Abstract
Copper is an essential micronutrient required for oxygen-dependent enzymes, yet excesses of the metal is a toxicant. The tug-of-war between these copper activities is balanced by chaperones and membrane transporters, which control copper distribution and availability. The P-type ATPase transporters, ATP7A and ATP7B, regulate cytoplasmic copper by pumping copper out of cells or into the endomembrane system. Mutations in ATP7A and ATP7B cause diseases that share neuropsychiatric phenotypes, which are similar to phenotypes observed in mutations affecting cytoplasmic trafficking complexes required for ATP7A/B dynamics. Here, we discuss evidence indicating that phenotypes associated to genetic defects in trafficking complexes, such as retromer and the adaptor complex AP-1, result in part from copper dyshomeostasis due to mislocalized ATP7A and ATP7B.
Copper is a double-edged sword in biological systems. On one side, copper is an essential micronutrient required for the activity of diverse, fundamental, and conserved enzymes that catalyze oxygen-dependent reactions. These enzymes include monooxygenases present in the endomembrane system, the superoxide dismutase SOD1, and the cytochrome oxidase complex IV in the mitochondrial respiratory chain. On the other hand, copper in excess is a noxious agent due to the oxidative capacity of copper in biological systems. To balance these opposing forces prokaryotic and eukaryotic cells have evolved sophisticated mechanisms to control copper availability and to move copper across membranes and in between compartments. Among these molecules are the P-type ATPase transporters, ATP7A and ATP7B, that deliver copper into the lumen of organelles along the exocytic and endocytic route. The biological impact of copper becomes evident from defects in these transporter genes. Mutations in ATP7A result in organismal depletion of copper due to impaired intestinal copper intake [1–4]. In contrast, ATP7B mutations lead to systemic accumulation of copper due to impaired excretion by the liver [1–4]. These genetic diseases have distinctive pathologies and manifestations yet they share neurodegeneration as a cardinal feature [5]. We argue that the study of rare ATP7A/B copper transporter genetic disorders points to mechanisms of neurodegeneration likely shared with widespread sporadic neurodegenerative and neurodevelopmental diseases [6]. Here, we elaborate on this contention focusing on the interactomes of these copper transporters, which enrich genes implicated in their trafficking as well as in neurodegenerative and neurodevelopmental diseases [7,8].
Genetic disorders of copper P-type ATPases.
ATP7A is polytopic transmembrane protein encoded in the X chromosome (Fig. 3A). ATP7A genetic defects cause the X-linked disorders: occipital horn syndrome (OMIM 304150); spinal muscular atrophy; distal, X-linked 3 (SMAX3, OMIM 300489); and Menkes disease (OMIM 309400). The ATP7B gene is encoded in human chromosome 13 and defects in this gene cause Wilson’s disease (OMIM 277900) [1,2]. SMAX3, Menkes, and Wilson share neurodegeneration as a phenotype but differ in their copper-dependent pathophysiology. SMAX3-causing ATP7A mutations do not decrease systemic copper, yet cause non-demyelinating spinomuscular atrophy [9–11]. In contrast, null ATP7A mutations cause Menkes disease resulting in a multisystemic copper deficiency. Menkes manifests soon after birth with hypotonia, focal and generalized seizures, impaired cognitive development, and brain atrophy (Fig. 1A). Systemic features associated with Menkes disease include pili torti (twisted hairs, Fig. 2A), hypopigmentation (Fig. 2B), laxity of the skin (cutis laxa, Fig. 2C) and joints, reduced bone density (Fig. 2D), bladder diverticula (Fig. 2E), aneurysms, and vascular tortuosity in brain arteries (Fig. 1B). These clinical features are attributable to defects in diverse cuproenzymes that traverse the secretory pathway and remain as inactive apoenzymes in the disease state [1–3]. Menkes neurodegeneration is a childhood affliction most prominent in the cerebral cortex but affects other forebrain structures to variable degrees [5]. Neurodegeneration also extends to the cerebellum where remaining Purkinje cells display abnormal positioning and cell architecture sometimes referred as ‘willow tree’ Purkinje cells (Fig. 1C) [12–16]. An important phenotype observed in Purkinje cell perikarya is the presence of increased numbers of distended mitochondria (Fig. 1D). A reasonable model for the genesis of the Menkes’ enlarged mitochondria phenotype is a defect in oxidative phosphorylation. This contention is based on the well-established fact that copper is required for the assembly and activity of complex IV of the mitochondrial respiratory chain [17]. However, measurements of respiratory chain levels or activity in Menkes brain or muscle provide ambiguous answers [18–20]. Moreover, ATP levels in brain tissue defective in ATP7A are normal [21]. Thus, the key questions of how copper depletion leads to enlarged mitochondria phenotypes in Menkes disease and whether dysfunctional mitochondria make pathogenic contributions to Menkes disease still remain unanswered [16].
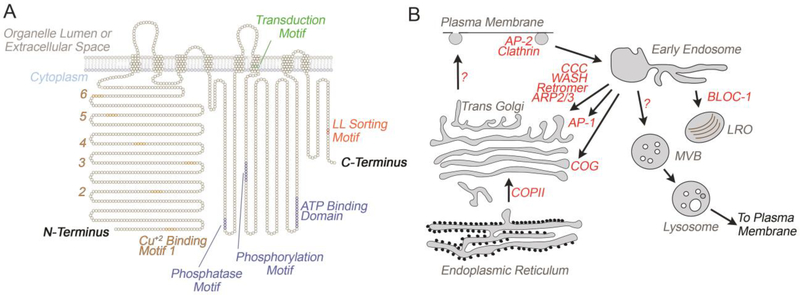
Figure 3. ATP7A/B topology and trafficking routes.
A) Model depicts the topology and relevant motifs present in the primary sequence of ATP7A. Note the presence of six copper binding motifs in the N-terminus and the di-leucine sorting motif in the C-terminus. Image adapted from [1]. B) Pathways and trafficking complexes utilized by ATP7A/B in cells. Note that while the COG complex is required for ATP7A traffic, its placement as an independent route and in endosome to Golgi recycling is speculative [7]. LRO refers to lysosome-related organelles, such as the lysosome [51].
