Abstract
Purpose:
We examined the prognostic impact of circulating tumor cells (CTCs) and disseminated tumor cells (DTCs) detected at the time of surgery in 742 untreated early breast cancer patients.
Experimental Design:
DTCs in bone marrow were enumerated using the EPCAM-based immunomagnetic enrichment and flow cytometry (IE/FC) assay. CTCs in blood were enumerated either by IE/FC or CellSearch. Median follow-up was 7.1 years for distant recurrence-free survival (DRFS) and 9.1 years for breast cancer-specific survival (BCSS) and overall survival (OS). Cox regressions were used to estimate hazard ratios for DRFS, BCSS and OS in all patients as well as in hormone receptor-positive (HR-positive, 87%) and HR-negative (13%) subsets.
Results:
In multivariate models, CTC-positivity by IE/FC was significantly associated with reduced BCSS in both all (n=288, p=0.0138) and HR-positive patients (n=249, p=0.0454). CTC-positivity by CellSearch was significantly associated with reduced DRFS in both all (n=380, p=0.0067) and HR-positive patients (n=328, p=0.0002). DTC status, by itself, was not prognostic; however, when combined with CTC status by IE/FC (n=273), double positivity (CTC+/DTC+, 8%) was significantly associated with reduced DRFS (p=0.0270), BCSS (p=0.0205), and OS (p=0.0168). In HR-positive patients, double positivity (9% of 235) was significantly associated with reduced DRFS (p=0.0285), BCSS (p=0.0357), and OS (p=0.0092).
Conclusions:
Detection of CTCs in HR-positive early breast cancer patients was an independent prognostic factor for DRFS (using CellSearch) and BCSS (using IE/FC). Simultaneous detection of DTCs provided additional prognostic power for outcome, including OS.
INTRODUCTION
Recurrence of breast cancer after initial treatment with surgery and adjuvant therapies remains the major cause of mortality from this disease (1). Mechanisms involved in the persistence of breast cancer cells and their spread to distant sites are not fully understood (2). Accumulated evidence demonstrates that the presence of cancer cells in hematopoietic compartments (blood and bone marrow) is associated with poor clinical outcome (3–6). Methods for reliable detection of these tumor cells have been actively pursued in the last decade (7,8).
Cancer cells in blood and bone marrow—referred to as circulating tumor cells (CTCs) and disseminated tumor cells (DTCs), respectively—can be detected using immunocytochemical and nucleic acid-based assays (e.g., reverse transcription-polymerase chain reaction) (9–11). Pooled analysis of data from several studies have now shown that the presence of DTCs is a strong predictor of poor outcomes (6). Despite demonstrated clinical significance, testing for DTCs has not yet become a standard component of disease staging. Lack of a standard DTC methodology has been one of the issues hampering adoption (12).
The CellSearch (Veridex LLC) system is currently the only US Food and Drug Administration-cleared system for detection of EPCAM-positive CTCs (13). Studies using CellSearch have demonstrated that enumeration (counting) of CTCs can provide prognostic information in patients with early (5,14,15) and advanced (13,16,17) breast cancer.
We have described an EPCAM-based immunomagnetic enrichment/flow cytometry (IE/FC) for enumeration and isolation of CTCs (18–20) in blood, and have applied this method as well to DTCs (18,21,22) in bone marrow.
We hypothesize that CTCs, DTCs and simultaneous detection of these cell at the time of surgery are associated with worse outcome. To address this hypothesis, we prospectively enumerated CTCs and DTCs from each patient immediately prior to breast cancer surgery. CTC enumeration was performed first by IE/FC and then by CellSearch; DTC enumeration was performed by IE/FC. With long patient follow-up (up to a median of 13.3 years), we analyzed the prognostic significance of CTC and DTC status in these patients. To our knowledge, our study is the first to report on synchronous detection of CTC and DTC in a large cohort using the same quantitative assay system.
METHODS
Patients.
Early breast cancer patients who were scheduled to undergo breast cancer surgery at the University of California San Francisco (UCSF) were recruited to participate in this study. The parent study included prospective collection of samples from both treatment-naïve and neoadjuvant-treated breast cancer patients. In this report, we excluded the neoadjuvant cohort to rule out potential confounding effects of neoadjuvant therapy on the levels of CTCs and DTCs. The study was performed with Institutional Review Board (UCSF Committee on Human Research) approval, and informed written consent was obtained from each patient. The study was performed in accordance with the Declaration of Helsinki.
Specimen collection of IE/FC.
Bone marrow samples were collected via a unilateral bone marrow aspiration from the posterior superior iliac crest while patients were under anesthesia prior to surgery. Two 5 mL samples were withdrawn from one site in posterior iliac crest. Peripheral blood was obtained on the same day, either in the preoperative setting or at the same time as bone marrow aspiration. Bone marrow (~4 mL) and peripheral blood (~10 mL) samples were drawn into tubes containing ethylenediaminetetraacetic acid (EDTA) for IE/FC (see below). Samples were processed within 24 hours after collection.
IE/FC assay.
Enumeration of CTCs (by IE/FC and CellSearch) and DTCs was performed by investigator JHS who was blinded to the study endpoints.
Blood and bone marrow samples were subjected to the IE/FC assay to enumerate CTCs and DTCs, respectively (18–20,22). Briefly, two distinct monoclonal antibodies against EPCAM, one conjugated to immunomagnetic beads (MJ37) and the other conjugated to phycoerythrin (EBA-1) were added to whole blood or bone marrow. The sample was then placed in a magnet to capture cells labeled with the magnetic bead-antibody conjugated. The supernatant containing cells that were unbound (including red blood cells) was aspirated. Magnetic separation was repeated twice to further enrich for EPCAM-expressing cells. A nucleic acid dye (Thioflavin-T, BD Biosciences) and a monoclonal antibody to the leukocyte-specific marker CD45 (2D1) conjugated to peridinin-chlorophyll-protein-Cy5.5 were added to the sample. The enriched sample was transferred to a BD TruCount™ (BD Biosciences) tube, and flow cytometric analysis was performed using the BD FACSCalibur™ (BD Biosciences). CTCs and DTCs were defined as nucleated cells that are EPCAM-positive and CD45-negative.
CellSearch assay.
In 2004, the CellSearch system was cleared by the US Food and Drug Administration for enumeration of CTCs. We amended our study protocol to utilize CellSearch for CTC enumeration in place of the IE/FC starting August 2005.
Peripheral blood was collected into CellSave preservative tubes (Menarini) for CellSearch analysis. CTCs were enumerated in 7.5 mLs of blood using the Circulating Tumor Cell Kit (Menarini) following manufacturer’s instructions without modifications (23). Briefly, the sample was subjected to immunomagnetic enrichment using beads coated with monoclonal antibody against EPCAM and then CTCs were detected by fluorescence microscopy. CTCs were defined as nucleated cells that are cytokeratin-positive and CD45-negative. CellSearch results were expressed as CTC/mL for direct comparison with IE/FC.
Study design.
Samples were prospectively collected between April 27, 1999 until June 19, 2012. Survival analysis was performed on follow-up data available as of December 30, 2017. The median follow-up times for DRFS and BCSS/OS for all patients in the study were 7.1, and 9.1 years, respectively. In subset analyses, median follow-up duration for BCSS/OS reached 13.3 years (Table 1).
