Abstract
Epidermal growth factor receptor (EGFR)‐tyrosine kinase inhibitors (TKIs) are the standard of care for non‐small‐cell lung cancer (NSCLC) patients harboring EGFR mutations. However, almost all patients develop resistance after approximately 1 y of treatment, with >50% of cases due to the T790M secondary mutation of the EGFR gene. A large global Phase III study (AURA3) demonstrated that osimertinib significantly prolonged progression‐free survival (PFS) over platinum‐doublet chemotherapy in patients with T790M‐positive NSCLC who had progressed on previous EGFR‐TKI therapy. However, it is not clear whether efficacy or safety of osimertinib in Japanese patients is similar to the overall population. We report a pre‐planned subgroup analysis of pooled Phase II data from the AURA Extension and AURA2 trials to investigate the efficacy and safety of osimertinib in Japanese patients. This study included 81 Japanese patients. Patients were administered 80 mg osimertinib orally once daily until disease progression. The main endpoints were objective response rate (ORR), PFS, and safety. The ORR was 63.6% and median PFS was 13.8 mo. Overall survival rate at 36 mo was 54.0%. The most common all‐cause adverse events (AEs) were rash (grouped term; 65.4%), diarrhea (51.9%), paronychia (grouped term; 49.4%), and dry skin (grouped term; 39.5%). Most AEs were grade 1‐2. Five patients (6.2%) developed interstitial lung disease, resulting in two deaths (2.5%). Osimertinib demonstrated favorable ORR and PFS in Japanese patients, similar to the overall population. Additionally, osimertinib has good efficacy and a manageable safety profile in Japanese patients with NSCLC who had acquired resistance due to the T790M mutation.
Keywords: acquired resistance, EGFR mutation, non‐small‐cell lung cancer, osimertinib, tyrosine kinase inhibitor
Abbreviations
- AE
adverse event
- CI
confidence interval
- DCR
disease control rate
- DoR
duration of response
- EGFR
epidermal growth factor receptor
- EGFRm
epidermal growth factor receptor sensitizing/activating gene mutations
- ILD
interstitial lung disease
- NSCLC
non‐small‐cell lung cancer
- ORR
objective response rate
- OS
overall survival
- PFS
progression‐free survival
- PS
performance status
- TKI
tyrosine kinase inhibitor
- WHO
World Health Organization
1. INTRODUCTION
Lung cancer is a leading cause of cancer death in many developed countries, including Japan.1 Non‐small‐cell lung cancer accounts for 85%‐90% of lung cancers2 with approximately 70% of NSCLC patients diagnosed with advanced or metastatic disease that is not amenable to surgical resection.3 For these patients, treatment can be guided by the presence of driver gene mutations such as EGFR sensitizing/activating gene mutations (EGFRm), because EGFRm increases the sensitivity of NSCLC to EGFR‐TKIs. EGFRm are found in approximately 30% and 10% of East‐Asian and Western NSCLC patients, respectively.4, 5 The superiority of EGFR‐TKI therapy over platinum‐doublet chemotherapy for patients with EGFRm‐positive advanced NSCLC has been demonstrated in several Phase III studies in Japan as well as other countries.6, 7, 8, 9, 10, 11, 12 These studies showed that the median PFS was approximately 10 mo in the EGFR‐TKI group and approximately 6 mo in the standard platinum‐doublet chemotherapy group.
Despite good initial responses to EGFR‐TKIs, resistance inevitably develops within 1 y of treatment. The most prevalent cause of resistance to EGFR‐TKIs is the development of an EGFR T790M secondary mutation.13, 14
Osimertinib (AZD9291) is an irreversible EGFR‐TKI that is selective for EGFR‐TKI sensitizing (EGFRm) and EGFR T790M resistance mutations, while sparing wild‐type EGFR.15 Some studies have shown that osimertinib is effective in T790M‐positive NSCLC patients who had previously progressed on EGFR‐TKIs.16, 17, 18, 19 The Phase III AURA3 study showed osimertinib to have greater efficacy than combination platinum‐based chemotherapy in an international cohort of T790M‐positive NSCLC patients with disease progression after EGFR‐TKI therapy.19 However, at present it is unclear whether osimertinib provides the same clinically meaningful response and survival rates in Japanese patients.
This report describes a pre‐planned subgroup analysis of Japanese patients from an analysis of pooled phase II data from the AURA Extension and AURA2 clinical trials.20 This analysis was performed to determine the efficacy and safety of osimertinib in a subgroup of Japanese patients with T790M‐positive NSCLC who had progressed on EGFR‐TKI treatment.
2. MATERIALS AND METHODS
2.1. Ethics
The study was carried out according to Good Clinical Practice; the laws and regulatory requirements of all participating countries; and the ethical principles originating in the Declaration of Helsinki. All patients provided written informed consent. The Institutional Review Board/Independent Ethics Committee at each site approved the study prior to commencement. The participating investigators and study sites are listed in Table S1. Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca's data‐sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
2.2. Trial design
Details on two multicenter, single‐arm, open‐label, Phase II clinical trials (AURA Extension and AURA2) of osimertinib (AstraZeneca) for the treatment of patients with T790M‐positive advanced NSCLC were previously reported.17, 18 The study designs of AURA Extension and AURA2, and the initial pooled analysis20 are illustrated in Figure S1. Briefly, both AURA Extension and AURA2 included patients with documented confirmation of radiological progression following either a single prior therapy with an EGFR‐TKI (2nd‐line cohort) or treatment with at least two prior lines of therapy, including at least one EGFR‐TKI (≥3rd‐line cohort). Patients in the ≥3rd‐line cohort of AURA2 were required to have received platinum‐based doublet chemotherapy as one of the prior lines of therapy. In both studies, eligible patients received 80 mg osimertinib orally once daily and continued until Response Evaluation Criteria in Solid Tumors 1.1 (RECIST1.1) defined progression or until a treatment discontinuation criterion was met.
