Abstract
This study aimed to evaluate the feasibility of combining helical tomotherapy (HT) and intensity‐modulated proton therapy (IMPT) in treating patients with nasopharynx cancer (NPC). From January 2016 to March 2018, 98 patients received definitive radiation therapy (RT) with concurrent chemotherapy (CCRT). Using simultaneous integrated boost and adaptive re‐plan, 3 different dose levels were prescribed: 68.4 Gy in 30 parts to gross tumor volume (GTV), 60 Gy in 30 parts to high‐risk clinical target volume (CTV), and 36 Gy in 18 parts to low‐risk CTV. In all patients, the initial 18 fractions were delivered by HT, and, after rival plan evaluation on the adaptive re‐plan, the later 12 fractions were delivered either by HT in 63 patients (64.3%, HT only) or IMPT in 35 patients (35.7%, HT/IMPT combination), respectively. Propensity‐score matching was conducted to control differences in patient characteristics. In all patients, grade ≥ 2 mucositis (69.8% vs 45.7%, P = .019) and grade ≥ 2 analgesic usage (54% vs 37.1%, P = .110) were found to be less frequent in HT/IMPT group. In matched patients, grade ≥ 2 mucositis were still less frequent numerically in HT/IMPT group (62.9% vs 45.7%, P = .150). In univariate analysis, stage IV disease and larger GTV volume were associated with increased grade ≥ 2 mucositis. There was no significant factor in multivariate analysis. With the median 14 month follow‐up, locoregional and distant failures occurred in 9 (9.2%) and 12 (12.2%) patients without difference by RT modality. In conclusion, comparable early oncologic outcomes with more favorable acute toxicity profiles were achievable by HT/IMPT combination in treating NPC patients.
Keywords: acute toxicity, nasopharyngeal neoplasms, proton therapy, radiotherapy, survival
1. INTRODUCTION
Intensity‐modulated radiation therapy (IMRT) has been the standard technique when treating most head and neck cancer (HNC) patients.1, 2 Since 2011, when IMRT for HNC was first recognized as standard practice by the Korean National Health Insurance system, we began to use helical tomotherapy (HT) when treating HNC patients.3 Our policy of definitive radiation therapy (RT) for loco‐regionally advanced HNC, including nasopharynx cancer (NPC), was to deliver 30 fractions of HT with a simultaneous integrate boost (SIB), in which an adaptive re‐plan was applied to accommodate body contour changes during the later 12 fractions, and concurrent administration of systemic therapy.4, 5
Proton beam therapy (PBT), by virtue of Bragg‐Peak, can frequently generate more favorable dosimetric profiles when compared with other up‐to‐date photon‐based RT techniques, including IMRT, in many cancer types.6 Although this involves higher cost for installation and operation of the PBT facility, PBT is known to offer promising cost‐effectiveness when treating the most high‐risk HNC patients, as targets are almost always closely surrounded by many critical normal structures.7, 8 Our institute started the clinical operation of 2 rotating PBT gantries, both with intensity modulated proton therapy (IMPT) capability, in December 2015.9
For HNC patients, there are some studies that reported that the longer the time between diagnosis and initiation of treatment, the worse oncologic outcomes would be.10, 11 Because high demand for PBT outstripped the limited resources at our institute, the average waiting time before commencing PBT after therapeutic decision was around 4 wk or longer, while that for HT was usually up to a week. To avoid this undesirable long waiting interval for PBT to start, we designed an alternative RT schedule to combine HT and IMPT: starting RT with HT to deliver an initial 18 fractions; and switching to IMPT during the later 12 fractions of the adapted re‐plan. The current study assesses these early clinical outcomes in treating NPC patients, including acute toxicity profiles following combined HT and IMPT, compared with HT alone throughout the RT course.
2. MATERIAL AND METHODS
2.1. Patients
From January 2016 to March 2018, 118 patients diagnosed with NPC received definitive RT with concurrent systemic therapy (CCRT). After excluding 20 patients (18 who received RT not as the upfront definitive modality and 2 who had non‐squamous cell carcinoma), 98 patients were included in the current study. Routine diagnostic neck computed tomography (CT) and whole body 5‐fluoro‐deoxyglucose positron emission tomography with CT (PET‐CT) were obtained in all patients, and magnetic resonance imaging (MRI) was obtained in 87 patients (88.8%) to determine whether to invade the skull base. Stages were assigned according to the 7th edition American Joint Committee on Cancer (AJCC) staging manual.12 We retrospectively reviewed the medical records of these patients after Institutional Review Board approval (IRB No. SMC 2018‐01‐116).
