Abstract
Adult T‐cell leukemia/lymphoma (ATLL) is a mature T‐cell neoplasm, and is divided into 2 indolent (smoldering and chronic) and 2 aggressive (acute and lymphoma) clinical subtypes. Based on previous integrated molecular analyses suggesting the importance of the JAK‐STAT pathway in ATLL, we attempted to clarify the clinicopathological significance of this pathway. Clinical and morphological findings were reviewed in 116 cases with ATLL. The nuclear localizations of phosphorylated STAT3 (pSTAT3), pSTAT5, and pSTAT6 were analyzed by immunohistochemistry. Targeted sequencing was undertaken on the portion of STAT3 encoding the Src homology 2 domain. Expression of pSTAT3 was observed in 43% (50/116) of ATLL cases, whereas pSTAT5 and pSTAT6 were largely undetected. Cases with the lymphoma type showed significantly less frequent pSTAT3 expression (8/45, 18%) than those with the other subtypes (41/66, 62%; P < .001). STAT3 mutations were detected in 36% (10/28) and 19% (12/64) of cases with the smoldering and aggressive types of ATLL, respectively. The correlation between STAT3 mutation and pSTAT3 expression was not significant (P = .07). Both univariate and multivariate analysis revealed that pSTAT3 expression was significantly associated with better overall survival and progression‐free survival in the smoldering type of ATLL, whereas STAT3 mutation was not related to a line of clinical outcome. Collectively, our data show that only the lymphoma type showed a low prevalence of tumor cells positive for pSTAT3 expression, and raises the possibility that pSTAT3 expression is a novel biomarker to predict better prognosis in the smoldering type of ATLL.
Keywords: adult T‐cell leukemia/lymphoma, immunohistochemistry, phosphorylated STAT3, Shimoyama classification, STAT3 mutation
1. INTRODUCTION
Adult T‐cell leukemia/lymphoma (ATLL) is a malignant peripheral T‐cell neoplasm caused by human T‐cell leukemia virus type I (HTLV‐1).1 According to the Shimoyama classification, ATLL is classified into 4 disease subtypes: smoldering, chronic, lymphoma, and acute.2, 3 The acute, lymphoma, and chronic types, when accompanied by unfavorable prognostic factors (hypoalbuminemia, high serum blood urea nitrogen, or high serum lactate dehydrogenase), are regarded as aggressive forms of the disease, and generally have an adverse clinical course.4 In contrast, the indolent type of ATLL, which includes the smoldering type and the chronic type without unfavorable factors, usually presents with a slower clinical course and progresses to an aggressive type of ATLL following additional genetic alterations.5, 6 The prognosis of each clinical subtype varies, and is estimated by clinical parameters of the ATLL prognostic index (ATL‐PI) or the indolent ATL‐PI (iATL‐PI) for the aggressive or indolent type, respectively.4, 7 Kataoka et al8 recently reported that several genetic alterations, including IRF4 amplification, 9p24 (PD‐L1) amplification, or 9p21 (CDKN2A) deletion, were significantly associated with poor prognosis in patients with the indolent type of ATLL. However, there is no fully established prognostic index that takes into account the molecular backgrounds of ATLL, and the molecular mechanisms responsible for such clinical diversity in ATLL are largely unknown.
The JAK‐STAT signaling pathway plays important roles in various critical cellular processes, including survival, proliferation, and differentiation.9 Activation of this pathway is usually induced by the binding of cytokines, such as interleukin (IL)‐6, IL‐10, and IL‐15, to common gamma receptors, followed by phosphorylation of the JAK and STAT proteins. Phosphorylated STAT proteins (pSTATs) then dimerize and migrate into the nucleus, and regulate the transcription of various target genes.10, 11 Seven known mammalian STAT family members have been identified, and their abnormal activation has been reported in various malignant tumors such as melanoma,12 lung cancer,13 ovarian cancer,14 colon cancer,15 and prostate cancer.16 In malignant lymphoma, abnormal activation or genetic alterations of STAT3, STAT5, and STAT6 have been reported.17, 18, 19, 20, 21, 22, 23, 24 In particular, the constitutive activation of the JAK‐STAT pathway was identified in leukemic cells of ATLL patients, indicating its involvement in the acceleration of the cell cycle.25 Furthermore, the recent development of next‐generation sequencing technologies has expanded our knowledge concerning genomic abnormalities in the molecular pathogenesis of ATLL. Kataoka et al26 reported that STAT3 is one of the most frequently mutated genes in ATLL, affecting 21% of all patients. They also found that STAT3 mutation was detected significantly more frequently in the indolent type than the aggressive type, suggesting that the pertinent mutation was associated with a slowly progressive clinical course in ATLL.8 STAT3 mutations were also identified in cases with indolent granular lymphocytic leukemia of both T cell and natural killer cell origin.27 Zhang et al28 reported the antitumor efficacy of JAK‐STAT pathway inhibition in both in vitro and in vivo models of the indolent type of ATLL. Although these findings strongly suggest a pivotal role for the JAK‐STAT pathway, the relationship between the activation of this pathway and the diverse clinicopathological subtypes of ATLL, particularly the indolent type, has not been previously examined.
In this study, we determine the clinicopathological relevance of JAK‐STAT pathway activation in patients with ATLL, with a particular emphasis on the impact of STAT3 mutation or pSTAT3 expression on the prognosis of the smoldering type.
2. MATERIALS AND METHODS
2.1. Patients and samples
Archival formalin‐fixed/paraffin‐embedded (FFPE) samples from 153 patients with ATLL who were diagnosed between 1986 and 2017 were obtained from the Ryukyu University Hospital (Nishihara, Japan) and the Okinawa Prefectural Nanbu Medical Center and Children's Medical Center (Haebaru, Japan). All samples were reviewed and diagnosed as ATLL based on the presence of anti‐HTLV‐1 Ab and histological consistency. Patients were classified into the following 4 ATLL clinical subtypes based on the Shimoyama classification: acute, lymphoma, chronic, and smoldering types.2 Briefly, among the aggressive types of ATLL, the acute type is characterized by multiorgan invasion, including peripheral blood, whereas the lymphoma type lacks leukemic involvement. The diagnosis of the acute type is based on the exclusion of the other subtypes. The diagnosis of the lymphoma type requires histological confirmation of tumor cell involvement in lymph nodes. Among the indolent types of ATLL, the chronic type shows more evident lymphocytosis than the smoldering type. In this study, however, all 3 patients with chronic type of ATLL were regarded as having the aggressive type due to the presence of unfavorable prognostic factors. Thus, all indolent‐type cases were classified as the smoldering type in this study. Cases with only cutaneous lesions, the so‐called “cutaneous type,” were included in the smoldering type in accordance with previous reports.29, 30, 31 We defined disease progression as the shift from the smoldering type to the acute or lymphoma type based on the Shimoyama classification criteria. Patients were excluded from the study if tissue samples could not be evaluated before cytotoxic chemotherapy. Thus, 116 of the 153 originally enrolled patients were analyzed. STAT3 mutation was analyzed in 92 samples from which good‐quality DNA was obtained. This study was approved by the institutional ethics committees of the Graduate School of Medicine and the School of Health Science at the University of the Ryukyus and the Okinawa Prefectural Nanbu Medical Center and Children's Medical Center. This study was carried out in accordance with the Declaration of Helsinki.