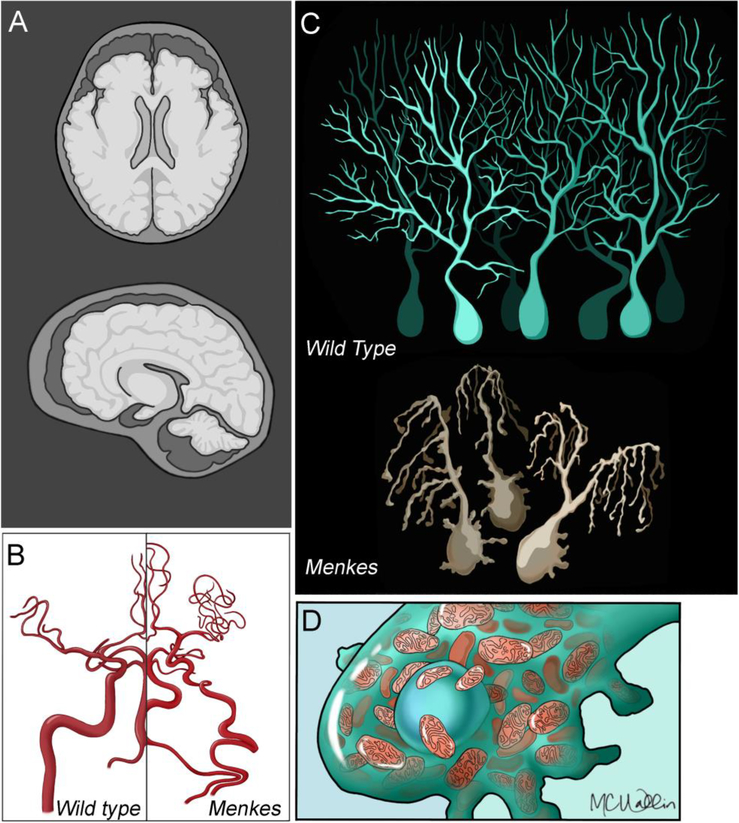
Figure 1. Menkes Disease Central Nervous System Pathology.
Images depict in A, the characteristic atrophy of the cortex and cerebellum in Menkes patients (light gray colored brain) as compare to wild type individuals (dark brain colored brain). Note the mark atrophy of the cerebellum. B) Vascular tortuosity of brain arteries in Menkes subjects. C) Depicts Purkinje cerebellar cells in wild type and Menkes patients. Menkes Purkinje cells are dystrophic and adopt the willow tree dendritic morphology with ectopic dendrites emerging around the cell body. D) Purkinje cells in Menkes patients accumulate distended mitochondria in the cell body.
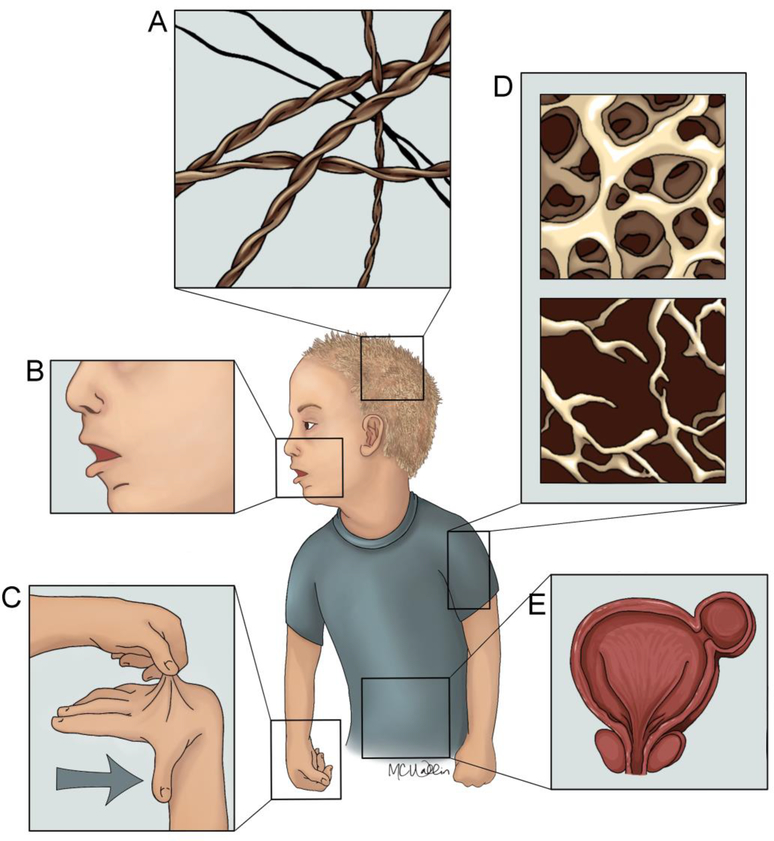
Figure 2. Systemic Features of Menkes Disease.
Menkes disease patients are characterized by: A) pili torti, coarse and sparse hair, B) hypopigmentation, C) cutis laxa, D) osteoporosis, and E) diverticula of the bladder and colon (not shown).
In contrast with Menkes disease, Wilson disease results from the abnormal accumulation of copper due to defective copper excretion into the bile. Liver and brain are the most affected tissues. Disease phenotypes include cirrhosis and chronic hepatitis, neurodegeneration, parkinsonian features, seizures, and psychiatric symptoms such as psychosis. Additional characteristics are the Kayser–Fleischer ring, a deposition of copper that creates a gold-brown halo around the edge of the cornea as well as decreased serum levels of the copper carrier protein ceruloplasmin [1–3]. Wilson disease neurodegeneration differs from Menkes disease neurodegeneration in that Wilson chiefly affects the developed brain. Wilson’s neurodegeneration encompasses the striatum and pallidum and to a minor degree cerebral cortex, brainstem, and dentate nucleus. The differences in regional neurodegeneration between Menkes disease and Wilson disease also extend to a more pronounced glial pathology and signs of inflammation in Wilson disease [22]. At the cellular level, null mutations in ATP7B lead to distended mitochondria in liver and neurons with an accumulation of these engorged organelles in the perikaryon [23,24]. While the mechanisms leading to the distended mitochondrial phenotype in Menkes Purkinje cells remains unclear, mitochondrial phenotypes in Wilson likely correspond to the final stages of mitochondria damaged by copper overload mechanisms [23].
Trafficking mechanisms of copper P-type ATPases.
ATP7A and B localize to the Golgi apparatus at steady state and cycle between the plasma membrane and the Golgi complex in non-specialized cells (Fig. 3B) [1–3]. However, ATP7B is also present in intracellular vesicles distinct from the Golgi complex in specialized secretory and epithelial cells [3,25,26]. The basolateral activity of ATP7A in enterocytes and the canalicular activity of ATP7B in hepatocytes determine intake and excretion of copper for the whole organism, respectively. Loss of these polarized activities are the primary determinant in the pathogenesis of the Menkes and Wilson diseases [27]. We refer the reader to an excellent overview of ATP7A/B traffic in enterocytes and hepatocytes and other polarized cells [3]. In fibroblasts, ATP7A and ATP7B exit the Golgi complex in route to the cell surface via post-Golgi vesicles. However, ATP7B expressed in hepatocytes is delivered to the canalicular surface by lysosomes (Fig. 3B) [28]. While this model has been challenged [29], the contention that ATP7B uses lysosomes to reach the canalicular membrane has received additional support including unbiased mass spectrometry analysis of isolated lysosomes [30–32]. ATP7A/B are retrieved back from the plasma membrane to the Golgi complex via endosomes (Fig. 3B). At steady state, translocation of these transporters from the Golgi to the plasma membrane proceeds slowly. However, copper binding to the ATP7A/B cytosolic N-termini induces surface translocation. This process requires first the deglutathionylation of cysteine residues in ATP7A/B by binding of GRLX1, thus leaving the N-terminal cysteines available for copper binding [33,34]. Next, it follows the formation of an ATP7A/B catalytic, phosphorylated intermediary generated during the pumping of copper into the trans-Golgi (TGN) network lumen, which is necessary for Golgi exit [35–38]. After copper binding, these transporters behave as regulated secretory cargoes traversing to the cell surface.