2.2. Treatment scheme
Dose planning was generated twice in all patients: the first one for the initial 18 fractions; and the second one for the later 12 fractions from the adaptive re‐plan, respectively. Three levels of target volume were manually contoured on the simulation CT images according to our institutional protocol.4, 5 Gross tumor volume (GTV) of the primary tumor and metastatic lymph nodes were delineated based on both clinical examination findings and all available diagnostic images. High‐risk clinical target volume (CTV) was to encompass the immediately adjacent regions to the primary GTV including the sphenoid sinus, posterior part of nasal cavity, and skull base in case of skull base invasion, and the lymphatic levels containing the nodal GTV. Low‐risk CTV was to include one lymphatic station level away from the high‐risk CTV. When a patient had evidence of ipsilateral lymph node involvement based on clinical imaging studies, low‐risk CTV usually covered the contralateral upper neck, but not the contralateral lower neck. By applying these SIB and the adaptive re‐plan policy, 3 different dose levels were prescribed: 68.4 Gy in 30 fractions (2.2 Gy × 18 fractions + 2.4 Gy × 12 fractions) to the GTV; 60 Gy in 30 fractions to the high‐risk CTV; and 36 Gy in 18 fractions to the low‐risk CTV, respectively.
As previously mentioned, all patients started RT by HT, and 2 sets of adaptive re‐plans, for the rival comparison purpose, were generated: one by HT; and the other by IMPT, respectively (Figure 1). The principles of target volume delineation and dose constraints to the organs at risk were identical between the 2 treatment plans. An HT planning system (TomoTherapy®) and a PBT planning system (RayStation®, RaySearch Laboratories AB) were used to generate the HT and IMPT plans, respectively. The relative biological effective for PBT was 1.1. Although the comparative evaluation of the rival plans usually revealed more favorable saving of the oral cavity structures by the IMPT plans, the actual assignment was also influenced by the availability of the IMPT gantry. Therefore, 63 patients had to continue HT (HT only, 64.3%), while 35 were able to be switched into the IMPT (HT/IMPT combination, 35.7%), respectively.
Figure 1.
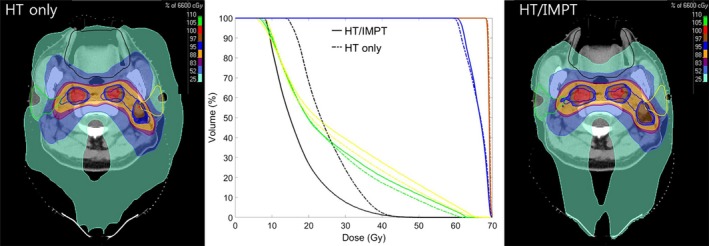
Comparison of dose distribution on axial view and dose volume histogram for gross tumor volume (red), clinical target volume (blue), oral cavity (black), and bilateral parotid gland (green and yellow) between HT only (dotted lines) and HT/IMPT combination (solid lines). HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy
Most patients (94 patients, 95.9%) received 2 cycles of intravenous cisplatin (100 mg/m2) at 3 wk intervals, and 4 received 6 weekly intravenous cisplatin (30 mg/m2) considering their age or medical co‐morbidities. All patients completed the planned chemotherapy cycles, except one who received 3‐weekly chemotherapy. No patient was given induction or adjuvant chemotherapy in relation to the current CCRT.
2.3. Evaluation of acute side effects, response, and subsequent follow‐up
During the RT course, all patients were examined weekly to evaluate acute toxicities and tumor response. Acute toxicities including oral mucositis, radiation dermatitis, and weight loss were graded according to Common Terminology Criteria for Adverse Events (CTCAE) ver. 4.03.13 In addition, analgesic usage during the RT course was evaluated according to the grading system that represented our pain control policy: grade 0 was defined as no need of analgesics; grade 1 was non‐regular use of non‐opioid analgesics only for 4 wk or shorter; grade 2 was regular use of non‐opioid analgesics with or without intermittent local anesthetic spray for longer than 4 wk; and grade 3 was additional use of opioids to non‐opioid analgesics with or without intermittent local anesthetic spray.
