Abstract
Previous studies have shown sex‐related differences in the incidence of adverse events following treatment with fluoropyrimidines, however the mechanism of this difference is unknown. We examined sex‐related differences in the safety of S‐1 plus oxaliplatin (SOX) and S‐1 plus cisplatin (CS) in 663 metastatic gastric cancer patients taking part in a phase III study. The incidences of leukopenia (odds ratio [OR] 1.9; P = .015), neutropenia (OR 2.2; P = .002), nausea (OR 2.0; P = .009), and vomiting (OR 2.8; P < .001) were increased in women versus men treated with SOX, while vomiting (OR 2.9; P < .001) and stomatitis (OR 1.8; P = .043) were increased in women versus men treated with CS. In contrast, male patients treated with CS experienced thrombocytopenia more often (OR 0.51; P = .009). The mean relative dose intensity of S‐1 in SOX was 75.4% in women and 81.4% in men (P = .032). No difference in efficacy was observed between women and men undergoing either regimen. Sex‐related differences in adverse reactions during SOX and CS treatment were confirmed in this phase III study. Further translational research studies are warranted to pursue the cause of this difference.
Keywords: fluorouracil, gastric cancer, oxaliplatin, S‐1, sex
1. INTRODUCTION
Gastric cancer is the 3rd leading cause of cancer‐related deaths worldwide.1 Fluoropyrimidines have been used as key drugs for patients with metastatic gastric cancer for more than half a century. It is known that female patients treated with fluoropyrimidines develop leukopenia, stomatitis, diarrhea, nausea, vomiting, and alopecia more often and more severely than male patients.2, 3, 4, 5, 6 Dose modification and the administration schedule of 5‐fluorouracil (5‐FU), a fluoropyrimidine, and optimum supportive therapies for female patients are not under current consideration and are not implemented in clinical practice, even though sex‐related differences in adverse reactions to fluoropyrimidines have been previously reported. Lower clearance of 5‐FU, could reduce the activity of dihydropyrimidine dehydrogenase (DPD), which is the initial enzyme in catabolism of 5‐FU.2, 7 Polymorphisms in DPD or thymidylate synthase5, 8 are thought to be possible causes of sex‐related differences in adverse events following fluoropyrimidine treatment, although the fundamental cause of this perceived difference is not yet known. Furthermore, a previous investigation into DPD expression and activity in the human liver did not reveal any sex‐related differences.5
Sex‐related differences in the toxicity of anticancer agents have not only been observed for 5‐FU treatment, but also for cisplatin and adriamycin, and in other anticancer agents.9 Female patients had significantly higher rates of vomiting and nausea, however the cause of this sex‐related difference is also unknown.10 Conversely, body mass index was inversely correlated with a decrease in platelet count following cisplatin and etoposide treatment, whereas there was no significant effect of sex on this related parameter.11
S‐1 is an oral combination preparation consisting of tegafur, a prodrug of 5‐FU, and the modulators gimeracil and oteracil potassium. Gimeracil prevents the degradation of 5‐FU by reversibly inhibiting DPD which is the primary metabolizing enzyme of fluorouracil, and oteracil potassium inhibits the activity of 5‐FU in the gastrointestinal tissue and decreases gastrointestinal toxicity.12 In patients with compromised renal function, gimeracil clearance is decreased, leading to high concentrations of 5‐FU in the blood and an increased risk of 5‐FU‐related side effects.13 We examined the incidence of diarrhea in metastatic colorectal cancer patients treated with S‐1 plus oxaliplatin (SOX) and plus bevacizumab, according to renal function in the previous SOFT trial.14 The incidence of grade 3, or higher, diarrhea among patients with a creatinine clearance rate (CCr) of <70 mL/min before treatment exceeded 20% and tended to be higher than the incidence among patients with a CCr of ≥70 mL/min. Another study, G‐SOX, compared treatment with SOX and treatment with S‐1 plus cisplatin (CS) and demonstrated comparable results for both treatments in progression‐free survival (PFS) and overall survival (OS).15 In this study, we aimed to evaluate and compare the safety and efficacy of the SOX and CS therapies in female and male patients with metastatic gastric cancer.
2. MATERIALS AND METHODS
2.1. Patients
The G‐SOX trial was a randomized, open‐label, phase III study that compared the efficacy and safety of the SOX and CS treatment regimens in patients with curatively unresectable, advanced, or recurrent gastric cancer.15 In total, 685 randomized patients were studied and data collected from January 2010 until October 2011. The SOX regimen was confirmed to be non‐inferior to the CS regimen. In the SOX regimen, S‐1 was given orally for the 1st 2 wk of a 3‐wk cycle, and oxaliplatin was infused at 100 mg/m2 on day 1. In the CS regimen, S‐1 was given for the 1st 3 wk of a 5‐wk cycle, and cisplatin was administered at 60 mg/m2 on day 8. The CCr was estimated using the Cockcroft‐Gault equation. The proportion of female patients treated with SOX and CS was 24.0% (81/338) and 27.5% (92/335) in the safety analysis set (SAF), and 24.3% (81/333) and 27.3% (90/330) in the full analysis set (FAS), respectively.
2.2. Statistical analysis
Median OS and PFS were estimated using the Kaplan‐Meier method. Differences in therapeutic efficacy between SOX and CS were tested using the log‐rank test. Statistical significance was considered to be at a value of P < .05. The Cox proportional hazards model was used to estimate hazard ratios (HRs) of SOX compared with CS with 95% confidence intervals (CIs). Efficacy was analyzed in the FAS, which included patients who met the main inclusion criteria and none of the exclusion criteria in the SAF.
