Abstract
Germline mutations in BRCA1/2 are risk factors for pancreatic ductal adenocarcinoma (PDAC). The aim of this study was to evaluate whether results of surveillance for PDAC in high-risk individuals (HRI) differ between those with and without a pathogenic BRCA1/2 mutation. This prospective study was conducted within the Pancreatic Tumor Registry at a major cancer center. There were 83 HRI with ≥1 first degree relative with PDAC who underwent surveillance and testing for pathogenic germline mutations in BRCA1/2. A secondary analysis includes 18 HRI with known mutations in BRCA1/2 but with weaker family history. HRI were evaluated over time using magnetic resonance cholangiopancreatography (MRCP) and endoscopic ultrasound (EUS) when indicated by MRCP findings. We reviewed imaging results, blinded to mutation status. Demographic information was obtained from interviewer-administered questionnaires. The outcome was the proportion with any pancreatic abnormality identified at initial or follow-up surveillance. Among the 83 HRI in the main analysis, 48 had a mutation in BRCA1/2 and 35 did not. Overall, 16/48 (33%) BRCA1/2 positive and 13/35 (37%) BRCA1/2 negative participants had pancreatic abnormalities on imaging; in each group, all but one finding was an intraductal papillary mucinous neoplasm. Among those with pathogenic mutations but weaker family history, results were similar: 7/18 (39%) with pancreatic abnormalities. Results of surveillance for pancreatic abnormalities on imaging are similar regardless of BRCA1/2 mutation status. While the results from this small study need confirmation in other studies, at present there doesn’t appear to be increased yield from targeting individuals with BRCA1/2 mutations for surveillance.
Introduction
Pancreatic ductal adenocarcinoma (PDAC) remains a highly lethal disease and is the third leading cause of cancer death in the United States(1). SEER data placed the age-adjusted incidence rate at 12.5 per 100,000 in 2010–2014, with the mortality rate nearing that at 10.9 per 100,000 (2). This poor outcome is attributed to the advanced stage of this disease that is almost always present when patients present with symptoms of PDAC such as abdominal pain or jaundice. Screening for some other gastrointestinal cancers can be effective in improving cancer mortality, as has been shown for colorectal cancer. While the approximately 30% reduction in colorectal cancer mortality over the last several decades is attributable to several factors, there is little doubt that screening programs have made a significant impact (3). There is some evidence that when PDAC is found incidentally, before symptoms develop, survival can be dramatically improved. Patients with small (<2 cm) pancreatic cancers or those without nodal disease have demonstrated improved 5-year survival rates following resection of PDAC (4). Recent studies on long-term surveillance programs show higher resectability rates for asymptomatic screen-detected PDAC compared to symptomatic PDAC and improved 3-year and 5-year survival rates (5,6).
The detection of PDAC precursor lesions is an area of interest in preventing the development of pancreatic cancer in high-risk individuals. These lesions include intraductal papillary mucinous neoplasms (IPMN) and pancreatic intraepithelial neoplasia (Pan-IN). IPMNs can be observed with cross-sectional imaging such as magnetic resonance cholangiopancreatography (MRCP), and endoscopy such as endoscopic ultrasound (EUS), while Pan-IN lesions cannot (7). Family history is an established risk factor for PDAC (8), as are smoking (9), obesity (10,11), diabetes (12), chronic pancreatitis (13), and pathogenic mutations in several genes, including BRCA1/2 (14-16).
Mutations in BRCA2 are the most common germline mutations influencing risk of PDAC with prevalence estimates ranging from approximately 2–19% (17,18). BRCA1 mutations also contribute, albeit less, to overall prevalence of inherited predisposition to PDAC (18,19). The relative risk of pancreatic cancer for carriers of BRCA1 mutations has ranged from 0.8 to 4.7 (14,18,20-26). In studies of carriers of BRCA2 mutations, the reported range is somewhat higher, from 2.0 to 21, with most results around 3 to 6 (14,18,20-24,27,28).
Hoping to identify precursor lesions such as IPMN or early PDAC in individuals at higher risk for development of PDAC, we began a surveillance program within the framework of our larger familial pancreatic tumor registry study at Memorial Sloan Kettering Cancer Center in 2002. The MSKCC Pancreatic Tumor Registry (29) includes both PDAC patients and at-risk family members, including those with germline mutations known to increase PDAC risk.
The question leading to the present study was whether, among healthy individuals with positive family history of PDAC, those with known pathogenic mutations in BRCA1/2 would have more pancreatic abnormalities compared to individuals who have undergone germline genetic testing with no mutations in BRCA1/2 identified.
