Abstract
This systematic review identifies models of service co-location, a structural intervention strategy to remove barriers to HIV care and services, and examines their associations with HIV care outcomes. A cumulative database (e.g., MEDLINE, EMBASE) of HIV, AIDS, and STI literature was systematically searched and manual searches were conducted to identify relevant studies. Thirty-six studies were classified into six models of co-location: HIV care co-located with multiple ancillary services, tuberculosis (TB) care, non-HIV specific primary care, drug abuse treatment, prevention of mother to child transmission programs (PMTCT), and mental health care. More evidence of a positive association was seen for linkage to care and antiretroviral therapy (ART) uptake than for retention and viral suppression. Models of co-location that addressed HIV and non-HIV medical care issues (i.e., co-location with non-HIV specific primary care, PMTCT, and TB) had more positive associations, particularly for linkage to care and ART uptake, than other co-location models. While some findings are encouraging, more research with rigorous study designs is needed to strengthen the evaluation of, and evidence for, service co-location.
Keywords: systematic review, HIV care outcomes, service co-location
INTRODUCTION
Antiretroviral therapy (ART) has been proven not only to improve health outcomes for persons with HIV (PWH) but also to substantially reduce the risk of sexual transmission of HIV (Cohen et al., 2011). With the advent of this powerful biomedical prevention tool, HIV prevention planners are now poised to maximize the number of PWH who are linked to and retained in care for sustained viral suppression. Addressing structural-level barriers to care, particularly access to HIV care, through service integration (CDC, 2009; CDC, 2012; World Health Organization (WHO), 2012), may be one of the important strategies to consider.
Co-location of services (hereafter referred to as “service co-location”) is one model of service integration or one of the steps towards comprehensive service integration (Carter et al., 2013; Sweeney et al., 2012; Sylla, Bruce, Kamarulzaman & Altice, 2007). Also referred to as a “one-stop shopping” model of service delivery, service co-location aims to facilitate patients’ access to multiple services by offering them onsite in a single location (Carter et al., 2013; Johnson et al., 2003). Thus, it is expected to remove structural/physical barriers to care and services (e.g., lack of transportation, services provided in separate locations) and to simultaneously address multiple co-occurring clinical and social service needs of PWH (Bauman et al., 2013; Mizuno et al., 2015). A previous meta-analysis (Suthar, Rutherford, Horvath, Doherty, & Negussie, 2014) has shown that integrating ART into maternal, newborn and child health care, tuberculosis (TB), and opiate substitution therapy services was associated with improvements in ART coverage. To our knowledge, no systematic review has comprehensively examined the associations between a variety of service co-location models and HIV care outcomes, including linkage to HIV care, retention in HIV care, ART uptake, and viral suppression among PWH.
The purpose of this systematic review is to examine the following research questions: 1) what models of service co-location have been studied; and 2) is service co-location positively associated with better HIV care outcomes (e.g., higher rates of linkage, retention, ART uptake, and viral suppression)?
METHODS
Database and search strategy
We searched the CDC’s Prevention Research Synthesis (PRS) project cumulative database of HIV, AIDS, and STI literature (Reference Hidden). The database was comprised of citations located through four comprehensive search strategies implemented annually to identify 1) behavioral risk reduction interventions; 2) medication adherence interventions; 3) linkage to, retention in and re-engagement in HIV medical care interventions; and 4) HIV prevention related systematic reviews. The four strategies were used in systematic searches in MEDLINE, CINAHL, EMBASE, Global Health, PsycINFO and Sociological Abstracts databases and in on-going manual searches (reference list checks, hand searches, etc.). Librarians with systematic search experience developed and tested each search in MEDLINE and translated the searches to the other databases (DeLuca et al., 2011). As of April 2016, these searches collected over 77,000 citations from 1988 through 2016. (See Appendix for the MEDLINE search for each of the four strategies).
For this review, we developed a list of more than 60 co-location terms to search title, abstract and keywords of each citation in the PRS database as of April 2016 (see Appendix for full list of terms used to search the PRS database). Additional manual searches for this review were performed by checking the references of pertinent studies and the references of previously published systematic reviews on the HIV care to obtain relevant studies.
Study selection criteria
Studies were eligible for this systematic review if they: 1) examined service co-location, defined as offering at least one other service (e.g., medical care for another disease or condition, ancillary service such as case management, transportation, insurance assistance, etc.) at the same facility as PWH received their HIV medical care (Liau et al., 2013); 2) reported any relevant HIV care outcome: linkage to care (e.g., newly-diagnosed PWH having their first HIV care visit), retention in care (e.g., having at least two HIV medical visits within a specified period of time), uptake of ART (e.g., initiating ART or time from diagnosis to initiated ART), and viral suppression (HIV viral load below a cut-off point defined by the authors of primary studies); and 3) conducted a statistical test to assess the association between service co-location and at least one of the aforementioned outcomes. Studies were excluded if they 1) only reported results from qualitative studies; 2) were systematic reviews, commentaries, editorials, or conference abstracts; or 3) were written in non-English languages. Citations were screened initially by title/abstract and included citations were further screened with full reports to confirm eligibility.
