Abstract
Introduction
Loss of mobility is common in advanced dementia and has important negative consequences related to fall risk, loss of independence, and lack of participation in meaningful activities. The causes of decline are multifactorial, including disease-specific changes in motor function, behavior, and cognition. To optimize clinical management of mobility, there is a need to better characterize capacity for safe and independent mobility. This study aimed to identify key factors that impact on mobility in dementia.
Methods
Expert input was gathered using a modified Delphi consensus approach. The primary criterion for participation was specialist knowledge in mobility or dementia, either as a clinician or a researcher. Participants rated elements of mobility for importance and feasibility of assessment in advanced dementia and prioritized items for inclusion in a mobility staging tool. Descriptive statistics and qualitative content analysis were used to summarize responses.
Results
Thirty-six experts completed the first survey with an 80% retention rate over three rounds. One-third of 61 items reached consensus for being both important and feasible to assess, representing five categories of elements. Items reaching agreement for a staging tool included walking, parkinsonism, gait, impulsivity, fall history, agitation, transfers, and posture control.
Discussion
This study highlights the need for a multidimensional, dementia-specific approach to mobility assessment. Results have implications for development of assessment methods and management guidelines to support the clinical care of mobility impairment in people with dementia.
Keywords: Mobility, Cognitive impairment, Dementia, Assessment, Modified Delphi, Gait, Falls
1. Introduction
Gradual loss of safe and independent mobility is a common feature of the advanced stages of dementia that impacts everyday function, safety, caregiving, and quality of life [1]. Falls also become increasingly common as dementia progresses. Each year, 40%–60% of individuals with advanced dementia fall [1], with a significant impact on morbidity and mortality, health care costs, and caregiver distress. The causes of mobility decline in dementia are multifactorial, including neurodegenerative changes, cerebrovascular disease, and age-related musculoskeletal or sensory changes. Cognitive changes and the presence of behavioral symptoms associated with dementia can also contribute to mobility deficits in this population.
Mobility, defined as moving from one position to another [2], requires the capacity to sit, stand, transition from one posture to another, and walk. Impaired mobility is a key intrinsic risk factor for falls, and as such, mobility and falls are often considered together [3], [4]. However, unlike fall risk status, which often relates to clinical targets surrounding a person's safety (e.g., avoidance of injury, significant morbidity, or mortality), individuals' capacity for mobility reflects additional important outcomes such as independence and participation in meaningful activities. For people with dementia (PWD), targeting assessment and management of mobility impairment may help to limit the use of inappropriate, mobility-limiting interventions and prevent premature or excess disability related to inactivity [5], [6].
Studies of mobility in healthy older adults have identified multiple factors that impact mobility status [7], [8], [9]. For example, lower limb strength, balance, reaction time, vision, pain, cognitive function, and health status are contributors to Timed-Up-and-Go (TUG) performance in healthy older adults [7] and multiple physiological and psychological processes predict sit-to-stand performance [8]. Key factors that specifically impact mobility in PWD have been less studied. Owing to the coupling of mobility and fall risk, however, insight into factors important for determining fall risk may inform factors impacting mobility status. Studies of the determinants of fall risk in PWD have also revealed the need for a multidimensional approach that encompasses several “categories” of factors [10], with some factors shown to vary between cognitively healthy and impaired older adults [11].
In a recent scoping review, we identified a lack of assessment tools that provide an overall profile of mobility in PWD inclusive of important disease-specific factors, as well as a lack of tools feasible for use in people with advanced dementia [6]. For these individuals, all of whom are likely to be deemed “high risk” for falls, there is an important need to shift focus from falls risk to mobility status and consider outcomes beyond safety including autonomy, comfort, and symptom management [5], [12]. To address this need, we are working to develop a multidimensional mobility staging tool for PWD, which can be used for both research and clinical purposes. Clinically, the staging tool will help to monitor changes in mobility status and mark important transitions in function over time, allowing for an appropriate shift in clinical management.
The principal objective of this study is to identify important elements of mobility, feasible to assess in people with advanced dementia, as determined by clinical and academic experts, in order to inform development of the mobility staging tool.
2. Methods
2.1. Design
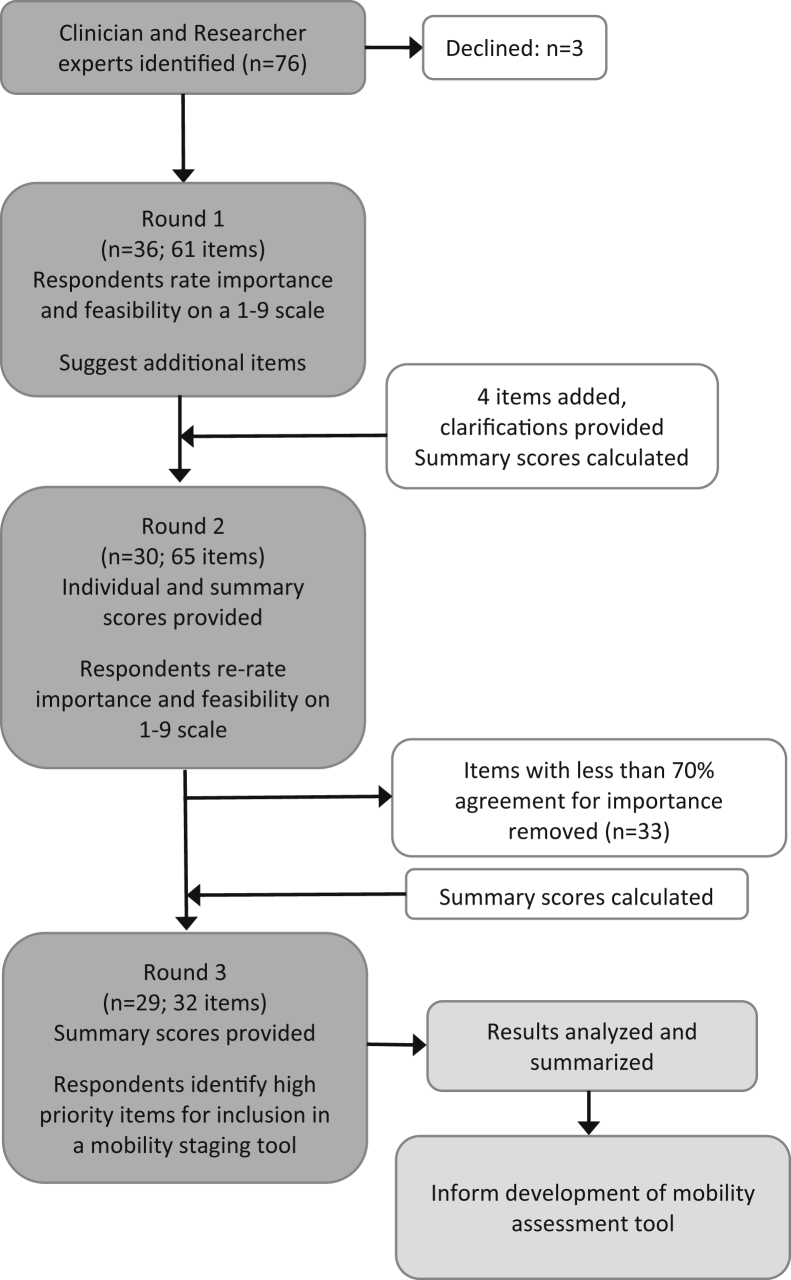
A cross-sectional, Web-based survey was conducted using a modified Delphi procedure with three rounds, between March and July 2018 (Fig. 1). The modified Delphi consensus technique allows for anonymous participation and is a widely used method to obtain unbiased expert consensus [13]. Four commonly accepted characteristics of the Delphi method were incorporated into the study including anonymity, iteration, controlled feedback, and statistical analyses of the group response [14].