Materials and Methods
High-Risk Individuals (HRI)
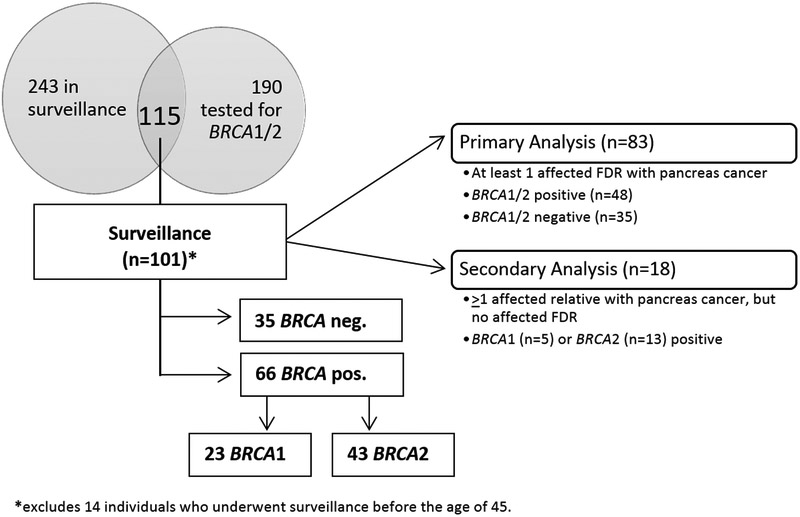
The MSKCC Pancreatic Tumor Registry opened enrollment in May 2003. As of June 30, 2017, the Registry had enrolled 554 relatives of pancreatic cancer patients. Of these, 243 HRI agreed to take part in surveillance with MRCP, with some participants undergoing CT in select circumstances. Regarding germline genetic test results, 190 of the 554 HRI have known BRCA1/2 mutation status. This analysis is based on individuals who have both undergone surveillance and have known BRCA1/2 mutation status (Figure 1).
Figure 1.
Overview of Subjects in Analysis
The eligibility criteria for HRI changed over the course of the Registry study, reflecting what we and others learned about conducting surveillance in this population. In general, changes resulted in requiring stronger family history and older age at beginning surveillance. Other changes reflected the growing numbers of HRI undergoing genetic testing for mutations in BRCA1/2 and genes involved in other rare cancer syndromes. When enrollment began in May 2003, we included relatives with ≥1 first degree relative (FDR) and ≥ 1 other affected relative. In 2011, we changed eligibility requirements to require HRI to have ≥2 affected FDRs, while individuals with known genetic syndromes were required to have ≥1FDR or ≥1 SDR (second-degree relative) with PDAC. The main analysis in this report is based on those individuals who had ≥1 FDR with pancreatic cancer, had surveillance at age ≥45 years, and had testing for pathogenic mutations in BRCA1/2 (n=83). We also report separately on a group of 18 HRI with known BRCA1/2 mutations and second-degree, but not first-degree, relatives with pancreatic cancer who underwent surveillance at age ≥45.
HRI in this report were not tested for all PDAC susceptibility genes. However, any HRI with known pathogenic mutations in other PDAC susceptibility genes were excluded from the present analysis.
HRI are identified in several ways: by study or clinical staff if they are related to a patient with pancreatic cancer; by referral from the MSKCC Clinical Genetics Service; or by self-referral after finding our Registry on the internet. HRI identified by study and clinical staff are referred to the Clinical Genetics Service for genetic counseling if they have not been seen there previously.
Data collection
All aspects of the study were performed after approval by the MSKCC institutional review board and conducted in accordance with the U.S. Common Rule. After providing informed written consent, all participants in the Registry are personally interviewed by a trained research study assistant. The interview covers established and potential risk factors for PDAC. Respondents also complete a detailed questionnaire to ascertain personal and family history of cancer. The family history questionnaire includes questions on the birthplace and religion of each grandparent to assess genetically high-risk populations such as individuals with Ashkenazi Jewish ancestry. Alternatively, if a participant was already seen by the MSKCC Clinical Genetics Service, the pedigree created during that consultation by a board-certified genetic counselor is used. Follow-up questionnaires are administered approximately every two years to update information on lifestyle exposures (such as smoking, body mass index, and diabetes) and family history of pancreatic and other cancers. For this analysis, we used data from the baseline questionnaire, except for personal and family history of cancer, which was from updated family history questionnaires.
Surveillance
HRI are offered surveillance with MRCP/CT at enrollment and at one-year intervals. Those with cysts and/or pancreatic lesions identified on screening may undergo follow-up imaging every 6–9 months at the discretion of the treating physician. We utilize MRCP as the primary surveillance tool because of its sensitivity and because there is no associated radiation exposure. By choice, per treating physician’s recommendation, or as follow-up for other medical issues, some participants also had abdominal and pelvic CT scans during the period of surveillance (n=13/83 in main analysis; 5/18 in secondary analysis). EUS and/or surgery are recommended if the MRCP indicates an abnormal finding in the pancreas, such as a large or enlarging pancreatic cyst size ≥3 cm, cyst size that increases over surveillance intervals, a dilated main pancreatic duct, or the presence of a solid component in the pancreas on imaging. In December 2012, the surveillance protocol was updated to recommend that HRI with pathogenic germline mutations in BRCA1/2 undergo annual EUS evaluations scheduled approximately six months after MRCP.
Data analysis
We compared the characteristics of HRI with and without pathogenic mutations in the BRCA1/2 genes, separately and together; Table 1 summarizes these results with p-values from chi-square tests. We determined the proportion with an abnormality on MRCP/CT within those groups and whether abnormalities noted were prevalent or incident. We further compared the characteristics of those HRI with and without findings of IPMN, the most common lesions observed. We described the lesions found and the outcome of follow-up for those with screen-detected IPMNs. We used logistic regression to estimate odds ratios (OR) to evaluate the independent influence of BRCA1/2 mutation status and characteristics associated with presence of IPMNs.
