] were used as the ligand for DDRs.
The IgG-like collagen receptors consist of GPVI, LAIR-1 and OSCAR. Like DDRs, the glycoprotein VI (GPVI) dimers on resting platelets have a high affinity for collagen[115][108]. Upon first contact of the platelet with the immobilized collagenous substrate, GPVI dimers cluster via their D2 domain[116]. Over the course of ~3 minutes, small GPVI clusters form throughout the platelet surface while expanding and coalescing and plateau around the 3 minute mark at into clusters of size ~10,000–20,000 nm2[117]. The number of clusters and cluster density is dependent upon the nature of the collagenous substrate. Recombinant GPVI-Fc (Revacept) bound to collagen fibrils can also be clustered by the addition of anti-Fc antibody, indicating that the dimers bind in close proximity along the collagen fibril[118] reminiscent of the linear clusters formed by DDRs. The leukocyte-associated immunoglobulin-like receptor 1 (LAIR-1) is present on immune cells and consists of the Immune Receptor Tyrosine based Inhibition Motif (ITIM)[119] which is also present in DDRs. The ITIM signal can be induced in vitro via antibody-induced cross-linking/clustering of LAIR-1, but much less is known about the clustering and activation of this receptor. Collagen fibrils also have the potential to bind and cluster multiple copies of the osteoclast-associated IgG-like receptor (OSCAR) to transduce its activating ITAM signaling. Many binding sites for OSCAR on the collagen triple helix have been identified and the cooperative binding of several copies of ligand to several copies of OSCAR could lead to receptor clustering and activation[109]. Further studies are needed to understand the clustering and oligomerization of LAIR-1 and OSCAR in response to collagen.
10. Conclusions and Perspectives
Taken together, the biological roles of DDRs should be interpreted in the context of their cellular and extracellular milieu. The macro-molecular assemblies formed by DDRs are modulated by a multitude of factors including receptor isoforms, receptor domains, post-translational modifications, supramolecular state of the ligand and cell-membrane components. DDR1 and DDR2 exhibit key differences in the domains responsible for dimer formation pre-ligand binding. However, the dimeric state exhibits enhanced binding to collagen for both DDR1 and DDR2 as compared to monomers. The morphological state of the ligand (collagen) also plays a major role in inducing higher order oligomers of DDRs. DDR1 differs from DDR2 by forming globular-shaped clusters upon stimulation with monomeric collagen. However, both DDR1 and DDR2 exhibit a similar response to collagen fibrils by forming linear, filamentous-shaped clusters along the collagen fibril. These two different macro-molecular assemblies of DDRs formed upon ligand binding appear to be a prerequisite for receptor phosphorylation but differ in their downstream pathways. Recent years have witnessed a number of small molecule inhibitors targeting the DDR kinase domain to inhibit DDR signaling. Modulating the oligomeric state of the receptor by targeting its ECD could serve as another therapeutic avenue to inhibit DDR signaling. Further studies are needed to characterize these macro-molecular DDR assemblies and design avenues to attenuate their formation. Thus much work remains at the cell-matrix interface.
Highlights.
Oligomeric status of discoidin domain receptors (DDRs) dictates their ligand binding
Supramolecular state of ligand(collagen) modulates distinct macro-molecular assemblies
Macro-molecular DDR-collagen assemblies are locales for receptor phosphorylation
DDRs also interact with other receptors and cell-surface molecules
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Lemmon MA, Schlessinger J, Cell signaling by receptor tyrosine kinases., Cell 141 (2010) 1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Leitinger B, Hohenester E, Mammalian collagen receptors, Matrix Biol 26 (2007) 146–155. doi: 10.1016/j.matbio.2006.10.007. [DOI] [PubMed] [Google Scholar]
- [3].Endres NF, Barros T, Cantor AJ, Kuriyan J, Emerging concepts in the regulation of the EGF receptor and other receptor tyrosine kinases, Trends Biochem. Sci 39 (2014) 437–446. doi: 10.1016/j.tibs.2014.08.001. [DOI] [PubMed] [Google Scholar]
- [4].Hubbard SR, Juxtamembrane autoinhibition in receptor tyrosine kinases, Nat. Rev. Mol. Cell Biol 5 (2004) 464–470. doi: 10.1038/nrm1399. [DOI] [PubMed] [Google Scholar]