The first and second clinical and response evaluations were done by CT within 1 month and PET‐CT within 4 months of completion of CCRT, respectively. The early response was assigned according to the PET response criteria in solid tumors (PERCIST) that reflected the metabolic tumor response.14 Subsequent follow‐up evaluations were arranged regularly at every 3−4 month intervals during the first 2 years with CT of the neck and necessary laboratory and imaging studies. Locoregional failure was defined as the reappearance or development of new lesions at the primary site and/or the regional lymphatics.
2.4. Statistical analysis
The chi‐squared test or the Fisher's exact test for categorical variables and the independent t test for continuous variables were used for the comparison of the patient characteristics and acute toxicities between the 2 treatment groups. Multivariate analysis was performed using logistic regression analysis. The clinical and treatment‐related factors that showed a P‐value < .1 on the univariate analyses were included in the multivariate analyses after excluding the possible confounding variables. The Kaplan‐Meier method was used to calculate the progression‐free survival (PFS), and log‐rank test was used to compare the oncologic outcomes between the 2 treatment groups. The propensity‐score matching method was used to account for differences in baseline clinical characteristics and treatment‐related factors between the 2 treatment groups. The propensity score was calculated using the multivariate logistic regression model. The variables that showed statistically significant differences in the patients’ characteristics (stage, initial GTV volumes, laterality of lymph node (LN) involvement) were included in the model. Then, the matching algorithm was used to match the patients who received HT only and those who were given the HT/IMPT combination, within a caliper of .2 standard deviations of the logit of the propensity score with matching ratio of 1:1. A P‐value < .05 was considered statistically significant. Statistical analysis was performed using SPSS ver. 22.0 software (SPSS Inc.).
3. RESULTS
3.1. Patient characteristics
Table 1 shows the characteristics of the patients. In all patients, the median age was 50 years (range: 19–80 years) and the majority was male (80.6%). While, most demographic profiles were comparable between the 2 groups, however, patients in the HT/IMPT combination group presented more frequently with lower AJCC stages (P = .001), and smaller initial GTV (P < .001). Most patients (94 patients, 95.9%) received bilateral neck irradiation. After propensity‐score matching, there was no significant difference in clinical and treatment‐related factors between the 2 groups.
Table 1.
Baseline demographic and clinical characteristics
| All patients (n = 98) | Matched patients (n = 70) | |||||
|---|---|---|---|---|---|---|
| HT only (n = 63) | HT/IMPT (n = 35) | P‐value | HT only (n = 35) | HT/IMPT (n = 35) | P‐value | |
| Age (y) | ||||||
| Median (range) | 51 (19–80) | 50 (24–66) | ||||
| Means ± SD | 50.7 ± 13.5 | 47.9 ± 10.8 | .312 | 51.8 ± 12.9 | 47.9 ± 10.8 | .182 |
| Gender | ||||||
| Male | 50 (79.4%) | 29 (82.9%) | .675 | 26 (74.3%) | 29 (82.9%) | .382 |
| Female | 13 (20.6%) | 6 (17.1%) | 9 (25.7%) | 6 (17.1%) | ||
| ECOG | ||||||
| 0–1 | 62 (98.4%) | 35 (100%) | 35 (100%) | 35 (100%) | – | |
| 2 | 1 (1.6%) | – | – | – | ||
| Smoking history | ||||||
| Yes | 38 (66.7%) | 15 (65.2%) | 1.000 | 18 (60%) | 15 (65.2%) | .698 |
| Not | 19 (33.3%) | 8 (34.8%) | 12 (40%) | 8 (34.8%) | ||
| Stage | ||||||
| I | – | – | .001 | – | – | .070 |
| II | 8 (12.7%) | 16 (45.7%) | 7 (20%) | 16 (45.7%) | ||
| III | 23 (36.5%) | 8 (22.9%) | 13 (37.1%) | 8 (22.9%) | ||
| IV | 32 (50.8%) | 11 (31.4%) | 15 (42.9%) | 11 (31.4%) | ||
| Initial GTV volume (cc) | ||||||
| Means + SD | 50.3 ± 28.6 | 30.7 ± 18.4 | <.001 | 40.1 ± 28.9 | 30.7 ± 18.4 | .111 |
| Neck irradiation | ||||||
| Ipsilateral | 1 (1.6%) | 3 (8.6%) | .129 | 1 (2.9%) | 3 (8.6%) | .614 |
| Bilateral | 62 (98.4%) | 32 (91.4%) | 34 (97.1%) | 32 (91.4%) | ||
Abbreviations: CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy; LN, lymph node; RT, radiotherapy; SD, standard deviation.