2.2. Histological and immunohistochemical evaluation
All FFPE samples were stained with H&E. Each case was subclassified into 1 of 4 morphological types: anaplastic large cell, pleomorphic large cell, pleomorphic medium cell, and small cell.32, 33 When at least 2 samples were available from a given patient, the sample excised at the earliest time point was used for the morphological classification. The Abs used for this analysis included anti‐pSTAT3 (phosphorylated on tyrosine Tyr 705, clone D3A7, catalog number 9145; Cell Signaling Technology Japan), anti‐pSTAT5 (phosphorylated on Tyr 694, clone C11C5, catalog number 9359; Cell Signaling Technology Japan), and anti‐pSTAT6 (phosphorylated on Tyr 641, clone pY641.18, catalog number sc‐101808; Santa Cruz Biotechnology). A 30% cut‐off was chosen for pSTATs based on previous studies.34, 35, 36, 37 Notably, some cutaneous samples showed uneven staining with regard to the location of lymphoma cell infiltration. These samples were regarded as positive when pSTAT expression was observed in more than 30% of the lymphoma cells in Pautrier's microabscesses, even if fewer than 30% of lymphoma cells in other sites detected pSTAT expression.
2.3. Mutation analysis
Targeted deep sequencing was undertaken on exons 20 and 21 of the STAT3 gene, corresponding to the Src homology 2 (SH2) domain of STAT3, which is a mutation hotspot.26 Genetic analysis of other genes associated with the JAK‐STAT pathway was not undertaken in this study because of their very low mutation frequency.26 In our analysis, DNA was extracted from FFPE samples with a QIAamp DNA FFPE Tissue Kit (catalog number 56404; Qiagen) and quantitated using a Qubit dsDNA HS Assay Kit (catalog number Q32854; Thermo Fisher Scientific). Overhang PCR was carried out with Trans Taq DNA Polymerase High Fidelity (catalog number AP131; TransGen Biotech) and primers harboring adapter sequences. Each primer sequence utilized in this study is listed in Table S1. Second‐step PCR was carried out using the Nextera XT Index Polymerase Kit (catalog number 15055294; Illumina) followed by purification of the libraries with an AMPure XP Kit (catalog number A63881; Beckman Coulter). A MiSeq instrument was used for the sequencing reaction (MiSeq Control Software 2.5; Illumina). All of these procedures were carried out according to Illumina's methods and recommendations (http://jp.support.illumina.com).
After sequencing, each FASTQ file was processed using the Variant Studio software (Illumina). STAT3 variants were selected when they showed a variant allele frequency higher than 2% and more than 10 variant read‐outs in at least 20 total reads. Only mutations already reported in COSMIC (http://cancer.sanger.ac.uk/cosmic) were considered to be significant STAT3 mutations.
2.4. Clinicopathological evaluation and statistical analysis
Patients’ clinicopathological characteristics were analyzed using Pearson's χ2 or Fisher's 2‐sided exact test. The Kaplan‐Meier method was used to analyze patient survival data and the log‐rank test was used to determine significant differences. To evaluate the prognostic impact of different variables, a Cox proportional hazards model was used for multivariate analysis. Overall survival (OS) was defined as the period from the day of diagnosis to the day of last follow‐up or to the day of death from any cause. Progression‐free survival (PFS) in patients with indolent (smoldering)‐type ATLL was defined as the period from the day of diagnosis to the day of progression to an aggressive type of ATLL. Patients still alive were censored at the last follow‐up date. All statistical analyses were undertaken using Stata Software, release 13 (Stata Corporation). P < .05 was considered statistically significant.
3. RESULTS
3.1. Clinicopathological characteristics of patients
Table 1 shows the proportion of each clinical subtype and summarizes the clinical characteristics of the patients with the smoldering and aggressive (acute, lymphoma, and unfavorable chronic) types of ATLL. The median age was 66 years (range, 30‐87 years) and the median follow‐up period was 11.7 months (range, 0.06‐295 months). None of the patients with the aggressive type of ATLL had been previously diagnosed with the indolent type. All 3 patients with the chronic type had unfavorable prognostic factors.38 The median survival period was 7.8 months (range, 0.23‐138 months) for the acute type, 6.8 months (range, 0.1‐95.6 months) for the lymphoma type, 18 months (range, 8.7‐76.5 months) for the chronic type with unfavorable prognostic factors, and 26.7 months (range, 0.3‐295 months) for the smoldering type. Patients with the smoldering type of ATLL showed significantly better OS than those with the aggressive type (Figure S1, P < .001). Both prognostic indexes, ATL‐PI and iATL‐PI, were significantly correlated with OS (data not shown).
Table 1.
Clinical characteristics of patients with smoldering or aggressive types of adult T‐cell leukemia/lymphoma (ATLL) in the cohort analyzed in the present study
| Smoldering type (N = 38) | Aggressive type (acute type; N = 25, lymphoma type; N = 45, chronic type; N = 3) | |||
|---|---|---|---|---|
| N | Percentage | N | Percentage | |
| Sex, male (female) | 23 (15) | 60 (40) | 44 (29) | 60 (40) |
| Age, median (range) | 65.8 (30‐87) | — | 67.0 (35‐87) | — |
| Follow‐up periods (mean, mo) (range) | 46 (0.3‐295) | — | 16.5 (0.06‐126) | — |
| Ann Arbor staging (3‐4) | 6/38 | 16 | 57/67 | 85 |
| ECOG performance status (2‐4) | 1/35 | 3 | 34/64 | 53 |
| B symptoms | 0/36 | 0 | 15/73 | 20 |
| Leukemic manifestation | 6/38 | 16 | 28/73 | 38 |
| Skin involvement | 37/38 | 97 | 21/73 | 29 |
| ATL‐PI or iATL‐PI (inter or more) | 25/35 | 71 | 62/73 | 85 |
| Hypoalbuminemia (<4 g/dL) | 5/35 | 14 | 43/65 | 66 |
| Hypercalcemia (>11 mg/dL) | 0/15 | 0 | 10/71 | 14 |
| Elevated LDH (>240 U/mL) | 12/34 | 35 | 55/67 | 82 |
| Elevated soluble IL‐2 receptor (>2.0 × 104 U/mL) | 2/35 | 6 | 29/65 | 45 |
| Treatment | ||||
|---|---|---|---|---|
| VCAP‐AMP‐VECP | 5/38 | 13 | 14/73 | 19 |
| CHOP/CHOP‐like | 14/38 | 37 | 34/73 | 47 |
| Others | 19/38 | 50 | 22/73 | 30 |
| HSCT | 2/38 | 5 | 4/73 | 5 |
| Mogamulizumab | 8/38 | 21 | 15/73 | 21 |
| Topical therapies | 12/38 | 32 | 0/73 | 0 |
| Observation, best supportive care | 3/38 | 8 | 3/73 | 4 |
Among 116 of the total cases, clinical subtypes of 5 cases were not determined. B symptoms refer to systemic symptoms including fever which come and go over several weeks, drenching night sweat, and unexplained weight loss at least 10% of body weight within the preceding 6 mo. Mild chemotherapies include oral anticancer agents. Topical therapies include ointment therapy or UV irradiation.