Table 1.
Characteristics of HRI tested for BRCA1 and BRCA2 mutations
| Total (83) (n, %) |
BRCA positive (48) (n, %) |
BRCA negative (35) (n, %) |
P-value | |
|---|---|---|---|---|
| Age at First Surveillance | ||||
| 45-49 | 24 (29) | 10 (21) | 14 (40) | 0.15 |
| 50-59 | 39 (47) | 26 (54) | 13 (37) | |
| 60-69 | 20 (24) | 12 (25) | 8 (23) | |
| Median Age (Range) | 54 (45-69) | 55 (45-67) | 53 (45-69) | |
| Sex | ||||
| Male | 21 (25) | 16 (33) | 5 (14) | 0.05 |
| Female | 62 (75) | 32 (67) | 30 (86) | |
| Race | ||||
| White | 77 (93) | 46 (96) | 31 (89) | 0.23 |
| African American, Other | 6 (7) | 2 (4) | 4 (11) | |
| Ethnicity | ||||
| Non-Hispanic | 80 (96) | 48 (100) | 32 (91) | 0.04 |
| Hispanic | 3 (4) | 0 | 3 (9) | |
| Ancestrya | ||||
| ≥2 Ashkenazi Jewish grandparents | 45 (54) | 31 (65) | 14 (40) | 0.03 |
| No Ashkenazi Jewish grandparents | 38 (46) | 17 (35) | 21 (60) | |
| Years of Education | ||||
| High school or less | 4 (5) | 2 (4) | 2 (6) | 0.93 |
| College | 23 (28) | 13 (27) | 10 (29) | |
| Graduate school | 56 (67) | 33 (69) | 23 (66) | |
| Smoking | ||||
| Never | 54 (65) | 34 (71) | 20 (57) | 0.20 |
| Past or current | 29 (35) | 14 (29) | 15 (43) | |
| BMI | ||||
| Normal or underweight (<25) | 57 (69) | 30 (63) | 27 (77) | 0.16 |
| Overweight or obese (≥25) | 26 (31) | 18 (38) | 8 (23) | |
| Diabetes | ||||
| No | 78 (94) | 46 (96) | 32 (91) | 0.40 |
| Yes | 5 (6) | 2 (4) | 3 (9) | |
| Personal History of Cancer | ||||
| None | 44 (53) | 22 (46) | 22 (63) | 0.12 |
| ≥1 diagnosis | 39 (47) | 26 (54) | 13 (37) | |
| Family history of PDAC | ||||
| ≥2 FDR | 17 (20) | 6 (13) | 11 (31) | <0.0001 |
| 1 FDR and ≥1SDR | 30 (36) | 10 (21) | 20 (57) | |
| 1 FDR only b | 36 (43) | 32 (67) | 4 (11) | |
| BRCA Mutation Status | ||||
| BRCA Positive | 48 (58) | 48 (100) | NA | NA |
| BRCA1 | 18 (22) | 18 (38) | NA | |
| BRCA2 | 30 (36) | 38 (62) | NA | |
| BRCA1/2 Negative | 35 (42) | NA | 35 (100) |
There were no HRI with only 1 Ashkenazi Jewish grandparent
Includes 1 HRI whose FDR had early-onset PDAC, and 3 HRI with 1 affected FDR and an additional affected relative (not SDR)
NA, Not applicable
Results
Characteristics of HRI
As shown in Table 1, the median age of HRI with at least one FDR was 54 (range 45 to 69) and 75% (62/83) of participants were women. Nearly all were white (77/83, 93%) and non-Hispanic (80/83, 96%), and over half (45/83, 54%) had 2 or more Ashkenazi Jewish grandparents. Most participants were college educated (79/83, 95%). Most were of normal weight or were underweight (57/83, 69%); 35% (29/83) had ever smoked cigarettes (only 4% were current smokers) and 6% (5/83) had been diagnosed with diabetes. Nearly half (39/83, 47%) had a personal history of cancer. Most of the reported cancers were breast cancer in women (n=25). Overall, 20% (17/83) had ≥2 FDRs with pancreatic cancer and 36% (30/83) had 1 FDR and ≥1 SDR. The median length of total time in surveillance for the 83 HRI was 48 months (range 0 to 149). Most participants (77%) had three or more screening events (i.e. MRCP, CT, or EUS) over the course of surveillance.
As shown in Table 1, HRI who tested positive for pathogenic mutations in BRCA1/2 were more likely to be of Ashkenazi Jewish descent (65% vs 40%, p=0.03). They were also more likely to be male (33% vs 14%, p=0.05) and to be non-Hispanic (100% vs 91%, p=0.04). Those who were BRCA1/2 negative had stronger family history, with 88% having at least 1 FDR and another first or second degree relative affected, compared to 34% of those who were BRCA1/2 positive (p<0.0001); this reflects differences in eligibility requirements as described above. BRCA2 mutations were more common (n=30) than BRCA1 mutations (n=18) (Table 1). Most mutations (29 of 48) were the Ashkenazi Jewish founder mutations: c.68_69delAG (n=6) and c.5266dupC (n=5) in BRCA1 and c.5946delT (n=18) in BRCA2. Median length in surveillance was longer for BRCA1/2-negative HRI (median 60 months, range 0–149) than for BRCA1/2-positive (median 36 months, range 0–127), again reflecting changes in eligibility over time.