3.2. Toxicities
The acute toxicity profiles are summarized in Table 2 and illustrated in Figure 2. Among all patients, grade ≥ 2 dermatitis, mucositis, weight loss, and analgesic usage were observed in 65 (66.3%), 60 (61.2%), 50 (51.0%) and 47 patients (48.0%), respectively. Grade ≥ 2 mucositis was significantly less frequent in the HT/IMPT combination group (69.8% vs 45.7%, P = .019) (Figure 2A). Grade ≥ 2 analgesic usage was also less frequent, although not significantly (54% vs 37.1%, P = .110). Numerically, grade ≥ 3 dermatitis was more frequent (3.2% vs 14.3%, P = .094), but ≥ 3 mucositis (17.5% vs 11.4%) and analgesic usage (17.5% vs 11.4%) was less frequent in the HT/IMPT combination group. No patient underwent gastrostomy tube feeding during or after CCRT. Among the matched patients, grade ≥ 2 dermatitis, mucositis, weight loss and analgesic usage were observed in 44 (62.9%), 38 (54.3%), 33 (47.1%), and 28 patients (40%), respectively. As in all patients, grade ≥ 2 mucositis (62.9% vs 45.7%, P = .150) and analgesic usage (42.9% vs 37.1%, P = .626) were less frequent numerically in the HT/IMPT combination group (Figure 2B).
Table 2.
Acute toxicity distribution
| All patients (n = 98) | Matched patients (n = 70) | |||||
|---|---|---|---|---|---|---|
| HT only (n = 63) | HT/IMPT (n = 35) | P‐value | HT only (n = 35) | HT/IMPT (n = 35) | P‐value | |
| Dermatitis | ||||||
| Grade 0 | 10 (15.9%) | 7 (20%) | .079 | 8 (22.9%) | 7 (20%) | .453 |
| Grade 1 | 9 (14.3%) | 7 (20%) | 4 (11.4%) | 7 (20%) | ||
| Grade 2 | 42 (66.7%) | 16 (45.7%) | 21 (60%) | 16 (45.7%) | ||
| Grade 3 | 2 (3.2%) | 5 (14.3%) | 2 (5.7%) | 5 (14.3%) | ||
| Mucositis | ||||||
| Grade 0 | – | – | .063 | – | – | .358 |
| Grade 1 | 19 (30.2%) | 19 (54.3%) | 13 (37.1%) | 19 (54.3%) | ||
| Grade 2 | 33 (52.4%) | 12 (34.3%) | 17 (48.6%) | 12 (34.3%) | ||
| Grade 3 | 11 (17.5%) | 4 (11.4%) | 5 (14.3%) | 4 (11.4%) | ||
| Weight loss | ||||||
| Grade 0 | 6 (9.5%) | 2 (5.7%) | .659 | 5 (14.3%) | 2 (5.7%) | .484 |
| Grade 1 | 23 (36.5%) | 16 (45.7%) | 13 (37.1%) | 16 (45.7%) | ||
| Grade 2 | 34 (54%) | 17 (45.6%) | 17 (48.6%) | 17 (45.6%) | ||
| Grade 3 | – | – | – | – | ||
| Analgesic usage | ||||||
| Grade 0 | 5 (7.9%) | 8 (22.9%) | .179 | 3 (8.6%) | 8 (22.9%) | .382 |
| Grade 1 | 24 (38.1%) | 14 (40%) | 17 (48.6%) | 14 (40%) | ||
| Grade 2 | 24 (38.1%) | 9 (25.7%) | 12 (34.3%) | 9 (25.7%) | ||
| Grade 3 | 10 (15.9%) | 4 (11.4%) | 3 (8.6%) | 4 (11.4%) | ||
Abbreviations: HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy.
Figure 2.