Abbreviations: AMP, doxorubicin, ranimustine, and prednisone; ATL‐PI, ATLL prognostic index for aggressive type patients; CHOP, cyclophosphamide, doxorubicin, vincristine, and prednisone; HSCT, hematopoietic stem cell transplantation; iATL‐PI, ATLL prognostic index for patients with indolent type; IL‐2, interleukin‐2; LDH, lactate dehydrogenase; VCAP, vindesine, cyclophosphamide, doxorubicin, and prednisone; VECP, vindesine, etoposide, carboplatin, and prednisone.
Notably, the samples analyzed in this study were obtained at the moment of diagnosis in all 73 patients with the aggressive type. By contrast, 42% (16/38) of the samples from patients with the smoldering type of ATLL were obtained at a median time of 36 months (range, 18‐296 months) after diagnosis. With regard to disease progression, 55% (21/38) of patients originally diagnosed with the smoldering type of ATLL progressed to the aggressive type, and the median period from the primary diagnosis to progression was 11.1 months (range, 1.2‐263 months).
The examined tumor sites were from the lymph nodes (47 samples), skin (45 samples), gastrointestinal tract (15 samples), bone marrow (4 samples), soft tissue (2 samples), and other sites (3 samples). A majority of cases with the smoldering type (34/38) were analyzed based on skin samples. Although the infiltration of tumor cells in lymph nodes was histologically confirmed in all cases with the lymphoma type, 18% (8/45) of these cases were evaluated using samples obtained from organs other than lymph nodes because FFPE samples of lymph nodes were not available (Figure S2). Morphologically, 84% (59/70) of patients with the aggressive type were classified as having the pleomorphic large cell type or anaplastic large cell type, whereas cases with the smoldering type were more frequently classified as having the pleomorphic medium cell or small cell type (P < .01, Tables S2 and S3).
3.2. Phosphorylated STAT immunohistochemistry in ATLL lymphoma cells and its relationship with clinicopathological findings
Nuclear pSTAT3 expression was observed in 43% (50/116) of the analyzed ATLL cases, whereas pSTAT5 and pSTAT6 were only observed in 3% (3/116) and 0% (0/116) of cases, respectively. Regarding pSTAT3 expression, 56% (14/25) of the acute type, 67% (2/3) of the chronic type, 66% (25/38) of the smoldering type, and 18% (8/45) of the lymphoma type cases were positive (Figure 1). These data indicated that pSTAT3 expression was significantly less common in cases with the lymphoma type than in those with the other types (Table 2, P < .001). Among the 42 cases with lymph node samples, 37 and 5 were diagnosed with the lymphoma and acute types, respectively. Fourteen percent (5/37) of cases with the lymphoma type showed pSTAT3 expression, whereas 60% (3/5) with the acute type showed pSTAT3 expression (P = .04). Therefore, the lower frequency of pSTAT3 expression observed in cases with the lymphoma type did not seem to be related to topographic location in the lymph nodes, but rather to the clinical ATLL subtype. Furthermore, the pleomorphic medium cell morphology was associated with more frequent pSTAT3 expression than the other morphologies (Table S4, P = .02). This result might be explained by the fact that patients with the smoldering and acute types were likely to have the pleomorphic medium cell type. Interestingly, in 27 of the 28 cases with cutaneous samples, lymphoma cells located in Pautrier's microabscesses showed equal (16 cases) or more frequent (11 cases) pSTAT3 expression relative to tumor cells in other sites, including the dermis and s.c. tissue in skin samples (Figure 2).
Figure 1.
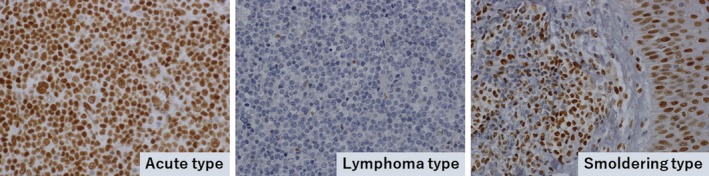
Phosphorylated STAT3 (pSTAT3) expression in adult T‐cell leukemia/lymphoma (ATLL). Left panel, acute type sample obtained from a lymph node lesion in a 72‐y‐old man. Almost all of the tumor cells showed nuclear expression of pSTAT3. Middle panel, in contrast, the lymphoma type sample obtained from a lymph node lesion in a 66‐y‐old man showed very few pSTAT3‐positive cells. Right panel, smoldering type sample obtained from a skin lesion in a 56‐y‐old woman. Infiltrating ATLL cells in the dermis (left side) were positive for pSTAT3 as well as normal epithelial cells (right side). Original magnifications of all images, ×400
Table 2.
Rate of phosphorylated STAT3 (pSTAT3)‐positive cases in each clinical subtype of adult T‐cell leukemia/lymphoma
| pSTAT3 (+), N = 50 | pSTAT3 (−), N = 66 | Percentage | P value | STAT3 mut, N = 22 | STAT3 wt, N = 70 | Percentage | P value | |
|---|---|---|---|---|---|---|---|---|
| Acute type | 14 | 11 | 56 | < .001a | 5 | 15 | 25 | 0.30a |
| Lymphoma type | 8 | 37 | 18 | 7 | 30 | 19 | ||
| Chronic type | 2 | 1 | 67 | 0 | 2 | 33 | ||
| Smoldering type | 25 | 13 | 66 | 10 | 18 | 36 | ||
| Unknown | 1 | 4 | 25 | 0 | 5 | 0 | ||
| Total | 50 | 66 | N = 116 | 22 | 70 | N = 92 |
Abbreviation: mut, mutant.