Pathogenic ATP7A mutations prevent gene expression, impair copper pump activity into the Golgi lumen, and/or trap ATP7A at alternative subcellular compartments along the exocytic and endocytic route [39–42]. Similarly, ATP7B pathogenic mutations and alleles associated with disease alter ATP7B expression and subcellular localization along the exocytic and endocytic route while concomitantly impairing ATP7B copper pump function [24,43–45]. These observations argue that similar to ATP7A/B genetic defects, mutations affecting complexes necessary for trafficking of ATP7A and ATP7B should also alter cellular and organismal copper homeostasis.
The cytosolic complexes required for the traffic of the ATP7 family of copper transporters from the plasma membrane to endosomes and from endosomes towards the Golgi complex have been mostly characterized for ATP7A (Fig. 3B). ATP7A and/or B require the adaptor complex AP-2 and clathrin for endocytosis. Retrieval to the Golgi complex or transport along endosomal compartments requires the concerted effort of a number of factors. These complexes include the adaptor complex AP-1, retromer, the Arp2/3 complex and its activating complex WASH, BLOC-1, and the CCC (COMMD/CCDC22/CCDC93) complexes (Figs. 3B–4A). AP-1 is required for endosome to Golgi retrieval of both ATP7A and B. The retromer is needed for ATP7A endosome to Golgi retrograde traffic [7,46–55] (Figs. 3B–4A). The sorting signals required for ATP7A/B binding to these sorting complexes remain elusive except for the di-leucine [DE]XXXL[LI] sorting motif in the C-termini of these transporters. This signal binds to the adaptor complex AP-1 (Fig. 3A) [56–58].
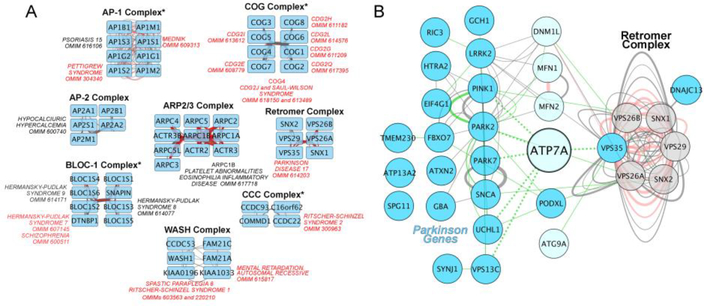
Figure 4. ATP7A, trafficking complexes, and Parkinson’s associated genes interactomes.
A) Trafficking complexes and their subunits were obtained from The Comprehensive Resource of Mammalian Protein Complexes (CORUM, http://mips.helmholtz-muenchen.de/corum/). Interactomes for each complex were defined using Genemania (http://genemania.org/) and data analyzed and graphed using Cytoscape. We identified diseases associated to trafficking complexes by searching the Online Mendelian Inheritance in Man database (OMIM, https://www.omim.org/) with each subunit gene. All diseases are listed with their OMIM number and those with neurological and/or neurodevelopmental phenotypes are listed in red font. Asterisks indicate genetic defects in complex subunits where the copper status of either cells or tissues have been analyzed. AP-1 or CCC deficiency increase cellular copper [46,62]. BLOC-1 mutations do not affect cellular copper in cells or tissues [50]. COG mutations decrease copper in cells likely due to combined effects in CTR1 and ATP7A [7]. B) Interactome of ATP7A and Parkinson’s genes. An updated and curated list of Parkinson’s genes was obtained from Lunati et al. [98]. Parkinson’s genes are depicted as cyan nodes. Retromer subunits are grey nodes. All other nodes are light cyan. Interactome was generated as in A. In A-B, edges represent biochemical interactions (grey), predicted interactions by yeast two hybrid (red), and genetic interactions (green) obtained from Genemania.
Although we have a rich catalog of complexes required for ATP7A sorting and targeting, we do not know the precise subcellular compartment where some of these complexes operate due to P-type ATPase trafficking complexity. First, trafficking mechanisms may depend on whether basal or copper stimulated traffic is studied. For example, the AP-1 adaptor complex is required for endosome to Golgi retrieval of both ATP7A and B. During constitutive ATP7A recycling AP-1 is required, whereas copper-regulated recycling seems to follow an AP-1 independent route [54]. Second, cell-type specific subcellular location of these trafficking complexes and/or the post-sorting fate of copper transporters is likely to determine the final destination of these transmembrane proteins. This is the case for BLOC-1 and the WASH complex in pigmentary and non-pigmentary cells. The absence of BLOC-1 prevents the endosome to melanosome delivery of ATP7A in pigmentary cells. However, even though BLOC-1 and WASH interact, WASH deficiency does not affect pigmentation in melanocytes [47,59,60]. These results are in contrast with the phenotypes in non-pigmentary cell lines where WASH disruption causes an increased cell surface expression of ATP7A [47,51]. One of the best characterized trafficking complexes is the retromer which is needed for ATP7A endosome to Golgi retrograde trafficking in multiple cell types (Figs. 3B–4A) [7,46–54]. Finally, the formation of supra-complexes may alter ATP7A sorting and targeting. These supra-complexes include retromer-WASH, WASH-CCC, BLOC-1-WASH, and BLOC-1-COG. While there is no evidence yet of a role for the retromer-like complex, retriever, in ATP7A traffic; since retriever can form complexes with the CCC and WASH complexes, it is possible that retriever may squelch away CCC and/or WASH from ATP7A enriched domains in endosomes [61]. These layers of complexity have not been studied systematically for any endosome cargo.
Genetic diseases of copper P-type ATPase trafficking.
Mutations in each one of the cytosolic complexes that regulate ATP7A sorting and targeting cause diverse neurodegenerative and neurodevelopmental disorders (Fig. 4A red font). For example, mutations in genes encoding subunits of the AP-1, COG, CCC, and WASH complexes cause syndromic forms of intellectual disability, microcephaly, and neuroanatomical defects in humans. Among these genetic diseases, alterations in copper homeostasis or the expression of copper-sensitive molecules have been demonstrated in mutations affecting mammalian AP-1, COG, and BLOC-1 complexes [4,7,47,50,62,63].