- [5].Paul MD, Hristova K, The RTK Interactome: Overview and Perspective on RTK Heterointeractions, Chem. Rev (2018). doi: 10.1021/acs.chemrev.8b00467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Johnson JD, Edman JC, Rutter WJ, A receptor tyrosine kinase found in breast carcinoma cells has an extracellular discoidin I-like domain., Proc. Natl. Acad. Sci. U. S. A 90 (1993) 5677–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Fukuzawa M, Ochiai H, Different subcellular localizations of discoidin I monomer and tetramer in Dictyostelium discoideum cells: Using conformation-specific monoclonal antibodies, Exp. Cell Res 204 (1993) 61–72. doi: 10.1006/excr.1993.1009. [DOI] [PubMed] [Google Scholar]
- [8].Mathieu SV, Aragão KS, Imberty A, Varrot A, Discoidin I from Dictyostelium discoideum and Interactions with Oligosaccharides: Specificity, Affinity, Crystal Structures, and Comparison with Discoidin II, J. Mol. Biol 400 (2010) 540–554. doi: 10.1016/j.jmb.2010.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shrivastava A, Radziejewski C, Campbell E, Kovac L, McGlynn M, Ryan TE, Davis S, Goldfarb MP, Glass DJ, Lemke G, Yancopoulos GD, An Orphan Receptor Tyrosine Kinase Family Whose Members Serve as Nonintegrin Collagen Receptors, Mol. Cell 1 (1997) 25–34. doi: 10.1016/S1097-2765(00)80004-0. [DOI] [PubMed] [Google Scholar]
- [10].Alves F, Saupe S, Ledwon M, Schaub F, Hiddemann W, Vogel WF, Identification of two novel, kinase-deficient variants of discoidin domain receptor 1: differential expression in human colon cancer cell lines., FASEB J 15 (2001) 1321–3. http://www.ncbi.nlm.nih.gov/pubmed/11344127 (accessed November 2, 2015). [DOI] [PubMed] [Google Scholar]
- [11].Fu H-L, Sohail A, Valiathan RR, Wasinski BD, Kumarasiri M, V Mahasenan K, Bernardo MM, Tokmina-Roszyk D, Fields GB, Mobashery S, Fridman R, Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases., J. Biol. Chem 288 (2013) 12114–29. doi: 10.1074/jbc.M112.409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Curat CA, Eck M, Dervillez X, Vogel WF, Mapping of epitopes in discoidin domain receptor 1 critical for collagen binding., J. Biol. Chem 276 (2001) 45952–8. doi: 10.1074/jbc.M104360200. [DOI] [PubMed] [Google Scholar]
- [13].Noordeen NA, Carafoli F, Hohenester E, Horton MA, Leitinger B, A transmembrane leucine zipper is required for activation of the dimeric receptor tyrosine kinase DDR1., J. Biol. Chem 281 (2006) 22744–51. doi: 10.1074/jbc.M603233200. [DOI] [PubMed] [Google Scholar]
- [14].Mihai C, Chotani M, Elton TS, Agarwal G, Mapping of DDR1 distribution and oligomerization on the cell surface by FRET microscopy., J. Mol. Biol 385 (2009) 432–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fu H-L, Valiathan RR, Payne L, Kumarasiri M, V Mahasenan K, Mobashery S, Huang P, Fridman R, Glycosylation at Asn211 regulates the activation state of the discoidin domain receptor 1 (DDR1)., J. Biol. Chem 289 (2014) 9275–87. doi: 10.1074/jbc.M113.541102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Abdulhussein R, Koo DHH, Voge WF, Identification of disulfide-linked dimers of the receptor tyrosine kinase DDR1, J. Biol. Chem 283 (2008) 12026–12033. doi: 10.1074/jbc.M704592200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xu H, Abe T, Liu JKH, Zalivina I, Hohenester E, Leitinger B, Normal activation of discoidin domain receptor 1 mutants with disulfide cross-links, insertions, or deletions in the extracellular juxtamembrane region: mechanistic implications., J. Biol. Chem 289 (2014) 13565–74. doi: 10.1074/jbc.M113.536144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Juskaite V, Corcoran DS, Leitinger B, Collagen induces activation of DDR1 through lateral dimer association and phosphorylation between dimers, Elife 6 (2017). doi: 10.7554/eLife.25716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Finger C, Escher C, Schneider D, The single transmembrane domains of human receptor tyrosine kinases encode self-interactions, Sci. Signal 2 (2009) ra56. doi: 10.1126/scisignal.2000547. [DOI] [PubMed] [Google Scholar]
