Abstract
Nivolumab is a human monoclonal antibody against the immune checkpoint receptor programmed death‐1, inhibiting binding to programmed death‐ligand 1 or 2 (PD‐L1 or PD‐L2). This phase 2 study evaluated the efficacy and safety of nivolumab in patients with advanced/recurrent uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma (STS). Patients received nivolumab 240 mg at 2‐week intervals. Primary endpoint was objective response rate; secondary endpoints included overall survival, progression‐free survival, and safety. PD‐L1 expression and microsatellite‐instability (MSI) status were analyzed as potential efficacy biomarkers. Objective response rate was 25%, 23%, and 0% in patients with cervical cancer (n = 20), corpus cancer (n = 22), and STS (n = 21), respectively. The lower 80% confidence intervals of objective response rates in patients with cervical or corpus cancer exceeded the threshold rate (5%); the primary endpoint was met in cervical and corpus cancer, but not in STS. Median progression‐free survival was 5.6, 3.4, and 1.4 months, and 6‐month overall survival was 84%, 73%, and 86% in cervical cancer, corpus cancer, and STS, respectively. The objective response rate was higher in patients with cervical cancer with PD‐L1‐positive (n = 5/15; 33%) versus PD‐L1‐negative (n = 0/5; 0%) tumors. The two patients with corpus cancer classified as MSI‐high responded; the six patients classified as microsatellite stable did not respond. Overall, nivolumab showed acceptable toxicity in all cohorts, with evidence of clinical activity in uterine cervical or corpus cancer, but not in STS. PD‐L1 expression in cervical cancer and MSI‐high in corpus cancer may predict clinical activity of nivolumab in these cancers.
Keywords: nivolumab, programmed death‐1, soft tissue sarcoma, uterine cervical cancer, uterine corpus cancer
Abbreviations
- AE
adverse event
- CI
confidence interval
- CR
complete response
- DCR
disease control rate
- FAS
full analysis set
- HPV
human papilloma virus
- MSI
microsatellite instability
- ORR
objective response rate
- OS
overall survival
- PD
progressive disease
- PD‐1
programmed death‐1
- PD‐L1
programmed death‐ligand 1
- PD‐L2
programmed death‐ligand 2
- PFS
progression‐free survival
- PR
partial response
- SAS
safety analysis set
- SD
stable disease
- STS
soft tissue sarcoma
1. INTRODUCTION
Programmed death‐1 is an immune checkpoint receptor expressed by activated T cells.1 Binding of the ligands PD‐L1 or PD‐L2 to PD‐1 suppresses T‐cell function leading to immune escape, where tumor cells can proliferate undetected by immunosurveillance.1 Clinical trials have shown antibodies against PD‐1 or PD‐L1 to be effective in blocking tumor immune evasion and inducing tumor regression in several types of cancer, including melanoma, non‐small cell lung cancer, and renal cell cancer.1 Some studies of PD‐L1 expression have reported PD‐L1 positivity in uterine cervical cancer, uterine corpus cancer, and STS,2, 3, 4 suggesting that these tumor types are potentially responsive to PD‐1/PD‐L1 blockade therapy. In support of this, the anti‐PD‐1 antibody pembrolizumab and the anti‐PD‐L1 antibody atezolizumab have shown antitumor activity in patients with uterine cervical cancer and/or patients with uterine corpus cancer.5, 6, 7, 8
Nivolumab is a human monoclonal antibody against PD‐1 that inhibits the binding of PD‐1 to PD‐L1 or PD‐L2, thereby enhancing the immune response to tumors.1 Nivolumab has been approved for the treatment of many tumor types in many countries. The aim of this phase 2 study was to evaluate the efficacy and safety of nivolumab in patients with advanced or recurrent uterine cervical cancer, uterine corpus cancer, or STS, which will assist in the decision to proceed to late‐phase clinical trials. An exploratory analysis of PD‐L1 expression, HPV genotype, and MSI status as potential biomarkers for efficacy was also carried out.
2. MATERIALS AND METHODS
2.1. Study design
This was a prospective, multicenter, open‐label, phase 2 study to evaluate the efficacy and safety of nivolumab in Japanese patients with advanced/recurrent uterine cervical cancer, uterine corpus cancer, or STS.
2.2. Patients
Patients aged ≥18 years with histologically confirmed advanced or recurrent uterine cervical cancer, uterine corpus cancer, or STS not curable by surgical or radiation therapy were eligible. Other main inclusion criteria were: ≥1 previous chemotherapy regimen for advanced/recurrent uterine cervical or corpus cancer, or ≥2 previous chemotherapy regimens for advanced/recurrent STS; ≥1 measurable lesion as defined in the RECIST guidelines, version 1.1;9 ECOG performance status score of 0 or 1; and adequate hematological, hepatic, and renal function.
Main exclusion criteria were previously receiving antibodies against PD‐1, PD‐L1, PD‐L2, CD137, or CTLA‐4, or other therapeutic antibodies or pharmacotherapies for regulation of T cells, and receiving systemic corticosteroids or immunosuppressants within 28 days before enrollment.
2.3. Treatment
Nivolumab 240 mg was given i.v. over 30 minutes at 2‐week intervals, with no less than 10 days between doses. Treatment continued until CR or PD, unacceptable toxicity, investigator decision, or withdrawal of consent.
2.4. Study endpoints and assessments
Primary endpoint was ORR, based on the investigator's assessment and defined as the percentage of patients with a best overall response of CR or PR per RECIST.