Table 1. Characteristics, follow-up, and outcomes of patients enrolled in the study.
Treatment-naïve patients with early breast cancer were recruited for simultaneous testing for CTCs in blood and DTCs in bone marrow collected immediately prior to surgery.
| Clinical Variable | n=742 |
|---|---|
| Age at Diagnosis median [range] | 53 [25-82] |
| Tumor size (cm) at surgery median [range] | 1.5 [0-24] |
| Pathologic stage | |
| Stage 0 | 2% (15/738) |
| Stage 1 | 56% (416/738) |
| Stage II | 33% (245/738) |
| Stage III | 8% (62/738) |
| Receptor status | |
| HR+ (ER+ or PR+) | 87% (645/737) |
| HER2+ | 12% (91/737) |
| Subtype | |
| HR+HER2+ | 10% (68/711) |
| HR+HER2− | 78% (556/711) |
| HR-HER2+ | 3% (23/711) |
| HR-HER2− | 9% (64/711) |
| Nodal status | |
| Node-negative | 71% (512/719) |
| Node-positive | 29% (207/719) |
| Grade | |
| 1 | 33% (235/704) |
| 2 | 45% (317/704) |
| 3 | 22% (152/704) |
| Events | |
| Distant recurrence | 9% (65/742) |
| Breast cancer specific death | 6% (40/720) |
| Death (any cause) | 13% (97/742) |
| Length of follow-up for DRFS | median years [range] |
| All patients | 7.1 [0.09-18.5] |
| CTC subset by CellSearch | 6.4 [0.16-13.8] |
| CTC subset by IE/FC | 9.8 [0.09-18.5] |
| DTC subset | 7.5 [0.09-18.5] |
| CTC and DTC by IE/FC | 9.8 [0.09-18.5] |
| Length of follow-up for BCSS/OS | |
| All patients | 9.1 [0.71-18.5] |
| CTC subset by CellSearch | 7.5 [0.71-15.0] |
| CTC subset by IE/FC | 13.3 [1.93-18.5] |
| DTC subset | 9.8 [1.55-18.5] |
| CTC and DTC by IE/FC | 13.3 [1.93-18.5] |
The primary clinical endpoints for the survival analysis included: distant recurrence-free survival (DRFS), breast cancer-specific survival (BCSS) and overall survival (OS). Survival was measured from the date of diagnosis to the corresponding event in question. Patients lost to follow-up were censored at the time of their last visit. Covariates examined in survival models include age at diagnosis, tumor size at surgery, stage, grade, hormone-receptor, HER2, and nodal status.
Statistical Methods.
To determine thresholds for CTC- and DTC-positivity by IE/FC, cutoffs were initially based on mean CTC/mL and DTC/mL in controls (see above) plus two standard deviations. To find optimal thresholds for association with outcome, we performed cutoff optimization with Monte-Carlo cross validation. First, half of the cases (balanced for number of events) were sub-sampled and used to derive a threshold between the 20% and 80% percentile that yielded the maximum Kaplan-Meier curve separation (i.e., minimum log rank p-value) for DRFS. We chose DRFS because we expected this was most likely to reflect breast cancer-specific outcome, while giving us more power to detect outcome differences (i.e., more events) than BCSS within our follow-up period. The threshold was then applied to the remaining half of the cases. The log rank p-values were assessed in the test set. The above procedure was repeated 1000 times. The log rank p-values for the test set over the 1000 iterations were combined using the logit method and the threshold with the lowest combined p-value was selected.
Univariate and multivariate Cox proportional hazards regression analysis was performed to calculate hazard ratios (HR) and 95% confidence intervals. Kaplan-Meier survival curves were plotted and p-values were calculated using the log rank test. The R package “survival” was used for Cox proportional hazards model, Kaplan-Meier survival analysis, Wald and log rank tests.
RESULTS
Study design and patient characteristics
Of the 1121 early stage breast cancer patients enrolled in the study, 742 were treatment-naïve, i.e., did not receive neoadjuvant therapy (Supplementary Figure 1A). The 379 patients who did receive neoadjuvant therapy were excluded from this analysis.
All patients underwent blood and/or bone marrow sampling in the operating room immediately prior to breast surgery (Supplementary Figure 2A). 71% patients were subsequently found to be lymph node-negative, and 87% were hormone-receptor positive (Table 1).
The median follow-up times for DRFS and BCSS/OS for all patients in the study were 7.1, and 9.1 years, respectively (Table1). In subset analyses, median follow-up duration for BCSS/OS reached 13.3 years.
The study initially used only IE/FC analysis of CTCs and DTCs. In 2004, the CellSearch system was granted clearance by the US Food and Drug Administration for enumeration of CTCs in breast cancer. Based on this, the study protocol was amended to replace IE/FC with CellSearch for CTC enumeration (starting August 2005). This explains the shorter follow-up duration among patients whose CTCs were enumerated by CellSearch. The final analysis therefore includes two separate CTC detection strategies: an EPCAM-positive cytokeratin approach (CellSearch) and a dual epitope EPCAM-based approach (IE/FC).
Comparison of levels of DTCs vs. CTCs in early breast cancer patients
CTCs in blood and DTCs in bone marrow were enumerated from samples collected immediately prior to surgery. The frequency distribution of CTC and DTC counts per mL are shown in Supplementary Figure 2B. The mean concentration of tumor cells in the bone marrow was significantly higher than that in blood (23.31 DTCs/mL vs. CellSearch: 0.09 CTCs/mL and IE/FC: 1.01 CTCs/mL, T-tests, p<0.001) (Supplementary Table 1). The range of DTCs (0-4743.20, median 6.73 DTC/mL) was similarly larger than CTC range by IE/FC assay or CellSearch (0-33.74, median 0.34 CTC/mL and. 0-6.67, median 0 CTC/mL, respectively).
In addition to tumor cells per mL of bone marrow vs. blood, we also compared the number of tumor cells per 106 mononuclear cells (MNCs) in blood (n=73 by IE/FC) and bone marrow (n=184) samples. Comparison of tumor cells/106 MNC data between compartments confirmed a significantly higher tumor cell/MNC ratio in the bone marrow, which was nearly 5-fold higher than in blood (0.23/106 MNCs in bone marrow vs. 0.05/106 MNCs in blood, Wilcoxon rank-sum test, p<0.001) (Supplementary Figure 3).
Patient samples analyzed by IE/FC were scored as positive for CTCs and DTCs using thresholds based on two standard deviations above the mean background levels in controls, i.e., >0.54 CTCs/mL of blood and >4.16 DTCs/mL of bone marrow. Using these cut points, 38% and 68% of patients were considered positive for CTCs and DTCs, respectively (Supplementary Table 1). The percent CTC detection rate by CellSearch was 23% ( cutoff ≥1 CTC per 7.5 mLs of blood).
Threshold optimization for survival analysis
We performed Monte-Carlo cross validation to find optimal cutoffs for prognostication in patients analyzed by IE/FC (see Methods). Threshold optimization yielded the following cutoffs: >0.44 cells/mL for CTCs and >18.61 cells/mL for DTCs. Using these thresholds, percent positivity for CTCs increased from 38% to 41%, while percent positivity for DTCs decreased from 68% to 19% (Supplementary Table 1). For CellSearch, we used the previously validated cutoffs of ≥1 CTC and ≥2 CTC per 7.5 mLs of blood (5,14,24). Percent positivity decreased from 23% to 9% using the latter cutoff.