- [20].Russ WP, Engelman DM, TOXCAT: A measure of transmembrane helix association in a biological membrane, Proc. Natl. Acad. Sci 96 (1999) 863–868. doi: 10.1073/pnas.96.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Carafoli F, Mayer MC, Shiraishi K, Pecheva MA, Chan LY, Nan R, Leitinger B, Hohenester E, Structure of the discoidin domain receptor 1 extracellular region bound to an inhibitory Fab fragment reveals features important for signaling, Structure 20 (2012) 688–697. doi: 10.1016/j.str.2012.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu WWH, Wong JP, Kast J, Molday RS, RS1 a discoidin domain-containing retinal cell adhesion protein associated with X-linked retinoschisis, exists as a novel disulfide-linked octamer, J. Biol. Chem 280 (2005) 10721–10730. doi: 10.1074/jbc.M413117200. [DOI] [PubMed] [Google Scholar]
- [23].Phan TN, Wong EL, Park SY, Kim HJ, Yang BS, Defective Ca2+ binding in a conserved binding site causes incomplete N-glycan processing and endoplasmic reticulum trapping of discoidin domain receptors, Biosci. Biotechnol. Biochem 79 (2015) 574–580. doi: 10.1080/09168451.2014.987208. [DOI] [PubMed] [Google Scholar]
- [24].Alves F, Vogel W, Mossie K, Millauer B, Höfler H, Ullrich A, Distinct structural characteristics of discoidin I subfamily receptor tyrosine kinases and complementary expression in human cancer., Oncogene 10 (1995) 609–18. [PubMed] [Google Scholar]
- [25].Leitinger B, Molecular analysis of collagen binding by the human discoidin domain receptors, DDR1 and DDR2. Identification of collagen binding sites in DDR2., J. Biol. Chem 278 (2003) 16761–9. doi: 10.1074/jbc.M301370200. [DOI] [PubMed] [Google Scholar]
- [26].Flynn LA, Blissett AR, Calomeni EP, Agarwal G, Inhibition of Collagen Fibrillogenesis by Cells Expressing Soluble Extracellular Domains of DDR1 and DDR2, J. Mol. Biol 395 (2010). doi: 10.1016/j.jmb.2009.10.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Grither WR, Longmore GD, Inhibition of tumor–microenvironment interaction and tumor invasion by small-molecule allosteric inhibitor of DDR2 extracellular domain, Proc. Natl. Acad. Sci 115 (2018) E7786–E7794. doi: 10.1073/pnas.1805020115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Kim D, Ko P, You E, Rhee S, The intracellular juxtamembrane domain of discoidin domain receptor 2 (DDR2) is essential for receptor activation and DDR2-mediated cancer progression, Int. J. Cancer 135 (2014) 2547–2557. doi: 10.1002/ijc.28901. [DOI] [PubMed] [Google Scholar]
- [29].Yeung D, Shankar N, Sohail A, Weiss B, Wang C, Herr AB, Fridman R, Agarwal G, Clustering, Spatial Distribution, and Phosphorylation of Discoidin Domain Receptors 1 and 2 in Response to Soluble Collagen I, J. Mol. Biol 431 (2019) 368–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Agarwal G, Kovac L, Radziejewski C, Samuelsson SJ, Binding of discoidin domain receptor 2 to collagen I: an atomic force microscopy investigation., Biochemistry 41 (2002) 11091–11098. doi: 10.1021/bi020087w. [DOI] [PubMed] [Google Scholar]
- [31].Agarwal G, Mihai C, Iscru DF, Interaction of discoidin domain receptor 1 with collagen type 1., J. Mol. Biol 367 (2007) 443–455. doi: 10.1016/j.jmb.2006.12.073. [DOI] [PubMed] [Google Scholar]
- [32].Perez JL, Jing SQ, Wong TW, Identification of two isoforms of the Cak receptor kinase that are coexpressed in breast tumor cell lines., Oncogene 12 (1996) 1469–77. [PubMed] [Google Scholar]
- [33].Vogel W, Brakebusch C, Fässler R, Alves F, Ruggiero F, Pawson T, Discoidin domain receptor 1 is activated independently of β1 integrin, J. Biol. Chem 275 (2000) 5779–5784. doi: 10.1074/jbc.275.8.5779. [DOI] [PubMed] [Google Scholar]
- [34].Olaso E, Ikeda K, Eng FJ, Xu L, Wang LH, Lin HC, Friedman SL, DDR2 receptor promotes MMP-2-mediated proliferation and invasion by hepatic stellate cells, J. Clin. Invest 108 (2001) 1369–1378. doi: 10.1172/JCI200112373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Croissant C, Tuariihionoa A, Bacou M, Souleyreau W, Sala M, Henriet E, Bikfalvi A, Saltel F, Auguste P, DDR1 and DDR2 physical interaction leads to signaling interconnection but with possible distinct functions, Cell Adhes. Migr 12 (2018) 324–334. doi: 10.1080/19336918.2018.1460012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ricard-Blum S, The collagen family., Cold Spring Harb. Perspect. Biol 3 (2011) a004978. doi: 10.1101/cshperspect.a004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Fu H-L, Valiathan RR, Arkwright R, Sohail A, Mihai C, Kumarasiri M, Mahasenan KV, Mobashery S, Huang P, Agarwal G, Fridman R, Discoidin domain receptors: Unique receptor tyrosine kinases in collagen-mediated signaling, J. Biol. Chem 288 (2013). doi: 10.1074/jbc.R112.444158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Abdulhussein R, McFadden C, Fuentes-Prior P, Vogel WF, Exploring the collagen-binding site of the DDR1 tyrosine kinase receptor, J. Biol. Chem 279 (2004) 31462–31470. doi: 10.1074/jbc.M400651200. [DOI] [PubMed] [Google Scholar]