Our paper focuses on the findings and follow-up of HRI with presumed IPMN. For the remaining 54 HRI in the main cohort without identified pancreatic lesions, the median months of follow up was 36 months (35 for the BRCA1/2 positive, 66.5 for the BRCA1/2 negative) and the median number of screening events was 5 (3.5 for the BRCA1/2 positive and 5.5 for the BRCA1/2 negative).
Abnormalities found on MRCP/CT
Overall, about one-third (29/83, 35%) of HRI with known BRCA1/2 mutation status were found to have an abnormality on MRCP/CT (Table 2); nearly all of these (27/83, 33%) were interpreted as branch-duct IPMN. The proportion with IPMN was similar for those with (31%) and without (34%) any pathogenic BRCA mutation and for those with mutations in BRCA1 (33%) and in BRCA2 (30%). Most IPMN (overall, 21/27, 78%) were prevalent lesions, found on initial surveillance, and this did not vary by presence or absence of BRCA1/2 mutations.
Table 2.
Pancreatic abnormalities identified in HRI testing positive or negative for BRCA mutations
| BRCA Positive |
BRCA Negative (35) (n, %) |
|||
|---|---|---|---|---|
| Total (48) (n, %) |
BRCA1 (18) (n, %) |
BRCA2 (30) (n, %) |
||
| Any abnormality on MRCP/CT | 16 (33) | 13 (37) | ||
| Abnormality identified as IPMN | 15 (31) | 6 (33) | 9 (30) | 12 (34) |
| Prevalent (found on first MRCP/CT) | 11 (23) | 5 (28) | 6 (20) | 10 (29) |
| Incident (found on subsequent MRCP/CT) | 4 (8) | 1 (6) | 3 (10) | 2 (6) |
Note: abnormalities other than branch-duct IPMNs were one BRCA-positive patient with chronic pancreatitis and one BRCA-negative patient with metastatic serous carcinoma of mullerian origin to pancreas.
For the remaining 2 HRI with abnormalities found on surveillance, one, with a BRCA2 mutation, was identified as having chronic pancreatitis (confirmed by EUS); the other, with no mutations in BRCA1/2, was found to have a pancreatic mass that was confirmed to be metastases of high-grade serous carcinoma of mullerian origin.
Secondary analysis in BRCA1/2 positive HRI with weaker family history
The 18 HRI in this additional group had at least one affected SDR, but no affected FDR; most (14/18, 78%) had only one SDR. Overall, these HRI were similar demographically to the BRCA1/2 positive HRI with stronger family history, described above, except that a non-statistically significant higher proportion were aged ≥60 (45% vs 25%, p=0.28). The proportion of this subgroup who were found to have abnormalities was 39%; all were considered to be IPMNs. Median length in surveillance was longer for those with negative findings (median 39 months, range 21–110) than those with positive (median 1 month, range 0–102). Five of the 11 HRI with negative findings were recently recruited and had only 1 screen.
Follow-up of presumed IPMNs
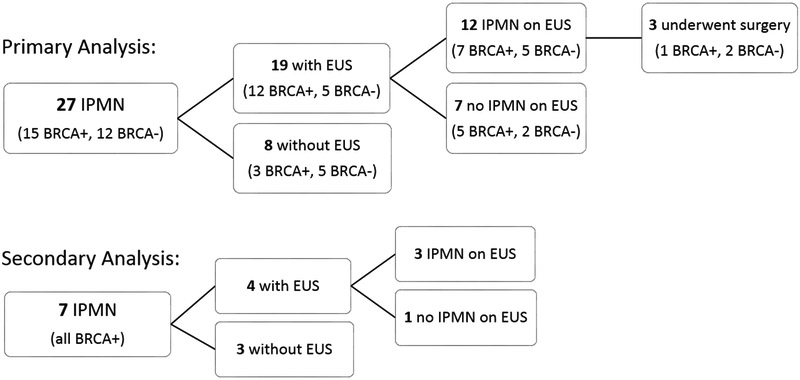
Our follow-up of the 27 HRI with presumed branch-duct IPMN in the main analysis is summarized in Supplemental Table 1. EUS was recommended and undertaken for 19 (12 BRCA1/2-positive and 7 BRCA1/2-negative) and confirmed pancreatic lesions in 12 of these cases (7 in BRCA1/2-positive and 5 in BRCA1/2-negative individuals) (Figure 2). EUS did not visualize the pancreatic lesions initially identified on imaging for 7 HRI, likely due to the small size of these pancreatic cysts, which ranged from 0.3 to 1.4 cm on imaging. These small cysts were again noted on follow-up MRCP for all 7 patients, and they continue to be monitored with MRCP for any changes.
Figure 2.