A particularly interesting group of mutations affecting copper homeostasis are those targeting the clathrin adaptor AP-1 sigma 1 subunit, encoded by the gene AP1S1 in humans. AP1S1 defects cause mental retardation, enteropathy, deafness, peripheral neuropathy, ichthyosis, and keratoderma (MEDNIK, OMIM 609313). The MEDNIK syndrome combines clinical and biochemical phenotypes found in Menkes and Wilson diseases including hypocupremia, hypoceruloplasminemia, liver copper accumulation and intrahepatic cholestasis. [4,62,64,65]. MEDNIK patient fibroblasts display abnormal subcellular distribution of ATP7A, which is routed to the cell surface instead of the Golgi complex [62]. Since normal subcellular distribution of ATP7B requires AP-1, it is likely that defective ATP7B function is also impaired in MEDNIK patients although this idea has not been tested yet [56]. One important feature of MEDNIK syndrome is that it can be ameliorated by oral zinc acetate therapy to reduce systemic copper overload [62]. Oral zinc supplementation is a standard treatment that decreases the burden of copper in Wilson disease. These MEDNIK zinc therapy outcomes are important as they strongly argue for an involvement of copper in the pathogenesis of MEDNIK syndrome [66,67]. However, while copper dyshomeostasis contributes to MEDNIK phenotypes, we contend that copper-unrelated membrane protein cargoes sensitive to AP1S1 mutations likely contribute to MEDNIK disease manifestations that do not have parallels in Menkes or Wilson disease.
The MEDNIK syndrome offers conceptual insight that goes beyond MEDNIK itself. This syndrome illustrates the idea that phenotypes originating from genetic defects of trafficking complexes are a collage of pathomechanisms caused by the defective function of membrane cargoes such as membrane ATPases. In the next section, we expand on this idea by discussing evidence supporting the case for the retromer complex in Parkinson’s disease and its cargoes, including ATP7A.
Mutations in the retromer complex and the mechanisms of Parkinson’s disease.
Genetic defects in the retromer subunits VPS26A, VPS29, and VPS35 (also known as PARK17, Parkinson’s disease gene 17, OMIM 614203) cause or associate with Parkinson’s disease in humans [68,69]. Retromer complex mutations that affect sorting of membrane protein cargoes and/or the activity controlling the fusion-fission cycle of mitochondria are associated with Parkinson’s disease.
Retromer cargoes relevant for Parkinson’s disease include the mannose-6-phospate receptor (IGFR2), ATG9A, LAMP2a (LAMP2), and synaptic neurotransmitter receptors (Fig. 4A–B) [48,69–72]. Retromer defects alter the sorting of ATG9A and LAMP2 impairing autophagy and chaperone-mediated autophagy, respectively. These autophagic mechanisms ameliorate Parkinson’s disease progression in mouse and Drosophila models by removing protein aggregates and damaged organelles [73–76]. Protein aggregates and damaged organelles can be induced by noxious agents, such as copper, or by alleles in genes that modulate the extent of protein aggregation, such as EIF4G1, a genetic interactor of retromer complex subunits and its cargoes [77,78]. Once protein aggregates or damaged organelles are captured by the autophagy machinery, they are destined for degradation within lysosomes. VPS35 mutations decrease endosome to Golgi retrieval of the mannose-6-phospate receptor (IGFR2), a receptor that is destined for lysosomal degradation in mutant cells [69,70]. This in turn, impairs delivery of hydrolases from the Golgi to the lysosome lumen. Thus, the defective sorting of lysosomal hydrolases in retromer-deficient cells is a compounding factor for the accumulation of protein aggregates or damaged organelles found in Parkinson’s disease [79].
Changes in copper content and copper buffering factors are common in the Parkinson’s disease brain [80–82]. Copper is a powerful trigger of Parkinson’s molecular pathology including alpha-synuclein aggregation as well as oxidative damage of proteins, lipids, and mitochondria [23,49,83,84]. Genetic evidence supports a function of the retromer in maintaining copper homeostasis in Saccharomyces cerevisiae. Deletion of the yeast retromer subunits vps26, vps29, or vps35 confer metal-specific sensitivity to copper. Importantly, the copper sensitivity phenotype in the vps35Δ strain can be reverted by re-expression of the wild type yeast gene but not by vps35 mutants carrying Parkinson’s causing mutations [85]. The mechanism by which retromer mutants increase copper sensitivity has not been uncovered but it is reasonable to hypothesize that the yeast ATP7A homologue, CCC2/YDR270W, or other retromer cargoes are involved indirectly or directly, respectively. ATP7A is a retromer cargo and associates with the retromer interacting complexes WASH and CCC in mammals [7,46–48]. Despite being a retromer cargo, ATP7A defects have not been implicated as a possible Parkinson’s pathogenesis mechanism [69,86–88].
Parkinson’s genes, the retromer complex, and copper transporters converge on mitochondria.
Retromer subunits are required to maintain mitochondria dynamics, function, and quality control mechanisms [89,90]. VPS35 Parkinson’s causing mutations or null alleles modify mitochondrial fusion and fission dynamics inducing organelle fragmentation. Mitochondrial fragmentation results from two mechanisms downstream of VPS35: impaired mitochondrial fusion due to defective mitofusin activity (MFN2) and increased fission activity due to persistent activity of dynamin-1-like protein (DNML1) on the mitochondrial membrane [89,90]. Mitochondrial depolarization and fragmentation mark organelles for autophagic destruction, a pathway controlled by the Parkinson’s disease genes parkin (PARK2) and PINK1 [75,91]. This parkin (PARK2) and PINK1 pathway intersects with retromer-dependent mechanisms as indicated by genetic interactions between Drosophila Vps35 and parkin [92].
Since VPS35, parkin (PARK2) and PINK1-dependent mitophagy mechanisms genetically interact, it is plausible that the P-type ATPases ATP7A and ATP7B will also interact with Parkinson’s causing genes and genes required for mitochondria quality control. This possibility is supported by curated biochemical and genetic interactomes linking ATP7A and Parkinson’s causing genes (Fig. 4B). We identified biochemical interactions between ATP7A and the Parkinson’s genes VPS35, VPS13C and PARK7, and genetic interactions between ATP7A and PARK5/UCHL1 in mammals and Drosophila [7,8]. Furthermore, our data indicate that Drosophila ATP7 genetically interacts with the Drosophila orthologues of PARK2 and PINK1 in a cell autonomous manner in neurons (C. Hartwig, unpublished data). Similarly, recent and exciting work in ATP7B deficiency models demonstrate that hepatocytes respond to copper-induced mitochondrial damage by upregulating PARK2-PINK1 mitophagy machinery as a protective mechanism [93]. Collectively these findings add a novel copper-dependent dimension to Parkinson’s disease pathogenesis.