The incidence of adverse events in female or male patients during the 1st treatment cycle was compared between the two regimens using Fisher's exact test and logistic regression. Multivariate analyses for toxicities were also carried out using a logistic regression model. Adverse events were assessed in accordance with the Common Terminology Criteria for Adverse Events version 3.0. Furthermore, treatment delivery was evaluated for both women and men in both treatment groups. Statistical analyses were performed using SAS version 9.3 software (SAS Institute).
3. RESULTS
Baseline characteristics of all patients enrolled in the G‐SOX study were comparable between the two sexes and treatment groups. The numbers of histologically undifferentiated type gastric cancer were higher in women than in men (Table 1).
Table 1.
Baseline characteristics in male and female patients
| SOX | CS | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male (n = 257) | Female (n = 81) | P a | Male (n = 243) | Female (n = 92) | P a | |||||
| n | % | n | % | n | % | n | % | |||
| Age | ||||||||||
| <65 | 111 | 43.2 | 42 | 51.9 | .201 | 109 | 44.9 | 53 | 57.6 | .038 |
| ≥65 | 146 | 56.8 | 39 | 48.1 | 134 | 55.1 | 39 | 42.4 | ||
| ECOG performance status | ||||||||||
| 0 | 183 | 71.2 | 56 | 63.5 | .414 | 177 | 72.8 | 59 | 64.1 | .109 |
| 1 | 72 | 28.0 | 23 | 28.4 | 62 | 25.5 | 33 | 35.9 | ||
| 2 | 2 | 0.8 | 2 | 2.5 | 4 | 1.6 | 0 | 0 | ||
| Unresectable | 207 | 80.5 | 71 | 87.7 | .182 | 199 | 81.9 | 78 | 84.8 | .628 |
| Recurrent | 50 | 19.5 | 10 | 12.3 | 44 | 18.1 | 14 | 15.2 | ||
| Adjuvant chemotherapy (+) | 24 | 9.3 | 7 | 8.6 | 20 | 8.2 | 9 | 9.8 | ||
| Adjuvant chemotherapy (‐) | 26 | 10.1 | 3 | 3.7 | 24 | 9.9 | 5 | 5.4 | ||
| Tumor histology | ||||||||||
| Differentiated type | 125 | 48.6 | 29 | 35.8 | .055 | 119 | 49.0 | 29 | 31.5 | .005 |
| Undifferentiated type | 132 | 51.4 | 52 | 64.2 | 124 | 51.0 | 63 | 68.5 | ||
| Primary tumor | ||||||||||
| ‐ | 66 | 25.7 | 13 | 16.0 | .097 | 62 | 25.5 | 17 | 18.5 | .196 |
| + | 191 | 74.3 | 68 | 84.0 | 181 | 74.5 | 75 | 81.5 | ||
| No. of metastatic sites | ||||||||||
| 1 | 87 | 33.9 | 22 | 27.2 | .377 | 78 | 32.1 | 26 | 28.3 | .455 |
| 2 | 105 | 40.9 | 33 | 40.7 | 107 | 44.0 | 36 | 39.1 | ||
| ≥3 | 59 | 23.0 | 24 | 29.6 | 57 | 23.5 | 27 | 29.3 | ||
| Metastatic site b | ||||||||||
| Liver | 104 | 40.5 | 22 | 27.2 | 101 | 41.6 | 30 | 32.6 | ||
| Lung | 32 | 12.5 | 5 | 6.2 | 27 | 11.1 | 8 | 8.7 | ||
| Lymph node | 225 | 87.5 | 74 | 91.4 | 214 | 88.1 | 79 | 85.9 | ||
| Peritoneum | 45 | 17.5 | 21 | 25.9 | 44 | 18.1 | 21 | 22.8 | ||
Abbreviations: CS, cisplatin plus S‐1; ECOG, Eastern Cooperative Oncology Group; SOX, S‐1 plus oxaliplatin.
aFisher's exact test; comparing proportion of each characteristic.
bPatients can be included in more than one category.
3.1. Safety
Adverse events are listed in Table 2. The median CCrs of female patients in the SOX group and the CS group were 72.9 mL/min (range, 36.5‐137.7 mL/min) and 76.8 mL/min (41.7‐211.0 mL/min), and those of male patients were 75.8 mL/min (33.7‐189.2 mL/min) and 79.4 mL/min (41.1‐151.2 mL/min), respectively. Female patients treated with SOX developed leukopenia, neutropenia, nausea, and vomiting significantly more frequently than male patients, while women treated with CS demonstrated vomiting and stomatitis in the 1st treatment cycle more often, regardless of renal function, as compared with men. In contrast, male patients undergoing CS therapy experienced thrombocytopenia in the 1st cycle more often compared with female patients. Sex was an independent predictive marker of those toxicities (Table 3). Thrombocytopenia following CS treatment was also observed more frequently in patients aged 70 years or older as measured by multivariate analysis.
Table 2.