Secondary endpoints included best overall response, DCR, OS, PFS, duration of response, and maximum percentage change in tumor size and percentage change over time. For best overall response, CR and PR needed to be confirmed by two consecutive assessments carried out at least 4 weeks apart. For SD, an overall response of SD (or better) must have been documented at least once, without PD at any time point, from the start of treatment until after the day 43 assessment. DCR was defined as the percentage of patients with best overall response of CR, PR, or SD. Duration of response was measured (in patients with a best overall response of CR or PR) from the date of first documentation of confirmed CR/PR to the date of first documentation of PD or death from any cause, whichever was earlier.
Tumor response was assessed every 6 weeks by chest, abdominal, and pelvic computed tomography/magnetic resonance imaging or other imaging examinations, according to RECIST.9
2.5. Exploratory biomarker study
2.5.1. Programmed death‐ligand 1 expression
Tumor tissue specimens were collected during the screening period (or a preserved specimen was used), stained for PD‐L1, and assessed by a pathologist at the central laboratory designated by the sponsor. PD‐L1 positivity was defined as PD‐L1 expression (detected by the 28‐8 PharmDx assay; Dako, Glostrup, Denmark) in ≥1% of tumor cells (tumor proportion score ≥1%).
2.5.2. Human papilloma virus genotyping and MSI testing
Human papilloma virus genotype testing10 was offered to patients with uterine cervical cancer. MSI testing (MSI Analysis System; Promega, Madison, WI, USA) was offered to patients with uterine cervical or corpus cancer. For both tests, a tumor tissue specimen was collected during the screening period or a preserved specimen was used.
2.6. Safety
Adverse events were classified according to the Japanese translation of the NCI‐Common Terminology Criteria for Adverse Events (NCI‐CTCAE), version 4.0.
2.7. Statistical methods
A sample size of 18 for each cancer type was calculated to allow a ≥70% probability of the lower limit of the 80% CI of the expected ORR exceeding the threshold ORR. To allow for approximately 10% dropout after enrollment, the target sample size was set to 20 for each cancer type.
Based on previous studies in melanoma, non‐small cell lung cancer, and renal cell cancer,11, 12, 13, 14 the expected ORR and threshold ORR required to show nivolumab efficacy in uterine cervical cancer, uterine corpus cancer, and STS were set at 19.2% and 5%, respectively. With these expected and threshold ORRs, the probability of the lower limit of the 80% CI of the observed ORR exceeding the threshold ORR was calculated using the Clopper‐Pearson method.
Data obtained up to the cut‐off date (August 18, 2017) were analyzed. The FAS was used for the efficacy endpoints and consisted of patients who received ≥1 dose of nivolumab and were considered Good Clinical Practice (GCP) compliant. The SAS consisted of patients who received ≥1 dose of nivolumab.
3. RESULTS
3.1. Baseline characteristics
The FAS and SAS included 63 and 64 patients, respectively. Baseline characteristics and pathological classification are shown in Tables 1 and 2, respectively. Most patients with uterine cervical cancer had squamous cell carcinoma (14/20 patients); most patients with uterine corpus cancer had endometrial carcinoma (21/23 patients, of whom 15 patients had endometrioid adenocarcinoma; Table 2). The most common type of STS was liposarcoma (8/21 patients; Table 2). Median (range) duration of treatment was 5.4 (1.0‐13.9), 2.4 (0.4‐13.4), and 2.6 (0.5‐13.6) months in patients with cervical cancer, corpus cancer, and STS, respectively. Median (range) duration of follow up was 8.6 (1.4‐13.7), 6.8 (1.6‐13.2), and 10.2 (2.7‐13.4) months in patients with cervical cancer, corpus cancer, and STS, respectively, and 8.9 (1.4‐13.7) months in the FAS.
Table 1.
Baseline characteristics of patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma
| Characteristic |
Uterine cervical cancer N = 20 |
Uterine corpus cancer N = 23 |
Soft tissue sarcoma N = 21 |
|---|---|---|---|
| Median age, y (range) | 50 (32‐68) | 58 (33‐74) | 51 (36‐77) |
| Female, n (%) | 20 (100) | 23 (100) | 11 (52) |
| ECOG PS, n (%) | |||
| 0 | 15 (75) | 19 (83) | 17 (81) |
| 1 | 5 (25) | 4 (17) | 4 (19) |
| Disease status, n (%) | |||
| III | 1 (5) | 0 (0) | 0 (0) |
| IV | 7 (35) | 10 (43) | 5 (24) |
| Recurrent | 12 (60) | 13 (57) | 16 (76) |
| No. of organs with metastases, n (%) | |||
| <2 | 9 (45) | 4 (17) | 9 (43) |
| 2 | 7 (35) | 9 (39) | 5 (24) |
| ≥3 | 4 (20) | 10 (43) | 7 (33) |
| Prior radiotherapy, n (%) | 17 (85) | 4 (17) | 9 (43) |
| Prior drug therapy, n (%) | 20 (100) | 23 (100) | 21 (100) |
| Prior regimens of chemotherapy,a n (%) | |||
| 1 | 7 (35) | 9 (39) | NA |
| 2 | 5 (25) | 10 (43) | 12 (57) |
| ≥3 | 8 (40) | 4 (17) | 9 (43) |
NA, not applicable; PS, performance status.
Included molecular targeted therapy; did not include any neoadjuvant or adjuvant chemotherapy for the target disease that was followed by a confirmed recurrence ≥52 wks after the last dosing.
Table 2.
