Abstract
We report a morphological manipulation of cell division which was achieved by changing the environment from isotonic to highly hypotonic. Cells at telophase were observed to undergo a morphological reversal to anaphase, with the contractile ring being reopened and the cell shape reversing from dumb-bell back to spherical. Once restored to isosmotic environment, the reversed cells would either continue to divide or instead to form binuclear cells that further proliferated in runaway fashions. The immunofluorescent staining of tubulins and myosin II indicated that the hypotonic stress affected the accumulation of tubulins and myosin II at the contractile ring. Distinct from previous studies using specific chemical reagents, the present study provides a simple method to manipulate cell division. The morphological reversal is the adaption of dividing cells to the environmental change. The observation opens a new window to understand cell division mechanisms and runaways.
Keywords: Bioengineering, Hypotonicity, Osmotic, Division, Cytokinesis, Binuclear
1. Introduction
Together with the level change of proteins [1, 2, 3, 4, 5], the mitotic process is accompanied by morphological transformation and volume change. Cell volume decreases at first during the transition from prophase to metaphase, with a 20–50% volume loss and recovers before abscission at the end of cytokinesis [6]. The volume control is a process co-regulated by osmotic pressure and actomyosin cortex and it is to complete different physiological processes and to meet stimulus from the external environment [7]. The morphological change involves central spindle assembly, actomyosin contractile ring assembly, cytokinetic furrow ingression and abscission [8]. Blocking and inhibiting the function of certain cytoskeleton will interrupt the normal process of cytokinesis, showing the role of cytoskeleton in eukaryotic cell mitosis. Cytochalasin B, a well-known chemical inhibitor of microfilaments, has been reported to preclude the formation of furrow and contraction of contractile ring during cytokinesis, resulting in a binuclear cell [9]. Inhibition of microtubules with nocodazole, which depolymerize tubulins, can block the onset of cytokinesis if cells are treated in prometaphase, however has less influence for those having already started contraction [9].
The effects of abnormal tonicity on cell cycles have long been investigated. For cell division, hypertonicity was found to inhibit normal mitosis of chick cells [13, 14] and HeLa cells [15, 16]. After brief exposure to hypotonic solutions cultures of human lymphocytes and PtK2 cells revealed a significant increase in the frequency of anaphase cells [17]. Hypotonic treatment was also found to interrupt normal mitosis, to inhibit or influence cell division at pre-prophase, metaphase or anaphase [14]. Hypotonic treatment could make chromatid pairs scattered throughout the cells at prophase or metaphase, which might be related with the mitosis inhibition [14]. By using hypotonic culture medium or saline solutions Nowak observed chromosomal aberrations in V79 cells that chromosomes would expand and spindle microtubules would depolymerize [18]. The hypotonic influences were also found reversible [19].
Here we report a simple hypotonic method that achieves a morphological reversal of cytokinesis. Hypotonic shock refers to an environment medium that is lower in solutes than that of the fluid inside of a cell. It is widely accepted that water will flow across the cell membrane into that cell from the surrounding hypotonic environment eventually causing the cell to swell and burst. Here we show that when treat dividing mammalian cells with highly hypotonic medium, the cytokinetic furrows would regress and the cells were morphologically reversed back to spherical shape. After the environment restored to isosmotic, some of the reversed cells went onto with a secondary cytokinesis; and some stopped the cytokinesis and became binuclear cells. We employed immunofluorescence to find the change of cytoskeleton of the aimed cells.
2. Experimental
Human cervical cancer cell HeLa, human ovarian cancer cell SiHa and mouse fibroblast cell NIH-3T3 were cultured in isosmotic DMEM (Dulbecco's Modified Eagle Medium, Sigma) with 10% fetal bovine serum (Hyclone, Logan, UT), 100 U/ml Penicillin-Streptomycin solution (Hyclone, Logan, UT) and 0.25% trypsin (Hyclone, Logan, UT). Cultures were maintained at 37 °C with 5% CO2 as gas atmosphere. Hypotonic treatment was achieved by replacing DMEM medium by hypotonic solution. The hypotonic solution was made by diluting isosmotic phosphate buffered saline (PBS) solution using deionized water. Concentration gradient was 5%, 10%, 20%, 30%.