Association between CTCs/DTCs and clinical variables
No significant association was observed between CTCs/DTCs and standard clinicopathologic variables using the initial thresholds. With the optimized cutoffs, we observed a significant association between CTC-positivity (by CellSearch) and HER2-positivity (Fisher’s Exact p=0.011) (Supplementary Table 2). We also found that patients who were positive for CTCs (by IE/FC) had numerically larger mean tumor size compared to those who were CTC-negative (T-test p=0.05).
Survival analysis based on initial thresholds for IE/FC
Of the 742 patients in the study, 65 (9%) experienced a distant recurrence and 97 (13%) died during the study; 40 (6%) were breast cancer-specific deaths. (Table 1). We performed univariate Cox regression analysis to evaluate the prognostic significance of established clinicopathologic variables in our study population. As expected, tumor size, nodal status, grade and pathological stage were strong predictors for all survival endpoints (Supplementary Table 3). HER2 status was prognostic for DRFS and OS, while age at diagnosis and hormone receptor status were prognostic for OS.
We evaluated CTC and DTC levels as continuous variables vs. outcome. CTCs by IE/FC was prognostic for BCSS (HR 1.25, p=0.0119), while DTCs were prognostic for BCSS (HR 1.2 p<0.0001) and OS (HR 1.19 p<0.0001) (Supplementary Table 4).
Next, we used the initial cutoffs of >0.54 cells/mL for CTCs and >4.16 cells/mL for DTCs to dichotomize patients into positive and negative groups. Univariate Cox regression analysis revealed no significant correlation between DTCs and clinical outcomes. In contrast, patients positive for CTCs by IE/FC had significantly reduced DRFS (HR 1.96, p=0.0420), and BCSS (HR 2.73, p=0.0172) (Supplementary Table 4).
Survival analysis based on optimized thresholds
To evaluate the prognostic impact of CTCs and DTCs using the optimized thresholds described above, we performed univariate Cox regression and Kaplan-Meier analyses. We also performed multivariate Cox regression analyses to adjust for age, tumor size, nodal status, hormone receptor/HER2 status, grade and pathological stage. The median follow-up times for each patient subset are shown in Table 1.
CTCs, IE/FC.
Patients positive for CTCs (>0.44 CTCs/mL) had significantly reduced DRFS (HR 2.16 p=0.0189), BCSS (HR 3.63, p=0.0021), and OS (HR 1.79, p=0.0235) (Figure 1A). In multivariate models, CTCs remained prognostic for BCSS (HR 3.54, p=0.0138), and OS (HR 1.89, p=0.0301) (Table 2, Supplementary Table 5).
Figure 1. Survival curves according to CTC and DTC status in all patients.
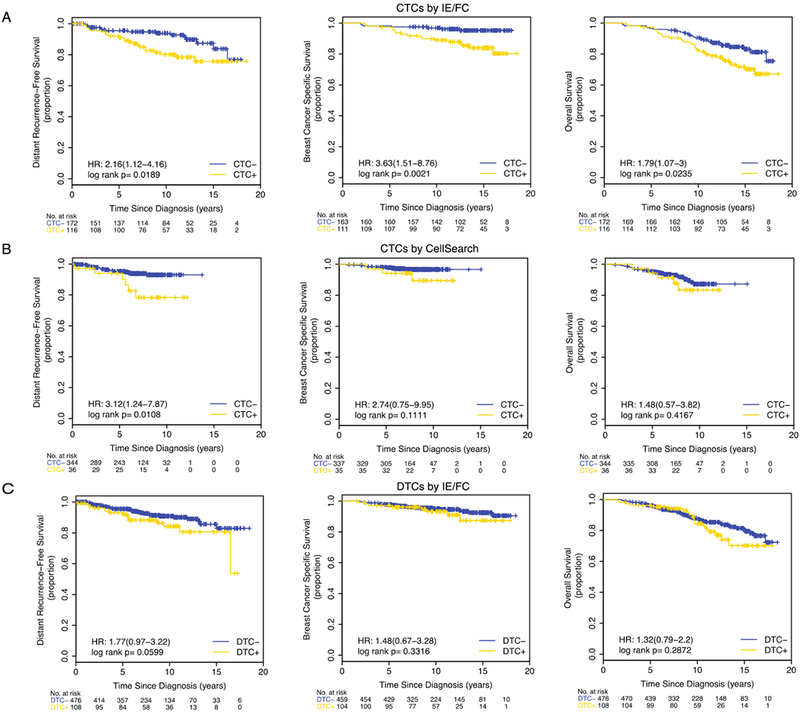
Kaplan-Meier plots are shown for the following subsets: (A) CTCs by IE/FC (cutoff >0.44 CTC per mL), (B) CTCs detected by CellSearch (cutoff≥2 CTC per 7.5 mLs), and (C) DTCs by IE/FC (cutoff >18.61 DTC per mL).
Table 2. CTC and DTC detection and clinical outcomes.
Multivariate Cox regression analysis was performed to estimate hazard ratios (HR) and 95% confidence intervals (CI) for distant recurrence-free survival (DRFS), breast cancer-specific survival (BCSS), and overall survival (OS). The model adjusted for age at diagnosis, tumor size, nodal status, hormone receptor (HRS) and HER2 status, grade and pathological stage. (also see Supplementary Table 5). Numbers in bold (Wald p <0.05) were considered statistically significant. HRS-hormone receptor-positive status, HR-hazard ratio, CI-confidence interval.
| DRFS | BCCS | OS | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell type | Method | Cutoff | Groups | HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value |
| CTC | IE/FC | >0.44 cells/mL | All n=288 | 1.92[0.93-3.95] | 0.0759 | 3.54[1.29-9.72] | 0.0138 | 1.89[1.06-3.39] | 0.0301 |
| HRS-positive n=249 | 1.75 [0.84-3.64] | 0.1317 | 2.80 [1.02-7.69] | 0.0454 | 1.72 [0.93-3.17] | 0.0830 | |||
| CTC | CellSearch | ≥2 cells per 7.5 mLs | All n=380 | 4.93[1.56-15.6] | 0.0067 | 4.50[0.76-26.5] | 0.0962 | 1.62[0.53-4.89] | 0.3924 |
| HRS-positive n=328 | 21.2 [4.25-105.3] | 0.0002 | 9.94 [1.43-69.18] | 0.0204 | 2.04 [0.62-6.73] | 0.2398 | |||
| DTC | IE/FC | >18.61 cells/mL | All n=584 | 1.46[0.75-2.81] | 0.2631 | 1.48[0.64-3.42] | 0.3542 | 1.24[0.71-2.15] | 0.4491 |
| HRS-positive n=507 | 1.40[0.69-2.83] | 0.3563 | 1.56 [0.64-3.83] | 0.3263 | 1.46 [0.81-2.63] | 0.2103 | |||
CTCs, CellSearch.
