Abstract
Background
Influenza is a highly infectious viral disease that is particularly common in the winter months. Oscillococcinum® is a patented homeopathic medicine that is made from a 1% solution of wild duck heart and liver extract, which is then serially diluted 200 times with water and alcohol.
Objectives
To determine whether homeopathic Oscillococcinum® is more effective than placebo in the prevention and/or treatment of influenza and influenza‐like illness in adults or children.
Search methods
We searched CENTRAL (2014, Issue 8), MEDLINE (1966 to August week 4, 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (4 September 2014), AMED (2006 to September 2014), Web of Science (1985 to September 2014), LILACS (1985 to September 2014) and EMBASE (1980 to September 2014). We contacted the manufacturers of Oscillococcinum® for information on further trials.
Selection criteria
Randomised, placebo‐controlled trials of Oscillococcinum® in the prevention and/or treatment of influenza and influenza‐like illness in adults or children.
Data collection and analysis
Three review authors independently extracted data and assessed risk of bias in the eligible trials.
Main results
No new trials were included in this 2014 update. We included six studies: two prophylaxis trials (327 young to middle‐aged adults in Russia) and four treatment trials (1196 teenagers and adults in France and Germany). The overall standard of trial reporting was poor and hence many important methodological aspects of the trials had unclear risk of bias. There was no statistically significant difference between the effects of Oscillococcinum® and placebo in the prevention of influenza‐like illness: risk ratio (RR) 0.48, 95% confidence interval (CI) 0.17 to 1.34, P value = 0.16. Two treatment trials (judged as 'low quality') reported sufficient information to allow full data extraction: 48 hours after commencing treatment, there was an absolute risk reduction of 7.7% in the frequency of symptom relief with Oscillococcinum® compared with that of placebo (risk difference (RD) 0.077, 95% CI 0.03 to 0.12); the RR was 1.86 (95% CI 1.27 to 2.73; P value = 0.001). A significant but lesser effect was observed at three days (RR 1.27, 95% CI 1.03 to 1.56; P value = 0.03), and no significant difference between the groups was noted at four days (RR 1.11, 95% CI 0.98 to 1.27; P value = 0.10) or at five days (RR 1.06, 95% CI 0.96 to 1.16; P value = 0.25). One of the six studies reported one patient who suffered an adverse effect (headache) from taking Oscillococcinum®.
Authors' conclusions
There is insufficient good evidence to enable robust conclusions to be made about Oscillococcinum® in the prevention or treatment of influenza and influenza‐like illness. Our findings do not rule out the possibility that Oscillococcinum® could have a clinically useful treatment effect but, given the low quality of the eligible studies, the evidence is not compelling. There was no evidence of clinically important harms due to Oscillococcinum®.
Plain language summary
Homeopathic Oscillococcinum® for preventing and treating influenza and influenza‐like illness
Review question To determine whether homeopathic Oscillococcinum® is more effective than placebo in the prevention and/or treatment of influenza and influenza‐like illness in adults or children.
Background Influenza ('the flu') is a highly infectious viral respiratory disease. Other than treatments for complications (such as pneumonia), the conventional medical strategies for the prevention or treatment of flu are not entirely effective or satisfactory. Oscillococcinum® is a highly diluted homeopathic preparation manufactured from wild duck heart and liver, which may be reservoirs of flu viruses. Some people take Oscillococcinum® regularly over the winter months either to prevent flu or as a treatment for flu symptoms.
Study characteristics We included six studies, which comprised two prevention trials (a total of 327 young to middle‐aged adults in Russia) and four treatment trials (a total of 1196 teenagers and adults in France and Germany).
Key results The findings from the two prevention trials did not show that Oscillococcinum® can prevent the onset of flu. Although the results from the four other clinical trials suggested that Oscillococcinum® relieved flu symptoms at 48 hours, this might be due to bias in the trial methods. One patient reported headache after taking Oscillococcinum®. The evidence is current to September 2014.
Quality of the evidence The overall standard of research reporting was poor, and thus many aspects of the trials' methods and results were at unclear risk of bias. We therefore judged the evidence overall as low quality, preventing clear conclusions from being made about Oscillococcinum® in the prevention or treatment of flu and flu‐like illness.
Summary of findings
for the main comparison.
| Oscillococcinum®compared with placebo for treatment of influenza | ||||||
|
Patients/sample: participants aged over 12 years, with influenza‐like illness Settings: general or specialist practices, France and Germany Intervention: Oscillococcinum® twice a day for 5 days; Oscillococcinum® 3 times a day for 3 days Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Oscillococcinum® | |||||
| Absence of symptoms at 48 hours ‐ patient assessment | 90 per 1000 | 167 per 1000 (114 to 245) | RR 1.86 (1.27 to 2.73) | 796 (2 studies) | ⊕⊕⊝⊝ low | |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: we are very uncertain about the estimate. | ||||||
The assumed risk is taken as that of the patients in the placebo groups of the two relevant trials. In the absence of information from other sources, calculations for low‐, medium‐ and high‐risk populations have not been calculated.
Background
Description of the condition
Influenza is a highly infectious and prevalent viral disease that is particularly common in the autumn and winter months in temperate regions of the world; annual epidemics are associated, worldwide, with three to five million cases of severe disease and one quarter to half a million deaths per annum (WHO 2009). In high‐income countries, most deaths occur among people aged 65 or older. The 2008‐2009 pandemic strain of H1N1 ('swine flu') virus was highly infectious but of relatively low pathogenicity. However, the risk of a pandemic of a more virulent H5N1 ('avian flu') strain persists. Though several prescription‐only agents can prevent or reduce the duration of influenza, much influenza is treated in the community without the involvement of a physician.
Description of the intervention
Oscillococcinum® is a patented homeopathic medicine that is commercially available over‐the‐counter in many countries. The rationale for its use in influenza is not the standard homeopathic principle of 'let like be cured by like', but the related principle of 'isopathy': that a medicine derived from the causative agent of the disease, or from a product of the disease process, is used to treat the condition (Swayne 2000). The medicine is manufactured from wild duck's heart and liver, which may be reservoirs and vectors of influenza viruses (CDCP 2010; Watanabe 2011; Woo 2011).
Homeopathic medicines are prepared in a flask by a process of serial dilution with succussion (vigorous shaking with impact against an elastic stop) at each stage. Oscillococcinum® is made by the 'Korsakovian' or single‐flask method. An extract of the duck liver and heart (Anas barbariae hepatis et cordis extractum HPUS) is shaken in a flask and then poured off. A water/alcohol mixture is added to dilute the liquid, which remains on the walls of the flask (approximately 1%). This new dilution is succussed and poured off. The process is carried out serially a total of 200 times, to give a '200K' dilution or 'potency' (HPUSA 2012).
The product Oscillococcinum® is manufactured only in the 200K formulation and by one company with exclusive rights to the 'Oscillococcinum®' registered trade name. A number of other preparations of Anas barbariae hepatis et cordis extractum are also available; their formulation is similar to that of Oscillococcinum® but the precise differences in extraction and preparation are unknown and so they have potentially different attributes of biological activity.
How the intervention might work
A 200K potency is so dilute that a typical dose is unlikely to contain any molecules of the starting material (Kayne 2006). The use of high dilutions, including 'ultra‐molecular' dilutions such as 200K, is the reason that homeopathy is sometimes viewed as implausible.
Nevertheless, there is some evidence from in vitro biological models that ultra‐molecular homeopathic dilutions elicit physiological effects. A total of some 1500 experiments have been reported (Clausen 2011). In a systematic review of in vitro biological experiments with ultra‐molecular dilutions, 73% showed biological effects; many of the experiments were of high quality (Witt 2007). Seventy‐three per cent of replication experiments were positive, though no positive experimental result was stable enough to be reproduced by all research groups. The best established such model is based on inhibition of basophil activation by high dilutions of histamine; there are multiple independent and multi‐centre reproductions of this model (Endler 2010; Ste Laudy 2009).
Physical research suggests that ultra‐molecular homeopathic dilutions may possess anomalous water structure. Nuclear magnetic resonance (NMR) studies suggest the presence in ultra‐molecular dilutions of stable supra‐molecular structures, involving nanobubbles of atmospheric gases and highly ordered water around them (Demangeat 2004; Demangeat 2009). Low temperature thermoluminescence experiments on the properties of ultra‐molecular dilutions show that a 'signature' of lithium is detectable in ultra‐molecular lithium chloride (Rey 2003; Van Wijk 2006). Rational hypotheses have been advanced to explain the mechanism of action of homeopathic or ultra‐low‐dose interventions on the immune system (Bellavite 2007) or in prothrombosis (Eizayaga 2011), but it remains unknown how such ultra‐dilute physical properties might enable the physiological effects noted in the other biological models above.
Why it is important to do this review
We reviewed randomised controlled trials (RCTs) of Oscillococcinum® for the prevention and treatment of influenza or influenza‐like illness (ILI). We defined ILI as symptoms of influenza, such as cough, fever, chills and muscle pain, without a need for virological confirmation of influenza virus infection. There is uncertainty as to the extent of similarity of related preparations (see above), therefore this review focuses solely on the registered product Oscillococcinum®.
Existing prevention and treatment strategies for influenza or ILI are not entirely effective or satisfactory. Immunisation provides moderately effective protection, though evidence is lacking in adults aged 65 years or older (Osterholm 2011). There is a delay of several months between identification of the epidemic strain and the vaccine becoming available in adequate amounts (WHO 2006). The adamantanes, amantadine and rimantadine, are only active against influenza A, and drug resistance is widespread (Jefferson 2009b); their use is recommended only in emergencies when all other measures have failed. Neuraminidase inhibitors (oseltamivir (Tamiflu®) and zanamivir (Relenza®) are moderately effective in reducing the duration of influenza symptoms (Jefferson 2009a; Jefferson 2014; Wang 2012). They may be effective in preventing laboratory‐confirmed influenza, but not ILI (Jefferson 2009a; Jefferson 2014). Both drugs are associated with adverse effects (Jefferson 2014). Alternative or additional prevention and treatment strategies are therefore of interest.
Objectives
To determine whether homeopathic Oscillococcinum® is more effective than placebo in the prevention and/or treatment of influenza and influenza‐like illness in adults or children.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) with a placebo control.
Types of participants
Patients of any age (adults or children) wishing to prevent, or presenting with, influenza or ILI (symptoms of influenza such as cough, fever, chills and muscle pain, in the absence of virological evidence of infection).
Types of interventions
Oscillococcinum® in any regime. All other formulations of Anas barbariae hepatis et cordis extractum and medicines made from homeopathically prepared influenza virus, influenza vaccine or avian liver, are not included. Previous versions of this review, Vickers 2000, Vickers 2004 and Vickers 2006, included one study on 'Anas barbariae 200 CH' that was not defined by the authors as Oscillococcinum® (Attena 1995); as stated above (Description of the intervention), such a preparation may have properties that differ importantly from those of true Oscillococcinum®. Previous versions of the review also included two studies on Mucococcinum (Nollevaux 1990; Rottey 1995), a preparation comprising a variety of inactivated viruses and bacteria prepared homeopathically to a 200K potency, which is clearly different from Oscillococcinum®.
Types of outcome measures
Any measure of influenza severity or duration, except laboratory findings (for example, antibody titres).
Primary outcomes
Primary outcome measures for prophylaxis studies:
Occurrence of influenza (either symptomatic or laboratory‐confirmed)
Primary outcome measures for treatment studies:
Patient‐reported absence of influenza symptoms at 48 hours
Secondary outcomes
Secondary outcome measures for prophylaxis studies:
Adverse events
Secondary outcome measures for treatment studies:
Adverse events
Physician assessment of symptoms at 48 hours
Patient‐reported symptom relief after more than 48 hours
Concomitant medication
Search methods for identification of studies
Electronic searches
For this 2014 update we searched the Cochrane Central Register of Controlled Trials (CENTRAL 2014, Issue 8) (accessed 5 September 2014), which contains the Acute Respiratory Infections Group's Specialised Register, MEDLINE (July 2012 to August week 4, 2014), MEDLINE In‐Process & Other Non‐Indexed Citations (4 September 2014), AMED (August 2012 to September 2014), Web of Science (August 2012 to September 2014), LILACS (August 2012 to September 2014) and EMBASE (August 2012 to September 2014). Previously we searched CENTRAL (2012, Issue 7), MEDLINE (Ovid) (January 2006 to July week 4, 2012), MEDLINE In‐Process & Other Non‐Indexed Citations (6 August 2012), AMED (2006 to August 2012), Web of Science (1985 to August 2012), LILACS (1982 to August 2012) and EMBASE.com (January 2006 to August 2012). There were no language or publication restrictions. (Details of earlier searches are in Appendix 1).
We used the search strategy described in Appendix 2 to search MEDLINE and CENTRAL. We combined the MEDLINE search strategy with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the terms to search the other databases (see Appendix 3).
Searching other resources
We searched the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) and ClinicalTrials.gov trials registries for completed and ongoing studies (latest search 8 September 2014). We contacted the manufacturers of Oscillococcinum® for information, which was provided. The manufacturers of Oscillococcinum® were aware of one paper, originally published in Russian in 2005, which is a randomised controlled trial of the preventive effects of Oscillococcinum® in influenza. This paper has been translated into English and its findings are reflected in this review update.