2.3. Patients
This was a pre‐planned analysis required by the local health authority in Japan (Ministry of Health, Labor and Welfare, Japan) for submission to the Japan Common Technical Document. The analysis included Japanese patients who were enrolled in the AURA Extension and AURA2 studies.17, 18 Inclusion and exclusion criteria for this study have been previously described.20 In brief, patients in both studies were aged ≥ 18 y (≥20 y in Japan) with a histologically or cytologically confirmed diagnosis of NSCLC, documented evidence of EGFRm and a World Health Organization (WHO) PS of 0 or 1. EGFR T790M status was confirmed by a central laboratory using the cobas® EGFR Mutation Test (Roche Molecular Systems) from a biopsy taken after disease progression on the most recent treatment regimen. Patients with asymptomatic, stable central nervous system metastases that did not require corticosteroids for at least 4 wk before the 1st dose of study treatment, were eligible for enrollment.
2.4. Endpoints
The primary endpoint was the ORR evaluated according to RECIST v1.1 by a blinded independent central review committee, and the secondary efficacy endpoints were the DoR, DCR, PFS, and OS. Computerized tomography and magnetic resonance imaging were done at baseline and every 6 wk following the 1st osimertinib dose to measure the change in lesion size.
Safety was assessed in terms of AEs, deaths, clinical chemistry, hematology, and urinalysis, vital signs, physical examination, weight, electrocardiogram, and PS. Presence of ILD was also assessed and reported.
2.5. Statistical methods
The full analysis set consisted of all patients randomized in the study. The safety analysis set consisted of all enrolled patients that received at least one dose of osimertinib. The evaluable for response analysis set consisted of all patients in the full analysis set that had measurable disease at baseline, as assessed by the blinded independent central review committee. The ORR was defined as the percentage of patients with at least one response of complete response or partial response (as determined by the blinded independent central review committee) that was confirmed ≥4 wk later. All analyses were pre‐planned, except for the analysis of time to the onset of ILD, which was conducted post hoc. Statistical analyses were performed using SAS software version 9.2 (SAS Institute, Inc.).
3. RESULTS
3.1. Patients
The 1st and last patients of the general population were enrolled on 14 May 2014 and 21 October 2014, respectively, in AURA Extension and on 13 June 2014 and 27 October 2014, respectively, in AURA2. For this subgroup analysis of the Japanese population, the data cut‐off date for the pooled analysis was 1 November 2016 for the RECIST endpoint; a later long‐term data cut‐off date of 1 May 2018 was used for the OS and safety analysis. In total, 873 patients were enrolled (401 in AURA Extension and 472 in AURA2) and 411 patients were assigned treatment (201 in AURA Extension and 210 in AURA2). This subgroup analysis included 81 Japanese patients from centers in Japan who were treated with osimertinib (35 in AURA Extension and 46 in AURA2). These patients were included in the full analysis set. Seventy‐seven participants were included in the evaluable analysis response set. Four patients were excluded from the evaluable for response analysis set because they did not have measurable disease at baseline.
In the Japanese subgroup, the median age of patients was 66 y (range 35‐87 y); 33.3% were male and 66.7% were female (Table 1). Brain metastasis was detected in 32 patients (39.5%). Central cobas tissue testing prior to enrollment identified the T790M mutation in 80 patients (98.8%). Fifty‐six (69.1%) had an exon 19 deletion, 25 (30.9%) had L858R, and one (1.2%) had an exon 20 insertion. The last treatment before enrollment was an EGFR‐TKI in 61 patients (75.3%). The most recent prior EGFR‐TKI was used for ≥6 mo in 59 (72.8%) patients.
Table 1.
Patient disposition (full analysis set, n = 81)
| Characteristic | n = 81 |
|---|---|
| Age, y | |
| Mean (SD) | 65 (11.5) |
| Median (range) | 66 (35‐87) |
| Sex, n (%) | |
| Men | 27 (33.3) |
| Women | 54 (66.7) |
| Smoking status, n (%) | |
| Never | 57 (70.4) |
| Current/former | 24 (29.6) |
| WHO performance status, n (%) | |
| 0 | 29 (35.8) |
| 1 | 52 (64.2) |
| Histology, n (%) | |
| Adenocarcinomaa | 77 (95.1) |
| Squamous cell carcinoma | 1 (1.2) |
| Adenosquamous carcinoma | 1 (1.2) |
| Other | 2 (2.5) |
| EGFR mutation, n (%) | |
| T790M | 80 (98.8)b |
| Exon 19 deletion | 56 (69.1) |
| L858R | 25 (30.9) |
| Exon 20 insertion | 1 (1.2) |
| Overall disease classification, n (%) | |
| Metastatic | 78 (96.3) |
| Locally advanced | 3 (3.7) |
| Metastases, n (%) | |
| Brain | 32 (39.5) |
| Visceral | 37 (45.7) |
| Prior EGFR‐TKI treatment, n (%) | |
| Gefitinib | 65 (80.2) |
| Erlotinib | 41 (50.6) |
| Afatinib | 1 (1.2) |
| Prior platinum‐containing doublet chemotherapy, n (%) | 62 (76.5) |
Abbreviations: EGFR, epidermal growth factor receptor; SD, standard deviation; TKI, tyrosine kinase inhibitor; WHO, World Health Organization.
Includes acinar, papillary, bronchioloalveolar, solid with mucus formation, and not otherwise specified.
One Japanese patient in the AURA2 study failed the initial screening and the patient's data were included. The patient was rescreened and entered the study.