Using the cutoff of ≥1 CTC per 7.5 mLs, no significant correlation between CTCs and any of the survival endpoints was observed (Supplementary Figure 4A). However, when the cutoff of ≥2 CTCs per 7.5 mLs was used, we observed significantly shorter DRFS in patients who were CTC-positive compared to those who were CTC-negative (HR 3.12, p=0.0108) (Figure 1B). Multivariate analyses confirmed the prognostic significance of CTCs in predicting DRFS (HR 4.93, p=0.0067) (Table 2, Supplementary Table 5).
DTCs, IE/FC.
Survival analysis suggested a trend towards shorter DRFS in DTC-positive patients (log rank p=0.0599) (Figure 1C). Univariate Cox regression analysis also showed a trend towards reduced DRFS in DTC-positive patients compared to those who were DTC-negative (HR 1.77, p=0.0634) (Supplementary Table 4).
Synchronous detection of CTCs and DTCs by IE/FC predicts poor clinical outcomes
Next, we examined whether simultaneous detection of CTCs and DTCs predicted survival. Paired CTC (by CellSearch) and DTC data was available for 246 patients. Results of the survival analysis was inconclusive due to the relatively small size of the double positive group [4 CTC+/DTC+ (2%) vs. 183 CTC-/DTC- (74%), 38 CTC-/DTC+ (15%), and 21 CTC+/DTC- (9%)].
Using the optimized cutoffs for IE/FC, we categorized the 273 patients with paired DTC and CTC data (by IE/FC) into 4 groups: 136 CTC-/DTC- (50%), 26 CTC-/DTC+ (10%), 88 CTC+/DTC- (32%), and 23 CTC+DTC+ (8%). We found that the CTC+DTC+ group had the highest proportion of distant recurrence and deaths (Supplementary Figure 5A). Log rank tests revealed significant differences in DRFS (p=0.0048), BCSS (p=0.0106) and OS (p=0.0132) among the four groups (Figure 2A). Multivariate analysis showed that patients who were positive for both CTCs and DTCs (CTC+/DTC+) had inferior DRFS (HR 3.09, p=0.0270), BCSS (HR 4.55, p=0.0205), and OS (HR 2.70, p=0.0168) compared to those in the CTC-DTC- group (Table 3).
Figure 2. Synchronous detection of CTCs and DTCs by IE/FC identifies patients with increased risk of distant recurrence and death.
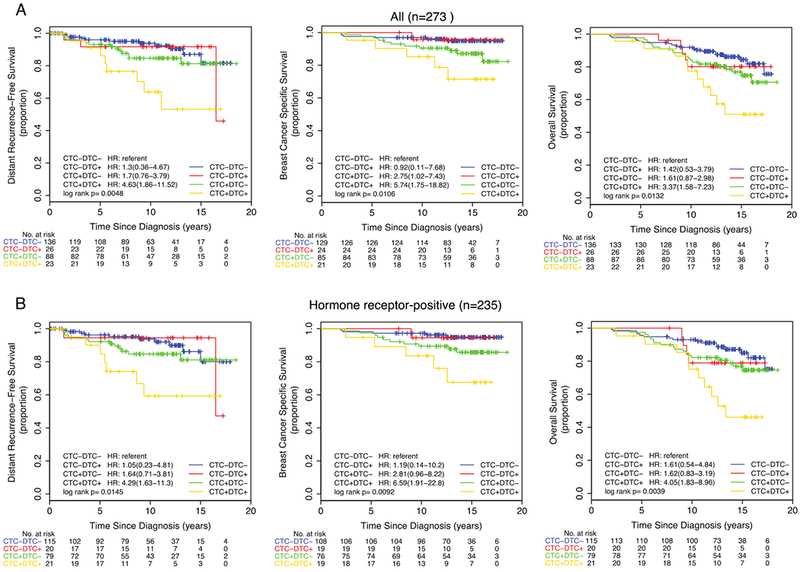
(A) All patients (n=273), (B) Hormone receptor-positive subset (n=235). Dichotomization into positive and negative status was based on the optimized cutoff value of >0.44 CTCs per mL and >18.61 DTCs per mL. Kaplan-Meier plots for distant recurrence-free survival (DRFS), breast cancer-specific survival (BCSS), and overall survival (OS) are shown.
Table 3. Simultaneous detection of CTCs in blood and DTCs in bone marrow by IE/FC predicts increased risked of distant recurrence and death.
Multivariate Cox regression analysis was performed to estimate hazard ratios (HR) and 95% confidence intervals (CI) for distant recurrence-free survival (DRFS), breast cancer-specific survival (BCSS), and overall survival (OS). The model adjusted for age at diagnosis, tumor size, nodal status, hormone receptor (HRS) and HER2 status, grade and pathological stage. CTC- and DTC- positivity were determined based on the optimal cutoffs of >0.44 CTCs/mL and 18.61 DTCs/mL. Numbers in bold are considered statistically significant (Wald p <0.05).
| All patients with paired data (n=273) | HR-positive patients with paired data (n=235) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DRFS | BCSS | OS | DRFS | BCSS | OS | |||||||
| HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | HR [95% CI] | p-value | |
| CTC−DTC+ vs. CTC−DTC− | 1.14 [0.30-4.34] | 0.8515 | 0.72[0.08-6.39] | 0.7693 | 1.03[0.32-3.28] | 0.9589 | 0.94 [0.2-4.51] | 0.9405 | 0.98 [0.11-8.73] | 0.9878 | 1.54 [0.43-5.59] | 0.5104 |
| CTC+DTC− vs. CTC−DTC− | 1.64[0.69-3.88] | 0.2597 | 2.74[0.89-8.44] | 0.0789 | 1.68[0.86-2.39] | 0.1299 | 1.38 [0.58-3.3] | 0.4672 | 2.16 [0.68-6.84] | 0.1892 | 1.48 [0.72-3.07] | 0.2898 |
| CTC+DTC+ vs. CTC−DTC− | 3.09[1.14-8.40] | 0.0270 | 4.55[1.26-16.39] | 0.0205 | 2.70[1.20-6.09] | 0.0168 | 3.05 [1.12-8.25] | 0.0285 | 3.90 [1.1-13.84] | 0.0355 | 3.03 [1.32-6.98] | 0.0091 |
| Age at Diagnosis (continuous) | 1.02[0.98-1.06] | 0.3342 | 1.02[0.97-1.07] | 0.4775 | 1.05[1.02-1.08] | 0.001 | 1.04 [0.99-1.08] | 0.0925 | 1.04 [0.99-1.10] | 0.1158 | 1.06 [1.02-1.1] | 0.0011 |
| Tumor size (cm) at surgery (continuous) | 1.10[0.89-1.36] | 0.3587 | 1.08[0.84-1.40] | 0.5303 | 0.95[0.74-1.22] | 0.6934 | 1.11 [0.9-1.37] | 0.3437 | 1.12 [0.87-1.44] | 0.3918 | 0.97 [0.76-1.24] | 0.8244 |
| Stage ll/lll vs. Stage 0/1 | 2.83[0.93-8.55] | 0.0658 | 2.36[0.55-10.12] | 0.2462 | 2.43 [1.02-5.78] | 0.0449 | 2.21 [0.65-7.51] | 0.2020 | 1.24 [0.25-6.0] | 0.7920 | 1.73 [0.62-4.82] | 0.2935 |
| HRS+ vs. HRS− | 2.31[0.47-11.45] | 0.3055 | 2.84[0.31-25.75] | 0.3523 | 1.08[0.39-2.95] | 0.8855 | ||||||
| HER2+ VS.HER2− | 1.85[0.78-4.41] | 0.1645 | 1.49[0.53-4.16] | 0.4518 | 1.76[0.83-3.73] | 0.1438 | 2.5 [1.05-5.95] | 0.0383 | 1.82 [0.64-5.23] | 0.2643 | 2.55 [1.17-5.56] | 0.0188 |
| Node+ vs. Node− | 1.61[0.61-4.24] | 0.3320 | 3.40[0.93-12.40] | 0.0643 | 1.59[0.73-3.46] | 0.2436 | 2.51 [0.84-7.5] | 0.0996 | 6.77 [1.44-31.85] | 0.0155 | 2.45 [0.97-6.19] | 0.0576 |
| Grade 2 vs. Grade 1 | 1.47[0.59-3.65] | 0.4039 | 2.77[0.80-9.55] | 0.1063 | 1.31[0.65-2.64] | 0.443 | 1.53 [0.61-3.83] | 0.3607 | 2.82 [0.82-9.77] | 0.1015 | 1.2 [0.57-2.51] | 0.6292 |
| Grade 3 vs. Grade 1 | 1.63[0.62-4.29] | 0.3256 | 3.20[0.87-11.79] | 0.0807 | 1.46[0.66-3.25] | 0.3508 | 1.51 [0.57-4.04] | 0.4095 | 3.28 [0.87-12.33] | 0.0783 | 1.38 [0.60-3.17] | 0.4472 |
Clinical significance of CTCs and DTCs by hormone receptor status
The study cohort consisted of 87% hormone receptor-positive (n=645) and 13% hormone receptor-negative patients (n=92) (Table 1, Supplementary Figure 1B). We evaluated the prognostic impact of CTCs and DTCs in these two subsets using the same analysis approach performed on the entire cohort. The number of distant recurrences and deaths in each group are found in Supplementary Table 6.