Fig. 1.
Study flow chart.
2.2. Participant recruitment
A snowball, criterion-based sampling approach was used in this study. The primary criterion for participation was specialist knowledge in mobility and/or dementia, gained through experience or qualifications. Two categories of experts were recruited: clinicians and academic researchers.
To begin, a steering group was assembled from the Principal Investigator's academic network in Toronto, Canada. In addition to being participants in the study, the group helped to identify additional experts. A knowledge resource nomination worksheet was populated with experts identified through the steering group's professional networks, review of key literature, and internet searching. Individuals were contacted by email to participate and asked to nominate others for inclusion in the study. The invitation included the study's objective and rationale and details of the study format. Previous research suggests that group sizes of 10 to 40 participants are feasible for Delphi processes [15], [16]. Seventy-six experts were invited to participate, representing the fields of dementia care, movement science, fall prevention, geriatric medicine, geriatric psychiatry, and behavioral neurology.
2.3. Data collection and analysis
The study was approved by the University Health Network Research Ethics Board. Questionnaires were developed and managed using Droupal™ Webforms (www.droupal.org); a web-based application hosted by University Health Network on a secure server. For each round, participants were linked to the questionnaire via personalized emails and given approximately four weeks to respond. On initial login to the survey site, participants provided their informed consent. Descriptive statistics were used to provide central tendencies, frequencies, and ranges of responses. Qualitative content analysis, including development of a coding framework [17], was used to analyze free-text comments.
2.3.1. Round 1 questionnaire
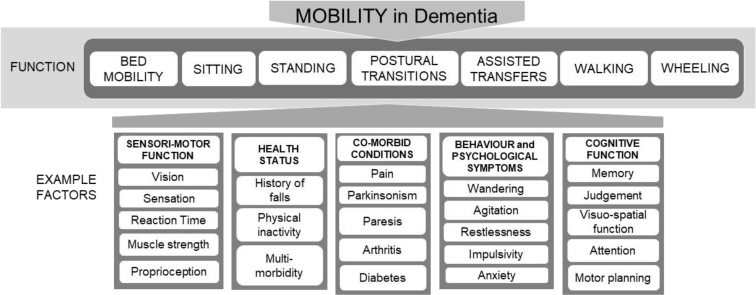
The study team developed the round 1 questionnaire based on a conceptual model of mobility in dementia informed by clinical expertise and published literature (Fig. 2). The questionnaire was organized into two sections: the first focused on functional mobility (e.g., sitting, standing, and walking) and the second targeted health status or intrinsic factors relevant to mobility impairment in dementia. Section 2 was categorized into five domains: sensorimotor function, health status, specific comorbid conditions, behavioral and psychological symptoms, and cognitive function. Participants were introduced to the model, provided with definitions of mobility and advanced dementia and reminded of the format and goals of the study. Advanced dementia was defined as Functional Assessment Staging Test stage 6 or higher [18].
Fig. 2.
Conceptual model of mobility.
For each item, participants were asked to rate the importance and feasibility of assessment, using 9-point Likert scales with ratings from 1 (not important or not feasible) to 9 (very important or highly feasible). In rating importance, participants were asked to indicate the importance of each item to the assessment of mobility in advanced dementia. In rating feasibility, participants were asked to indicate how feasible it would be for a skilled clinician experienced in dementia to assess each item. Participants had the option to choose “don't know.” Ratings of importance were solicited before ratings of feasibility. At the end of each section, participants were given opportunity to provide free-text comment regarding their ratings and to suggest additional factors for consideration. At the end of the questionnaire, participants were asked to indicate their primary group membership (clinician, researcher – dementia, researcher – mobility, or other) and assessment experience (mobility assessment, cognitive assessment, behavior assessment). The questionnaire was piloted with the steering group to ensure clarity and finalized to include seven functional elements and 54 intrinsic factors.
2.3.2. Round 2 questionnaire
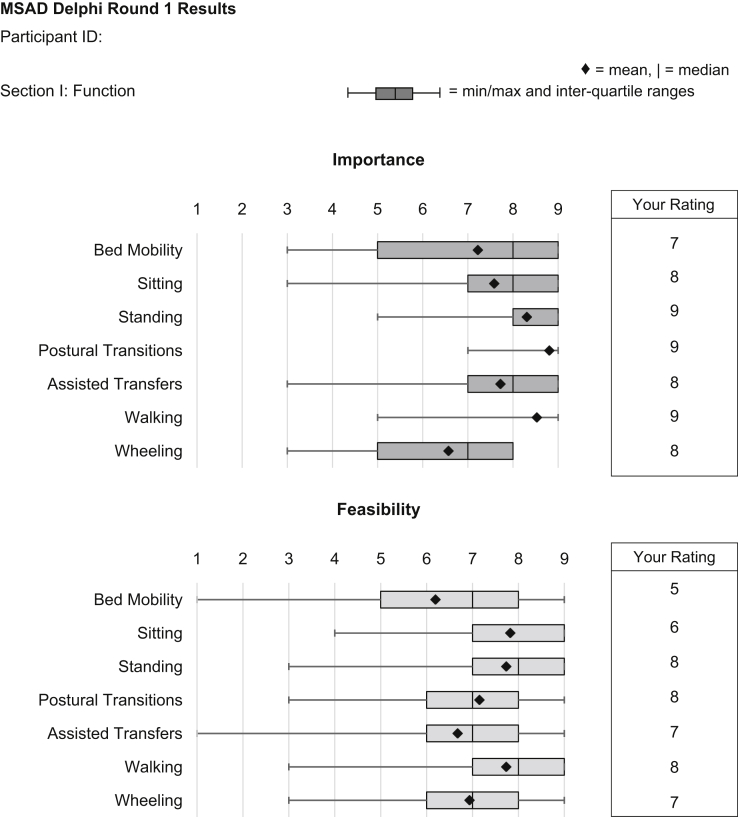
The round 2 questionnaire was intended to give participants an opportunity to re-rate the importance and feasibility of each item presented in round 1 in view of the group's results. All round 1 participants were invited to participate in round 2. To assist in completing this questionnaire, participants were provided with personalized reports, which included their round 1 ratings and group statistics including mean, median, minimum and maximum values, and interquartile ranges (IQR) (Fig. 3). Based on a thematic summary of participant comments, the second questionnaire was edited for clarity including an expanded description of “skilled clinician.” For the remainder of the study, a skilled clinician was defined as “someone who has skills which could be used to assess aspects of mobility in dementia including physiotherapists, occupational therapists, neurologists, and some other medical specialists, and primary care physicians or nurses with expertise in care of the elderly.” Participants were also told that the term “skilled clinician” was being used to reflect that not all clinicians in each category will necessarily have these particular skills or expertise and to note that not all skilled clinicians will have the skills or knowledge of an expert clinician. See Supplementary Material for full description. Following review of respondent recommendations, four new factors were included for rating. Similar to round 1, participants were given opportunity to provide free-text comments regarding their ratings. Participants were also reminded to use the full range of the scale [19].