EUS among high-risk individuals with IPMN from primary and secondary analyses
Surgery was recommended for 4 HRI. Patient 1, with a mutation in BRCA2, underwent surgery due to increasing size cystic lesions throughout the pancreas. Surgical pathology showed IPMN with low grade dysplasia and chronic pancreatitis in the remaining pancreas. This patient presented approximately 9 months later with jaundice and metastatic cancer in her liver; liver biopsy was consistent with pancreaticobiliary origin. Cross-sectional imaging of the remaining pancreas showed no pancreatic mass to account for this metastatic disease. The patient is now deceased.
Baseline MRCP for Patient 2, BRCA1/2 negative, identified cystic lesions in the pancreatic head and neck. Surgery was later recommended due to increasing size of the cystic lesions and dilatation of the main pancreatic duct. Pathology revealed multifocal IPMN of borderline malignant potential. The patient was followed closely after surgery, with MRCP showing no evidence of suspicious lesion in the pancreatic remnant, until expiring from uterine cancer about 6 years later.
Patient 3, also BRCA1/2 negative, had surgery after MRCP noted a cystic lesion in the tail of the pancreas. EUS with FNA (fine needle aspiration) identified mucinous cells consistent with a mucinous neoplasm. The decision to move forward with surgery was primarily patient-driven; despite a lack of worrisome features such as increased cyst size and ductal dilatation, the patient opted for surgery given her family history of PDAC. Pathology showed PanIN-2, focal fibrosis and chronic inflammation. The patient is in good health 9 years after surgery, with follow-up MRCPs showing no new or suspicious lesions in the residual portion of the pancreas.
MRCP identified few cystic lesions in the pancreatic body and uncinate process of Patient 4, a BRCA1 mutation carrier. EUS confirmed these lesions and the patient was referred to surgery to discuss these findings given her strong family history and BRCA1 mutation; given the options to undergo surgery as a prophylactic measure overall or continue watchful surveillance of her cysts, the patient chose the latter. She continues to be under surveillance 10 years later with no significant change in the identified lesions.
One HRI, Patient 6, was scheduled for a repeat EUS for follow-up on a screen-identified oval mass at the time of writing; subsequent scans for all remaining HRI with pancreatic abnormalities in this analysis did not indicate significant changes in the identified lesions.
In the secondary analysis of HRI known to have BRCA1/2 mutations, we identified 7 cases with screen-identified lesions (Figure 2). EUS confirmed the pancreatic lesions in 3 of these cases; all were recommended to continue surveillance to monitor their presumed IPMN (see Supplemental Table 2).
Other factors related to presence of IPMNs in surveillance participants
We investigated characteristics of HRI that might have influenced the presence of IPMNs. Table 3 shows characteristics of those with (n=27) and those without IPMNs (n=56); none of these differences reached statistical significance in chi-square tests. Logistic regression models, with presence of IPMN as the outcome, found no significant associations either. Odds ratios were close to 1 for presence of BRCA1/2 mutations and borderline significant for increasing age (Table 4).
Table 3.
Selected characteristics of HRI with and without IPMN
| IPMN (27) (n, %) |
No IPMN (56) (n, %) |
P-value | |
|---|---|---|---|
| Age at First Surveillance | |||
| 45-49 | 6 (22) | 18 (32) | 0.15 |
| 50-59 | 11 (41) | 28 (50) | |
| 60-69 | 10 (37) | 10 (18) | |
| Sex | |||
| Male | 7 (26) | 14 (25) | 0.93 |
| Female | 20 (74) | 42 (75) | |
| Ancestry | |||
| ≥2 Ashkenazi Jewish grandparents | 18 (67) | 27 (48) | 0.11 |
| No Ashkenazi Jewish grandparentsa | 9 (33) | 29 (52) | |
| Smoking | |||
| Ever | 12 (44) | 17 (30) | 0.21 |
| Never | 15 (56) | 39 (70) | |
| BMI | |||
| Normal or underweight (<25) | 17 (63) | 40 (71) | 0.44 |
| Overweight or obese (≥25) | 10 (37) | 16 (29) | |
| Personal History of Cancer | |||
| None | 15 (56) | 29 (52) | 0.75 |
| ≥1 diagnosis | 12 (44) | 27 (48) | |
| Family history of PDAC | |||
| ≥2 FDR | 6 (22) | 11 (20) | 0.42 |
| 1 FDR and ≥1SDR | 12 (44) | 18 (32) | |
| 1 FDR only | 9 (33) | 27 (48) |
No HRI had only 1 Ashkenazi Jewish grandparent
Table 4.