Data collection and analysis
Selection of studies
The three review authors independently applied prospective inclusion and exclusion criteria to the literature identified. There were no disagreements about study inclusion. In particular, there was no dissent in the decision to include only those studies that investigated trademarked Oscillococcinum®.
Data extraction and management
The three review authors independently extracted data. We extracted the following data on the trial participants from included trials: inclusion and exclusion criteria and method and place of recruitment (for example, primary care). We extracted separately, by group, the following data on trial participants: number randomised, number of withdrawals, age and gender. We recorded, by group, the number of participants and number of events, or the mean and standard deviation (SD) for each outcome measure. We turned ordinal scales into binomial variables by regarding each participant as 'improved' or 'not improved', as appropriate. If variables were reported more than once per follow‐up day, we selected the results for the evening thereof. We recorded details of the treatment given and adverse events reported for the experimental and comparison groups.
In this update, we did not attempt to contact trial authors to provide data or other information that was missing from their trial reports. 'Mean time to recovery' (the main outcome measure selected by the authors of the previous versions of this review) cannot be extracted from the original trial reports, and so 'absence of patient‐assessed influenza symptoms at 48 hours' was the most appropriate measure available to us as 'primary outcome' ‐ see Types of outcome measures. As was the case in previous versions of the review, both published and unpublished studies were eligible for data extraction (see Electronic searches); thus, our adjusted approach does not render this review update any more or less prone to publication bias than its predecessors. We contacted the manufacturers of Oscillococcinum® for trial reports. We resolved disagreements between review authors by consensus.
Assessment of risk of bias in included studies
We used the following methodological categories to appraise each paper:
Sequence generation (was the allocation sequence adequately generated by, for example, a computerised random number generator?)
Allocation concealment of treatment (was treatment allocation concealed until each new patient had been unambiguously entered into the trial?)
Blinding of (a) participants and personnel; and (b) blinding of outcome assessors (was knowledge of the allocated interventions adequately prevented during the study?)
Incompleteness of outcome data (were incomplete or missing outcome data adequately addressed? Were there systematic differences in withdrawals from the trial?)
Free from selective reporting (have all the measures described in the paper's Methods been reported in the Results?)
Other potential threats to validity (was the study apparently free of other problems that could put it at risk of bias? (e.g. was there extreme baseline imbalance in the groups' participants?)).
We judged each category using the Cochrane 'Risk of bias' tool (Higgins 2011). 'Low risk' of bias indicates our opinion that the plausibly postulated bias was unlikely to alter the results seriously; 'high risk' of bias, on the other hand, indicates that plausibly postulated bias seriously weakened our confidence in the results. We judged a category 'unclear risk' if there was insufficient or no information on which to assess whether or not an important risk of bias existed. Two review authors (RTM, JF) independently judged each trial. After the first independent assessments, there was 64% accord between the two review authors across all six categories for the seven eligible papers. We readily resolved the areas of disagreement by discussion, including input from the third review author (PF).
No trial was excluded from the review if we judged it 'high risk of bias', but we regarded the findings from any such trial with increased caution.
Measures of treatment effect
We used relative treatment effect (risk ratio, RR) as the measure of choice (dichotomous data). For primary outcomes (in cases for which the RR data showed a statistically significant difference), we then also examined the absolute risk reduction (risk difference, RD). We calculated number needed to treat to benefit (NNTB) in the standard way, as a reciprocal of the risk difference. At a population level, there would be significant social gains from at least a 5% difference in the proportion of individuals prevented from acquiring influenza symptoms or in the proportion of those achieving symptom relief at 48 hours (see Implications for practice). We therefore regard the minimal clinically important benefit as a difference of 5% favouring Oscillococcinum® (i.e. RD > 0.05, corresponding to NNTB < 20). Correspondingly, we regard the minimal clinically important harm as a difference of 5% favouring placebo.
Unit of analysis issues
All eligible trials were of parallel‐group design. There were no issues in connection with non‐standard designs, such as cross‐over trials and cluster‐randomised trials.
Dealing with missing data
We noted missing data in the description of each trial. In all analyses, we have used the per‐protocol sample sizes in order to remain consistent with the data presentation and analyses in the original papers.
Assessment of heterogeneity
We used the fixed‐effect meta‐analysis model by default. We used the random‐effects model in cases where statistical heterogeneity was evident (visually, where I2 statistic > 50% and/or where Chi2 > number of degrees of freedom (df)). In such cases, we applied the more conservative of the two results.
Assessment of reporting biases
We have not specifically addressed publication bias in view of the detailed 'Risk of bias' assessment per trial and the small number of eligible trials overall.
Data synthesis
We assumed there was sufficient clinical homogeneity in influenza symptoms and in the prescription of Oscillococcinum® in the trials to enable the fixed‐effect meta‐analysis model to be used by default (see also Assessment of heterogeneity). We usually considered a meta‐analysis was appropriate when a given type of data was available from more than a single trial report.
Subgroup analysis and investigation of heterogeneity
We analysed prophylaxis and treatment trials in two distinct categories, with the investigation of heterogeneity for each particular analysis carried out as above.
One paper presented subgroup analyses on the effect of age of patient and severity of symptoms after 48 hours of treatment (Ferley 1989); that analysis was not a planned part of the study's protocol. We undertook Chi2 analysis on these data to examine more directly the comparative influence of younger/older age and of illness severity on symptom relief at 48 hours.
Sensitivity analysis
We did not carry out sensitivity analyses.
Results
Description of studies
For this 2014 update, we obtained a total of 453 records from the electronic database searches. We identified no new trials for inclusion.
Results of the search
Each of the six eligible trials (see below) reported outcomes that reflected the presence or absence of influenza or ILI, compatible with our designated primary outcomes both for prophylaxis and treatment studies.
For prophylaxis trials, 'number of subjects who fell ill' was the common outcome identifiable across the two studies concerned (Selkova 2005a; Selkova 2005b). Neither study reported adverse events.
For treatment trials, symptom relief was reported in a number of different ways. Indeed, a primary outcome measure was defined clearly by the original authors in just one of the treatment studies (Ferley 1989): the proportion of patients that reported absence of symptoms (complete resolution of five defined cardinal symptoms and rectal temperature < 37.5 °C) within 48 hours of treatment with Oscillococcinum®. The same paper examined patient‐reported symptom relief over a period of seven days following treatment, while other treatment trials, including Papp 1998, selected two to five days or more. Physician assessments of symptoms at 48 hours were also used in one paper (Papp 1998). Both these treatment trials could be included in a meta‐analysis of the primary outcome measure: patient‐assessed absence of influenza symptoms at 48 hours after receiving Oscillococcinum® or placebo (see also Notes in Characteristics of included studies: Papp 1998).
As secondary outcomes from treatment, we have included 'proportion of patients reporting absence of symptoms by three days of treatment', 'proportion of patients reporting absence of symptoms by four days of treatment' and 'proportion of patients reporting absence of symptoms by five days of treatment'. Three trials reported outcomes for individual symptoms of influenza, such as fever, chills, aches or cough (Casanova 1984; Casanova 1988; Papp 1998).
Additional secondary outcomes from the treatment studies included: physician‐assessed absence of symptoms at 48 hours; physician‐assessed improvement at 48 hours (see also Notes in the Characteristics of included studies table: Papp 1998); use of concomitant medication (Ferley 1989; Papp 1998). Only one study assessed and reported adverse events (Papp 1998).
Included studies
We included six studies in this review: two prophylaxis trials, published in a single paper by Selkova (Selkova 2005a; Selkova 2005b), with a total of 327 participants, and four treatment trials (Casanova 1984; Casanova 1988; Ferley 1989; Papp 1998), with a total of 1196 participants. All six trials compared trademarked Oscillococcinum® (Boiron) to a placebo.
Only one of the treatment trials explicitly reported that participants were accrued during an outbreak of influenza (Ferley 1989). Participants were generally recruited from primary care settings. Some trials included both children (older than 12 years) and adults. Inclusion and exclusion criteria were sometimes not described. In two of the four treatment trials, participants had to meet a defined standard for influenza‐like illness (for example, rectal temperature greater than 38 °C and at least two episodes of headache, stiffness, lumbar and articular pain or shivers). Exclusion criteria in these two trials were prior duration of symptoms for longer than 24 hours, immune deficiency, influenza vaccination or immunostimulant treatment.
Details are given in the Characteristics of included studies table.
Excluded studies
Three studies included in the previous version of the review, Vickers 2006, have been excluded in this update because we have now focused solely on patented Oscillococcinum®. Attena 1995 used a 200C potency of extract of liver and heart from Anas barbariae (not Oscillococcinum®); it was published as a letter to the Editor of a peer‐reviewed journal. Nollevaux 1990 and Rottey 1995 used a preparation of inactivated viruses and bacteria prepared to a 200K potency.
Risk of bias in included studies
The standard of trial reporting was poor or very poor. In only two trials was there sufficient information to enable data extraction of the main outcome (defined above), though some of the necessary data were extractable solely from the graphical illustrations in those papers (Ferley 1989; Papp 1998). Four studies were published in the non‐peer‐reviewed literature, in France or in Russia (Casanova 1984; Casanova 1988; Selkova 2005a; Selkova 2005b). One of the above trials was reported in a general medical magazine rather than in a scientific journal; accordingly, this trial was reported very briefly and most of the important experimental details were missing (Casanova 1984). No details of exclusions and withdrawals were given in four trials (Casanova 1984; Casanova 1988; Selkova 2005a; Selkova 2005b). The sample sizes in the trials by Selkova 2005a, Casanova 1984 and Casanova 1988 are suspiciously round numbers (100, 100, 300 respectively).
Overall, the extent of methodological bias in this set of trials is difficult to determine, as illustrated by the high frequency of the judgement 'unclear' using the 'Risk of bias' tool in RevMan 2014 (Figure 1). Specific examples of plausible bias per assessment domain are given below.
1.
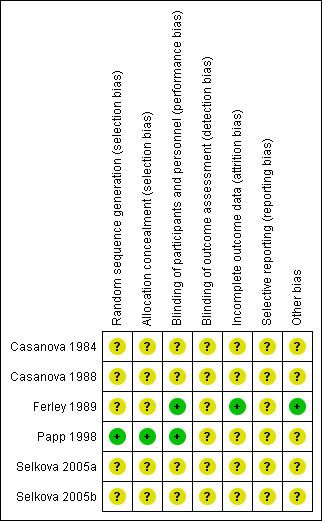
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The procedures for sequence generation and allocation concealment were adequately reported in just one paper (Papp 1998). Allocation procedures were not reported in any of the other papers; risk of bias arising from such deficiencies of reporting and/or prosecution of the trials is therefore impossible to ascertain.
Blinding
Blinding of participants and personnel was generally adequate overall, explicitly so in two trials (Ferley 1989; Papp 1998). Homeopathic medicines are generally impossible to distinguish from matching placebos because they have no inherent taste, smell or obvious adverse effects such as dry mouth, and this also is the case for Oscillococcinum®. Thus it is unlikely that bias from this source was introduced during the trials. Adequacy of blinding of outcome assessment was unclear for all trials.
Incomplete outcome data
We judged only one trial at low risk of bias in this domain (Ferley 1989). In one study, up to 10% of participants did not complete the trial; moreover, the paper did not describe details of the group‐specific reasons for drop‐out and attrition numbers throughout the paper are confused and confusing (Papp 1998). We assessed Papp 1998 as having 'unclear' risk of attrition bias, as well as all the remaining studies.
Selective reporting
All studies were 'unclear' risk of bias in this assessment domain. Amongst other areas of lack of clarity, in one of the papers the authors state that statistical analysis was "based on the mean date of elimination of symptoms" but their graphical and statistical data do not reveal such a time point; nor is it possible to extract data from the information provided in the paper (Papp 1998). Without making a number of assumptions about the precise data, it is also not possible to derive 'mean time to recovery from symptoms' from the other trial that presented time‐related information (Ferley 1989). Other concerns about statistical analysis/presentation in those two papers are summarised in the Characteristics of included studies table.
Another research group conducted two trials (Casanova 1984; Casanova 1988). The first of those trials reported data for patient assessment, chills, aches, rhinitis, night cough, day cough and fever; the second trial reported data only for temperature, chills and aches. We do not know if data on rhinitis, cough and patient assessment were recorded in the second trial but not reported. Moreover, the length of follow‐up varied between the two trials. The first reported data for day eight; the second for day four. We do not know if data were recorded daily but only the most favourable comparisons were reported. Given those considerations, the outcomes for individual symptoms are more likely to be biased than those for presence or absence of influenza or use of concomitant medication.
Other potential sources of bias
Only two of the trials presented baseline information about the study participants (Ferley 1989; Papp 1998). We nevertheless labelled Papp 1998 'unclear' risk of bias in this domain because of shortfalls in the clarity of its statistical presentation (see also Characteristics of included studies table).
Effects of interventions
See: Table 1
Prophylaxis trials
Primary outcome
Occurrence of influenza
The data on occurrence of influenza‐like illness displayed considerable heterogeneity between trials (Chi2 = 1.49, df = 1; I2 statistic = 33%). Using a random‐effects model for statistical analysis, the mean risk ratio (RR) of influenza‐like illness occurring in participants receiving treatment was 0.48 (95% confidence interval (CI) 0.17 to 1.34; P value = 0.16) (Analysis 1.1; Figure 2).