Fig. 3.
Sample report of round 1 results for Section I (Functional Mobility).
2.3.3. Round 3 questionnaire
The goal of round 3 was to identify those factors deemed highest priority for inclusion in a mobility staging tool for advanced dementia. All round 1 participants were invited to participate in round 3. Only those items that reached consensus in round 2 were included in round 3. Consensus was defined as greater than or equal to 70 percent agreement that an item was important, as indicated by a rating of seven or higher on the 9-point scale. For each domain, participants were asked to select a minimum of one and maximum of three items. Participants were asked to consider both the importance and feasibility of assessment when choosing high-priority items. To assist in decision-making, participants were provided with round 2 median and IQRs for importance and feasibility of each item, as well as a “total score” equating to a sum of the median scores for importance and feasibility (maximum possible score of 18 per item).
3. Results
3.1. Participants and response rates
Of the 76 experts who were invited to participate, 36 completed the first round of data collection (47%). Thirty first-round respondents completed round two (83% retention) and 29 completed round 3 (80% retention). Participants represented the fields of family medicine, occupational therapy, physical therapy, geriatrics, nursing, geriatric psychiatry, rehabilitation science, neuropsychology, neurology, and neuroscience. Clinician participants were distributed as follows: family medicine (n = 3), occupational therapy (n = 1), physical therapy (n = 6), geriatrics (n = 2), nursing (n = 2), geriatric psychiatry (n = 4), neuropsychology (n = 1), and neurology (n = 1). Participant characteristics, including primary area of expertise, assessment experience, and geographical representation for each round of data collection are reported in Table 1. The proportion of respondents within each category did not vary significantly across rounds. Approximately 50% of the participants were clinicians. A majority of respondents indicated that they had assessment experience in two or more areas of interest (mobility, cognition, or behavior). Across all rounds, a minimum of 20% of respondents were located outside of Canada.
Table 1.
Participant characteristics by survey round
| Characteristic | Round 1 (n = 36) (%) | Round 2 (n = 30) (%) | Round 3 (n = 29) (%) |
|---|---|---|---|
| Primary area of expertise | |||
| Clinician | 20 (55) | 18 (60) | 16 (55) |
| Researcher – dementia | 6 (17) | 4 (13) | 5 (17) |
| Researcher – mobility | 10 (28) | 8 (27) | 8 (28) |
| Assessment experience | |||
| Mobility assessment | 3 (8) | 3 (10) | 3 (10) |
| Cognitive assessment | 1 (3) | 1 (3) | 1 (3) |
| Behavioral assessment | 1 (3) | 0 | 0 |
| Two of the above | 11 (30.5) | 8 (27) | 9 (31) |
| Three of the above | 16 (44) | 14 (47) | 12 (41) |
| Not indicated | 4 (11) | – | – |
| Geographical representation | |||
| Local (Greater Toronto Area) | 17 (47) | 15 (50) | 16 (55) |
| National (Canada) | 9 (25) | 7 (23) | 7 (24) |
| International | 10 (28) | 8 (27) | 6 (21) |
3.2. Participant responses
3.2.1. First-round responses
Missing data were limited to 34 of 4392 possible ratings across all items and participants (0.8%). Among feasibility ratings, 123 were rated as “don't know” (5.6%). Median and IQR results for each item in the first round of data collection are reported in Table 2. Nineteen items reached consensus for both importance and feasibility. Within the functional mobility domain, these included sitting, standing, and walking. Across domains of intrinsic factors, items included gait and postural control (sensorimotor), gait aid use, fall history, medications, multimorbidity, and physical activity (health status), contractures, paresis, parkinsonism, postural hypotension, and stroke (specific comorbid conditions), agitation (behavioral and psychological symptoms), and apraxia, attention, and judgment (cognitive symptoms). No item reached consensus for being unimportant or not feasible.
Table 2.