- [39].Ichikawa O, Osawa M, Nishida N, Goshima N, Nomura N, Shimada I, Structural basis of the collagen-binding mode of discoidin domain receptor 2, EMBO J 26 (2007) 4168–4176. doi: 10.1038/sj.emboj.7601833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Carafoli F, Bihan D, Stathopoulos S, Konitsiotis AD, Kvansakul M, Farndale RW, Leitinger B, Hohenester E, Crystallographic insight into collagen recognition by discoidin domain receptor 2., Structure 17 (2009) 1573–81. doi: 10.1016/j.str.2009.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Yeung D, Chmielewski D, Mihai C, Agarwal G, Oligomerization of DDR1 ECD affects receptor-ligand binding., J. Struct. Biol 183 (2013) 495–500. doi: 10.1016/j.jsb.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G, Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1., J. Mol. Biol 361 (2006) 864–76. doi: 10.1016/j.jmb.2006.06.067. [DOI] [PubMed] [Google Scholar]
- [43].Konitsiotis AD, Raynal N, Bihan D, Hohenester E, Farndale RW, Leitinger B, Characterization of high affinity binding motifs for the discoidin domain receptor DDR2 in collagen., J. Biol. Chem 283 (2008) 6861–8. doi: 10.1074/jbc.M709290200. [DOI] [PubMed] [Google Scholar]
- [44].Leitinger B, Steplewski A, Fertala A, The D2 period of collagen II contains a specific binding site for the human discoidin domain receptor, DDR2, J. Mol. Biol 344 (2004) 993–1003. doi: 10.1016/j.jmb.2004.09.089. [DOI] [PubMed] [Google Scholar]
- [45].Xu H, Raynal N, Stathopoulos S, Myllyharju J, Farndale RW, Leitinger B, Collagen binding specificity of the discoidin domain receptors: binding sites on collagens II and III and molecular determinants for collagen IV recognition by DDR1., Matrix Biol 30 (2011) 16–26. doi: 10.1016/j.matbio.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Bhadriraju K, Chung K-H, Spurlin TA, Haynes RJ, Elliott JT, Plant AL, The relative roles of collagen adhesive receptor DDR2 activation and matrix stiffness on the downregulation of focal adhesion kinase in vascular smooth muscle cells., Biomaterials 30 (2009) 6687–94. doi: 10.1016/j.biomaterials.2009.08.036. [DOI] [PubMed] [Google Scholar]
- [47].Gao H, Chakraborty G, Zhang Z, Akalay I, Gadiya M, Gao Y, Sinha S, Hu J, Jiang C, Akram M, Brogi E, Leitinger B, Giancotti FG, Multi-organ Site Metastatic Reactivation Mediated by Non-canonical Discoidin Domain Receptor 1 Signaling, Cell 166 (2016) 47–62. doi: 10.1016/j.cell.2016.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Luczynski MT, Harrison PT, Lima N, Krasny L, Paul A, Huang PH, Spatial localisation of Discoidin Domain Receptor 2 (DDR2) signalling is dependent on its collagen binding and kinase activity, Biochem. Biophys. Res. Commun 501 (2018) 124–130. doi: 10.1016/j.bbrc.2018.04.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Blissett AR, Garbellini D, Calomeni EP, Mihai C, Elton TS, Agarwal G, Regulation of Collagen Fibrillogenesis by Cell-surface Expression of Kinase Dead DDR2, J. Mol. Biol 130 (2009). doi: 10.1016/j.jmb.2008.10.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Agarwal G, DDRs and Collagen Fibrillogenesis, in: Discoidin Domain Recept. Heal. Dis, 2016: pp. 23–56.
- [51].Coelho N, Arora P, van Putten S, Boo S, Petrovic S, Lin A, Hinz B, McCulloch C, Discoidin Domain Receptor 1 Mediates Myosin-Dependent Collagen Contraction, Cell Rep 18 (2017) 1774–1790. [DOI] [PubMed] [Google Scholar]
- [52].Foehr ED, Tatavos A, Tanabe E, Raffioni S, Goetz S, Dimarco E, De Luca M, Bradshaw RA, Discoidin domain receptor 1 (DDR1) signaling in PC12 cells: activation of juxtamembrane domains in PDGFR/DDR/TrkA chimeric receptors., FASEB J 14 (2000) 973–81. http://www.ncbi.nlm.nih.gov/pubmed/10783152. [DOI] [PubMed] [Google Scholar]
- [53].L’hôte CGM, Thomas PH, Ganesan TS, Functional analysis of discoidin domain receptor 1: effect of adhesion on DDR1 phosphorylation., FASEB J 16 (2002) 234–236. [DOI] [PubMed] [Google Scholar]
- [54].Ram R, Lorente G, Nikolich K, Urfer R, Foehr E, Nagavarapu U, Discoidin domain receptor-1a (DDR1a) promotes glioma cell invasion and adhesion in association with matrix metalloproteinase-2, J. Neurooncol 76 (2006) 239–248. doi: 10.1007/s11060-005-6874-1. [DOI] [PubMed] [Google Scholar]
