Abstract
In human and dogs, bladder cancer (BC) is the most common neoplasm affecting the urinary tract. Dog BC resembles human muscle‐invasive BC in histopathological characteristics and gene expression profiles, and could be an important research model for this disease. Cancer patient‐derived organoid culture can recapitulate organ structures and maintains the gene expression profiles of original tumor tissues. In a previous study, we generated dog prostate cancer organoids using urine samples, however dog BC organoids had never been produced. Therefore we aimed to generate dog BC organoids using urine samples and check their histopathological characteristics, drug sensitivity, and gene expression profiles. Organoids from individual BC dogs were successfully generated, expressed urothelial cell markers (CK7, CK20, and UPK3A) and exhibited tumorigenesis in vivo. In a cell viability assay, the response to combined treatment with a range of anticancer drugs (cisplatin, vinblastine, gemcitabine or piroxicam) was markedly different in each BC organoid. In RNA‐sequencing analysis, expression levels of basal cell markers (CK5 and DSG3) and several novel genes (MMP28,CTSE,CNN3,TFPI2,COL17A1, and AGPAT4) were upregulated in BC organoids compared with normal bladder tissues or two‐dimensional (2D) BC cell lines. These established dog BC organoids might be a useful tool, not only to determine suitable chemotherapy for BC diseased dogs but also to identify novel biomarkers in human muscle‐invasive BC. In the present study, for the 1st time, dog BC organoids were generated and several specifically upregulated organoid genes were identified. Our data suggest that dog BC organoids might become a new tool to provide fresh insights into both dog BC therapy and diagnostic biomarkers.
Keywords: biomarker, bladder cancer, dog, organoid, RNA‐seq
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- BC
bladder cancer
- COX
cyclooxygenase
- CSCs
cancer stem cells
- PC
prostate cancer
- PCA
principle component analysis
- SMA
smooth muscle actin
1. INTRODUCTION
Bladder cancer comprises approximately 1‐2% of all naturally occurring cancers in dogs, a similar rate to that found in human.1, 2 While more than 90% of BC found in dogs consist of intermediate‐ to high‐grade invasive urothelial carcinomas,3, 4, 5 low‐grade, superficial transitional cell carcinoma (TCC) is very rare. Diagnosis of dog BC is usually at the late stage, and is then often impossible to treat.4, 6, 7, 8
Although several experimental models of BC exist, including carcinogen‐induced mouse models and genetically engineered mice, they do not completely reflect the characteristics of invasive or metastatic human BC.9 Interestingly, naturally occurring dog BC closely mimics human muscle‐invasive BC in its cellular and molecular characteristics including histopathological characteristics, biological behavior, local cancer invasion, distant metastases, molecular features, response to chemotherapy, and prognosis,10, 11, 12, 13 suggesting that dog BC could be a relevant model for muscle‐invasive human BC.10, 14 Therefore, identification of diagnostic markers and investigation of the mechanisms involved in dog BC might be beneficial not only for the veterinary clinic but also for human patients with muscle‐invasive BC.
Three‐dimensional (3D) culture (organoids), derived from self‐renewing stem cells, typically recapitulates in vivo architecture, functions, and genetic and molecular signatures of the parent tumors. This technique holds great promise for use in medical research into the development of new personalized therapy, especially for cancer.15, 16
In a previous study, we established a culture method for dog prostate cancer (PC) organoids using urine samples from PC diseased dogs.17 The organoids recapitulated the tumor microenvironment of dog PC tissues and showed tumorigenesis in vivo. Furthermore, this model could be used to investigate sensitivity of anticancer drugs.
Conversely, dog BC organoids had never been established. We therefore collected urine samples from BC–diseased dogs and cultured these samples using the previously described urine‐derived organoid culture method. Here, for the 1st time, dog BC organoids were generated and demonstrated that BC organoids could be useful for analysis of tumorigenesis and to determine the sensitivity of anticancer drugs. We also identified novel diagnostic marker candidates (MMP28, CTSE, CNN3, TFPI2, COL17A1, and AGPAT4) by analyzing RNA‐seq data from urine‐derived BC organoids.
2. MATERIALS AND METHODS
2.1. Materials
To generate dog BC organoids, cells from urine samples were mixed with Matrigel (BD Bioscience) and cultured with stem cell‐stimulated medium, as described previously.17 Anticancer drugs used were as follows: piroxicam; gemcitabine; vinblastine (Cayman); and cisplatin (WAKO). Antibody sources used were as follows: E‐cadherin (R&D System); CK7; Ki67 (Novus); CK20; UPK3; MMP28; TFPI2; AGPAT4 (Bioss); vimentin (Sigma‐Aldrich); α‐smooth muscle actin (SMA) (DAKO); CK5; CNN3 (GeneTex, Inc.); and CTSE (Bioworld Technology, Inc.). Fluorescent secondary antibodies used were as follows: Alexa Fluor™ 488 donkey anti‐goat IgG; Alexa Fluor 488™ goat anti‐rabbit IgG; Alexa Fluor 488™ goat anti‐mouse IgG; (Thermo Fisher Scientific Inc.); Biotinylated goat anti‐mouse IgG (Vector Laboratories, Inc.). Horseradish peroxidase (HRP)‐conjugated anti‐rabbit IgG (Cayman); and HRP‐conjugated anti‐mouse IgG (Millipore).
2.2. Cell culture
Dog two‐dimensional (2D) urothelial carcinoma cell lines were purchased from COSMO BIO CO., Ltd, and cultured in Dulbecco's Modified Eagle's Medium containing 10% fetal bovine serum (FBS; Thermo Fisher Scientific Inc.).
2.3. Generation of urine sample‐derived BC organoids
Between February 2017 and October 2018, 17 urine and blood samples were collected from dogs diagnosed with BC based on their clinical symptoms and cytological examinations (Figure S1). Sample collection was a cooperation between the Animal Medical Center, Faculty of Agriculture, Tokyo University of Agriculture and Technology (Tokyo, Japan), the Department of Small Animal Clinical Science, Joint Faculty of Veterinary Medicine (Tokyo, Japan), the Laboratory of Veterinary Radiology, Graduate School of Life and Environmental Sciences, Osaka Prefecture University (Osaka, Japan), Laboratory of Veterinary Clinical Oncology, Faculty of Applied Biological Sciences, Gifu University (Gifu, Japan), Laboratory of Veterinary Surgery, Graduate School of Agricultural and Life Sciences, University of Tokyo (Tokyo, Japan) and Laboratory of Small Animal Surgery 2 School of Veterinary Medicine, Kitasato University (Aomori, Japan). Written informed consent for this study was obtained from all dog owners, and the study was conducted under direction from the Institute Animal Care and Use Committee of Tokyo University of Agriculture and Technology approval (Approval number: 0016012). In total, 12 urine samples were successfully expanded using the 3D organoid culture method, as described previously.17 Among these, four organoid lines (Ors 1‐4) were used for experiments; dog information is listed in Table 1.
Table 1.
Sample information
| Case ID | Age (y old) | Breed | Sex | Sample date | Muscle‐invasive or not | Prior therapy | Other information |
|---|---|---|---|---|---|---|---|
| BC17004 | 11 | ShiTzu | Male (not castrated) | 12‐10‐2017 | Muscle‐invasive | Piroxicam, Enrofloxacin | This sample was used as Or 1 |
| BC18004 | 12 | Miniature Dachshund | Female (spayed) | 02‐05‐2018 | Muscle‐invasive | Alendronate, Prednisolone | This sample was used as Or 2 |
| BC18005 | 11 | Mix | Female (spayed) | 11‐05‐2018 | Muscle‐invasive | Piroxicam, Misoprostol | This sample was used as Or 3 |
| BC18006 | 12 | Miniature Dachshund | Female (spayed) | 26‐06‐2018 | Muscle‐invasive | Prednisolone, Lansoprazole, Orbifloxacin | This sample was used as Or 4 |
2.4. Passaging of BC organoids
After 7‐14 d of culture, BC organoids were passaged into a new well at a ratio of 1:3‐4. To dissolve Matrigel, 5 mmol/L EDTA/phosphate‐buffered saline (PBS) was added to each well and the culture plate was placed on ice for 90 min. The cell suspension was collected into a 15‐mL tube and then centrifuged at 600 g for 3 min. Cell pellets were washed with PBS and then trypsinized at 37°C for 5 min. Vigorous pipetting was performed and 100 μL of FBS was added to the tube to neutralize trypsination. Cell pellets were collected by centrifugation and were mixed with new Matrigel on ice. Matrigel‐containing cells were dropped into a 24‐well plate, solidified in a CO2 incubator at 37°C for 30 min, and then culture medium was added to each well.
2.5. Collection of normal bladder tissue
Four healthy female beagle dogs (3 y old) were obtained from the Research Institute for Animal Science in Biochemistry and Toxicology (Tokyo, Japan). These canines were accorded recommendations listed in the “Guide for the Care and Use of Laboratory Animals” approved by the Faculty of Agriculture, Tokyo University of Agriculture and Technology. A catheter was inserted through the urethra, normal bladder tissues were isolated by scratching the mucosal membrane of the bladder and then used for RNA preparation. Blood samples were also collected and used for RNA preparation. The study was conducted under the approval of the Institute Animal Care and Use Committee of Tokyo University of Agriculture and Technology (Approval number: 30‐55).
2.6. Hematoxylin and eosin staining of BC organoids
Hematoxylin and eosin (H&E) staining of BC organoids was performed as described previously.17, 18 Bladder cancer organoids were fixed in 4% paraformaldehyde (PFA) at room temperature for 2‐3 h, and then embedded in paraffin. After deparaffinization, 4‐μm‐thick sections were stained with H&E. Images were obtained using a light microscope (BX‐52; Olympus).