Lymphoma type was compared with all other types (χ2 test).
Figure 2.
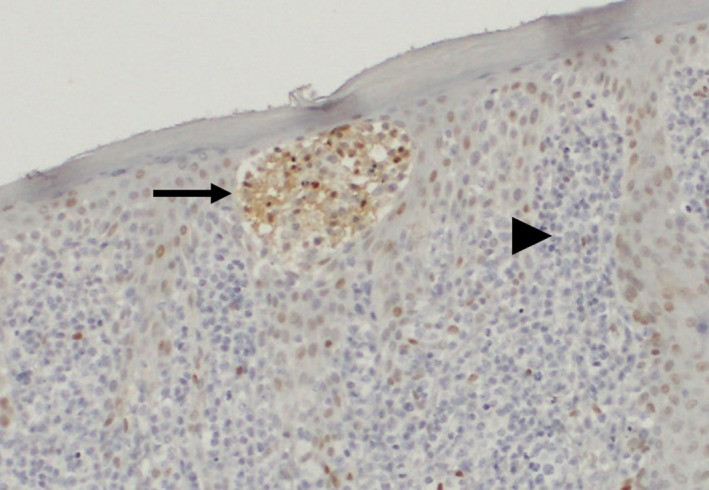
Representative image of phosphorylated STAT3 (pSTAT3)‐positive lymphoma cells in Pautrier's microabscess. The sample was obtained from a skin lesion in a 51‐y‐old man with smoldering type adult T‐cell leukemia/lymphoma (ATLL). The aggregated lymphoma cells in a Pautrier's microabscess (arrow) showed stronger pSTAT3 expression compared with those in the dermis (arrowhead) (original magnification, ×200). This finding indicates that extracellular stimuli such as cytokines might influence pSTAT3 expression in ATLL cells located in Pautrier's microabscess
Next, we analyzed the effects of disease progression on pSTAT3 expression. Fifty‐five percent (21/38) of patients with the smoldering type of ATLL showed disease progression. In this group, pSTAT3 nuclear expression was observed in 43% (6/14) and 64% (9/14) of patients whose samples were obtained before and after progression, respectively. Among the 7 cases whose samples were obtained both before and after progression, the level of pSTAT3 expression changed from negative to positive in 2 cases, from positive to negative in 1 case, and was unchanged in 4 cases. Notably, 83% (5/6) of the cases who progressed to the lymphoma type showed significantly more frequent pSTAT3 expression than the cases with the lymphoma type who had not been previously diagnosed with the indolent type of ATLL (18%, 8/45; P < .01).
Table 3 shows the association between patients’ clinical features and pSTAT3 expression. The pSTAT3‐positive group had better performance status (PS) than the pSTAT3‐negative group. Regarding prognosis, the pSTAT3‐positive group had significantly better OS than the pSTAT3‐negative group (Figure 3A). This is likely due to the fact that more than half of the patients with the smoldering type were in the pSTAT3‐positive group. Therefore, we also evaluated the prognostic impact of pSTAT3 expression separately in the aggressive and smoldering types. In this analysis, pSTAT3‐positive patients with the smoldering type showed significantly better OS and PFS than pSTAT3‐negative patients, whereas this association was not observed in patients with the aggressive type (Figures 3B‐D and S3). Univariable analysis showed that positive pSTAT3 expression, age greater than 70 years, and iATL‐PI had a trend to impact prognosis. In multivariable analysis, age greater than 70 years and positive pSTAT3 expression was associated with poor and favorable prognostic significance, respectively, for both OS and PFS in the patients with the smoldering type (Table 4).
Table 3.
Clinical characteristics of adult T‐cell leukemia/lymphoma cases with regard to phosphorylated STAT3 (pSTAT3) expression
| pSTAT3 (+) | pSTAT3 (−) | P value | |
|---|---|---|---|
| N, Patients (male : female) | 49 (31:18) | 62 (35:27) | |
| Age (range) | 66 (30‐87) | 71 (35‐87) | .51 |
| White blood cell (/mm3) (range) | 7.8 × 103 (3.5‐123 × 103) | 7.7 × 103 (2.7‐80 × 103) | .77 |
| Hemoglobin (g/dL) (range) | 13.9 (4.3‐14.4) | 13.3 (7.7‐16.9) | .67 |
| Platelet (/mm3) (range) | 22.4 × 104 (8.1‐37.4 × 104) | 21.3 × 104 (8.4‐62.2 × 104) | .44 |
| Ann Arbor staging (3‐4), N (%) | 25/49 (50%) | 36/55 (65%) | .14 |
| ECOG performance status (2‐4), N (%) | 9/45 (20%) | 25/53 (47%) | <.01 |
| B symptoms, N (%) | 5/49 (10%) | 10/60 (17%) | .33 |
| Albumin (g/dL) (range) | 4.1 (1.7‐4.8) | 3.8 (2.2‐4.9) | .57 |
| Elevated serum LDH (>240 U/mL), N (%) | 26/44 (59%) | 40/56 (71%) | .20 |
| Hypercalcemia (>11 mg/dL), N (%) | 5/38 (13%) | 5/47 (11%) | .72 |
| Elevated serum IL‐2 receptor (>2.0 × 104 U/dL), N (%) | 12/42 (29%) | 19/58 (33%) | .66 |
B symptoms refer to systemic symptoms of fever which come and go over several weeks, drenching night sweat, and unexplained weight loss at least 10% of body weight within the preceding 6 mo.
Abbreviations: IL‐2, interleukin‐2; LDH, lactate dehydrogenase.
Figure 3.
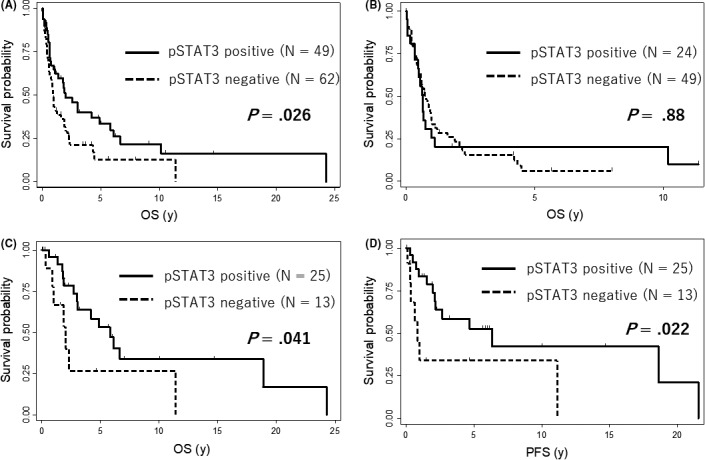
Impact of phosphorylated STAT3 (pSTAT3) expression on survival in adult T‐cell leukemia/lymphoma (ATLL). A, Overall survival (OS) according to pSTAT3 expression (patients with positive pSTAT3 expression, N = 49; patients with negative pSTAT3 expression, N = 62) in all analyzed cases. B, Overall survival (OS) according to pSTAT3 expression in cases with aggressive type ATLL (patients with positive pSTAT3 expression, N = 24; patients with negative pSTAT3 expression, N = 49). C,D, OS and progression‐free survival (PFS) according to pSTAT3 expression in cases with smoldering type ATLL (patients with positive pSTAT3 expression, N = 25; patients with negative pSTAT3 expression, N = 13)
Table 4.