1.1. Analysis.
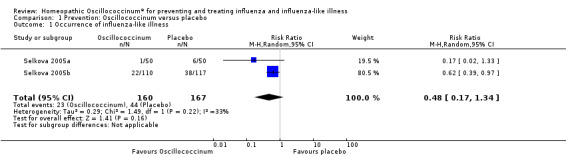
Comparison 1 Prevention: Oscillococcinum versus placebo, Outcome 1 Occurrence of influenza‐like illness.
2.
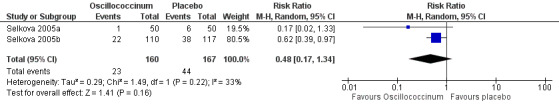
Forest plot of comparison: 1 Prevention: Oscillococcinum versus placebo. Outcome 1: Occurrence of influenza‐like illness.
Secondary outcome
Adverse events
Adverse events were not reported in either of the eligible trials.
Treatment trials
Primary outcomes
Patient‐reported absence of symptoms at 48 hours
Data from two trials showed that the mean RR for symptom absence was 1.86 (95% CI 1.27 to 2.73) (Analysis 2.1), statistically significant in favour of Oscillococcinum® (P value = 0.001; Figure 3). The two trials comprised a total of 796 participants. The mean proportion of patients who reported absence of influenza symptoms was 36/401 (= 9.0%) in the placebo groups and 66/395 (= 16.7%) in the Oscillococcinum® groups (Ferley 1989; Papp 1998), a mean difference of 7.7%. Correspondingly, the risk difference (RD) was 0.077 (95% CI 0.03 to 0.12) and so the number needed to treat to benefit (NNTB) was 13 (95% CI 9 to 34); the 95% confidence limits include the pre‐defined minimal clinically important benefit (RD 0.05, NNTB 20) ‐ see Measures of treatment effect.
2.1. Analysis.
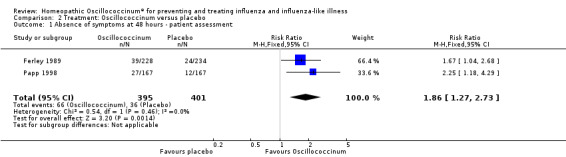
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 1 Absence of symptoms at 48 hours ‐ patient assessment.
3.
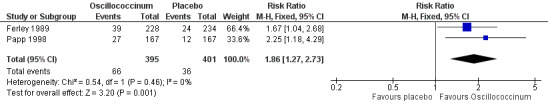
Forest plot of comparison: 2 Treatment: Oscillococcinum versus placebo. Outcome 1: Absence of symptoms at 48 hours ‐ patient assessment.
Subgroup analysis
Effect of age of patient (Analysis 2.2): for participants aged 12 to 29 years, the effect of Oscillococcinum® was RR 3.08, 95% CI 1.32 to 7.23, whereas for participants aged > 30 years, the effect was RR 1.26, 95% CI 0.59 to 2.70. Direct comparison between the two groups of participants showed that the higher frequency of symptom absence in younger participants did not reach the level of statistical significance (Chi2 = 3.188; P value = 0.07).
Effect of severity of illness (Analysis 2.3): for participants with mild to moderate symptoms, the effect of Oscillococcinum® was RR 2.08, 95% CI 1.19 to 3.61, whereas for participants with severe symptoms, the effect was RR 0.88, 95% CI 0.33 to 2.32. Direct comparison between the two groups of participants showed that symptom relief did not occur significantly more frequently in the subgroup with mild to moderate symptoms compared to those with severe symptoms (Chi2 = 1.784; P value = 0.18).
2.2. Analysis.
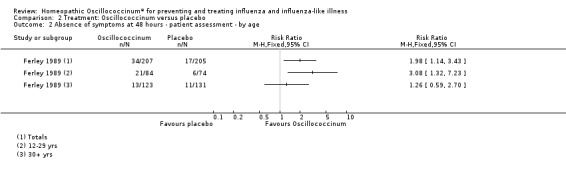
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 2 Absence of symptoms at 48 hours ‐ patient assessment ‐ by age.
2.3. Analysis.
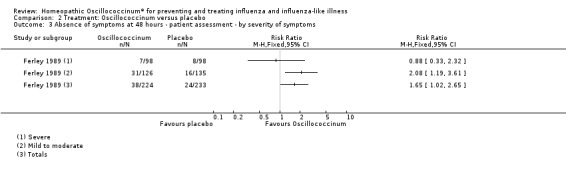
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 3 Absence of symptoms at 48 hours ‐ patient assessment ‐ by severity of symptoms.
Patient‐reported absence of specified symptoms at 48 hours
Data are derived from the trials reported by Casanova 1984, Casanova 1988 and/or Papp 1998.
Fitness for work at day two is presented in Analysis 2.4 (RR 1.80, 95% CI 0.99 to 3.26; P value = 0.05). Fitness for work at day four is shown in Analysis 2.5 (RR 1.04, 95% CI 0.83 to 1.30; P value = 0.74).
2.4. Analysis.
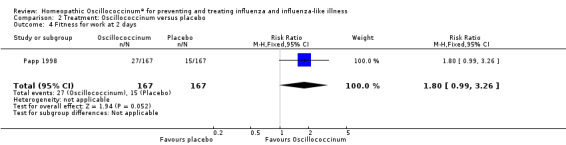
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 4 Fitness for work at 2 days.
2.5. Analysis.
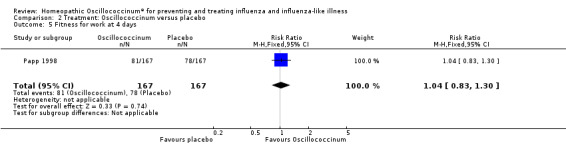
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 5 Fitness for work at 4 days.
Results for individual symptoms (each reported at 48 hours) are presented in Analyses 2.6 to 2.17. Most analyses showed symptom changes in favour of Oscillococcinum®: Analysis 2.6 (no chills: 1.30, 95% CI 1.04 to 1.63; P value = 0.02); Analysis 2.7 (no fever: 1.98, 95% CI 1.34 to 2.92; P value = 0.0006); Analysis 2.9 (no general aches: 1.73, 95% CI 1.16 to 2.59; P value = 0.007); Analysis 2.11 (no backache: 1.27, 95% CI 1.00 to 1.61; P value = 0.05); Analysis 2.12 (no spinal pain: 1.27, 95% CI 1.02 to 1.58; P value = 0.03); Analysis 2.13 (no muscle pain: 1.47, 95% CI 1.10 to 1.97; P value = 0.01); Analysis 2.14 (no articular pain: 1.40, 95% CI 1.09 to 1.80; P value = 0.009); Analysis 2.16 (no day cough: 2.00, 95% CI 1.20 to 3.31; P value = 0.008); Analysis 2.17 (temperature: mean difference ‐0.50 degrees, 95% CI ‐0.67 to ‐0.33; P value < 0.00001). The remaining analyses showed symptom changes in favour of placebo: Analysis 2.8 (no rhinitis: RR 1.33, 95% CI 0.66 to 2.70; P value = 0.42); Analysis 2.10 (no headache: 1.20, 95% CI 0.88 to 1.63; P value = 0.25); Analysis 2.15 (no night cough: 1.44, 95% CI 0.73 to 2.84; P value = 0.29).
2.6. Analysis.
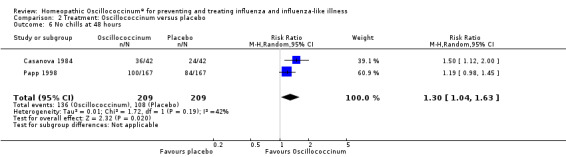
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 6 No chills at 48 hours.
2.7. Analysis.
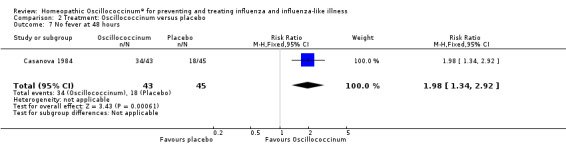
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 7 No fever at 48 hours.
2.9. Analysis.
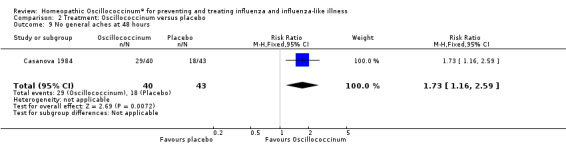
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 9 No general aches at 48 hours.
2.11. Analysis.
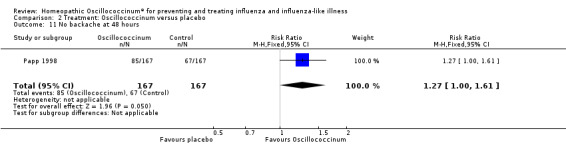
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 11 No backache at 48 hours.
2.12. Analysis.
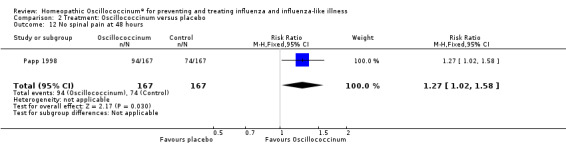
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 12 No spinal pain at 48 hours.
2.13. Analysis.
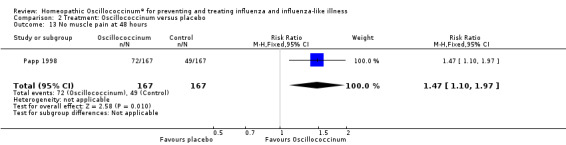
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 13 No muscle pain at 48 hours.
2.14. Analysis.
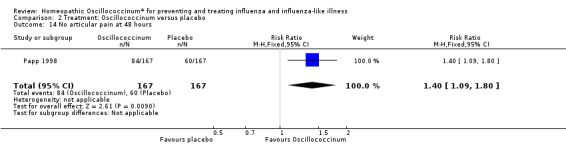
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 14 No articular pain at 48 hours.
2.16. Analysis.
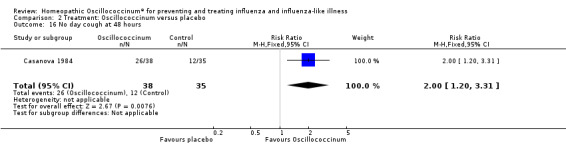
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 16 No day cough at 48 hours.
2.17. Analysis.
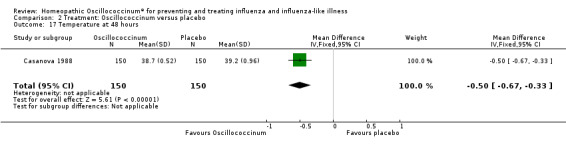
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 17 Temperature at 48 hours.
2.8. Analysis.
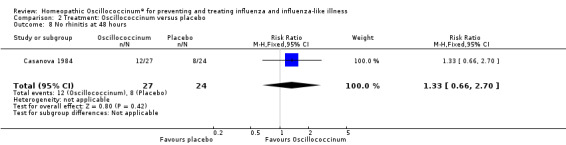
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 8 No rhinitis at 48 hours.
2.10. Analysis.
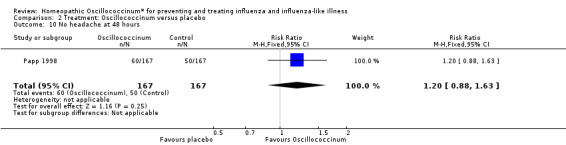
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 10 No headache at 48 hours.
2.15. Analysis.
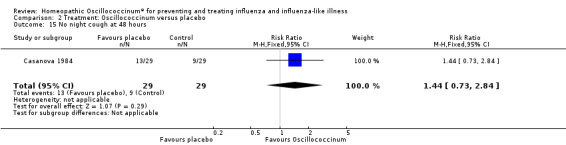
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 15 No night cough at 48 hours.
Secondary outcomes
Adverse events
One patient taking Oscillococcinum® reported a headache that 'might' have been due to the trial medication (Papp 1998). None of the other eligible trials of Oscillococcinum® reported adverse events (Casanova 1984; Casanova 1988; Ferley 1989).
Physician assessment of symptoms at 48 hours
Physician assessment of improvement in symptoms at 48 hours was reported in one paper (Papp 1998): mean RR in favour of Oscillococcinum® was 1.07, 95% CI 0.98 to 1.18, which is not statistically significant (P value = 0.13) (Analysis 2.18). Physician assessment of participants' absence of symptoms at 48 hours was reported in one paper (Papp 1998): mean RR in favour of Oscillococcinum® was 1.28, 95% CI 0.79 to 2.06, which is not statistically significant (P value = 0.31) (Analysis 2.19).
2.18. Analysis.
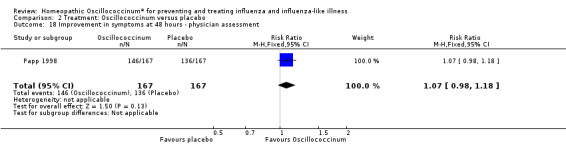
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 18 Improvement in symptoms at 48 hours ‐ physician assessment.