- [55].Huang H, Svoboda RA, Lazenby AJ, Saowapa J, Chaika N, Ding K, Wheelock MJ, Johnson KR, Up-Regulation of N-Cadherin by Collagen I-activated discoidin domain receptor 1 in pancreatic cancer requires the adaptor molecule Shc, J. Biol. Chem 291 (2016) 23208–23223. doi: 10.1074/jbc.M116.740605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Borza CM, Pozzi A, Discoidin domain receptors in disease, Matrix Biol 34 (2014) 185–192. doi: 10.1016/j.matbio.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Perez JL, Shen X, Finkernagel S, Sciorra L, Jenkins NA, Gilbert DJ, Copeland NG, Wong TW, Identification and chromosomal mapping of a receptor tyrosine kinase with a putative phospholipid binding sequence in its ectodomain., Oncogene 9 (1994) 211–9. http://www.ncbi.nlm.nih.gov/pubmed/8302582. [PubMed] [Google Scholar]
- [58].Wall SJ, Werner E, Werb Z, DeClerck YA, Discoidin domain receptor 2 mediates tumor cell cycle arrest induced by fibrillar collagen., J. Biol. Chem 280 (2005) 40187–94. doi: 10.1074/jbc.M508226200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Yang K, Kim JH, Kim HJ, Park IS, Kim IY, Yang BS, Tyrosine 740 phosphorylation of discoidin domain receptor 2 by Src stimulates intramolecular autophosphorylation and Shc signaling complex formation, J. Biol. Chem 280 (2005) 39058–39066. doi: 10.1074/jbc.M506921200. [DOI] [PubMed] [Google Scholar]
- [60].Xu L, Jensen H, Johnston JJ, Di Maria E, Kloth K, Cristea I, Sapp JC, Darling TN, Huryn LA, Tranebjærg L, Cinotti E, Kubisch C, Rødahl E, Bruland O, Biesecker LG, Houge G, Bredrup C, Recurrent, Activating Variants in the Receptor Tyrosine Kinase DDR2 Cause Warburg-Cinotti Syndrome, Am. J. Hum. Genet 103 (2018) 976–983. doi: 10.1016/j.ajhg.2018.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Farndale RW, Lisman T, Bihan D, Hamaia S, Smerling CS, Pugh N, Konitsiotis A, Leitinger B, de Groot PG, Jarvis GE, Raynal N, Cell-collagen interactions: the use of peptide Toolkits to investigate collagen-receptor interactions., Biochem. Soc. Trans 36 (2008) 241–50. doi: 10.1042/BST0360241. [DOI] [PubMed] [Google Scholar]
- [62].Orgel JPRO, Madhurapantula RS, A structural prospective for collagen receptors such as DDR and their binding of the collagen fibril, BBA-Mol Cell Res (2019). [DOI] [PubMed]
- [63].Hogrebe NJ, Reinhardt JW, Tram NK, Debski AC, Agarwal G, Reilly MA, Gooch KJ, Independent control of matrix adhesiveness and stiffness within a 3D self-assembling peptide hydrogel, Acta Biomater 70 (2018) 110–119. doi: 10.1016/j.actbio.2018.01.031. [DOI] [PubMed] [Google Scholar]
- [64].An B, Abbonante V, Xu H, Gavriilidou D, Yoshizumi A, Bihan D, Farndale RW, Kaplan DL, Balduini A, Leitinger B, Brodsky B, Recombinant collagen engineered to bind to discoidin domain receptor functions as a receptor inhibitor, J. Biol. Chem 291 (2016) 4343–4355. doi: 10.1074/jbc.M115.674507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Belfiore A, Malaguarnera R, Nicolosi ML, Lappano R, Ragusa M, Morrione A, Vella V, A novel functional crosstalk between DDR1 and the IGF axis and its relevance for breast cancer, Cell Adhes. Migr 12 (2018) 305–314. doi: 10.1080/19336918.2018.1445953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Vella V, Malaguarnera R, Nicolosi ML, Palladino C, Spoleti C, Massimino M, Vigneri P, Purrello M, Ragusa M, Morrione A, Belfiore A, Discoidin domain receptor 1 modulates insulin receptor signaling and biological responses in breast cancer cells, Oncotarget 8 (2017). doi: 10.18632/oncotarget.18020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Malaguarnera R, Nicolosi ML, Sacco A, Morcavallo A, Vella V, Voci C, Spatuzza M, Xu S-Q, Iozzo RV, Vigneri R, Morrione A, Belfiore A, Novel cross talk between IGF-IR and DDR1 regulates IGF-IR trafficking, signaling and biological responses, Oncotarget 6 (2015). doi: 10.18632/oncotarget.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Iwai LK, Chang F, Huang PH, Phosphoproteomic analysis identifies insulin enhancement of discoidin domain receptor 2 phosphorylation, Cell Adhes. Migr 7 (2013) 161–164. doi: 10.4161/cam.22572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Kim D, You E, Min NY, Lee KH, Kim HK, Rhee S, Discoidin domain receptor 2 regulates the adhesion of fibroblasts to 3D collagen matrices, Int. J. Mol. Med 31 (2013) 1113–1118. doi: 10.3892/ijmm.2013.1320. [DOI] [PubMed] [Google Scholar]