Pathological classification of patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma
| Pathological classification | n (%) |
|---|---|
| Uterine cervical cancer (N = 20) | |
| Squamous cell carcinoma | 14 (70) |
| Adenocarcinoma | 5 (25) |
| Adenosquamous carcinoma | 1 (5) |
| Uterine corpus cancer (N = 23) | |
| Endometrioid adenocarcinomaa | 15 (65) |
| Serous adenocarcinoma | 5 (22) |
| Carcinosarcoma | 2 (9) |
| Undifferentiated endometrial carcinoma | 1 (4) |
| Soft tissue sarcoma (N = 21) | |
| Liposarcomaa | 8 (38) |
| Leiomyosarcoma | 3 (14) |
| Myofibrosarcoma | 2 (10) |
| Undifferentiated pleomorphic sarcoma | 1 (5) |
| Angiosarcoma | 1 (5) |
| Unknownb | 6 (29) |
Grade 1/2: 10 patients; Grade 3: 5 patients.
Included dedifferentiated liposarcoma (2 patients), myxoid liposarcoma (2 patients), well‐differentiated liposarcoma (1 patient), myxoid/round cell liposarcoma (1 patient), liposarcoma (1 patient), and pleomorphic sarcoma (1 patient).
Included differentiated but lineage‐unspecified sarcomas (2 patients) and undifferentiated or unclassifiable sarcomas (4 patients).
3.2. Efficacy
The ORR was 25%, 23%, and 0% in patients with cervical cancer, corpus cancer, and STS, respectively (Table 3). The lower limit of the 80% CI of the ORR in patients with cervical or corpus cancer exceeded the threshold ORR (5%) (Table 3); thus, the primary endpoint was met in these cancer types, but not in STS. The ORR for cervical and corpus cancer also exceeded the expected ORR of 19.2%. No CR was observed. DCR was 75%, 68%, and 48% in patients with cervical cancer, corpus cancer, and STS, respectively (Table 3).
Table 3.
Response and survival rates of nivolumab‐treated patients by HPV, PD‐L1, and MSI status
| Efficacy parameter | Uterine cervical cancer | Uterine corpus cancer | Soft tissue sarcoma |
|---|---|---|---|
| Total, n | 20 | 22 | 21 |
| ORR, n | 5 | 5 | 0 |
| % (80% CI) | 25 (13‐41) | 23 (11‐38) | 0 (0‐10) |
| DCR, n | 15 | 15 | 10 |
| % (80% CI) | 75 (59‐87) | 68 (52‐81) | 48 (32‐64) |
| Median OS, mo (80% CI) | NE (NE‐NE) | 8.7 (7.1‐NE) | NE (10.8‐NE) |
| 6‐month OS, % (80% CI) | 84 (70‐92) | 73 (58‐83) | 86 (72‐93) |
| Median PFS, mo (80% CI) | 5.6 (2.8‐7.1) | 3.4 (2.0‐5.4) | 1.4 (1.4‐2.8) |
| Median DoR, mo (80% CI) | NE (3.0‐NE) | NE (NE‐NE) | NA |
| Subgroups | |||
| PD‐L1 ≥1%, n | 15 | 8 | 1 |
| ORR, n | 5 | 2 | 0 |
| % (80% CI) | 33 (17‐53) | 25 (7‐54) | 0 (0‐90) |
| 6‐month OS, % (80% CI) | 86 (69‐94) | 63 (37‐80) | 100 (100‐100) |
| Median PFS, mo (80% CI) | 5.5 (2.8‐7.1) | 3.5 (1.5‐5.9) | 5.6 (NE‐NE) |
| PD‐L1 <1%, n | 5 | 14 | 20 |
| ORR, n | 0 | 3 | 0 |
| % (80% CI) | 0 (0‐37) | 21 (8‐42) | 0 (0‐11) |
| 6‐month OS, % (80% CI) | 80 (45‐94) | 79 (60‐89) | 85 (71‐93) |
| Median PFS, mo (80% CI) | 6.2 (1.4‐7.1) | 3.3 (2.0‐9.1) | 1.4 (1.4‐2.8) |
| HPV positive,a n | 9 | NA | NA |
| ORR, n | 3 | NA | NA |
| % (80% CI) | 33 (13‐60) | NA | NA |
| 6‐month OS, % (80% CI) | 100 (100‐100) | NA | NA |
| Median PFS, mo (80% CI) | 7.1 (4.4‐NE) | NA | NA |
| HPV negative, n | 3 | NA | NA |
| ORR, n | 1 | NA | NA |
| % (80% CI) | 33 (3‐80) | NA | NA |
| 6‐month OS, % (80% CI) | 67 (23‐89) | NA | NA |
| Median PFS, mo (80% CI) | 6.2 (1.4‐NE) | NA | NA |
| MSI‐H, n | 0 | 2 | NA |
| ORR, % (80% CI) | NA | 100 (32‐100) | NA |
| 6‐month OS, % (80% CI) | NA | 100 (100‐100) | NA |
| Median PFS, mo (80% CI) | NA | NE (NE‐NE) | NA |
| MSS,b n | 8 | 6 | NA |
| ORR, % (80% CI) | 25 (7‐54) | 0 (0‐32) | NA |
| 6‐month OS, % (80% CI) | 88 (62‐96) | 83 (52‐95) | NA |
| Median PFS, mo (80% CI) | 5.9 (2.5‐7.1) | 2.2 (1.4‐4.0) | NA |
CI, confidence interval; DCR, disease control rate; DoR, duration of response; HPV, human papillomavirus; MSI‐H, high‐level microsatellite instability; MSI‐L, low‐level microsatellite instability; MSS, microsatellite stable; NA, not applicable; NE, not estimable; ORR, objective response rate; OS, overall survival; PD‐L1, programmed death‐ligand 1; PFS, progression‐free survival.
HPV16 and/or HPV18 positive.
No patient with cervical cancer or corpus cancer was classified as MSI‐L.