2.19. Analysis.
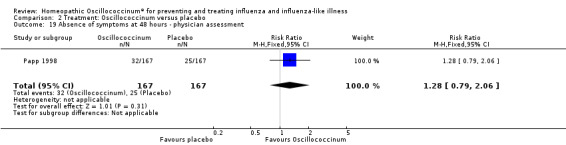
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 19 Absence of symptoms at 48 hours ‐ physician assessment.
Patient‐reported symptom relief after more than 48 hours
Day 3 (Analysis 2.20; Figure 4): the RR of relief from influenza symptoms was 1.27, 95% CI 1.03 to 1.56, statistically significantly in favour of Oscillococcinum® (P value = 0.03).
Day 4 (Analysis 2.21; Figure 5): the RR of relief from influenza symptoms was 1.11, 95% CI 0.98 to 1.27, which is not statistically significant (P value = 0.10).
Day 5 (Analysis 2.22; Figure 6): the RR of relief from influenza symptoms was 1.06, 95% CI 0.96 to 1.16, which is not statistically significant (P value = 0.25).
2.20. Analysis.
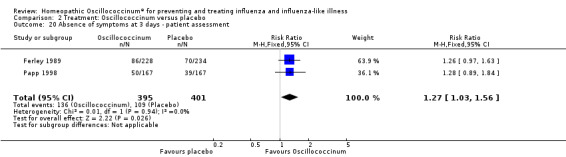
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 20 Absence of symptoms at 3 days ‐ patient assessment.
4.
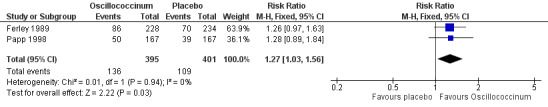
Forest plot of comparison: 2 Treatment: Oscillococcinum versus placebo. Outcome 20: Absence of symptoms at 3 days ‐ patient assessment.
2.21. Analysis.
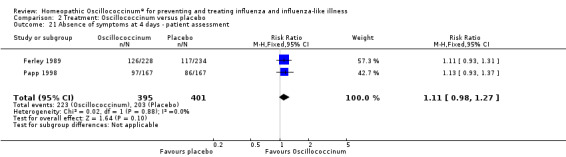
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 21 Absence of symptoms at 4 days ‐ patient assessment.
5.
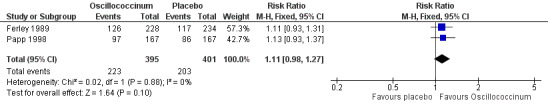
Forest plot of comparison: 2 Treatment: Oscillococcinum versus placebo. Outcome 21: Absence of symptoms at 4 days ‐ patient assessment.
2.22. Analysis.
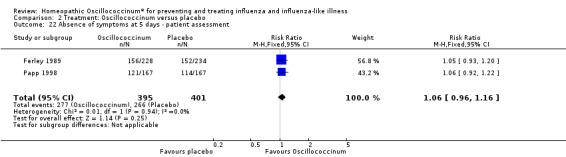
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 22 Absence of symptoms at 5 days ‐ patient assessment.
6.
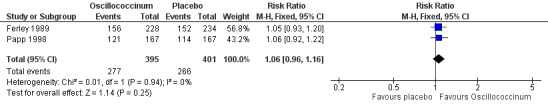
Forest plot of comparison: 2 Treatment: Oscillococcinum versus placebo. Outcome 22: Absence of symptoms at 5 days ‐ patient assessment.
Concomitant medication
There was less increased use of concomitant medication over the trial period in the Oscillococcinum® group compared with the placebo group: RR 0.61, 95% CI 0.40 to 0.92; P value = 0.02 (Analysis 2.23). Medication use for pain or fever was significantly less in the Oscillococcinum® group: RR 0.82, 95% CI 0.67 to 1.00; P value = 0.05 (Analysis 2.24). There was no inter‐group difference in the use of medication for cough or coryza: RR 0.96, 95% CI 0.76 to 1.21; P value = 0.72 (Analysis 2.25) or of antibiotics: RR 0.87, 95% CI 0.47 to 1.62; P value = 0.67 (Analysis 2.26).
2.23. Analysis.
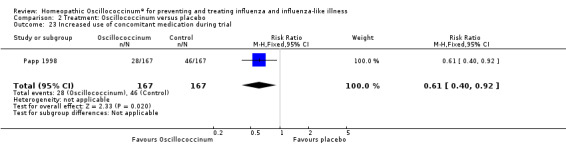
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 23 Increased use of concomitant medication during trial.
2.24. Analysis.
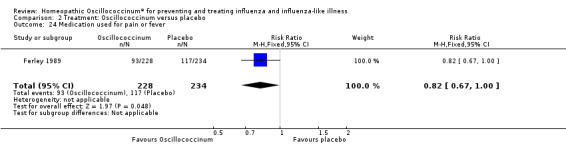
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 24 Medication used for pain or fever.
2.25. Analysis.
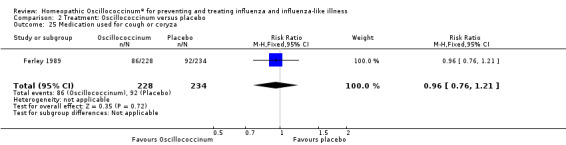
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 25 Medication used for cough or coryza.
2.26. Analysis.
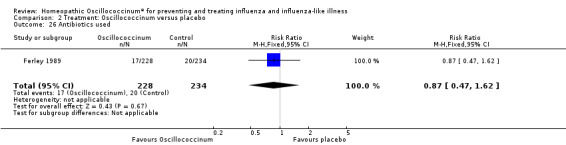
Comparison 2 Treatment: Oscillococcinum versus placebo, Outcome 26 Antibiotics used.
Discussion
Overall, the risk of bias in the six included trials was unclear, and so the statistical findings from this systematic review must be viewed with caution. Only two treatment trials contained some domains that we judged to have low risk of bias (Ferley 1989; Papp 1998), but each of those trials also included domains in which lack of clarity prevented clear judgement. Due to this unclear reporting, we felt obliged to judge even these two studies 'low quality of evidence' (GRADE Working Group ‐ see Table 1).
The evidence, which is limited to two studies with unclear risk of bias, did not support a preventive effect of Oscillococcinum® in influenza and influenza‐like illness (risk ratio (RR) 0.48, 95% confidence interval (CI) 0.17 to 1.34; P value = 0.16). The results were skewed by the extremely diverse data reported in the two prophylaxis studies (Selkova 2005a; Selkova 2005b); the larger of the two trials (n = 227) obtained positive findings. Further prophylaxis research on Oscillococcinum® might thus be indicated. Since the single eligible prophylaxis paper to date did not report adverse events, any new research in this area should include such assessment. This is especially important given the high frequency of adverse reactions reported in a non‐eligible prophylaxis trial on an Oscillococcinum‐like homeopathic product (Attena 1995).
Oscillococcinum® appeared to have a modest effect on influenza and influenza‐like illness in the first two days of treatment (as assessed by the patient). At 48 hours, the RR of 1.86 (95% CI 1.27 to 2.73) indicated a statistically significant effect of Oscillococcinum®, and the 95% CIs of the risk difference (RD) (0.03 to 0.12) and the number needed to treat to benefit (NNTB) (9 to 34) suggested that the additional treatment benefit would potentially be of clinical importance at population level (limits defined as RD 0.05, NNTB 20). Four to five days after the start of treatment, the difference between Oscillococcinum® and placebo dwindled to statistical non‐significance, which may be deemed consistent with the self limiting natural course of the illness.
The limited available evidence suggested that, at 48 hours, the following symptoms were most responsive to treatment: chills, fever, general aches, backache, spinal pain, muscle pain, articular pain and day cough. A key limitation in interpreting these findings is that one of the trials was not published in a standard medical journal, contained little experimental detail, did not report withdrawals and analysed a suspiciously rounded number of participants (Casanova 1984).
There were data from only one trial on the effects of Oscillococcinum® with respect to age of patient or severity of illness (Ferley 1989). Though Ferley 1989 found a better response to treatment at 48 hours in people aged less than 30 years and in participants with less severe symptoms, these findings were based on unplanned subgroup analyses; our Chi2 test analyses did not support the researchers' original conclusions.
Although there were insufficient data to determine clearly the effect of Oscillococcinum® on concomitant medication, one trial noted a lesser increase in the overall use of concomitant medication during the study period (Papp 1998), while another trial reported a decreased use of analgesic and antipyretic medication (Ferley 1989).
It is obvious that doubts remain about the reliability and the clinical importance of the findings reported. A question as scientifically controversial as whether or not a highly diluted homeopathic medicine is equivalent to placebo will require many more statistically robust data. As adjudged above, further research is very likely to have an important impact on the confidence in, and the magnitude of, the estimate of treatment effects (GRADE Working Group grade of evidence: low quality). To confirm or refute the existing evidence, it is therefore concluded that additional research on Oscillococcinum® prevention and/or treatment is necessary ‐ see Authors' conclusions below.
Summary of main results
The evidence from two studies with unclear risk of bias did not support a significant preventive effect of Oscillococcinum® (RR 0.48, 95% CI 0.17 to 1.34; P value = 0.16). Two studies with low quality of evidence suggested that 48 hours after commencing treatment, the effect of Oscillococcinum® on patient‐reported symptom relief was significantly greater than that of placebo (RR 1.86, 95% CI 1.27 to 2.73; P value = 0.001); the RD was 0.077, 95% CI 0.03 to 0.12, indicating that at population level the symptom improvement in early influenza‐like illness (ILI) might potentially be clinically useful. There was no evidence of clinically important harms. Adverse effects of the intervention have been reported by one patient in one study.
Overall completeness and applicability of evidence
The trials reviewed here were sampled from general primary care populations and did not specifically focus on target sub‐populations for treatment of influenza, such as individuals with severe disease or at high risk of complications, including older people, pregnant and post‐partum women and those with chronic medical conditions. The incidence of complications of ILI in such groups, and indeed the general population, is of interest but was not investigated in the trials reviewed.
As discussed above, the intervention covered by this review is narrower than in the previous versions: it includes only trademarked Oscillococcinum®, whereas the earlier versions included other, similar, products. However, Oscillococcinum® is the most widely used product and has been the subject of considerable research. In one of the papers included in the previous versions (but which we have excluded), there was ambiguity about the precise nature of the medicinal product (Attena 1995). By removing such uncertainty, we therefore believe we have included only exactly relevant interventions.
From the viewpoint of external validity (generalisability), it is important to note that the majority of ILIs are not true, virologically confirmed, influenza (Jefferson 2009a). Furthermore, ILI and laboratory‐confirmed influenza are clinically indistinguishable (Call 2005). In the 2009‐2010 influenza season in the USA, which included the H1N1 'swine flu' pandemic, overall only 21% of specimens tested positive for influenza viruses, rising to around 40% at the peak of the pandemic (CDCP 2011). Clinical trials of neuraminidase inhibitors tend to include a higher proportion of true influenza than is encountered in routine practice. For people exposed to influenza, neuraminidase inhibitors reduce their chance of developing true, laboratory‐confirmed, influenza, but not ILI (Jefferson 2009a; Jefferson 2014). However, as stated, true influenza is a small component of ILI (Jefferson 2009a). Neuraminidase inhibitors are moderately effective in shortening the duration of influenza symptoms (Jefferson 2009a; Jefferson 2014; Wang 2012). Regarding neuraminidase inhibitors in epidemic and pandemic situations, there are concerns about the availability of adequate supplies and, in low‐income countries, their affordability. Given those limitations of conventional drugs, and that the studies included in the current review investigated ILI, not virologically confirmed influenza, the advent of higher‐quality data on alternative or additional options, such as Oscillococcinum®, would be of interest.
Quality of the evidence
The two prophylaxis trials comprised a total of 327 participants; the quality of their reporting was poor and the studies may have been underpowered. The two treatment trials in which our ascribed 'primary outcome measure' was reported (patient‐reported absence of symptoms at 48 hours) comprised a total of 796 participants: overall, these trials were of unclear risk of bias (GRADE Working Group: 'low quality of evidence' ‐ Table 1). The available body of evidence therefore does not enable robust conclusions about the impact of Oscillococcinum® in preventing and/or treating influenza or influenza‐like illness.
Potential biases in the review process
Some trials of homeopathy are published in the 'grey' literature, which is not indexed in medical bibliographic databases; such was the case with several of the trials reviewed here. We have made efforts to search comprehensively and have identified and included two new trials (Selkova 2005a; Selkova 2005b), which are not indexed in the standard medical bibliographic databases. Nevertheless it remains possible that we have missed some trials, though we consider it unlikely that we have failed to identify any study of sufficient quality that would influence our findings importantly. We assessed the eligible trials rigorously for risk of bias. We identified all five original studies (six trials) as lacking clarity in some or all assessment domains. We rated no trial overall as low risk of bias and so the conclusions about trial results drawn from this review are necessarily cautious in nature.
Agreements and disagreements with other studies or reviews
Three other reviews have appraised the evidence of Oscillococcinum® or Oscillococcinum‐like homeopathic preparations (Bornhöft 2006; Marrari 2012; Ulbricht 2011). The first review cited Ferley 1989 and Papp 1998 as showing 'significance for homeopathy' and categorised each of those studies as having unclear external validity. The second review appraised the evidence both from the original randomised controlled trials and the earlier reviews: its findings and conclusions are broadly in line with this Cochrane Review, though it regarded the paper by Papp 1998 as "well‐designed and well‐reported". The third review concluded that, given the available evidence including its high benefit/risk ratio, Oscillococcinum® should be assigned the classification "generally proven". None of these three reviews cited the paper by Selkova (Selkova 2005a; Selkova 2005b). The present review is less positively positioned than any of those above.