Adverse events during the first cycle of treatment with S‐1 plus oxaliplatin or S‐1 plus cisplatin by creatinine clearance
| SOX | CS | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Female (n = 81) | Male (n = 257) | Fisher P a | OR, 95%CI | P | Female (n = 92) | Male (n = 243) | Fisher P a | OR, 95%CI | P | |||||
| n | % | n | % | n | % | n | % | |||||||
| Leukopenia | 33 | 41 | 68 | 27 | .018 | 1.9, 1.1‐3.2 | .015 | 46 | 50 | 121 | 50 | 1.0 | 1.0, 0.62‐1.6 | .97 |
| CCr 70 mL/min > | 16 | 46 | 29 | 30 | .098 | 2.0, 0.91‐4.4 | .086 | 23 | 64 | 50 | 60 | 0.84 | 1.2, 0.52‐2.6 | .71 |
| 70 mL/min≤ | 17 | 37 | 38 | 24 | .092 | 1.9, 0.92‐3.7 | .085 | 23 | 42 | 71 | 45 | 0.75 | 0.88, 0.47‐1.6 | .69 |
| Neutropenia | 42 | 52 | 84 | 33 | .0024 | 2.2, 1.3‐3.7 | .002 | 49 | 53 | 150 | 62 | 0.17 | 0.71, 0.44‐1.1 | .16 |
| CCr 70 mL/min> | 22 | 63 | 36 | 37 | .0098 | 2.9, 1.3‐6.5 | .009 | 25 | 69 | 58 | 70 | 1.0 | 0.98, 0.42‐2.3 | .96 |
| 70 mL/min≤ | 20 | 44 | 48 | 30 | .11 | 1.8, 0.90‐3.5 | .099 | 24 | 44 | 91 | 58 | 0.085 | 0.57, 0.31‐1.1 | .075 |
| Thrombocytopenia | 16 | 20 | 66 | 26 | .30 | 0.71, 0.39‐1.3 | .28 | 31 | 34 | 121 | 50 | 0.0097 | 0.51, 0.31‐0.85 | .009 |
| CCr 70 mL/min> | 5 | 14 | 26 | 27 | .17 | 0.46, 0.16‐1.3 | .15 | 17 | 47 | 46 | 55 | 0.43 | 0.72, 0.33‐1.6 | .41 |
| 70 mL/min≤ | 11 | 24 | 39 | 25 | 1.0 | 0.96, 0.45‐2.1 | .92 | 14 | 26 | 75 | 48 | 0.0044 | 0.38, 0.19‐0.75 | .005 |
| Nausea | 46 | 57 | 103 | 40 | .010 | 2.0, 1.2‐3.3 | .009 | 55 | 60 | 123 | 51 | 0.14 | 1.5, 0.89‐2.4 | .13 |
| CCr 70 mL/min> | 21 | 60 | 41 | 42 | .077 | 2.1, 0.95‐4.6 | .067 | 23 | 64 | 44 | 53 | 0.32 | 1.6, 0.70‐3.5 | .27 |
| 70 mL/min≤ | 25 | 54 | 61 | 39 | .064 | 1.9, 0.98‐3.7 | .059 | 32 | 58 | 78 | 49 | 0.28 | 1.4, 0.77‐2.7 | .26 |
| Vomiting | 30 | 37 | 45 | 18 | <.001 | 2.8, 1.6‐4.8 | <.001 | 38 | 41 | 48 | 20 | <0.001 | 2.9, 1.7‐4.8 | <.001 |
| CCr 70 mL/min> | 13 | 37 | 22 | 22 | .12 | 2.0, 0.89‐4.7 | .093 | 20 | 56 | 21 | 25 | 0.0029 | 3.7, 1.6‐8.4 | .002 |
| 70 mL/min≤ | 17 | 37 | 23 | 15 | .0014 | 3.4, 1.6‐7.2 | .001 | 18 | 33 | 27 | 17 | 0.021 | 2.4, 1.2‐4.7 | .016 |
| Diarrhea | 30 | 37 | 76 | 30 | .22 | 1.4, 0.83‐2.4 | .21 | 37 | 40 | 78 | 32 | 0.20 | 1.4, 0.87‐2.3 | .16 |
| CCr 70 mL/min> | 13 | 37 | 37 | 38 | 1.0 | 0.97, 0.44‐2.2 | .95 | 17 | 47 | 29 | 35 | 0.22 | 1.7, 0.75‐3.7 | .21 |
| 70 mL/min≤ | 17 | 37 | 39 | 25 | .13 | 1.8, 0.89‐3.6 | .10 | 20 | 36 | 48 | 30 | 0.41 | 1.3, 0.69‐2.5 | .41 |
| Stomatitis | 6 | 7 | 24 | 9 | .82 | 0.78, 0.31‐2.0 | .60 | 29 | 32 | 50 | 21 | 0.043 | 1.8, 1.0‐3.0 | .036 |
| CCr 70 mL/min> | 3 | 9 | 13 | 13 | .56 | 0.61, 0.16‐2.3 | .47 | 10 | 28 | 23 | 28 | 1.0 | 1.0, 0.42‐2.4 | .99 |
| 70 mL/min≤ | 3 | 7 | 11 | 7 | 1.0 | 0.93, 0.25‐3.5 | .92 | 19 | 35 | 26 | 17 | 0.0070 | 2.7, 1.3‐5.4 | .006 |
Abbreviations: 95%CI, 95% confidence interval; CCr, creatinine clearance rate; CS, cisplatin plus S‐1; OR, odds ratio; SOX, S‐1 plus oxaliplatin.
a Fisher's exact test; comparing frequency of adverse events.
Table 3.