The 6‐month OS rate, median PFS, and Kaplan‐Meier curves for OS and PFS for the three cancer cohorts are shown in Table 3 and Figure 1. Median duration of response could not be estimated for the patients with cervical or corpus cancer who responded (Table 3).
Figure 1.
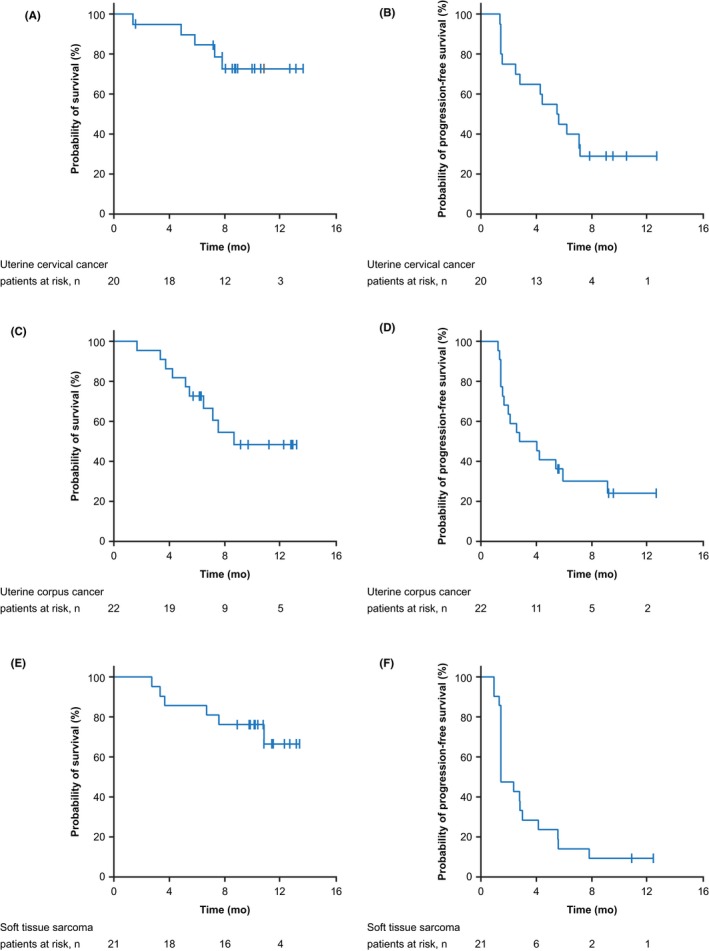
Overall survival (A, C, E) and progression‐free survival (B, D, F) in patients with uterine cervical cancer (A, B), uterine corpus cancer (C, D), and soft tissue sarcoma (E, F)
Tumor size was reduced with nivolumab treatment in more than half of patients with cervical cancer and in almost half of patients with corpus cancer (Figure 2A,C). Tumor size reduction was maintained in most patients with cervical or corpus cancer who responded (Figure 2B,D). Only a few patients with STS had reduced tumor size; however, a large proportion of patients with STS survived for several months with SD (Figure 2E,F). At the time of data cut‐off, 4/5 and 5/5 patients with cervical and corpus cancer, respectively, who responded to nivolumab had not yet progressed.
Figure 2.
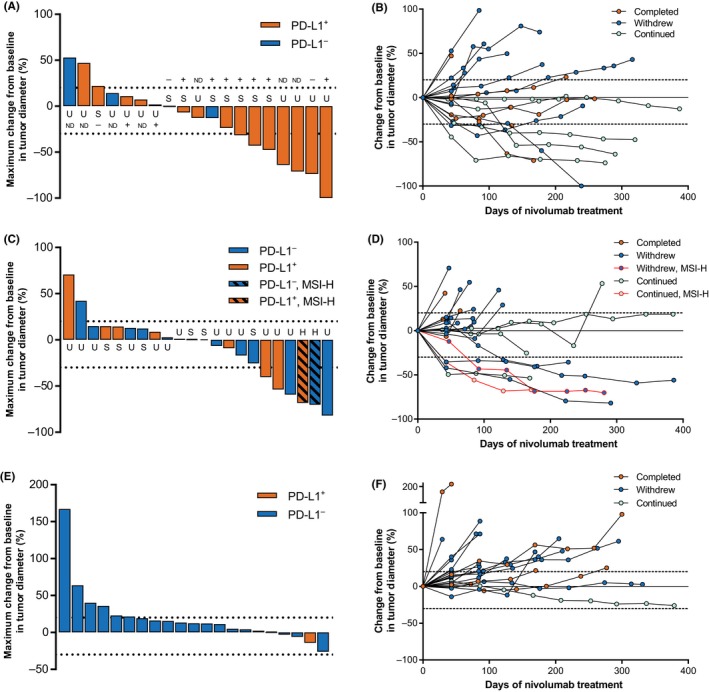
Maximum percentage change in tumor size at best response (A, C, E) and change in tumor size over time (B, D, F) in patients with uterine cervical cancer (A, B), uterine corpus cancer (C, D), and soft tissue sarcoma (E, F). Dotted lines indicate +20% and −30% change in tumor size. In the uterine cervical cancer cohort (A), “+” indicates human papilloma virus (HPV)‐positive tumor, “−” indicates HPV‐negative tumor, and ND indicates HPV status could not be determined. H, high‐level microsatellite instability; MSI‐H, high‐level microsatellite instability; ND, not determined; PD‐L1+, programmed death‐ligand 1‐positive; PD‐L1−, programmed death‐ligand 1‐negative; S, microsatellite stable; U, microsatellite instability status unknown
3.3. Subgroup analyses
3.3.1. Histopathology
Among patients with cervical cancer, 4/14 patients with squamous cell carcinoma and 1/6 patients with adenocarcinoma or adenosquamous carcinoma responded. Among patients with corpus cancer, 5/14 evaluable patients with endometrioid adenocarcinoma (grade 1/2: 3 patients; grade 3: 2 patients) responded; neither of the two patients with carcinosarcoma responded.