3. Results and discussion
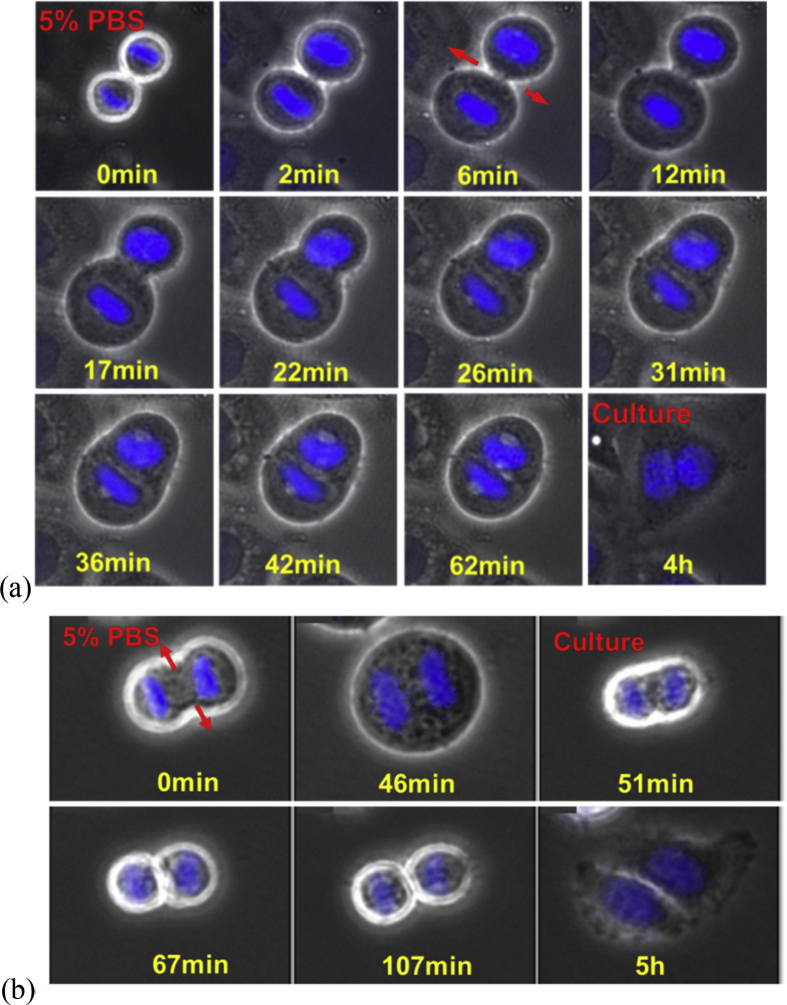
The hypotonic treatment was conducted after the cells entered telophase when the cytokinetic furrows had been formed. A typical result is shown in Fig. 1(a). At time zero, the target HeLa cell had come to its telophase. The two sets of daughter chromosomes had arrived at the pole of the spindle and decondensed. A new nuclear envelope reassembled around each set, completing the formation of the two nuclei. Then we replaced the medium by highly hypotonic i.e. 5% PBS solution. The highly hypotonic environment led to an immediate increase in the cell volume since water flew into the cell (Fig. 1(a), 0–6 min). After about 10 min of the hypotonic treatment, the midbody disappeared and the cytokinetic furrow started to retract. At the same time the newly formed nuclear envelope dissolved. The retraction lasted for tens of minutes until the furrow thoroughly disappear (Fig. 1(a), 42 min). After that, the cell further adjusted its shape to spherical (Fig. 1(a), 62 min) and the chromosomes aligned near the metaphase plate. The cell was therefore backing to its anaphase in morphology. Another example is shown in Fig. 1(b). The hypotonic treatment was started at 0 min and stopped at 46 min. It is seen that the cell has been reversed to spherical at 46 min.
Fig. 1.
The morphological change of HeLa cells undergoing division in highly hypotonic environment. The red arrows indicate the retracting of the cytokinetic furrow: (a) The HeLa cell was cultured in isotonic DMEM till its telophase and then the medium was replaced by 5% PBS at time 0 min. Morphological reversal occurred. At 62 min the cell was cultured again in isotonic DMEM and the cell finally became binuclear. (b) The HeLa cell was cultured in isotonic DMEM till its telophase and then the medium was replaced by 5% PBS at time 0 min. Morphological reversal occurred. At 46 min the cell was cultured again in isotonic DMEM and the cell started a secondary division and finally finished the division.
The reversal process occurred only in highly hypotonic i.e. low concentration solutions. In the literatures usually a more moderate hypotonicity was employed, with PBS concentrations >40% [10, 11, 12]. In our experiments as shown in Tables 1, 2, and 3, lower PBS concentration i.e. greater hypotonicity led to higher possibility of cell reversal. For HeLa cells, 20% and lower PBS solutions were appropriate for reversal observation and for SiHa even lower, 5%. In the tables, the target cell number was the number of living cells that were not washed away or dead during the hypotonic treatment. The treatment was mostly done in room condition i.e. 25 °C and CO2 0.03–0.04%. To check the influence of treatment condition i.e. temperature and gas atmosphere on reversal, we also conducted experiments in incubator at 37 °C with 5% CO2, as shown in Table 2. The reversal results were similar to those conducted in room condition. Especially for 20% and 30% PBS, there was barely difference between the results of the two kinds of conditions. On the other hand, we noticed that for 10% and lower PBS the treated cells were easier to be washed away in incubator than in room condition. In room condition, 11% and 15% of HeLa cells were washed away after the treatment of 10% and 5% PBS respectively, while in incubator it was 45% and nearly 100% washed away.
Table 1.
Relationship between PBS concentration and possibility of morphological reversal (HeLa cells, in room condition).
| PBS Concentration | Target cell Number | Reversal number/percentage |
|---|---|---|
| 30% | 97 | 19/17.8% |
| 20% | 78 | 39/50.0% |
| 15% | 83 | 49/59.0% |
| 10% | 59 | 50/84.7% |
| 5% | 27 | 26/96.3% |
Table 2.