Future Perspectives
Menkes and Wilson disease are models to understand fundamental pathogenic mechanisms common to more prevalent disorders. The established model is that copper behaves as an enzymatic cofactor or noxious agent. However, copper can also operate as an allosteric modulator of signal transduction or a second messenger capable of integrating the metabolic activity of the Golgi complex, mitochondria, and plasma membrane metal transport [94–97]. These emerging concepts along with the established models of copper action open unexplored possibilities to understand how copper dyshomeostasis, caused either by defective pumps or their trafficking complexes, may contribute to the pathogenesis of a wide range of neurological disorders.
Acknowledgements
This work was supported by a grant from the National Institutes of Health R01AG060285 to VF R15AR070505 to AVM, and R01GM67169 to CJF. CH is supported by the FIRST postdoctoral fellowship NIH 5K12GM000680. We thank members of the Faundez Lab for their comments and insight.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kaler SG: ATP7A-related copper transport diseases-emerging concepts and future trends. Nat Rev Neurol 2011, 7:15–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lutsenko S, Barnes NL, Bartee MY, Dmitriev OY: Function and regulation of human copper-transporting ATPases. Physiol Rev 2007, 87:1011–1046. [DOI] [PubMed] [Google Scholar]
- 3.Polishchuk R, Lutsenko S: Golgi in copper homeostasis: a view from the membrane trafficking field. Histochem Cell Biol 2013, 140:285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaler SG: Inborn errors of copper metabolism. Handb Clin Neurol 2013, 113:1745–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zlatic S, Comstra HS, Gokhale A, Petris MJ, Faundez V: Molecular basis of neurodegeneration and neurodevelopmental defects in Menkes disease. Neurobiol Dis 2015, 81:154–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winckler B, Faundez V, Maday S, Cai Q, Guimas Almeida C, Zhang H: The Endolysosomal System and Proteostasis: From Development to Degeneration. J Neurosci 2018, 38:9364–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Comstra HS, McArthy J, Rudin-Rush S, Hartwig C, Gokhale A, Zlatic SA, Blackburn JB, Werner E,Petris M, D’Souza P, et al. : The interactome of the copper transporter ATP7A belongs to a network of neurodevelopmental and neurodegeneration factors. Elife 2017, 6.* provide the first comprehensive interactomes of ATP7A in mammalian cells and Drosophila demonstrating an enrichment of gene implicated in neurological and neuropsychiatric disorders.
- 8.Zlatic SA, Vrailas-Mortimer A, Gokhale A, Carey LJ, Scott E, Burch R, McCall MM, Rudin-Rush S, Davis JB, Hartwig C, et al. : Rare Disease Mechanisms Identified by Genealogical Proteomics of Copper Homeostasis Mutant Pedigrees. Cell Syst 2018, 6:368–380 e366.* provide the first comprehensive interactomes of ATP7A in mammalian cells and Drosophila demonstrating an enrichment of gene implicated in neurological and neuropsychiatric disorders.
- 9.Kennerson ML, Nicholson GA, Kaler SG, Kowalski B, Mercer JF, Tang J, Llanos RM, Chu S, Takata RI, CE Speck-Martins, et al. : Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am J Hum Genet 2010, 86:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takata RI, Speck Martins CE, Passosbueno MR, Abe KT, Nishimura AL, Da Silva MD, Monteiro A Jr., Lima MI, Kok F, Zatz M: A new locus for recessive distal spinal muscular atrophy at Xq13.1-q21. J Med Genet 2004, 41:224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodgkinson VL, Dale JM, Garcia ML, Weisman GA, Lee J, Gitlin JD, Petris MJ: X-linked spinal muscular atrophy in mice caused by autonomous loss of ATP7A in the motor neuron. J Pathol 2015, 236:241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghatak NR, Hirano A, Poon TP, French JH: Trichopoliodystrophy. II. Pathological changes in skeletal muscle and nervous system. Arch Neurol 1972, 26:60–72. [DOI] [PubMed] [Google Scholar]
- 13.Hirano A, Llena JF, French JH, Ghatak NR: Fine structure of the cerebellar cortex in Menkes Kinky-hair disease. X-chromosome-linked copper malabsorption. Arch Neurol 1977, 34:52–56. [DOI] [PubMed] [Google Scholar]
- 14.Vagn-Hansen L, Reske-Nielsen E, Lou HC: Menkes’ disease--a new leucodystrophy (?). A clinical and neuropathological review together with a new case. Acta Neuropathol 1973, 25:103–119. [DOI] [PubMed] [Google Scholar]
- 15.Purpura DP, Hirano A, French JH: Polydendritic Purkinje cells in X-chromosome linked copper malabsorption: a Golgi study. Brain Res 1976, 117:125–129. [DOI] [PubMed] [Google Scholar]
- 16.Troost D, van Rossum A, Straks W, Willemse J: Menkes’ kinky hair disease. II. A clinicopathological report of three cases. Brain Dev 1982, 4:115–126. [DOI] [PubMed] [Google Scholar]
- 17.Timon-Gomez A, Nyvltova E, Abriata LA, Vila AJ, Hosler J, Barrientos A: Mitochondrial cytochrome c oxidase biogenesis: Recent developments. Semin Cell Dev Biol 2018, 76:163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sparaco M, Hirano A, Hirano M, DiMauro S, Bonilla E: Cytochrome C oxidase deficiency and neuronal involvement in Menkes’ kinky hair disease: immunohistochemical study. Brain Pathol 1993, 3:349–354. [DOI] [PubMed] [Google Scholar]
- 19.Pedespan JM, Jouaville LS, Cances C, Letellier T, Malgat M, Guiraud P, Coquet M, Vernhet I,Lacombe D, Mazat JP: Menkes disease: study of the mitochondrial respiratory chain in three cases. Eur J Paediatr Neurol 1999, 3:167–170. [DOI] [PubMed] [Google Scholar]
- 20.Kuznetsov AV, Clark JF, Winkler K, Kunz WS: Increase of flux control of cytochrome c oxidase in copper-deficient mottled brindled mice. J Biol Chem 1996, 271:283–288. [DOI] [PubMed] [Google Scholar]
- 21.Kunz WS, Kuznetsov AV, Clark JF, Tracey I, Elger CE: Metabolic consequences of the cytochrome c oxidase deficiency in brain of copper-deficient Mo(vbr) mice. J Neurochem 1999, 72:1580–1585. [DOI] [PubMed] [Google Scholar]
- 22.Poujois A, Mikol J, Woimant F: Wilson disease: brain pathology. Handb Clin Neurol 2017, 142:77–89. [DOI] [PubMed] [Google Scholar]
- 23.Zischka H, Lichtmannegger J, Schmitt S, Jagemann N, Schulz S, Wartini D, Jennen L, Rust C,Larochette N, Galluzzi L, et al. : Liver mitochondrial membrane crosslinking and destruction in a rat model of Wilson disease. J Clin Invest 2011, 121:1508–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong Y, Shi SS, Chen S, Ni W, Zhu M, Wu ZY: The discrepancy between the absence of copper deposition and the presence of neuronal damage in the brain of Atp7b(−/−) mice. Metallomics 2015, 7:283–288. [DOI] [PubMed] [Google Scholar]
- 25.Barnes N, Tsivkovskii R, Tsivkovskaia N, Lutsenko S: The copper-transporting ATPases, menkes and wilson disease proteins, have distinct roles in adult and developing cerebellum. J Biol Chem 2005, 280:9640–9645. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt K, Ralle M, Schaffer T, Jayakanthan S, Bari B, Muchenditsi A, Lutsenko S: ATP7A and ATP7B copper transporters have distinct functions in the regulation of neuronal DOPAMINE-beta-HYDROXYLASE. J Biol Chem 2018, 10.1074/jbc.RA118.004889.* presents evidence that the functions of ATP7A and ATP7B are distinct in loading apoenzymes with copper at the Golgi complex.