3.3.2. Programmed death‐ligand 1 status
The ORR in cervical cancer was higher in patients with PD‐L1‐positive tumors (33%) than in those with PD‐L1‐negative tumors (0%), despite similar PFS and OS (Table 3, Figure 3A,B). In contrast, the ORR in corpus cancer was similar regardless of PD‐L1 status (positive, 25% vs negative, 21%), as was PFS (Table 3, Figure 3D). Only one patient with STS (leiomyosarcoma) showed PD‐L1 expression ≥1% (Table 3, Figure 3E,F). Examples of PD‐L1 immunostaining in cervical cancer and corpus cancer are shown in Figure 4.
Figure 3.
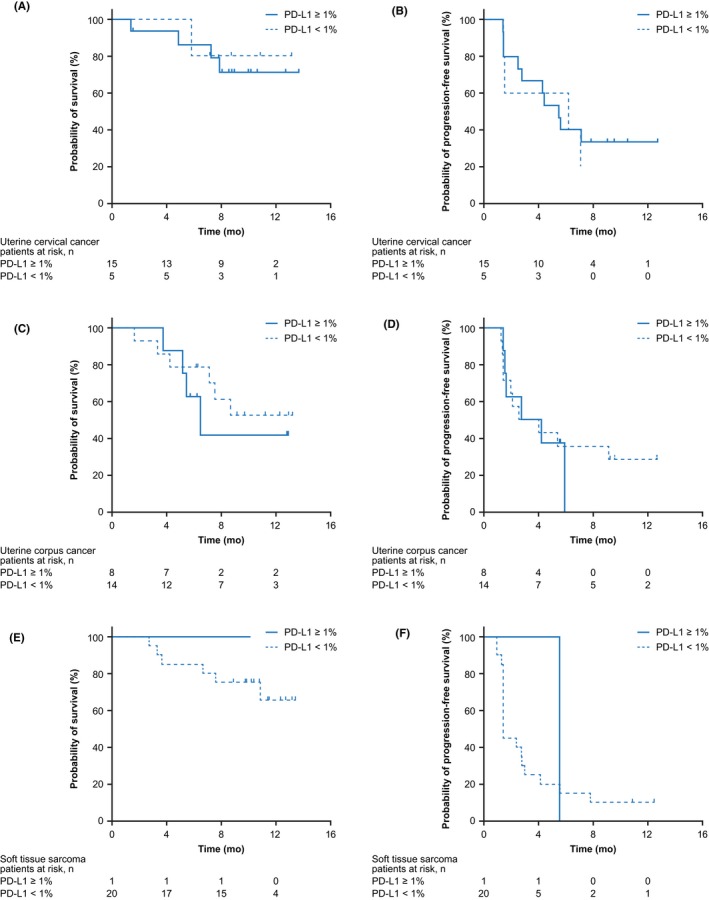
Overall survival (A, C, E) and progression‐free survival (B, D, F) in patients with uterine cervical cancer (A, B), uterine corpus cancer (C, D), and soft tissue sarcoma (E, F) by programmed death‐ligand 1 (PD‐L1) expression status
Figure 4.
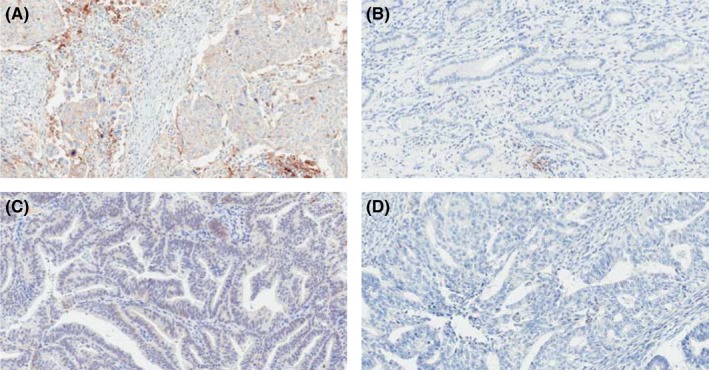
Programmed death‐ligand 1 (PD‐L1) immunostaining in uterine cervical cancer (A, B) and uterine corpus cancer (C, D). Tumor samples in panels A and C were classified as PD‐L1‐positive tumors; the tumor samples in panels B and D were classified as PD‐L1‐negative tumors
3.3.3. Human papilloma virus genotype status
The ORR and PFS were similar between patients with HPV‐positive and HPV‐negative cervical cancer (Table 3). Approximately half of patients with cervical cancer who had HPV‐positive and PD‐L1‐positive tumors (3/7 patients) or who had HPV‐negative and PD‐L1‐positive tumors (1/2 patients) responded to nivolumab treatment.
3.3.4. Microsatellite‐instability status
Microsatellite‐instability status was determined for eight patients with cervical cancer, all of whom were classified as microsatellite stable (MSS); the ORR in these eight patients was 25% (Table 3). MSI status was determined for eight patients with corpus cancer: the ORR was higher in patients classified as MSI‐high (2/2 patients, 100%) than in patients classified as MSS (0/6 patients, 0%) (Table 3).
3.4. Safety
The most common treatment‐related AEs were increased aspartate aminotransferase, hypothyroidism, and pruritus in the cervical cancer cohort, pruritus in the corpus cancer cohort, and rash and pruritus in the STS cohort (Table 4). There were few grade 3 treatment‐related AEs, including immune‐related AEs. There were no treatment‐related type 1 diabetes events. One sudden death, not immune‐related or related to nivolumab, occurred in a patient with cervical cancer.
