Abstract
Objective:
Thiamine deficiency may propagate lactate production by limiting pyruvate dehydrogenase activity, and studies suggest benefit for thiamine administration in septic adults. We studied the effect of thiamine on physiological and clinical outcomes for children with septic shock and hyperlactatemia.
Design:
Retrospective matched cohort study
Setting:
Single academic pediatric intensive care unit
Patients:
Six thiamine-treated cases and nine matched controls
Measurements and Main Results:
The primary outcome was change in blood lactate from pre-thiamine (T0, cases) or maximum (T0, controls) lactate through 24 hours later (T24). Secondary outcomes were change in lactate over 48 (T48) and 72 (T72) hours, time to lactate normalization, changes in vasoactive-inotrope score, organ dysfunction severity (dPELOD-2 score), and creatinine, PICU length of stay, and hospital mortality. Lactate was >5 mmol/L for a median of 39 hours (range 16.1-64.3) prior to thiamine administration for cases compared to 3.4 hours (range 0-22.9) prior to maximum lactate for controls (p=0.002). There was no difference in median (IQR) change in lactate from T0 to T24 between thiamine-treated cases and controls (−9.0, −17.0 to −5.0 versus −7.2, −9.0 to −5.3 mmol/L, p=0.78), with both groups exhibiting a rapid decrease in lactate. There were also no differences in secondary outcomes between groups.
Conclusions:
Treatment of pediatric septic shock with thiamine was followed by rapid improvement in physiological and clinical outcomes after prolonged hyperlactatemia. Although we are not able to infer that thiamine provided benefit over usual care, the rapid decline in lactate after thiamine despite a prolonged period of hyperlactatemia raises the possibility that thiamine helped to reverse lactate production.
Keywords: sepsis, thiamine, pediatric, lactic acidosis, mitochondria, metabolic
INTRODUCTION
Early in septic shock hyperlactatemia largely reflects tissue hypoxia, but persistence of lactic acidosis may signify the inability of tissues to utilize oxygen for mitochondrial oxidative phosphorylation (1). Thiamine is a co-factor for pyruvate dehydrogenase (PDH), which metabolizes pyruvate to fuel oxidative phosphorylation (2). Acute thiamine deficiency can cause an acquired form of PDH deficiency (2-5), and the resultant mitochondrial dysfunction can precipitate a cellular bioenergetic crisis that contributes to organ dysfunction (1, 6). When PDH activity is limited by thiamine availability, pyruvate is alternatively metabolized through lactate dehydrogenase to lactate. Thus, acute thiamine deficiency can precipitate refractory lactic acidosis despite restoration of tissue oxygen delivery (3, 7).
Prior studies suggest benefit of thiamine administration in adult sepsis (8-11), but there are no studies of thiamine treatment in pediatric septic shock. We therefore investigated our experience of the effect of thiamine on outcomes for children with septic shock and persistent hyperlactatemia.
METHODS
We performed a retrospective matched cohort study of patients ≤18 years treated for septic shock in the Children’s Hospital of Philadelphia (CHOP) Pediatric Intensive Care Unit (PICU) between January 1, 2012 and May 15, 2018 (12). Cases had arterial/venous blood lactate ≥5 mmol/L prior to intravenous administration of thiamine for the purpose of reversing hyperlactatemia. Control patients with septic shock, lactate ≥5 mmol/L, and no exposure to thiamine were matched to cases on age, sex, race, illness severity, lactate, presence of arterial catheter, and mechanical ventilation. Exclusion criteria were thiamine for an indication other than hyperlactatemia, intestinal ischemia/infarction, renal replacement therapy, liver failure, or metabolic disorder. The study was approved by the CHOP Institutional Review Board.