Multivariable and univariable analyses of covariates in patients with adult T‐cell leukemia/lymphoma (ATLL) by Cox proportional hazards model
| Survival | Covariate | HR | 95% CI | P value |
|---|---|---|---|---|
| Univariable analysis for each covariate in patients with smoldering type | ||||
| OS | pSTAT3 (pos. vs neg.) | 0.39 | 0.15‐0.99 | .041 |
| OS | Age ≥70 years | 5.44 | 1.94‐15.30 | .001 |
| OS | iATL‐PI: high risk (5.79 ≥ 1.51 × log10 (sIL‐2R [U/mL]) | 4.07 | 1.12‐14.80 | .033 |
| PFS | pSTAT3 (pos. vs neg.) | 0.34 | 0.13‐0.89 | .027 |
| PFS | Age ≥70 years | 4.12 | 0.15‐11.70 | .008 |
| PFS | iATL‐PI: high risk (5.79 ≥ 1.51 × log10(sIL2R[U/mL]) | 3.13 | 0.86‐11.50 | .085 |
| Multivariable analysis for pSTAT3, age, and iATL‐PI as covariates in patients with smoldering type | ||||
| OS | pSTAT3 (pos. vs neg.) | 0.41 | 0.13‐0.98 | .046 |
| OS | Age ≥70 years | 2.73 | 1.50‐13.50 | .007 |
| OS | iATL‐PI: high risk (5.79 ≥ 1.51 × log10 (sIL‐2R [U/mL]) | 1.37 | 0.77‐10.70 | .170 |
| PFS | pSTAT3 (pos. vs neg.) | 0.29 | 0.099‐0.84 | .023 |
| PFS | Age ≥70 years | 3.07 | 1.22‐10.90 | .020 |
| PFS | iATL‐PI: high risk (5.79 ≥ 1.51 × log10 (sIL‐2R [U/mL]) | 3.65 | 0.77‐12.30 | .110 |
Abbreviations: CI, confidence interval; HR, hazard ratio; iATL‐PI, indolent ATLL prognostic index; neg., negative; OS, overall survival; PFS, progression‐free survival; pos., positive; pSTAT3, phosphorylated STAT3; sIL‐2, serum interleukin‐2.
3.3. STAT3 mutation and its relationship with clinicopathological findings
Based on our immunohistochemical staining results, STAT3 seems to play a critical role in the activation of the JAK‐STAT pathway in ATLL. In diffuse large B‐cell lymphoma, the Toll‐like receptor pathway, suppressor of cytokine signaling 1 (SOCS1) mutations, and Epstein‐Barr virus infection are representative molecular abnormalities related to STAT3 activation.39, 40, 41 STAT3 mutations and JAK3 mutations are the most common genomic abnormalities associated with STAT3 activation in ATLL.26 The Toll‐like receptor pathway, SOCS1 mutations, and Epstein‐Barr virus infection were found to be very rare in ATLL.26 Additionally, the frequency of JAK3 mutations (2.1%) was much lower than that of STAT3 mutations (21%).26 Therefore, we focused on genetic mutations of STAT3. STAT3 mutations in the SH2 domain were detected in 24% (22/92) of all analyzed ATLL cases. Thirty‐six percent (10/28) of cases with the smoldering type and 19% (12/64) of those with the aggressive type had STAT3 mutations. In contrast with our immunohistochemistry results, there was no significant difference in the frequency of STAT3 mutations between the lymphoma type and other ATLL types (P = .30). Fifty‐five percent (12/22) of cases with STAT3 mutations were positive for pSTAT3, compared to 33% (23/70) of those with WT STAT3; however, this difference did not reach statistical significance (P = .07). Furthermore, only 29% (2/7) of the lymphoma type cases with mutated STAT3 showed pSTAT3 expression, a lower percentage than in all the other ATLL types combined (10/15, 67%). There was no significant correlation between STAT3 mutation and prognosis in either the aggressive or smoldering type of ATLL, in contrast to the results of pSTAT3 immunohistochemistry (Figure S4). Additionally, no significant differences were observed in STAT3 mutation according to PS, age, serum albumin, serum soluble IL‐2 receptor, or Ann Arbor stage, all of which are clinical factors included in ATL‐PI or iATL‐PI (data not shown).
4. DISCUSSION
The major findings in this study were that pSTAT3 positivity was substantially lower in patients with the lymphoma type than in those with other clinical subtypes of ATLL, and that pSTAT3 positivity could predict favorable prognosis in patients with the smoldering type of ATLL.
In our data, 40% of ATLL cases showed nuclear expression of pSTAT3, whereas pSTAT5 or pSTAT6 expression was rarely observed, suggesting that activation of STAT3 is the primary effector in the JAK‐STAT pathway in ATLL. It has been reported that STAT3, STAT5, and STAT6 are the predominant effectors of the JAK‐STAT pathway in malignant lymphoma.42 In fact, Takemoto et al25 used EMSA to show that the JAK‐STAT pathway was frequently activated in leukemia cells in ATLL. The activation of STAT3 has been identified in 40% and 50% of cases with diffuse large B‐cell lymphoma and extranodal natural killer/T‐cell lymphoma, respectively.17, 18 In contrast, STAT5 and STAT6 are frequently activated in cutaneous T‐cell lymphoma (CTCL) and primary mediastinal large B‐cell lymphoma, respectively.19, 20 The dysregulation status of various STATs and their close association with different diseases suggest a distinct mechanism of the JAK‐STAT pathway activation in each type of lymphoma, including ATLL.