Median scores for item importance and feasibility, group consensus ratings (importance), and group agreement with respect to inclusion in a mobility staging tool for advanced dementia (MSAD)
| Category and Items | Importance (median [IQR limits]) |
Feasibility (median [IQR limits]) |
Consensus importance (round 2) (%) | Consensus feasibility (round 2) (%) | Agreement (round 3) (%) | ||
|---|---|---|---|---|---|---|---|
| Round 1 | Round 2 | Round 1 | Round 2 | ||||
| Section I: Function | |||||||
| Bed Mobility | 8 [5–9] | 8 [5.75–8.25] | 7 [5–8] | 7 [5–8] | 67 | 52 | – |
| Sitting | 8 [7–9] | 8 [6.75–9] | 9 [7–9] | 8 [7–9] | 75 | 77 | 17 |
| Standing | 9 [8–9] | 8 [8–9] | 8 [7–9] | 8.5 [7–9] | 97 | 86 | 52 |
| Postural Transitions | 9 [9] | 9 [8.75–9] | 7 [6–8] | 7 [6.5–8] | 97 | 74 | 69 |
| Assisted Transfers | 8 [7–9] | 8 [8–9] | 7 [6–8] | 7 [6–8] | 89 | 68 | 76 |
| Walking | 9 [9] | 9 [8–9] | 8 [7–9] | 8 [7.5–9] | 92 | 86 | 96 |
| Wheeling | 7 [5–8] | 6.5 [5.75–7.25] | 7 [6–8] | 7 [6–8] | 50 | 73 | – |
| Section II: Factors | |||||||
| Sensorimotor function | |||||||
| Coordination | 7 [6.5–8] | 7 [7–8] | 6 [5–8] | 6 [5–7] | 78 | 43 | 28 |
| Gait (e.g., speed, symmetry) | 9 [8–9] | 8 [8–9] | 8 [7–9] | 8 [7–9] | 94 | 89 | 86 |
| Hearing | 6 [5–7.5] | 5.5 [5–7] | 6 [4–7] | 6 [4–7] | 30 | 36 | – |
| Muscular endurance | 6 [5–7.5] | 6 [5.75–7] | 5 [3–7] | 5 [3.5–7] | 47 | 31 | – |
| Muscle strength | 8 [7–9] | 8 [7–9] | 7 [6–8.25] | 7 [6–8] | 83 | 64 | 55 |
| Muscle tone | 7 [5.5–7] | 7 [6–7] | 8 [5.5–8.5] | 7 [5.5–8] | 56 | 63 | – |
| Peripheral sensation | 7 [5–8] | 6.5 [6–7.25] | 6 [3.5–8] | 6 [4–7] | 50 | 40 | – |
| Postural control/balance | 9 [8–9] | 9 [8–9] | 8 [7–8.5] | 7 [7–8] | 92 | 83 | 76 |
| Proprioception | 7 [6–8] | 7 [6–8] | 6 [3–8] | 5.5 [3–7] | 56 | 39 | – |
| Reaction time | 7 [6–7.5] | 7 [5–7] | 5 [4–8] | 5 [4–7] | 53 | 37 | – |
| Range of motion | 7 [6–7] | 7 [6–7] | 8 [7–9] | 8 [7–8] | 56 | 83 | – |
| Sensory integration | 7 [5–8] | 6 [5–8] | 4.5 [3–6.25] | 4 [3–5] | 44 | 15 | – |
| Vision | 8 [7–9] | 8 [6–8.25] | 7 [5–8] | 6 [5–7] | 72 | 48 | 14 |
| Health status | |||||||
| Fear of falling | 7.5 [6–9] | 7 [6–9] | 6 [4–8] | 6 [4–7.75] | 70 | 44 | 7 |
| Gait aid use | 9 [7.75–9] | 8 [7.75–9] | 8 [7–9] | 8 [7.75–9] | 86 | 83 | 52 |
| History of falls | 9 [8–9] | 9 [8–9] | 8 [6.75–9] | 8 [7–9] | 92 | 80 | 83 |
| Medication(s) | 8 [8–9] | 8 [8–9] | 9 [8–9] | 9 [8–9] | 89 | 97 | 48 |
| Multimorbidity | 8 [6–9] | 7.5 [6.75–9] | 8 [8–9] | 8 [8–9] | 75 | 94 | 24 |
| Physical activity level | 8 [7–9] | 8 [7–8] | 7 [6.5–8] | 7 [6.5–8] | 92 | 74 | 24 |
| Frailty | – | 7.5 [6.25–8] | – | 7 [6.75–8] | 73 | 75 | 45 |
| Type of dementia | – | 7 [5–8] | – | 7 [6–7] | 53 | 57 | – |
| Specific comorbid conditions | |||||||
| Arthritis | 8 [6–9] | 7 [6–8.25] | 8 [7–9] | 8 [7–9] | 70 | 89 | 7 |
| Cardiovascular conditions | 7 [5.75–8] | 7 [6–7.25] | 8 [6.5–9] | 8 [6.5–9] | 56 | 76 | – |
| Contractures | 8 [7–9] | 8 [7–9] | 8 [7–9] | 8 [7–9] | 86 | 96 | 14 |
| Diabetes | 6 [5–7] | 6 [4.75–7] | 8 [7–9] | 8 [7–9] | 33 | 90 | – |
| Edema | 6 [4.75–7] | 6 [4.75–7] | 8 [8–9] | 8 [8–9] | 30 | 96 | – |
| Joint pain | 8 [7–9] | 8 [7–9] | 7 [6–8] | 7 [6–8] | 92 | 66 | 24 |
| Osteoporosis | 6 [5–7] | 6 [5–7] | 8 [6–8.75] | 8 [6.5–9] | 36 | 76 | – |
| Paresis | 8 [8–9] | 8 [7.75–9] | 8 [7–9] | 8 [7–9] | 89 | 93 | 45 |
| Parkinsonism | 9 [8–9] | 9 [8–9] | 8 [7–9] | 8 [7–9] | 92 | 96 | 90 |
| Postural hypotension | 8 [7–9] | 8 [7–9] | 7 [6.5–9] | 7 [7–9] | 83 | 79 | 48 |
| Respiratory problems | 6 [5–7] | 6 [5–7] | 8 [7–8] | 8 [7–8] | 44 | 79 | – |
| Stroke | 8 [7–9] | 7 [7–9] | 8 [7–9] | 8 [7–8.5] | 80 | 86 | 21 |
| Urinary incontinence | 6 [4.5–7.5] | 6 [5–7] | 8 [7–8] | 8 [7–8.75] | 37 | 93 | – |
| Vestibular dysfunction | 7.5 [6–9] | 7 [6–8] | 6 [4–7.25] | 5 [3.5–6.5] | 61 | 23 | – |
| Foot care | – | 7 [6–7] | – | 7 [7–8] | 60 | 89 | – |
| Generalized pain | – | 7 [7–8] | – | 7 [6–7.5] | 80 | 52 | 38 |
| Behavioral and psychological symptoms | |||||||
| Aggression | 7 [5–9] | 7 [5.75–8.25] | 8 [8–9] | 8 [8–9] | 64 | 94 | – |
| Agitation | 8 [6.75–9] | 8 [6–9] | 8 [8–9] | 8 [8–9] | 72 | 94 | 79 |
| Anxiety | 7.5 [6–9] | 7 [6–8] | 8 [7–8] | 8 [7–8] | 69 | 82 | – |
| Apathy | 7 [5–7.25] | 6.5 [5–7] | 7 [6–8] | 7 [7–8] | 50 | 76 | – |
| Delusions | 6 [5–7] | 6 [5–7] | 7 [6–8] | 7 [6–7.25] | 36 | 65 | – |
| Depression | 7 [5–8] | 6 [5–7.25] | 7 [6–8] | 7 [6–8] | 47 | 66 | – |
| Disinhibition | 6 [5–7] | 6 [5–7] | 8 [7–8.25] | 8 [7–8] | 30 | 84 | – |
| Hallucination | 6.5 [5–7] | 6 [5–7] | 7 [6–8] | 7 [6–8] | 44 | 64 | – |
| Impulsivity | 8.5 [7.75–9] | 8.5 [7–9] | 8 [7–8] | 8 [7–8] | 80 | 82 | 86 |
| Restlessness | 7.5 [6–8.25] | 7 [6–8] | 8 [8–9] | 8 [8–9] | 67 | 91 | – |
| Sleep disturbance(s) | 7 [5–8] | 6 [5–7.25] | 8 [7–8] | 8 [7–8] | 47 | 85 | – |
| Wandering | 7 [5.75–8] | 7 [6–8] | 8 [7.5–9] | 8 [8–9] | 61 | 86 | – |
| Wayfinding | 7 [5–8] | 7 [5–8] | 8 [6–9] | 7 [6–8] | 53 | 69 | – |