For hormone receptor-positive patients, we observed the following:
CTCs, IE/FC.
Patients positive for CTCs (>0.44 CTCs/mL) had significantly reduced DRFS (HR 2.12 p=0.0311), BCSS (HR 3.78 p=0.0028), and OS (HR 1.88 p=0.0233) (Figure 3A). After adjusting for potential confounders, CTCs remained prognostic for BCSS (HR 2.80, p=0.0454) (Table 2, Supplementary Table 5).
Figure 3. Survival curves according to CTC and DTC status in hormone receptor-positive patients.
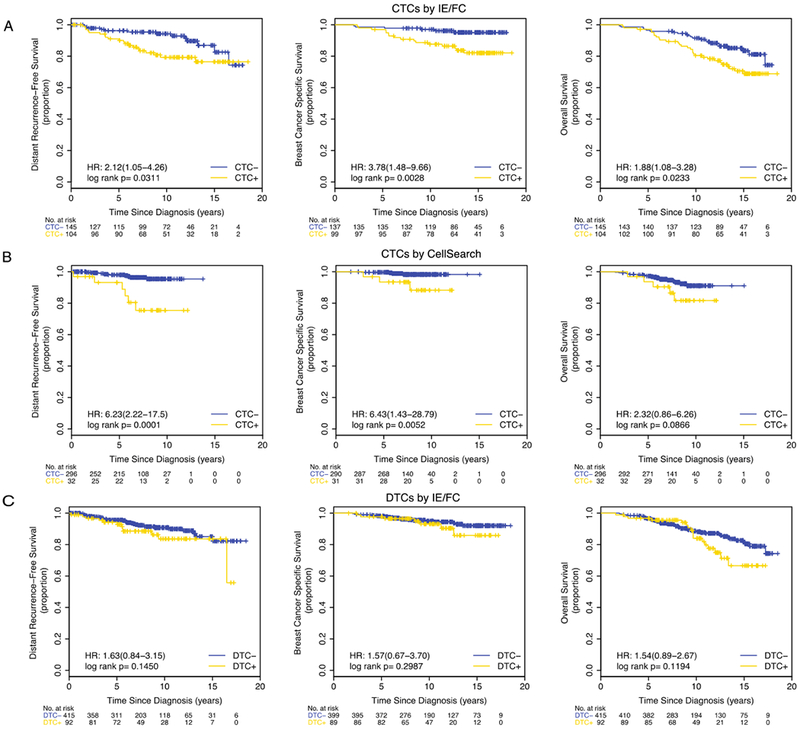
Kaplan-Meier plots are shown for the following subsets: (A) CTCs by IE/FC (cutoff >0.44 CTC per mL), (B) CTCs detected by CellSearch (cutoff ≥2 CTC per 7.5 mLs), and (C) DTCs by IE/FC (cutoff >18.61 DTC per mL).
CTCs, CellSearch.
Using the cutoff of ≥1 CTC per 7.5 mLs, we observed a significant association between CTC-positivity and reduced DRFS (HR 2.88, p=0.0322) (Supplementary Figure 4B). Similarly, CTC-positivity using the cutoff of ≥2 CTC per 7.5 mLs was significantly associated with shorter DRFS (HR 6.23, p=0.0001) and BCSS (HR 6.43, p=0.0052) (Figure 3B). After multivariate analyses, CTCs remained significant predictors of DRFS (HR 21.2, p=0.0002) and BCSS (HR 9.94, p=0.0204) (Table 2, Supplementary Table 5).
DTCs, IE/FC.
No significant prognostic impact was observed for DTCs (Figure 3C, Table 2, Supplementary Table 5).
Synchronous detection of CTCs and DTCs by IE/FC in hormone receptor-positive patients.
Using the optimized cutoffs for IE/FC, we categorized the 235 patients with paired DTC and CTC data (by IE/FC) into 4 groups: 116 CTC-DTC- (49%), 20 CTC-/DTC+ (9%), 78 CTC+/DTC- (33%), and 21 CTC+/DTC+ (9%). The CTC+/DTC+ group had the highest proportion of distant recurrence and deaths (Supplementary Figure 5B). Log rank tests revealed significant differences in DRFS (p=0.0145), BCSS (p=0.0092) and OS (p=0.0039) among the four groups (Figure 2B). Multivariate analysis showed that patients who were positive for both CTCs and DTCs (CTC+/DTC+) had inferior DRFS (HR 3.05, p=0.0285), BCSS (HR 3.90, p=0.0355), and OS (HR 3.03, p=0.0091) compared to those in the CTC-/DTC- group (Table 3).
We did not observe significant correlation between CTCs and DTCs vs. survival endpoints in the much smaller hormone receptor-negative subset (Supplementary Figure 6).
DISCUSSION
CTC and DTC assessment can facilitate precision medicine-based management of early breast cancer patients by identifying those with increased risk of metastatic recurrence. In this study, we used two clinically validated, EPCAM-based rare cell detection platforms for CTC enumeration: CellSearch (13–17,25,26) and IE/FC (18–22). Our previous studies in triple-negative metastatic breast cancer have demonstrated high concordance between these methods (20). A major difference between the two is that IE/FC has been validated for detection of both CTCs (18–20) and DTCs (18,21,22), while the current configuration of CellSearch only allows for CTC enumeration. One advantage of CTC or DTC detection by IE/FC is that it can be performed in concert with fluorescence-activated cell sorting (FACS) for isolation of the tumor cells. This capability can in turn provide detailed genomic and phenotypic profiling for personalized medicine applications (19,22,27–31).