- 27.Wang Y, Zhu S, Hodgkinson V, Prohaska JR, Weisman GA, Gitlin JD, Petris MJ: Maternofetal and neonatal copper requirements revealed by enterocyte-specific deletion of the Menkes disease protein. Am J Physiol Gastrointest Liver Physiol 2012, 303:G1236–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polishchuk EV, Concilli M, Iacobacci S, Chesi G, Pastore N, Piccolo P, Paladino S, Baldantoni D,van ISC, Chan J, et al. : Wilson disease protein ATP7B utilizes lysosomal exocytosis to maintain copper homeostasis. Dev Cell 2014, 29:686–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lalioti V, Peiro R, Perez-Berlanga M, Tsuchiya Y, Munoz A, Villalba T, Sanchez C, Sandoval IV: Basolateral sorting and transcytosis define the Cu+-regulated translocation of ATP7B to the bile canaliculus. J Cell Sci 2016, 129:2190–2201. [DOI] [PubMed] [Google Scholar]
- 30.Chapel A, Kieffer-Jaquinod S, Sagne C, Verdon Q, Ivaldi C, Mellal M, Thirion J, Jadot M, Bruley C, Garin J, et al. : An extended proteome map of the lysosomal membrane reveals novel potential transporters. Mol Cell Proteomics 2013, 12:1572–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chandhok G, Horvath J, Aggarwal A, Bhatt M, Zibert A, Schmidt HH: Functional analysis and drug response to zinc and D-penicillamine in stable ATP7B mutant hepatic cell lines. World J Gastroenterol 2016, 22:4109–4119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Polishchuk EV, Polishchuk RS: The emerging role of lysosomes in copper homeostasis. Metallomics 2016, 8:853–862. [DOI] [PubMed] [Google Scholar]
- 33.Lim CM, Cater MA, Mercer JF, La Fontaine S: Copper-dependent interaction of glutaredoxin with the N termini of the copper-ATPases (ATP7A and ATP7B) defective in Menkes and Wilson diseases. Biochem Biophys Res Commun 2006, 348:428–436. [DOI] [PubMed] [Google Scholar]
- 34.Singleton WC, McInnes KT, Cater MA, Winnall WR, McKirdy R, Yu Y, Taylor PE, Ke BX,Richardson DR, Mercer JF, et al. : Role of glutaredoxin1 and glutathione in regulating the activity of the copper-transporting P-type ATPases, ATP7A and ATP7B. J Biol Chem 2010, 285:27111–27121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petris MJ, Voskoboinik I, Cater M, Smith K, Kim BE, Llanos RM, Strausak D, Camakaris J, Mercer JF: Copper-regulated trafficking of the Menkes disease copper ATPase is associated with formation of a phosphorylated catalytic intermediate. J Biol Chem 2002, 277:46736–46742. [DOI] [PubMed] [Google Scholar]
- 36.Cater MA, Forbes J, La Fontaine S, Cox D, Mercer JF: Intracellular trafficking of the human Wilson protein: the role of the six N-terminal metal-binding sites. Biochem J 2004, 380:805–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strausak D, La Fontaine S, Hill J, Firth SD, Lockhart PJ, Mercer JF: The role of GMXCXXC metal binding sites in the copper-induced redistribution of the Menkes protein. J Biol Chem 1999, 274:11170–11177. [DOI] [PubMed] [Google Scholar]
- 38.Voskoboinik I, Strausak D, Greenough M, Brooks H, Petris M, Smith S, Mercer JF, Camakaris J: Functional analysis of the N-terminal CXXC metal-binding motifs in the human Menkes copper-transporting P-type ATPase expressed in cultured mammalian cells. J Biol Chem 1999, 274:22008–22012. [DOI] [PubMed] [Google Scholar]
- 39.Vulpe C, Levinson B, Whitney S, Packman S, Gitschier J: Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase. Nat Genet 1993, 3:7–13. [DOI] [PubMed] [Google Scholar]
- 40.Tumer Z: An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Hum Mutat 2013, 34:417–429. [DOI] [PubMed] [Google Scholar]
- 41.Kim BE, Smith K, Petris MJ: A copper treatable Menkes disease mutation associated with defective trafficking of a functional Menkes copper ATPase. J Med Genet 2003, 40:290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petris MJ, Mercer JF: The Menkes protein (ATP7A; MNK) cycles via the plasma membrane both in basal and elevated extracellular copper using a C-terminal di-leucine endocytic signal. Hum Mol Genet 1999, 8:2107–2115. [DOI] [PubMed] [Google Scholar]
- 43.Payne AS, Kelly EJ, Gitlin JD: Functional expression of the Wilson disease protein reveals mislocalization and impaired copper-dependent trafficking of the common H1069Q mutation. Proc Natl Acad Sci U S A 1998, 95:10854–10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forbes JR, Cox DW: Copper-dependent trafficking of Wilson disease mutant ATP7B proteins. Hum Mol Genet 2000, 9:1927–1935. [DOI] [PubMed] [Google Scholar]
- 45.Huster D, Hoppert M, Lutsenko S, Zinke J, Lehmann C, Mossner J, Berr F, Caca K: Defective cellular localization of mutant ATP7B in Wilson’s disease patients and hepatoma cell lines. Gastroenterology 2003, 124:335–345. [DOI] [PubMed] [Google Scholar]
- 46.Phillips-Krawczak CA, Singla A, Starokadomskyy P, Deng Z, Osborne DG, Li H, Dick CJ, Gomez TS, Koenecke M, Zhang JS, et al. : COMMD1 is linked to the WASH complex and regulates endosomal trafficking of the copper transporter ATP7A. Mol Biol Cell 2015, 26:91–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ryder PV, Vistein R, Gokhale A, Seaman MN, Puthenveedu M, Faundez V: The WASH Complex, an Endosomal Arp2/3 Activator, Interacts with the Hermansky-Pudlak Syndrome Complex BLOC-1 and its Cargo Phosphatidylinositol-4-kinase Type II Alpha. Mol Biol Cell 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Steinberg F, Gallon M, Winfield M, Thomas EC, Bell AJ, Heesom KJ, Tavare JM, Cullen PJ: A global analysis of SNX27-retromer assembly and cargo specificity reveals a function in glucose and metal ion transport. Nat Cell Biol 2013, 15:461–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aboud AA, Tidball AM, Kumar KK, Neely MD, Han B, Ess KC, Hong CC, Erikson KM, Hedera P,Bowman AB: PARK2 patient neuroprogenitors show increased mitochondrial sensitivity to copper. Neurobiol Dis 2015, 73:204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gokhale A, Vrailas-Mortimer A, Larimore J, Comstra HS, Zlatic SA, Werner E, Manvich DF,Iuvone PM, Weinshenker D, Faundez V: Neuronal copper homeostasis susceptibility by genetic defects in dysbindin, a schizophrenia susceptibility factor. Hum Mol Genet 2015, 24:5512–5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setty SR, Tenza D, Sviderskaya EV, Bennett DC, Raposo G, Marks MS: Cell-specific ATP7A transport sustains copper-dependent tyrosinase activity in melanosomes. Nature 2008, 454:1142–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holloway ZG, Velayos-Baeza A, Howell GJ, Levecque C, Ponnambalam S, Sztul E, Monaco AP: Trafficking of the Menkes copper transporter ATP7A is regulated by clathrin-, AP-2-, AP-1-, and Rab22-dependent steps. Mol Biol Cell 2013, 24:1735–1748, S1731–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chesi G, Hegde RN, Iacobacci S, Concilli M, Parashuraman S, Festa BP, Polishchuk EV, Di Tullio G, Carissimo A, Montefusco S, et al. : Identification of p38 MAPK and JNK as new targets for correction of Wilson disease-causing ATP7B mutants. Hepatology 2016, 63:1842–1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hirst J, Borner GH, Antrobus R, Peden AA, Hodson NA, Sahlender DA, Robinson MS: Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr Biol 2012, 22:1711–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lalioti V, Hernandez-Tiedra S, Sandoval IV: DKWSLLL, a versatile DXXXLL-type signal with distinct roles in the Cu(+)-regulated trafficking of ATP7B. Traffic 2014, 15:839–860. [DOI] [PubMed] [Google Scholar]