- [70].Kim HG, Hwang SY, Aaronson SA, Mandinova A, Lee SW, DDR1 receptor tyrosine kinase promotes prosurvival pathway through Notch1 activation, J. Biol. Chem 286 (2011) 17672–17681. doi: 10.1074/jbc.M111.236612. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [71].Shitomi Y, Thogersen IB, Ito N, Leitinger B, Enghild JJ, Itoh Y, ADAM10 controls collagen signaling and cell migration on collagen by shedding the ectodomain of discoidin domain receptor 1 (DDR1), Mol. Biol. Cell 26 (2014) 659–673. doi: 10.1091/mbc.E14-10-1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Johnson N, Březinová J, Stephens E, Burbridge E, Freeman M, Adrain C, Strisovsky K, Quantitative proteomics screen identifies a substrate repertoire of rhomboid protease RHBDL2 in human cells and implicates it in epithelial homeostasis, Sci. Rep 7 (2017). doi: 10.1038/s41598-017-07556-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vogel WF, Ligand-induced shedding of discoidin domain receptor 1., FEBS Lett 514 (2002) 175–80. http://www.ncbi.nlm.nih.gov/pubmed/11943146 (accessed June 1, 2014). [DOI] [PubMed] [Google Scholar]
- [74].Wang C-Z, Yeh Y-C, Tang M-J, DDR1/E-cadherin complex regulates the activation of DDR1 and cell spreading, Am. J. Physiol. Physiol 297 (2009) C419–C429. doi: 10.1152/ajpcell.00101.2009. [DOI] [PubMed] [Google Scholar]
- [75].Dejmek J, Dib K, Jönsson M, Andersson T, WNT-5A and G-protein signaling are required for collagen-induced DDR1 receptor activation and normal mammary cell adhesion, Int. J. Cancer 103 (2003) 344–351. doi: 10.1002/ijc.10752. [DOI] [PubMed] [Google Scholar]
- [76].Delos Santos RC, Garay C, Antonescu CN, Charming neighborhoods on the cell surface: Plasma membrane microdomains regulate receptor tyrosine kinase signaling, Cell. Signal 27 (2015) 1963–1976. doi: 10.1016/j.cellsig.2015.07.004. [DOI] [PubMed] [Google Scholar]
- [77].Pike LJ, Growth factor receptors, lipid rafts and caveolae: An evolving story, Biochim. Biophys. Acta - Mol. Cell Res 1746 (2005) 260–273. doi: 10.1016/j.bbamcr.2005.05.005. [DOI] [PubMed] [Google Scholar]
- [78].Lee AG, Lipid-protein interactions in biological membranes: A structural perspective, Biochim. Biophys. Acta - Biomembr 1612 (2003) 1–40. doi: 10.1016/S0005-2736(03)00056-7. [DOI] [PubMed] [Google Scholar]
- [79].Smith AW, Lipid-protein interactions in biological membranes: A dynamic perspective, Biochim. Biophys. Acta - Biomembr 1818 (2012) 172–177. doi: 10.1016/j.bbamem.2011.06.015. [DOI] [PubMed] [Google Scholar]
- [80].Hedger G, Sansom MSP, Koldsø H, The juxtamembrane regions of human receptor tyrosine kinases exhibit conserved interaction sites with anionic lipids, Sci. Rep 5 (2015). doi: 10.1038/srep09198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Yang JC, Zhang Y, He SJ, Li MM, Cai XL, Wang H, Xu LM, Cao J, TM4SF1 Promotes Metastasis of Pancreatic Cancer via Regulating the Expression of DDR1, Sci. Rep 7 (2017). doi: 10.1038/srep45895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Castro-Sanchez L, Soto-Guzman A, Navarro-Tito N, Martinez-Orozco R, Salazar EP, Native type IV collagen induces cell migration through a CD9 and DDR1-dependent pathway in MDA-MB-231 breast cancer cells, Eur. J. Cell Biol 89 (2010) 843–852. doi: 10.1016/j.ejcb.2010.07.004. [DOI] [PubMed] [Google Scholar]
- [83].Grove J, Metcalf DJ, Knight AE, Wavre-Shapton ST, Sun T, Protonotarios ED, Griffin LD, Lippincott-Schwartz J, Marsh M, Flat clathrin lattices: stable features of the plasma membrane, Mol. Biol. Cell 25 (2014) 3581–3594. doi: 10.1091/mbc.E14-06-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Couet J, Li S, Okamoto T, Ikezu T, Lisanti MP, Identification of peptide and protein ligands for the caveolin- scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins, J. Biol. Chem 272 (1997) 6525–6533. doi: 10.1074/jbc.272.10.6525. [DOI] [PubMed] [Google Scholar]
- [85].Vihanto MM, Caveolin-1 is required for signaling and membrane targeting of EphB1 receptor tyrosine kinase, J. Cell Sci 119 (2006) 2299–2309. doi: 10.1242/jcs.02946. [DOI] [PubMed] [Google Scholar]