| Cognitive function | |||||||
| Agnosia | 7.5 [6–8.25] | 7 [6–8] | 6.5 [5–7] | 6 [5–7] | 56 | 31 | – |
| Apraxia | 8 [7.75–9] | 8 [7.75–9] | 7 [6–8] | 7 [6–7] | 83 | 54 | 55 |
| Attention | 8 [7–9] | 8 [7–8] | 8 [6.5–8] | 7 [6–8] | 80 | 68 | 59 |
| Judgment | 8 [7–8] | 7 [6–8] | 7 [6–8] | 7 [6–7.25] | 72 | 56 | 45 |
| Memory | 6 [5–6.25] | 6 [5–6] | 8 [7–8] | 7.5 [7–8] | 19 | 80 | – |
| Motor planning | 8 [7–9] | 8 [7–9] | 7 [5.5–7.5] | 6 [5–7] | 80 | 44 | 38 |
| Processing speed | 7 [6–7.25] | 7 [6–7] | 6 [5–7.75] | 6 [5–7] | 56 | 38 | – |
| Visuospatial ability | 8 [7–9] | 8 [7–8] | 6 [5–8] | 6 [5–7] | 80 | 45 | 62 |
Items in bold represent those that reached consensus (≥ 70%) for importance and agreement as high priority for inclusion in an MSAD.
Participant comments consisted of requests for clarification or reflected the context-dependency of responses. Requests for clarification focused on the conditions of assessment and on the term “skilled clinician.” The round 2 questionnaire was edited for clarity based on these comments (see Supplementary Material). In regard to context, both clinician and researcher respondents commented on the importance of assessment type, emphasizing the value of observational assessment and access to collateral sources such as health records or a caregiver to maximize feasibility. Respondents also commented on the importance of setting for feasibility of assessment and suggested that consideration should be given to the skills of the assessor (see Supplementary Material for sample comments).
Twenty-two suggestions were made regarding additional factors to include in the study. A majority could be accounted for within existing items, resulting in four new factors for inclusion: frailty, type of dementia, foot care, and generalized pain.
3.2.2. Second-round responses
Missing data included 68 of 3660 possible ratings across all items and participants (1.8 %). Among feasibility ratings, the proportion of “don't know” responses decreased to 3.5%. Of a possible 130 ratings, the number of items that were re-rated ranged from zero to 95 per participant (mean 35 ± 28). Both clinician (CLIN) and researcher (RES) groups re-rated approximately 30% of items within a domain for both importance and feasibility (CLIN: mean 31% ± 8.3 for importance, 26.8% ± 6.5 for feasibility; RES: mean 29.3% ± 3.7 for importance, 30% ± 6.2 for feasibility).
Median and IQR ratings for each item in the second round of data collection are reported in Table 2. In total, 32 items representing all domains reached consensus for being important to the assessment of mobility in advanced dementia. Thirty-six items reached consensus for feasibility. Twenty items reached consensus for both importance and feasibility (Table 2). No item reached consensus for being unimportant or not feasible. Median consensus ratings within a domain were generally higher for RES than CLIN for both importance and feasibility. A breakdown of consensus ratings by group is reported in Table 3. The difference between groups was most evident in ratings of feasibility: RES consistently rated factors higher on feasibility of assessment compared with CLIN, with the exception of sensorimotor function, which was rated low for feasibility by both groups. Across all domains, 31 and 45 items reached consensus for feasibility among CLIN and RES, respectively. The group effect for rating of importance was less consistent. Overall, RES reached full agreement (100%) for 11 ratings and CLIN reached full agreement for two.
Table 3.
Breakdown of consensus ratings by primary group membership
| Group | Importance |
Feasibility |
||
|---|---|---|---|---|
| Median consensus rating | No. items reaching consensus (%) | Median consensus rating | No. items reaching consensus (%) | |
| Section I: Function | ||||
| All | 88.9 | 5 (71.4) | 74.3 | 5 (71.4) |
| CLIN (n = 20) | 90 | 5 (71.4) | 72.2 | 4 (57.1) |
| RES (n = 16) | 87.5 | 6 (85.7) | 86.7 | 6 (85.7) |
| Section II: Factors | ||||
| Sensorimotor function | ||||
| All | 55.6 | 5 (38.5) | 42.8 | 3 (23.1) |
| CLIN | 55 | 5 (38.5) | 42.1 | 4 (30.8) |
| RES | 62.5 | 5 (38.5) | 46.7 | 3 (23.1) |
| Health Status | ||||
| All | 80.6 | 6 (75) | 77.8 | 6 (75) |
| CLIN | 77.5 | 6 (75) | 80.6 | 5 (62.5) |
| RES | 87.5 | 7 (87.5) | 80.8 | 7 (87.5) |
| Specific comorbid conditions | ||||
| All | 65.3 | 7 (43.8) | 85.5 | 13 (81.2) |
| CLIN | 62.8 | 8 (50) | 73 | 9 (56.2) |
| RES | 67.7 | 6 (37.5) | 93.8 | 14 (87.5) |
| Behavioural and psychological symptoms | ||||
| All | 52.8 | 2 (15.4) | 82.4 | 9 (69.2) |
| CLIN | 45 | 4 (30.8) | 77.8 | 8 (61.5) |
| RES | 56.2 | 1 (7.7) | 87.5 | 12 (92.3) |
| Cognitive function | ||||
| All | 76.4 | 5 (62.5) | 50 | 1 (12.5) |
| CLIN | 67.5 | 5 (62.5) | 39.5 | 1 (12.5) |
| RES | 81.2 | 5 (62.5) | 64.3 | 3 (37.5) |
| Totals | ||||
| All | 32 (49.2) | 37 (56.9) | ||
| CLIN | 33 (50.8) | 31 (47.7) | ||
| RES | 30 (46.2) | 45 (69.2) | ||
Abbreviations: CLIN, clinician; RES, researcher.