Data abstraction
Using a standardized coding form, three coders abstracted the following information from each of the eligible studies based on full reports: study characteristics (e.g., location, objectives, setting, design, sample size, outcome measures, and limitations); participant characteristics; description of service co-location (e.g., services offered in addition to HIV medical care);and study findings (i.e., statistical tests and results of associations between service co-location and HIV care outcomes). The coders then met to compare abstracted information and resolved discrepancies via group consensus.
Determination of co-location models
Two coders independently reviewed the description of service co-location across all eligible studies and jointly came up with six co-location models that included two or more primary studies: HIV care co-located with 1) multiple ancillary services, 2) TB care, 3) non-HIV specific primary care, 4) drug abuse treatment, 5) prevention of mother to child transmission (PMTCT) services, and 6) mental health care. The pair together applied the coding scheme to each eligible study, based on which co-located services were described in a given study. Two other team members verified the coding of the co-location models.
Determination of study rigor
Few randomized controlled trials (RCTs) have been conducted to examine the effects of service co-location on HIV care outcomes, which may reflect the current state of the science. We opted to broadly assess the risk of bias, and used a three-tiered system (Liau et al., 2013) that classifies evidence based on the rigor of study designs. This three-tiered system was previously used to evaluate the research literature on engagement in care, which was at a comparable level of development as the co-location literature.
Three coders classified the eligible studies into one of the three tiers. RCTs were considered the most rigorous (Tier I) while studies that used a non-randomized comparison – either comparing pre- and post-outcomes or comparing against a separate group without randomization – were designated as Tier II. Studies that determined correlational associations from observational data were considered the least rigorous (Tier III) in terms of reducing selection bias. We summarized the findings (i.e., positive association, null association, and mixed findings defined below) with indication of tiers.
Determination of study finding per outcome per study
Due to the heterogeneity in co-location models, study designs, outcome measures, analytic approaches, and the presentation of statistical results across studies, we conducted a qualitative synthesis rather than a meta-analysis. We used the following rules to determine a finding for each outcome per study. If unadjusted and adjusted findings were both reported, we focused on the adjusted findings. When studies reported multiple findings for a specific outcome due to utilizing equally valid multiple measures, we designated the evidence as: 1) a “positive association” if >50% of the results showed statistically significant (p<0.05) positive associations between co-location and the outcome; 2) a “mixed result” if 50% of the results showed statistically significant positive associations; and 3) a “null association” if <50% of the results showed statistically significant positive associations.
RESULTS
Study characteristics
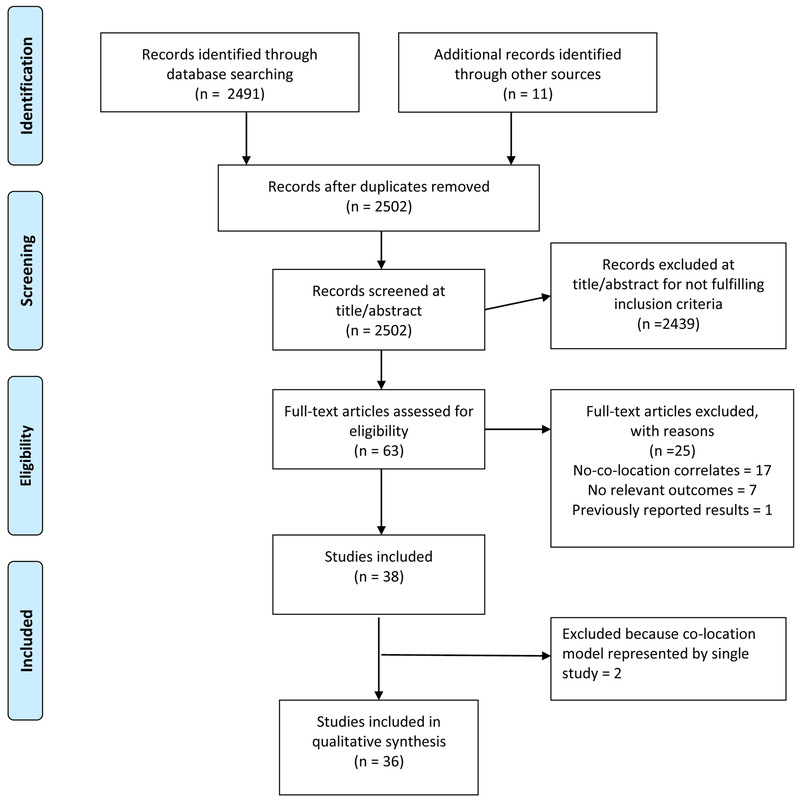
The study selection process is summarized in Figure 1. Among the 36 studies included, 15 studies (42%) were from the United States [U.S.] and 21 were international studies. U.S. studies were conducted in large cities with high HIV prevalence. International studies were primarily conducted in African countries (e.g., Kenya, Mozambique, South Africa, and Zambia). The study setting included various types of clinics and facilities such as HIV clinics, TB clinics, methadone maintenance clinics, medical centers, and antenatal clinics.