- [86].Sarabipour S, Hristova K, Mechanism of FGF receptor dimerization and activation, Nat. Commun 7 (2016). doi: 10.1038/ncomms10262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY, An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly, Nat. Struct. Mol. Biol 17 (2010) 398–402. doi: 10.1038/nsmb.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Nikolov DB, Himanen JP, Xu K, Mensinga A, Lackmann M, Atapattu L, Walker JR, Dhe-Paganon S, Yermekbayeva L, Rajashankar KR, Janes PW, Architecture of Eph receptor clusters, Proc. Natl. Acad. Sci 107 (2010) 10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Yarden Y, Schlessinger J, Self-Phosphorylation of Epidermal Growth Factor Receptor: Evidence for a Model of Intermolecular Allosteric Activation, Biochemistry 26 (1987) 1434–1442. doi: 10.1021/bi00379a034. [DOI] [PubMed] [Google Scholar]
- [90].Chung I, Akita R, Vandlen R, Toomre D, Schlessinger J, Mellman I, Spatial control of EGF receptor activation by reversible dimerization on living cells, Nature 464 (2010) 783–787. doi: 10.1038/nature08827. [DOI] [PubMed] [Google Scholar]
- [91].Sinclair JKL, Walker AS, Doerner AE, Schepartz A, Mechanism of Allosteric Coupling into and through the Plasma Membrane by EGFR, Cell Chem. Biol 25 (2018) 857–870.e7. doi: 10.1016/j.chembiol.2018.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].King C, Hristova K, Direct measurements of VEGF:VEGFR2 binding affinities reveal the coupling between ligand binding and receptor dimerization, J. Biol. Chem (2019) jbc.RA119.007737. doi: 10.1074/jbc.ra119.007737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yarden Y, Sliwkowski MX, Untangling the ErbB signalling network, Nat. Rev. Mol. Cell Biol 2 (2001) 127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- [94].Jones RB, Gordus A, Krall JA, MacBeath G, A quantitative protein interaction network for the ErbB receptors using protein microarrays, Nature 439 (2006) 168–174. doi: 10.1038/nature04177. [DOI] [PubMed] [Google Scholar]
- [95].Chen Z-Y, A Novel Endocytic Recycling Signal Distinguishes Biological Responses of Trk Neurotrophin Receptors, Mol. Biol. Cell 16 (2005) 5761–5772. doi: 10.1091/mbc.e05-07-0651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y, Pelton JG, Shan Y, Shaw DE, Wemmer DE, Groves JT, Kuriyan J, Conformational coupling across the plasma membrane in activation of the EGF receptor, Cell 152 (2013) 543–556. doi: 10.1016/j.cell.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Needham SR, Roberts SK, Arkhipov A, Mysore VP, Tynan CJ, Zanetti-Domingues LC, Kim ET, Losasso V, Korovesis D, Hirsch M, Rolfe DJ, Clarke DT, Winn MD, Lajevardipour A, Clayton AHA, Pike LJ, Perani M, Parker PJ, Shan Y, Shaw DE, Martin-Fernandez ML, EGFR oligomerization organizes kinase-active dimers into competent signalling platforms, Nat. Commun 7 (2016). doi: 10.1038/ncomms13307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Clayton AHA, Walker F, Orchard SG, Henderson C, Fuchs D, Rothacker J, Nice EC, Burgess AW, Ligand-induced dimer-tetramer transition during the activation of the cell surface epidermal growth factor receptor-A multidimensional microscopy analysis, J. Biol. Chem 280 (2005) 30392–30399. doi: 10.1074/jbc.M504770200. [DOI] [PubMed] [Google Scholar]
- [99].Zhang X, Gureasko J, Shen K, Cole PA, Kuriyan J, An Allosteric Mechanism for Activation of the Kinase Domain of Epidermal Growth Factor Receptor, Cell 125 (2006) 1137–1149. doi: 10.1016/j.cell.2006.05.013. [DOI] [PubMed] [Google Scholar]
- [100].Mieruszynski S, Tarantino C, Chiu CL, Ojosnegros S, Hortigüela V, Raya A, Lakadamyali M, Cutrale F, Fraser SE, Seriola A, Otterstrom JJ, Martínez E, Rodríguez D, Eph-ephrin signaling modulated by polymerization and condensation of receptors, Proc. Natl. Acad. Sci 114 (2017) 13188–13193. doi: 10.1073/pnas.1713564114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Salaita K, Nair PM, Petit RS, Neve RM, Das D, Gray JW, Groves JT, Restriction of receptor movement alters cellular response: Physical force sensing by EphA2, Science (80-. ) 327 (2010) 1380–1385. doi: 10.1126/science.1181729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Xu Q, Lin WC, Petit RS, Groves JT, EphA2 receptor activation by monomeric ephrin-A1 on supported membranes, Biophys. J 101 (2011) 2731–2739. doi: 10.1016/j.bpj.2011.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Nikolov DB, Xu K, Himanen JP, Eph/ephrin recognition and the role of Eph/ephrin clusters in signaling initiation, Biochim. Biophys. Acta - Proteins Proteomics 1834 (2013) 2160–2165. doi: 10.1016/j.bbapap.2013.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Domigan CK, Ziyad S, Iruela-Arispe ML, Canonical and Noncanonical Vascular Endothelial Growth Factor Pathways, Arterioscler. Thromb. Vasc. Biol 35 (2015) 30–39. doi: 10.1161/ATVBAHA.114.303215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Lee HH, Wang YN, Hung MC, Non-canonical signaling mode of the epidermal growth factor receptor family, Am. J. Cancer Res 5 (2015) 2944–2958. [PMC free article] [PubMed] [Google Scholar]