Relationship between PBS concentration and possibility of morphological reversal (HeLa cells, in incubator).
| PBS Concentration | Target cell Number | Reversal number/percentage |
|---|---|---|
| 30% | 55 | 12/21.8% |
| 20% | 68 | 36/52.9% |
| 10% | 58 | 45/77.6% |
Table 3.
Relationship between PBS concentration and possibility of morphological reversal (SiHa cells, in room condition).
| PBS Concentration | Target cell Number | Reversal number/percentage |
|---|---|---|
| 30% | 10 | 0/0% |
| 20% | 74 | 1/1.3% |
| 10% | 24 | 4/14.7% |
| 5% | 84 | 49/57.0% |
We restored the environment to be isotonic to continue the culture after the cells had been reversed back to a spherical shape. The cells then first shrank in volume due to the water loss to the environment and then moved on in two different directions. A big portion of them, as shown in the last picture in Fig. 1(a), completely stopped the cytokinesis and became binuclear cells. The binuclear cell then grew and reproduced in disordered fashion. While a smaller portion of the reversed cells, as shown in Fig. 1(b), would quickly have the cytokinetic furrow again and continue to finish the cytokinesis. This secondary division could be finished in tens of minutes. The two daughter cells could further divide into four after hours. The fact that cells could have the secondary division indicates that the reversal due to the high hypotonicity is reversible. A quantification of binucleated cells and cells which succeed to divide after restauration of isotonic conditions were discussed in Table 4.
Table 4.
Culture results of cells with morphology reversal after restauration of isotonic conditions (5% PBS hypotonic treatment).
| Cell Type | cell Number | Dead | Alive |
|
|---|---|---|---|---|
| binucleated | Secondary division | |||
| SiHa | 7 | 1/17.8% | 3/42.9% | 3/42.9% |
| HeLa | 25 | 1/4.0% | 16/64.0% | 8/32.0% |
Cell number: Morphological reversal cell number which were still alive after changing medium for reculture.
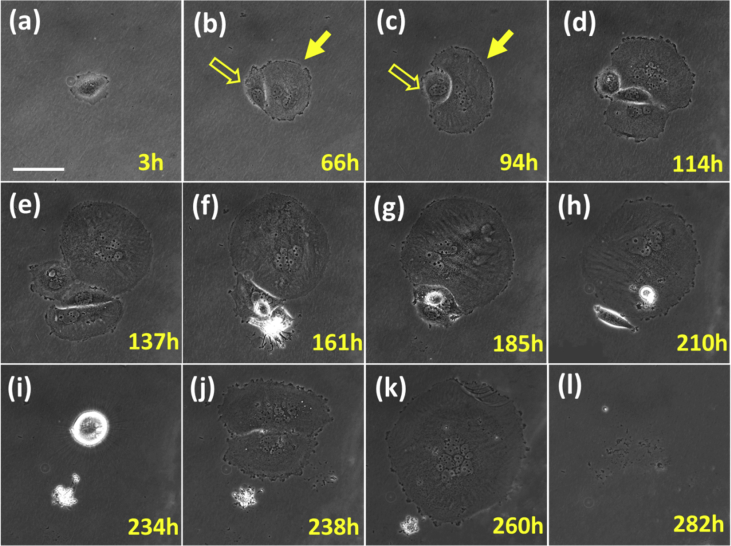
A big portion of reversed cells could not have the secondary division but form binuclear cells that further reproduced in a runaway fashion. Fig. 2 shows a typical result. The binuclear cell waited for a very long time, 66 h of culture in isotonic DMEM, to start a division. The binuclear cell was huge as about 100 μm in diameter and the division was asymmetrical: One small daughter and one big daughter were produced as shown at 66 h in Fig. 2(b). The small one eventually died at about 161 h. The big one continued to divide in a disordered manner. At about 234 h, a small daughter cell remained at the location and then it grew huge again and divided into two daughters after 4 h. We observed a huge multinucleate cell at 260 h with a diameter of over 250 μm. No cell was observed at the same location after 282 h.
Fig. 2.
A culture process in isotonic DMEM of a binuclear cell after the morphological reversal in 5% PBS. The culture was started at 0 h. The binuclear cell was growing and proliferating in a disordered fashion. Multinucleate cells were produced. Scale bar = 100 μm.