Table 4.
Summary of treatment‐related adverse events reported in ≥2 patients in any cohort
| Treatment‐related AE, n (%) |
Uterine cervical cancer N = 20 |
Uterine corpus cancer N = 23 |
Soft tissue sarcoma N = 21 |
|||
|---|---|---|---|---|---|---|
| All grades | Grade 3‐4a | All grades | Grade 3‐4b | All grades | Grade 3‐4 | |
| Any treatment‐related AE | 13 (65) | 4 (20) | 14 (61) | 4 (17) | 9 (43) | 0 |
| Increased AST | 3 (15) | 0 | 2 (9) | 0 | 1 (5) | 0 |
| Hypothyroidism | 3 (15) | 0 | 2 (9) | 0 | 1 (5) | 0 |
| Pruritus | 3 (15) | 0 | 3 (13) | 0 | 2 (10) | 0 |
| Increased ALT | 2 (10) | 0 | 1 (4) | 0 | 1 (5) | 0 |
| Anemia | 2 (10) | 0 | 0 | 0 | 0 | 0 |
| Arthralgia | 2 (10) | 0 | 0 | 0 | 0 | 0 |
| Diarrhea | 2 (10) | 0 | 2 (9) | 0 | 1 (5) | 0 |
| Pyrexia | 2 (10) | 0 | 0 | 0 | 0 | 0 |
| Increased lipase | 2 (10) | 1 (5) | 2 (9) | 1 (4) | 0 | 0 |
| Malaise | 2 (10) | 0 | 2 (9) | 0 | 0 | 0 |
| Rash maculopapular | 2 (10) | 1 (5) | 1 (4) | 0 | 0 | 0 |
| Decreased appetite | 1 (5) | 0 | 2 (9) | 0 | 0 | 0 |
| Increased γ‐glutamyl transferase | 1 (5) | 1 (5) | 2 (9) | 0 | 1 (5) | 0 |
| Rash | 1 (5) | 0 | 0 | 0 | 2 (10) | 0 |
| Increased blood creatine phosphokinase | 0 | 0 | 2 (9) | 1 (4) | 0 | 0 |
| Fatigue | 0 | 0 | 2 (9) | 0 | 0 | 0 |
| Injection‐site reaction | 0 | 0 | 2 (9) | 0 | 0 | 0 |
| Proteinuria | 0 | 0 | 2 (9) | 0 | 1 (5) | 0 |
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Additional grade 3 treatment‐related AE (1 patient): spondylitis.
Additional grade 3 treatment‐related AE (1 patient each): adrenal insufficiency, drug‐induced liver injury.
4. DISCUSSION
This prospective study showed that nivolumab has acceptable toxicity, with evidence of clinical activity, in patients with uterine cervical or corpus cancer. However, based on ORR, our study did not show clinical activity of nivolumab in STS. Given that the observed ORRs in uterine cervical and corpus cancers exceeded the expected ORR of 19.2%, these results support proceeding to a larger and/or randomized phase 2 or 3 trial of nivolumab in these cancers.
In this study of nivolumab, antitumor activity was observed in patients with uterine cervical or corpus cancer. In patients with cervical cancer, the ORR was 25% (80% CI: 13%‐41%). In the published literature, ORRs of 26% have been reported in the ongoing phase 1/2 CheckMate 358 study of nivolumab in patients with virus‐associated cancers, including cervical cancer,15 17% in the phase 1b KEYNOTE‐028 study of pembrolizumab in patients with PD‐L1‐positive advanced cervical cancer,5 13% in the phase 2 KEYNOTE‐158 study of pembrolizumab in patients with advanced cervical cancer (16% in PD‐L1‐positive patients),6 4% in the phase 2 NRG‐GY002 study of nivolumab in patients with persistent/recurrent cervical cancer,16 and 3% in a phase 1/2 study examining ipilimumab (a humanized monoclonal antibody against CTLA‐4) in patients with recurrent HPV‐related cervical cancer.17 Median PFS in patients with cervical cancer was 5.6 months in the current study and 5.5 months in the CheckMate 358 study.15 In patients with corpus cancer, the ORR was 23% (80% CI: 11%‐38%). In the published literature, ORRs of 13% have been reported in the KEYNOTE‐028 study of pembrolizumab in patients with PD‐L1‐positive endometrial cancer7 and in a phase 1a study of atezolizumab in patients with endometrial cancer.8
Our subgroup analyses suggested that PD‐L1 expression and MSI status may be useful biomarkers for clinical response to nivolumab in uterine cervical and corpus cancers, respectively. In patients with cervical cancer, the ORR was higher in patients with PD‐L1‐positive tumors (33%) than in those with PD‐L1‐negative tumors (0%), whereas in patients with corpus cancer, patients with PD‐L1‐positive and PD‐L1‐negative tumors responded similarly (25% vs 21%). In a phase 1a study in advanced/recurrent endometrial cancer, the clinical benefit of atezolizumab appeared to increase with higher PD‐L1 levels.8 In contrast, both the CheckMate 358 study15 and the phase 1/2 ipilimumab study17 found no relationship between PD‐L1 expression and response rates. We note that there did not appear to be a correlation between PD‐L1 expression and the neutrophil‐to‐lymphocyte ratio in the current study (data not shown).