- [106].Carpenter G, Pozzi A, Cell responses to growth factors: The role of receptor tyrosine kinase intracellular domain fragments, Sci. Signal 5 (2012). doi: 10.1126/scisignal.2003526. [DOI] [PubMed] [Google Scholar]
- [107].Humphries MJ, Integrin Structure, Biochem. Soc. Trans 28 (2015) 311–340. doi: 10.1042/bst0280311. [DOI] [PubMed] [Google Scholar]
- [108].Jung SM, Moroi M, Soejima K, Nakagaki T, Miura Y, Berndt MC, Gardiner EE, Howes JM, Pugh N, Bihan D, Watson SP, Farndale RW, Constitutive dimerization of glycoprotein VI (GPVI) in resting platelets is essential for binding to collagen and activation in flowing blood, J. Biol. Chem 287 (2012) 30000–30013. doi: 10.1074/jbc.M112.359125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Barrow AD, Raynal N, Andersen TL, Slatter DA, Bihan D, Pugh N, Cella M, Kim T, Rho J, Negishi-Koga T, Delaisse JM, Takayanagi H, Lorenzo J, Colonna M, Farndale RW, Choi Y, Trowsdale J, OSCAR is a collagen receptor that costimulates osteoclastogenesis in DAP12-deficient humans and mice, J. Clin. Invest 121 (2011) 3505–3516. doi: 10.1172/JCI45913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jokinen J, Dadu E, Nykvist P, Käpylä J, White DJ, Ivaska J, Vehviläinen P, Reunanen H, Larjava H, Häkkinen L, Heino J, Integrin-mediated cell adhesion to type I collagen fibrils, J. Biol. Chem 279 (2004) 31956–31963. doi: 10.1074/jbc.M401409200. [DOI] [PubMed] [Google Scholar]
- [111].Connors WL, Jokinen J, White DJ, Puranen JS, Kankaanpää P, Upla P, Tulla M, Johnson MS, Heino J, Two synergistic activation mechanisms of α2β1 integrin-mediated collagen binding, J. Biol. Chem 282 (2007) 14675–14683. doi: 10.1074/jbc.M700759200. [DOI] [PubMed] [Google Scholar]
- [112].Mercier I, Lechaire JP, Desmouliere A, Gaill F, Aumailley M, Interactions of human skin fibroblasts with monomeric or fibrillar collagens induce different organization of the cytoskeleton, Exp. Cell Res 225 (1996) 245–256. doi: 10.1006/excr.1996.0174. [DOI] [PubMed] [Google Scholar]
- [113].Kawakami K, Tatsumi H, Sokabe M, Dynamics of integrin clustering at focal contacts of endothelial cells studied by multimode imaging microscopy., J. Cell Sci 114 (2001) 3125–35. http://www.ncbi.nlm.nih.gov/pubmed/11590239. [DOI] [PubMed] [Google Scholar]
- [114].Humphries JD, Wang P, Streuli C, Geiger B, Humphries MJ, Ballestrem C, Vinculin controls focal adhesion formation by direct interactions with talin and actin, J. Cell Biol 179 (2007) 1043–1057. doi: 10.1083/jcb.200703036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Jung SM, Tsuji K, Moroi M, Glycoprotein (GP) VI dimer as a major collagen-binding site of native platelets: Direct evidence obtained with dimeric GPVI-specific Fabs, J. Thromb. Haemost 7 (2009) 1347–1355. doi: 10.1111/j.1538-7836.2009.03496.x. [DOI] [PubMed] [Google Scholar]
- [116].O’Connor MN, Smethurst PA, Davies LW, Joutsi-Korhonen L, Onley DJ, Herr AB, Farndale RW, Ouwehand WH, Selective blockade of glycoprotein VI clustering on collagen helices, J. Biol. Chem 281 (2006) 33505–33510. doi: 10.1074/jbc.M606480200. [DOI] [PubMed] [Google Scholar]
- [117].Poulter NS, Pollitt AY, Owen DM, Gardiner EE, Andrews RK, Shimizu H, Ishikawa D, Bihan D, Farndale RW, Moroi M, Watson SP, Jung SM, Clustering of glycoprotein VI (GPVI) dimers upon adhesion to collagen as a mechanism to regulate GPVI signaling in platelets, J. Thromb. Haemost 15 (2017) 549–564. doi: 10.1111/jth.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Jamasbi J, Megens RTA, Bianchini M, Uhland K, Münch G, Ungerer M, Sherman S, Faussner A, Brandl R, John C, Buchner J, Weber C, Lorenz R, Elia N, Siess W, Cross-Linking GPVI-Fc by Anti-Fc Antibodies Potentiates Its Inhibition of Atherosclerotic Plaque- and Collagen-Induced Platelet Activation, JACC Basic to Transl. Sci 1 (2016) 131–142. doi: 10.1016/j.jacbts.2016.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Meyaard L, LAIR and collagens in immune regulation, Immunol. Lett 128 (2010) 26–28. doi: 10.1016/j.imlet.2009.09.014. [DOI] [PubMed] [Google Scholar]