Multivariate analyses for adverse events during the first cycle of treatment with S‐1 plus oxaliplatin and S‐1 plus cisplatin
| Leukopenia | Neutropenia | Thrombocytopenia | Nausea | Vomiting | Diarrhea | Stomatitis | |
|---|---|---|---|---|---|---|---|
| S‐1 plus oxaliplatin | |||||||
| Sex, female vs male | |||||||
| OR, 95%CI | 1.9, 1.1‐3.3 | 2.2, 1.3‐3.7 | 0.70, 0.39‐1.32 | 2.0, 1.2‐3.3 | 2.8, 1.6‐4.9 | 1.3, 0.78‐2.3 | 0.73, 0.28‐1.9 |
| P value | .015 | .0036 | .25 | .0096 | .0004 | .30 | .51 |
| CCr, 70 mL/min ≤ vs 70 mL/min > | |||||||
| OR, 95%CI | 0.78, 0.43‐1.4 | 0.55, 0.31‐0.97 | 0.82, 0.43‐1.5 | 0.94, 0.55‐1.6 | 0.68, 0.35‐1.3 | 0.56, 0.32‐1.0 | 0.52, 0.20‐1.3 |
| P value | .41 | .039 | .53 | .83 | .25 | .053 | .17 |
| BMI, median ≤ vs median > per gender | |||||||
| OR, 95%CI | 0.72, 0.43‐1.2 | 0.81, 0.50‐1.3 | 1.2, 0.67‐2.0 | 0.76, 0.48‐1.2 | 0.85, 0.48‐1.5 | 1.3, 0.78‐2.1 | 1.2, 0.55‐2.8 |
| P value | .20 | .40 | .61 | .26 | .59 | .33 | .60 |
| Age, 70 ≤ vs 70 > | |||||||
| OR, 95%CI | 0.91, 0.50‐1.6 | 0.63, 0.36‐1.1 | 0.64, 0.34‐1.2 | 1.1, 0.63‐1.9 | 0.77, 0.40‐1.5 | 0.95, 0.53‐1.7 | 1.0, 0.41‐2.6 |
| P value | .75 | .12 | .18 | .79 | .45 | .86 | .93 |
| PS, 1 or 2 vs 0 | |||||||
| OR, 95%CI | 0.90, 0.53‐1.5 | 0.60, 0.36‐1.0 | 0.92, 0.53‐1.6 | 0.92, 0.57‐1.5 | 1.8, 1.0‐3.1 | 1.1, 0.63‐1.8 | 2.0, 0.90‐4.3 |
| P value | .69 | .057 | .78 | .75 | .040 | .84 | .088 |
| Peritoneal dissemination, yes vs no | |||||||
| OR, 95%CI | 0.97, 0.53‐1.8 | 1.1, 0.62‐1.9 | 1.1, 0.58‐2.1 | 1.3, 0.74‐2.3 | 0.80, 0.40‐1.6 | 1.3, 0.75‐2.4 | 1.4, 0.56‐3.5 |
| P value | .92 | .75 | .78 | .36 | .52 | .32 | .47 |
| S‐1 plus cisplatin | |||||||
| Sex, female vs male | |||||||
| OR, 95%CI | 0.98, 0.60‐1.6 | 0.66, 0.40‐1.1 | 0.52, 0.31‐0.88 | 1.4, 0.86‐2.3 | 2.8, 1.7‐4.9 | 1.5, 0.87‐2.4 | 1.9, 1.1‐3.4 |
| P value | .94 | .11 | .014 | .17 | .0001 | .15 | .022 |
| CCr, 70 mL/min ≤ vs 70 mL/min > | |||||||
| OR, 95%CI | 0.59, 0.36‐0.99 | 0.60, 0.35‐1.0 | 0.78, 0.47‐1.3 | 0.74, 0.44‐1.2 | 0.55, 0.31‐0.98 | 0.91, 0.54‐1.6 | 0.94, 0.52‐1.7 |
| P value | .044 | .06 | .35 | .25 | .042 | .74 | .84 |
| BMI, median ≤ vs median > per gender | |||||||
| OR, 95%CI | 0.67, 0.42‐1.1 | 0.53, 0.33‐0.85 | 0.83, 0.52‐1.3 | 1.2, 0.76‐1.9 | 0.85, 0.49‐1.5 | 0.68, 0.42‐1.1 | 0.77, 0.44‐1.3 |
| P value | .086 | .0084 | .43 | .44 | .55 | .12 | .34 |
| Age, 70 ≤ vs 70 > | |||||||
| OR, 95%CI | 1.3, 0.77‐2.2 | 0.98, 0.57‐1.7 | 1.9, 1.1‐3.2 | 0.84, 0.51‐1.4 | 1.1, 0.59‐1.9 | 1.2, 0.72‐2.1 | 1.8, 1.0‐3.2 |
| P value | .33 | .94 | .016 | .51 | .85 | .46 | .045 |
| PS, 1 or 2 vs 0 | |||||||
| OR, 95%CI | 1.0, 0.60‐1.6 | 1.5, 0.87‐2.4 | 0.89, 0.54‐1.5 | 1.1, 0.67‐1.8 | 1.1, 0.60‐1.9 | 1.4, 0.84‐2.3 | 1.3, 0.74‐2.3 |
| P value | .91 | .15 | .64 | .71 | .86 | .20 | .36 |
| Peritoneal dissemination, yes vs no | |||||||
| OR, 95%CI | 1.6, 0.91‐2.8 | 0.93, 0.53‐1.7 | 1.0, 0.59‐1.8 | 1.5, 0.85‐2.6 | 1.2, 0.65‐2.3 | 0.76, 0.42‐1.4 | 0.82, 0.41‐1.6 |
| P value | .11 | .81 | .91 | .16 | .54 | .38 | .56 |
The median BMI of female patients treated with S‐1 plus oxaliplatin or S‐1 plus cisplatin were 20.4 kg/m2, and the BMI of males were 21.6 kg/m2.
Abbreviations: BMI, body mass index; CCr, creatinine clearance; CI, confidence interval; OR, odds ratio; PS performance status.