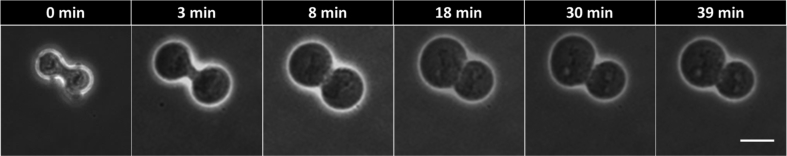
Flavopiridol can induce the degradation of cyclin B and therefore mitotic cells treated with flavopiridol will undergo a premature mitotic exit accompanied by cytokinesis. Here we added 5 μM and 10 μM flavopiridol to the 5% hypotonic PBS medium to see if flavopiridol can push the cells to go through the cytokinesis despite trend of the morphological reversal. The results found that flavopiridol did affect the reversal. The percentage of reversal was lower for 10 μM than 5 μM flavopiridol, referring to Table 5. As shown in Fig. 3, the contractile ring became wider and wider at first, showing a reversal process, but then was stopped and behaved in back-and-forth mode indicating the competition of the two factors i.e. flavopiridol’ s pushing to mitotic exit and hypotonicity ‘s reversing. Note that flavopiridol induced more death of the cells, as shown in Table 5. We speculated that the morphological reversal was an adaptation of the dividing cells to the hypotonicity stress; flavopiridol forced the cell to go through the exit and thus harmed the adaptation, which therefore led to more cell death.
Table 5.
Flavopiridol test (HeLa cells treated with 5% PBS with flavopiridol).
| Concentration | Target No. | Reversed | Washed away | Alive |
|
|---|---|---|---|---|---|
| Binuclear | Continue to divide | ||||
| 5 uM | 12 | 10, 83.3% | 5, 41.6% | 5/41.6% | |
| 3/60.0% | 2/40.0% | ||||
| 10 uM | 8 | 3, 37.5% | 0, 0.0% | 3/37.5% | |
| 2/66.7% | 1/33.3% | ||||
Washed away: Morphological reversal cells disappeared after re-culture in DMEM. The percentage is total target number divided by washed-away number.
Alive: Morphological reversal cells were still alive after changing medium for re-culture. The percentage is total target number divided by alive number.
Binuclear and Divided: The percentage is alive number divided by binuclear or divided number.
Fig. 3.
HeLa cells treated with 5% PBS with 5 uM flavopiridol being added. The cytokinetic furrow got wider at the beginning and then stopped and contracted later, indicating that low concentration flavopiridol might have a competition with the hypotonic stress on the cell cycle. Scale bar = 20 um.
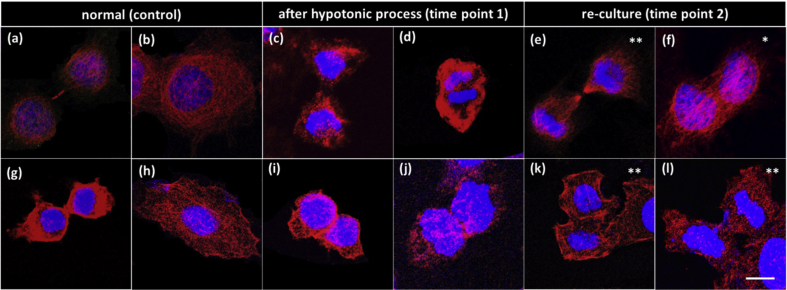
We have conducted immunofluorescence tests on tubulins and myosin to have an idea about the cell cytoskeleton change during the reversal. As shown in Fig. 4, the first row shows immunofluorescence test results for tubulins as well as DNA (Fig. 4(a)–(f)). The pattern of tubulins was a little different at timepoint 1 (Fig. 4(c) and (d)) from the control (Fig. 4(a) and (b)). Tubulins were not arranged as ordered and tended to form small patches. Fig. 4(d) shows an accumulation of tubulins at cell border. The change of tubulin pattern might result from the hypotonic stimulation. At timepoint 2, Figs. 4(e) and (f) show that there is no significant difference between a going-on-division cell (Fig. 4(e)) and a normal dividing cell (Fig. 4(a)). In a binuclear cell (Fig. 4(f)), tubulins showed an ordered and directional arrangement. The second row shows results for myosin II as well as DNA (Fig. 4(g)–(l)). At timepoint 1 myosin II accumulated like spots and patches (Fig. 4(j)) which might indicate the change induced by hypotonicity. The results for going-on-division cells (Fig. 4(k) and 4(l)) were again alike a normal adherent cell (Fig. 4(h)).
Fig. 4.
Immunofluorescence results of tubulins (a-f, red) and myosin II (g-l, red) and DNA (blue) of HeLa cells. Normal HeLa cells were taken as the control (a, b, g, h). The timing when the reversed cells were just back to isotonic DMEM culture was taken as time point 1, then 40 min after was taken as time point 2. The symbol “*” represents results of binuclear cells and “**” represents results of resuming-to-divide cells. Scale bar = 20 μm.
The results showed that tubulins and myosin tended to form small patches under hypotonicity stress, indicating that the hypotonic stress might affect the accumulation of tubulins and myosin II at the contractile ring and interrupted the contraction process.