Authors' conclusions
Implications for practice.
As stated in Measures of treatment effect, at a population level there would be significant social gains from at least a 5% absolute increase in the proportion of individuals achieving symptom relief at 48 hours. Our findings do not rule out the possibility that Oscillococcinum® could have such impact but, given the low quality of the eligible studies, the evidence is not compelling. It seems clear that Oscillococcinum® provides no additional benefit beyond the third day of treatment, and this is consistent with the self limiting natural course of the disease. There is no evidence of clinically important harms.
Implications for research.
Overall, the findings from this review have been obtained from trials of low quality. Confirmatory placebo‐controlled studies of high quality therefore seem warranted to study the efficacy of Oscillococcinum® both in prevention and treatment of influenza and influenza‐like illness (ILI).
The existing research studies on influenza prophylaxis using Oscillococcinum® are very poorly reported and robust conclusions cannot be drawn from them. Nevertheless, if it were effective for prevention, Oscillococcinum® might be an interesting intervention. It would not suffer from the lag of several months between the identification of the epidemic strain and large‐scale production of a vaccine and it might be effective against ILI which, even in an epidemic, forms the bulk of clinically diagnosed influenza. These considerations would need to be balanced against the scale and expense of the trials required to answer this question adequately, especially given the highly equivocal nature of the current data.
The two treatment trials, Ferley 1989 and Papp 1998, yielded combined data that showed an absolute risk reduction of 7.7%; the total sample size of 796 participants provided sufficient statistical power to detect that substantial treatment effect. However, the quality of the evidence from these treatment trials is considerably less than robust, and further research is indicated. Based on the combined data from the control arms of the treatment trials by Ferley 1989 and Papp 1998 (in which the frequency of improvement in the placebo group was 9%), we conducted a sample size calculation (Altman 1991) for patient‐reported symptom relief at 48 hours as the main outcome measure, with an absolute improvement in frequency of symptom relief of 5% as the minimal clinically important benefit (see Implications for practice above), power set at 90% and a 5% level of statistical significance: the required sample is 1600 (i.e. 800 patients per group), which is twice the size of Ferley's and Papp's trials combined. Ideally, such a 'definitive' trial of Oscillococcinum® treatment should also plan subgroup analyses to investigate Ferley's tentative finding of a greater effect in patients under 30 years of age and in those with less severe symptoms. Due to its very large size, such a trial would obviously require substantial financial and organisational resources.
Feedback
Reported side effects, 4 March 2003
Summary
Were there any side effects reported, relating to this study (or any known side effects related to this product)?
Cindy Haberfield
Reply
Adverse events are discussed in the review. A review on the safety of homeopathy is available at: http://climed.epm.br/homeopatia/SafetyHomeopathyReview2000.pdf
Contributors
Andrew Vickers
Homeopathic Oscillococcinum® for preventing and treating influenza and influenza‐like illness, 8 January 2013
Summary
Comment: I do not believe that this is an appropriate subject for the Cochrane library, or that the reviewers are appropriate, or that the conclusion is appropriate.
Oscillococcinum was invented between 1917 and around 1925 by one Joseph Roy (1891‐1978), a French physician on military duty during the Spanish flue epidemic of 1917 onwards. It is based on his purported discovery using (optical) microscopy of a bacterium in the blood of flu victims, which he named oscillococcus due to its motion. He later identified this bacterium in patients with many other disorders, and posted it as a causative agent in herpes, chicken pox, shingles, eczema, rheumatism, tuberculosis, measles, and cancer. He then searched for the bacterium in animals, finding it eventually in the liver of a Long Island duckling. The remedy Oscillococcinum(R) is prepared from the heart and liver of a Muscovy duck, and the label states: "Active ingredient: Anas Barbariae Hepatis et Cordis Extractum (extract of Muscovy Duck liver and heart) 200CK HPUS 1×10^−400 g; Inactive ingredient: 0.85 g sucrose, 0.15 g lactose.[1]
There are a number of fundamental issues to be addressed before one even considers evaluating this commercial and trademarked product:
1. The oscillococcus bacterium does not exist. Whatever Roy saw, it was not an oscillococcus bacterium.
2. Influenza is not caused by a bacterium. The virus that causes flu is not visible in an optical microscope.
3. The large list of other proposed conditions, which are now known to have entirely different causes, indicates systemic false attribution.
4. The idea of a preparation from a source believed to contain the actual pathogen, albeit incorrectly identified, is not consistent with the (unproven) homeopathic principle of similia similibus curentur; this particular version of the archaic principle of sympathetic magic is based on *like* not *same* curing same. There is no inferential link, even according to the odd doctrines of homeopathy, linking duck liver and influenza. There is no record of consumption of duck liver, a common foodstuff, causing flu‐like symptoms in healthy individuals.
5. The ingredients listed are preposterous. There is no instrument known to science that is capable of measuring dilutions of one part in ten to the minus four hundredth power, this is billions of orders of magnitude greater than the number of atoms in the known universe. No objective test is identified (or indeed plausible) that shows Oscillococcinum tablets to be anything other than 100.0% sugar.
The authors, who have an ideological disposition towards homeopathy, have in effect taken a number of weak observational studies of a substance that is objectively indistinguishable from a sugar pill, for which there is no credible reason to believe any relevant effect should arise, even according to the principles of homeopathy, and concluded from these that their findings "do not rule out the possibility that Oscillococcinum® could have a clinically useful treatment effect".
Observational studies are, by their very nature, not capable of refuting the null hypothesis. The typical test of significance, P=0.05, explicitly allows for the fact that a false result is not only possible but expected. No such study can rule out a possibility of clinical effect.
The consensus from systematic reviews is that the effects of homeopathic remedies are placebo effects.[2]
This review could therefore be summarised thus:
Three authors with a known predisposition towards homeopathy[3], reviewed the literature supporting a commercial product whose manufacturers have recently settled class actions accepting that their claims cannot be substantiated as made[4]. The evidence was found to be weak, consistent with the documented correlation between study quality and negative results for homeopathy[5]. Reviewing a number of studies which are not capable, by design, of ruling out clinical effect, the authors conclude that these poor quality studies do not rule out clinical effect.
The value of this negative finding, spun by the authors as cautiously positive, would appear to be negligible compared with the vastly more robust finding, based n a much broader evidence base, that the effects of homeopathy are placebo effects [6]. This study is already being presented by homeopaths as cautiously supportive, and as an indication of the need for "more research" [7].
Can we really not just have a moratorium on tooth fairy science [8] in the Cochrane library?
1. The True Story of Oscillococcinum, Jan Willem Nienhuys, Homeopwatch, August 2003 http://www.homeowatch.org/history/oscillo.html
2. A systematic review of systematic reviews of homeopathy, Ernst E, Br J Clin Pharmacol. 2002 Dec;54(6):577‐82.
3. See author affiliations
4. Class action settlement agreement: Galluci and others v. Boiron Inc and Boiron USA Inc., US District Court, Southern District of California case 3:11‐cv‐02039‐JAH‐NLS http://www.gilardi.com/boironsettlement/pdf/BRGL_SettlementAgreement.pdf
5. Impact of study quality on outcome in placebo‐controlled trials of homeopathy. Linde K, Scholz M, Ramirez G, Clausius N, Melchart D, Jonas WB. J Clin Epidemiol. 1999 Jul;52(7):631‐6.
6. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo‐controlled trials of homoeopathy and allopathy. Shang A, Huwiler‐Müntener K, Nartey L, Jüni P, Dörig S, Sterne JA, Pewsner D, Egger M. Lancet. 2005 Aug 27‐Sep 2;366(9487):726‐32.
7. e.g. Homoeopathic Oscillococcinum for preventing and treating influenza and influenza‐like syndromes, Vickers A, Smith C, The Homeopathic College http://www.thehomeopathiccollege.org/portfolio‐view/homoeopathic‐oscillococcinum‐for‐preventing‐and‐treating‐influenza‐and‐influenza‐like‐syndromes/
8. Evidence‐Based Medicine, Tooth Fairy Science, and Cinderella Medicine, Hall, H, Skeptic Vol 17, No. 1, p. 4‐5.
I agree with the conflict of interest statement below: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Guy Chapman Role: Skeptical activist
Reply
We appreciate Mr Chapman's description of the interesting history of the 1917 observation that led to the development of Oscillococcinum®. Dr Roy's mischaracterisation of his observations might perhaps be forgiven, given that viruses were only first visualised after the development of the transmission electron microscope in 1932.[i] Prior to that time, experimental transmission of influenza symptoms from infected to uninfected animals was attributed to an 'ultrafiltered material'.[ii] The annals of medicine are replete with medicines that changed direction during their development: e.g. sildenafil citrate (Viagra), which failed in its trials as an anti‐hypertensive, but found new life in one of its 'adverse effects'.
Our review makes the following points about Oscillococcinum®: "The rationale for its use in influenza is not the standard homeopathic principle of 'let like be cured by like', but the related principle of 'isopathy': that a medicine derived from the causative agent of the disease, or from a product of the disease process, is used to treat the condition". And the assertion that there is 'no inferential link [between] duck liver and influenza' is simply false: it is 'hypothesised that wild aquatic birds are the primordial reservoir of all influenza A viruses'.[iii] However, water fowl do not generally become ill with the virus they harbour.
In our review, we directly address the subject of high dilution (How the intervention might work). Studies in recent decades with a variety of instruments have demonstrated the ability to distinguish various homeopathic medicines as well as different potencies of the same medicines. Recent studies reveal that homeopathic remedies contain nanoparticles of source materials formed by mechanical grinding in lactose and/or succussion (forceful agitation) in ethanolic solutions combined with silica nanostructures formed during succussions in glass.[iv] Other studies using various physical and physico‐chemical methods have demonstrated persistent structural modifications as a result of homeopathic preparation methods.e.g. [v], [vi], [vii] These technologies have not yet been applied to Oscillococcinum®, but the assertion that no such instrument exists is incorrect.
Similarly, it is unclear why Mr Chapman characterises randomised controlled trials as 'observational studies'. Consistent with standard Cochrane methods, our conclusions were based on experimental research: i.e. randomised controlled trials, not observational studies. We refer him to further information on the subject of clinical study design.[viii] Given his expressed concerns about conflict of interest, it is interesting that he nevertheless accepts the evidence from an article published in 2002 by a 'trained homeopath' (see Conflict of interest in his reference no. 2). Even in 2002, when that article was published, there was no 'consensus' of evidence from systematic reviews of homeopathy. More recently, systematic reviews have reported positive conclusions about homeopathy in several medical conditions.e.g. [ix], [x]
We are open‐minded scientists who strive to know the facts about homeopathy and, most importantly, its potential contribution to the welfare of patients. Our review was conducted to rigorous standards of objectivity, as required by the Cochrane Collaboration. The conclusions are supported by correct interpretation of the statistical facts, and recognising the limitations of the original clinical trials. Mr Chapman's reference to the previous version (and authorship) of our review is bizarre, especially since the conclusions of our updated version are considerably more cautious than those of its predecessor. And, as we state in the section Agreements and disagreements with other studies, our conclusions are also less positively positioned than those of other previous reviews of Oscillococcinum. We stand by our
statement (Implications for research): "The two treatment trials (Ferley 1989, Papp 1998) yielded combined data that showed an absolute risk reduction of 7.7%; the total sample size of 796 participants provided sufficient statistical power to detect that substantial treatment effect. However, the quality of the evidence from these treatment trials is considerably less than robust, and further research is indicated." As scientists, we want to see much more robust evidence before making any definitive pronouncement about the efficacy or otherwise of Oscillococcinum®.
Boiron's expedient settlement of a harassing suit does not confirm that 'their claims cannot be substantiated as made'. Indeed, careful reading of the settlement of Gallucci v. Boiron Inc. et al. (the California class action suit that refers), reveals in Terms and Conditions of Settlement 2.1: 'Defendants deny the material factual allegations and legal claims asserted by the Representative Plaintiffs in the Litigation, including any and all charges of wrongdoing or liability arising out of any of the conduct, statements, acts or omissions alleged, or that could have been alleged, in the Litigation'.[1]
Mr Chapman challenges the Cochrane Collaboration's criteria for subject selection, the reviewers' credentials and the statistical conclusions from factual data. These are serious allegations. Does he therefore: advocate arbitrary removal of other subject areas from Cochrane's formal review process (e.g. acupuncture, cognitive behaviour therapy, physiotherapy); dispute that an expert in a particular branch of medicine or science is appropriate to review research in that specialist field; dismiss the robust statistical methods that are accepted by the entire scientific community?
The respondent is well‐known for his anti‐homeopathy views,[2] and has posted identical allegations as above on his personal website.[3] Unfortunately he appears to be unaware of the difference between experimental and observational studies or of physical research on high dilutions. He substitutes these gaps in his knowledge with rhetoric about 'tooth‐fairy science'.