Figure 1.
Study Flow Diagram
Over one-half (k=19) of the studies assessed ART uptake as an outcome, and 42% (k=15) assessed viral suppression as an outcome. Eight studies assessed retention in care and six assessed linkage to care outcomes. These care outcomes were operationalized in a variety of ways; this was particularly true for the retention outcome. Only three studies (8%) were RCTs (Tier I), 15 studies (42%) had a comparison (Tier II) and 18 studies (50%) reported correlational data (Tier III). Sample sizes varied substantially across studies (range: 24 to 36,411).
Models of service co-location
Supplementary table summarizes characteristics of the 36 studies by six models of service co-location, presented in descending order by the number of studies that were conducted, and within a model by outcome. The model “HIV care co-located with multiple ancillary services” was the most frequently reported (k=11) and these studies were conducted mostly in the U.S. (k=9/11, 82%). Four out of the six studies of the model “HIV care co-located with drug abuse treatment” and both studies of the model “HIV care co-located with mental health care” were conducted in the U.S. “HIV care co-located with TB care” (k=8), “HIV care co-located with non-HIV specific primary care” (k=6), and “HIV care co-located with PMTCT” (k=3) were examined only in international studies.
Associations between service co-location and HIV care outcomes
Table 1 summarizes the 48 sets of findings reported in the 36 studies. Twenty-nine (60%) were positive associations between service co-location and HIV care outcomes, 18 (38%) were null associations, and one (2%) was of mixed findings. Fifty percent of the Tier I study findings showed positive associations, compared to 63% of the Tier II study findings and 60% of the Tier III study findings showing positive associations. Sixty-eight per cent of international studies’ findings and 50% of U.S. findings were positive associations.
Table 1.
Summary of overall findings (association between co-location of services and HIV continuum of care outcomes) (Total set of findings = 48 from 36 studies)
| Positive Association | Null Association | Mixed findings | Total by outcome | |
|---|---|---|---|---|
| Linkage to care | 6(100%)a | 0 | 0 | 6 |
| Retention in care | 3(37.5%)a | 4 (50%)a | 1(12.5%)a | 8 |
| ART uptake | 14 (74%)a | 5 (26%)a | 0 | 19 |
| Viral suppression | 6(40%)a | 9 (60%)a | 0 | 15 |
| Total by association |
29 (60%)a Tier I: 3 (50%)a Tier II: 12 (63%)a Tier III: 14 (60%)a US: 10 (50%)a International: 19 (68%)a |
18 (38%)a Tier I: 3 (50%)a Tier II: 6 (37%)a Tier III: 9 (36%)a US: 10 (50%)a International: 8 (29%)a |
1(2%)a Tier III: 1 (4%)a US: 0 (0%) International: 1(3%)a |
48 |
Column percentage
With regard to each HIV care outcome, all (100%) of the six findings that reported linkage to care outcomes and almost three-quarters (74%) of the 19 findings that reported ART uptake were positive associations. For the eight findings on retention in care, 50% showed null associations, 37.5% showed positive associations, 12.5% was of mixed results. For the 15 findings on viral suppression, 60% showed null associations and 40% showed positive associations.
Differences by co-location model
Table 2 summarizes the associations between co-location and HIV care outcomes by the six co-location models by counting the number of findings with positive association, null association or mixed findings. Eight of the 10 findings (80%) from the model “HIV care co-located with non-HIV specific primary care” were positive associations. Among these eight positive findings, three were for linkage to care, one was for retention, and four were for ART uptake. Four of the five findings (80%) of “HIV care co-located with PMTCT” were positive associations; two positive findings were for linkage and the other two were for ART uptake. Six out of the eight findings (75%) of “HIV care co-located with TB treatment” were positive associations; all six positive associations were for ART uptake. Five of the nine findings (56%) of the model “HIV care co-located with drug abuse treatment” were positive associations; one positive association was for retention, two for ART uptake, and two for viral suppression. Five (38.5%) of the 13 findings of the model “HIV care co-located with multiple ancillary services” were positive associations; one positive association was for linkage, one for retention, and three for viral suppression. Findings from the models “HIV care co-located with mental health care” showed more null associations than positive associations (see Table 2).
Table 2.