The effects of abnormal tonicity on cell cycles have long been investigated. For cell division, hypertonicity was found to inhibit normal mitosis of chick cells [13, 14] and HeLa cells [15, 16]. After brief exposure to hypotonic solutions cultures of human lymphocytes and PtK2 cells revealed a significant increase in the frequency of anaphase cells [17]. Hypotonic treatment was also found to interrupt normal mitosis, to inhibit or influence cell division at pre-prophase, metaphase or anaphase [14]. Hypotonic treatment could make chromatid pairs scattered throughout the cells at prophase or metaphase, which might be related with the mitosis inhibition [14]. By using hypotonic culture medium or saline solutions Nowak observed chromosomal aberrations in V79 cells that chromosomes would expand and spindle microtubules would depolymerize [18]. The hypotonic influences were also found reversible [19]. In our experiment, we also observed the quickly volume increase both of the cell and the chromosomes in the first 2 or 3 min immediately after the hypo-osmotic shock (Fig. 1(a)).
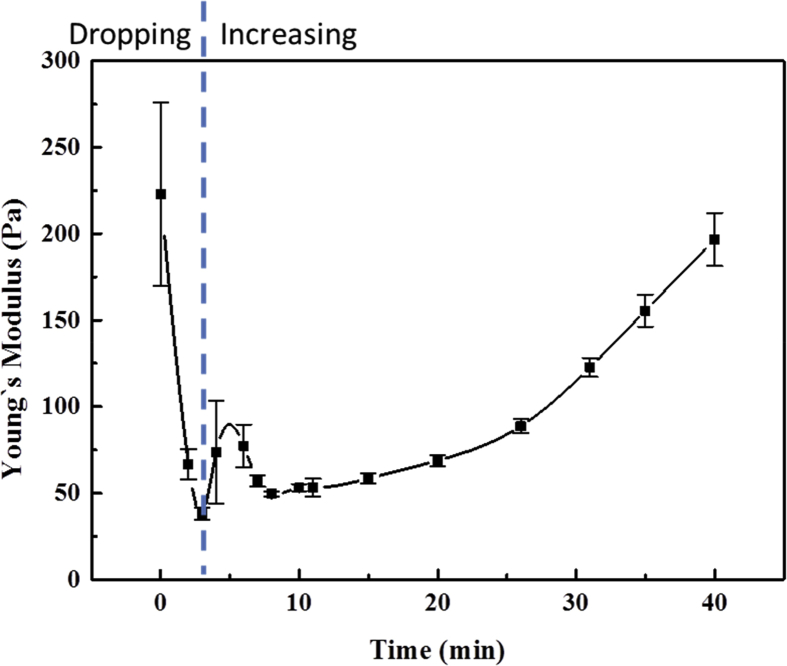
The observed morphological reversal can be divided into two key steps. The first step was the disfunction i.e. loosing contractility of the cytokinetic furrow; the second step was the cell shape rounding up from dumb-bell to spherical. Regarding the first step, as well known, the cytokinesis furrow is composed of myosin and bundles of F-actin. The myosin like motors intermediates the gliding of the F-actin and makes the furrow to contract. The observed furrow with loosing contractility under high hypotonicity is related to the disfunction of these two components. Regarding myosin, we have conducted immunofluorescence tests on tubulins and myosin of cytokinetic cells, Figs. 4(i),(j). The results have showed that tubulins and myosin formed small patches under the hypotonicity stress. Since myosin has been well identified as the force generator in furrow contraction [20, 21, 22, 23, 24, 25], the furrow would then lose the constriction force. Regarding F-actin, a series of studies in various cells types have suggested that F-actin network would be disrupted at the onset of osmotic swelling under hypotonicity stress [26, 27, 28, 29, 30]. For example Pedersen et al. [31] demonstrated a decrease in cellular F-actin content within 3 min with a hypotonic challenge. In our cases as a proof, we used atomic force microscopy to measure the cell stiffness. As shown in Fig. 5, the stiffness decreased sharply in the first 2–3 min when was the onset of osmotic swelling, indicating the disruption of F-actin since the membrane stiffness of living cells depends on the submembrane F-actin cytoskeleton as proved by Maa et al. [32].
Fig. 5.
Typical AFM result of cell stiffness change during hypotonic treatment. The error bar stands for the standard deviation of the data points.
Based on the above analysis, the cleavage furrow lost the contractility under hypotonicity due to the disfunction of its myosin and F-actin. Then the second step, i.e. morphological reversal occurred. Still due to the hypotonicity, water kept fluxing into the cell and gradually increased the intracellular hydrostatic pressure. In Fig. 5, at time 0 the culture medium was replaced by a 5% PBS solution. HeLa cells at metaphase were measured. Micro-sized blunt tip was used. The measurement was conducted at room temperature 25 °C. The value of Young's modulus at each data point was the average of 8–16 data points detected by AFM within 15 s on an area of about 2*2 μm2 on the cell surface. The Young's modulus decreased sharply in the first 2–3mins, indicating the disruption of submembrane F-actin cytoskeleton. The gradual increase of the stiffness in the AFM result, Fig. 5, lasting for 30–50 min in different cases, is the proof for the hydrostatic pressure increase due to water influx.
It has been know that hydrodynamic pressure can mechanically control cell shapes [33]. The increased intracellular pressure swelled the cell, pushed open the furrow which had no contractility, thus rounded the cell up and finally a cell with double nucleus was formed. The rounding up process was also lasting for 30–50 min as shown in Fig. 1(a) (b), consistent with the time lasting for the pressure increase.