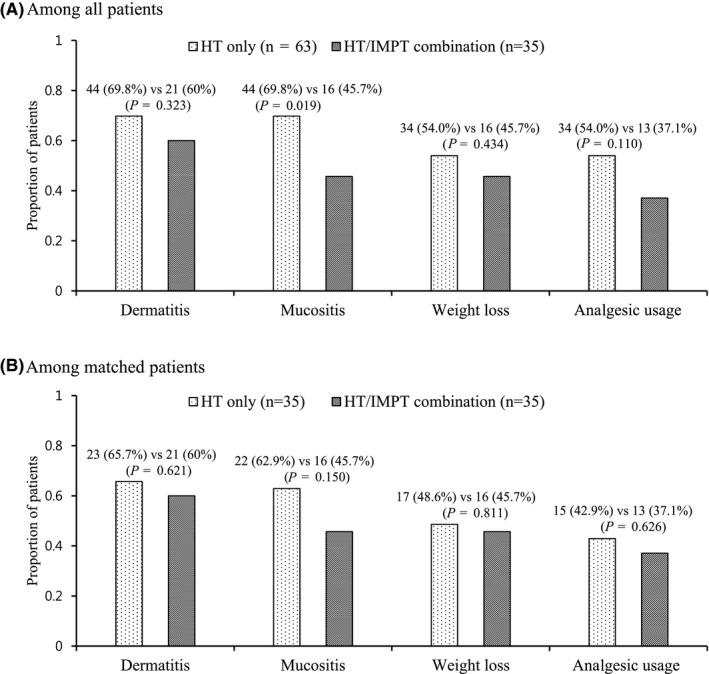
Grade 2 or higher toxicity distribution by treatment group among all patients (A), and matched patients (B). HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy
In univariate analyses of demographic‐, clinical‐, and treatment‐related factors with toxicity profiles, grade ≥ 2 dermatitis was more frequent in patients of a younger age (P = .057) and more limited smoking history (86.8% vs 59.3%, P = .005). Grade ≥ 2 mucositis was more frequent in patients with stage IV disease (78.1% vs 52.7%, P = .051), and larger GTV volume (P = .010) (Table 3). Grade ≥ 2 weight loss was more frequent in patients with stage IV disease (62.8% vs 41.8%, P = .039), larger GTV volume (P = .004), and bilateral neck irradiation (53.2% vs 0%, P = .054). Grade ≥ 2 analgesic usage was more frequent in patients with larger GTV volume (P = .097). In multivariate analyses of the related factors with grade ≥ 2 mucositis, there were no significant factors including RT modality (HT only vs HT/IMPT combination), stages (I–III vs IV), and GTV volume (Table 3).
Table 3.
Univariate and multivariate analyses of clinical and treatment‐related factors associated with grade ≥ 2 mucositis
| Factors | Grade ≥ 2 mucositis in all patients (n = 60) | Grade ≥ 2 mucositis in matched cohort (n = 38) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| n (%) | P‐value | HR (95% CI) | P‐value | n (%) | P‐value | HR (95% CI) | P‐value | |
| Age | ||||||||
| (Years, means ± SD) | 49.3 ± 14.0 | .684 | 49.3 ± 14.1 | .668 | ||||
| Gender | ||||||||
| Male | 47 (59.5%) | .473 | 29 (52.7%) | .616 | ||||
| Female | 13 (68.4%) | 9 (60%) | ||||||
| Performance status | ||||||||
| ECOG 0 or 1 | 59 (60.8%) | 1.000 | 38 (54.3%) | – | ||||
| ECOG 2 | 1 (100%) | – | ||||||
| Smoking history | ||||||||
| Yes | 33 (62.3%) | .951 | 17 (51.5%) | .582 | ||||
| No | 17 (63%) | 12 (60%) | ||||||
| AJCC stage | ||||||||
| I–III | 29 (52.7%) | .051 | 1 | .294 | 20 (45.5%) | .054 | 1 | .196 |
| IV | 31 (72.1%) | 1.649 (.648–4.196) | 18 (69.2%) | 2.079 (.686–6.303) | ||||
| GTV volume | ||||||||
| (cc, means ± SD) | 48.9 ± 27.5 | .010 | 1.015 (.995–1.036) | .133 | 40.2 ± 26.7 | .078 | 1.012 (.988–1.038) | .330 |
| Bilateral neck irradiation | ||||||||
| Yes | 58 (61.7%) | .640 | 36 (54.5%) | 1.000 | ||||
| No | 2 (50%) | 2 (50%) | ||||||
| RT modality | ||||||||
| HT only | 44 (69.8%) | .019 | 1 | .166 | 22 (62.9%) | .150 | 1 | .276 |
| HT/IMPT combination | 16 (45.7%) | .521 (.208–1.309) | 16 (45.7%) | .576 (.213–1.555) | ||||
Abbreviations: AJCC, American Joint Committee on Cancer; CCRT, concurrent chemoradiotherapy; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy; LN, lymph node; RT, radiotherapy; SD, standard deviation.