3.2.3. Third-round responses
Of the 32 items included in round 3, participant agreement that an item was high priority for inclusion in a mobility staging tool for advanced dementia ranged from 7% to 96%. Eight items reached agreement of 70% or greater, representing all domains except cognitive function (Table 2). These items included walking (96%), parkinsonism (90%), gait (86%), impulsivity (86%), fall history (83%), agitation (79%), transfers (76%), and posture control (76%), with postural transitions (69%) closely following. Of these items, all but transfers reached consensus for feasibility although it narrowly missed the criterion (69%). Visuospatial ability was ranked the highest priority cognitive function (62%).
4. Discussion
In this study, we engaged clinicians and academic researchers in the areas of mobility and dementia to identify key factors that impact mobility in people with advanced dementia. Participants were instructed to take a broader perspective on mobility in dementia, by considering independence, autonomy, and engagement in meaningful activities as important outcomes that overlap with, but are separate from falls risk, and to consider the feasibility of assessment in the advanced dementia population. The conceptual model of mobility used to guide this study considered functional mobility separate from intrinsic factors that can affect a person's mobility status (Fig. 2). The limited number of additional items identified by participants suggests that the consensus exercise was grounded in a comprehensive model. The results of this study have important implications for development of assessment protocols and guidelines to support treatment and clinical care of mobility decline in PWD.
Overall, consensus was reached on 32 items important to assess in PWD with eight items selected as high priority for inclusion in a mobility staging tool. These high priority items represented five different categories. Although many of the selected items are already routinely included in clinical assessments of mobility or fall risk, for example walking and transfer ability, gait and balance impairments, parkinsonism, and fall history, others are specific to dementia, namely the behavioral symptoms of impulsivity and agitation.
Walking and ability to transfer are important for defining an individual's degree of independence in mobility. In the present study, both were deemed feasible to assess in people with advanced dementia. Clinically, they can be evaluated by observation and rated based on degree of assistance required as in the Rating Scale for Gait Evaluation–Cognitive Deterioration [6], [20]. Wheeled mobility was not rated highly as a factor to consider. This finding could reflect, in part, the view that mobility requires ambulation, highlighting a gap in research and clinical practice regarding the use of wheelchairs to support independent mobility in PWD [21].
Gait and balance impairment were also deemed feasible to assess in PWD. Separate from fall risk, the quality of gait and ability to maintain stability provide important information about individuals' health status and are predictors of medical events, decline, and death [22], [23], [24], [25]. Although tools such as the Berg Balance Scale [26] or the Performance Oriented Mobility Assessment [27] are commonly used to identify impairments in these areas, a recent review by our group suggested that a majority of existing tools designed to assess balance, gait, and mobility are not feasible to administer in people with advanced dementia because of their performance-based approach and the need for comprehension, attention, motivation, and complex motor skill execution during testing [6]. The importance of parkinsonism in staging mobility in this population reflects its significant impact on the performance of functional mobility tasks (transferring, walking, standing), and its role as a marker for accelerated mobility decline [28], [29]. Similarly, a history of falls is a powerful predictor of future falls in PWD, and from a staging perspective, people who fall are also at higher risk of progression in mobility loss [30], [31].
Behavioral features of dementia are less typically included in mobility assessments. In this study, both impulsivity and agitation were rated as factors to consider in a staging tool. The care and attention to safety used by an individual when moving is a reflection of their impulsivity, which is a contributor to fall risk in PWD living in long-term care [19], [32]. A fall-related impulsive behavior scale has been validated in the dementia population [32]. Agitation is a marker for a more rapid decline in functional status and a neuropsychiatric symptom, which contributes to fall risk, with the relationship between agitation and these outcomes both direct and indirect through the use of psychotropic medications [19], [33], [34], [35].
Although all eight highest priority factors correspond to commonly identified predictors of fall risk [19], [36], [37], many factors known to be significant independent predictors of falls in PWD were not ranked high priority despite reaching consensus for importance. One explanation for this finding is that priority ratings were influenced by the feasibility of assessing a particular factor in people with advanced dementia, which participants were instructed to consider alongside its importance. For example, visuospatial abilities and sensory impairments were rated very highly for importance but were viewed as less feasible to assess in this population, reflecting a lack of assessment methods developed and validated in people with advanced dementia [38], [39]. Interestingly, across all domains, there was a bias toward lower median ratings and fewer items reaching consensus for clinicians versus researchers. Lower feasibility ratings among clinicians may be attributable to the unique perspective afforded by expert clinical judgment. Clinician respondents also commented on practical experiences that informed their responses such as lack of availability of skilled clinicians or resources, or patient-centered barriers to assessment.
A second explanation for the exclusion of a number of well-known fall risk factors from the high priority list is that, as instructed, participants selected factors they felt were particularly important for assessing the stage of mobility impairment as distinct from risk. Staging, which we described as “a standardized, shorthand expression of patient function in various domains of related activities” [40], is function-focused and allows interventions to be tailored to the abilities of the individual to help manage risk in balance with other outcomes. Risk assessment, on the other hand, tends to have a deficit focus and aims to identify and mitigate hazards with a focus on prevention. It is thus important that the final selection of these eight high priority items be interpreted in the context of the study goal, which was to inform the development of a mobility staging tool in dementia. The broader list of 32 items reaching consensus for importance, and their ratings of feasibility of assessment also have future value in informing the development of tools for comprehensive mobility assessment in this population, for example, by informing what constructs to include. This information, combined with additional literature and clinical expertise informing frequency of assessment, conditions for triggering assessment, etc., has the potential to advance clinical care guidelines about best practices for mobility assessment in PWD. The results of this study also serve to highlight gaps where feasible assessment methods do not currently exist and as such opportunities for tool development.
4.1. Limitations
Although the modified Delphi approach is commonly used to obtain unbiased expert consensus, we do acknowledge that the approach is not completely objective. Although the pool of nominated participants was intended to be representative, not all types of mobility specialists were reflected in our group and it is possible that participants in this study do not share the opinions of all those who assess mobility. Furthermore, the number of participants was relatively small although it fell within the suitable range for Delphi consensus exercises [14], [15], and equal representation of clinicians and academic researchers with a breadth of expertise and geographical location may be considered a strength.
5. Conclusions
Identifying key factors that impact mobility in advanced dementia has important consequences for clinical management; impacting clinicians' ability to monitor changes over time, direct therapeutic interventions, and guide caregiver decision-making. This consensus exercise has identified several important mobility factors deemed feasible to assess in individuals with advanced dementia. These items will be used as the foundation for a staging tool to help characterize and monitor transitions in mobility function. Results of this study also provide a foundation for development of other assessment protocols and clinical care guidelines that will help to promote mobility and identify opportunities for clinical intervention and support. Future work will focus on determining how to reliably assess these factors and how to stage mobility function using this multidimensional approach.
