Short abstract
Objective
This study was performed to determine the effect of dexmedetomidine (DEX) administration on myocardial damage in cardiac surgery with sevoflurane postconditioning.
Methods
We retrospectively examined all cardiac valve replacement surgeries from 1 April 2016 to 30 April 2017. Eligible patients were divided into two groups based on whether DEX was infused. DEX infusion was permitted only between intubation and the beginning of cardiopulmonary bypass (CPB). Sevoflurane was inhaled via the standard postconditioning procedure starting at aortic declamping. The cardiac troponin I (cTnI) level was measured at different time points. The postoperative outcomes and complications were also analyzed.
Results
One hundred patients were included in the study (DEX group, n = 53; non-DEX group, n = 47). Increased cTnI levels were significantly correlated with the New York Heart Association classification, CPB time, and DEX use. DEX use and the CPB time were potential independent factors contributing to changes in the cTnI level. The cTnI level at 6, 12, and 24 hours postoperatively was remarkably lower in the DEX than non-DEX group by 1.14, 7.83, and 5.86 ng/mL, respectively.
Conclusions
DEX decreased the cTnI level after CPB when sevoflurane postconditioning was used, especially at 6, 12, and 24 hours postoperatively.
Keywords: Dexmedetomidine, sevoflurane, troponin, cardiopulmonary bypass, myocardial injury, postconditioning
Introduction
According to a previous report, 1 million patients worldwide benefit from cardiac surgery each year,1 with a 4% incidence of high-risk 30-day mortality.2 Perioperative cardiac ischemia is inevitable in cardiac surgery with or without cardiopulmonary bypass (CPB) and directly causes postoperative myocardial dysfunction and arrhythmia if perioperative CPB is involved. Moreover, reperfusion injury theoretically causes myocardial tissue deterioration, which results in the presence of certain detectable enzymes in the first several days after surgery. Cardiac troponin I (cTnI) is a highly sensitive and specific marker that is used as the gold standard diagnostic marker of myocardial infarction with coronary artery bypass grafting (CABG); cTnI is also a remarkable predictor of the outcomes of cardiac patients.3–5 Numerous studies have concluded that an elevated postoperative cTnI level is correlated with morbidity and mortality; additionally, an elevated postoperative cTnI concentration is an independent risk factor for serious outcomes after cardiac surgery, such as a prolonged length of stay in the intensive care unit (ICU) and prolonged hospitalization.6–14 In 2004, the American Heart Association/American College of Cardiology first regarded cTnI as a cardiac biomarker for predicting the patient prognosis.15 A meta-analysis also confirmed its prognostic value for all-cause mortality after cardiac surgery.9 cTnI reportedly has the strongest association with mortality at 18 to 24 h after surgery and can predict short-, medium-, and long-term mortality.11–13 Hence, if cTnI can be decreased at an early stage postoperatively, then patient outcomes may be effectively improved. Increasingly more clinical studies are now focusing on this issue.