Microsatellite instability has frequently been observed in endometrial adenocarcinomas.18 In patients with corpus cancer in the current study, the ORR was higher in patients with MSI‐high tumors (100%) than in those with MSS tumors (0%), although it should be noted that only two patients were determined to be MSI‐high. In the KEYNOTE‐028 study, only 1/24 patients with PD‐L1‐positive endometrial cancer was classified as MSI‐high; this patient had a best response of PD.7 Microsatellite instability has also been detected in cervical squamous cell carcinoma;18 however, the potential role of MSI as a biomarker in patients with cervical cancer could not be examined in the current study because the eight patients for whom MSI status was determined were all classified as MSS. We note that the US FDA recently granted accelerated approval to pembrolizumab for the treatment of advanced solid tumors in patients with the MSI‐high biomarker and to nivolumab for the treatment of colorectal cancer that has progressed after standard chemotherapy in patients with the MSI‐high biomarker.19 These regulatory approvals mark the first approvals of a cancer treatment based on a common biomarker rather than the site of origin of the tumor.19
Given the small number of patients with uterine cervical cancer for whom HPV status could be determined (12 of 20 patients), it is difficult to evaluate the relationship between HPV status and response to nivolumab in the current study. Studies investigating the relationship between HPV status and response to nivolumab have had mixed results.20
None of the patients with STS in our study responded to nivolumab, consistent with a previous finding that nivolumab did not show efficacy in 12 patients with advanced uterine leiomyosarcoma.21 In contrast, a phase 2 study reported that 7/40 patients (18%) with STS responded to pembrolizumab.22 In that study, 4/10 patients with undifferentiated pleomorphic sarcoma responded (3 PR, 1 CR); two of the four responders expressed PD‐L1.22 In our study, only one patient with STS (leiomyosarcoma) had a PD‐L1‐positive tumor. Enrollment of larger numbers of patients with various subtypes of STS, in particular those with PD‐L1 expression, may show clinical activity of nivolumab in these cancers.
The safety profile of nivolumab in the present study was favorable and consistent with that observed in previous studies.11, 12, 13, 14
Limitations of the current study included the small number of patients, especially for the subgroup/biomarker analyses, and the lack of a comparator treatment arm.
In conclusion, this study shows that nivolumab has acceptable toxicity in the three cohorts studied, with evidence of clinical activity, based on ORR, in patients with uterine cervical or corpus cancer, but not in patients with STS. This study provides supportive evidence for a larger and/or randomized phase 2 or 3 study of nivolumab in the treatment of uterine cervical and corpus cancer. Confirmation of predictive markers such as PD‐L1 or MSI‐high, as well as assessment of the efficacy of nivolumab in combination with chemotherapy, would assist the development of effective immunotherapy of uterine cervical and corpus cancers.
CONFLICTS OF INTEREST
K. Tamura has received research funding from Ono Pharmaceutical, AstraZeneca, Chugai Pharmaceutical, Daiichi Sankyo, Eisai, Eli Lilly, Kyowa Pharmaceutical Inc., MSD, Novartis, and Pfizer. K. Hasegawa has received honoraria from Chugai Pharmaceutical. N. Katsumata has no relationships to disclose. K. Matsumoto has received research funding from Ono Pharmaceutical, MSD, and Novartis and honoraria from Chugai Pharmaceutical and Kyowa Hakko Kirin. H. Mukai has received honoraria from AstraZeneca, Pfizer, and Taiho Pharmaceutical. S. Takahashi has received research funding from Ono Pharmaceutical, AstraZeneca, Daiichi Sankyo, Eisai, IQVIA, MSD, Quintiles, and Taiho Pharmaceutical and honoraria from Bayer, Bristol‐Myers Squibb, Eisai, and Taiho Pharmaceutical. H. Nomura has no relationships to disclose. H. Minami has received institutional research funding from Ono Pharmaceutical, Astellas Pharma, Bristol‐Myers Squibb, Chugai Pharmaceutical, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Kyowa Hakko Kirin, Novartis, Sanofi, and Taiho Pharmaceutical and honoraria from Ono Pharmaceutical, Bayer, Bristol‐Myers Squibb, Celgene, Chugai Pharmaceutical, Daiichi Sankyo, Dainippon Sumitomo Pharma, Eisai, Eli Lilly, Janssen, Kowa Pharmaceutical, Kyowa Hakko Kirin, Merck Serono, Novartis, Otsuka Pharmaceutical, Pfizer, Sanofi, Shire, Taiho Pharmaceutical, and Takeda Pharmaceutical, and participated in consultancies for Ono Pharmaceutical and Merck Serono. This work was supported by Ono Pharmaceutical and Bristol‐Myers Squibb. The study sponsor was involved in study design, writing of the report, and in the decision to submit the article for publication. Study drug was provided by Ono Pharmaceutical. AC Medical Inc. and A2 Healthcare Corporation collected and analyzed the data in conjunction with the Ono Pharmaceutical Data Management and Statistics groups. All authors had full access to all of the data in the study and had final responsibility for the decision to submit for publication.
DATA AVAILABILITY STATEMENT
Qualified researchers may request Ono Pharmaceutical to disclose individual patient‐level data from clinical studies through the following website: Clinical Study Data Request.com. For more information on Ono Pharmaceutical's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.
ETHICAL APPROVAL
This study was approved by each study site's Institutional Review Board and conducted in accordance with the principles of the Declaration of Helsinki and GCP guidelines, and with local laws and regulations. The study was registered at the Japan Pharmaceutical Information Center (www.japic.org; JapicCTI‐163212). All patients provided written informed consent.
ACKNOWLEDGMENTS
The authors thank all study participants, investigators, and study teams, as well as Toshihiro Yoshikawa (Ono Pharmaceutical) for statistical support and Yusuke Takahashi (Ono Pharmaceutical) for reviewing the manuscript and providing constructive comments. Medical writing support was provided by Tomo Sawado, PhD, and Rebecca Lew, PhD, CMPP, of ProScribe – Envision Pharma Group and funded by Ono Pharmaceutical.