2.7. Immunofluorescence staining of BC organoids
Immunofluorescence staining of BC organoids was performed as described previously.17, 18 Organoid cells were fixed in 4% PFA for 1 h, dehydrated with 30% sucrose solution at 4°C overnight, then embedded in OCT compound. Frozen sections were made and blocked with 1.5% normal goat serum (NGS)/PBS at room temperature for 1 h. Subsequently, sections were incubated with a primary antibody (E‐cadherin; 1:200, CK7;1:100, CK20;1:200, UPK3A;1:200, vimentin; 1:200, α‐SMA; 1:200, ki67; 1:100, MMP28;1:200, CTSE; 1:200, CNN3; 1:200, TFPI2; 1:200, and AGPAT4; 1:200) at 4°C overnight, and then with a secondary antibody for 1 h and finally observed using a confocal microscope (LSM 800; ZEISS).
2.8. Mouse xenograft assay
Mouse xenograft assay of BC organoids was performed as described previously.17 C.B‐17/IcrHsd‐Prkdc scid mice were obtained from Japan SLC. Male immunodeficient mice (6 wk old) were housed under specific pathogen‐free conditions. Here, 1 × 106 cells of BC organoids were subcutaneously injected into the back of mice. At 6 wk later, the organoid‐derived tumors were isolated and used for H&E and immunofluorescence staining. All studies involving mice were conducted according to the Guide to Animal Use and Care, Tokyo University of Agriculture and Technology, and approved by the ethics committee (Approval number: 29‐92).
2.9. Cell viability assay of BC organoids
Cell viability assay of organoids was performed as described previously.17, 19 Taking into consideration the therapeutic dose in clinic and pharmacokinetics in dogs,10, 20, 21, 22, 23, 24, 25 we determined the appropriate concentration to be used for each drug in the cell viability assay. Briefly, 5 × 103 cells of each BC organoid were seeded into 10 μL Matrigel in a 96‐well culture plate and incubated for 24 h. Next, cells were treated with anticancer drugs at variable concentrations for 3 d. Cell viability was examined using an alamar blue kit (Thermo Fisher Scientific Inc.). Fluorescence (emission wavelength; 585 nm) was read on a microplate reader (TECAN).
2.10. RNA‐sequencing analysis
Total RNA was extracted from normal bladder tissues from healthy dogs, BC organoid samples and 2D urothelial carcinoma cell lines using the NucleoSpin kit (TaKaRa Bio Inc.) according to the manufacturer's instructions. Total extracted RNA (10 ng) for each sample was used to generate the sequencing libraries. RNA‐seq was performed at the Research and Education Center for Prevention of Global Infectious Disease of Animals, Tokyo University of Agriculture and Technology (Tokyo, Japan). A Ribo‐Zero Human Kit (Illumina) and a TruSeq Stranded Total RNA Library Prep Kit (Illumina) were used for library preparation, followed by sequencing (7.5 million single‐end reads) on an Illumina MiSeq instrument. Initial quality control of RNA‐seq data (FASTQ) for each sample was performed using cutadapt (version 1.8.3) and cmpfastq_pe.pl software. Reads were mapped to the reference genome (CamFam 3.1) using the STAR (version 2.5.1b) software. PCA was performed to display differences between samples. Fragments per kilobase of transcript per million mapped reads were normalized using the trimmed mean of M value method.
2.11. Quantitative real‐time polymerase chain reaction
Total RNA was extracted from normal bladder tissues, organoid samples, 2D urothelial carcinoma cell lines, and blood samples using the NucleoSpin kit (Takara Bio Inc) according to the manufacturer's instructions. First‐strand cDNA was synthesized using a QuantiTect Reverse Transcription Kit (QIAGEN). Quantitative real‐time PCR was performed using a QuantiTect SYBR I Kit (QIAGEN) and a StepOnePlus Real‐Time PCR System (Applied Biosystems). The ΔΔCq method was used for quantification. Specific primers used for dog CK5, DSG3, GATA3, ERBB2, MMP28, CTSE, CNN3, TFPI2, COL17A1, AGPAT4, GAPDH, CK15, TGM2, GJB2, IL1R2, COL5A2, CDH3, CHST4, and ADORA2B are listed in Table 2.
Table 2.
Primers for real‐time quantitative PCR analysis
| Primer | Sequence | |
|---|---|---|
| CK5 | Forward | 5′‐CAAGGTCCTGGACACCAAGT‐3′ |
| Reverse | 5′‐ATGCTGTCCAGCTGTCTCCT‐3′ | |
| DSG3 | Forward | 5′‐CCTTGGGTTGTTGCAGTTTT‐3′ |
| Reverse | 5′‐ATCGATCCCGAGGCTTATCT‐3′ | |
| GATA3 | Forward | 5′‐GTCCCTCCAGCCCTTTCTAC‐3′ |
| Reverse | 5′‐GGCAAACGTCATTTTGCTTT‐3′ | |
| ERBB2 | Forward | 5′‐CCCCGAGAGTATGTGAAGGA‐3′ |
| Reverse | 5′‐ACTTCCAGATGGGCATGAAG‐3′ | |
| MMP28 | Forward | 5′‐GAGGCGTAAGAAACGCTTTG‐3′ |
| Reverse | 5′‐ATTGCTCCACAGTTGGAAGG‐3′ | |
| CTSE | Forward | 5′‐CAGACCTTTGTGAACGCAGA‐3′ |
| Reverse | 5′‐GTGGTCATAGCCTCCGAAAA‐3′ | |
| CNN3 | Forward | 5′‐TCCCAAAATGCAAACTGACA‐3′ |
| Reverse | 5′‐CGCAGTACTTGGGGTCGTAT‐3′ | |
| TFPI2 | Forward | 5′‐AACGCCAACAACTTCGAAAC‐3′ |
| Reverse | 5′‐AGCAGAGCACAGTCCCTCAT‐3′ | |
| COL17A1 | Forward | 5′‐CGGGAGATCCAGCAGTACAT‐3′ |
| Reverse | 5′‐GACGCAGATACTGCCTCACA‐3′ | |
| AGPAT4 | Forward | 5′‐AATTCCTGTGCCATCTCGTC‐3′ |
| Reverse | 5′‐CACCACTCCAGCAACATCAC‐3′ | |
| GAPDH | Forward | 5′‐AACTCCCTCAAGATTGTCAGCAA‐3′ |
| Reverse | 5′‐CATGGATGACTTTGGCTAGAGGA‐3′ | |
| CK15 | Forward | 5′‐AGACAGTGGACGGGAAAGTG‐3′ |
| Reverse | 5′‐ACCCTCTGAAAGCAGGGACT‐3′ | |
| TGM2 | Forward | 5′‐TCAGAAAGGATGGGATGAGG‐3′ |
| Reverse | 5′‐AGGTGGGGTTTCACACTCAG‐3′ | |
| GJB2 | Forward | 5′‐CCCATCTCTCACATCCGACT‐3′ |
| Reverse | 5′‐CAAAGATGACCCGGAAGAAA‐3′ | |
| IL1R2 | Forward | 5′‐AGACGAGAATGTGGGTCCAG‐3′ |
| Reverse | 5′‐TCTGGCAGTGCAGATGTAGG‐3′ | |
| COL5A2 | Forward | 5′‐CCTCAGGGAATTGATGGAGA‐3′ |
| Reverse | 5′‐CTGCCCTTGTAAGCCTTGAG‐3′ | |
| CDH3 | Forward | 5′‐GGAGCCTATCACCTGCCATA‐3′ |
| Reverse | 5′‐TGTCCGGAGAGGAGAGAGAA‐3′ | |
| CHST4 | Forward | 5′‐TGCTCAAGGAGGTACGCTTT‐3′ |
| Reverse | 5′‐TAGCGTTCCCTCAGTGCTTT‐3′ | |
| ADORA2B | Forward | 5′‐AAGCTGTTGCCTCGTGAAGT‐3′ |
| Reverse | 5′‐AGCCAGCACAGAGCAAAAAT‐3′ |
2.12. Immunohistochemical staining of organoids
Immunohistochemical staining of BC organoids was performed as described previously.17 After deparaffinization, sections were treated with 1% peroxidase for 30 min, then blocked with 1.5% NGS/PBS for 30 min. Sections were then incubated with primary antibodies (CK5; 1:200) at 4°C overnight followed by incubation with biotinylated secondary antibody/PBS (1:500) for 30 min. Slides were treated with a solution of ABC kit (Vector Laboratories) for 30 min and then with a solution of DAB for 3‐5 min. Images were obtained using a light microscope (BX‐52).
2.13. Western blotting
Western blotting was performed as described previously.26 Protein lysates were obtained by homogenizing normal bladder tissues, organoid samples, and 2D urothelial carcinoma cell lines with Cell Lysis Reagent (Sigma‐Aldrich) containing 1% protease inhibitor cocktail (Sigma‐Aldrich). Loading proteins (10 μg) were separated by SDS‐PAGE (7.5%) and transferred to a nitrocellulose membrane (Wako). After blocking with 0.5% skimmed milk, the membranes were incubated with primary antibody (CK5; 1:500, VCP; 1:500) at 4°C overnight followed by incubation with the secondary antibody (1:10 000 dilution, 1 h) and ECL Prime (GE Healthcare). Results were obtained with FujiFilm LAS‐3000 and quantified using ImageJ densitometry analysis software (National Institutes of Health).
2.14. Statistical analysis
Data shown are means ± SEM. Statistical evaluations were performed using one‐way analysis of variance (ANOVA) followed by Bonferroni's test. P‐values ≤0.05 were considered to be statistically significant.