- 56.Jain S, Farias GG, Bonifacino JS: Polarized sorting of the copper transporter ATP7B in neurons mediated by recognition of a dileucine signal by AP-1. Mol Biol Cell 2015, 26:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yi L, Kaler SG: Direct interactions of adaptor protein complexes 1 and 2 with the copper transporter ATP7A mediate its anterograde and retrograde trafficking. Hum Mol Genet 2015, 24:2411–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhu S, Shanbhag V, Hodgkinson VL, Petris MJ: Multiple di-leucines in the ATP7A copper transporter are required for retrograde trafficking to the trans-Golgi network. Metallomics 2016, 8:993–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delevoye C, Heiligenstein X, Ripoll L, Gilles-Marsens F, Dennis MK, Linares RA, Derman L,Gokhale A, Morel E, Faundez V, et al. : BLOC-1 Brings Together the Actin and Microtubule Cytoskeletons to Generate Recycling Endosomes. Curr Biol 2016, 26:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tyrrell BJ, Woodham EF, Spence HJ, Strathdee D, Insall RH, Machesky LM: Loss of strumpellin in the melanocytic lineage impairs the WASH Complex but does not affect coat colour. Pigment Cell Melanoma Res 2016, 29:559–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McNally KE, Faulkner R, Steinberg F, Gallon M, Ghai R, Pim D, Langton P, Pearson N, Danson CM, Nagele H, et al. : Retriever is a multiprotein complex for retromer-independent endosomal cargo recycling. Nat Cell Biol 2017, 19:1214–1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Martinelli D, Travaglini L, Drouin CA, Ceballos-Picot I, Rizza T, Bertini E, Carrozzo R, Petrini S,de Lonlay P, El Hachem M, et al. : MEDNIK syndrome: a novel defect of copper metabolism treatable by zinc acetate therapy. Brain 2013, 136:872–881. [DOI] [PubMed] [Google Scholar]
- 63.Malinouski M, Hasan NM, Zhang Y, Seravalli J, Lin J, Avanesov A, Lutsenko S, Gladyshev VN: Genome-wide RNAi ionomics screen reveals new genes and regulation of human trace element metabolism. Nat Commun 2014, 5:3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Montpetit A, Cote S, Brustein E, Drouin CA, Lapointe L, Boudreau M, Meloche C, Drouin R,Hudson TJ, Drapeau P, et al. : Disruption of AP1S1, causing a novel neurocutaneous syndrome, perturbs development of the skin and spinal cord. PLoS Genet 2008, 4:e1000296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Martinelli D, Dionisi-Vici C: AP1S1 defect causing MEDNIK syndrome: a new adaptinopathy associated with defective copper metabolism. Ann N Y Acad Sci 2014, 1314:55–63. [DOI] [PubMed] [Google Scholar]
- 66.Brewer GJ, Hill GM, Prasad AS, Cossack ZT, Rabbani P: Oral zinc therapy for Wilson’s disease. Ann Intern Med 1983, 99:314–319. [DOI] [PubMed] [Google Scholar]
- 67.Hoogenraad TU: Paradigm shift in treatment of Wilson’s disease: zinc therapy now treatment of choice. Brain Dev 2006, 28:141–146. [DOI] [PubMed] [Google Scholar]
- 68.Kumar KR, Weissbach A, Heldmann M, Kasten M, Tunc S, Sue CM, Svetel M, Kostic VS, Segura-Aguilar J, Ramirez A, et al. : Frequency of the D620N mutation in VPS35 in Parkinson disease. Arch Neurol 2012, 69:1360–1364. [DOI] [PubMed] [Google Scholar]
- 69.McMillan KJ, Korswagen HC, Cullen PJ: The emerging role of retromer in neuroprotection. Curr Opin Cell Biol 2017, 47:72–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seaman MN: Cargo-selective endosomal sorting for retrieval to the Golgi requires retromer. J Cell Biol 2004, 165:111–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jimenez-Orgaz A, Kvainickas A, Nagele H, Denner J, Eimer S, Dengjel J, Steinberg F: Control of RAB7 activity and localization through the retromer-TBC1D5 complex enables RAB7-dependent mitophagy. EMBO J 2018, 37:235–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams ET, Glauser L, Tsika E, Jiang H, Islam S, Moore DJ: Parkin mediates the ubiquitination of VPS35 and modulates retromer-dependent endosomal sorting. Hum Mol Genet 2018, 27:3189–3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lynch-Day MA, Mao K, Wang K, Zhao M, Klionsky DJ: The role of autophagy in Parkinson’s disease. Cold Spring Harb Perspect Med 2012, 2:a009357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Narendra D, Tanaka A, Suen DF, Youle RJ: Parkin is recruited selectively to impaired mitochondria and promotes their autophagy. J Cell Biol 2008, 183:795–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pickrell AM, Youle RJ: The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron 2015, 85:257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orenstein SJ, Kuo SH, Tasset I, Arias E, Koga H, Fernandez-Carasa I, Cortes E, Honig LS, Dauer W, Consiglio A, et al. : Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci 2013, 16:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dhungel N, Eleuteri S, Li LB, Kramer NJ, Chartron JW, Spencer B, Kosberg K, Fields JA, Stafa K,Adame A, et al. : Parkinson’s disease genes VPS35 and EIF4G1 interact genetically and converge on alpha-synuclein. Neuron 2015, 85:76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Khurana V, Peng J, Chung CY, Auluck PK, Fanning S, Tardiff DF, Bartels T, Koeva M, Eichhorn SW, Benyamini H, et al. : Genome-Scale Networks Link Neurodegenerative Disease Genes to alpha-Synuclein through Specific Molecular Pathways. Cell Syst 2017, 4:157–170 e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shahmoradian SH, Lewis AJ, Genoud C, Graff-Meyer A, Hench J, Moors T, Schweighauser G,Wang J, Goldie KN, Suetterlin R, et al. : Lewy pathology in Parkinson’s disease consists of a crowded organellar, membranous medley. bioRxiv 2018.** describes ultrastractural details of the Lewi bodies in Parkinson’s disease as aggregates of mebranous organelles including autophagosomes and mitochondria.