Multivariate analysis of factors related to presence of IPMN
| Odds Ratio | 95% CI | |
|---|---|---|
| Age at first surveillance (per year) | 1.07 | 1.0-1.2 |
| ≥2 Ashkenazi Jewish grandparents | 2.7 | 0.86-8.2 |
| Ever smoked | 2.1 | 0.69-6.5 |
| Family history of PDAC | ||
| 1 FDR only | 1 (reference) | |
| 1 FDR and ≥1 SDR | 1.7 | 0.45-6.2 |
| ≥2 FDR | 1.4 | 0.31-6.6 |
| BRCA1/2 mutation status | 0.89 | 0.26-3.0 |
CI, confidence interval
Discussion
Our Registry population and close coordination with the Clinical Genetics Service provide an opportunity to evaluate our hypothesis that surveillance in individuals with pathogenic mutations in BRCA1/2 might be beneficial to them by identifying pancreatic lesions that could be characterized as premalignant. Our comparison group is unique: those known not to have mutations in either of these genes. We found no difference in pancreatic abnormalities between those with and without mutations. Results were similar in those with mutations in BRCA1 and BRCA2. In an additional subset of 18 HRI who were BRCA1/2 positive but had weaker family history, the proportion of abnormalities found on MRCP was also similar. Based on our study, HRI with mutations in BRCA1/2 do not appear to be more likely to have pancreatic abnormalities on surveillance. Since this report is based on a small number of HRI, research should be continued on outcomes of surveillance programs among the BRCA positive population as well as the general high-risk population. We do not recommend changes in clinical practice at this time.
A recent study (5), which focused on neoplastic progression in high-risk individuals undergoing surveillance, included 41 HRI with known mutations in BRCA1/2 or PALB2; two of the 41 HRI showed neoplastic progression, a similar proportion to that among all HRI (n=354) in that study. Bartsch et al (30) studied a group of individuals at risk for pancreatic cancer based on their family history and BRCA1/2/PALB2 mutation status in three European centers. Their study included 17 individuals with known mutations. In contrast to our study, they observed a higher proportion with potentially significant lesions compared to all others, 18% vs 6%.
The overall proportion of HRI in our study with positive findings on MRCP was 35%; this was higher than the 17% reported in our previous study of a more broadly defined group of at-risk relatives (31) but consistent in other studies, where prevalence of lesions has ranged from 32% to 53% (30,32,33).
It is not clear why in this high-risk cohort of patients with pathogenic BRCA1/2 mutations, our imaging findings are no different from those individuals with BRCA–negative results. It may be that BRCA1/2 mutations are more strongly associated with Pan-INs, which cannot be detected with the imaging modalities used in our study. Although most of the lesions found in this study were prevalent (found at initial surveillance), it is possible that more incident lesions or changes in existing lesions would be found with longer follow-up (34,35).
This study has several limitations. Because the study was conducted at a tertiary referral hospital, the HRI included here do not come from a well-defined population and results may not be generalizable to the overall population. As in other studies (5,18,36,37) our population is not diverse in terms of race; in contrast to other studies, our population includes a relatively large proportion of Ashkenazi Jews. These characteristics reflect both our local population and the self-selection of HRI for this program. We do not have information on reasons for undergoing testing for mutations in BRCA1/2 and our data do not allow us to disentangle the roles of family history, personal history, and Ashkenazi Jewish background in their decisions. HRI included in this analysis were not tested for all possible PDAC susceptibility genes. Some of the HRI had testing only for the 3 Ashkenazi Jewish founder mutations. However, among the thirteen HRI of Ashkenazi background who tested negative for BRCA1/2, ten provided documentation that they had more thorough testing with sequencing and/or large rearrangement analyses for mutations, making it unlikely that misclassification influenced the results. Another limitation is that our eligibility criteria changed over the study period. Though inclusion guidelines for the Registry have been consistent with past consensus and present recommendations for who should be screened (38), criteria changes have resulted in differences between those with and without BRCA1/2 mutations in family history and length of follow-up. However, since family history was not related to presence of IPMNs, and most lesions were found on the initial scan and did not change in subsequent scans, these factors are unlikely to have influenced results.
This study includes only a subset of HRI included in our Registry, and a larger analysis of findings in all surveillance participants is underway. The contribution of the present analysis is the direct comparison of those with mutations in BRCA1/2 to those known not to have mutations, with no differences noted based on surveillance with dedicated MRCP/CT and EUS. What is needed is a biomarker for PDAC that can reproducibly stratify PDAC risk and aide in identifying individuals who are in the early stage of developing a pancreatic neoplasm from those who are not. Work in the identification of such biomarkers is currently ongoing, and it is hoped that this will make an impact on the early identification of individuals at high risk for this disease.
Supplementary Material
Acknowledgements
This research was supported in part by the NIH/NCI Cancer Center Support Grant P30 CA008748. The following authors were directly supported by the grant: A. Saldia, S. Olson, P. Nunes, X. Liang, M. Samson, E. Salo-Mullen, V. Marcell, Z. Stadler, P. Allen, K. Offit, R. Kurtz.
Abbreviations:
- EUS
endoscopic ultrasound
- FDR
first degree relative
- HRI
high-risk individual
- IPMN
intraductal papillary mucinous neoplasms
- MRCP
magnetic resonance cholangiopancreatography
- Pan-IN
pancreatic intraepithelial neoplasia
- PDAC
pancreatic ductal adenocarcinoma
- SDR
second degree relative
Footnotes
Conflict of Interest: The authors declare no potential conflicts of interest
References
- 1.Cancer Facts & Figures 2018. American Cancer Society; 2018.