Research in context.
-
1.
Systematic review: A scoping review conducted by the authors (Van Ooteghem K, Musselman K, Gold D, Marcil MN, Keren R, Tartaglia MC, et al. Evaluating Mobility in Advanced Dementia: A Scoping Review and Feasibility Analysis. The Gerontologist. 2018;23(11):1018-33; https://doi.org/10.1093/geront/gny068.) informed the conceptual model of mobility that was used to guide this study. The review identified mobility assessment tools used in dementia and the elements of mobility evaluated. It also established the need to consider feasibility of assessment in people with advanced dementia.
-
2.
Interpretation: Key factors identified through the consensus exercise represented different domains, supporting the need for a multidimensional approach to assessment inclusive of dementia-specific factors. Fewer factors reached consensus for feasibility among health care providers versus researchers, demonstrating the significance of input from clinical experts. Factors that reached agreement as high priority for inclusion in a mobility staging tool may represent a minimum set of essential measures deemed feasible to assess in people with advanced dementia.
-
3.
Future directions: Future work should determine how to adequately assess key factors and how to integrate the results of assessment into practice.
Acknowledgments
The authors acknowledge the following individuals for their participation in the consensus exercise: Charlene Chu, Kim Delbaere, Rosalie Freund-Heritage, Liz Inness, Zahnoor Ismail, Linda Lee, Stephen Lord, Andrea Moser, Susan Muir-Hunter, Dimity Pond, Lily Spanjevic, Asenath Steiman, Carolyn Sterke, David Tang-Wai, Sarah Tyson, Lisa Van Bussel, Joe Verghese, Rosalie Wang, and Aleksandra Zecevic, as well as those participants who preferred to remain anonymous.
This work was supported by the Alzheimer's Association [grant number AARG-16-442805] to A.I.
Footnotes
A.J.F. receives grant support from the U.S. National Institutes of Health, the Patient-Centered Outcomes Research Institute, the Canadian Institutes of Health Research, Brain Canada, and the Alzheimer's Association. A.I. receives grant support from the Alzheimer's Association, Canadian Center for Aging and Brain Health Innovation, and Brain Canada. A.M. receives grant support from the Canadian Institutes of Health Research, the Heart and Stroke Foundation of Canada, the Natural Sciences and Engineering Research Council of Canada, and the Ministry of Research and Innovation (Ontario). All other authors have no conflicts of interest to report.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2019.07.002.
Supplementary Data
References
- 1.Goin L., Duke L., Hollawell D., Horton A., Voytek M.W. Ambulation and Mobility. In: Martin G.A., Sabbagh M.N., editors. Palliative care for advanced Alzheimer's and dementia: Guidelines and standards for evidence-based care. Springer Publishing Company; New York, NY: 2010. pp. 131–145. [Google Scholar]
- 2.Tyson S.C., Connell L. The psychometric properties and clinical utility of measures of walking and mobility in neurological conditions: a systematic review. Clin Rehabil. 2009;23:1018–1033. doi: 10.1177/0269215509339004. [DOI] [PubMed] [Google Scholar]
- 3.Taylor M.E., Delbaere K., Lord S.R., Mikolaizak A.S., Brodaty H., Close J.C. Neuropsychological, physical, and functional mobility measures associated with falls in cognitively impaired older adults. J Gerontol A Biol Sci Med Sci. 2014;69:987–995. doi: 10.1093/gerona/glt166. [DOI] [PubMed] [Google Scholar]
- 4.Kikkert L.H.J., de Groot M.H., van Campen J.P., Beijnen J.H., Hortobagyi T., Vuillerme N. Gait dynamics to optimize fall risk assessment in geriatric patients admitted to an outpatient diagnostic clinic. PLoS One. 2017;12:e0178615. doi: 10.1371/journal.pone.0178615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iaboni A., Van Ooteghem K., Marcil M.N., Cockburn A., Flint A.J., Grossman D. A palliative approach to falls in advanced dementia. Am J Geriatr Psychiatry. 2018;26:407–414. doi: 10.1016/j.jagp.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Van Ooteghem K., Musselman K., Gold D., Marcil M.N., Keren R., Tartaglia M.C. Evaluating mobility in advanced dementia: a scoping review and feasibility analysis. Gerontologist. 2018;23:1018–1033. doi: 10.1093/geront/gny068. [DOI] [PubMed] [Google Scholar]
- 7.Kwan M.M., Lin S.I., Chen C.H., Close J.C., Lord S.R. Sensorimotor function, balance abilities and pain influence Timed Up and Go performance in older community-living people. Aging Clin Exp Res. 2011;23:196–201. doi: 10.1007/BF03324960. [DOI] [PubMed] [Google Scholar]
- 8.Lord S.R., Murray S.M., Chapman K., Munro B., Tiedemann A. Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people. J Gerontol A Biol Sci Med Sci. 2002;57:M539–M543. doi: 10.1093/gerona/57.8.m539. [DOI] [PubMed] [Google Scholar]
- 9.Chen H.Y., Tang P.F. Factors contributing to single- and dual-task timed “Up & Go” test performance in middle-aged and older adults who are active and dwell in the community. Phys Ther. 2016;96:284–292. doi: 10.2522/ptj.20140292. [DOI] [PubMed] [Google Scholar]
- 10.Whitney J., Close J.C., Jackson S.H., Lord S.R. Understanding risk of falls in people with cognitive impairment living in residential care. J Am Med Dir Assoc. 2012;13:535–540. doi: 10.1016/j.jamda.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Fernando E., Fraser M., Hendriksen J., Kim C.H., Muir-Hunter S.W. Risk factors associated with falls in older adults with dementia: a systematic review. Physiother Can. 2017;69:161–170. doi: 10.3138/ptc.2016-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Growdon M.E., Shorr R.I., Inouye S.K. The tension between promoting mobility and preventing falls in the hospital. JAMA Intern Med. 2017;177:759–760. doi: 10.1001/jamainternmed.2017.0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hsu C.C.S., Sandford B.A., editors. The Delphi Technique: Making Sense of Consensus. Practical Assessment Research and Evaluation; 2007. [Google Scholar]
- 14.von der Gracht H.A. Consensus measurement in Delphi studies. Technol Forecast Social Change. 2012;79:1525–1536. [Google Scholar]