3. RESULTS
3.1. Generation of dog BC organoids
Urine sample‐derived dog PC organoids were generated by our group in a previous study. As most dogs with BC also exhibit invasion and metastasis,1, 27 we hypothesized that urine cells from BC diseased dogs would be useful for organoid culture. Urine samples from BC diseased dogs were collected and used to generate urine‐derived organoids (Figure 1A). Urine cells were cultured using the method reported previously.17 Cells from the urine samples from each BC diseased dog gradually formed organoids (Figure 1B) and were continually propagated by serial passage. The efficacy in establishing continually propagated organoid lines from BC dogs was approximately 70% (12 lines from 17 attempted samples). We observed that urine‐derived organoids from each sample had spheroidal structures (Figure 1C), and a similar histology to dog urothelial carcinomas reported previously27 (Figure 1D). For the immunofluorescence assay, expression of an epithelial cell marker, E‐cadherin, was observed in all organoids (Figure 1E). Expression of urothelial cell markers, CK 7 (Figure 1F), CK20 (Figure 1G), and UPK3A, (Figure 1H) was also observed in each organoid strain (Figure S2). Similarly to previously found for urine sample‐derived dog PC organoids, we observed that a fibroblast cell marker, vimentin, (Figure 1I) and a myofibroblast marker, α‐SMA, (Figure 1J) were expressed around the organoids. Expression of a proliferation cell marker, Ki67, (Figure 1K) was also observed in the organoids. These results suggested that urine sample‐derived organoids from BC diseased dogs could recapitulate the characteristics of dog BC tissues.
Figure 1.
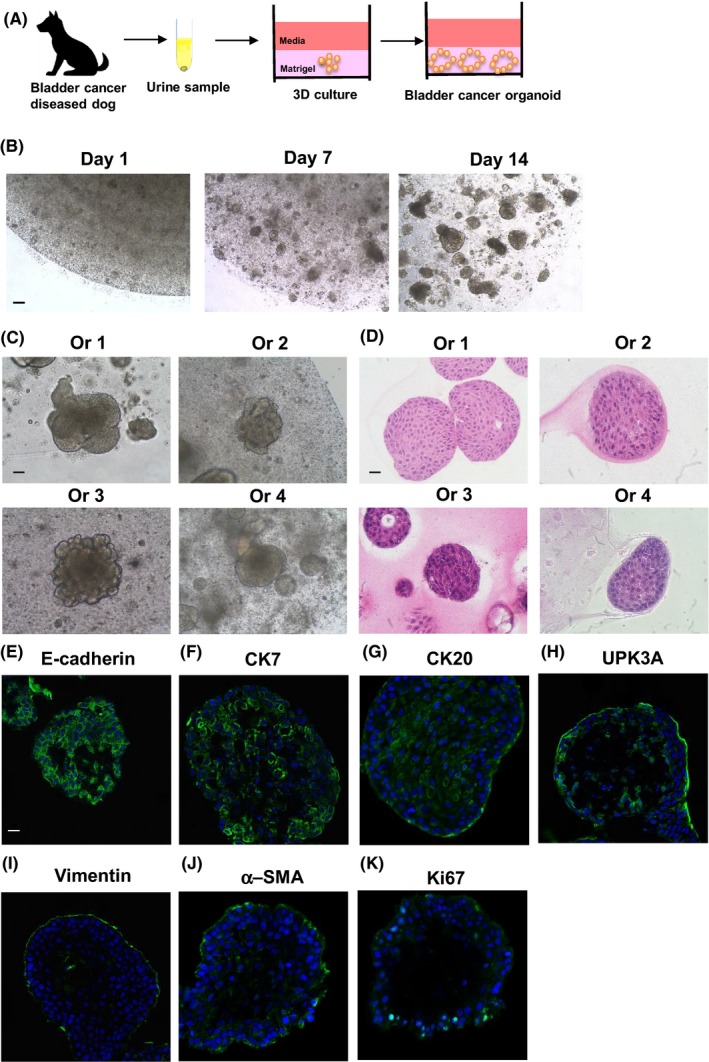
Generation of dog BC organoids. (A) Schematic experimental design of a procedure for generation of dog BC organoids using urine samples. (B) Images of a representative growing process were taken at days 1, 7, and 14 after seeding the cells. Scale bar: 500 μm. Representative images for phase‐contrast (C) and hematoxylin and eosin (H&E) staining (D) (Culture days 14‐21) of each dog‐derived organoids (Ors 1‐4) were shown. Scale bar: 200 μm (C), 50 μm (D). Expression of an epithelial cell marker, E‐cadherin (E), urothelial cell markers, CK7 (F), CK20 (G), and UPK3A (H), a fibroblast cell marker, vimentin (I), a myofibroblast marker, α‐smooth muscle actin (SMA) (J), and a proliferating cell marker, Ki67 (K) in the organoids. Representative photomicrographs were shown (n = 4). Scale bar: 50 μm (E‐K).
3.2. Tumorigenesis induced by BC organoids
We next examined the ability of dog BC organoids to form tumors in vivo. Injection of the organoids into immunodeficient mice successfully generated tumors within 6 wk (Figure 2A). Cell clusters resembling the structure of organoids were observed in the BC organoid injection‐derived tumor tissues (Figure 2B). As the xenografted tumor tissues contained many extracellular components, it was implied that BC organoids secreted cytokines to promote the proliferation of mesenchymal cells which resulted in tumor tissue occupation of mesenchymal components. Immunofluorescence staining was performed to identify the cell components of the tumor tissues. E‐cadherin‐ (Figure 2C), CK7‐ (Figure 2D), CK20‐ (Figure 2E), UPK3A‐ (Figure 2F), and Ki67‐positive cells (Figure 2G) were observed in the tumor tissues. These findings suggested that urine‐derived dog BC organoids could reproduce tumor tissues in vivo and show similar features to the organoid cells.
Figure 2.
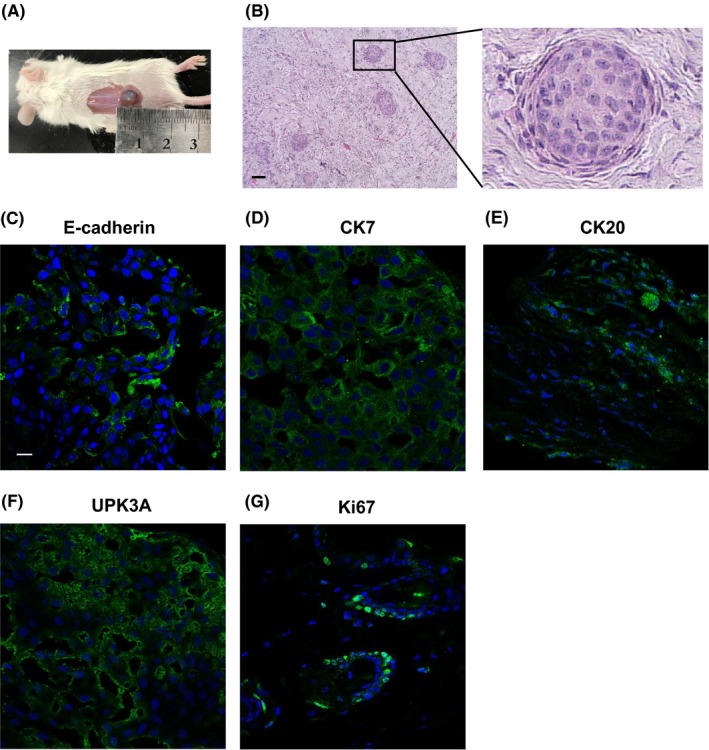
Tumorigenesis induced by BC organoids. The trypsinized BC organoid cells were subcutaneously injected into the back of NOD/SCID mice (n = 4). At 6 wk later, the formed tumors were stained with H&E and immunofluorescence recorded. (A) Observation of BC organoid injection‐induced tumor formation. (B) Representative images with H&E staining of the tumor tissues were shown. The enlarged image is shown on the right. Scale bar: 100 μm. E‐cadherin (C), CK7 (D), CK20 (E), UPK3A (F), and Ki67 (G) expression is shown. Representative photomicrographs are shown (n = 4). Scale bar: 50 μm.
3.3. Effects of anticancer drugs on BC organoids
To demonstrate the use of BC organoids as a preclinical model for checking the sensitivity of anticancer drugs, we performed a 96‐well Matrigel cell viability assay, as described previously.18, 19 Treatment with a nonselective cyclooxygenase (COX) inhibitor, piroxicam, had no effect on the cell viability of each BC organoid (Figure 3A,B). Treatment with a pyrimidine antimetabolite, gemcitabine, had no effect on the cell viability of organoids, expect for the 2nd BC organoid (Or 2) (Figure 3A,C). Treatment with a DNA‐damaging agent, cisplatin, or a microtubule inhibitor, vinblastine, decreased the cell viability of organoids in a dose‐dependent manner (Figure 3A,D,E).
Figure 3.