- 2.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, et al. SEER Cancer Statistics Review, 1975–2014. April 2017 ed. Bethesda MD: National Cancer Institute; 2016. [Google Scholar]
- 3.Lin JS, Piper MA, Perdue LA, Rutter C, Webber EM, O’Connor E, et al. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews Screening for Colorectal Cancer: A Systematic Review for the US Preventive Services Task Force. Rockville (MD): Agency for Healthcare Research and Quality (US); 2016. [PubMed] [Google Scholar]
- 4.de Jong MC, Li F, Cameron JL, Wolfgang CL, Edil BH, Herman JM, et al. Re-evaluating the impact of tumor size on survival following pancreaticoduodenectomy for pancreatic adenocarcinoma. Journal of surgical oncology 2011;103(7):656–62 doi 10.1002/jso.21883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Canto MI, Almario JA, Schulick RD, Yeo CJ, Klein A, Blackford A, et al. Risk of Neoplastic Progression in Individuals at High Risk for Pancreatic Cancer Undergoing Long-term Surveillance. Gastroenterology 2018;155(3):740–51 e2 doi 10.1053/j.gastro.2018.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vasen H, Ibrahim I, Ponce CG, Slater EP, Matthai E, Carrato A, et al. Benefit of Surveillance for Pancreatic Cancer in High-Risk Individuals: Outcome of Long-Term Prospective Follow-Up Studies From Three European Expert Centers. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2016;34(17):2010–9 doi 10.1200/jco.2015.64.0730. [DOI] [PubMed] [Google Scholar]
- 7.Distler M, Aust D, Weitz J, Pilarsky C, Grutzmann R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. BioMed research international 2014;2014:474905 doi 10.1155/2014/474905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klein AP, Brune KA, Petersen GM, Goggins M, Tersmette AC, Offerhaus GJ, et al. Prospective risk of pancreatic cancer in familial pancreatic cancer kindreds. Cancer research 2004;64(7):2634–8. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Duan H, Yang X, Guo J. Cigarette smoking and the risk of pancreatic cancer: a case–control study. Medical Oncology 2014;31(10):184 doi 10.1007/s12032-014-0184-4. [DOI] [PubMed] [Google Scholar]
- 10.Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, et al. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Annals of oncology : official journal of the European Society for Medical Oncology 2012;23(4):843–52 doi 10.1093/annonc/mdr398. [DOI] [PubMed] [Google Scholar]
- 11.Genkinger JM, Kitahara CM, Bernstein L, Berrington de Gonzalez A, Brotzman M, Elena JW, et al. Central adiposity, obesity during early adulthood, and pancreatic cancer mortality in a pooled analysis of cohort studies. Annals of oncology : official journal of the European Society for Medical Oncology 2015;26(11):2257–66 doi 10.1093/annonc/mdv355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson SH, Xu Y, Herzog K, Saldia A, DeFilippis EM, Li P, et al. Weight Loss, Diabetes, Fatigue, and Depression Preceding Pancreatic Cancer. Pancreas 2016;45(7):986–91 doi 10.1097/mpa.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Midha S, Sreenivas V, Kabra M, Chattopadhyay TK, Joshi YK, Garg PK. Genetically Determined Chronic Pancreatitis but not Alcoholic Pancreatitis Is a Strong Risk Factor for Pancreatic Cancer. Pancreas 2016;45(10):1478–84 doi 10.1097/mpa.0000000000000684. [DOI] [PubMed] [Google Scholar]
- 14.Mersch J, Jackson MA, Park M, Nebgen D, Peterson SK, Singletary C, et al. Cancers associated with BRCA1 and BRCA2 mutations other than breast and ovarian. Cancer 2015;121(2):269–75 doi 10.1002/cncr.29041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salo-Mullen EE, O’Reilly EM, Kelsen DP, Ashraf AM, Lowery MA, Yu KH, et al. Identification of germline genetic mutations in patients with pancreatic cancer. Cancer 2015;121(24):4382–8 doi 10.1002/cncr.29664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Holter S, Borgida A, Dodd A, Grant R, Semotiuk K, Hedley D, et al. Germline BRCA Mutations in a Large Clinic-Based Cohort of Patients With Pancreatic Adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2015;33(28):3124–9 doi 10.1200/jco.2014.59.7401. [DOI] [PubMed] [Google Scholar]