In this study, CTCs and DTCs were simultaneously enumerated in a cohort of early breast cancer patients. We found 12 studies published between 1997-2018 that reported simultaneous CTC and DTC detection at the time of breast surgery (9,10,32–41) (Supplementary Table 7). Of these, two assessed the prognostic impact of combined CTC and DTC detection (9,34); however, these two studies did not compare quantitative results (e.g., tumor cells per mL or tumor cells per 10^6 leukocytes) for both DTCs and CTCs. A fully quantitative assay, like IE/FC, is of interest because it enables enumeration of tumor cells from each compartment, i.e., blood and bone marrow, and can report both CTCs/DTCs per mL as well as CTCs/DTCs per 106 MNCs.
We found that DTCs were present at generally higher levels than CTCs, including higher mean concentration and larger range. The higher levels of DTCs in marrow relative to CTCs in blood suggests that tumor cell dissemination is not merely stochastic, and that there may be an intrinsic difference in the biology of tumor cell localization to each compartment.
CTC detection by IE/FC was performed at the study outset, and thus the median follow-up for this cohort was particularly long (13.3 years). We found that CTC-positivity by IE/FC in all patients as well as in the hormone receptor-positive group was significantly associated with reduced BCSS and OS.
CTC detection by CellSearch, which was implemented later in the study, was significantly associated with poor DRFS (median follow-up 6.4 years) in all patients, as well as the hormone receptor-positive subset. Janni and colleagues using the CellSearch system previously demonstrated that CTCs in hormone receptor-positive early breast cancer are a significant prognostic factor for OS (5). In addition, Sparano et al (42) recently reported that detection of CTCs by CellSearch five years after diagnosis of hormone receptor-positive breast cancer is associated with increased recurrence risk.
We observed a DTC-positivity rate of 68% before cutoff optimization. In a large study that used ICC-based assay for detection of DTCs, the detection rate was 31% (6). The higher detection rate by IE/FC compared to the standard ICC method is likely due, at least in part, to the total number of cells analyzed in each assay. The standard ICC protocol for DTC detection typically examines about 4-8 million mononuclear cells per sample (11), while IE/FC examines approximately 176 million mononuclear cells per sample (4 mLs of bone marrow); this is a >20-fold larger number of cells analyzed compared to that of the standard ICC assay.
DTC positivity by itself was not significantly correlated with survival in this study. However, when CTC and DTC status (both by IE/FC) were simultaneously considered, we found that positive detection for both CTCs and DTCs (CTC+DTC+) in all patients as well as in the hormone receptor-positive subset, was significantly associated with poor outcome. CTC+DTC+ patients had significantly shorter DRFS, BCSS, and OS compared to those who were CTC-DTC-. These results suggest that assessment of CTC and DTC status at surgery in early breast cancer patients may help identify those who are at increased risk of distant recurrence and death due to breast cancer.
Our study observed that detection of CTCs in HR-positive early breast cancer patients was an independent prognostic factor for DRFS (using CellSearch) and BCSS (using IE/FC). Simultaneous detection of DTCs provided additional prognostic power for outcome, including OS. These results are consistent with previous reports in which detection of DTCs (3,6) and CTCs (5,14,15,24,43) have separately been demonstrated to have prognostic significance in early breast cancer. Although these methods have not yet become standard clinical tests for early breast cancer, it is possible that they may provide information about metastatic potential that complements existing tumor profiling assays.
Molecular characterization of CTCs and DTCs may provide novel insights into mechanisms of tumor dormancy, metastatic spread, and cancer recurrence. In this regard, we have previously reported strategies for molecular characterization of IE/FC-isolated CTCs and DTCs, and have confirmed the malignant nature of these cells (19,22,27–31).
In addition to EPCAM, cytokeratin expression has also been used for detecting cancer cells in the blood (e.g., CellSearch) and bone marrow (e.g., standard immunocytochemistry, ICC) (11,44). In this study, we used the CellSearch system for CTC detection, which combines anti-EPCAM immunomagnetic enrichment with anti-cytokeratin ICC. In addition, we utilized the IE/FC strategy which is based on dual epitope EPCAM capture. This approach offers several potential advantages: First, the assay configuration targets EPCAM in both immunomagnetic enrichment and flow cytometric steps, using two independent monoclonal antibodies. This eliminates the possibility of missing tumor cells that fail to show adequate expression of two different antigens, such as EPCAM and selected cytokeratins, especially since breast cancer cells vary in their cytokeratin expression profile (45). Note that EPCAM-negative tumors will be missed by any strategy relying upon anti-EPCAM enrichment; however, since 90% of invasive breast cancer expressed EPCAM (46), and primary and metastatic breast cancer cells overexpress EPCAM by 100-1000 fold (47), there is only a small possibility of missing breast cancer tumor cells. The IE/FC assay configuration also obviates the need for a permeabilization step to stain for intracellular cytokeratin antigens. Since detergent-based permeabilization may affect the suitability of cells for downstream analyses, the assay described here minimizes such manipulation by direct staining of intact cells prior to acquisition. Nonetheless, tumor cells that express EPCAM at low levels — e.g., those undergoing epithelial-mesenchymal transition (EMT) — will be missed by IE/FC and CellSearch, and thus represents a limitation of this study. While numerous clinical studies have demonstrated that EPCAM-positive CTCs are unequivocally associated with poor response and survival (5,14,15), the clinical relevance of EPCAM-negative tumor cells in circulation remains unclear (48).
In summary, we show that CTC detection either by CellSearch or IE/FC are adverse prognostic factors for distant recurrence and death. We also demonstrate the feasibility of simultaneous enumeration of CTCs and DTCs using the same quantitative IE/FC approach. With long follow-up (up to a median of 13.3 years), we show that detection of CTCs and DTCs at the time of surgery in hormone receptor-positive early breast cancer patients is an independent prognostic factor for distant recurrence and breast cancer-specific death. Given the lack of early endpoints in this low-risk subtype, liquid biopsy may be an important consideration for future studies. Validation in an independent cohort is warranted to confirm the results of this study.
Supplementary Material
STATEMENT OF TRANSLATIONAL RELEVANCE.
Biomarkers for robust risk stratification are needed for optimal cancer management and treatment selection. Liquid biopsy-based markers, e.g., circulating tumor cells (CTC) in blood and disseminated tumor cells (DTC) in bone marrow may have the potential to address this need. To our knowledge, we report for the first time, the assessment of prognostic impact of synchronous detection of CTCs and DTCs in a large patient cohort with long clinical follow-up. Using the same assay system, we observed that CTCs and DTCs detected at surgery in untreated early breast cancer patients significantly predicted distant recurrence and breast cancer-specific death. Liquid biopsy can in principle complement tissue-based prognostic markers to identify patients who have elevated risk of metastatic relapse and death due to breast cancer.
ACKNOWLEDGMENTS
We acknowledge the outstanding assistance of Margot Paisley, Alvina Leung, Alison Lozner, Teresa Seo, Kavitha Krishnan, Laura Petrillo, Amy Moore, Jasmine Wong, Hope Timberlake, and Richard Hwang in coordinating the clinical studies; Ann Griffin and Joseph McGuire for information on patient follow-up.