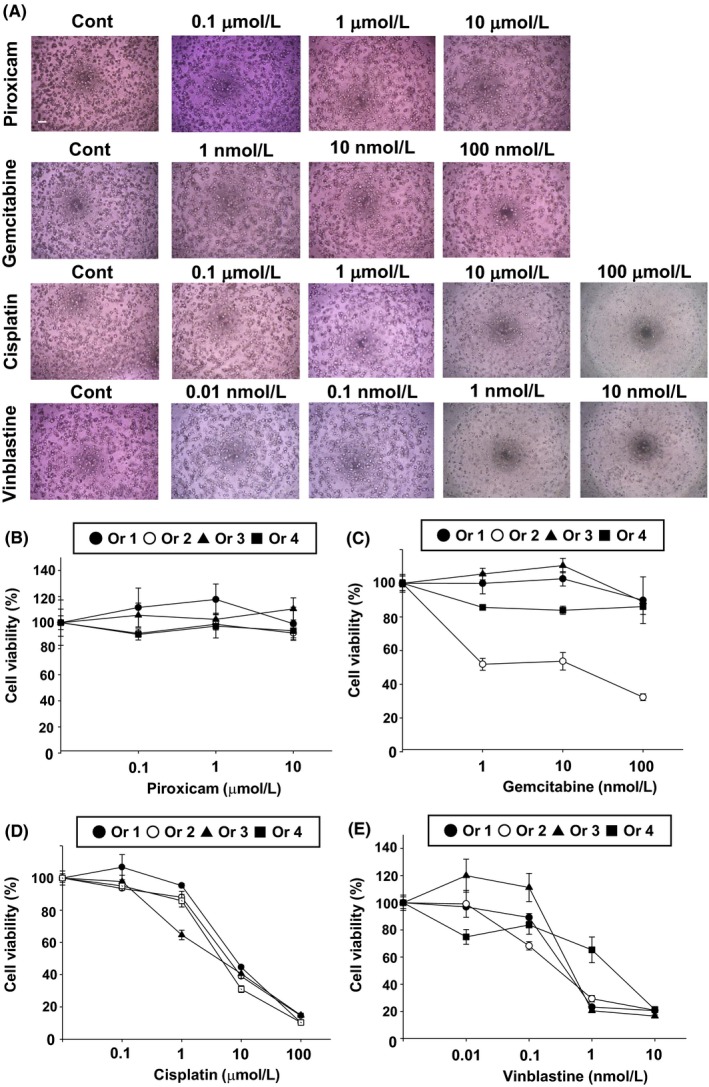
Effects of anticancer drugs on BC organoids. After BC organoids were trypsinized and seeded into Matrigel, they were treated with piroxicam (0.1‐10 μmol/L), gemcitabine (1‐100 nmol/L), cisplatin (0.1‐100 μmol/L) or vinblastine (0.01‐10 nmol/L) for 3 d (n = 6 each for four organoids [Ors 1‐4]). (A) Representative phase‐contrast images of BC organoids treated with piroxicam, gemcitabine, cisplatin or vinblastine are shown. Scale bar: 500 μm. (B‐E) Cell viability was assessed using an alamarblue assay, and 100% represents cell viability for each control. Results are expressed as mean ± SEM.
3.4. Effects of combination treatment with anticancer drugs on BC organoids
In the veterinary clinic, multiple anticancer drug therapies are used to treat BC diseased dogs.28, 29, 30, 31, 32 However, it is difficult to predict which combination therapies will be effective for each BC diseased dog. We therefore investigated the response to several patterns of co‐treatment with anticancer drugs using BC organoids. In three out of four BC organoids (Ors 1, 2, and 3), co‐treatment with gemcitabine and cisplatin significantly improved responses compared with cisplatin alone, while it had no additional effects on one organoid (Or 4) (Figure 4A). In two out of four BC organoids (Or 1 and Or 3), co‐treatment with piroxicam and cisplatin significantly improved response compared with cisplatin treatment alone, while it had no additional effects on other organoids (Ors 2 and 4) (Figure 4A). In three out of four BC organoids (Ors 1, 2 and 3), co‐treatment of gemcitabine with vinblastine significantly improved response compared with vinblastine treatment alone, while this had no additional effect on one organoid (Or 4) (Figure 4B). In one out of four BC organoids (Or 3), co‐treatment with piroxicam and vinblastine significantly improved response compared with vinblastine treatment alone, while it had no additional effect on other organoids (Or 1, 2, 4) (Figure 4B). These results suggested that response to co‐treatment with anticancer drugs is different in each BC organoid, and that cell viability testing using BC organoids might become a useful tool for measuring differences in the sensitivity of combination therapies.
Figure 4.
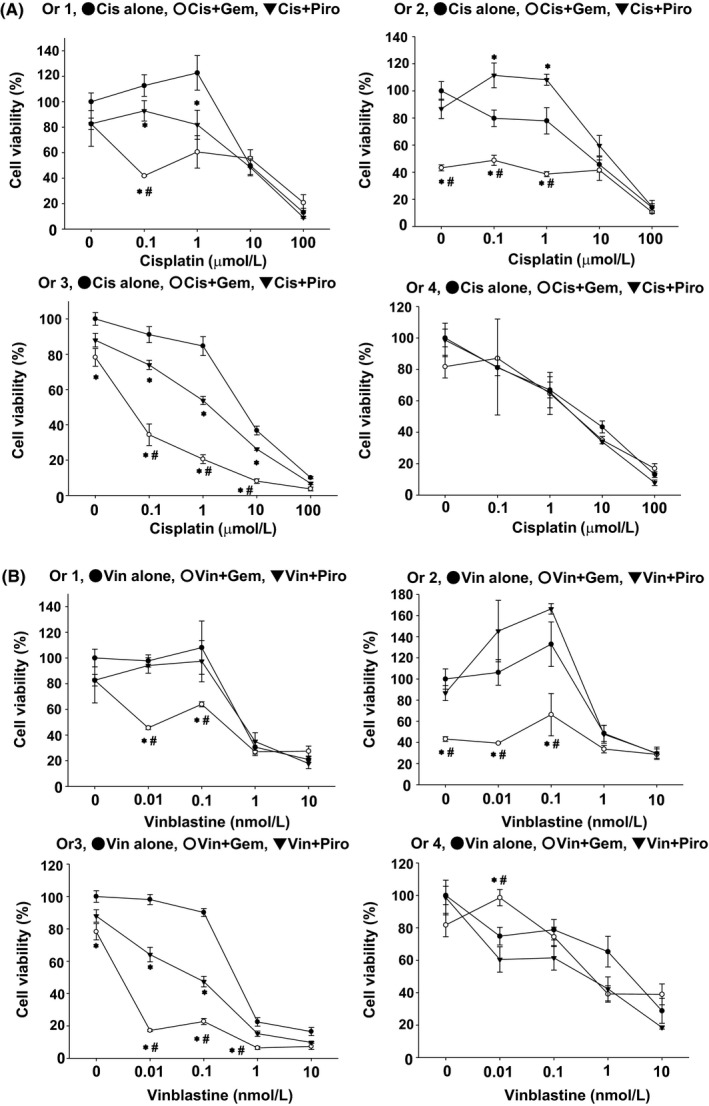
Effects of combination treatment with anticancer drugs on BC organoids. After BC organoids were trypsinized and seeded into Matrigel, they were treated with cisplatin (A) or vinblastine (B) in the presence or absence of piroxicam (10 μmol/L) or gemcitabine (100 nmol/L) for 3 d (n = 3‐6 each for four organoids [Ors 1‐4]). Cell viability was determined using an alamar blue assay; 100% represents cell viability of each control. Results were expressed as mean ± SEM *P ≤ 0.05 vs Cis alone; # P ≤ 0.05 vs Cis + Piro (A). *P ≤0.05 vs Vin alone; # P ≤ 0.05 vs Vin + Piro (B).
3.5. RNA‐sequencing analysis of BC organoids
We next investigated the difference between gene expression profiles from normal bladder tissues, 2D BC cell lines, and BC organoid samples using RNA‐seq analysis. Expression of each sample was clearly separated in a PCA plot (Figure 5A). Differential expression analysis showed that several genes were differentially regulated in BC organoid samples compared with normal bladder tissues or 2D BC cell lines (Figure 5B). In muscle‐invasive human BC, a basal‐like subtype showed a more aggressive phenotype than a luminal‐like subtype.33, 34 However, the main subtype of dog BC remains unclear. We therefore compared the expression levels of basal or luminal cell markers in BC organoids using a heat map (Figure 5C). Expression of basal cell markers, including CK5, p63, CD44, and DSG3, was upregulated in BC organoids compared with normal bladder tissues or 2D BC cell lines, while expression of luminal cell markers, including GATA3, FABP4, ERBB2, and FOXA1, was not upregulated. To validate the RNA‐seq data, we performed quantitative real‐time PCR. Expression of CK5 and DSG3 mRNA was significantly upregulated in the BC organoids compared with normal bladder tissues or 2D BC cell lines (Figure 5D), while expression of GATA3 and ERBB2 mRNA was significantly downregulated compared with normal bladder tissues (Figure 5E). We further observed that CK5 protein was expressed in BC organoids as determined by immunohistochemistry (Figure 5F). Furthermore, we confirmed by western blotting that the expression of CK5 in BC organoids was significantly increased compared with 2D cells (Figure 5G, H). These results indicated that the main type of dog BC might be a basal‐like subtype, as is the case for human muscle‐invasive BC.
Figure 5.