- 15.Khodyakov D., Hempel S., Rubenstein L., Shekelle P., Foy R., Salem-Schatz S. Conducting online expert panels: a feasibility and experimental replicability study. BMC Med Res Methodol. 2011;11:174. doi: 10.1186/1471-2288-11-174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinha I.P., Smyth R.L., Williamson P.R. Using the Delphi technique to determine which outcomes to measure in clinical trials: recommendations for the future based on a systematic review of existing studies. PLos Med. 2011;8:e1000393. doi: 10.1371/journal.pmed.1000393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh H.F., Shannon S.E. Three approaches to qualitative content analysis. Qual Health Res. 2005;19:1277–1288. doi: 10.1177/1049732305276687. [DOI] [PubMed] [Google Scholar]
- 18.Auer S., Reisberg B. The GDS/FAST staging system. Int Psychogeriatr. 1997;9:167–171. doi: 10.1017/s1041610297004869. [DOI] [PubMed] [Google Scholar]
- 19.Fitch K., Bernstein S.J., Aguilar M.D., Burnand B., LaCalle J.R., Lazaro P. RAND Corporation; Santa Monica, CA: 2001. The RAND/UCLA Appropriateness Method User's Manual. Available at https://www.rand.org/pubs/monograph_reports/MR1269.html. Accessed January 20, 2019. [Google Scholar]
- 20.Martinez-Martin P., Osa-Ruiz E., Gomez-Conesa A., Olazaran J. A rating scale for gait evaluation in cognitive deterioration (RSGE-CD): validation study. J Alzheimers Dis. 2012;31:543–553. doi: 10.3233/JAD-2012-120271. [DOI] [PubMed] [Google Scholar]
- 21.Mortenson W.B., Miller W.C., Backman C.L., Oliffe J.L. Predictors of mobility among wheelchair using residents in long-term care. Arch Phys Med Rehabil. 2011;92:1587–1593. doi: 10.1016/j.apmr.2011.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mahmoudi R., Novella J.L., Manckoundia P., Ahssaini F., Lang P.O., Blanchard F. Is functional mobility an independent mortality risk factor in subjects with dementia? Maturitas. 2017;103:65–70. doi: 10.1016/j.maturitas.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 23.Buchman A.S., Dawe R.J., Leurgans S.E., Curran T.A., Truty T., Yu L. Different combinations of mobility metrics derived from a wearable sensor are associated with distinct health outcomes in older adults. J Gerontol A Biol Sci Med Sci. 2019 doi: 10.1093/gerona/glz160. pii: glz160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Toots A., Rosendahl E., Lundin-Olsson L., Nordström P., Gustafson Y., Littbrand H. Usual gait speed independently predicts mortality in very old people: a population-based study. J Am Med Dir Assoc. 2013;14:529.e1–529.e6. doi: 10.1016/j.jamda.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 25.Koutsavlis A.T., Wolfson C. Elements of mobility as predictors of survival in elderly patients with dementia: findings from the Canadian Study of Health and Aging. Chronic Dis Can. 2000;21:93–103. [PubMed] [Google Scholar]
- 26.Berg K., Wood-Dauphinee S., Williams J.I., Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–311. [Google Scholar]
- 27.Tinetti M.E. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc. 1986;34:119–126. doi: 10.1111/j.1532-5415.1986.tb05480.x. [DOI] [PubMed] [Google Scholar]
- 28.Scarmeas N., Albert M., Brandt J., Blacker D., Hadjigeorgiou G., Papadimitriou A. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilson R.S., Schneider J.A., Beckett L.A., Evans D.A., Bennett D.A. Progression of gait disorder and rigidity and risk of death in older persons. Neurology. 2002;58:1815–1819. doi: 10.1212/wnl.58.12.1815. [DOI] [PubMed] [Google Scholar]
- 30.Deandrea S., Bravi F., Turati F., Lucenteforte E., La Vecchia C., Negri E. Risk factors for falls in older people in nursing homes and hospitals. A systematic review and meta-analysis. Arch Gerontol Geriatr. 2013;56:407–415. doi: 10.1016/j.archger.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 31.Panel on Prevention of Falls in Older Persons, American Geriatrics Society and British Geriatrics Society Summary of the updated American Geriatrics Society/British Geriatrics Society clinical practice guideline for prevention of falls in older persons. J Am Geriatr Soc. 2011;59:148–157. doi: 10.1111/j.1532-5415.2010.03234.x. [DOI] [PubMed] [Google Scholar]
- 32.Whitney J., Jackson S.H.D., Close J.C.T., Lord S.R. Development and validation of a fall-related impulsive behaviour scale for residential care. Age Ageing. 2013;42:754–758. doi: 10.1093/ageing/aft130. [DOI] [PubMed] [Google Scholar]
- 33.Rosenberg P.B., Mielke M.M., Han D., Leoutsakos J.S., Lyketsos C.G., Rabins P.V. The association of psychotropic medication use with the cognitive, functional, and neuropsychiatric trajectory of Alzheimer's disease. Int J Geriatr Psychiatry. 2012;27:1248–1257. doi: 10.1002/gps.3769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sylliaas H., Selbaek G., Bergland A. Do behavioural disturbances predict falls among nursing home residents? Aging Clin Exp Res. 2012;24:251–256. doi: 10.1007/BF03325253. [DOI] [PubMed] [Google Scholar]
- 35.Roitto H.M., Kautiainen H., Ohman H., Savikko N., Strandberg T.E., Raivio M. Relationship of neuropsychiatric symptoms with falls in Alzheimer's disease – does exercise modify the risk? J Am Geriatr Soc. 2018;66:2377–2381. doi: 10.1111/jgs.15614. [DOI] [PubMed] [Google Scholar]
- 36.Bauermeister S., Sutton G., Mon-Williams M., Wilkie R., Graveson J., Cracknell A. Intraindividual variability and falls in older adults. Neuropsychology. 2017;31:20–27. doi: 10.1037/neu0000328. [DOI] [PubMed] [Google Scholar]
- 37.Kallin K., Gustafson Y., Sandman P.-O., Karlsson S. Factors associated with falls among older, cognitively impaired people in geriatric care settings: a population-based study. Am J Geriatr Psychiatry. 2005;13:501–509. doi: 10.1176/appi.ajgp.13.6.501. [DOI] [PubMed] [Google Scholar]
- 38.Wittich W., Höbler F., Jarry J., McGilton K.S. Recommendations for successful sensory screening in older adults with dementia in long-term care: a qualitative environmental scan of Canadian specialists. BMJ Open. 2018;8:e019451. doi: 10.1136/bmjopen-2017-019451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Campos J.L., Höbler F., Bitton E., Labreche T., McGilton K.S., Wittich W. Screening for vision impairments in individuals with dementia living in long-term care: a scoping review. J Alzheimers Dis. 2019;68:1039–1049. doi: 10.3233/JAD-181129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stineman M.G., Ross R.N., Fiedler R., Granger C.V., Maislin G. Functional independence staging: conceptual foundation, face validity, and empirical derivation. Arch Phys Med Rehabil. 2003;84:29–37. doi: 10.1053/apmr.2003.50061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.