This work was supported by University of California BioStar (S97-49, B99-55); National Institutes of Health/National Cancer Institute (U54 CA90788, Early Detection Research Network (EDRN) U01 CA111234); Breast Cancer Research Foundation [MJM (BCRF-17-140) and DMW and LVV (BCRF-17-162)].
Footnotes
Conflict of interest: JWP has received research funding from Veridex (now Menarini), the rest of the co-authors have declared no conflict interest.
REFERENCES
- 1.Pan H, Gray R, Braybrooke J, Davies C, Taylor C, McGale P, et al. 20-Year Risks of Breast-Cancer Recurrence after Stopping Endocrine Therapy at 5 Years. N Engl J Med 2017;377(19):1836–46 doi 10.1056/NEJMoa1701830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sosa MS, Bragado P, Aguirre-Ghiso JA. Mechanisms of disseminated cancer cell dormancy: an awakening field. Nat Rev Cancer 2014;14(9):611–22 doi 10.1038/nrc3793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naume B, Synnestvedt M, Falk RS, Wiedswang G, Weyde K, Risberg T, et al. Clinical outcome with correlation to disseminated tumor cell (DTC) status after DTC-guided secondary adjuvant treatment with docetaxel in early breast cancer. J Clin Oncol 2014;32(34):3848–57 doi 10.1200/JCO.2014.56.9327. [DOI] [PubMed] [Google Scholar]
- 4.Bidard FC, Peeters DJ, Fehm T, Nole F, Gisbert-Criado R, Mavroudis D, et al. Clinical validity of circulating tumour cells in patients with metastatic breast cancer: a pooled analysis of individual patient data. Lancet Oncol 2014;15(4):406–14 doi 10.1016/S1470-2045(14)70069-5. [DOI] [PubMed] [Google Scholar]
- 5.Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, Fehm T, et al. Pooled Analysis of the Prognostic Relevance of Circulating Tumor Cells in Primary Breast Cancer. Clin Cancer Res 2016;22(10):2583–93 doi 10.1158/1078-0432.CCR-15-1603. [DOI] [PubMed] [Google Scholar]
- 6.Braun S, Vogl FD, Naume B, Janni W, Osborne MP, Coombes RC, et al. A pooled analysis of bone marrow micrometastasis in breast cancer. N Engl J Med 2005;353(8):793–802. [DOI] [PubMed] [Google Scholar]
- 7.Magbanua MJ, Das R, Polavarapu P, Park JW. Approaches to isolation and molecular characterization of disseminated tumor cells. Oncotarget 2015;6(31):30715–29 doi 10.18632/oncotarget.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene 2016;35(10):1216–24 doi 10.1038/onc.2015.192. [DOI] [PubMed] [Google Scholar]
- 9.Molloy TJ, Bosma AJ, Baumbusch LO, Synnestvedt M, Borgen E, Russnes HG, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res 2011;13(3):R61 doi 10.1186/bcr2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ismail MS, Wynendaele W, Aerts JL, Paridaens R, Gaafar R, Shakankiry N, et al. Detection of micrometastatic disease and monitoring of perioperative tumor cell dissemination in primary operable breast cancer patients using real-time quantitative reverse transcription-PCR. Clin Cancer Res 2004;10(1 Pt 1):196–201. [DOI] [PubMed] [Google Scholar]
- 11.Fehm T, Braun S, Muller V, Janni W, Gebauer G, Marth C, et al. A concept for the standardized detection of disseminated tumor cells in bone marrow from patients with primary breast cancer and its clinical implementation. Cancer 2006;107(5):885–92 doi 10.1002/cncr.22076. [DOI] [PubMed] [Google Scholar]
- 12.Park JW. Disseminated tumor cells: the method is the message. Breast Cancer Res Treat 2010. doi 10.1007/s10549-010-1107-5. [DOI] [PubMed] [Google Scholar]
- 13.Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med 2004;351(8):781–91. [DOI] [PubMed] [Google Scholar]
- 14.Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol 2012;13(7):688–95 doi 10.1016/S1470-2045(12)70209-7. [DOI] [PubMed] [Google Scholar]
- 15.Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst 2014;106(5) doi 10.1093/jnci/dju066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cristofanilli M, Hayes DF, Budd GT, Ellis MJ, Stopeck A, Reuben JM, et al. Circulating tumor cells: a novel prognostic factor for newly diagnosed metastatic breast cancer. J Clin Oncol 2005;23(7):1420–30. [DOI] [PubMed] [Google Scholar]
- 17.Liu MC, Shields PG, Warren RD, Cohen P, Wilkinson M, Ottaviano YL, et al. Circulating tumor cells: a useful predictor of treatment efficacy in metastatic breast cancer. J Clin Oncol 2009;27(31):5153–9 doi 10.1200/JCO.2008.20.6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Magbanua MJM, Solanki TI, Ordonez AD, Hsiao F, Park JW. Enumeration of Circulating Tumor Cells and Disseminated Tumor Cells in Blood and Bone Marrow by Immunomagnetic Enrichment and Flow Cytometry (IE/FC). Methods Mol Biol 2017;1634:203–10 doi 10.1007/978-1-4939-7144-2_17. [DOI] [PubMed] [Google Scholar]
- 19.Magbanua MJM, Rugo HS, Wolf DM, Hauranieh L, Roy R, Pendyala P, et al. Expanded Genomic Profiling of Circulating Tumor Cells in Metastatic Breast Cancer Patients to Assess Biomarker Status and Biology Over Time (CALGB 40502 and CALGB 40503, Alliance). Clin Cancer Res 2018;24(6):1486–99 doi 10.1158/1078-0432.CCR-17-2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Magbanua MJ, Carey LA, DeLuca A, Hwang J, Scott JH, Rimawi MF, et al. Circulating tumor cell analysis in metastatic triple-negative breast cancers. Clin Cancer Res 2015;21(5):1098–105 doi 10.1158/1078-0432.CCR-14-1948. [DOI] [PubMed] [Google Scholar]
- 21.Campbell MJ, Scott J, Maecker HT, Park JW, Esserman LJ. Immune dysfunction and micrometastases in women with breast cancer. Breast Cancer Res Treat 2005;91(2):163–71 doi 10.1007/s10549-004-7048-0. [DOI] [PubMed] [Google Scholar]
- 22.Magbanua MJM, Rugo HS, Hauranieh L, Roy R, Scott JH, Lee JC, et al. Genomic and expression profiling reveal molecular heterogeneity of disseminated tumor cells in bone marrow of early breast cancer. npj Breast Cancer 2018;4(1):31 doi 10.1038/s41523-018-0083-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res 2007;13(3):920–8 doi 10.1158/1078-0432.CCR-06-1695. [DOI] [PubMed] [Google Scholar]
- 24.Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating Tumor Cells in Breast Cancer Patients Treated by Neoadjuvant Chemotherapy: A Meta-analysis. J Natl Cancer Inst 2018. doi 10.1093/jnci/djy018. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Riethdorf S, Wu G, Wang T, Yang K, Peng G, et al. Meta-analysis of the prognostic value of circulating tumor cells in breast cancer. Clin Cancer Res 2012;18(20):5701–10 doi 10.1158/1078-0432.CCR-12-1587. [DOI] [PubMed] [Google Scholar]
- 26.Ignatiadis M, Xenidis N, Perraki M, Apostolaki S, Politaki E, Kafousi M, et al. Different prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancer. J Clin Oncol 2007;25(33):5194–202 doi 10.1200/JCO.2007.11.7762. [DOI] [PubMed] [Google Scholar]