3.2. Efficacy
For RECIST endpoints, the data cut‐off date was 1 November 2016. The median duration of osimertinib treatment, including interruptions, was 15.2 mo (range: 0.0‐29.2 mo). The ORR in the Japanese population was 63.6% (95% confidence interval [CI] 51.9‐74.3) (Table 2). Of the 49 Japanese patients with an overall response, the median DoR was 15.2 mo (95% CI 9.7‐not calculable) (Figure 1A). The proportion of patients whose response was maintained at 12 mo was 61.4% (95% CI 45.7‐73.7).
Table 2.
Tumor responses (evaluable response analysis set, n = 77)
| Total (n = 77) | Second‐line (n = 11) | Third‐line or later (n = 66) | |
|---|---|---|---|
| Objective response rate, n (%) | 49 (63.6) | 9 (81.8) | 40 (60.6) |
| Best objective response, n (%) | |||
| Complete response | 1 (1.3) | 0 (0.0) | 1 (1.5) |
| Partial response | 48 (62.3) | 9 (81.8) | 39 (59.1) |
| Stable disease ≥ 6 wk | 23 (29.9) | 2 (18.2) | 21 (31.8) |
| Progression | 5 (6.5) | 0 (0.0) | 5 (7.6) |
| Time to onset of response (wk), mediana | 6.1 | 6.0 | 6.1 |
| Duration of response from onset (mo), median (95% CI)a | 15.2 (9.7 to NC) | NC (7.8 to NC) | 15.2 (11.1 to NC) |
| Patients remaining in response, n (%)a | |||
| >3 mo | 46 (93.9) | 8 (88.9) | 38 (95.0) |
| >6 mo | 38 (77.6) | 8 (88.9) | 30 (75.0) |
| >9 mo | 29 (59.2) | 6 (66.7) | 23 (57.5) |
| >12 mo | 25 (51.0) | 4 (44.4) | 21 (52.5) |
| Disease control rate, n (%) | 72 (93.5) | 11 (100.0) | 61 (92.4) |
| Best percent change from baseline in target lesion, median (range) | −48.40 (−100.0 to 20.0) | −52.90 (−100.0 to −17.0) | −47.10 (−100.0 to 20.0) |
Responses were assessed by blinded independent central review committee.
Abbreviations: CI, confidence interval; NC, not calculable.
Patients with an objective response (2nd‐line, n = 9; 3rd‐line or later, n = 40).
Figure 1.
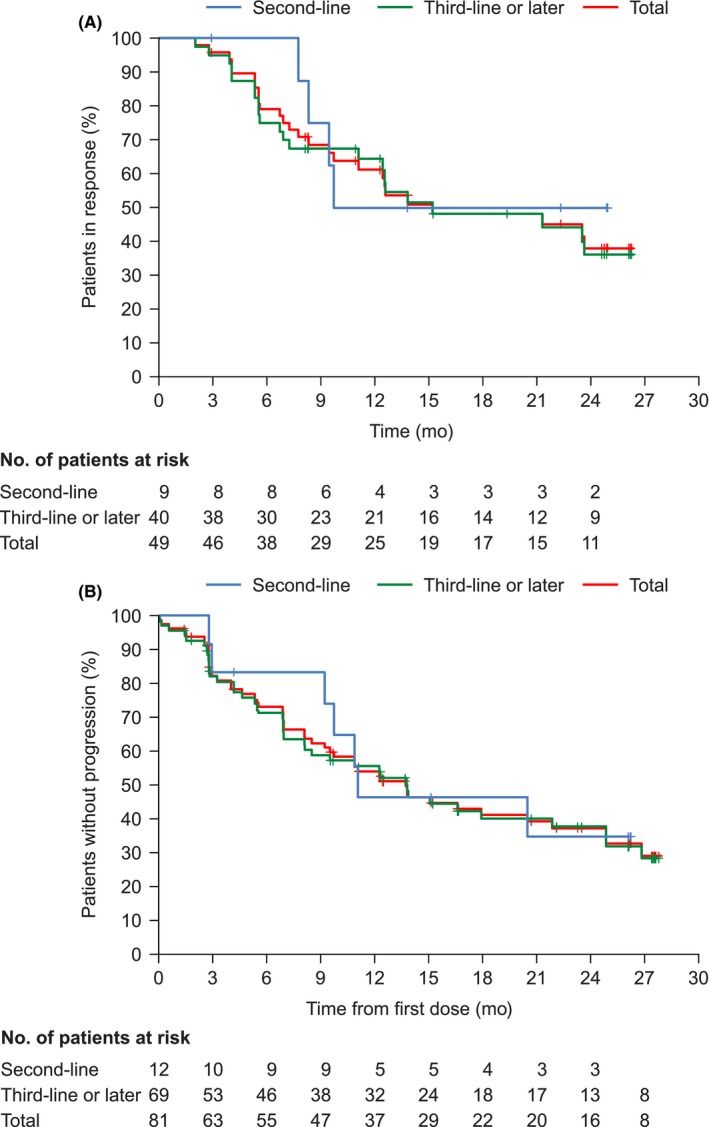
Kaplan‐Meier plots of the duration of response (A) and progression‐free survival (B). The duration of response was assessed in the evaluable response analysis set (n = 49). Progression‐free survival was assessed in the full analysis set (n = 81). Data cut‐off date: 1 November 2016
The DCR was 93.5% (95% CI 85.5‐97.9) (Table 2), and the median PFS was 13.8 mo (95% CI 9.2‐21.9) (Figure 1B). After 12 mo of treatment, 54.0% of patients were alive and progression‐free. Figure 2 shows the best percent change from baseline in the target lesion size according to the best objective response. The best percent change from baseline in the target lesion size was −48.4% (range −100.0 to 20.0) (Table 2).