- 80.Gaggelli E, Kozlowski H, Valensin D, Valensin G: Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis). Chem Rev 2006, 106:1995–2044. [DOI] [PubMed] [Google Scholar]
- 81.Lan AP, Chen J, Chai ZF, Hu Y: The neurotoxicity of iron, copper and cobalt in Parkinson’s disease through ROS-mediated mechanisms. Biometals 2016, 29:665–678. [DOI] [PubMed] [Google Scholar]
- 82.Davies KM, Bohic S, Carmona A, Ortega R, Cottam V, Hare DJ, Finberg JP, Reyes S, Halliday GM, Mercer JF, et al. : Copper pathology in vulnerable brain regions in Parkinson’s disease. Neurobiol Aging 2014, 35:858–866. [DOI] [PubMed] [Google Scholar]
- 83.Paik SR, Shin HJ, Lee JH, Chang CS, Kim J: Copper(II)-induced self-oligomerization of alpha-synuclein. Biochem J 1999, 340 (Pt 3):821–828. [PMC free article] [PubMed] [Google Scholar]
- 84.Rasia RM, Bertoncini CW, Marsh D, Hoyer W, Cherny D, Zweckstetter M, Griesinger C, Jovin TM, Fernandez CO: Structural characterization of copper(II) binding to alpha-synuclein: Insights into the bioinorganic chemistry of Parkinson’s disease. Proc Natl Acad Sci U S A 2005, 102:4294–4299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sowada N, Stiller B, Kubisch C: Increased copper toxicity in Saccharomyces cerevisiae lacking VPS35, a component of the retromer and monogenic Parkinson disease gene in humans. Biochem Biophys Res Commun 2016, 476:528–533. [DOI] [PubMed] [Google Scholar]
- 86.Wang S, Bellen HJ: The retromer complex in development and disease. Development 2015, 142:2392–2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Small SA, Petsko GA: Retromer in Alzheimer disease, Parkinson disease and other neurological disorders. Nat Rev Neurosci 2015, 16:126–132. [DOI] [PubMed] [Google Scholar]
- 88.Zhang H, Huang T, Hong Y, Yang W, Zhang X, Luo H, Xu H, Wang X: The Retromer Complex and Sorting Nexins in Neurodegenerative Diseases. Front Aging Neurosci 2018, 10:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC: VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep 2015, 12:1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X: Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med 2016, 22:54–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pickles S, Vigie P, Youle RJ: Mitophagy and Quality Control Mechanisms in Mitochondrial Maintenance. Curr Biol 2018, 28:R170–R185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Malik BR, Godena VK, Whitworth AJ: VPS35 pathogenic mutations confer no dominant toxicity but partial loss of function in Drosophila and genetically interact with parkin. Hum Mol Genet 2015, 24:6106–6117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Polishchuk EV, Merolla A, Lichtmannegger J, Romano A, Indrieri A, Ilyechova EY, Concili M, De Cegli R, Crispino R, Mariniello M, et al. : Activation of autophagy, observed in liver tissues from patients with Wilson disease and from ATP7B-deficient animals, protects hepatocytes from copper-induced apoptosis. Gastroenterology 2019, in press.** describes a protective role of autophagy and mitophagy genes in ATP7B deficiency.
- 94.Krishnamoorthy L, Cotruvo JA Jr., Chan J, Kaluarachchi H, Muchenditsi A, Pendyala VS, Jia S, Aron AT, Ackerman CM, Wal MN, et al. : Copper regulates cyclic-AMP-dependent lipolysis. Nat Chem Biol 2016, 12:586–592.** describe novel modes of copper action as a ‘second messenger’ or allosteric modulator of enzymatic reactions.
- 95.Brady DC, Crowe MS, Turski ML, Hobbs GA, Yao X, Chaikuad A, Knapp S, Xiao K, Campbell SL,Thiele DJ, et al. : Copper is required for oncogenic BRAF signalling and tumorigenesis. Nature 2014, 509:492–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baker ZN, Jett K, Boulet A, Hossain A, Cobine PA, Kim BE, El Zawily AM, Lee L, Tibbits GF, PetrisMJ, et al. : The mitochondrial metallochaperone SCO1 maintains CTR1 at the plasma membrane to preserve copper homeostasis in the murine heart. Hum Mol Genet 2017, 26:4617–4628.** describe novel modes of copper action as a ‘second messenger’ or allosteric modulator of enzymatic reactions.
- 97.Hlynialuk CJ, Ling B, Baker ZN, Cobine PA, Yu LD, Boulet A, Wai T, Hossain A, El Zawily AM,McFie PJ, et al. : The Mitochondrial Metallochaperone SCO1 Is Required to Sustain Expression of the High-Affinity Copper Transporter CTR1 and Preserve Copper Homeostasis. Cell Rep 2015, 10:933–943. [DOI] [PubMed] [Google Scholar]
- 98.Lunati A, Lesage S, Brice A: The genetic landscape of Parkinson’s disease. Rev Neurol (Paris) 2018, 174:628–643. [DOI] [PubMed] [Google Scholar]