- 27.Gulbahce N, Magbanua MJM, Chin R, Agarwal MR, Luo X, Liu J, et al. Quantitative Whole Genome Sequencing of Circulating Tumor Cells Enables Personalized Combination Therapy of Metastatic Cancer. Cancer Res 2017;77(16):4530–41 doi 10.1158/0008-5472.CAN-17-0688. [DOI] [PubMed] [Google Scholar]
- 28.Lang JE, Ring A, Porras T, Kaur P, Forte VA, Mineyev N, et al. RNA-Seq of Circulating Tumor Cells in Stage II-III Breast Cancer. Annals of surgical oncology 2018;25(8):2261–70 doi 10.1245/s10434-018-6540-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magbanua MJ, Sosa EV, Roy R, Eisenbud LE, Scott JH, Olshen A, et al. Genomic profiling of isolated circulating tumor cells from metastatic breast cancer patients. Cancer Res 2013;73(1):30–40 doi 10.1158/0008-5472.CAN-11-3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paszek MJ, DuFort CC, Rossier O, Bainer R, Mouw JK, Godula K, et al. The cancer glycocalyx mechanically primes integrin-mediated growth and survival. Nature 2014;511(7509):319–25 doi 10.1038/nature13535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ring A, Mineyev N, Zhu W, Park E, Lomas C, Punj V, et al. EpCAM based capture detects and recovers circulating tumor cells from all subtypes of breast cancer except claudin-low. Oncotarget 2015;6(42):44623–34 doi 10.18632/oncotarget.5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walter VP, Taran FA, Wallwiener M, Hahn M, Brucker SY, Hartkopf AD. Simultaneous detection of circulating and disseminated tumor cells in primary breast cancer patients following neoadjuvant chemotherapy. Arch Gynecol Obstet 2018;297(3):785–90 doi 10.1007/s00404-018-4669-9. [DOI] [PubMed] [Google Scholar]
- 33.Kasimir-Bauer S, Reiter K, Aktas B, Bittner AK, Weber S, Keller T, et al. Different prognostic value of circulating and disseminated tumor cells in primary breast cancer: Influence of bisphosphonate intake? Sci Rep 2016;6:26355 doi 10.1038/srep26355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kasimir-Bauer S, Bittner AK, Konig L, Reiter K, Keller T, Kimmig R, et al. Does primary neoadjuvant systemic therapy eradicate minimal residual disease? Analysis of disseminated and circulating tumor cells before and after therapy. Breast Cancer Res 2016;18(1):20 doi 10.1186/s13058-016-0679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hartkopf AD, Wallwiener M, Hahn M, Fehm TN, Brucker SY, Taran FA. Simultaneous Detection of Disseminated and Circulating Tumor Cells in Primary Breast Cancer Patients. Cancer research and treatment : official journal of Korean Cancer Association 2016;48(1):115–24 doi 10.4143/crt.2014.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schindlbeck C, Andergassen U, Hofmann S, Juckstock J, Jeschke U, Sommer H, et al. Comparison of circulating tumor cells (CTC) in peripheral blood and disseminated tumor cells in the bone marrow (DTC-BM) of breast cancer patients. Journal of cancer research and clinical oncology 2013;139(6):1055–62 doi 10.1007/s00432-013-1418-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mathiesen RR, Borgen E, Renolen A, Lokkevik E, Nesland JM, Anker G, et al. Persistence of disseminated tumor cells after neoadjuvant treatment for locally advanced breast cancer predicts poor survival. Breast Cancer Res 2012;14(4):R117 doi 10.1186/bcr3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fehm T, Hoffmann O, Aktas B, Becker S, Solomayer EF, Wallwiener D, et al. Detection and characterization of circulating tumor cells in blood of primary breast cancer patients by RT-PCR and comparison to status of bone marrow disseminated cells. Breast Cancer Res 2009;11(4):R59 doi 10.1186/bcr2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benoy IH, Elst H, Philips M, Wuyts H, Van Dam P, Scharpe S, et al. Real-time RT-PCR detection of disseminated tumour cells in bone marrow has superior prognostic significance in comparison with circulating tumour cells in patients with breast cancer. Br J Cancer 2006;94(5):672–80 doi 10.1038/sj.bjc.6602985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berois N, Varangot M, Aizen B, Estrugo R, Zarantonelli L, Fernandez P, et al. Molecular detection of cancer cells in bone marrow and peripheral blood of patients with operable breast cancer. Comparison of CK19, MUC1 and CEA using RT-PCR. Eur J Cancer 2000;36(6):717–23. [DOI] [PubMed] [Google Scholar]
- 41.Schoenfeld A, Kruger KH, Gomm J, Sinnett HD, Gazet JC, Sacks N, et al. The detection of micrometastases in the peripheral blood and bone marrow of patients with breast cancer using immunohistochemistry and reverse transcriptase polymerase chain reaction for keratin 19. Eur J Cancer 1997;33(6):854–61. [DOI] [PubMed] [Google Scholar]
- 42.Sparano J, O’Neill A, Alpaugh K, Wolff AC, Northfelt DW, Dang CT, et al. Association of Circulating Tumor Cells With Late Recurrence of Estrogen Receptor-Positive Breast Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol 2018. doi 10.1001/jamaoncol.2018.2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Franken B, de Groot MR, Mastboom WJ, Vermes I, van der Palen J, Tibbe AG, et al. Circulating tumor cells, disease recurrence and survival in newly diagnosed breast cancer. Breast Cancer Res 2012;14(5):R133 doi 10.1186/bcr3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coumans FA, Doggen CJ, Attard G, de Bono JS, Terstappen LW. All circulating EpCAM+CK+CD45- objects predict overall survival in castration-resistant prostate cancer. Ann Oncol 2010;21(9):1851–7 doi 10.1093/annonc/mdq030. [DOI] [PubMed] [Google Scholar]
- 45.Jarasch ED, Nagle RB, Kaufmann M, Maurer C, Bocker WJ. Differential diagnosis of benign epithelial proliferations and carcinomas of the breast using antibodies to cytokeratins. Hum Pathol 1988;19(3):276–89. [DOI] [PubMed] [Google Scholar]
- 46.Spizzo G, Went P, Dirnhofer S, Obrist P, Simon R, Spichtin H, et al. High Ep-CAM expression is associated with poor prognosis in node-positive breast cancer. Breast Cancer Res Treat 2004;86(3):207–13. [DOI] [PubMed] [Google Scholar]
- 47.Osta WA, Chen Y, Mikhitarian K, Mitas M, Salem M, Hannun YA, et al. EpCAM is overexpressed in breast cancer and is a potential target for breast cancer gene therapy. Cancer Res 2004;64(16):5818–24. [DOI] [PubMed] [Google Scholar]
- 48.de Wit S, van Dalum G, Lenferink AT, Tibbe AG, Hiltermann TJ, Groen HJ, et al. The detection of EpCAM(+) and EpCAM(−) circulating tumor cells. Sci Rep 2015;5:12270 doi 10.1038/srep12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