Figure 2.
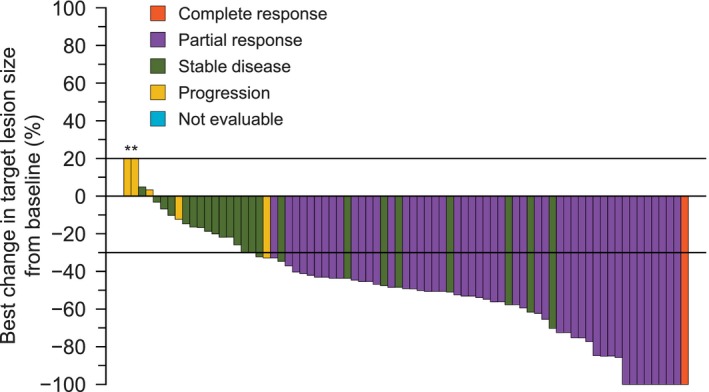
Waterfall plot of the best percentage change in target lesion size from baseline. Evaluable response analysis set (n = 77). *If the patient had died, had new lesions or progression of non‐target lesions, had withdrawn due to disease progression, and had no evaluable target lesion (before or at progression) assessments, the best change was imputed as 20%
For OS analysis, the data cut‐off date was 1 May 2018. The median duration of osimertinib treatment, including interruptions, was 15.2 mo (range: 0.0‐46.6 mo). At the data cut‐off, seven patients were continuing treatment and 74 patients had discontinued treatment for the following reasons: 49 because of objective disease progression, 11 because of an AE, one because of consent withdrawal, and 13 for other reasons. The other reasons for discontinued treatment were physician's decision to switch to other cancer therapy (n = 4), clinical progression (n = 3), death (n = 1), switched to another clinical trial (n = 1), next cancer therapy (n = 3) and investigator's decision (n = 1). The median OS was 37.5 mo (95% CI 24.21‐not calculable). The OS rates at 12, 24, and 36 mo were 86.1% (95% CI 76.35‐92.07), 62.1% (95% CI 50.23%‐71.95), and 54.0% (95% CI 42.08‐64.41), respectively (Figure 3A). The OS rates were similar between 2nd‐ and 3rd‐line or later treatment cohorts: at 12 mo, 83.3% (95% CI 48.17‐95.55) and 86.7% (95% CI 75.90‐92.82), at 24 mo, 66.7% (95% CI 33.70‐85.97) and 61.3% (95% CI 48.23‐71.97), and at 36 mo, 50.0% (95% CI 20.85‐73.61) and 54.7% (95% CI 41.72‐66.01), respectively (Figure 3B).
Figure 3.
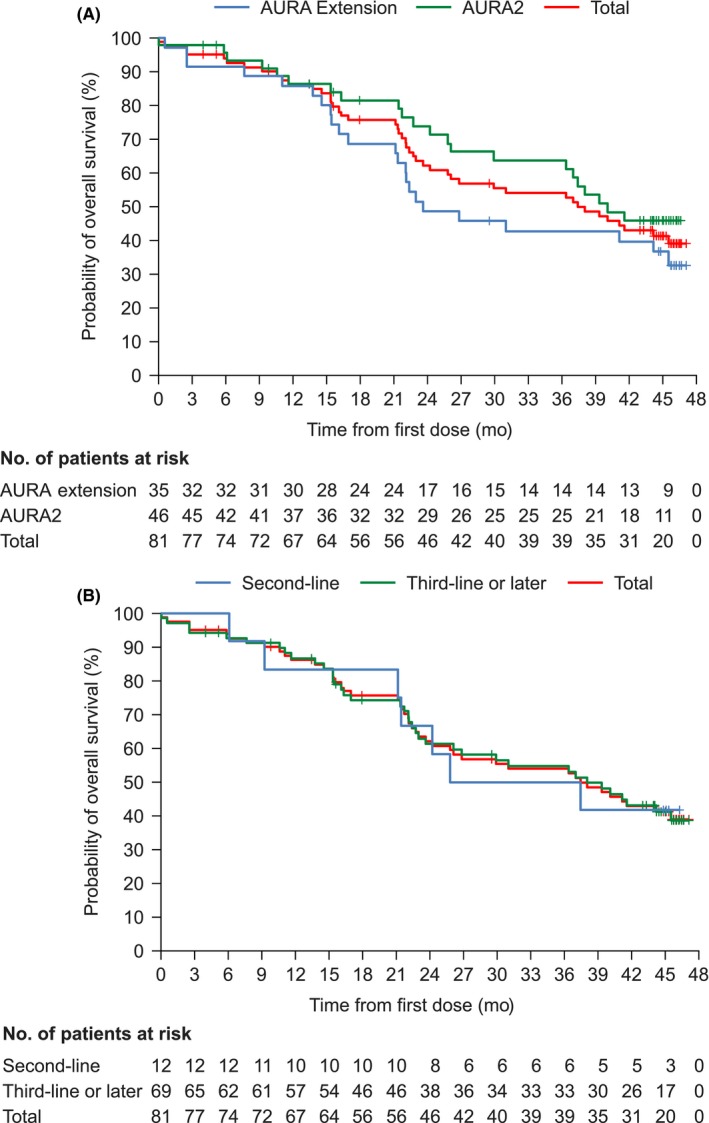
Kaplan‐Meier plots of the probability of overall survival by AURA Extension and AURA2 cohorts (A) and by line of treatment (B). Full analysis set (n = 81). Data cut‐off date: 1 May 2018
3.3. Safety
For the safety analysis, the data cut‐off date was 1 May 2018. A summary of the AEs in the patient population is given in Table 3. Most patients experienced at least one AE (98.8%), and a grade ≥ 3 AE was experienced by 51.9% of patients. A serious AE was reported by 35.8% of patients, and an AE led to death in 4.9% of the patient population. AEs leading to discontinuations that also led to death, occurred in three of the 11 patients. All‐cause AEs in ≥10% of patients and all‐cause selected AEs are shown in Table 4. The most common all‐cause AEs were rash (grouped term; 65.4%), diarrhea (51.9%), paronychia (grouped term; 49.4%), dry skin (grouped term; 39.5%), white blood cell count decreased (32.1%), and stomatitis (30.9%) (Table 4). The most common all‐cause grade ≥ 3 AEs were white blood cell count decreased (8.6%) and neutrophil count decreased (7.4%), followed by anemia (4.9%), rash (grouped term; 3.7%), and alanine aminotransferase increased (3.7%). All‐cause selected AEs were electrocardiogram QT prolonged (7.4%), ILD (grouped term; 6.2%), and hyperglycemia (1.2%). Of these, three cases of ILD (grouped term; 3.7%) and one case of electrocardiogram QT prolonged (1.2%) were grade ≥ 3 AEs. Possibly related AEs and possibly related grade ≥ 3 AEs occurred in 96.3% and 35.8% of patients, respectively. The most common possibly related AEs are shown in Table S2.