Summary of findings (association between co-location of services and HIV continuum of care outcomes) by model of co-location (Total set of findings = 48 from 36 studies)
| Multiple ancillary services |
TB care | Non-HIV primary specific primary care |
Drug abuse treatment |
PMTCT | Mental health care |
|
|---|---|---|---|---|---|---|
| Significant positive association | ||||||
| Linkage to care | 1 | 3 | 2 | |||
| Retention in care | 1 | 1 | 1 | |||
| ART uptake | 6 | 4 | 2 | 2 | ||
| Viral | 3 | 2 | 1 | |||
| Subtotal | 5 (38.5%) | 6 (75.0%) | 8 (80.0%) | 5 (55.6%) | 4 (80.0%) | 1 (33.3%) |
| Null association | ||||||
| Linkage to care | ||||||
| Retention in care | 1 | 1 | 1 | 1 | ||
| ART uptake | 1 | 1 | 1 | 1 | 1 | |
| Viral | 5 | 1 | 3 | |||
| Subtotal | 7(53.8%) | 2 (25%) | 2 (20.0%) | 4 (44.4%) | 1 (20.0%) | 2 (66.7%) |
| Mixed findings | ||||||
| Linkage to care | ||||||
| Retention in care | 1 | |||||
| ART uptake | ||||||
| Viral | ||||||
| Subtotal | 1 (7.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Total count | 13 | 8 | 10 | 9 | 5 | 3 |
DISCUSSION
Summary of findings and implications
Overall, there were more positive associations (60%) than null (38%) or mixed (2%) associations between service co-location and HIV care outcomes. More evidence of a positive association was seen for linkage (100%) and ART uptake (74%) outcomes, while more evidence of a null or mixed association was seen for retention (62.5%) and viral suppression (60%) outcomes. Tier II and III study findings were more likely to be positive than more rigorous Tier I study findings, and international studies’ findings were more likely to be positive than U.S. findings.
The co-location models that showed more (>70%) positive associations were those that also addressed non-HIV medical care issues, i.e., co-location with non-HIV specific primary care, with PMTCT, and with TB care, showing associations particularly with linkage and ART uptake outcomes. These were all tested in international settings, and this may explain one of the reasons why the international studies’ findings showed more positive associations. It is noteworthy that the models of co-location with TB care and with drug abuse treatment were likely to be tested with more rigorous study designs (Tier II as opposed to Tier III) suggesting that the evidence for these models may be stronger. The findings for these models are consistent with findings of a meta-analysis conducted by Suthar and colleagues (2014). However, we also found inconclusive evidence that these models are effective in improving retention in care. It is plausible that service integration itself may not solve problems such as shortages of human resources and inadequate infrastructure that are often the main challenges in resource-limited international settings (Turan et al., 2015).
The models of co-location with drug abuse treatment and with mental health care were primarily tested in the U.S. The association between the model of co-location with drug abuse treatment and ART uptake appears slightly stronger than the association with retention in care or viral suppression. The model of co-location with mental health care showed more null than positive associations; however, the evidence is only based on three findings. A plausible reason is that some mentally ill patients might have already been well-connected to both mental health and HIV care and, as a result, service co-location might have a negligible effect for such a group (Sullivan et al., 2006). The model of co-location with multiple ancillary services was also primarily tested in the U.S. Although the model would be expected to simultaneously and holistically address multiple health and social needs of PWH, we observed fewer positive findings (38.5%) than null findings (53.8%).
Our review found more null (60%) than positive (40%) associations for viral suppression outcomes. It is not surprising as viral suppression is a more “distal” outcome in the sense that it requires PWH to be retained in HIV care and to strictly adhere to a medication regimen, thus merely co-locating services may not be sufficient to achieve this outcome. A significantly higher proportion of findings from U.S. studies (60%) than from international studies (10.7%) assessed viral suppression as an outcome, which may also explain one of the reasons why the U.S. studies’ findings showed fewer positive associations.
Limitations of the studies reviewed
Only three of the studies we reviewed were randomized controlled trials and most studies were not specifically designed to directly test whether service co-location improves HIV care outcomes. As noted above, cross-examination of associations and rigor of study design suggests that positive associations tended to be observed in less rigorous Tier II and Tier III studies. In addition, potential bias associated with study methodology (e.g., use of not strictly comparable comparison groups, selection bias and non-generalizability, missing data or poor data quality, small sample sizes) has been noted as a limitation in many of the studies. We also observed a lack of standardization for outcome measures, particularly for retention outcomes across these studies.