The incidences of nausea and vomiting were higher in women, even though aprepitant was not commonly administered to patients treated with SOX. Aprepitant was given to 9.3% of male patients and 11.1% of female patients in the SOX group, and 74.5% of male patients and 73.9% of female patients in the CS group. Despite aprepitant administration, all grades of vomiting were still observed. Although the incidence of vomiting decreased to 22% (2/9) for women and 17% (4/24) for men, there was no difference in the incidence of vomiting between women and men (P = 1.0). The incidence of vomiting (39%; 28/72) in women was significantly higher than that in men (18%; 41/233) (P = .0003) in the SOX group when aprepitant was not given (Table 4). The mean relative dose intensities (RDIs) for S‐1 during the three cycles of SOX were significantly lower in women (75.4%) than that in men (81.4%) (P = .032), while the RDIs for S‐1 during the two cycles of CS were 84.0% in women and 79.6% in men (P = .081) (Table 5). The reasons for the dose reduction of oxaliplatin did not differ between female patients or male patients treated with SOX (Table 6).
Table 4.
The incidence of any grade of nausea and vomiting by S‐1 plus oxaliplatin (SOX) and S‐1 plus cisplatin (CS)
| Arm | Aprepitant (1st cycle) | Female | Male | Fisher | ||
|---|---|---|---|---|---|---|
| n | % | n | % | P | ||
| Nausea | ||||||
| SOX | No | 41 | 57 | 94 | 40 | .015 |
| Yes | 5 | 56 | 9 | 38 | .44 | |
| CS | No | 14 | 58 | 32 | 52 | .64 |
| Yes | 41 | 60 | 91 | 50 | .20 | |
| Vomiting | ||||||
| SOX | No | 28 | 39 | 41 | 18 | .0003 |
| Yes | 2 | 22 | 4 | 17 | 1.0 | |
| CS | No | 13 | 54 | 15 | 24 | .011 |
| Yes | 25 | 37 | 33 | 18 | .0038 | |
Table 5.
Total dose and relative dose intensity
| SOX | P | CS | P | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | ||||
| (n = 221) | (n = 73) | (n = 221) | (n = 81) | ||||
| S‐1 | |||||||
| RDI (%) | Median | 83.7 | 75.0 | .029a | 83.3 | 83.3 | .411a |
| [Range] | [17.0‐114.5] | [13.0‐100] | [2.4‐112.9] | [11.9‐101.7] | |||
| Mean, SD | 81.4, 20.6 | 75.4, 21.6 | .032b | 79.6, 22.7 | 84.0, 18.0 | .081b | |
| Oxaliplatin/cisplatin | |||||||
| RDI (%) | Median | 98.3 | 75.0 | .077a | 87.5 | 87.5 | .958a |
| [Range] | [0‐100] | [28.0‐100] | [0‐134.6] | [0‐102.9] | |||
| Mean, SD | 83.5, 20.0 | 79.1, 20.0 | .100b | 80.3, 30.2 | 82.1, 27.2 | .636b | |
SOX for 3 cycles, CS for 2 cycles.
Abbreviations: CS; S‐1 plus cisplatin, RDI, relative dose intensity; SD, standard deviation; SOX, S‐1 plus oxaliplatin.
Wilcoxon rank sum test.
t‐test.
Table 6.
Reasons for dose reduction of oxaliplatin in S‐1 plus oxaliplatin (SOX)
| Dose reduction of oxaliplatina | Male | Female | ||
|---|---|---|---|---|
| (n = 257) | (n = 81) | |||
| n | % | n | % | |
| Thrombocytopenia: ≥75 000/mm3 (≤Grade 1) is not met by day 29 | 40 | 15.6 | 12 | 14.8 |
| Thrombocytopenia: <25 000/mm3 (Grade 4) | 2 | 0.8 | 0 | 0 |
| Thrombocytopenia: platelet transfusion was performed | 1 | 0.4 | 0 | 0 |
| Neutropenia: <500/mm3 (Grade 4) | 1 | 0.4 | 1 | 1.2 |
| Febrile neutropenia: neutrophil count < 1000/mm3 and fever (axillary temperature) ≥ 38.0°C | 0 | 0 | 1 | 1.2 |
| Diarrhea: ≥Grade 3 | 10 | 3.9 | 4 | 4.9 |
| Stomatitis: ≥Grade 3 | 1 | 0.4 | 0 | 0 |
| Sensory neuropathy (Grade 2) | 33 | 12.8 | 7 | 8.6 |
| Investigator's judgment | 59 | 23.0 | 23 | 28.4 |
Patients can be included in more than one category.
3.2. Efficacy
In female patients, the median OS was 14.4 mo for SOX and 12.6 mo for CS (HR 0.812, 95% CI 0.577‐1.143; P = .233) (Figure 1), while the median PFS was 5.5 mo for SOX and 4.1 mo for CS (HR 0.877, 95% CI 0.621‐1.237; P = .454) (Figure 2). In the male patients, the median OS was 14.3 mo for SOX and 14.2 mo for CS (HR 0.976, 95% CI 0.800‐1.190; P = .808) (Figure 1), while median PFS was 5.4 mo for SOX and 5.5 mo for CS (HR 0.952, 95% CI 0.778‐1.165; P = .633) (Figure 2). The response rates were 49.4% (95% CI 38.1‐60.7) in female patients and 54.8% (95% CI 48.4‐61.0) in male patients for SOX (P = .443), and 46.7% (95% CI 36.1‐57.5) in female patients and 52.9% (95% CI 46.4‐59.4) in male patients for CS (P = .325). No significant differences in efficacy with regard to OS, PFS, and response rate were identified between the sexes in either treatment group.
Figure 1.