Mr Chapman's submitted comments could be summarised thus:
A respondent with a known predisposition to condemn homeopathy finds that the Cochrane Collaboration must adopt a prejudiced approach to subject selection, and ensure that reviewers take a close‐minded attitude to the available evidence by ignoring factual evidence provided by standard statistical methods and risk‐of‐bias assessments. Furthermore, he advises reviewers to ignore modern interpretations of drug discovery and to disregard the difference between experiment and observation. He advocates selective reading and referencing from the wider research literature, where conclusions against homeopathy must always prevail in the face of any evidence to the contrary that may emerge.
References
1. Roingeard P. Viral detection by electron microscopy: past, present and future. Biol Cell 2008; 100: 491–501. (doi:10.1042/BC20070173)
2. Shope RE. Swine influenza. III. Filtration experiments and aetiology. J Exp Med 1931; 54: 373–380.
3. Murphy BR, Webster RG. Orthomyxoviruses. In: Fields BN, Knipe DM, Howley PM (eds). Fields' Virology. Philadelphia: Lippincott‐Raven, 1996: 1353–1445.
4. Bell IR, Schwartz GE. Adaptive network nanomedicine: an integrated model for homeopathic medicine. Front Biosci (Schol Ed) 2013; S5: 685–708.
5. van Wijk R, Bosman S, van Wijk EP. Thermoluminescence in ultra‐high dilution research. J Altern Complement Med 2006; 12: 437–443.
6. Elia V, Napoli E, Niccoli M, Nonatelli L, Ramaglia A, Ventimiglia E. New physico‐chemical properties of extremely diluted aqueous solutions. J Therm Anal Calor 2004; 78: 331–342.
7. Demangeat J.‐L. NMR water proton relaxation in unheated and heated ultrahigh aqueous dilutions of histamine: Evidence for an air‐dependent supramolecular organization of water. J Mol Liq 2009; 144: 32–39.
8. Editorial commentary. Why comparisons are essential. The James Lind Library, 2007 (www.jameslindlibrary.org)
9. Jacobs J, Jonas WB, Jimenez‐Perez M, Crothers D. Homeopathy for childhood diarrhea: combined results and metaanalysis from three randomized, controlled clinical trials. Pediatr Infect Dis J 2003; 22: 229–234.
10. Schneider B, Klein P, Weiser M. Treatment of vertigo with a homeopathic complex remedy compared with usual treatments: a meta‐analysis of clinical trials. Arzneimittelforschung 2005; 55: 23–29.
11. Gallucci S et al. v. Boiron Inc. et al. Class Action Settlement Agreement: Case No. 3:11‐cv‐02039‐JAH‐NLS. United States District Court: Southern District of California, 2012. (http://www.gilardi.com/boironsettlement/pdf/BRGL_SettlementAgreement.pdf)
12. Guy Chapman's Blahg. (http://www.chapmancentral.co.uk/blahg/?s=homeopathy)
13. Chapman G. Tooth fairy science in the Cochrane Library. (http://www.chapmancentral.co.uk/blahg/2013/01/tooth‐fairy‐science‐in‐the‐cochrane‐library/)
Contributors
RT Mathie, J Frye, P Fisher
What's new
| Date | Event | Description |
|---|---|---|
| 5 September 2019 | Amended | One of the review authors (JF) amended her declaration of interest statement in response to a request by the Cochrane Funding Arbiters (FAs). The FAs do not consider the grant a Clause 2 (employment) or Clause 3 (financial support) conflict as Standard Homeopathic does not manufacture the intervention or the comparator. |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 1, 2000
| Date | Event | Description |
|---|---|---|
| 5 September 2014 | New search has been performed | Searches conducted. We identified no new trials for inclusion. |
| 5 September 2014 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 16 April 2013 | Feedback has been incorporated | Feedback comment and reply added to the review. |
| 20 November 2012 | Amended | Headings for Figures have been correctly labelled. |
| 7 August 2012 | New citation required but conclusions have not changed | A new team of authors has taken over this previously withdrawn review. |
| 7 August 2012 | New search has been performed | Due to this review update's focus on registered trademark Oscillococcinum®, the conclusions on prevention are based on different studies from those in previous versions of this review (i.e. Selkova 2005a and Selkova 2005b instead of Attena 1995 and Nollevaux 1990). Nevertheless, the results of our meta‐analyses are similar to the previous publication of this review, Vickers 2006, and the fundamental conclusion is unchanged: current evidence does not support a preventive effect of Oscillococcinum® in influenza and influenza‐like illness. Our focus solely on reported trial data, without reference to data or other information that was missing from the original trial reports, has had an impact on our reporting of the main outcome measure for the treatment studies. Mean duration of influenza illness cannot be extracted from the two relevant papers, Ferley 1989 and Papp 1998, so patient‐reported symptom relief at 48 hours is the most closely comparable primary outcome measure we can report. Again, the adjusted focus does not alter the fundamental conclusion: Oscillococcinum® may have a beneficial treatment effect above that of placebo but the clinical importance of any such effect is unclear. Rigorous assessment of the eligible studies has designated the evidence overall as 'low quality'. |
| 19 April 2008 | Amended | Converted to new review format. |
| 27 February 2006 | New search has been performed | Searches conducted. Electronic literature searches were repeated in February 2006; one additional trial was found and excluded. |
| 24 June 2003 | New search has been performed | Searches conducted. |
| 10 March 2003 | Feedback has been incorporated | Response to feedback added to review. |
| 4 March 2003 | Feedback has been incorporated | Feedback added to review. |
| 27 February 2001 | New search has been performed | Searches conducted. |
| 20 February 1999 | New search has been performed | Searches conducted. |
Notes
The original review was withdrawn from The Cochrane Library, 2009, Issue 3 as the authors were unable to update it. This review has been updated by a new team of authors.
Acknowledgements
The authors are grateful to: Elizabeth Dooley, Managing Editor, Cochrane Acute Respiratory Infections (ARI) Group, for her skill and attentiveness in overseeing this review update; Sarah Thorning, Cochrane ARI Group, for undertaking the literature search updates; Phil Wiffen, UK Cochrane Centre, for general guidance in meta‐analysis methods; and Maria Ximena Rojas Reyes, Collaborator Centre for the Ibero‐American Cochrane Center, Pontificia Universidad Javeriana, Bogotá, Colombia, for kind assistance with the 'Summary of findings' table. We appreciate the input of individuals who refereed our first submitted manuscript: Anne Lyddiatt (Consumer referee), Edzard Ernst (External Referee), Mark Jones (Statistical Editor) and Taixiang Wu (Contact Editor).
Appendices
Appendix 1. Details of earlier searches
In 1999, the registry of randomised trials for the Complementary Medicine Field of The Cochrane Collaboration was searched using the terms "homeopathy" with "influenza", "respiratory tract", "infection", "cough", "virus" and "fever". This registry had then recently benefited from incorporating trials found during an extremely comprehensive systematic review of homeopathy (Linde 1997) and it was considered unlikely that further studies existed. Homeopathic manufacturers were contacted for information about other trials.
For the first update of this review, published in Issue 1, 2004, the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 2, 2003), MEDLINE (January 1966 to June 2003) and EMBASE (1980 to June 2003) were searched, but no new trials were found. There were no language restrictions.
For the Vickers 2006 update, the search remained focused on the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library Issue 1, 2006), MEDLINE (January 1966 to February 2006) and EMBASE (1980 to February 2006). See below for details of MEDLINE search strategy. The manufacturers of Oscillococcinum® were contacted for information, which was provided. There were no language restrictions.
MEDLINE (Ovid)
#1. exp HOMEOPATHY/ #2. homeopath$.mp. #3. homoeopath$.mp. #4. oscillococcinum.mp. #5. or/1‐4 #6. exp INFLUENZA/ #7. influenza.mp. #8. flu.mp. #9. exp COUGH/ #10. cough$.mp. #11. exp VIRUSES/ #12. virus$.mp. #13. exp Respiratory Tract Infections/ #14. exp Respiratory System/ #15. respiratory tract$.mp. #16. exp INFECTION/ # 17. infection$.mp. # 18. exp FEVER/ # 19. fever$.mp. # 20. or/6‐19 # 21. 5 and 20 # 22. limit 21 to yr=2003‐2006
Appendix 2. MEDLINE (Ovid)
1 oscillococcinum.tw,nm. 2 "anas barbariae hepatis et cordis extractum".tw,nm. 3 Homeopathy/ 4 homeopath*.tw. 5 homoeopath*.tw. 6 oscillo*.tw,nm. 7 or/3‐6 8 Influenza, Human/ 9 exp Influenzavirus A/ 10 exp Influenzavirus B/ 11 influenza*.tw. 12 flu.tw. 13 Cough/ 14 cough*.tw. 15 sore throat*.tw. 16 exp Viruses/ 17 virus*.tw. 18 Respiratory Tract Infections/ 19 Respiratory System/ 20 exp Infection/ 21 infection*.tw. 22 (respiratory adj3 (infection* or tract or acute or symptom*)).tw. 23 exp Fever/ 24 fever*.tw. 25 runny nose*.tw. 26 Headache/ 27 headache*.tw. 28 (pain adj2 (limb* or joint*)).tw. 29 or/8‐28 30 7 and 29 31 1 or 2 or 30
Appendix 3. Other search strategies
MEDLINE In‐Process & Other Non‐Indexed Citations (Ovid)
1 oscillo*.tw. 2 "anas barbariae".tw. 3 (homeopath* or homoeopath*).tw. 4 or/1‐3 5 influenza.tw. 6 flu.tw. 7 influenzavirus.tw. 8 cough*.tw. 9 sore throat*.tw. 10 virus*.tw. 11 respiratory tract infection*.tw. 12 respiratory infection*.tw. 13 fever*.tw. 14 runny nose*.tw. 15 headache*.tw. 16 (pain adj2 (limb* or joint*)).tw. 17 infection*.tw. 18 or/5‐17 19 4 and 18
EMBASE.com
31. #27 AND #30 30. #28 OR #29 29. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross‐over':ab,ti OR 'cross over':ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR volunteer*:ab,ti OR ((singl* OR doubl*) NEAR/2 (blind* OR mask*)):ab,ti 28. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp 27. #1 OR #2 OR #26 26. #6 AND #25 25. #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 24. (pain NEAR/2 (limb* OR joint*)):ab,ti 23. headache*:ab,ti 22. 'headache'/exp 21. 'runny nose':ab,ti OR 'runny noses':ab,ti 20. 'fever'/de 19. (respiratory NEAR/3 (infection* OR tract OR acute OR symptom*)):ab,ti 18. infection*:ab,ti 17. 'infection'/de 16. 'respiratory system'/de 15. 'respiratory tract infection'/de OR 'upper respiratory tract infection'/de OR 'lower respiratory tract infection'/de OR 'viral respiratory tract infection'/de 14. virus*:ab,ti 13. 'virus'/exp 12. 'sore throat':ab,ti OR 'sore throats':ab,ti 11. cough*:ab,ti 10. 'coughing'/de 9. influenza*:ab,ti OR flu:ab,ti 8. 'influenza virus a'/exp OR 'influenza virus b'/exp OR 'swine influenza virus'/de OR 'influenza virus c'/de 7. 'influenza'/exp 6. #3 OR #4 OR #5 5. oscillo*:ab,ti 4. homeopath*:ab,ti OR homoeopath*:ab,ti 3. 'homeopathy'/de 2. 'anas barbariae hepatis':ab,ti 1. oscillococcinum:ab,ti
AMED (Ovid)
1 oscillococcinum.tw. 2 exp homeopathy/ 3 (homeopath* or homoeopath*).tw. 4 oscillo*.tw. 5 or/2‐4 6 influenza/ 7 (influenza* or flu).tw. 8 cough/ 9 cough*.tw. 10 sore throat*.tw. 11 viruses/ 12 virus*.tw. 13 respiratory tract infections/ 14 respiratory system/ 15 exp infection/ 16 infection*.tw. 17 (respiratory adj3 (infection* or acute or tract or symptom*)).tw. 18 fever/ 19 fever*.tw. 20 runny nose*.tw. 21 headache/ 22 headache*.tw. 23 (pain* adj2 (limb* or joint*)).tw. 24 or/6‐23 25 5 and 24 26 1 or 25
Web of Science (Thomson Reuters) and LILACS (BIREME) were searched using the term 'oscillococcinum'.