The primary outcome was the change in blood lactate over 24 hours. For cases, change in lactate was anchored from the lactate measured most proximal to first thiamine administration (T0) and followed to the lactate closest to 24 hours after first thiamine administration (T24). For controls, T0 was anchored to the maximum lactate. Secondary outcomes were change in lactate over 48 (T48) and 72 (T72) hours, time to lactate <2 mmol/L after T0 (normalization), change in vasoactive-inotrope score (VIS) (13), daily Pediatric Logistic Organ Dysfunction (dPELOD)-2 score (14), and creatinine over 72 hours, PICU length of stay (LOS), extracorporeal membrane oxygenation (ECMO) rescue, and hospital mortality.
Analyses were performed using R 2.13.1 (R Foundation) and STATA (Version 12.1, College Station, TX). Medians with range or interquartile range (IQR) and frequencies were compared using Wilcoxon rank sum and Fisher’s exact tests, respectively. We used an optimal matching algorithm (cardinality matching) based on integer programming to pair cases and controls on pre-specified covariates (15), followed by regression models to adjust for residual covariate imbalances. We first used linear regression to adjust for imbalanced covariates after matching and then applied a Wilcoxon test to the regression model residuals to estimate the difference in medians with 95% confidence interval (CI) between groups for the change in outcomes from T0 to T24, T48, and T72. Statistical significance was defined as a p<0.05.
RESULTS
Six cases and nine controls met inclusion criteria. All patients achieved acceptable hemodynamic targets by T0, as indicated by blood pressure and central venous oxygen saturation (Table 1). Imbalances in site of infection, comorbid genetic syndrome, initial blood lactate and serum creatinine, and treatment with epinephrine necessitated adjustment in statistical models.
Table 1:
Patient Characteristics
| Variablea | Controls (n=9) | Cases (n=6) | P Value |
|---|---|---|---|
| Age, years | 13 (6-16) | 11 (5-12) | 0.32 |
| Sex, male | 3 (33) | 2 (33) | 0.99 |
| Race | 0.99 | ||
| White | 6 (67) | 4 (66) | |
| Black | 2 (22) | 1 (17) | |
| Other | 1 (11) | 1 (17) | |
| Primary Site of Infection | 0.24 | ||
| Blood | 2 (22) | 1 (17) | |
| Respiratory | 3 (33) | 0 | |
| Gastrointestinal | 1 (11) | 1 (17) | |
| Other | 2 (22) | 0 | |
| Unknown | 1 (11) | 4 (67) | |
| Comorbid conditions | |||
| Cancer | 3 (33) | 3 (50) | 0.62 |
| Prematurity <32 weeks | 0 | 0 | -- |
| Chronic ventilation | 0 | 0 | -- |
| Chronic kidney disease | 0 | 0 | -- |
| Congenital heart disease | 1 (11) | 0 | 1.0 |
| Static encephalopathy | 2 (22) | 1 (17) | 1.0 |
| Ventriculoperitoneal shunt | 1 (11) | 1 (17) | 1.0 |
| Genetic syndrome | 3 (33)b | 0 | 0.23 |
| Parenteral nutrition-dependent | 1 (11) | 1 (17) | 1.0 |
| PRISM-III score | 19 (17-22) | 18 (14-27) | 0.81 |
| PIM-2 risk of mortality, % | 1.5 (1.4, 4.4) | 3.6 (2-4.6) | 0.41 |
| Lactate, admission | 3.7 (2.2-9.4) | 7.5 (3.1-10.2) | 0.41 |
| Creatinine, admission | 1.2 (0.6-1.6) | 0.6 (0.3-0.8) | 0.26 |
| Variables at T0 | |||
| Systolic blood pressure, mm Hg | 97 (87-108) | 102 (98-112) | 0.35 |
| Diastolic blood pressure, mm Hg | 50 (48-62) | 60 (50-70) | 0.34 |
| ScvO2, % | 81 (71-87)c | 77 (63-80)c | 0.28 |
| PELOD-2 score | 12 (11-14) | 8 (5-13) | 0.09 |
| Vasoactive-inotrope score | 12 (10-15) | 12.5 (8-104) | 0.65 |
| Epinephrine therapy | 7 (78) | 3 (50) | 0.33 |
| Mechanical ventilation | 6 (67) | 5 (83) | 0.60 |
| Lactate | 11.0 (9.9-12.1) | 11.7 (9.4-22) | 0.72 |
PRISM, pediatric risk of mortality; PIM, pediatric index of mortality; ScvO2, central venous oxygen saturation; PELOD, pediatric logistic organ dysfunction
Data are presented as median (interquartile range) or n (%)
Genetic syndromes included trisomy 21, Aicardi syndrome, and paroxysmal nocturnal hemoglobinuria
ScvO2 measured in six cases and seven controls
Lactate was >5 mmol/L for median of 39 hours (range 16.1–64.3) prior to thiamine administration in cases and 3.4 hours (0–22.9) prior to maximum lactate in controls (p=0.002). The thiamine dose ranged from 1–5 mg/kg/day, was administered 1–2 times/day, and duration of therapy was a median of 9 (range 7–30) days. No adverse effects were attributable to thiamine.