Table 3.
Adverse events
| Number of patients, n (%) (n = 81) | |
|---|---|
| Any AE | 80 (98.8) |
| Any grade ≥ 3 AE | 42 (51.9) |
| Any AE leading to death | 4 (4.9) |
| Any AE leading to dose reduction | 8 (9.9) |
| Any AE leading to treatment discontinuation | 11 (13.6) |
| Any serious AE | 29 (35.8) |
| Any possibly related AE | 78 (96.3) |
| Any possibly related grade ≥ 3 AE | 29 (35.8) |
| Any possibly related AE leading to treatment discontinuation | 7 (8.6) |
| Any possibly related serious AE | 16 (19.8) |
AEs were assessed by the investigator.
Cut‐off date used was 1 May 2018.
Abbreviation: AE, adverse event.
Table 4.
All‐cause AEs in ≥10% of patients and all‐cause selected AEs
| Number of patients, n (%) (N = 81) | ||||
|---|---|---|---|---|
| Total | Grade 1 | Grade 2 | Grade ≥ 3 | |
| All‐cause AEs in ≥10% of patients | ||||
| Rash (grouped term) | 53 (65.4) | 33 (40.7) | 17 (21.0) | 3 (3.7) |
| Diarrhea | 42 (51.9) | 34 (42.0) | 6 (7.4) | 2 (2.5) |
| Paronychia (grouped term) | 40 (49.4) | 17 (21.0) | 23 (28.4) | 0 |
| Dry skin (grouped term) | 32 (39.5) | 24 (29.6) | 8 (9.9) | 0 |
| Stomatitis | 25 (30.9) | 18 (22.2) | 7 (8.6) | 0 |
| White blood cell count decreased | 26 (32.1) | 4 (4.9) | 15 (18.5) | 7 (8.6) |
| Platelet count decreased | 21 (25.9) | 18 (22.2) | 2 (2.5) | 1 (1.2) |
| Nasopharyngitis | 22 (27.2) | 10 (12.3) | 12 (14.8) | 0 |
| Nausea | 16 (19.8) | 13 (16.0) | 2 (2.5) | 1 (1.2) |
| Pyrexia | 16 (19.8) | 14 (17.3) | 2 (2.5) | 0 |
| Anemia | 14 (17.3) | 4 (4.9) | 6 (7.4) | 4 (4.9) |
| Constipation | 15 (18.5) | 10 (12.3) | 5 (6.2) | 0 |
| Neutrophil count decreased | 14 (17.3) | 2 (2.5) | 6 (7.4) | 6 (7.4) |
| Aspartate aminotransferase increased | 13 (16.0) | 9 (11.1) | 2 (2.5) | 2 (2.5) |
| Upper respiratory tract infection | 13 (16.0) | 2 (2.5) | 10 (12.3) | 1 (1.2) |
| Alanine aminotransferase increased | 12 (14.8) | 7 (8.6) | 2 (2.5) | 3 (3.7) |
| Vomiting | 12 (14.8) | 10 (12.3) | 1 (1.2) | 1 (1.2) |
| Decreased appetite | 10 (12.3) | 4 (4.9) | 4 (4.9) | 2 (2.5) |
| Fatigue | 9 (11.1) | 6 (7.4) | 2 (2.5) | 1 (1.2) |
| All‐cause selected AEs | ||||
| ILD (grouped term) | 5 (6.2) | 2 (2.5) | 0 | 3 (3.7) |
| Hyperglycemia | 1 (1.2) | 0 | 1 (1.2) | 0 |
| Electrocardiogram QT prolonged | 6 (7.4) | 4 (4.9) | 1 (1.2) | 1 (1.2) |
Adverse events were assessed by the investigator. In this pooled analysis, there were 2 (5.7%) patients who presented grade 5 AEs that occurred in the AURA Extension study. These corresponded to two cases of ILD.
Cut‐off date used was 1 May 2018.
Abbreviations: AEs, adverse events; ILD, interstitial lung disease.