Few studies have analyzed the clinicopathological features of patients in relation to the subtype of ATLL. In one report, Mihashi et al43 showed that patients with the aggressive type showed a higher expression of MYC and a lower expression of F‐box/WD repeat‐containing protein 7 (FBXW7) than patients with the indolent type. In this study, pSTAT3 expression differed between the acute and lymphoma types. Although these types are distinguished based on their different clinical symptoms, both have similar morphological features and prognosis.5 Our previous report described the biological differences between the acute and lymphoma types based on the results of a comprehensive genomic analysis.44 Several clinical trials have revealed that the response rate to interferon‐α/zidovudine or anti‐CC chemokine receptor 4 (CCR4) Ab was significantly higher in patients with the acute, chronic, or smoldering type than in those with the lymphoma type.45, 46 These results indicate that the lymphoma type might represent a distinct molecular pathophysiology. Ramos et al47 indicated that c‐Rel and interferon regulatory factor‐4 (IRF4) might play an important role behind the different responses of the acute and lymphoma types to combination therapy. The distinct molecular pathophysiology of the lymphoma type, including pSTAT3 expression that was revealed in this study, might also alter responsiveness to a variety of drugs, as shown with interferon‐α/zidovudine or anti‐CCR4 Ab.45, 46
In this study, we also observed that 29% (6/21) of cases with disease progression showed clinical features corresponding to the lymphoma type. In fact, the positive rate of pSTAT3 in these cases (5/6, 83%) was significantly higher than that in cases with the lymphoma type who had not been previously diagnosed with the indolent type (referred to as “de novo” lymphoma, 8/45 cases). During disease progression, patients seemed to retain the pathological characteristics of the indolent stage even though the clinical manifestations of the disease were similar to those of patients with the de novo lymphoma type. We speculate that STAT3 activation is one of the key factors in the multistep progression from the indolent type to the lymphoma‐like type, whereas the de novo lymphoma type may depend on pSTAT3‐independent pathways (Figure 4). The underlying mechanisms of the pSTAT3‐dependent and ‐independent pathways involved in the molecular pathophysiology of ATLL await further investigation.
Figure 4.
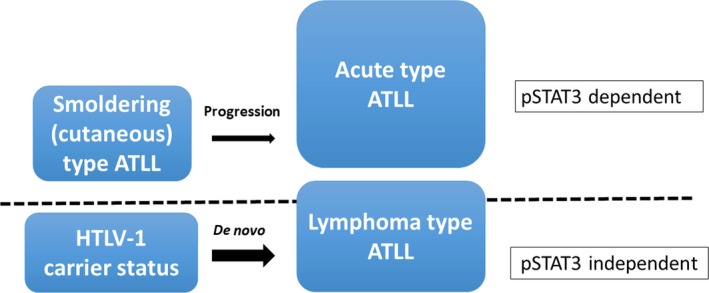
Schematic representation of the 2 different oncogenic pathways involved in the lymphoma type of adult T‐cell leukemia/lymphoma (ATLL). Nuclear phosphorylated STAT3 (pSTAT3) expression is the distinguishing characteristic between the progressed lymphoma type (expresses pSTAT3, similar to acute and smoldering types) and de novo lymphoma type (does not express pSTAT3), suggesting the existence of at least 2 oncogenic molecular mechanisms. HTLV‐1, human T‐cell leukemia virus type I
In addition to pSTAT3 expression, we explored the role of STAT3 mutations in each ATLL subtype. Hotspot mutations located in the SH2 domain of STAT3 were detected in 24% of the analyzed ATLL cases. Patients with the smoldering type showed STAT3 mutations more frequently than patients with the aggressive type. These results are consistent with those of a previous study.8 In our data, pSTAT3 expression showed an association with the occurrence of STAT3 mutations (P = .07). In our results, approximately two‐thirds of patients with pSTAT3 expression showed no STAT3 mutations. Although the precise mechanism of STAT3 activation in such cases remains unknown, extracellular stimuli in the tumor microenvironment might be involved. In this study, higher pSTAT3 expression levels in lymphoma cells in the skin were associated with specific locations, particularly Pautrier's microabscesses (Figure 2). In this site, Langerhans cells were reported to typically activate lymphoma cells through T‐cell receptor signaling in CTCL.48 Furthermore, thymic stromal lymphoproteins derived from keratinocytes around Pautrier's microabscesses exaggerated tumor cell growth through the activation of the JAK‐STAT pathway in CTCL.49 The importance of such extracellular stimuli for JAK‐STAT pathway activation was also found in ALK‐negative anaplastic large cell lymphoma.50 Further research, such as a comparative analysis of gene expression between cases with and without STAT3 mutation, is required to elucidate the mechanism of pSTAT3 expression. We also showed that pSTAT3 expression was significantly associated with better PS and favorable prognosis in the smoldering type of ATLL, whereas STAT3 mutation was not. This result suggests that pSTAT3 expression reflects the underlying molecular pathogenesis more directly than STAT3 mutation, at least in the smoldering type of ATLL. Multidisciplinary approaches, including pathological assessment, remain critical, even though precision medicine mainly based on comprehensive genomic analysis is rapidly being applied to clinical practice.
There are several limitations to this study. First, the molecular mechanisms underlying the difference in expression levels of pSTAT3 between the lymphoma type and other subtypes were not clarified. According to a previous study, the lymphoma type of ATLL showed no genomic abnormalities or dysregulated gene expression that might have inhibited STAT3.24 Ideally, experiments using cell lines derived from each subtype should be undertaken to address this issue. However, almost all established ATLL cell lines are derived from the acute type. Another limitation of this study is that it evaluated only FFPE tissue samples, and not peripheral blood samples. Although further studies, including those analyzing samples obtained from peripheral blood, are required to reinforce our results, this study could highlight a pivotal role for STAT3 activation in distinct subtypes of ATLL.
In conclusion, in this study we reported in detail the clinicopathological significance of the JAK‐STAT pathway in ATLL. Our data showed that pSTAT3 was mainly involved in the pathogenesis of the smoldering, chronic, and acute types of ATLL. Our results also showed that pSTAT3 expression, and not STAT3 mutation, predicted favorable clinical outcome exclusively in the smoldering type. Collectively, this study sheds light on the molecular pathogenesis of ATLL, thereby highlighting pSTAT3 as a potentially novel biomarker for the prognosis of the smoldering type of ATLL.
DISCLOSURE
The authors declare no potential conflicts of interest.
Supporting information
ACKNOWLEDGMENTS
The authors thank Professor Elias Campo for his grateful advice for this study. The authors thank Chikako Nagamine, Mutsumi Isa, and Ryosuke Kimura for their technical support and Pathology Unit staff of Ryukyu University Hospital and Okinawa Prefectural Nanbu Medical Center and Children's Medical Center for their kind assistance. This study was supported by “the Spatiotemporal Genomics Project” promoted by the University of the Ryukyus. This work was also supported by Grants‐in‐Aid for Scientific Research (KAKENHI) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (15K08371 to KK), Okinawa Prefecture (to KK), the Ichiro Kanehara Foundation (to KK), the Yasuda Medical Foundation (to KK), the Mochida Memorial Foundation for Medical and Pharmaceutical Research (to KK), the Japan Leukemia Research Fund (to KK), The Okinawa Internal Medicine Research Promotion Society (to KK), and Okinawa Medical Science Research Foundation (to KM).