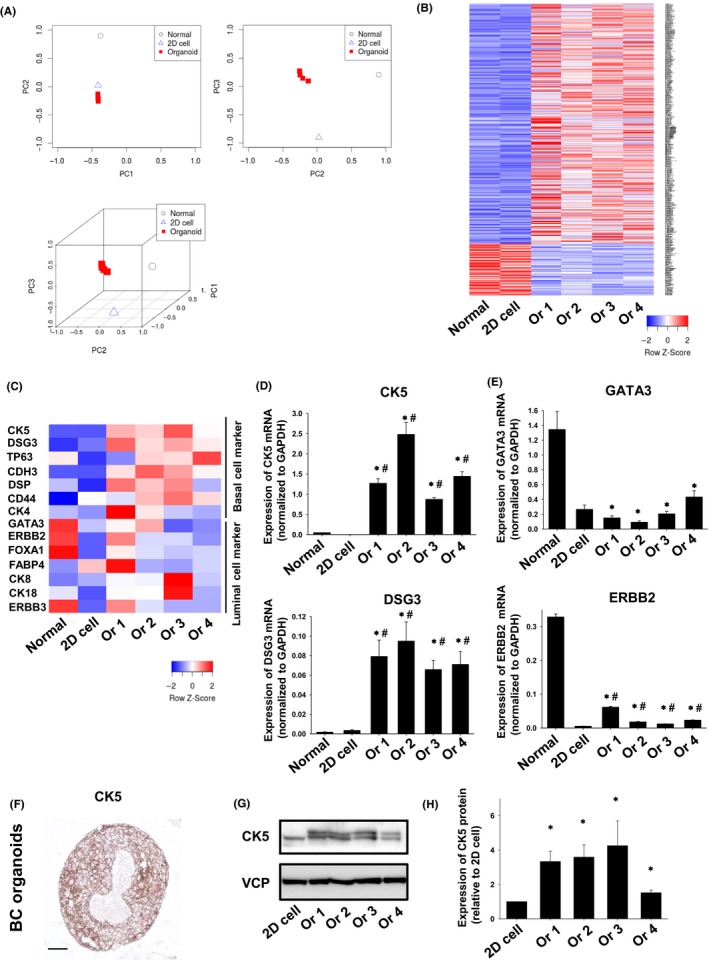
RNA sequencing analysis of BC organoids. (A) PCA plot of normal bladder tissues, 2D BC cell lines, and BC organoids. (B) Hierarchical clustering of differentially expressed genes in normal bladder tissues, 2D BC cell lines, and BC organoids. Genes shown in blue are downregulated, while genes shown in red are upregulated. (C) Heatmap analysis of basal cell‐ and luminal cell‐related genes in normal bladder tissues, 2D BC cell lines, and BC organoids. Genes shown in blue are downregulated, while genes shown in red are upregulated. Expression of basal cell markers (D) and luminal cell markers (E) in normal bladder tissues, 2D BC cell lines, and BC organoids were determined by quantitative real‐time PCR (n = 4). Expression levels of CK5,DSG3 (D), GATA3, and ERBB2 (E) are quantified based on the ratio of their expression level to that of GAPDH. Results are expressed as mean ± SEM *P ≤ 0.05 vs Normal; # P ≤ 0.05 vs 2D cell. (F) Protein expression of CK5 in BC organoids. Representative photomicrographs are shown (F; n = 4). Scale bar: 100 μm. (G) Expression of CK5 in 2D BC cell lines and BC organoids was determined by western blotting. Equal protein loading was confirmed using total VCP antibody. (H) Expression level of CK5 was analyzed using an ImageJ software and quantified (n = 4). Results are expressed as mean ± SEM *P ≤ 0.05 vs 2D cell.
3.6. Searching for novel diagnostic makers using BC organoids
To explore novel diagnostic marker genes using dog BC organoids, we analyzed RNA‐seq data and picked up the top 30 highly upregulated genes in dog BC organoids compared with normal bladder tissues (Table 3) or 2D BC cell lines (Table 4). To validate the RNA‐seq data, we prepared an additional three strains of normal bladder tissue samples and performed quantitative real‐time PCR. Expression of MMP28 (Figure 6A), CTSE (Figure 6B), CNN3 (Figure 6C), TFPI2 (Figure 6D), COL17A1 (Figure 6E), and AGPAT4 (Figure 6F) mRNA was significantly upregulated in the BC organoid group compared with the normal bladder tissue group. Interestingly, the mRNA expression levels of these genes did not change in blood samples from each group (Figure S3). We also confirmed that expression levels of these genes were quite low in the 2D BC cell lines and significantly lower compared with each BC organoid (Figure 6A‐F). Other selected genes (CK15, TGM2, GJB2, IL1R2, COL5A2, CDH3, CHST4, and ADORA2B) were not upregulated in some BC organoids or upregulated even in normal bladder tissues (Figure S4). Finally, we observed that MMP28, CTSE, CNN3, TFPI2, and AGPAT4 proteins were expressed in BC organoids (Figure 6G).
Table 3.
Top 30 upregulated genes in dog bladder cancer organoids compared with normal bladder tissues
| Name | Normal | Or 1 | Or 2 | Or 3 | Or 4 | Fold increase (relative to normal) | |
|---|---|---|---|---|---|---|---|
| 1 | SLPI | 0.6 | 683.0 | 5.7 | 5.5 | 251.4 | 421.176 752 3 |
| 2 | S100A2 | 8.4 | 2312.6 | 2088.0 | 2545.5 | 1645.8 | 255.162 300 6 |
| 3 | LOC489428 | 1.1 | 643.5 | 34.0 | 281.9 | 7.3 | 215.324 602 4 |
| 4 | CTSE | 1.1 | 93.5 | 128.5 | 502.2 | 235.1 | 213.691 455 |
| 5 | CHST4 | 0.6 | 269.4 | 34.0 | 28.0 | 25.2 | 158.861 991 5 |
| 6 | ADORA2B | 0.6 | 15.8 | 88.8 | 107.5 | 135.9 | 154.983 282 1 |
| 7 | HK2 | 1.7 | 433.9 | 157.8 | 111.4 | 110.6 | 120.821 426 1 |
| 8 | FBLIM1 | 0.6 | 12.4 | 80.3 | 70.9 | 104.9 | 119.614 246 5 |
| 9 | MMP28 | 0.6 | 51.8 | 84.1 | 38.2 | 88.7 | 117.052 030 2 |
| 10 | TFPI2 | 2.8 | 184.8 | 326.0 | 35.0 | 735.5 | 114.153 761 3 |
| 11 | COL5A2 | 2.2 | 53.0 | 189.9 | 353.5 | 261.2 | 95.502 436 34 |
| 12 | IL1R2 | 1.1 | 27.0 | 144.6 | 39.7 | 188.7 | 89.106 915 88 |
| 13 | RHCG | 0.6 | 149.9 | 13.2 | 24.9 | 4.1 | 85.576 011 25 |
| 14 | CKMT1B | 0.6 | 46.2 | 24.6 | 58.4 | 59.4 | 83.998 604 46 |
| 15 | CK6A | 0.6 | 165.7 | 0.9 | 14.0 | 0.0 | 80.464 846 31 |
| 16 | CK5 | 38.7 | 3048.5 | 2702.1 | 4184.6 | 2154.3 | 78.051 089 03 |
| 17 | COL17A1 | 15.2 | 1056.0 | 833.3 | 1360.4 | 1074.7 | 71.347 086 34 |
| 18 | CK14 | 0.6 | 112.7 | 10.4 | 29.6 | 4.9 | 70.189 688 7 |
| 19 | FSCN1 | 1.1 | 59.7 | 84.1 | 57.6 | 104.1 | 68.062 078 44 |
| 20 | AGPAT4 | 1.7 | 58.6 | 140.8 | 83.3 | 132.6 | 61.668 709 28 |
| 21 | CK16 | 8.4 | 1890.0 | 41.6 | 10.9 | 56.1 | 59.353 708 39 |
| 22 | CDH3 | 1.7 | 82.3 | 132.3 | 106.7 | 74.0 | 58.691 222 64 |
| 23 | C9H17orf64 | 1.1 | 129.6 | 21.7 | 48.3 | 30.1 | 51.165 373 21 |
| 24 | ALDOC | 2.2 | 59.7 | 71.8 | 184.5 | 130.2 | 49.697 809 89 |
| 25 | CNN3 | 2.2 | 124.0 | 116.2 | 82.5 | 111.5 | 48.353 037 49 |
| 26 | ADM | 3.9 | 251.3 | 110.5 | 191.6 | 171.7 | 46.142 754 19 |
| 27 | ALDH1A3 | 0.6 | 21.4 | 25.5 | 10.9 | 43.1 | 44.966 681 58 |
| 28 | DDIT4 | 4.5 | 126.2 | 102.0 | 316.1 | 223.7 | 42.772 478 99 |
| 29 | PDPN | 1.1 | 23.7 | 30.2 | 37.4 | 93.6 | 41.169 345 72 |
| 30 | HIP1 | 2.8 | 30.4 | 142.7 | 81.0 | 196.9 | 40.177 382 67 |
Table 4.