Interstitial lung disease (by grouped term) developed in five patients (6.2%), and all of these discontinued the study treatment in accordance with the protocol for patients with confirmed ILD. In a post‐hoc analysis, the median time to the onset of ILD in the five patients was 79 d (range: 17‐230). Two patients who developed ILD died. One of these patients developed pneumonia on Day 229 after presenting with pyrexia, cough, and hypoxia. The study drug was discontinued, antibiotics were administered, and the diagnosis was changed to ILD. After discontinuing osimertinib, ILD progressed rapidly, and steroid pulse therapy was initiated. The patient died on Day 232. The 2nd Japanese patient that died was diagnosed with ILD on Day 47, after which the study drug was discontinued, and steroids, antibiotics, and antifungals were administered. The patient died 31 d after the last osimertinib dose. Regarding the three other patients with ILD, one was a female with grade 3 pneumonitis, which developed after 17 d on the study drug and was resolved after additional treatment (analgesics, antibiotics, beta‐lactamase inhibitors, and corticosteroids). One patient was a male with grade 1 ILD that was reported on Day 79, which was resolved with additional treatment (antibiotics). The last patient was a male with grade 1 ILD, which was reported on Day 85 and resolved after 17 d without additional treatment.
4. DISCUSSION
This was a subgroup pooled analysis of the AURA Extension and AURA2 clinical trials to determine the efficacy and safety of osimertinib for the treatment of Japanese patients with T790M‐positive NSCLC who had progressed on previous EGFR‐TKI treatment. Compared with the total population,20 Japanese patients treated with 80 mg osimertinib once daily achieved similar ORR and DCR, whereas the median PFS was slightly longer in the Japanese population and a larger proportion of the Japanese patients remained in response after 12 mo. The AEs induced by osimertinib in this subgroup were manageable. However, compared with the total population, ILD occurred in a greater proportion of Japanese patients. Previous studies have suggested that risk factors for ILD among Japanese patients treated with a 1st‐generation EGFR‐TKI, include: smoking history, poor PS, pre‐existing pulmonary fibrosis, and prior treatment with chemotherapy.21, 22 It is widely recognized that incidence of ILD is greater in Japan than elsewhere, but while there is no known mechanism for the higher level of ILD reporting in Japan, cultural and clinical practice differences may be contributing factors.23 Some studies have reported an incidence that is 13‐fold higher in Japan than in the US, but importantly, there are no major differences in the severity of ILD between Japan and the rest of the world.23, 24, 25, 26
Currently, the treatment options for patients who have progressed on EGFR‐TKIs are limited and several trials have been conducted to examine next‐step therapy for these patients. The response to osimertinib reported in this pooled analysis of Japanese patients in the AURA Extension and AURA2 clinical trials (ORR, 63.6%; median PFS, 13.8 mo) numerically exceed those reported in previous trials on other EGFR‐TKIs in NSCLC patients (ORR, 37%27 and 25.9%28 and median PFS, 6.627 and 7.0 mo28). Moreover, the response in the Japanese subgroup was favorable compared with that in the global population of this pooled analysis; the ORR was 66% (95% CI 61‐70) and the median PFS was 9.9 mo (95% CI 9.5‐12.3).20 Additionally, the median OS was 26.8 mo (95% CI 24.0‐29.1) in the global population20 compared with a median OS of 37.5 months (95% CI 24.21‐not calculable) in the Japanese subgroup. These subgroup results suggest durable efficacy of osimertinib for Japanese patients with NSCLC with acquired resistance to prior EGFR‐TKI therapy as reported in the global population.20
Although osimertinib showed a good efficacy profile, ILD occurred in a higher proportion of Japanese patients. However, the proportion of patients developing ILD in the Japanese subgroup is consistent with findings reported in previous studies of other anti‐cancer agents in Japanese lung cancer patients.23, 29 In addition, in the overall study populations of the AURA Extension and AURA2 studies, the median time to onset of ILD after initiation of osimertinib was significantly later (69 and 156 d, respectively) than after treatment with gefitinib and erlotinib (29 and 28 d, respectively).30, 31 Similarly, in this Japanese subgroup, the median time to onset was 79 d in the five patients that developed ILD.
In the Japanese subgroup, the most common all‐cause AEs were rash, diarrhea, paronychia, dry skin, stomatitis, and white blood cell count decreased, and most of the events were of low severity grade. The frequency of AEs leading to discontinuation was high in this study (13.6%), however, this is in part due to the protocol‐mandated discontinuation for ILD, regardless of severity. The most common all‐cause grade ≥ 3 AEs were white blood cell count decreased (seven patients [8.6%]), and neutrophil count decreased (six patients [7.4%]), followed by anemia (four patients [4.9%]). Possibly causally related grade ≥ 3 AEs were reported in 29 patients (35.8%) in the Japanese subgroup compared with 65 (16%) patients in the global population.20 In comparison with the AEs reported in previous studies of gefitinib in patients with NSCLC and EGFR mutation with those of the present subgroup analysis,32, 33 the AE profiles are similar for the two drugs.
The limitations of the AURA Extension and AURA2 study are that they were open‐label and uncontrolled; however, based on the appropriate statistical power for phase II trials, we consider that the proof of concept design of these studies was appropriate. Given that this was a subgroup analysis, the number of patients is relatively small and pooled from two different studies, meaning results should be interpreted with some caution.
This pooled analysis of two phase II trials suggests that osimertinib is associated with favorable ORR and PFS in Japanese patients with NSCLC who experienced disease progression on previous EGFR‐TKI therapy. Although ILD rates tended to be greater in Japanese patients than in the total population, the rates of other AEs were similar in both populations and most AEs were grade 1‐2.
In conclusion, this sub‐analysis of osimertinib 80 mg once daily in Japanese patients showed good efficacy and a manageable safety profile as previously shown in the global analysis. This demonstrates that osimertinib is an important treatment option for Japanese patients with NSCLC with acquired resistance to prior EGFR‐TKI therapy.