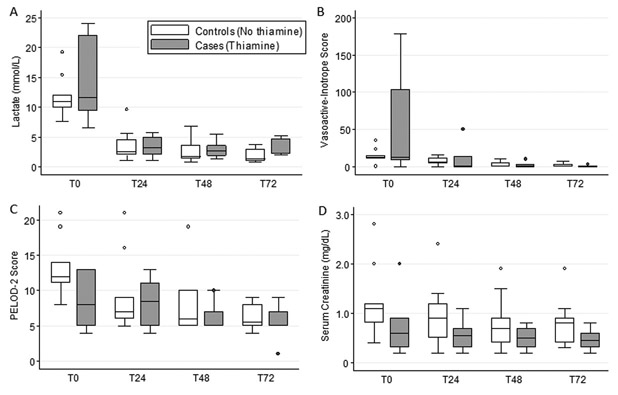
At T0, the median (IQR) lactate for thiamine-treated cases was 11.7 mmol/L (9.4–22.0) and for controls was 11.0 mmol/L (9.9–12.1; p=0.72). There was no difference in the median change in lactate from T0 to T24 between cases (−9.0, IQR −17.0 to −5.0) and controls (−7.2, IQR −9.0 to −5.3 mmol/L) with an adjusted difference in median change of −0.7, 95% CI −2.6, 2.0 (p=0.78). Notably, both groups exhibited a rapid fall in blood lactate (Figure 1A). There were also no between-group differences in lactate change at T48 or T72 (p>0.50 for both). Lactate normalized slightly slower in cases (5.8, IQR 1.8–8.9 days) than in controls (1.7, IQR 1.5–3.6 days), but this difference was not significant (p=0.35).
Figure 1: Blood Lactate Levels and Organ Dysfunction Over Time.
Lactate levels (A) at T0 (most proximal measurement prior to thiamine administration for cases and maximum measurement for controls) and at 24 (T24), 48 (T48), and 72 (T72) hours thereafter according to exposure group. Vasoactive-inotrope scores (B), Pediatric Logistic Organ Dysfunction (PELOD)-2 scores (C), and serum creatinine (D) at T0 through T72 according to exposure group. Vasoactive-inotrope score was calculated as (100 X epinephrine in mcg/kg/min) + (100 X norepinephrine in mcg/kg/min) + dopamine in mcg/kg/min + dobutamine in mcg/kg/min + (10 X vasopressin in mU/kg/min) + (10 X milrinone in mcg/kg/min). Grey boxes are thiamine-treated cases and white boxes are unexposed controls. Data are presented as boxplots with 25th percentile, median, and 75th percentile and whiskers representing the 10th and 90th percentiles. There were no differences in lactate levels between groups at individual timepoints (all p>0.05) and or in the adjusted median change in lactate from T0 to T48 (−0.4, 95% CI −1.9, 1.1; p=0.53) or to T72 between groups (−0.2, 95% CI −1.5, 1.3; p=0.78). There were no differences in vasoactive-inotrope scores, PELOD-2 scores, or creatinine between groups at individual timepoints (all p>0.05) or in the adjusted median changes from T0 through T72 between groups (all p>0.05). Analyses account for matching and are adjusted for residual imbalances in site of infection, comorbid genetic syndrome, initial blood lactate, initial serum creatinine, and treatment with epinephrine at T0.