Tamura K, Hasegawa K, Katsumata N, et al. Efficacy and safety of nivolumab in Japanese patients with uterine cervical cancer, uterine corpus cancer, or soft tissue sarcoma: Multicenter, open‐label phase 2 trial. Cancer Sci. 2019;110:2894–2904. 10.1111/cas.14148
Funding information
This work was supported by Ono Pharmaceutical and Bristol‐Myers Squibb. The study sponsor was involved in study design, writing of the report, and in the decision to submit the article for publication.
REFERENCES
- 1. Guo L, Zhang H, Chen B. Nivolumab as programmed death‐1 (PD‐1) inhibitor for targeted immunotherapy in tumor. J Cancer. 2017;8:410‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Reddy OL, Shintaku PI, Moatamed NA. Programmed death‐ligand 1 (PD‐L1) is expressed in a significant number of the uterine cervical carcinomas. Diagn Pathol. 2017;12:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mo Z, Liu J, Zhang Q, et al. Expression of PD‐1, PD‐L1 and PD‐L2 is associated with differentiation status and histological type of endometrial cancer. Oncol Lett. 2016;12:944‐950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim C, Kim EK, Jung H, et al. Prognostic implications of PD‐L1 expression in patients with soft tissue sarcoma. BMC Cancer. 2016;16:434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Frenel JS, Le Tourneau C, O'Neil B, et al. Safety and efficacy of pembrolizumab in advanced, programmed death ligand 1‐positive cervical cancer: results from the phase Ib KEYNOTE‐028 trial. J Clin Oncol. 2017;35:4035‐4041. [DOI] [PubMed] [Google Scholar]
- 6. Chung HC, Schellens JHM, Delord J‐P, Perets R, Italiano A, Shapira‐Frommer R. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase 2 KEYNOTE‐158 study. J Clin Oncol. 2018;36(Suppl 15):Abstr 5522. [Google Scholar]
- 7. Ott PA, Bang YJ, Berton‐Rigaud D, et al. Safety and antitumor activity of pembrolizumab in advanced programmed death ligand 1‐positive endometrial cancer: results from the KEYNOTE‐028 study. J Clin Oncol. 2017;35:2535‐2541. [DOI] [PubMed] [Google Scholar]
- 8. Fleming GF, Emens LA, Eder JP, Hamilton EP, Liu JF, Liu B. Clinical activity, safety and biomarker results from a phase Ia study of atezolizumab (atezo) in advanced/recurrent endometrial cancer (rEC). J Clin Oncol. 2017;35(Suppl 15):Abstr 5585. [Google Scholar]
- 9. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228‐247. [DOI] [PubMed] [Google Scholar]
- 10. Nishiwaki M, Yamamoto T, Tone S, et al. Genotyping of human papillomaviruses by a novel one‐step typing method with multiplex PCR and clinical applications. J Clin Microbiol. 2008;46:1161‐1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Weber JS, D'Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti‐CTLA‐4 treatment (CheckMate 037): a randomised, controlled, open‐label, phase 3 trial. Lancet Oncol. 2015;16:375‐384. [DOI] [PubMed] [Google Scholar]
- 12. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous‐cell non‐small‐cell lung cancer. N Engl J Med. 2015;373:123‐135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Borghaei H, Paz‐Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non‐small‐cell lung cancer. N Engl J Med. 2015;373:1627‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal‐cell carcinoma. N Engl J Med. 2015;373:1803‐1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hollebecque A, Meyer T, Moore KN, Machiels J‐PH, De Greve J, López‐Picazo JM. An open‐label, multicohort, phase I/II study of nivolumab in patients with virus‐associated tumors (CheckMate 358): efficacy and safety in recurrent or metastatic (R/M) cervical, vaginal, and vulvar cancers. J Clin Oncol. 2017;35(Suppl 15):Abstr 5504. [Google Scholar]
- 16. Santin A, Deng W, Frumovitz MM, et al. A phase II evaluation of nivolumab, a fully human antibody against PD‐1, in the treatment of persistent or recurrent cervical cancer. J Clin Oncol. 2018;36(Suppl 15):Abstr 5536. [Google Scholar]
- 17. Lheureux S, Butler MO, Clarke B, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus‐related cervical carcinoma. JAMA Oncol. 2018;4:e173776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bonneville R, Krook MA, Kautto EA, et al. Landscape of microsatellite instability across 39 cancer types. JCO Precis Oncol. 2017 10.1200/po.17.00073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yan L, Zhang W. Precision medicine becomes reality‐tumor type‐agnostic therapy. Cancer Commun. 2018;38:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oliva M, Spreafico A, Taberna M, et al. Immune biomarkers of response to immune‐checkpoint inhibitors in head and neck squamous cell carcinoma. Ann Oncol. 2019;30:57‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ben‐Ami E, Barysauskas CM, Solomon S, et al. Immunotherapy with single agent nivolumab for advanced leiomyosarcoma of the uterus: results of a phase 2 study. Cancer. 2017;123:3285‐3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft‐tissue sarcoma and bone sarcoma (SARC028): a multicentre, two‐cohort, single‐arm, open‐label, phase 2 trial. Lancet Oncol. 2017;18:1493‐1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Qualified researchers may request Ono Pharmaceutical to disclose individual patient‐level data from clinical studies through the following website: Clinical Study Data Request.com. For more information on Ono Pharmaceutical's Policy for the Disclosure of Clinical Study Data, please see the following website: https://www.ono.co.jp/eng/rd/policy.html.