4. Conclusions
As summary, hypotonicity quickly made the furrow lose the contractility by disrupting the myosin and F-actin, and then gradually rounded up the cell by increasing the hydrostatic pressure through water influx. The molecular mechanism of how hypotonicity disrupts myosin and F-actin, however, remains an open question despite the many studies. Overall the reversal can be regarded as the protection response of a cell to an emergency accident i.e. hypotonic shock in vitro. Enforcing the osmotically shocked cell to finish the cytokineisis would cause higher death ratio which have confirmed by our flavopiridol experiment as discussed.
On the other hand, the present study has showed that high hypotonicity can be a new approach to manipulate cytokinesis. Previous studies always used chemical reagents to manipulate cytokinesis. For example cytochalasin B and its derivative dihydrocytochalasin B (H2CB) could inhibit cytokinesis and form binuclear cells [9, 34, 35, 36]. Microtubule inhibitors colcemid, vinblastine [37] and nocodazole [9, 38] were discovered to inhibit cell division by affecting microtubule functions. Psychosine, a lipid metabolite, was found to affect actin reorganization and inhibit cytokinesis. Studies showed that it might account for the formation of multinuclear globoid cells in the brains of patients with globoid cell leukodystrophy [39]. 1-O-Octadecyl-2-O-methyl- glycero-3-phosphocholine (ET-18-OCH3) `s could inhibit cell division and the reason might be related to cytoskeletal effects [40]. Here we have shown that high hypotonicity can stop and reverse the cytokinesis. Moreover, the reversal could be reversible since the cytokinesis could be continued once the environment restored isosmotic. The reversal is an adaption to the environmental osmotic pressure change. We believe it opens a new window to understand division mechanisms, cell cycle reversibility as well as runaways. Overall the present study is reporting preliminary observations, and intensive study of molecular mechanisms is needed in the future to establish the link between physical phenomenon i.e. osmotic equilibration with a biochemical process i.e. the regulation of mitosis.
5. Materials & methods
5.1. Cells preparations
Human cervical cancer cell HeLa, human ovarian cancer cell SiHa and mouse fibroblast cell NIH-3T3 were cultured in DMEM (Dulbecco's Modified Eagle Medium, Sigma) with 10% fetal bovine serum (Hyclone, Logan, UT), 100U/ml Penicillin-Streptomycin solution (Hyclone, Logan, UT) and 0.25% trypsin (Hyclone, Logan, UT). Cultures were maintained at 37% with 5% CO2 as gas atmosphere.
5.2. Cell synchronization
Thymidine (TdR, Sigma) was used to synchronize HeLa cells. TdR power was dissolved in PBS solution with concentration of 100mM, restored at 4. The working concentration for cell culture was 2 mM. The time nodes for synchronization using TdR were 16h-9h–16h. 2mM TdR solution added in HeLa cells, cultured for 16h and released for 9h. 2mM TdR added for a second time for 16h and released for M phase of HeLa cells.
5.3. Hypotonic treatment
PBS hypotonic solution was diluted for gradient concentration using sterile diluted-ironed water and PBS (Phosphate Buffered Saline) solution. Concentration gradient was 5%, 10%, 20%, 30%. Cells in cytokinesis were chosen as targeted cells. Cells were washed with PBS solution twice and then added with hypotonic PBS solution. Then the cells were observed under optical microscopy and recorded cellular dynamics. Then hypotonic solution was removed after treatment and changed to isotonic culture medium for sequential culture.
5.4. Immunofluorescence staining
Cells were fixed with 3.7% paraformaldehyde (Sigma-Aldrich) and 0.2% Triton X-100 (Sigma-Aldrich). 30% stock solution was stored at 4. Cells were sealed with normal goat serum at room temperature. The primary antibody (anti-alpha tubulin antibody, mouse source, ab15426, Abcam) and secondary antibody (Goat anti-rabbit IgG H&L, Alexa Fluor 555, ab150078, Abcam) were diluted to final concentration (1/100, 1/10,1/500). DAPI (4′6-diamidino-2-phenylindole, 1 mg/ml) was diluted with PBS to 5 μg/ml. Fluorescent mounting medium ZLI-9556 (ZSGB-BIO, BJ) was used for slide mounting. Cells were treated with primary antibody which was diluted to final concentration for 14–16 h at 4. Then the primary antibody was removed with PBS solution and secondary antibody was added for 2h at room temperature in dark place. The nucleus was stained using DAPI for 5min and washed off with PBS. The slides were mounted and sealed.
5.5. AFM test
The experiment used a state-of-the-art TM-AFM (MFP-3D-BIO, Asylum Research) combined with an inverted optical microscope for the study. All the tests were performed under room temperature (25 °C) and finished within 1 h. Two types of probes were used for testing. The sharp tip used (TR400PSA, Oxford instruments) had a measured force constant of 20 mN/m with an opening angle of 35°. The tip radius was 20 nm. The other type i.e. microsphere tip consisted of a cantilever (PNP-TR-TL-Au, Nanoworld) with spring constant of 80 mN/m, and with a 10 microns diameter polystyrene bead adhered to the cantilever top.