Change in VIS, dPELOD-2, and creatinine did not differ between cases and controls (Figure 1B-D), nor did PICU LOS (adjusted difference in medians, 9 days fewer in controls [IQR 23 days fewer to 7 days longer]; p=0.39), ECMO rescue (33% versus 33%; p=0.99), or hospital mortality (17% versus 22%; p=0.99).
DISCUSSION
In six children with septic shock and hyperlactatemia, treatment with thiamine was followed by a rapid decrease in lactate and VIS and improvement in dPELOD-2. However, because a matched control group with hyperlactatemia not treated with thiamine exhibited similar outcomes, we are not able to conclude that thiamine conferred benefit over usual care.
Several studies have addressed potential benefit of thiamine in sepsis. Among septic adults with lactate >3 mmol/L, treatment with thiamine lowered lactate, reduced renal replacement therapy, and trended toward lower mortality in the subset with thiamine deficiency (8, 10). Treatment of adult sepsis with hydrocortisone, vitamin C, and thiamine was associated with a 31.9% absolute mortality reduction (9). More recently, very high-dose thiamine (up to 1500 mg/day) was associated with improved lactate clearance and lower mortality in septic adults (11). However, neither adult nor pediatric guidelines currently recommend thiamine in sepsis (16, 17).
There is biologic rationale for thiamine as an adjunctive therapy in pediatric septic shock, especially in those with persistent hyperlactatemia. First, acute thiamine deficiency is evident in 12.5–28.2% of critically ill children at presentation (5, 18), while others are at risk given the limited body storage and high turnover rate of thiamine (half-life <10 days), hypermetabolism, and limited nutrition during the initial phase of critical illness (5, 18, 19). Second, thiamine deficiency has been associated with mortality in children with septic shock (5). And third, similar to adults, mitochondrial dysfunction is evident in pediatric sepsis (20). Moreover, the rapid decline in lactate after thiamine despite a prolonged period of hyperlactatemia raises the possibility that thiamine helped to reverse lactate production in our study.
Limitations of this study include the small sample size, precluding strong conclusions about the utility of thiamine. We also could not confirm thiamine deficiency or differentiate “type B” lactic acidosis from ongoing tissue hypoxia. However, hemodynamic targets were consistent with reversal of circulatory shock at T0 in all cases. Although matching and statistical adjustment minimized differences between groups, we cannot rule out additional unmeasured confounding. While cases were treated as per adult studies (8, 9), there was variation in thiamine prescription and at least one study demonstrating benefit of thiamine in adult sepsis used substantially higher doses (11). Finally, we conservatively anchored T0 lactate to maximum values for controls, but this approach would have biased any difference in the primary outcome toward the null and increased type II error.
CONCLUSIONS
Although children with septic shock and hyperlactatemia exhibited rapid improvement in physiological and clinical outcomes after treatment with thiamine, we cannot infer benefit when compared to usual care in a matched control group. However, because cases had a prolonged period of hyperlactatemia that rapidly declined after starting thiamine, this therapy warrants further study in pediatric septic shock.
Acknowledgments
Financial support was provided by NIGMS K23GM110496 (SLW) and the Department of Anesthesiology and Critical Care at the Children’s Hospital of Philadelphia.
Disclosures: Drs. Weiss and Blowey’s institutions received funding from National Institute of General Medical Sciences K23GM110496. Drs. Weiss, Blowey, and Ganetzky received support for article research from the National Institutes of Health (NIH). Dr. Ganetzky received funding from the NIH. Dr. Sutton’s institution received funding from NIH National Heart, Lung, and Blood Institute R01; he received funding from Zoll Medical (speaking honoraria); and he disclosed he is a writing group member of the Pediatric Advanced Life Support Guidelines and Chair of the AHA’s Get With the Guidelines-Resuscitation Pediatric Research Task Force. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
This study was performed at the Children’s Hospital of Philadelphia.