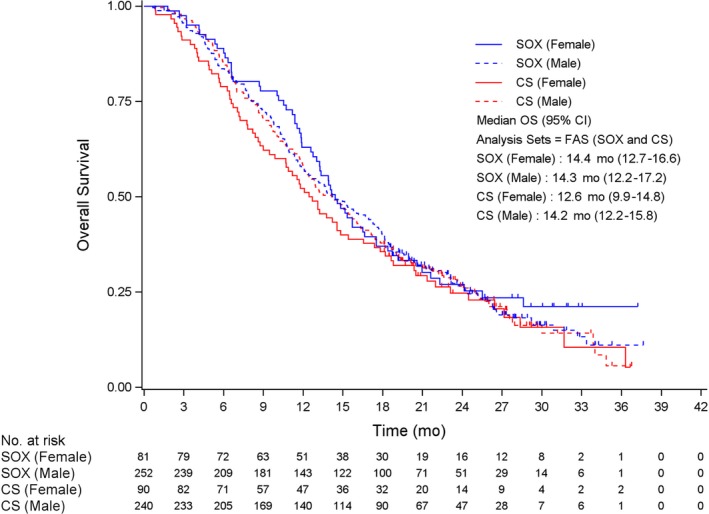
Overall survival (OS) according to sex and treatment arms. Checkmarks represent censored patients. CS, S‐1 plus cisplatin; FAS, full analysis set; SOX, S‐1 plus oxaliplatin.
Figure 2.
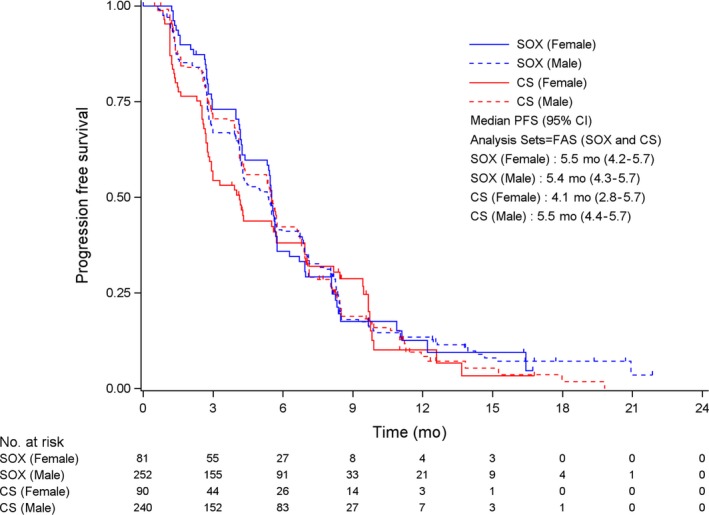
Progression‐free survival according to sex and treatment arms. Checkmarks represent censored patients. CS, S‐1 plus cisplatin; FAS, full analysis set; SOX, S‐1 plus oxaliplatin.
4. DISCUSSION
Leukopenia, neutropenia, nausea, and vomiting during the 1st cycle of SOX treatment, and vomiting and stomatitis during the 1st cycle of CS treatment were more frequently observed in female patients compared with male patients. In contrast, thrombocytopenia developed more often in male patients compared with female patients. However, no significant sex‐related difference was observed in the incidence of subjective adverse reactions such as diarrhea because patients could themselves temporarily stop oral S‐1 by assessing adequate self‐administration, in contrast with infused 5‐FU. There was also no difference in the incidence of leukopenia and neutropenia in patients treated with CS. The pharmacokinetics of 5‐FU after oral S‐1 administration could vary in patients because cisplatin can impair renal function and decrease 5‐FU clearance. This decrease is through reduced clearance of gimeracil, a DPD inhibitor, that leads to high concentrations of 5‐FU in the blood. Therefore, the effect of cisplatin treatment on sex‐related differences in adverse reactions induced by 5‐FU would be more variable compared with oxaliplatin treatment. Despite the observed differences between female patients and male patients of these toxicities, their cause was not clearly explained from this study on its own.
5‐Fluorouracil clearance is significantly lower in women than in men regardless of patient age and the given 5‐FU dose.2 Plasma samples from the 1st cycle of 391 female patients and 536 male patients treated with a 2400 mg/m2 continuous infusion of 5‐FU over 44‐48 h were tested for 5‐FU. These analyses indicated that, when comparing the proposed optimal area under the plasma drug concentration‐time curve (AUC) target range of 20‐30 mg h/L, women received supraoptimal doses compared with men (P = .0083).16 This higher plasma 5‐FU concentration was significantly related to severer neutropenia and stomatitis.17 More than 80% of a given dose of 5‐FU is rapidly catabolized to dihydrofluorouracil by DPD, the rate‐limiting enzyme of pyrimidine metabolism, and to inactive dihydrouracil.7 Toxicity from 5‐FU in women was found in a recent study to be independent of DPYD genotype. Female patients had a two‐fold higher risk for severe 5‐FU‐related toxicity compared with male patients and toxicity in women was independent of DPYD genotype because the OR for toxicity of 41.8 (95% CI, 9.2‐190, P < .0001) in men with DPYD polymorphism was much higher than the OR of 1.33 (95% CI, 0.34‐5.2, P = .68) in women. In addition, analysis from the same study of DPD expression and activity in the human liver did not reveal any sex‐related differences. Evidence for methylation of the DPYD promotor in the same DNA from the same human liver was not found.5 Pretherapeutic dihydrouracil concentration was significantly higher in female colorectal cancer patients compared with male patients, however the dihydrouracil/uracil ratio did not differ according to sex. A high level of uracil in females might therefore be associated with hematologic toxicity after 5‐FU‐based chemotherapy.