DISCLOSURE
These studies were designed under the responsibility of AstraZeneca. The studies were funded by AstraZeneca; osimertinib was provided by AstraZeneca; AstraZeneca contributed to the study design, the collection, analysis, and interpretation of data, the writing of the manuscript, and the decision to submit the manuscript for publication. All authors had full access to all of the data. Tomonori Hirashima declares receiving honoraria and research funding from Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim Co., Ltd., and MSD K.K.; research funding from Merck Serono Co., Ltd., and Taiho Pharmaceutical Co., Ltd., and honoraria from Kyowa Hakko Kirin Co., Ltd., Bristol‐Myers Squibb, Co., and Pfizer Inc. Miyako Satouchi declares receiving honoraria and research funding from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim, Co., Ltd., Eli Lilly Japan K.K., Bristol‐Myers Squibb Co., MSD K.K., Pfizer Inc., Taiho Pharmaceutical Co., Ltd., and Ono Pharmaceutical Co., Ltd., and research funding from Novartis Inc., and Astellas Pharma Inc. Toyoaki Hida declares receiving research funding from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., Boehringer Ingelheim Co., Ltd., Eli Lilly Japan K.K., Bristol‐Myers Squibb, MSD K.K., Pfizer Inc., Ono Pharmaceutical Co., Ltd., Novartis Inc., and Astellas Pharma Inc. Makoto Nishio declares receiving honoraria from Ono Pharmaceutical Co., Bristol‐Myers Squibb, Pfizer Inc., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Taiho Pharmaceutical Co., AstraZeneca K.K., Boehringer Ingelheim Co., Ltd., MSD K.K., Novartis Inc., Daiichi Sankyo and Merck Serono Co., Ltd., and research funding from MSD K.K., Novartis Inc., Ono Pharmaceutical Co., Ltd., Chugai Pharmaceutical Co., Ltd., Bristol‐Myers Squibb, Taiho Pharmaceutical Co., Eli Lilly Japan K.K., AstraZeneca K.K., Pfizer Inc., and Astellas Pharma Inc. Terufumi Kato declares receiving lecture fees and research funding from AstraZeneca, K.K. Hiroshi Sakai has no conflict of interest to disclose. Fumio Imamura declares receiving honoraria from AstraZeneca K.K., and Ono Pharmaceutical Co., Ltd., and research funding from Pfizer Inc., AstraZeneca K.K., Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Chugai Pharmaceutical Co., Ltd., Bristol‐Myers Squibb and MSD K.K. Katsuyuki Kiura declares receiving honoraria from Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Boehringer Ingelheim Co., Ltd., and Taiho Pharmaceutical Co., Ltd., and research funding from AstraZeneca K.K., Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Eli Lilly Japan K.K. Isamu Okamoto declares receiving honoraria and research funding from AstraZeneca K.K. Kazuo Kasahara declares receiving honoraria from AstraZeneca K.K., MSD K.K., Chugai Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., and Boehringer Ingelheim Co., Ltd., and research funding from AstraZeneca K.K Boehringer Ingelheim Co., Ltd., Ono Pharmaceutical Co., Ltd., Astellas Pharma Inc., and Eli Lilly Japan K.K. Hirohiko Uchida and Sarah L. Vowler declare employment and stock ownership from AstraZeneca K.K. Tetsuya Mitsudomi declares an advisory role for Astra Zeneca, Chugai Pharmaceutical Co., Ltd., and Boehringer Ingelheim Co., Ltd., honoraria from AstraZeneca K.K., Chugai Pharmaceutical Co., Ltd., and Boehringer Ingelheim Co., Ltd., and research funding from Chugai Pharmaceutical Co., Ltd., and Boehringer Ingelheim Co., Ltd.
Supporting information
ACKNOWLEDGMENTS
The authors wish to thank all of the study participants and the principal investigators (Table S1). The authors also wish to thank John Gibbins of Edanz Group for providing medical writing services, which were funded by AstraZeneca through EMC K.K. in accordance with Good Publication Practice (GPP3) guidelines (http://www.ismpp.org/gpp3).
Hirashima T, Satouchi M, Hida T, et al. Osimertinib for Japanese patients with T790M‐positive advanced non‐small‐cell lung cancer: A pooled subgroup analysis. Cancer Sci. 2019;110:2884‐2893. 10.1111/cas.14120
Clinical trial registration: NCT01802632 and NCT02094261 (ClinicalTrials.gov).