Data and analyses
Comparison 1. Prevention: Oscillococcinum versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Occurrence of influenza‐like illness | 2 | 327 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.17, 1.34] |
Comparison 2. Treatment: Oscillococcinum versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Absence of symptoms at 48 hours ‐ patient assessment | 2 | 796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [1.27, 2.73] |
| 2 Absence of symptoms at 48 hours ‐ patient assessment ‐ by age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Absence of symptoms at 48 hours ‐ patient assessment ‐ by severity of symptoms | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Fitness for work at 2 days | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.99, 3.26] |
| 5 Fitness for work at 4 days | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.83, 1.30] |
| 6 No chills at 48 hours | 2 | 418 | Risk Ratio (M‐H, Random, 95% CI) | 1.30 [1.04, 1.63] |
| 7 No fever at 48 hours | 1 | 88 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.98 [1.34, 2.92] |
| 8 No rhinitis at 48 hours | 1 | 51 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.66, 2.70] |
| 9 No general aches at 48 hours | 1 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.16, 2.59] |
| 10 No headache at 48 hours | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.88, 1.63] |
| 11 No backache at 48 hours | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.00, 1.61] |
| 12 No spinal pain at 48 hours | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.02, 1.58] |
| 13 No muscle pain at 48 hours | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [1.10, 1.97] |
| 14 No articular pain at 48 hours | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [1.09, 1.80] |
| 15 No night cough at 48 hours | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.73, 2.84] |
| 16 No day cough at 48 hours | 1 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.20, 3.31] |
| 17 Temperature at 48 hours | 1 | 300 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐0.67, ‐0.33] |
| 18 Improvement in symptoms at 48 hours ‐ physician assessment | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.98, 1.18] |
| 19 Absence of symptoms at 48 hours ‐ physician assessment | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.79, 2.06] |
| 20 Absence of symptoms at 3 days ‐ patient assessment | 2 | 796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.03, 1.56] |
| 21 Absence of symptoms at 4 days ‐ patient assessment | 2 | 796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.98, 1.27] |
| 22 Absence of symptoms at 5 days ‐ patient assessment | 2 | 796 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.96, 1.16] |
| 23 Increased use of concomitant medication during trial | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.40, 0.92] |
| 24 Medication used for pain or fever | 1 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.67, 1.00] |
| 25 Medication used for cough or coryza | 1 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.76, 1.21] |
| 26 Antibiotics used | 1 | 462 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.47, 1.62] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Casanova 1984.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the treatment of influenza‐like illness | |
| Participants | 100 participants with influenza‐like illness onset less than 48 hours previously No details of method of recruitment or exclusion criteria Average age of Oscillococcinum/placebo groups: 42/41 years Males:females in Oscillococcinum/placebo groups: 19:31/26:24 | |
| Interventions | Oscillococcinum®, 4 doses in over 2 days at 6‐hour intervals | |
| Outcomes | Participant global assessment of success; presence of chills, aches, rhinitis, night cough, day cough, fever at day 8 | |
| Notes | Reported in what appears to be a general medical magazine: very few experimental details given Research setting: France (unspecified location) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No statement about randomisation |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding procedure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information provided |
Casanova 1988.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the treatment of influenza | |
| Participants | 300 participants complaining of influenza No details of inclusion or exclusion criteria Average age of Oscillococcinum/placebo groups: 44/38 Males:females in Oscillococcinum/placebo: 61:89/56:94 | |
| Interventions | Oscillococcinum® twice a day for 3 to 4 days | |
| Outcomes | Temperature recorded twice a day for 4 days (data for evening of second day used for continuous outcome); presence of chills, aches at day 4 | |
| Notes | Inconsistency between text and Table 3: the data for day 4 in the table appear to have been transposed; the text values were selected Research setting: France (unspecified location) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No description of randomisation procedure |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding procedure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information provided |
Ferley 1989.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the treatment of influenza‐like illness | |
| Participants | 487 participants presenting in primary care with a complaint of influenza‐like illness Inclusion criteria: age older than 12 years; rectal temperature above 38 °C and at least 2 of headache, stiffness, lumbar and articular pain, shivers Exclusion criteria: duration more than 24 hours; immune deficiency; local infection; immunisation against influenza; depression; immunostimulant treatment Average age of Oscillococcinum/placebo groups: 34/35 Males:females in Oscillococcinum/placebo groups: 93:127/97:129 | |
| Interventions | Oscillococcinum® twice a day for 5 days | |
| Outcomes | Primary outcome measure (patient‐assessed): proportion of patients who recovered (defined as rectal temperature below 37.5 °C and complete resolution of all 5 symptoms) within 48 hours of treatment. Number of days to recovery; number of days to return to work; use of medication for pain or fever; use of medication for cough or sore throat; use of antibiotic medication; patient judgement of effectiveness of treatment | |
| Notes | Use of medication calculated from percentages given in text. Some minor inconsistencies between figures suggest a small amount of missing data Specific outcomes (temperature, symptoms including cough, coryza and fatigue) not reported per se Research setting: general practices in Rhône‐Alpes region, France |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on actual randomisation method |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical presentation of active drug and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low rate of attrition (and similar rate per group) |
| Selective reporting (reporting bias) | Unclear risk | Specific outcomes not reported: temperature, symptoms including cough, coryza and fatigue |
| Other bias | Low risk | Study appears to be free of other sources of bias (e.g. baseline imbalance) |
Papp 1998.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the treatment of influenza‐like illness | |
| Participants | 372 participants recruited in primary care or by internal medicine specialists
Inclusion criteria: rectal temperature above 38 °C; muscle pain or headache; one of shivering, cough, spinal pain, nasal irritation, malaise, thoracic pain, periarticular pain
Exclusion criteria: duration more than 24 hours; immune deficiency; local infection; immunisation against influenza; medical need for medication; immunostimulant or immunosuppressive treatment Use of analgesics, antibiotics or anti‐influenza agents in the first 48 hours was a post‐randomisation exclusion criterion Average age of Oscillococcinum/placebo groups: 35/35 Males:females in Oscillococcinum/placebo groups: 95:93/96:88 |
|
| Interventions | Oscillococcinum® 3 times a day for 3 days | |
| Outcomes | Whether patients' condition improved after 48 hours (physician‐assessed; authors' primary outcome); whether absence of symptoms after 48 hours (physician‐assessed); time to recovery (patient‐assessed; authors' primary outcome); use of concomitant medication during trial; total symptoms score; time to return to work; temperature and presence of aches, headache, shivers, back or side pain, joint pain, spinal pain, cough, rhinitis, sore throat on evening of day 2; fever calculated from temperature using normal distribution | |
| Notes | Some outcomes not clearly reported, including mean time to recovery or return to work. Not clear which data were analysed to obtain P value = 0.023: was it date of elimination of symptoms (though mean date per group is not provided in the main text) or presence of 'milder symptoms' at 48 hours (abstract)? Both options seem to reflect our stated primary outcome Physician‐assessed absence of patients' symptoms at 48 hours is also emphasised by Papp (P value = 0.0028). (This outcome measure is analogous to the primary outcome in the Ferley trial (though patient‐assessed in Ferley's case)). Patient‐assessed absence of symptoms at 48 hours in the Papp trial may be deduced from Figure 2 of their paper At 48 hours, improvement was reported by a total of 146/167 (87%) patients in the homeopathy group, compared with 136/167 (81%) in the placebo group (Table 2; statistical analysis not presented) Due to the above confusion, and to approximate, as closely as possible, the main outcome measure used by the previous authors of this review, we present 'patient‐assessed absence of symptoms at 48 hours' as the main outcome measure. Physician‐assessed absence of symptoms and physician‐assessed improvement at 48 hours are also presented (secondary outcome measures; Papp trial) Research setting: general or specialist practices, Germany Principal author (P Belon): employee of Boiron, the manufacturers of Oscillococcinum® |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Adequately described randomisation procedure |
| Allocation concealment (selection bias) | Low risk | Adequately described allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Identical presentation of active drug and placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderately high overall rate of attrition (approximately 10%); numbers stated in Methods do not reconcile with those in Results |
| Selective reporting (reporting bias) | Unclear risk | Lack of clarity regarding several outcomes – see Notes above |
| Other bias | Unclear risk | Insufficient information provided; statistical presentation unclear |
Selkova 2005a.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the prevention of influenza‐like illness | |
| Participants | 100 professional staff (average age, 50 years approximately) in outpatient health clinic, Moscow, Russia; those with influenza‐like symptoms in previous 2 days or have family contact/s displaying influenza‐like symptoms | |
| Interventions | Oscillococcinum®, prophylactically, once per week for 4 weeks | |
| Outcomes | Number of participants who fell ill with influenza symptoms | |
| Notes | Methodological details for each study are scantily described, but the tabulations of key results for each are clearly presented Research setting: outpatient health clinic, Moscow, Russia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on actual randomisation method |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding procedure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information provided |
Selkova 2005b.
| Methods | Randomised, placebo‐controlled trial of Oscillococcinum® in the prevention of influenza‐like illness | |
| Participants | 227 students (aged 16 to 22 years), at medical school, Kalouga, Russia; not vaccinated against influenza | |
| Interventions | Oscillococcinum®, prophylactically, once per week for 4 weeks | |
| Outcomes | Number of participants who fell ill with influenza symptoms | |
| Notes | Methodological details for each study are scantily described, but the tabulations of key results for each are clearly presented Research setting: Medical School, Kalouga, Russia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information on actual randomisation method. The description "two similar groups...were randomly constituted" is equivocal |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation procedure |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of blinding procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No description of blinding procedure |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information provided |
| Other bias | Unclear risk | Insufficient information provided |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Attena 1995 | Not Oscillococcinum® |
| Brydak 1999 | Not Oscillococcinum® |
| Bungetzianu 1985 | Not Oscillococcinum® |
| Ferley 1987 | Not Oscillococcinum® |
| Heilmann 1992 | Not Oscillococcinum® |
| Hourst 1982 | Not Oscillococcinum® |
| Lapitskaya 2010 | Not placebo‐controlled |
| Lecocq 1985 | Not Oscillococcinum® |
| Lewith 1989 | Not Oscillococcinum® |
| Masciello 1985 | Not placebo‐controlled |
| Nollevaux 1990 | Not Oscillococcinum® |
| Rabe 2004 | Not Oscillococcinum® |
| Rottey 1995 | Not Oscillococcinum® |
Differences between protocol and review
This review has focused explicitly and solely on registered trademark Oscillococcinum®. The rationale for this focus is described in the Description of the intervention and Types of interventions sections.
The original authors of published studies were not invited to clarify or provide missing data. See Data extraction and management section.
Contributions of authors
Andrew Vickers wrote the protocol and consequent text of the original review; he undertook the data analyses and, along with Claire Smith, the original data extractions. For the 2012 update, based on amended exclusion criteria for eligible studies, Robert Mathie undertook the 'Risk of bias' assessments, data extractions and analyses and led the drafting of the review text; Joyce Frye and Peter Fisher undertook the 'Risk of bias' assessments and co‐edited the review text. For this 2014 update, Robert Mathie screened the new search results, determining that none was eligible for inclusion; Joyce Frye and Peter Fisher agreed with those primary decisions.
Declarations of interest
All three review authors are research‐active in the field of homeopathy. They were members of the International Scientific Committee for Homeopathic Investigations (ISCHI), whose membership also included two employees of Boiron (the manufacturers of Oscillococcinum®), and whose committee activities ceased in July 2013. Progress with the Cochrane Review on Oscillococcinum® was presented briefly at ISCHI meetings in 2010 and 2011. The drafting of this Cochrane Review was carried out independently of those communications and of the authors' other ongoing research activity. ISCHI has not run or sponsored any research on Oscillococcinum®.
Robert T Mathie: Dr Mathie is Research Development Adviser, British Homeopathic Association. He was a member of the International Scientific Committee on Homeopathic Investigations, which ceased its committee activities in July 2013. Joyce Frye: Part of Dr Frye's salary was supported by a research grant from the Standard Homeopathic Company, paid to her employer, the Center for Integrative Medicine, Department of Family Medicine, University of Maryland, USA. Support ended in June 2013 when Dr Frye resigned from the University of Maryland. Standard Homeopathic Company does not manufacture Oscillococcinum or any similar product, and had no interest in the outcome of the review. Dr Frye received honoraria from the International Scientific Committee on Homeopathic Investigations, which was dissolved in July 2013. Peter Fisher: I am Expert Adviser on Complementary and Alternative Medicine to the National Institute for Health and Clinical Excellence (NICE), which may take an interest in the evidence in this review. I am Editor in Chief of an international, peer‐reviewed journal dedicated to homeopathy. All payments and reimbursements for lectures have been from universities or professional or learned societies. None of these lectures has been dedicated to the subject of this review. Some meetings have been supported by grants from commercial interests, including the manufacturer of the product that is the subject of the review.
Edited (no change to conclusions)
References
References to studies included in this review
Casanova 1984 {published data only}
- Casanova, Basquin, Mangenay, Pacotte, Questel [first initials not given]. Homeopathy, flu syndrome and double blinding [Homéopathie, syndrome grippal et double insu]. Tonus 1984:25‐6. [Google Scholar]
Casanova 1988 {published data only}
- Casanova P, Gerard R. Results of three years of randomised, multicentre studies on Oscillococcinum/placebo [Bilan de 3 années d'études randomisées multicentriques Oscillococcinum/placebo]. Proposta Omeopatica. Anno VI. Vol. 3, Milan: Boiron, 1988:14‐7. [Google Scholar]
Ferley 1989 {published data only}
- Ferley JP, Zmirou D, D'Adhemar D, Balducci F. A controlled evaluation of a homoeopathic preparation in the treatment of influenza‐like syndromes. British Journal of Clinical Pharmacology 1989;27:329‐35. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papp 1998 {published data only}
- Papp R, Schuback G, Beck E, Burkard G, Bengel J, Lehrl S, et al. Oscillococcinum in patients with influenza‐like syndromes: a placebo‐controlled double‐blind evaluation. British Homoeopathic Journal 1998;87:69‐76. [Google Scholar]
Selkova 2005a {published data only}
- Selkova EP, Semenenko TA, Gorbachev IA. Use of the medicine Oscillococcinum® for the prevention and treatment of influenza and acute respiratory viral infection. Infectionni Bolezni 2005;3:20‐4. [Google Scholar]
Selkova 2005b {published data only}
- Selkova EP, Semenenko TA, Gorbachev IA. Use of the medicine Oscillococcinum® for the prevention and treatment of influenza and acute respiratory viral infection. Infectionni Bolezni 2005;3:20‐4. [Google Scholar]
References to studies excluded from this review
Attena 1995 {published data only}
- Attena F, Toscano G, Agozzino E, Giudice N. A randomized trial in the prevention of influenza‐like syndromes by homoeopathic management. Revue d'Epidémiologie et de Santé Publique 1995;43:380‐2. [PubMed] [Google Scholar]
Brydak 1999 {published data only}
- Brydak LB, Denys A. The evaluation of humoral response and the clinical evaluation of a risk‐group patients’ state of health after administration of the homeopathic preparation Gripp‐Heel during the influenza epidemic season 1993/94. International Review of Allergology and Clinical Immunology 1999;5:223–7. [Google Scholar]
Bungetzianu 1985 {published data only}
- Bungetzianu G. The results obtained by the homeopathical dilution (15 CH) of an antiinfluenzal (Anti‐Flu) vaccine. Proceedings of the 40th Congress of the LMHI. Lyon, 1985:143.