3.3. Oncologic outcome
In 4 months after CCRT completion, the early response was excellent in both groups with an overall response rate and complete response rate of 100% and 84.7%, respectively. The median follow‐up time was 14 months (range, 5–33 mo). Among all patients, 22 patients (22.4%) had treatment failure: locoregional failure in 9 (9.2%); distant failure in 12 (12.2%); and both locoregional and distant failure in 1 patient (1.0%), respectively. The 1‐year PFS in HT alone and HT/IMPT combination groups were 81.0% and 87.1%, respectively (P = .912) (Figure 3A). Among the matched patients, 14 patients (20.0%) had treatment failure: locoregional failure in 6 (8.6%), distant failure in 7 (10.0%), and both locoregional and distant failure in 1 patient (1.4%). The 1‐year PFS in HT alone and HT/IMPT combination groups were 82.9% and 87.1%, respectively (P = .874) (Figure 3B).
Figure 3.
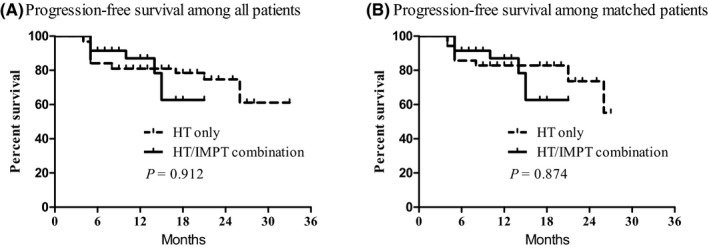
Progression‐free survival among all patients (A) and matched patients (B) according to treatment group. HT, helical tomotherapy; IMPT, intensity‐modulated proton therapy
4. DISCUSSION
PBT has widened the therapeutic window with the potential of saving more normal structures when treating HNC patients, when compared with other photon‐based RT techniques.7 Although there have been no prospective clinical trial data that directly compared IMPT and IMRT, there have been several dosimetric studies and retrospective reports.15, 16, 17, 18 Lewis and colleagues19 compared IMPT vs rival IMRT plans generated for 9 NPC patients who actually were treated with IMPT, and demonstrated significant lower doses to the normal tissues including the oral cavity using IMPT with similar conformality and homogeneity around the targets. Holliday and colleagues,20 in a case‐matched analysis of NPC patients who received either IMPT or IMRT, reported that the rate of feeding gastrostomy tube placement was significantly reduced following IMPT due to better sparing of the oral cavity mucosa. Although there are not much data on clinical efficacy, it is clear that dosimetric advantage appears to translate into improvements in acute toxicity profiles.7
Higher clinical demand than PBT resources could accommodate, however, often necessitate an undesirable long delay before treatment initiation for PBT as the initial modality. This long delay was known to have reduced oncologic outcomes,10, 11 and may not be easily tolerated by many HNC patients and their families, together with the physicians in charge. Induction chemotherapy before IMPT initiation, to bridge the gap, could also be considered. However, we would not recommend this approach, instead of upfront CCRT, based on 2 main reasons: first, no significant benefit of clinical outcomes; and second, increased risk of added morbidity and increased care cost.21 We generated RT plans both for HT and IMPT for the several HNC patients for dosimetric comparison purpose, and achieved more favorable saving of oral cavity structures in most cases by combining HT and IMPT, when compared with HT only (Figure 1 for example). Based on our dosimetric comparison results and to avoid any long delay, we decided to start RT using HT to deliver the initial 18 fractions and then switch to IMPT to deliver the later 12 fraction as the adaptive RT modality. The current study reports the early clinical outcomes, including acute toxicity profiles and response using this combination. Alhough we were not able to take full dosimetric advantage of IMPT throughout the RT course, we were able to avoid too long a delay before treatment initiation. In addition, we achieved more favorable acute toxicity profiles, especially grade ≥ 2 mucositis following combined HT and IMPT, without undue waiting, when compared with HT only, although with no statistical significance. Based on our observations, we speculate that the greater potential benefit of less frequent and less severe toxicity profiles could be achieved by increasing the proportion of IMPT.