Limitations of this review
First, although we searched a comprehensive HIV, AIDS and STI literature database using more than 60 search terms (see Appendix for complete list) that broadly cover the concept of service co-location, we might have missed studies due to the evolving array of terms used to describe the research. Additionally, the search located non-English language literature that we excluded at screening of title and abstract. Second, given the lack of studies with rigorous methodology, we opted to broadly assess the risk of bias based on study designs (i.e., a Tier system). Third, our methods to determine a study finding per outcome per study, specifically, the 50% cutoff point in case of multiple findings, is somewhat arbitrary. In addition, we treated different measures for the same outcome used across the studies equally when they could have different clinical meanings. Finally, due to the heterogeneity in study methods and outcome measures, we were unable to compare the strength of the association across studies. However, our findings were consistent with those of the meta-analysis conducted by Suthar and colleagues (2014) for the association between ART uptake and co-location of HIV/TB as well as co-location of HIV/non- HIV primary care.
Research gaps and future directions
Despite the limitations, our findings show that service co-location has the potential to improve some HIV care outcomes, especially for linkage to care and ART uptake. However, more research with rigorous study des igns is needed in order to strengthen the evaluation of, and evidence for, service co-location. Further examination of whether service co-location is the best way of improving retention in care, medication adherence, and ultimately viral suppression, is also a welcome quest. Research that identifies the specific types of ancillary services to co-locate, and determines what types of models work best for specific sub-populations, especially those requiring mental health care, will expand our understanding of how to use this structural intervention. Finally, studies that assess the relative cost-efficiency of these approaches (Sweeny et al., 2015) and answer program implementation questions (e.g., which co-located services should be provided by the same or different staff, what is the optimum number of staff needed and how should they be trained and supervised, how best to facilitate communication among staff providing different services, how service utilization data and medical records should be integrated into one data system, and how effective models of co-located services are adapted for different settings) will provide valuable insights for policy and program decisions.
CONCLUSION
This systematic review shows some evidence that service co-location that addresses HIV and non-HIV medical issues may improve linkage and ART uptake, but shows inconclusive evidence for retention and viral suppression. This review contributes to the evidence base demonstrating potential positive effects of structural interventions on HIV care outcomes.
Supplementary Material
Acknowledgments
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention.
REFERENCES
- Achmad YM, Istiqomah AN, Iskandar S, Wisaksana R, van Crevel R, & Hidayat T (2009). Integration of methadone maintenance treatment and HIV care for injecting drug users: A cohort study in Bandung, Indonesia. Acta Medica Indonesiana, 41 Suppl 1, 23–27 [PubMed] [Google Scholar]
- Adjorlolo-Johnson G, Wahl Uheling A, Ramachandran S, Strasser S, Kouakou J, Tindyebwa D, … Marlink R (2013). Scaling up pediatric HIV care and treatment in Africa: Clinical site characteristics associated with favorable service utilization. Journal of Acquired Immune Deficiency Syndromes, 62(1), e7–e13. doi: 10.1097/QAI.0b013e3182706401 [DOI] [PubMed] [Google Scholar]
- Altice FL, Bruce RD, Lucas GM, Lum PJ, Korthuis PT, Flanigan TP, … Finkelstein R (2011). HIV treatment outcomes among HIV-infected, opioid-dependent patients receiving buprenorphine/naloxone treatment within HIV clinical care settings: Results from a multisite study. Journal of Acquired Immune Deficiency Syndromes, 56 Suppl 1, S22–32. doi: 10.1097/QAI.0b013e318209751e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachireddy C, Soule MC, Izenberg JM, Dvoryak S, Dumchev K, & Altice FL (2014). Integration of health services improves multiple healthcare outcomes among HIV-infected people who inject drugs in Ukraine. Drug and Alcohol Dependence, 134, 106–114. doi: 10.1016/j.drugalcdep.2013.09.