Another recent study has shown that global capecitabine toxicities were associated with rare, functional DPYD alleles 2846T>A (minor allele frequency, 0.6%) and *2A (IVS14 + 1G>A, 0.4%) (combined OR, 5.51; P = .0013), the common TYMS polymorphism 5′VNTR2R/3R (47%), and a 3′UTR 6‐bp Indel (31%) (combined OR, 1.31; P = 9.4 × 10−6).8 The higher incidence of 5‐FU‐related toxicities is difficult to explain by these rare DPYD variants. In total, 3‐5% of Caucasians have reduced DPD activity,18 however DPYD variants in the Japanese population are somewhat different from the previously reported Caucasian variants. DPYD alleles 2303C>A and 103G>T might play important roles in 5‐FU‐related toxicity for Japanese patients.19 The sensitivity of DPYD genetic testing depends on the number of variants investigated. By combining the DPYD variants c.1905 + 1G>A (also known as DPYD * 2A, IVS14 + 1G>A), c.2846A>T, c.1679T>G, c.1129‐5923C>G, the 20%‐30% rate of early‐onset 5‐fluorouracil toxicities can be explained.20, 21 Patients without a DPYD decreased/no function variant may still experience severe toxicity due to other genetic, environmental, or other factors.21
Tegafur is converted to 5‐FU mainly by CYP2A6, and polymorphisms in CYP2A6 are thought to be related to 5‐FU toxicity.22 In addition, previous studies have suggested that CYP2A6 activity is higher in women and could be induced by estradiol via ERα, although further studies are required to confirm this suggestion.23 Sex differences are well known in disease manifestations and treatment effects such as in autoimmune or cardiovascular diseases, and reaction to vaccines. Despite these insights, the X chromosome is scrutinized less often in the current era of population genetics analyses due to unique statistical challenges,24 although genome analyses of sex chromosomes could resolve some profound medical questions such as described here. The reason for the higher incidence of thrombocytopenia in male patients treated with CS therapy could not be clearly understood. Thrombocytopenia after CS was not correlated with body mass index, this result differed from that of a previous study on cisplatin‐based therapy.11
In our study, no statistically significant relationship between treatment effects and sex was observed, although the incidence of adverse events was higher in female patients who more commonly have undifferentiated type adenocarcinoma with a worse prognosis compared with the differentiated type. Intensive antiemetic therapy with aprepitant should be considered because of the higher incidence of nausea and vomiting in SOX and vomiting in CS. It is difficult to reduce the starting dose of SOX for female patients due to their higher incidence of adverse events compared with male patients and because severe toxicities were rarely induced by SOX with 100 mg/m2 of oxaliplatin.15 Conversely, the oxaliplatin dose should rather be increased to the recommended dose of 130 mg/m2, as proposed by the phase I/II trial on SOX,25 along with full supportive antiemetic therapy for male patients. In conclusion, incidences of sex‐related differences in adverse reactions during treatment with SOX and CS were confirmed in the G‐SOX study. Further fundamental research studies are warranted to pursue the underlying cause.
DISCLOSURE
Yasuhide Yamada received remuneration from Janssen Pharmaceutical, and honoraria from Chugai and Taiho Pharmaceutical. Kazuhiko Nishikawa received honoraria from Chugai, Taiho Pharmaceutical and Yakult Honsha, and research grants from Taiho and Yakult Honsha. Nozomu Fuse received an honorarium from Taiho Pharmaceutical and research funding from Taiho Pharmaceutical and Yakult Honsha. Naotoshi Sugimoto received research funding from Yakult Honsha and Taiho Pharmaceutical. Kenji Amagai received research funding from Taiho Pharmaceutical. Akihito Tsuji received honoraria from Yakult Honsha and Taiho Pharmaceutical. Kensei Yamaguchi received an honorarium from Chugai and research grants from Chugai and Yakult Honsha. Shuichi Hironaka received honoraria from Yakult Honsha, Daiichi‐Sankyo and Taiho Pharmaceutical. Ichinosuke Hyodo received advisory fees from Yakult Honsha and Daiichi‐Sankyo. The other authors have no conflicts of interest to declare.
ACKNOWLEDGMENTS
All authors were involved in the conception and/or design of the study and in drafting and revising the manuscript for publication. Yamada, Koizumi, Nishikawa, Gotoh, Fuse, Sugimoto, Nishina, Amagai, Chin, Niwa, Tsuji, Imamura, Tsuda, Yasui, Fujii, Yamaguchi, Yasui, Hironaka, Shimada were involved with data collection. Yamada and Hyodo were involved in analyzing data. All authors were involved in the interpretation of data and approved the final version of the manuscript. We wish to thank all the patients, clinicians, and support staff who participated in this study. We also thank Hiroki Kageyama, Terukazu Mitome, Seiji Takahashi, and Chikuma Hamada for their helpful advice. G‐SOX trial was supported by Yakult Honsha.