Morichika K, Karube K, Kayo H, et al. Phosphorylated STAT3 expression predicts better prognosis in smoldering type of adult T‐cell leukemia/lymphoma. Cancer Sci. 2019;110:2982–2991. 10.1111/cas.14114
Funding information
University of the Ryukyus; Ministry of Education, Culture, Sports, Science and Technology of Japan, KAKENHI Grant/Award Number 15K08371; Okinawa Prefecture; Ichiro Kanehara Foundation; Yasuda Medical Foundation; Mochida Memorial Foundation for Medical and Pharmaceutical Research; Japan Leukemia Research Fund; The Okinawa Internal Medicine Research Promotion Society; Okinawa Medical Science Research Foundation
Contributor Information
Kennosuke Karube, Email: karube@med.u-ryukyu.ac.jp.
Hiroaki Masuzaki, Email: hiroaki@med.u-ryukyu.ac.jp.
REFERENCES
- 1. Swerdlow SH, Campo E, Harris NL, et al. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues, Revised 4th ed. Lyon: IARC Press; 2017:363‐367. [Google Scholar]
- 2. Shimoyama M. Diagnostic criteria and classification of clinical subtypes of adult T‐cell leukaemia‐lymphoma. A report from the Lymphoma Study Group (1984‐87). Br J Haematol. 1991;79:428‐437. [DOI] [PubMed] [Google Scholar]
- 3. Fukushima T, Nomura S, Shimoyama M, et al. Japan Clinical Oncology Group (JCOG) prognostic index and characterization of long‐term survivors of aggressive adult T‐cell leukaemia‐lymphoma (JCOG0902A). Br J Haematol. 2014;166:739‐748. [DOI] [PubMed] [Google Scholar]
- 4. Katsuya H, Yamanaka T, Ishitsuka K, et al. Prognostic index for acute‐ and lymphoma‐type adult T‐cell leukemia/lymphoma. J Clin Oncol. 2012;30:1635‐1640. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida N, Karube K, Utsunomiya A, et al. Molecular characterization of chronic‐type adult T‐cell leukemia/lymphoma. Cancer Res. 2014;74:6129‐6138. [DOI] [PubMed] [Google Scholar]
- 6. Katsuya H, Ishitsuka K, Utsunomiya A, et al. Treatment and survival among 1594 patients with ATL. Blood. 2015;126:2570‐2577. [DOI] [PubMed] [Google Scholar]
- 7. Katsuya H, Shimokawa M, Ishitsuka K, et al. Prognostic index for chronic‐ and smoldering‐type adult T‐cell leukemia‐lymphoma. Blood. 2017;130:39‐47. [DOI] [PubMed] [Google Scholar]
- 8. Kataoka K, Iwanaga M, Yasunaga JI, et al. Prognostic relevance of integrated genetic profiling in adult T‐cell leukemia/lymphoma. Blood. 2018;131:215‐225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Levy DE, Darnell JE Jr. Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651‐662. [DOI] [PubMed] [Google Scholar]
- 10. Stark GR, Darnell JE Jr. The JAK‐STAT pathway at twenty. Immunity. 2012;36:503‐514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med. 2013;368:161‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene. 2002;21:7001‐7010. [DOI] [PubMed] [Google Scholar]
- 13. Gao SP, Mark KG, Leslie K, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL‐6 production in human lung adenocarcinomas. J Clin Invest. 2007;117:3846‐3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dijkgraaf EM, Heusinkveld M, Tummers B, et al. Chemotherapy alters monocyte differentiation to favor generation of cancer‐supporting M2 macrophages in the tumor microenvironment. Cancer Res. 2013;73:2480‐2492. [DOI] [PubMed] [Google Scholar]
- 15. Orlovsky K, Theodor L, Malovani H, et al. Gamma interferon down‐regulates Fer and induces its association with inactive Stat3 in colon carcinoma cells. Oncogene. 2002;21:4997‐5001. [DOI] [PubMed] [Google Scholar]
- 16. Izumi K, Fang LY, Mizokami A, et al. Targeting the androgen receptor with siRNA promotes prostate cancer metastasis through enhanced macrophage recruitment via CCL2/CCR16‐induced STAT3 activation. EMBO Mol Med. 2013;5:1383‐1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Scuto A, Kujawski M, Kowolik C, et al. STAT3 inhibition is a therapeutic strategy for ABC‐like diffuse large B‐cell lymphoma. Cancer Res. 2011;71:3182‐3188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sim SH, Kim S, Kim TM, et al. Novel JAK3‐activating mutations in extranodal NK/T‐cell lymphoma, nasal type. Am J Pathol. 2017;187:980‐986. [DOI] [PubMed] [Google Scholar]
- 19. Litvinov IV, Tetzlaff MT, Thibault P, et al. Gene expression analysis in cutaneous T‐cell lymphomas (CTCL) highlights disease heterogeneity and potential diagnostic and prognostic indicators. Oncoimmunology. 2017;6:e1306618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guiter C, Dusanter‐Fourt I, Copie‐Bergman C, et al. Constitutive STAT6 activation in primary mediastinal large B‐cell lymphoma. Blood. 2004;104:543‐549. [DOI] [PubMed] [Google Scholar]
- 21. Martini M, Hohaus S, Petrucci G, et al. Phosphorylated STAT5 represents a new possible prognostic marker in Hodgkin lymphoma. Am J Clin Pathol. 2008;129:472‐477. [DOI] [PubMed] [Google Scholar]
- 22. Weniger MA, Melzner I, Menz CK, et al. Mutations of the tumor suppressor gene SOCS‐1 in classical Hodgkin lymphoma are frequent and associated with nuclear phospho‐STAT5 accumulation. Oncogene. 2006;25:2679‐2684. [DOI] [PubMed] [Google Scholar]
- 23. Mottok A, Renne C, Willenbrock K, et al. Somatic hypermutation of SOCS1 in lymphocyte‐predominant Hodgkin lymphoma is accompanied by high JAK2 expression and activation of STAT6. Blood. 2007;110:3387‐3390. [DOI] [PubMed] [Google Scholar]
- 24. Skinnider BF, Elia AJ, Gascoyne RD, et al. Signal transducer and activator of transcription 6 is frequently activated in Hodgkin and Reed‐Sternberg cells of Hodgkin lymphoma. Blood. 2002;99:618‐626. [DOI] [PubMed] [Google Scholar]
- 25. Takemoto S, Mulloy JC, Cereseto A, et al. Proliferation of adult T cell leukemia/lymphoma cells is associated with the constitutive activation of JAK/STAT proteins. Proc Natl Acad Sci USA. 1997;94:13897‐13902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kataoka K, Nagata Y, Kitanaka A, et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat Genet. 2015;47:1304‐1315. [DOI] [PubMed] [Google Scholar]