Top 30 upregulated genes in dog bladder cancer organoids compared with 2D cell lines
| Name | 2D cell | Or 1 | Or 2 | Or 3 | Or 4 | Fold increase (relative to 2D cell) | |
|---|---|---|---|---|---|---|---|
| 1 | CK5 | 1.9 | 3048.5 | 2702.1 | 4184.6 | 2154.3 | 1628.263 966 |
| 2 | TGM2 | 0.6 | 544.3 | 1382.2 | 242.2 | 1031.6 | 1293.094 044 |
| 3 | GJB2 | 0.6 | 871.2 | 300.4 | 122.3 | 127.7 | 574.395 25 |
| 4 | CK15 | 1.9 | 1165.3 | 646.2 | 1009.2 | 295.3 | 419.679 583 7 |
| 5 | ANXA6 | 0.6 | 20.3 | 334.5 | 245.3 | 372.6 | 392.993 828 9 |
| 6 | CDH13 | 0.6 | 10.1 | 171.0 | 142.5 | 405.2 | 294.471 941 |
| 7 | NDUFA4L2 | 0.6 | 313.3 | 85.0 | 161.2 | 78.9 | 257.961 221 9 |
| 8 | CP | 1.9 | 9.0 | 515.9 | 128.5 | 732.2 | 186.611 392 8 |
| 9 | COL5A2 | 1.2 | 53.0 | 189.9 | 353.5 | 261.2 | 173.245 696 5 |
| 10 | IL1R2 | 0.6 | 27.0 | 144.6 | 39.7 | 188.7 | 161.643 935 9 |
| 11 | CDH3 | 0.6 | 82.3 | 132.3 | 106.7 | 74.0 | 159.702 759 4 |
| 12 | CYP1B1 | 0.6 | 86.8 | 79.4 | 131.6 | 91.1 | 157.117 706 5 |
| 13 | AUTS2 | 0.6 | 105.9 | 115.3 | 58.4 | 102.5 | 154.392 274 5 |
| 14 | SEMA3G | 0.6 | 38.3 | 69.9 | 86.4 | 168.4 | 146.699 493 7 |
| 15 | S100A12 | 0.6 | 353.9 | 1.9 | 0.0 | 1.6 | 144.404 975 4 |
| 16 | CHST4 | 0.6 | 269.4 | 34.0 | 28.0 | 25.2 | 144.091 383 5 |
| 17 | ADORA2B | 0.6 | 15.8 | 88.8 | 107.5 | 135.9 | 140.573 307 2 |
| 18 | NCCRP1 | 1.2 | 293.0 | 205.0 | 126.1 | 65.9 | 139.414 207 3 |
| 19 | CYP1A1 | 9.3 | 668.3 | 1584.4 | 1497.4 | 954.3 | 126.721 804 2 |
| 20 | MMP28 | 0.6 | 51.8 | 84.1 | 38.2 | 88.7 | 106.168 812 4 |
| 21 | SCEL | 0.6 | 176.9 | 8.5 | 39.7 | 14.6 | 96.890 595 78 |
| 22 | C9H17orf64 | 0.6 | 129.6 | 21.7 | 48.3 | 30.1 | 92.816 278 39 |
| 23 | EGLN3 | 8.0 | 757.3 | 745.4 | 851.9 | 534.5 | 89.797 596 59 |
| 24 | FADS2 | 0.6 | 5.6 | 42.5 | 101.2 | 61.8 | 85.339 282 83 |
| 25 | GPR115 | 0.6 | 75.5 | 56.7 | 30.4 | 42.3 | 82.777 703 16 |
| 26 | SSPN | 0.6 | 4.5 | 36.8 | 54.5 | 74.0 | 68.646 626 33 |
| 27 | DKK3 | 0.6 | 4.5 | 9.4 | 58.4 | 87.9 | 64.737 402 18 |
| 28 | LOC100685649 | 6.8 | 1382.8 | 159.7 | 59.2 | 29.3 | 59.908 252 73 |
| 29 | ATP2C2 | 0.6 | 51.8 | 32.1 | 20.2 | 43.1 | 59.528 317 9 |
| 30 | FA2H | 1.2 | 91.3 | 35.0 | 42.8 | 57.8 | 45.826 176 65 |
Figure 6.
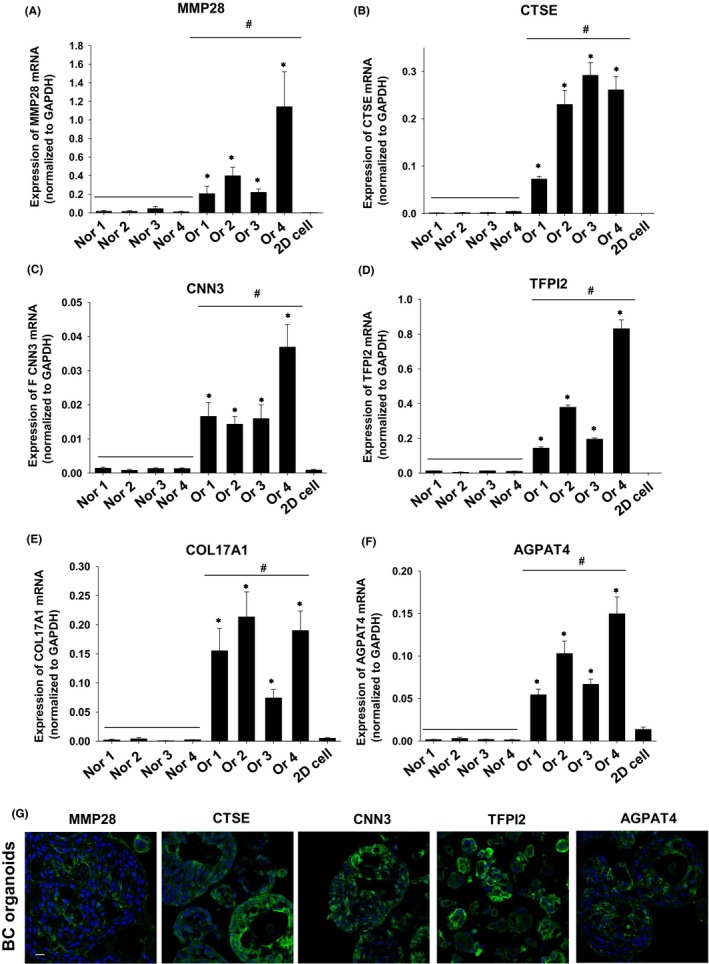
Search for novel diagnostic makers by using BC organoids. Expression of MMP28 (A), CTSE (B), CNN3 (C), TFPI2 (D), COL17A1 (E), and AGPAT4 (F) mRNA in normal bladder tissue (Nors 1‐4), BC organoids (Ors 1‐4), and 2D BC cell lines was determined by quantitative real‐time PCR (n = 4). Expression level of each gene was quantified based on the ratio of expression level to that of GAPDH. Results are expressed as mean ± SEM *P ≤ 0.05 vs 2D cell; # P ≤ 0.05 vs Normal. Protein expression of MMP28, CTSE, CNN3, TFPI2, and AGPAT4 in BC organoids (G). Representative photomicrographs are shown (G; n = 4). Scale bar: 50 μm.
4. DISCUSSION
In the current study, for the 1st time, dog BC organoids were generated and several genes specifically upregulated in the organoids were identified. The main findings of the current study are as follows: The organoids from BC diseased dogs expressed urothelial cell markers, and showed urothelial carcinoma‐like structures, which resembled the architecture of invasive type of BC in dogs (Figure 1). Injection of BC organoids into mice formed tumors (Figure 2). The responsiveness to combination treatment with anticancer drugs was markedly changed in each organoid (Figure 4). Many basal but not luminal cell markers were upregulated in the BC organoids (Figure 5). Several novel genes were specifically upregulated in the BC organoids (Figure 6).Collectively, our data suggest that dog BC organoids might become a new tool to provide new insights for both dog BC therapy and diagnostic markers.
Dog BC, also classified as TCC, is usually a high‐grade invasive cancer. Treatments for dogs with BC, such as surgery, radiation therapy, medical therapy, and local intravesical therapy were conducted. As BC diseased dogs usually have severe conditions and it was difficult to perform surgery or tissue biopsy prior to treatment, in the present study we could not obtain tissue samples to compare histology between original tumor tissues and generated organoids, as shown in our previous dog PC organoid paper.17 Furthermore, surgical resection in dogs with BC is limited due to the trigonal location (difficult for surgical operation) and metastases at the time of diagnosis.35 Therefore, systemic medical therapies, including chemotherapy agents (cisplatin, vinblastine, and gemcitabine) and COX inhibitors (piroxicam), are considered to be mainstay treatments.13, 35 In particular, oral piroxicam (0.3 mg/kg daily with food) is known to be a useful palliative treatment for dogs with BC, which provides excellent quality of life.13 The direct effects of these drugs on patient‐derived BC cells, however, have never been investigated. In the present study, for the 1st time, dog BC organoids were generated (Figures 1, 2) and showed that piroxicam had no effect on cell viability of dog BC organoids (Figure 3A,B). In contrast, treatment with cisplatin or vinblastine decreased cell viability of BC organoids in a dose‐dependent manner, similarly to the clinical use of cisplatin28, 36 and vinblastine37, 38 in the treatment of BC dogs. Interestingly, gemcitabine had no effect on most strains of dog BC organoids, except for the 2nd dog BC organoids (Figures2 and 3C). To clarify the difference in gemcitabine sensitivity, the expression levels of genes involved in gemcitabine metabolism (dCK, CDA, RRM1, RRM2, and SLC29A1) were measured using RNA‐seq (Figure S5). However, no correlation between the sensitivity and gene expression pattern in organoids was found. Further investigations using dog BC organoids are needed to measure drug sensitivity and to select a suitable medical therapy for each BC diseased dog.
In the treatment of dog BC, medical therapy is not usually curative, and resistance to one drug often develops. Once treatment is tolerated, dogs sequentially receive multiple different treatment protocols over the course of their disease.13 The pattern of combination chemotherapy such as with cisplatin and piroxicam,28, 29, 39 carboplatin and piroxicam,40 vinblastine and piroxicam41 has been reported. For example, piroxicam enhanced the antitumor activity of carboplatin40 and cisplatin28, 29, 39 in dog BC. Piroxicam also significantly increased the activity of vinblastine in dog BC.41 However, renal toxicity is especially problematic when cisplatin is combined with piroxicam.28 In a previous report, the combination of cisplatin and piroxicam led to effective remission rates, but caused renal, gastrointestinal, and bone marrow toxicities. Considering these studies, simultaneous treatment with multiple chemotherapeutic agents might be more toxic than curative. It also remains unknown whether it would be more appropriate to simultaneously combine multiple chemotherapy agents in dogs with BC. To solve these problems, we measured which anticancer drug combination treatment affected cell viability of BC organoids. Co‐treatment with gemcitabine and cisplatin or vinblastine was more effective than piroxicam (Figure 4) suggesting that, in the future, use of a cell viability assay for BC organoids would predict the most effective combination treatment for dogs.