REFERENCES
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69‐90. [DOI] [PubMed] [Google Scholar]
- 2. Cataldo VD, Gibbons DL, Pérez‐Soler R, Quintás‐Cardama A. Treatment of non small cell lung cancer with erlotinib or gefitinib. N Engl J Med. 2011;364:947‐955. [DOI] [PubMed] [Google Scholar]
- 3. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non‐small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Russo A, Franchina T, Ricciardi GR, et al. A decade of EGFR inhibition in EGFR‐mutated non‐small cell lung cancer (NSCLC): old successes and future perspectives. Oncotarget. 2015;6:26814‐26825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prabhakar CN. Epidermal growth factor receptor in non‐small cell lung cancer. Transl Lung Cancer Res. 2015;4:110‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Inoue A, Kobayashi K, Maemondo M, et al. Updated overall survival results from a randomized phase III trial comparing gefitinib with carboplatin‐paclitaxel for chemo‐naïve non‐small cell lung cancer with sensitive EGFR gene mutations (NEJ002). Ann Oncol. 2013;24:54‐59. [DOI] [PubMed] [Google Scholar]
- 7. Fukuoka M, Wu YL, Thongprasert S, et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol. 2011;29:2866‐2874. [DOI] [PubMed] [Google Scholar]
- 8. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31:3327‐3334. [DOI] [PubMed] [Google Scholar]
- 9. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): an open‐label, randomised phase 3 trial. Lancet Oncol. 2014;15:213‐222. [DOI] [PubMed] [Google Scholar]
- 10. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): a multicentre, open‐label, randomised phase 3 trial. Lancet Oncol. 2012;13:239‐246. [DOI] [PubMed] [Google Scholar]
- 11. Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): a multicentre, open‐label, randomised, phase 3 study. Lancet Oncol. 2011;12:735‐742. [DOI] [PubMed] [Google Scholar]
- 12. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121‐128. [DOI] [PubMed] [Google Scholar]
- 13. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR‐TKI therapy in 155 patients with EGFR‐mutant lung cancers. Clin Cancer Res. 2013;19:2240‐2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ercan D, Choi HG, Yun CH, et al. EGFR mutations and resistance to irreversible pyrimidine‐based EGFR inhibitors. Clin Cancer Res. 2015;21:3913‐3923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M‐mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014;4:1046‐1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jänne PA, Yang JC, Kim DW, et al. AZD9291 in EGFR inhibitor‐resistant non‐small‐cell lung cancer. N Engl J Med. 2015;372:1689‐1699. [DOI] [PubMed] [Google Scholar]
- 17. Yang JC, Ahn MJ, Kim DW, et al. Osimertinib in pretreated T790M‐positive advanced non‐small‐cell lung cancer: AURA Study Phase II extension component. J Clin Oncol. 2017;35:1288‐1296. [DOI] [PubMed] [Google Scholar]
- 18. Goss G, Tsai CM, Shephard FA, et al. Osimertinib for pretreated EGFR Thr790Met‐positive advanced non‐small‐cell lung cancer (AURA2): a multicentre, open‐label, single‐arm, phase 2 study. Lancet Oncol. 2016;17:1643‐1652. [DOI] [PubMed] [Google Scholar]
- 19. Mok TS, Wu Y‐L, Ahn M‐J, et al. Osimertinib or platinum‐pemetrexed in EGFR T790M‐positive lung cancer. N Engl J Med. 2017;376:629‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ahn MJ, Tsai CM, Shepherd FA, et al. Osimertinib in patients with T790M mutation‐positive advanced non‐small cell lung cancer: long term follow‐up from a pooled analysis of 2 Phase 2 studies. Cancer. 2019;125:892‐901. [DOI] [PubMed] [Google Scholar]
- 21. Akamatsu H, Inoue A, Mitsudomi T, et al. Interstitial lung disease associated with gefitinib in Japanese patients with EGFR‐mutated non‐small cell lung cancer: combined analysis of two Phase III trials (NEJ 002 and WJTOG 3405). Jpn J Clin Oncol. 2013;43:664‐668. [DOI] [PubMed] [Google Scholar]
- 22. Hotta K, Kiura K, Takigawa N, et al. Comparison of the incidence and pattern of interstitial lung disease during erlotinib and gefitinib treatment in Japanese patients with non‐small cell lung cancer: the Okayama Lung Cancer Study Group experience. J Thorac Oncol. 2010;5:179‐184. [DOI] [PubMed] [Google Scholar]
- 23. Azuma A, Kudoh S. High prevalence of drug‐induced pneumonia in Japan. JMAJ. 2007;50:405‐411. [Google Scholar]
- 24. Raghu G, Nyberg F, Morgan G. The epidemiology of interstitial lung disease and its association with lung cancer. Br J Cancer. 2004;91:S3‐S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kudoh S, Takeda K, Nakagawa K, et al. Phase III study of docetaxel compared with vinorelbine in elderly patients with advanced non‐small cell lung cancer: results of the West Japan Thoracic Oncology Group trial (WJTOG 9904). J Clin Oncol. 2006;24:3657‐3663. [DOI] [PubMed] [Google Scholar]
- 26. Koo LC, Clark JA, Quesenberry CP, et al. National differences in reporting pneumonia and pneumonia interstitial: an analysis of the WHO International Drug Monitoring Database on 15 drugs in nine countries for seven pulmonary conditions. Pharmacoepidemiol Drug Saf. 2005;14:775‐787. [DOI] [PubMed] [Google Scholar]
- 27. Hattori Y, Satouchi M, Shimada T, et al. A phase 2 study of bevacizumab in combination with carboplatin and paclitaxel in patients with non‐squamous non‐small‐cell lung cancer harboring mutations of epidermal growth factor receptor (EGFR) after failing first‐line EGFR‐tyrosine kinase inhibitors (HANSHIN Oncology Group 0109). Lung Cancer. 2015;87:136‐140. [DOI] [PubMed] [Google Scholar]
- 28. Yoshimura N, Okishio K, Mitsuoka S, et al. Prospective assessment of continuation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of pemetrexed. J Thorac Oncol. 2013;8:96‐101. [DOI] [PubMed] [Google Scholar]
- 29. Camus P, Kudoh S, Ebina M. Interstitial lung disease associated with drug therapy. Br J Cancer. 2004;91(suppl 2):S18‐S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Beom SH, Kim DW, Sim SH, et al. Gefitinib‐induced interstitial lung disease in Korean Lung cancer patients. Cancer Res Treat. 2016;48:88‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gemma A, Kudoh S, Ando M, et al. Final safety and efficacy of erlotinib in the phase 4 POLARSTAR surveillance study of 10 708 Japanese patients with non‐small‐cell lung cancer. Cancer Sci. 2014;105:1584‐1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380‐2388. [DOI] [PubMed] [Google Scholar]
- 33. Ichihara E, Hotta K, Nogami N, et al. Phase II trial of gefitinib in combination with bevacizumab as first‐line therapy for advanced non‐small cell lung cancer with activating EGFR gene mutations: the Okayama Lung Cancer Study Group Trial 1001. J Thorac Oncol. 2015;10:486‐491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