The AFM was operated in the force-mapping mode to measure the local Young's modulus. Force displacement curve were recorded at 1 Hz for determination of Young's modulus. The force curves were converted to force-indentation curves and fitted with Hertz model. For each time point, we recorded a force map of 44 force curves. Indentations were controlled under 600 nm to avoid damage to cell surface and substrate-induced effects.
Declarations
Author contribution statement
Chaoyu Huang: Performed the experiments, Analyzed and interpreted the data, Wrote the paper.
Yuhui Li: Performed the experiments, Analyzed and interpreted the data.
Hao Wang: Conceived and designed the experiments, Wrote the paper.
Funding statement
This work was supported by National Natural Science Foundation of China (No. 51622601).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.King R.W., Deshaies R.J., Peters J.-M., Kirschner M.W. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- 2.López-Avilés S., Kapuy O., Novák B., Uhlmann F. Irreversibility of mitotic exit is the consequence of systems-level feedback. Nature. 2009;459:592–595. doi: 10.1038/nature07984. 528 May 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak B., Tyson J.J., Gyorffy B., Csikasz-Nagy A. Irreversible cell-cycle transitions are due to systems-level feedback. Nat. Cell Biol. 2007;9:724–728. doi: 10.1038/ncb0707-724. [DOI] [PubMed] [Google Scholar]
- 4.Potapova T.A., Sivakumar S., Flynn J.N., Li R., Gorbsky G.J. Mitotic progression becomes irreversible in prometaphase and collapses when Wee1 and Cdc25 are inhibited. Mol. Biol. Cell. 2011;22:1191–1206. doi: 10.1091/mbc.E10-07-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Potapova Tamara A. The reversibility of mitotic exit in vertebrate cells. Nature. 2006;440:954–958. doi: 10.1038/nature04652. 913 April 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boucrot E., Kirchhausen T. Mammalian cells change volume during mitosis. PLoS One. 2008;3 doi: 10.1371/journal.pone.0001477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stewart M.P. Hydrostatic pressure and the actomyosin cortex drive mitotic cell rounding. Nature. 2011;469:226–230. doi: 10.1038/nature09642. [DOI] [PubMed] [Google Scholar]
- 8.Fededa J.P., Gerlich D.W. Molecular control of animal cell cytokinesis. Nat. Cell Biol. 2012;14 doi: 10.1038/ncb2482. [DOI] [PubMed] [Google Scholar]
- 9.Chen Z., Cai S., Jiang Q., Zhang C., Tang X. Roles for microtubule and microfilament cytoskeletons in animal cell cytokinesis. Chin. Sci. Bull. 2005;50:229–235. [Google Scholar]
- 10.Henson J.H. Confocal microscopic observation of cytoskeletal reorganizations in cultured shark rectal gland cells following treatment with hypotonic shock and high external K+ J. Exp. Zool. 1997;279:415–424. [PubMed] [Google Scholar]
- 11.Dartsch P.C., Kolb H.-A., Beckmann M., Lang F. Morphological alterations and cytoskeletal reorganization in opossum kidney (OK) cells during osmotic swelling and volume regulation. Histochem. Cell Biol. 1994;102:69–75. doi: 10.1007/BF00271051. [DOI] [PubMed] [Google Scholar]
- 12.Hoffmann E.K., Simonsen L.O. Membrane mechanisms in volume and pH regulation in vertebrate cells. Physiol. Rev. 1989;69:315–382. doi: 10.1152/physrev.1989.69.2.315. [DOI] [PubMed] [Google Scholar]
- 13.Wheatley D.N., Angus B. Inhibition of cell division in mammalian cell cultures by hypertonic medium. Experientia. 1973;29:1393. doi: 10.1007/BF01922837. [DOI] [PubMed] [Google Scholar]
- 14.Hughes A. Some effects of abnormal tonicity on dividing cells in chick tissue cultures. Q. J. Microscopicalence. 1952;93:207–219. [Google Scholar]
- 15.Stubblefield E., Mueller G.C. Effects of sodium chloride concentration on growth, biochemical composition, and metabolism of HeLa cells. Cancer Res. 1960;20:1646–1655. [Google Scholar]
- 16.Robbins E., Pederson T., Klein P. Comparison of mitotic phenomena and effects induced by hypertonic solutions in HeLa cells. J. Cell Biol. 1970;44:400. doi: 10.1083/jcb.44.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ford J.H., Congedi M.M. Rapid induction of anaphase in competent cells by hypotonic treatment. Cytobios. 1987;51:183–192. [PubMed] [Google Scholar]
- 18.Nowak C. Studies on the ability of hypotonic solutions to induce chromosomal aberrations in V 79 cells. Teratog. Carcinog. Mutagen. 1987;7:515. doi: 10.1002/tcm.1770070603. [DOI] [PubMed] [Google Scholar]