Understanding how tumors arise requires identification of the cancer cell origin.42 Although it is widely assumed that CSCs may arise from normal stem cells undergoing gene mutations43 via a complex mechanism,44 the origin of CSCs is still unclear.44, 45 In general, urothelial lesions are classified into basal and luminal types that have different gene expression patterns. Basal and luminal subtypes of BC show distinct clinical behaviors and responses to frontline chemotherapy.33, 46, 47 For example, muscle‐invasive basal BC is more aggressive with shorter survival time, high metastasis, and is more sensitive to cisplatin‐based chemotherapy compared with luminal cancers.33, 46 Furthermore, it was demonstrated that tumor‐propagating cells isolated from BC have a basal‐type characteristics in human48, 49, 50, 51 and mice,42 suggesting that the origins of muscle‐invasive BC are mainly basal cells. As it remains unclear which subtype of cells contributes to the development of dog BC, we examined the expression pattern of basal and luminal cell markers in BC organoids (Figure 5). Expression of several basal cell markers including CK5 and DSG3 was upregulated in most BC organoids. Conversely, luminal cell marker expression, including GATA3 and ERBB2, was downregulated. These data suggest that the cell origin of dog BC organoids might be basal cells, as is the case with muscle‐invasive human BC.
In the present study, we found that expression levels of MMP28, CTSE, CNN3, TFPI2, COL17A1, and AGPAT4 were specifically upregulated in dog BC organoids (Figure 6). MMP28 is a member of metalloproteinase family;52 it is expressed in many normal tissues and has a role in the production of cytokines and growth factors.53, 54 CTSE is an aspartic endopeptidase belonging to the cathepsin family of proteases and functions mainly to liberate peptide epitopes for antigen presentation.55, 56 CNN3 is involved in the regulation of cell migration57, 58 and the placentation process.59 TFPI2 encodes a Kunitz‐type serine proteinase inhibitor60 with inhibitory function against tumor growth and metastasis;61, 62 protecting the extracellular matrix of cancer cells from degradation and tumor invasion.63 COL17A1 encodes collagen XVII (COL17), a transmembrane protein that is an essential component of type I hemidesmosomes and acts as a cell‐matrix adhesion molecule.64 AGPAT4 functions mainly to convert lysophosphatidic acid to phosphatidic acid, the 2nd step in de novo phospholipid biosynthesis.65 In a previous study, expression levels of MMP28 were associated with human high‐grade BC.66 Overexpression of CTSE is associated with several types of cancer including human noninvasive BC.67 These studies suggested that genes identified in the present study may be promising biomarkers for dog BC. Our established dog BC organoid system could become in the near future a useful tool to identify further novel biomarkers of both dog and human BC.
In conclusion, for the 1st time, we produced dog BC organoids from urine samples. The organoids showed tumorigenesis in vivo and basal‐like subtype. It was also suggested that several genes might become biomarkers to predict the development of high‐grade BC. Further studies on the dog BC organoid contribution to the treatment and diagnosis of both dog and human BC.
DISCLOSURE
The authors have no conflict of interest.
Supporting information
ACKNOWLEDGMENTS
We thank Dr. Kuo and Dr. Salahudeen for kindly providing Wnt, Noggin and R‐spondin‐producing cells.
Elbadawy M, Usui T, Mori T, et al. Establishment of a novel experimental model for muscle‐invasive bladder cancer using a dog bladder cancer organoid culture. Cancer Sci. 2019;110:2806–2821. 10.1111/cas.14118
Mohamed Elbadawy and Tatsuya Usui contributed equally to this work.
Clinical Trial register and clinical registration number: 0016012 (Institute Animal Care and Use Committee of Tokyo University of Agriculture and Technology approval).
REFERENCES
- 1. Knapp DW, McMillan SK. Tumors of the urinary system In: MacEwen's W, ed. Small Animal Clinical Oncology, 5th ed St. Louis, MO: Elsevier–Saunders; 2013:572‐582. [Google Scholar]
- 2. Lerner SP, Schoenberg MP, Sternberg CN. Textbook of Bladder Cancer. Oxon: Taylor and Francis; 2006. [Google Scholar]
- 3. Patrick DJ, Fitzgerald SD, Sesterhenn IA, Davis CJ, Kiupel M. Classification of canine urinary bladder urothelial tumours based on the World Health Organization/International Society of Urological Pathology consensus classification. J Comp Pathol. 2006;135:190‐199. [DOI] [PubMed] [Google Scholar]
- 4. Valli VE, Norris A, Jacobs RM, et al. Pathology of canine bladder and urethral cancer and correlation with tumour progression and survival. J Comp Pathol. 1995;113:113‐130. [DOI] [PubMed] [Google Scholar]
- 5. Knapp D, McMillan S. Withrow and MacEwen's Small Animal Clinical Oncology. St Louis, MO: Elsevier‐Saunders; 2013. [Google Scholar]
- 6. Priester WA, McKay FW. The occurrence of tumors in domestic animals. Natl Cancer Inst Monogr. 1980;54:1‐210. [PubMed] [Google Scholar]
- 7. Mutsaers AJ, Widmer WR, Knapp DW. Canine transitional cell carcinoma. J Vet Intern Med. 2003;17:136‐144. [DOI] [PubMed] [Google Scholar]
- 8. Knapp D. Animal models of naturally occurring canine urinary bladder cancer In: Lerner S, Schoenberg M, Sternberg C, eds. Textbook of Bladder Cancer. Oxon: Taylor and Francis; 2006:171‐175. [Google Scholar]
- 9. Kobayashi T, Owczarek TB, McKiernan JM, Abate‐Shen C. Modelling bladder cancer in mice: opportunities and challenges. Nat Rev Cancer. 2015;15:42‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knapp DW, Glickman NW, Denicola DB, Bonney PL, Lin TL, Glickman LT. Naturally‐occurring canine transitional cell carcinoma of the urinary bladder A relevant model of human invasive bladder cancer. Urol Oncol. 2000;5:47‐59. [DOI] [PubMed] [Google Scholar]
- 11. Norris AM, Laing EJ, Valli VE, et al. Canine bladder and urethral tumors: a retrospective study of 115 cases (1980–1985). J Vet Intern Med. 1992;6:145‐153. [DOI] [PubMed] [Google Scholar]
- 12. Santos M, Dias Pereira P, Montenegro L, Faustino AM. Recurrent and metastatic canine urethral transitional cell carcinoma without bladder involvement. Vet Rec. 2007;160:557‐558. [DOI] [PubMed] [Google Scholar]
- 13. Knapp DW, Ramos‐Vara JA, Moore GE, Dhawan D, Bonney PL, Young KE. Urinary bladder cancer in dogs, a naturally occurring model for cancer biology and drug development. ILAR J. 2014;55:100‐118. [DOI] [PubMed] [Google Scholar]
- 14. Henry CJ, McCaw DL, Turnquist SE, et al. Clinical evaluation of mitoxantrone and piroxicam in a canine model of human invasive urinary bladder carcinoma. Clin Cancer Res. 2003;9:906. [PubMed] [Google Scholar]
- 15. Bartfeld S, Clevers H. Stem cell‐derived organoids and their application for medical research and patient treatment. J Mol Med (Berl). 2017;95:729‐738. [DOI] [PubMed] [Google Scholar]
- 16. Takahashi T. Organoids for drug discovery and personalized medicine. Annu Rev Pharmacol Toxicol. 2018; 59: 447-462. [DOI] [PubMed] [Google Scholar]
- 17. Usui T, Sakurai M, Nishikawa S, et al. Establishment of a dog primary prostate cancer organoid using the urine cancer stem cells. Cancer Sci. 2017;108:2383‐2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Usui TA‐O, Sakurai M, Enjoji S, et al. Establishment of a novel model for anticancer drug resistance in three‐dimensional primary culture of tumor microenvironment. Stem Cells Int. 2016;2016:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Usui T, Sakurai M, Umata K, et al. Hedgehog signals mediate anti‐cancer drug resistance in three‐dimensional primary colorectal cancer organoid culture. Int J Mol Sci. 2018;19:1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Galbraith EA, McKellar QA. Pharmacokinetics and pharmacodynamics of piroxicam in dogs. Vet Rec. 1991;128:561‐565. [DOI] [PubMed] [Google Scholar]
- 21. Freise KJ, Martin‐Jimenez T. Pharmacokinetics of gemcitabine and its primary metabolite in dogs after intravenous bolus dosing and its in vitro pharmacodynamics. J Vet Pharmacol Ther. 2006;29:137‐145. [DOI] [PubMed] [Google Scholar]
- 22. Cozzi PJ, Bajorin DF, Tong W, et al. Toxicology and pharmacokinetics of intravesical gemcitabine: a preclinical study in dogs. Clin Cancer Res. 1999;5:2629‐2637. [PubMed] [Google Scholar]
- 23. Achanta S, Ngo M, Veitenheimer A, Maxwell LK, Wagner JR. Simultaneous quantification of vinblastine and desacetylvinblastine concentrations in canine plasma and urine samples using LC‐APCI‐MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;913–914:147‐154. [DOI] [PubMed] [Google Scholar]
- 24. Sockalingam R, Filippich L, Charles B, Murdoch B. Cisplatin‐induced ototoxicity and pharmacokinetics: preliminary findings in a dog model. Ann Otol Rhinol Laryngol. 2002;111:745‐750. [DOI] [PubMed] [Google Scholar]
- 25. Yin JX, Wei Z, Xu JJ, Sun ZQ. In vivo pharmacokinetic and tissue distribution investigation of sustained‐release cisplatin implants in the normal esophageal submucosa of 12 beagle dogs. Cancer Chemother Pharmacol. 2015;76:525‐536. [DOI] [PubMed] [Google Scholar]