- 17.Grover S, Syngal S. Hereditary pancreatic cancer. Gastroenterology 2010;139(4):1076–80, 80 e1–2 doi 10.1053/j.gastro.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hu C, Hart SN, Polley EC, Gnanaolivu R, Shimelis H, Lee KY, et al. Association between inherited germline mutations in cancer predisposition genes and risk of pancreatic cancer. JAMA 2018;319(23):2401–9 doi 10.1001/jama.2018.6228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chaffee KG, Oberg AL, McWilliams RR, Majithia N, Allen BA, Kidd J, et al. Prevalence of germ-line mutations in cancer genes among pancreatic cancer patients with a positive family history. Genet Med 2018;20(1):119–27 doi 10.1038/gim.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Streff H, Profato J, Ye Y, Nebgen D, Peterson SK, Singletary C, et al. Cancer Incidence in First- and Second-Degree Relatives of BRCA1 and BRCA2 Mutation Carriers. The oncologist 2016;21(7):869–74 doi 10.1634/theoncologist.2015-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mocci E, Milne RL, Mendez-Villamil EY, Hopper JL, John EM, Andrulis IL, et al. Risk of pancreatic cancer in breast cancer families from the breast cancer family registry. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 2013;22(5):803–11 doi 10.1158/1055-9965.epi-12-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iqbal J, Ragone A, Lubinski J, Lynch HT, Moller P, Ghadirian P, et al. The incidence of pancreatic cancer in BRCA1 and BRCA2 mutation carriers. British journal of cancer 2012;107(12):2005–9 doi 10.1038/bjc.2012.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran A, O’Hara C, Khan S, Shack L, Woodward E, Maher ER, et al. Risk of cancer other than breast or ovarian in individuals with BRCA1 and BRCA2 mutations. Familial cancer 2012;11(2):235–42 doi 10.1007/s10689-011-9506-2. [DOI] [PubMed] [Google Scholar]
- 24.Risch HA, McLaughlin JR, Cole DE, Rosen B, Bradley L, Fan I, et al. Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. Journal of the National Cancer Institute 2006;98(23):1694–706 doi 10.1093/jnci/djj465. [DOI] [PubMed] [Google Scholar]
- 25.Brose MS, Rebbeck TR, Calzone KA, Stopfer JE, Nathanson KL, Weber BL. Cancer risk estimates for BRCA1 mutation carriers identified in a risk evaluation program. Journal of the National Cancer Institute 2002;94(18):1365–72. [DOI] [PubMed] [Google Scholar]
- 26.Thompson D, Easton DF. Cancer Incidence in BRCA1 mutation carriers. Journal of the National Cancer Institute 2002;94(18):1358–65. [DOI] [PubMed] [Google Scholar]
- 27.van Asperen CJ, Brohet RM, Meijers-Heijboer EJ, Hoogerbrugge N, Verhoef S, Vasen HF, et al. Cancer risks in BRCA2 families: estimates for sites other than breast and ovary. Journal of medical genetics 2005;42(9):711–9 doi 10.1136/jmg.2004.028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cancer risks in BRCA2 mutation carriers. Journal of the National Cancer Institute 1999;91(15):1310–6. [DOI] [PubMed] [Google Scholar]
- 29.2017 Memorial Sloan Kettering Cancer Center Pancreatic Tumor Registry. Memorial Sloan Kettering Cancer Center <https://www.mskcc.org/cancer-care/clinical-trials/02-102>.
- 30.Bartsch DK, Slater EP, Carrato A, Ibrahim IS, Guillen-Ponce C, Vasen HF, et al. Refinement of screening for familial pancreatic cancer. Gut 2016;65(8):1314–21 doi 10.1136/gutjnl-2015-311098. [DOI] [PubMed] [Google Scholar]
- 31.Ludwig E, Olson SH, Bayuga S, Simon J, Schattner MA, Gerdes H, et al. Feasibility and yield of screening in relatives from familial pancreatic cancer families. The American journal of gastroenterology 2011;106(5):946–54 doi 10.1038/ajg.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Sukhni W, Borgida A, Rothenmund H, Holter S, Semotiuk K, Grant R, et al. Screening for pancreatic cancer in a high-risk cohort: an eight-year experience. J Gastrointest Surg 2012;16(4):771–83 doi 10.1007/s11605-011-1781-6. [DOI] [PubMed] [Google Scholar]
- 33.Canto MI, Hruban RH, Fishman EK, Kamel IR, Schulick R, Zhang Z, et al. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012;142(4):796–804; quiz e14–5 doi 10.1053/j.gastro.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pergolini I, Sahora K, Ferrone CR, Morales-Oyarvide V, Wolpin BM, Mucci LA, et al. Long-term Risk of Pancreatic Malignancy in Patients With Branch Duct Intraductal Papillary Mucinous Neoplasm in a Referral Center. Gastroenterology 2017;153(5):1284–94 e1 doi 10.1053/j.gastro.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 35.Lawrence SA, Attiyeh MA, Seier K, Gonen M, Schattner M, Haviland DL, et al. Should Patients With Cystic Lesions of the Pancreas Undergo Long-term Radiographic Surveillance?: Results of 3024 Patients Evaluated at a Single Institution. Ann Surg 2017;266(3):536–44 doi 10.1097/SLA.0000000000002371. [DOI] [PubMed] [Google Scholar]
- 36.Shindo K, Yu J, Suenaga M, Fesharakizadeh S, Cho C, Macgregor-Das A, et al. Deleterious Germline Mutations in Patients With Apparently Sporadic Pancreatic Adenocarcinoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2017;35(30):3382–90 doi 10.1200/JCO.2017.72.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yurgelun MB, Chittenden AB, Morales-Oyarvide V, Rubinson DA, Dunne RF, Kozak MM, et al. Germline cancer susceptibility gene variants, somatic second hits, and survival outcomes in patients with resected pancreatic cancer. Genet Med 2018. doi 10.1038/s41436-018-0009-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, et al. International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut 2013;62(3):339–47 doi 10.1136/gutjnl-2012-303108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.