- 19.Brinkley B.R., Cox S.M., Pepper D.A. Structure of the mitotic apparatus and chromosomes after hypotonic treatment of mammalian cells in vitro. Cytogenet. Genome Res. 1980;26:165–174. doi: 10.1159/000131438. [DOI] [PubMed] [Google Scholar]
- 20.Mendes Pinto I., Rubinstein B., Kucharavy A., Unruh Jay R., Li R. Actin depolymerization drives actomyosin ring contraction during budding yeast cytokinesis. Dev. Cell. 2012;22:1247–1260. doi: 10.1016/j.devcel.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murthy K., Wadsworth P. Myosin-II-dependent localization and dynamics of F-actin during cytokinesis. Curr. Biol. Cb. 2005;15:724–731. doi: 10.1016/j.cub.2005.02.055. [DOI] [PubMed] [Google Scholar]
- 22.Straight A.F. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299:1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 23.Burgess D.R. Cytokinesis: new roles for myosin. Curr. Biol. Cb. 2005;15:310–311. doi: 10.1016/j.cub.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 24.Cheffings T.H., Burroughs N.J., Balasubramanian M.K. Actomyosin ring formation and tension generation in eukaryotic cytokinesis. Curr. Biol. Cb. 2016;26:R719. doi: 10.1016/j.cub.2016.06.071. [DOI] [PubMed] [Google Scholar]
- 25.Scholey J.M., Brustmascher I., Mogilner A. Cell division. Nature. 2003;422:746. doi: 10.1038/nature01599. [DOI] [PubMed] [Google Scholar]
- 26.Begg D.A., Salmon E.D., Hyatt H.A. The changes in structural organization of actin in the sea urchin egg cortex in response to hydrostatic pressure. J. Cell Biol. 1983;97:1795. doi: 10.1083/jcb.97.6.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Henson J.H. Relationships between the actin cytoskeleton and cell volume regulation. Microsc. Res. Tech. 1999;47:155. doi: 10.1002/(SICI)1097-0029(19991015)47:2<155::AID-JEMT7>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 28.Jorgensen N.K. Cell swelling activates cloned Ca(2+)-activated K(+) channels: a role for the F-actin cytoskeleton. Biochim. Biophys. Acta. 2003;1615:115–125. doi: 10.1016/s0005-2736(03)00237-2. [DOI] [PubMed] [Google Scholar]
- 29.Levitan I., Almonte C., Mollard P., Garber S.S. Modulation of a volume-regulated chloride current by F-actin. J. Membr. Biol. 1995;147:283. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- 30.Pritchard S., Guilak F. The role of F-actin in hypo-osmotically induced cell volume change and calcium signaling in anulus fibrosus cells. Ann. Biomed. Eng. 2004;32:103–111. doi: 10.1023/b:abme.0000007795.69001.35. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen S.F., Mills J.W., Hoffmann E.K. Role of the F-actin cytoskeleton in the RVD and RVI processes in ehrlich ascites tumor cells. Exp. Cell Res. 1999;252:63–74. doi: 10.1006/excr.1999.4615. [DOI] [PubMed] [Google Scholar]
- 32.Maa A., Lemaster E., Teng T., Lee J., Levitan I. Hypotonic challenge of endothelial cells increases membrane stiffness with No effect on tether force. Biophys. J. 2018;114:929. doi: 10.1016/j.bpj.2017.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y. Going with the flow: water flux and cell shape during cytokinesis. Biophys. J. 2017;113:2487–2495. doi: 10.1016/j.bpj.2017.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.RIDLER M.A.C., SMITH G.F. The response of human cultured lymphocytes to cytochalasin B. J. Cell Sci. 1968;3:595–602. doi: 10.1242/jcs.3.4.595. [DOI] [PubMed] [Google Scholar]
- 35.Carter S.B. Effects of cytochalasins on mammalian cells. Nature. 1967;213:261–264. doi: 10.1038/213261a0. [DOI] [PubMed] [Google Scholar]
- 36.Aubin J., Osborn M., Weber K. Inhibition of cytokinesis and altered contractile ring morphology induced by cytochalasins in synchronized PtK2 cells. Exp. Cell Res. 1981;136:63–79. doi: 10.1016/0014-4827(81)90038-0. [DOI] [PubMed] [Google Scholar]
- 37.Yang H., Ganguly A., Cabral F. Inhibition of cell migration and cell division correlates with distinct effects of microtubule inhibiting drugs. J. Biol. Chem. 2010;285:32242–32250. doi: 10.1074/jbc.M110.160820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wheatley S.P., Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J. Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kanazawa T. Inhibition of cytokinesis by a lipid metabolite, psychosine. J. Cell Biol. 2000;149:943–950. doi: 10.1083/jcb.149.4.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pushkareva M.Y., Janoff A.S., Mayhew E. Inhibition of cell division but not nuclear division by 1-O-octadecyl-2-O-methyl-Sn-glycero-3-phosphocholine. Cell Biol. Int. 1999;23:817–828. doi: 10.1006/cbir.1999.0478. [DOI] [PubMed] [Google Scholar]