Yamada Y, Koizumi W, Nishikawa K, et al. Sex differences in the safety of S‐1 plus oxaliplatin and S‐1 plus cisplatin for patients with metastatic gastric cancer. Cancer Sci. 2019;110:2875‐2883. 10.1111/cas.14117
REFERENCES
- 1. Ferlay J, Soerjomataram I, Dickshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359‐E386. [DOI] [PubMed] [Google Scholar]
- 2. Milano G, Etienne MC, Cassuto‐Viguier E, et al. Influence of sex and age on fluorouracil clearance. J Clin Oncol. 1992;10:1171‐1175. [DOI] [PubMed] [Google Scholar]
- 3. Sloan JA, Goldberg RM, Sargent DJ, et al. Women experience greater toxicity with fluorouracil‐based chemotherapy for colorectal cancer. J Clin Oncol. 2002;15:1491‐1498. [DOI] [PubMed] [Google Scholar]
- 4. Pal SK, Hurria A. Impact of age, sex, and comorbidity on cancer therapy and disease progression. J Clin Oncol. 2010;10:4086‐4093. [DOI] [PubMed] [Google Scholar]
- 5. Schwab M, Zanger UM, Marx C, et al. Role of genetic and nongenetic factors for fluorouracil treatment‐related severe toxicity: a prospective clinical trial by the German 5‐fluorouracil Toxicity Study Group. J Clin Oncol. 2008;26:2131‐2138. [DOI] [PubMed] [Google Scholar]
- 6. Chansky K, Benedetti J, Macdonald JS. Differences in toxicity between men and women treated with 5‐fluorouracil therapy for colorectal carcinoma. Cancer. 2005;103:1165‐1171. [DOI] [PubMed] [Google Scholar]
- 7. Heggie GD, Sommadossi JP, Cross DS, et al. Clinical pharmacokinetics of 5‐fluorouracil and its metabolites in plasma, urine, and bile. Cancer Res. 1987;7:2203‐2206. [PubMed] [Google Scholar]
- 8. Rosmarin D, Palles C, Church D, et al. Genetic markers of toxicity from capecitabine and other fluorouracil‐based regimens: investigation in the QUASAR2 study, systematic review, and meta‐analysis. J Clin Oncol. 2014;32:1031‐1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Singh S, Parulekar W, Murray N, et al. Influence of sex on toxicity and treatment outcome in small‐cell lung cancer. J Clin Oncol. 2005;23:850‐856. [DOI] [PubMed] [Google Scholar]
- 10. Liaw CC, Wang CH, Chang HK, et al. Gender discrepancy observed between chemotherapy‐induced emesis and hiccups. Support Care Cancer. 2001;9:435‐441. [DOI] [PubMed] [Google Scholar]
- 11. Miya T, Goya T, Yanagida O, et al. The influence of relative body weight on toxicity of combination chemotherapy with cisplatin and etoposide. Cancer Chemother Pharmacol. 1998;42:386‐390. [DOI] [PubMed] [Google Scholar]
- 12. Shirasaka T, Nakano K, Takechi T, et al. Antitumor activity of 1 M tegafur‐0.4 M 5‐chloro‐2,4‐dihydroxypyridine‐1 M potassium oxonate (S‐1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res. 1996;56:2602‐2606. [PubMed] [Google Scholar]
- 13. Ikeda M, Furukawa H, Imamura H, et al. Pharmacokinetic study of S‐1, a novel oral fluorouracil antitumor agent in animal model and in patients with impaired renal function. Cancer Chemother Pharmacol. 2002;50:25‐32. [DOI] [PubMed] [Google Scholar]
- 14. Yamada Y, Takahari D, Matsumoto H, et al. Leucovorin, fluorouracil, and oxaliplatin plus bevacizumab versus S‐1 and oxaliplatin plus bevacizumab in patients with metastatic colorectal cancer (SOFT): an open‐label, non‐inferiority, randomised phase 3 trial. Lancet Oncol. 2013;14:1278‐1286. [DOI] [PubMed] [Google Scholar]
- 15. Yamada Y, Higuchi K, Nishikawa K, et al. Phase III study comparing oxaliplatin plus S‐1 with cisplatin plus S‐1 in chemotherapy‐naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141‐148. [DOI] [PubMed] [Google Scholar]
- 16. Kaldate RR, Haregewoin A, Grier CE, et al. Modeling the 5‐fluorouracil area under the curve versus dose relationship to develop a pharmacokinetic dosing algorithm for colorectal cancer patients receiving FOLFOX6. Oncologist. 2012;17:296‐302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schneider M, Etienne MC, Milano G, et al. Phase II trial of cisplatin, fluorouracil, and pure l folinic acid for locally advanced head and neck cancer: a pharmacokinetic and clinical survey. J Clin Oncol. 1995;13:1656‐1662. [DOI] [PubMed] [Google Scholar]
- 18. Froehlich TK, Amstutz U, Aebi S, et al. Clinical importance of risk variants in the dihydropyrimidine dehydrogenase gene for the prediction of early‐onset fluoropyrimidine toxicity. Int J Cancer. 2015;136:730‐739. [DOI] [PubMed] [Google Scholar]
- 19. Amstutz U, Henricks LM, Offer SM, et al. Clinical pharmacogenetics implementation consortium (CPIC) guideline for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing: 2017 update. Clin Pharmacol Ther. 2018;103:210‐216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Etienne MC, Lagrange JL, Dassonville O, et al. Population study of dihydropyrimidine dehydrogenase in cancer patients. J Clin Oncol. 1994;12:2248‐2253. [DOI] [PubMed] [Google Scholar]
- 21. Maekawa K, Saeki M, Saito Y, et al. Genetic vvvariations and haplotype structure of the DPYD gene encoding dihydropyrimidine dehydrogenase in Japanese and their ethnic differences. J Hum Genet. 2007;52:804‐819. [DOI] [PubMed] [Google Scholar]
- 22. Fujita K, Yamamoto W, Endo S, et al. CYP2A6 and the plasma level of 5‐chloro‐2, 4‐dihydroxypyridine are determinants of the pharmacokinetic variability of tegafur and 5‐fluorouracil, respectively, in Japanese patients with cancer given S‐1. Cancer Sci. 2008;99:1049‐1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Higashi E, Fukami T, Itoh M, et al. Human CYP2A6 is induced by estrogen via estrogen receptor. Drug Metab Dispos. 2007;35:1935‐1941. [DOI] [PubMed] [Google Scholar]
- 24. Accounting for sex in the genome. Nat Med. 2017;23:1243. [DOI] [PubMed] [Google Scholar]
- 25. Yamada Y, Tahara M, Miya T, et al. Phase I/II study of oxaliplatin with oral S‐1 as first‐line therapy for patients with metastatic colorectal cancer. Br J Cancer. 2008;98:1034‐1038. [DOI] [PMC free article] [PubMed] [Google Scholar]