Ferley 1987 {published data only}
- Ferley JP, Poutignat N, Azzopardi Y, Charrel M, Zmirou D. Outpatient evaluation of the effect of a homeopathic complex remedy in the prevention of flu and flu‐like syndromes [Evaluation en médecine ambulatoire de l'activité d'un complexe homéopathique dans la prévention de la grippe et des syndromes grippaux]. Immunologie Médicale 1987;20:22‐8. [Google Scholar]
Heilmann 1992 {published data only}
- Heilmann A. An injectable combined medication (Engystol N) as a prevention against the flu [Ein injizierbares Kombinationspräparat (Engystol N) als Prophylaktikum des grippalen Infekts]. Biologische Medizin 1992;21:225‐9. [Google Scholar]
Hourst 1982 {published data only}
- Hourst P. Tentative appreciation of homeopathy's effectiveness [Tentative j'appréciation de l'effacacité de l'homéopathie]. Unpublished Dissertation, Pitie‐Salpetrière: Universite Pierre et Marie Curie 1982.
Lapitskaya 2010 {published data only}
- Lapitskaya A, Selkova E, Lytkina I, Grenkova T. Homeopathic medicine Oscillococcinum and influenza vaccine in preventing influenza‐like illnesses in children. Acta Pædiatrica 2010;99(Suppl 462):86‐7. [Google Scholar]
Lecocq 1985 {published data only}
- Lecocq P. Therapeutic means to treat flu [Les voies therapeutiques des syndromes grippaux]. Cahiers de Biothérapie 1985;87:65‐73. [Google Scholar]
Lewith 1989 {published data only}
- Lewith G, Brown PK, Tyrell DA. Controlled study of the effects of a homoeopathic dilution of influenza vaccine on antibody titres in man. Complementary Medical Research 1989;3:22‐4. [Google Scholar]
Masciello 1985 {published data only}
- Masciello E, Felisi E. Dilutions of material, with a high percentage of DNA and RNA, in the presentation of viral epidemics [Dilutions de matériel, à pourcentage élevé de ADN et ARN, dans la prévention des viroses epidemiques]. Proceedings of the 40th Congress of the Liga Medicorum Homoeopathica Internationalis, Lyon. Lyon, 1985:271‐4.
Nollevaux 1990 {published data only}
- Nollevaux MA, Danhier Ph, Fagard H. Double‐blind clinical observations versus placebo of Mucococcinum 200K in the preventive treatment of flu‐like symptoms [Observations cliniques en double aveugle contre placebo, de Mucococcinum 200K dans le traitement préventif des états grippaux]. Unpublished 1990.
Rabe 2004 {published data only}
- Rabe A, Weiser M, Klein P. Effectiveness and tolerability of a homoeopathic remedy compared with conventional therapy for mild viral infections. International Journal of Clinical Practice 2004;58(9):827‐32. [DOI] [PubMed] [Google Scholar]
Rottey 1995 {published data only}
- Rottey EED, Verleye GB, Liagre RLP. The effects of a homeopathic remedy made of micro‐organisms in the prevention of flu. A randomised double‐blind trial in GP practices [Het effect van een homeopathische bereiding van micro‐organismen bij de preventie van griepsymptomen. Een gerandomiseerd dubbel‐blind onderzoek in de huisartspraktijk]. Tijdschrift voor Integrale Geneeskunde 1995;11:54‐8. [Google Scholar]
Additional references
Altman 1991
- Altman DG. Sample size, hypothesis tests and power. Practical Statistics for Medical Research. London: Chapman & Hall, 1991:455‐60. [Google Scholar]
Bellavite 2007
- Bellavite P, Ortolani R, Pontarollo F, Pitari G, Conforti A. Immunology and homeopathy. 5. The rationale of the 'Simile'. Evidence‐based Complementary and Alternative Medicine: eCAM 2007;4:149‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bornhöft 2006
- Bornhöft G, Wolf U, Ammon K, Righetti M, Maxion‐Bergemann S, Baumgartner S, et al. Effectiveness, safety and cost‐effectiveness of homeopathy in general practice ‐ summarized health technology assessment. Forschende Komplementarmedizin 2006;13(Suppl 2):19‐29. [DOI] [PubMed] [Google Scholar]
Call 2005
- Call SA, Vollenweider MA, Hornung CA, Simel DL, McKinney WP. Does this patient have influenza?. JAMA 2005;293(8):987‐97. [DOI] [PubMed] [Google Scholar]
CDCP 2010
- Centers for Disease Control and Prevention. Key facts about avian influenza (bird flu) and highly pathogenic avian influenza A (H5N1) virus, 2010. http://www.cdc.gov/flu/avian/gen‐info/facts.htm (accessed 18 July 2012) 2010.
CDCP 2011
- Centers for Disease Control and Prevention. Seasonal influenza (flu), 7 November 2011. http://www.cdc.gov/flu/weekly/weeklyarchives2009‐2010/09‐10summary.htm (accessed 18 July 2012) 2011.
Clausen 2011
- Clausen J, Wijk R, Albrecht H. Review of the use of high potencies in basic research on homeopathy. Homeopathy 2011;100:288‐92. [DOI] [PubMed] [Google Scholar]
Demangeat 2004
- Demangeat J‐L, Gries P, Poitevin B, Droesbeke J‐J, Zahaf T, Maton F, et al. Low‐field NMR water proton longitudinal relaxation in ultrahighly diluted aqueous solutions of silica‐lactose prepared in glass material for pharmaceutical use. Applied Magnetic Resonance 2004;26:465‐81. [Google Scholar]
Demangeat 2009
- Demangeat J‐L. NMR water proton relaxation in unheated and heated ultrahigh aqueous dilutions of histamine: evidence for an air‐dependent supramolecular organization of water. Journal of Molecular Liquids 2009;144:32–9. [Google Scholar]
Eizayaga 2011
- Eizayaga FX, Aguejouf O, Desplat V, Doutremepuich C. Beneficial effect of ultra‐low‐dose aspirin in platelet activity alterations and haemorrhage observed in experimental portal hypertension. Thrombosis 2011 Nov 14 [Epub ahead of publication]. [DOI: 10.1155/2012/430460] [DOI] [PMC free article] [PubMed]
Endler 2010
- Endler PCK, Thieves K, Reich C, Matthiessen P, Bonamin L, Scherr C, et al. Repetitions of fundamental research models for homeopathically prepared dilutions beyond 10‐23. Homeopathy 2010;99:25‐36. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
HPUSA 2012
- Homeopathic Pharmacopoeia Convention of the United States. Monograph: Anas barbariae hepatis et cordis extractum. Southeastern, PA 2012.
Jefferson 2009a
- Jefferson T, Jones MA, Doshi P, Mar CB, Dooley L, Foxlee R. Neuraminidase inhibitors for preventing and treating influenza in healthy adults. BMJ 2009;339:b5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2009b
- Jefferson T, Demicheli V, Pietrantonj C, Rivetti D. Amantadine and rimantadine for influenza A in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD001169.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2014
- Jefferson T, Jones MA, Doshi P, Mar CB, Heneghan CJ, Hama R, et al. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD008965.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayne 2006
- Kayne SB. Homoeopathic Pharmacy. Edinburgh: Churchill Livingstone, 2006. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Linde 1997
- Linde K, Clausius N, Ramirez G, Melchart D, Eitel F, Hedges LV. Are the clinical effects of homeopathy placebo effects? A meta‐analysis of placebo‐controlled trials. Lancet 1997;350:834‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marrari 2012
- Marrari LA, Terzan L, Chaufferin G. Oscillococcinum for influenza treatment. Annali dell'Istituto Superiore di Sanità 2012;48:105–9. [DOI] [PubMed] [Google Scholar]
Osterholm 2011
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. RevIew Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rey 2003
- Rey L. Thermoluminescence of ultra‐high dilutions of lithium chloride and sodium chloride. Physica (A) 2003;323:67‐74. [Google Scholar]
Ste Laudy 2009
- Laudy J, Belon P. Inhibition of basophil activation by histamine: a sensitive and reproducible model for study of biological activity of high dilutions. Homeopathy 2009;98:186–97. [DOI] [PubMed] [Google Scholar]
Swayne 2000
- Swayne J. International Dictionary of Homeopathy. Edinburgh: Churchill Livingstone, 2000. [Google Scholar]
Ulbricht 2011
- Ulbricht C, Chao W, Clark A, Conquer J, Cook D, Cormier T, et al. Oscillococcinum (Anas barbariae hepatis et cordis extractum 200CK HPUS). An evidence‐based systematic review by the Natural Standard Research Collaboration. Alternative and Complementary Therapies 2011;17:41‐9. [Google Scholar]
Van Wijk 2006
- Wijk R, Bosman S, Wijk EP. Thermoluminescence in ultra‐high dilution research. Journal of Alternative and Complementary Medicine 2006;12:437–43. [DOI] [PubMed] [Google Scholar]
Wang 2012
- Wang K, Shun‐Shin M, Gill P, Perera R, Harnden A. Neuraminidase inhibitors for preventing and treating influenza in children. Cochrane Database of Systematic Reviews 2012, Issue 1. [DOI: 10.1002/14651858.CD002744.pub3] [DOI] [PubMed] [Google Scholar]
Watanabe 2011
- Watanabe Y, Ibrahim MS, Ellakany HF, Abd El‐Hamid HS, Ikuta K. Genetic diversification of H5N1 highly pathogenic avian influenza A virus during replication in wild ducks. Journal of General Virology 2011;92:2105‐10. [DOI] [PubMed] [Google Scholar]
WHO 2006
- World Health Organization: Regional Office for Europe's Health Evidence Network (HEN). How effective would antiviral vaccination and antiviral drug prevention and treatment strategies be for reducing the impact of the next influenza pandemic? 2006. http://www.euro.who.int/__data/assets/pdf_file/0003/74658/E88034.pdf (accessed 18 July 2012) 2006.
WHO 2009
- World Health Organization. Influenza (Seasonal); Fact Sheet No. 211, 2009. http://www.who.int/mediacentre/factsheets/fs211/en/ (accessed 18 July 2012) 2009.
Witt 2007
- Witt CM, Bluth M, Albrecht H, Weißhuhn T, Baumgartner S, Willich SN. The in vitro evidence for an effect of high homeopathic potencies – a systematic review of the literature. Complementary Therapies in Medicine 2007;15:128–38. [DOI] [PubMed] [Google Scholar]
Woo 2011
- Woo GH, Kim HY, Bae YC, Jean YH, Bak EJ, Kim MJ, et al. Comparative histopathological characteristics of highly pathogenic avian influenza (HPAI) in chickens and domestic ducks in 2008 Korea. Histology and Histopathology 2011;26:167‐75. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Mathie 2012
- Mathie RT, Frye J, Fisher P. Homeopathic Oscillococcinum® for preventing and treating influenza and influenza‐like illness. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD001957.pub5] [DOI] [PubMed] [Google Scholar]
Vickers 1998
- Vickers A, Smith C. Homeopathic Oscillococcinum for preventing and treating influenza and influenza‐like syndromes. Cochrane Database of Systematic Reviews 1998, Issue 3. [DOI: 10.1002/14651858.CD001957] [DOI] [PubMed] [Google Scholar]
Vickers 2000
- Vickers A, Smith C. Homeopathic Oscillococcinum for preventing and treating influenza and influenza‐like syndromes. Cochrane Database of Systematic Reviews 2000, Issue 1. [DOI: 10.1002/14651858.CD001957.pub4] [DOI] [PubMed] [Google Scholar]
Vickers 2004
- Vickers A, Smith C. Homeopathic Oscillococcinum for preventing and treating influenza and influenza‐like syndromes. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD001957.pub4] [DOI] [PubMed] [Google Scholar]
Vickers 2006
- Vickers A, Smith C. Homeopathic Oscillococcinum for preventing and treating influenza and influenza‐like syndromes. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD001957.pub4] [DOI] [PubMed] [Google Scholar]