Moreover, we reduced the direct cost of RT by upto 28% according to the Korean National Health Insurance plan, when compared with the whole RT course of 30 fractions of IMPT. Considering the resource limitation and, at the same time, the potential benefits, we recently modified the dose schedule to deliver 67.2 Gy in 28 parts: 18 HT + 10 IMPT or 16 HT + 12 IMPT. In addition, the interval of concurrent chemotherapy (cisplatin) was optionally chosen between the 2 schedules (3‐weekly vs weekly) considering the patients’ conditions, which could reduce the acute side effects. These changes are expected to increase PBT utilization efficiency, to reduce the acute toxicity risk and deliver optimal overall treatment cost without altering therapeutic efficacy, as they possess a comparable biologically equivalent dose as the higher systemic therapy dose intensity.
The current study, mainly because of its retrospective nature, has the weakness of unequal distribution of patients with respect to AJCC stage and GTV volume. A propensity‐score matching method was applied to overcome this innate selection bias. However, the relatively small sample size might still have masked the further potential benefits of combining HT and IMPT. In addition, the policy of selective neck irradiation with reduced elective radiation dose at our institution could have reduced oral cavity dose and led to decreased acute toxicities in all patients regardless of treatment group, when compared with previous studies.5, 19 Decreased toxicities in all patient groups might also have masked further potential benefits of the HT/IMPT combination.
The current study, however, is believed to have a few strong points. First, we avoided the undesirable long waiting time before treatment initiation. Second, this is the first study to report the planned sequential combination HT and IMPT. In previous studies, PBT was mainly used as a boost technique following or simultaneously with photon therapy,22, 23, 24 and also showed favorable toxicity and treatment results. Third, the acute toxicity profiles were, more or less, favorable following the HT/IMPT combination, while oncologic outcomes were all excellent and comparable. Fourth, we reduced the direct cost of RT by using the HT/IMPT combination, when compared with a whole course of IMPT. Longer term follow‐up and recruiting more patients, or a prospective randomized clinical trial, however, would be needed to document the issues on late toxicities as well as long‐term oncologic outcomes.
The current combination of HT and IMPT over the 6 wk RT course seemed feasible with respect to normal tissue sparing and avoidance of undesirable waiting times before treatment initiation. Excellent and comparable early oncologic outcomes with more favorable acute toxicity profiles were achieved using the HT/IMPT combination. Further effort to develop a more optimum and cost‐effective dose schedule may be warrantied.
DISCLOSURE
The authors have nothing to disclosure.
ACKNOWLEDGMENTS
None.