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman LJ, Braunstein S, Calderon Y, Chhabra R, Cutler B, Leider J, … Watnick D (2013). Barriers and facilitators of linkage to HIV primary care in New York City. Journal of Acquired Immune Deficiency Syndromes, 64 Suppl 1, S20–26. doi: 10.1097/QAI.0b013e3182a99c19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AJ, Bourgeois S, O'Brien N, Abelsohn K, Tharao W, Greene S, … Loutfy MR (2013). Women-specific HIV/AIDS services: Identifying and defining the components of holistic service delivery for women living with HIV/AIDS. Journal of the International AIDS Society, 16, 17433. doi: 10.7448/ias.16.1.17433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2009). Program collaboration and service integration: Enhancing the prevention and control of HIV/AIDS, viral hepatitis, sexually transmitted diseases, and tuberculosis in the United States. Atlanta (GA): U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Retrieved from: https://www.cdc.gov/nchhstp/programintegration/docs/207181-c_nchhstp_pcsi-whitepaper-508c.pdf [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2012). Integrated prevention services for HIV infection, viral hepatitis, sexually transmitted diseases, and tuberculosis for persons who use drugs illicitly: Summary guidance from CDC and the U.S. Department of Health and Human Services. MMWR: Recommendations and Reports, 61(RR-5), 1–40 [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC). (2016). Partnerships for Care (P4C): Health Departments and Health Centers Collaborating to Improve HIV Health Outcomes. Retrieved from https://www.cdc.gov/hiv/research/demonstration/p4c/index.html
- Centers for Disease Control and Prevention (CDC) and Health Resources and Services Administration (HRSA) P4C Federal Project Team. (2015, December). Partnerships for Care (P4C): Health Departments and Health Centers Collaborating to Improve HIV Health Outcomes. (Panel Session C03, Abstracts 1609, 1626, 1634, 1658). 2015 National HIV Prevention Conference, Atlanta, Georgia. [Google Scholar]
- Hidden Reference
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, … Fleming TR (2011). Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine, 365(6), 493–505. doi: 10.1056/NEJMoa1105243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman SM, Blashill AJ, Gandhi RT, Safren SA, & Freudenreich O (2012). Impact of integrated and measurement-based depression care: Clinical experience in an HIV clinic. Psychosomatics, 53(1), 51–57. doi: 10.1016/j.psym.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Craw JA, Gardner LI, Marks G, Rapp RC, Bosshart J, Duffus WA, … Schmitt K (2008). Brief strengths-based case management promotes entry into HIV medical care: Results of the antiretroviral treatment access study-II. Journal of Acquired Immune Deficiency Syndromes, 47(5), 597–606. doi: 10.1097/QAI.0b013e3181684c51 [DOI] [PubMed] [Google Scholar]
- Davila JA, Miertschin N, Sansgiry S, Schwarzwald H, Henley C, & Giordano TP (2013). Centralization of HIV services in HIV-positive African-American and Hispanic youth improves retention in care. AIDS Care, 25(2), 202–206. doi: 10.1080/09540121.2012.689811 [DOI] [PubMed] [Google Scholar]
- DeLuca J, Mullins MM, Lyles CM, Crepaz N, Kay LS, & Thadiparti S (2011). Developing a comprehensive search strategy for evidence-based systematic review. Evidence Based Library and Information Practice, 3, 3–32. doi: 10.18438/B8KP66 [DOI] [Google Scholar]
- Frick P, Tapia K, Grant P, Novotny M, & Kerzee J (2006). The effect of a multidisciplinary program on HAART adherence. AIDS Patient Care and STDS, 20(7), 511–524. doi: 10.1089/apc.2006.20.511 [DOI] [PubMed] [Google Scholar]
- Greig J, O'Brien DP, Ford N, Spelman T, Sabapathy K, & Shanks L (2012). Similar mortality and reduced loss to follow-up in integrated compared with vertical programs providing antiretroviral treatment in sub-saharan Africa. Journal of Acquired Immune Deficiency Syndromes, 59(5), e92–98. doi: 10.1097/QAI.0b013e31824206c7 [DOI] [PubMed] [Google Scholar]
- Higa DH, Crepaz N, Mullins MM, & Prevention Research Synthesis Project. (2016). Identifying best practices for increasing linkage to, retention, and re-engagement in HIV medical care: Findings from a systematic review, 1996-2014. AIDS and Behavior, 20(5), 951–966. doi: 10.1007/s10461-015-1204-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horberg MA, Hurley LB, Silverberg MJ, Kinsman CJ, & Quesenberry CP (2007). Effect of clinical pharmacists on utilization of and clinical response to antiretroviral therapy. Journal of Acquired Immune Deficiency Syndromes, 44(5), 531–539. doi: 10.1097/QAI.0b013e318031d7cd [DOI] [PubMed] [Google Scholar]
- Horberg MA, Hurley LB, Towner WJ, Allerton MW, Tang BT, Catz SL, … Quesenberry CP (2012). Determination of optimized multidisciplinary care team for maximal antiretroviral therapy adherence. Journal of Acquired Immune Deficiency Syndromes, 60(2), 183–190. doi: 10.1097/QAI.0b013e31824bd605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerga H, Spillane H, Guerrero W, Odongo A, & Varaine F (2010). Impact of introducing human immunodeficiency virus testing, treatment and care in a tuberculosis clinic in rural Kenya. International Journal of Tuberculosis and Lung Disease, 14(5), 611–615 [PubMed] [Google Scholar]
- Ikeda JM, Tellez CA, Hudes ES, Page K, Evans J, Racancoj O, & Hearst N (2014). Impact of integrating HIV and TB care and treatment in a regional tuberculosis hospital in rural Guatemala. AIDS and Behavior, 18 Suppl 1, S96–103. doi: 10.1007/s10461-013-0595-9 [DOI] [PubMed] [Google Scholar]
- Johnson RL, Botwinick G, Sell RL, Martinez J, Siciliano C, Friedman LB, … Bell D (2003). The utilization of treatment and case management services by HIV-infected youth. Journal of Adolescent Health, 33(2 Suppl), 31–38 [DOI] [PubMed] [Google Scholar]
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2014, October). 90-90-90 an ambitious treatment target to help end the AIDS epidemic. Retrieved from: http://www.unaids.org/en/resources/documents/2014/90-90-90.