REFERENCES
- 1.Singer M The role of mitochondrial dysfunction in sepsis-induced multi-organ failure. Virulence 2014;5(1):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Manzanares W, Hardy G. Thiamine supplementation in the critically ill. Curr Opin Clin Nutr Metab Care 2011;14(6):610–617. [DOI] [PubMed] [Google Scholar]
- 3.Donnino MW, Carney E, Cocchi MN, et al. Thiamine deficiency in critically ill patients with sepsis. J Crit Care 2010;25(4):576–581. [DOI] [PubMed] [Google Scholar]
- 4.Costa NA, Gut AL, de Souza Dorna M, et al. Serum thiamine concentration and oxidative stress as predictors of mortality in patients with septic shock. J Crit Care 2014;29(2):249–252. [DOI] [PubMed] [Google Scholar]
- 5.Lima LF, Leite HP, Taddei JA. Low blood thiamine concentrations in children upon admission to the intensive care unit: risk factors and prognostic significance. Am J Clin Nutr 2011;93(1):57–61. [DOI] [PubMed] [Google Scholar]
- 6.Leite HP, de Lima LF. Metabolic resuscitation in sepsis: a necessary step beyond the hemodynamic? J Thorac Dis 2016;8(7):E552–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klein M, Weksler N, Gurman GM. Fatal metabolic acidosis caused by thiamine deficiency. J Emerg Med 2004;26(3):301–303. [DOI] [PubMed] [Google Scholar]
- 8.Donnino MW, Andersen LW, Chase M, et al. Randomized, Double-Blind, Placebo-Controlled Trial of Thiamine as a Metabolic Resuscitator in Septic Shock: A Pilot Study. Crit Care Med 2016;44(2):360–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Marik PE, Khangoora V, Rivera R, et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest 2017;151(6):1229–1238. [DOI] [PubMed] [Google Scholar]
- 10.Moskowitz A, Andersen LW, Cocchi MN, et al. Thiamine as a Renal Protective Agent in Septic Shock. A Secondary Analysis of a Randomized, Double-Blind, Placebo-controlled Trial. Ann Am Thorac Soc 2017;14(5):737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woolum JA, Abner EL, Kelly A, et al. Effect of Thiamine Administration on Lactate Clearance and Mortality in Patients With Septic Shock. Crit Care Med 2018;46(11):1747–1752. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein B, Giroir B, Randolph A. International pediatric sepsis consensus conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005;6(1):2–8. [DOI] [PubMed] [Google Scholar]
- 13.Gaies MG, Gurney JG, Yen AH, et al. Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 2010;11(2):234–238. [DOI] [PubMed] [Google Scholar]
- 14.Leteurtre S, Duhamel A, Salleron J, et al. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med 2013;41(7):1761–1773. [DOI] [PubMed] [Google Scholar]
- 15.Zubizarreta JR, Paredes R, Rosenbaum PR. Matching for balance, pairing for heterogeneity in an observational study of the effectiveness of for-profit and not-for-profit high schools in Chile. Ann Appl Stat 2014;8(1):204–223. [Google Scholar]
- 16.Davis AL, Carcillo JA, Aneja RK, et al. The American College of Critical Care Medicine Clinical Practice Parameters for Hemodynamic Support of Pediatric and Neonatal Septic Shock: Executive Summary. Pediatr Crit Care Med 2017;18(9):884–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Crit Care Med 2017. [DOI] [PubMed] [Google Scholar]
- 18.Seear M, Lockitch G, Jacobson B, et al. Thiamine, riboflavin, and pyridoxine deficiencies in a population of critically ill children. J Pediatr 1992;121(4):533–538. [DOI] [PubMed] [Google Scholar]
- 19.Hiffler L, Rakotoambinina B, Lafferty N, et al. Thiamine Deficiency in Tropical Pediatrics: New Insights into a Neglected but Vital Metabolic Challenge. Front Nutr 2016;3:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss SL, Selak MA, Tuluc F, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med 2015;16(1):e4–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]