- 27. Koskela HL, Eldfors S, Ellonen P, et al. Somatic STAT3 mutations in large granular lymphocytic leukemia. N Engl J Med. 2012;366:1905‐1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zhang M, Mathews Griner LA, et al. Selective targeting of JAK/STAT signaling is potentiated by Bcl‐xL blockade in IL‐2‐dependent adult T‐cell leukemia. Proc Natl Acad Sci U S A. 2015;112:12480‐12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amano M, Kurokawa M, Ogata K, et al. New entity, definition and diagnostic criteria of cutaneous adult T‐cell leukemia/lymphoma: human T‐lymphotropic virus type 1 proviral DNA load can distinguish between cutaneous and smoldering types. J Dermatol. 2008;35:270‐275. [DOI] [PubMed] [Google Scholar]
- 30. Takahashi K, Tanaka T, Fujita M, et al. Cutaneous‐type adult T‐cell leukemia/lymphoma. A unique clinical feature with monoclonal T‐cell proliferation detected by Southern blot analysis. Arch Dermatol. 1988;124:399‐404. [DOI] [PubMed] [Google Scholar]
- 31. Tsukasaki K, Imaizumi Y, Tokura Y, et al. Meeting report on the possible proposal of an extranodal primary cutaneous variant in the lymphoma type of adult T‐cell leukemia‐lymphoma. J Dermatol. 2014;41:26‐28. [DOI] [PubMed] [Google Scholar]
- 32. Karube K, Aoki R, Sugita Y, et al. The relationship of FOXP3 expression and clinicopathological characteristics in adult T‐cell leukemia/lymphoma. Mod Pathol. 2008;21:617‐625. [DOI] [PubMed] [Google Scholar]
- 33. Miyoshi H, Kiyasu J, Kato T, et al. PD‐L1 expression on neoplastic or stromal cells is respectively a poor or good prognostic factor for adult T‐cell leukemia/lymphoma. Blood. 2016;128:1374‐1381. [DOI] [PubMed] [Google Scholar]
- 34. Gupta M, Maurer MJ, Wellik LE, et al. Expression of Myc, but not pSTAT3, is an adverse prognostic factor for diffuse large B‐cell lymphoma treated with epratuzumab/R‐CHOP. Blood. 2012;120:4400‐4406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Paik JH, Nam SJ, Kim TM, et al. Overexpression of sphingosine‐1‐phosphate receptor 1 and phospho‐signal transducer and activator of transcription 3 is associated with poor prognosis in rituximab‐treated diffuse large B‐cell lymphomas. BMC Cancer. 2014;14:911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu ZL, Song YQ, Shi YF, et al. High nuclear expression of STAT3 is associated with unfavorable prognosis in diffuse large B‐cell lymphoma. J Hematol Oncol. 2011;4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma J, Xing W, Coffey G, et al. Cerdulatinib, a novel dual SYK/JAK kinase inhibitor, has broad anti‐tumor activity in both ABC and GCB types of diffuse large B cell lymphoma. Oncotarget. 2015;6:43881‐43896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tsukasaki K, Hermine O, Bazarbachi A, et al. Definition, prognostic factors, treatment, and response criteria of adult T‐cell leukemia‐lymphoma: a proposal from an international consensus meeting. J Clin Oncol. 2009;27:453‐459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ngo VN, Young RM, Schmitz R, et al. Oncogenically active MYD88 mutations in human lymphoma. Nature. 2011;470:115‐119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kamizono S, Hanada T, Yasukawa H, et al. The SOCS box of SOCS‐1 accelerates ubiquitin‐dependent proteolysis of TEL‐JAK2. J Biol Chem. 2001;276:12530‐12538. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Luo F, Li L, et al. STAT3 activation induced by Epstein‐Barr virus latent membrane protein1 causes vascular endothelial growth factor expression and cellular invasiveness via JAK3 and ERK signaling. Eur J Cancer. 2010;46:2996‐3006. [DOI] [PubMed] [Google Scholar]
- 42. Younes A, Ansell S, Fowler N, et al. The landscape of new drugs in lymphoma. Nat Rev Clin Oncol. 2016;14:335‐346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mihashi Y, Mizoguchi M, Takamatsu Y, et al. C‐MYC and its Main Ubiquitin Ligase, FBXW7, Influence Cell Proliferation and Prognosis in Adult T‐cell Leukemia/Lymphoma. Am J Surg Pathol. 2017;41:1139‐1149. [DOI] [PubMed] [Google Scholar]
- 44. Oshiro A, Tagawa H, Ohshima K, et al. Identification of subtype‐specific genomic alterations in aggressive adult T‐cell leukemia/lymphoma. Blood. 2006;107:4500‐4507. [DOI] [PubMed] [Google Scholar]
- 45. Bazarbachi A, Plumelle Y, Carlos Ramos J, et al. Meta‐analysis on the use of zidovudine and interferon‐alfa in adult T‐cell leukemia/lymphoma showing improved survival in the leukemic subtypes. J Clin Oncol. 2010;28:4177‐4183. [DOI] [PubMed] [Google Scholar]
- 46. Yamamoto K, Utsunomiya A, Tobinai K, et al. Phase I study of KW‐0761, a defucosylated humanized anti‐CCR46 antibody, in relapsed patients with adult T‐cell leukemia‐lymphoma and peripheral T‐cell lymphoma. J Clin Oncol. 2010;28:1591‐1598. [DOI] [PubMed] [Google Scholar]
- 47. Ramos JC, Ruiz P Jr, Ratner L, et al. IRF‐4 and c‐Rel expression in antiviral‐resistant adult T‐cell leukemia/lymphoma. Blood. 2007;109:3060‐3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Edelson RL. Cutaneous T cell lymphoma: the helping hand of dendritic cells. Ann N Y Acad Sci. 2001;941:1‐11. [PubMed] [Google Scholar]
- 49. Takahashi N, Sugaya M, Suga H, et al. Thymic stromal chemokine TSLP acts through Th2 cytokine production to induce cutaneous T‐cell lymphoma. Cancer Res. 2016;76:6241‐6252. [DOI] [PubMed] [Google Scholar]
- 50. Chen J, Zhang Y, Petrus MN, et al. Cytokine receptor signaling is required for the survival of ALK‐ anaplastic large cell lymphoma, even in the presence of JAK1/STAT3 mutations. Proc Natl Acad Sci U S A. 2017;114:3975‐3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