- Joint United Nations Programme on HIV/AIDS (UNAIDS). (2010, September). Combination HIV prevention: Tailoring and coordinating biomedical, behavioural and structural strategies to reduce new HIV infections: A UNAIDS discussion paper. Retrieved from: http://www.unaids.org/sites/default/files/media_asset/JC2007_Combination_Prevention_paper_en_0.pdf.
- Kerschberger B, Hilderbrand K, Boulle AM, Coetzee D, Goemaere E, De Azevedo V, & Van Cutsem G (2012). The effect of complete integration of HIV and TB services on time to initiation of antiretroviral therapy: A before-after study. PloS One, 7(10), e46988. doi: 10.1371/journal.pone.0046988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killam WP, Tambatamba BC, Chintu N, Rouse D, Stringer E, Bweupe M, … Stringer JS (2010). Antiretroviral therapy in antenatal care to increase treatment initiation in HIV-infected pregnant women: A stepped-wedge evaluation. AIDS, 24(1), 85–91. doi: 10.1097/QAD.0b013e32833298be [DOI] [PubMed] [Google Scholar]
- Lahuerta M, Lima J, Nuwagaba-Biribonwoha H, Okamura M, Alvim MF, Fernandes R, … Nash D (2012). Factors associated with late antiretroviral therapy initiation among adults in Mozambique. PloS One, 7(5), e37125. doi: 10.1371/journal.pone.0037125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb MR, El-Sadr WM, Geng E, & Nash D (2012). Association of adherence support and outreach services with total attrition, loss to follow-up, and death among ART patients in sub-Saharan Africa. PloS One, 7(6), e38443. doi: 10.1371/journal.pone.0038443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambdin BH, Micek MA, Sherr K, Gimbel S, Karagianis M, Lara J, … Pfeiffer J (2013). Integration of HIV care and treatment in primary health care centers and patient retention in central Mozambique: A retrospective cohort study. Journal of Acquired Immune Deficiency Syndromes, 62(5), e146–152. doi: 10.1097/QAI.0b013e3182840d4e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn SD, Campbell L, Kaplan R, Little F, Morrow C, & Wood R (2011). Delays in starting antiretroviral therapy in patients with HIV-associated tuberculosis accessing non-integrated clinical services in a South African township. BMC Infectious Diseases, 11, 258. doi: 10.1186/1471-2334-11-258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon A, Caceres C, Fernandez E, Chausa P, Martin M, Codina C, … Garcia F (2011). A new multidisciplinary home care telemedicine system to monitor stable chronic human immunodeficiency virus-infected patients: A randomized study. PloS One, 6(1), e14515. doi: 10.1371/journal.pone.0014515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liau A, Crepaz N, Lyles CM, Higa DH, Mullins MM, DeLuca J, … Marks G(2013). Interventions to promote linkage to and utilization of HIV medical care among HIV-diagnosed persons: A qualitative systematic review, 1996-2011. AIDS and Behavior, 17(6), 1941–1962. doi: 10.1007/s10461-013-0435-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louwagie G, Girdler-Brown B, Odendaal R, Rossouw T, Johnson S, & Van der Walt M (2012). Missed opportunities for accessing HIV care among Tshwane tuberculosis patients under different models of care. International Journal of Tuberculosis and Lung Disease, 16(8), 1052–1058. doi: 10.5588/ijtld.11.0753 [DOI] [PubMed] [Google Scholar]
- Lucas GM, Chaudhry A, Hsu J, Woodson T, Lau B, Olsen Y, … Moore RD (2010). Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: A randomized trial. Annals of Internal Medicine, 152(11), 704–711. doi: 10.7326/0003-4819-152-11-201006010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas GM, Mullen BA, Weidle PJ, Hader S, McCaul ME, & Moore RD (2006). Directly administered antiretroviral therapy in methadone clinics is associated with improved HIV treatment outcomes, compared with outcomes among concurrent comparison groups. Clinical Infectious Diseases, 42(11), 1628–1635. doi: 10.1086/503905 [DOI] [PubMed] [Google Scholar]
- Ma A, Chen DM, Chau FM, & Saberi P (2010). Improving adherence and clinical outcomes through an HIV pharmacist's interventions. AIDS Care, 22(10), 1189–1194. doi: 10.1080/09540121003668102 [DOI] [PubMed] [Google Scholar]