- 26. Kake S, Usui T, Ohama T, Yamawaki H, Sato K. Death‐associated protein kinase 3 controls the tumor progression of A549 cells through ERK MAPK/c‐Myc signaling. Oncol Rep. 2017;37:1100‐1106. [DOI] [PubMed] [Google Scholar]
- 27. Meuten DJ, Meuten TLK. Tumors of the urinary system In: Meuten DJ, ed. Tumors in Domestic Animals, 5th ed Ames, IA: John Wiley & Sons Inc; 2017:665‐678. [Google Scholar]
- 28. Knapp DW, Glickman NW, Widmer WR, et al. Cisplatin versus cisplatin combined with piroxicam in a canine model of human invasive urinary bladder cancer. Cancer Chemother Pharmacol. 2000;46:221‐226. [DOI] [PubMed] [Google Scholar]
- 29. Greene SN, Lucroy MD, Greenberg CB, Bonney PL, Knapp DW. Evaluation of cisplatin administered with piroxicam in dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2007;231:1056‐1060. [DOI] [PubMed] [Google Scholar]
- 30. Marconato L, Zini E, Lindner D, Suslak‐Brown L, Nelson V, Jeglum AK. Toxic effects and antitumor response of gemcitabine in combination with piroxicam treatment in dogs with transitional cell carcinoma of the urinary bladder. J Am Vet Med Assoc. 2011;238:1004‐1010. [DOI] [PubMed] [Google Scholar]
- 31. Robat C, Burton J, Thamm D, Vail D. Retrospective evaluation of doxorubicin‐piroxicam combination for the treatment of transitional cell carcinoma in dogs. J Small Anim Pract. 2013;54:67‐74. [DOI] [PubMed] [Google Scholar]
- 32. Allstadt SD, Rodriguez CO Jr, Boostrom B, Rebhun RB, Skorupski KA. Randomized phase III trial of piroxicam in combination with mitoxantrone or carboplatin for first‐line treatment of urogenital tract transitional cell carcinoma in dogs. J Vet Intern Med. 2015;29:261‐267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Choi W, Porten S, Kim S, et al. Identification of distinct basal and luminal subtypes of muscle‐invasive bladder cancer with different sensitivities to frontline chemotherapy. Cancer Cell. 2014;25:152‐165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. N Cancer Genome Atlas Research . Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Fulkerson CM, Knapp DW. Management of transitional cell carcinoma of the urinary bladder in dogs: a review. Vet J. 2015;205:217‐225. [DOI] [PubMed] [Google Scholar]
- 36. Moore AS, Cardona A, Shapiro W, Madewell BR. Cisplatin (cisdiamminedichloroplatinum) for treatment of transitional cell carcinoma of the urinary bladder or urethra. A retrospective study of 15 dogs. J Vet Intern Med. 1990;4:148‐152. [DOI] [PubMed] [Google Scholar]
- 37. Knapp DW, Richardson RC, Chan TC, et al. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1994;8:273‐278. [DOI] [PubMed] [Google Scholar]
- 38. Arnold EJ, Childress MO, Fourez LM, et al. Clinical trial of vinblastine in dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 2011;25:1385‐1390. [DOI] [PubMed] [Google Scholar]
- 39. Mohammed SI, Craig BA, Mutsaers AJ, et al. Effects of the cyclooxygenase inhibitor, piroxicam, in combination with chemotherapy on tumor response, apoptosis, and angiogenesis in a canine model of human invasive urinary bladder cancer. Mol Cancer Ther. 2003;2:183‐188. [PubMed] [Google Scholar]
- 40. Boria PA, Glickman NW, Schmidt BR, et al. Carboplatin and piroxicam therapy in 31 dogs with transitional cell carcinoma of the urinary bladder. Vet Comp Oncol. 2005;3:73‐80. [DOI] [PubMed] [Google Scholar]
- 41. Knapp DW, Ruple‐Czerniak A, Ramos‐Vara JA, Naughton JF, Fulkerson CM, Honkisz SI. A nonselective cyclooxygenase inhibitor enhances the activity of vinblastine in a naturally‐occurring canine model of invasive urothelial carcinoma. Bladder Cancer. 2016;2:241‐250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Shin K, Lim A, Odegaard JI, et al. Cellular origin of bladder neoplasia and tissue dynamics of its progression to invasive carcinoma. Nat Cell Biol. 2014;16:469‐478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Passegue E, Jamieson CH, Ailles LE, Weissman IL. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc Natl Acad Sci USA. 2003;100(Suppl 1):11842‐11849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jordan CT. Cancer stem cells: controversial or just misunderstood? Cell Stem Cell. 2009;4:203‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bjerkvig R, Tysnes BB, Aboody KS, Najbauer J, Terzis AJ. Opinion: the origin of the cancer stem cell: current controversies and new insights. Nat Rev Cancer. 2005;5:899‐904. [DOI] [PubMed] [Google Scholar]
- 46. Choi W, Czerniak B, Ochoa A, et al. Intrinsic basal and luminal subtypes of muscle‐invasive bladder cancer. Nat Rev Urol. 2014;11:400‐410. [DOI] [PubMed] [Google Scholar]
- 47. McConkey DJ, Choi W, Dinney CP. Genetic subtypes of invasive bladder cancer. Curr Opin Urol. 2015;25:449‐458. [DOI] [PubMed] [Google Scholar]
- 48. Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor‐initiating cells. Proc Natl Acad Sci USA. 2009;106:14016‐14021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. He X, Marchionni L, Hansel DE, et al. Differentiation of a highly tumorigenic basal cell compartment in urothelial carcinoma. Stem Cells. 2009;27:1487‐1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yang YM, Chang JW. Bladder cancer initiating cells (BCICs) are among EMA‐CD44v6+ subset: novel methods for isolating undetermined cancer stem (initiating) cells. Cancer Invest. 2008;26:725‐733. [DOI] [PubMed] [Google Scholar]
- 51. Li C, Yang Z, Du Y, et al. BCMab1, a monoclonal antibody against aberrantly glycosylated integrin alpha3 beta1, has potent antitumor activity of bladder cancer in vivo. Clin Cancer Res. 2014;20:4001‐4013. [DOI] [PubMed] [Google Scholar]
- 52. Marchenko GN, Strongin AY. MMP‐28, a new human matrix metalloproteinase with an unusual cysteine‐switch sequence is widely expressed in tumors. Gene. 2001;265:87‐93. [DOI] [PubMed] [Google Scholar]
- 53. Jian P, Yanfang T, Zhuan Z, Jian W, Xueming Z, Jian N. MMP28 (epilysin) as a novel promoter of invasion and metastasis in gastric cancer. BMC Cancer. 2011;11:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Illman SA, Keski‐Oja J, Lohi J. Promoter characterization of the human and mouse epilysin (MMP‐28) genes. Gene. 2001;275:185‐194. [DOI] [PubMed] [Google Scholar]
- 55. Katunuma N, Matsunaga Y, Himeno K, Hayashi Y. Insights into the roles of cathepsins in antigen processing and presentation revealed by specific inhibitors. Biol Chem. 2003;384:883‐890. [DOI] [PubMed] [Google Scholar]
- 56. Conus S, Simon HU. Cathepsins and their involvement in immune responses. Swiss Med Wkly. 2010;140:w13042. [DOI] [PubMed] [Google Scholar]
- 57. Appel S, Allen PG, Vetterkind S, Jin JP, Morgan KG. h3/Acidic calponin: an actin‐binding protein that controls extracellular signal‐regulated kinase 1/2 activity in nonmuscle cells. Mol Biol Cell. 2010;21:1409‐1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Daimon E, Shibukawa Y, Wada Y. Calponin 3 regulates stress fiber formation in dermal fibroblasts during wound healing. Arch Dermatol Res. 2013;305:571‐584. [DOI] [PubMed] [Google Scholar]
- 59. Shibukawa Y, Yamazaki N, Kumasawa K, et al. Calponin 3 regulates actin cytoskeleton rearrangement in trophoblastic cell fusion. Mol Biol Cell. 2010;21:3973‐3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rollin J, Iochmann S, Blechet C, et al. Expression and methylation status of tissue factor pathway inhibitor‐2 gene in non‐small‐cell lung cancer. Br J Cancer. 2005;92:775‐783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lavergne M, Jourdan ML, Blechet C, et al. Beneficial role of overexpression of TFPI‐2 on tumour progression in human small cell lung cancer. FEBS Open Bio. 2013;3:291‐301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Wang G, Huang W, Li W, et al. TFPI‐2 suppresses breast cancer cell proliferation and invasion through regulation of ERK signaling and interaction with actinin‐4 and myosin‐9. Sci Rep. 2018;8:14402. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Gerecke C, Scholtka B, Lowenstein Y, et al. Hypermethylation of ITGA4, TFPI2 and VIMENTIN promoters is increased in inflamed colon tissue: putative risk markers for colitis‐associated cancer. J Cancer Res Clin Oncol. 2015;141:2097‐2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Borradori L, Sonnenberg A. Structure and function of hemidesmosomes: more than simple adhesion complexes. J Invest Dermatol. 1999;112:411‐418. [DOI] [PubMed] [Google Scholar]
- 65. Bradley RM, Marvyn PM, Aristizabal Henao JJ, et al. Acylglycerophosphate acyltransferase 4 (AGPAT4) is a mitochondrial lysophosphatidic acid acyltransferase that regulates brain phosphatidylcholine, phosphatidylethanolamine, and phosphatidylinositol levels. Biochem Biophys Acta. 2015;1851:1566‐1576. [DOI] [PubMed] [Google Scholar]
- 66. Wallard MJ, Pennington CJ, Veerakumarasivam A, et al. Comprehensive profiling and localisation of the matrix metalloproteinases in urothelial carcinoma. Br J Cancer. 2006;94:569‐577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wild PJ, Herr A, Wissmann C, et al. Gene expression profiling of progressive papillary noninvasive carcinomas of the urinary bladder. Clin Cancer Res. 2005;11:4415‐4429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