Park SG, Ahn YC, Oh D, et al. Early clinical outcomes of helical tomotherapy/intensity‐modulated proton therapy combination in nasopharynx cancer. Cancer Sci. 2019;110:2867–2874. 10.1111/cas.14115
REFERENCES
- 1. van der Veen J, Nuyts S. Can intensity‐modulated‐radiotherapy reduce toxicity in head and neck squamous cell carcinoma? Cancers. 2017;9(12):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gregoire V, Langendijk JA, Nuyts S. Advances in radiotherapy for head and neck cancer. J Clin Oncol. 2015;33(29):3277‐3284. [DOI] [PubMed] [Google Scholar]
- 3. Ahn YC. Introduction of intensity modulated radiation therapy. J Korean Med Assoc. 2011;54(11):1172‐1178. [Google Scholar]
- 4. Lee H, Ahn YC, Oh D, Nam H, Noh JM, Park SY. Tumor volume reduction rate during adaptive radiation therapy as a prognosticator for nasopharyngeal cancer. Cancer Res Treat. 2016;48(2):537‐545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho WK, Oh D, Lee E, et al. Feasibility of selective neck irradiation with lower elective radiation dose in treating nasopharynx cancer patients. Cancer Res Treat. 2019;51:603‐610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Mitin T, Zietman AL. Promise and pitfalls of heavy‐particle therapy. J Clin Oncol. 2014;32(26):2855‐2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leeman JE, Romesser PB, Zhou Y, et al. Proton therapy for head and neck cancer: expanding the therapeutic window. Lancet Oncol. 2017;18(5):e254‐e265. [DOI] [PubMed] [Google Scholar]
- 8. Verma V, Mishra MV, Mehta MP. A systematic review of the cost and cost‐effectiveness studies of proton radiotherapy. Cancer. 2016;122(10):1483‐1501. [DOI] [PubMed] [Google Scholar]
- 9. Chung K, Han Y, Kim J, et al. The first private‐hospital based proton therapy center in Korea; status of the proton therapy center at Samsung medical center. Radiat Oncol J. 2015;33(4):337‐343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Murphy CT, Galloway TJ, Handorf EA, et al. Survival impact of increasing time to treatment initiation for patients with head and neck cancer in the United States. J Clin Oncol. 2016;34(2):169‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Naghavi AO, Echevarria MI, Strom TJ, et al. Treatment delays, race, and outcomes in head and neck cancer. Cancer Epidemiol. 2016;45:18‐25. [DOI] [PubMed] [Google Scholar]
- 12. Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A, eds. AJCC Cancer Staging Manual, 7th ed New York, NY: Springer; 2010. [Google Scholar]
- 13. National Cancer Institute . Common Terminology Criteria for Adverse Events Version 4.0.
- 14. O JH, Lodge MA, Wahl RL. Practical PERCIST: a simplified guide to PET response criteria in solid tumors 1.0. Radiology. 2016;280(2):576‐584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Muzik J, Soukup M, Alber M. Comparison of fixed‐beam IMRT, helical tomotherapy, and IMPT for selected cases. Med Phys. 2008;35(4):1580‐1592. [DOI] [PubMed] [Google Scholar]
- 16. Widesott L, Pierelli A, Fiorino C, et al. Intensity‐modulated proton therapy versus helical tomotherapy in nasopharynx cancer: planning comparison and NTCP evaluation. Int J Radiat Oncol Biol Phys. 2008;72(2):589‐596. [DOI] [PubMed] [Google Scholar]
- 17. Romesser PB, Cahlon O, Scher E, et al. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity‐modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118(2):286‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stromberger C, Cozzi L, Budach V, et al. Unilateral and bilateral neck SIB for head and neck cancer patients: intensity‐modulated proton therapy, tomotherapy, and RapidArc. Strahlenther Onkol. 2016;192(4):232‐239. [DOI] [PubMed] [Google Scholar]
- 19. Lewis GD, Holliday EB, Kocak‐Uzel E, et al. Intensity‐modulated proton therapy for nasopharyngeal carcinoma: decreased radiation dose to normal structures and encouraging clinical outcomes. Head Neck. 2016;38(Suppl 1):E1886‐E1895. [DOI] [PubMed] [Google Scholar]
- 20. Holliday EB, Garden AS, Rosenthal DI, et al. Proton therapy reduces treatment‐related toxicities for patients with nasopharyngeal cancer: a case‐match control study of intensity‐modulated proton therapy and intensity‐modulated photon therapy. Int J Particle Ther. 2015;2(1):19‐28. [Google Scholar]
- 21. Zhang L, Jiang N, Shi Y, Li S, Wang P, Zhao Y. Induction chemotherapy with concurrent chemoradiotherapy versus concurrent chemoradiotherapy for locally advanced squamous cell carcinoma of head and neck: a meta‐analysis. Sci Rep. 2015;5:10798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Slater JD, Yonemoto LT, Mantik DW, et al. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62(2):494‐500. [DOI] [PubMed] [Google Scholar]
- 23. Takayama K, Nakamura T, Takada A, et al. Treatment results of alternating chemoradiotherapy followed by proton beam therapy boost combined with intra‐arterial infusion chemotherapy for stage III‐IVB tongue cancer. J Cancer Res Clin Oncol. 2016;142(3):659‐667. [DOI] [PubMed] [Google Scholar]
- 24. Chan A, Adams JA, Weyman E, et al. A phase II trial of proton radiation therapy with chemotherapy for nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2012;84(3):S151‐S152. [Google Scholar]