- Mizuno Y, Purcell DW, Knowlton AR, Wilkinson JD, Gourevitch MN, & Knight KR (2015). Syndemic vulnerability, sexual and injection risk behaviors, and HIV continuum of care outcomes in HIV-positive injection drug users. AIDS and Behavior, 19(4), 684–693. doi: 10.1007/s10461-014-0890-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer J, Montoya P, Baptista AJ, Karagianis M, Pugas Mde M, Micek M, … Gloyd S (2010). Integration of HIV/AIDS services into African primary health care: Lessons learned for health system strengthening in Mozambique - a case study. Journal of the International AIDS Society, 13, 3. doi: 10.1186/1758-2652-13-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SJ, De Palomo F, Little SE, & Woldehanna S (2013). Testing a service integration model: Results from the HIV/AIDS initiative, ConnectHIV. AIDS Care, 25(3), 317–325. doi: 10.1080/09540121.2012.712657 [DOI] [PubMed] [Google Scholar]
- Schulz SA, Draper HR, & Naidoo P (2013). A comparative study of tuberculosis patients initiated on ART and receiving different models of TB-HIV care. International Journal of Tuberculosis and Lung Disease, 17(12), 1558–1563. doi: 10.5588/ijtld.13.0247 [DOI] [PubMed] [Google Scholar]
- Schwartz AB, Tamuhla N, Steenhoff AP, Nkakana K, Letlhogile R, Chadborn TR, … Bisson GP (2013). Outcomes in HIV-infected adults with tuberculosis at clinics with and without co-located HIV clinics in Botswana. International Journal of Tuberculosis and Lung Disease, 17(10), 1298–1303. doi: 10.5588/ijtld.12.0861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen JL, Haug NA, Larios S, Gruber VA, Tulsky J, Powelson E, … Shapiro B (2012). Directly administered antiretroviral therapy: Pilot study of a structural intervention in methadone maintenance. Journal of Substance Abuse Treatment, 43(4), 418–423. doi: 10.1016/j.jsat.2012.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson K, Boulle A, Coetzee D, Abrams EJ, & Myer L (2010). Initiation of highly active antiretroviral therapy among pregnant women in Cape Town, South Africa. Tropical Medicine and International Health, 15(7), 825–832. doi: 10.1111/j.1365-3156.2010.02538.x [DOI] [PubMed] [Google Scholar]
- Sullivan G, Kanouse D, Young AS, Han X, Perlman J, & Koegel P (2006). Co-location of health care for adults with serious mental illness and HIV infection. Community Mental Health Journal, 42(4), 345–361. doi: 10.1007/s10597-006-9053-8 [DOI] [PubMed] [Google Scholar]
- Suthar AB, Rutherford GW, Horvath T, Doherty MC, & Negussie EK (2014). Improving antiretroviral therapy scale-up and effectiveness through service integration and decentralization. AIDS, 28 Suppl 2, S175–185. doi: 10.1097/qad.0000000000000259 [DOI] [PubMed] [Google Scholar]
- Sweeney S, Obure CD, Maier CB, Greener R, Dehne K, & Vassall A (2012). Costs and efficiency of integrating HIV/AIDS services with other health services: A systematic review of evidence and experience. Sexually Transmitted Infections, 88(2), 85–99. doi: 10.1136/sextrans-2011-050199 [DOI] [PubMed] [Google Scholar]
- Sylla L, Bruce RD, Kamarulzaman A, & Altice FL (2007). Integration and co-location of HIV/AIDS, tuberculosis and drug treatment services. International Journal on Drug Policy, 18(4), 306–312. doi: 10.1016/j.drugpo.2007.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsague L, Tsiouris FO, Carter RJ, Mugisha V, Tene G, Nyankesha E, … Abrams EJ (2010). Comparing two service delivery models for the prevention of mother-to-child transmission (PMTCT) of HIV during transition from single-dose nevirapine to multi-drug antiretroviral regimens. BMC Public Health, 10, 753. doi: 10.1186/1471-2458-10-753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turan JM, Onono M, Steinfeld RL, Shade SB, Owuor K, Washington S, … Cohen CR(2015). Implementation and Operational Research: Effects of Antenatal Care and HIV Treatment Integration on Elements of the PMTCT Cascade: Results From the SHAIP Cluster-Randomized Controlled Trial in Kenya. Journal of Acquired Immune Deficiency Syndromes, 69(5), e172–181. doi: 10.1097/qai.0000000000000678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Rie A, Patel MR, Nana M, Vanden Driessche K, Tabala M, Yotebieng M, & Behets F (2014). Integration and task shifting for TB/HIV care and treatment in highly resource-scarce settings: One size may not fit all. Journal of Acquired Immune Deficiency Syndromes, 65(3), e110–117. doi: 10.1097/01.qai.0000434954.65620.f3 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2012). WHO policy on collaborative TB/HIV activities: Guidelines for national programs and other stakeholders. Retrieved from http://www.who.int/tb/publications/2012/tb_hiv_policy_9789241503006/en/ [PubMed]
- Zaller N, Gillani FS, & Rich JD (2007). A model of integrated primary care for HIV-positive patients with underlying substance use and mental illness. AIDS Care, 19(9), 1128–1133. doi: 10.1080/09540120701335196 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.