Sevoflurane conditioning is a convenient and applicable strategy in the clinical setting. Sevoflurane protects perioperative organ function against ischemia/reperfusion (I/R) injury via nonanesthetic pharmacological properties. Experimental models have provided strong evidence of the efficacy of sevoflurane postconditioning, and many of the involved signaling pathways or molecular mechanisms have been elucidated.16–21 The use of sevoflurane during CPB is associated with reduced peak postoperative troponin levels.22 The related mechanisms induced by postconditioning have been described in detail along with analogous actions in humans,23 such as the generation of reactive oxygen species, actions on mitochondria, and the activation of cellular signaling pathways. Research has confirmed that inhaled anesthetics can provide more long-term benefits than can intravenous anesthetics. Dexmedetomidine (DEX) is a highly specific α2-adrenergic agonist that exhibits a broad spectrum of biological activities, including anti-inflammation, signal pathway modulation, and apoptotic and necrotic protection, as well as the ordinary properties applied in clinical anesthesia.24 Numerous animal models have demonstrated the organ-protective effects of DEX against I/R injury25–31 in intestinal, cerebral, myocardial, renal, pulmonary, and hepatic tissues. According to an increasing number of clinical studies,32–37 DEX has anti-inflammatory effects and shows remarkable myocardial protection characterized by obvious decreases in cTnI, cardiac troponin T, or creatine kinase isoenzyme MB without severe complications. The mechanism may involve several different signaling pathways such as high-mobility group box 1/Toll-like receptor 4/myeloid differentiation primary response factor 88/nuclear factor kappa-light-chain enhancer of activated B cells (HMGB1/TLR4/MyD88/NF-κB), cyrillic B, mitochondrial adenosine triphosphate-sensitive potassium channel, P38-mitogen activated protein kinase/thioredoxin-interacting protein (P38-MAPK/TXNIP), and 5′-adenosine monophosphate-activated protein kinase/phosphoinositide 3-kinase/protein kinase B/endothelial nitric oxide synthase (AMPK/PI3K/Akt/eNOS).38 Despite its widespread use in clinical practice, DEX has been only occasionally infused during cardiac surgery, especially when sevoflurane postconditioning was popular, and adverse outcomes of its combination use have been seldom reported. We hypothesized that DEX improves the effects of ordinary postconditioning of sevoflurane inhalation. The present study was conducted to confirm the influence of DEX in combination with sevoflurane postconditioning on myocardial injury following cardiac surgery, thus further clarifying the mechanism of sevoflurane postconditioning and improving the current understanding of organ protection by DEX.
Methods
Study population
The ethics committee of the Second Affiliated Hospital of Jiaxing University (tertiary, grade A class, public and teaching hospital) approved this retrospective study and waived the requirement for individual patient consent (JXEY-20180718H01). The present manuscript was written in line with the STROBE checklist for cohort studies. The inclusion criteria were the performance of cardiac surgery (mitral valve, tricuspid valve, and aortic valve surgeries) from 1 April 2016 to 30 April 2017 in the Second Affiliated Hospital of Jiaxing University; an age of >18 years; the absence of dramatic perioperative hemodynamic changes; and successful discharge from the hospital. The exclusion criteria were emergency surgery; New York Heart Association (NYHA) class IV heart failure; incomplete cTnI data (measured every 6 h after surgery in our unit); and coronary heart disease, perioperative coronary embolism, or a pre-existing high cTnI level (≥0.2 ng/ml).39 The patients’ baseline characteristics (age, sex, body mass index, left ventricular ejection fraction, NYHA class, type of surgery needed, preoperative medications, and coexisting disease), surgical procedure details (CPB time, cross-clamping time, cardioplegia dosage, and cardiac cardioversion), and perioperative cTnI levels were recorded by browsing the Medical Anesthesia Information System (primarily consisting of information on intraoperative data before ICU admission) and the Resident Information Service System (which included all electronic data from the operating room, as well as the ward and ICU data). The abovementioned variables were also chosen as potential confounders for the multiple regression analysis. After exclusion of ineligible patients, consecutive eligible patients were assigned to two groups: the non-DEX group (only sevoflurane postconditioning; this strategy was used in an ongoing clinical study) and the DEX group (DEX infusion was administered based on sevoflurane postconditioning). Electronic data were collected from the abovementioned databases by an independent inspector. Because this study was retrospective, no a priori statistical power analysis calculation was used to guide the sample size.
General anesthesia was induced with midazolam (average of 0.1 mg/kg), etomidate (average of 0.3 mg/kg), lidocaine (average of 0.5 mg/kg), sufentanil (average of 1 µg/kg), and rocuronium (average 0.6 mg/kg). Phenylephrine (20-µg single injection in our unit) was used intermittently to manage fluctuations in blood pressure. Ventilation parameters were adjusted to an end-tidal carbon dioxide pressure of 35 to 45 mmHg. Arteriovenous catheterization and transesophageal echocardiography were usually applied for hemodynamic and cardiac functional monitoring. DEX infusion was performed between intubation and the beginning of CPB; the specific dose was determined by an anesthesiologist, with a defined use of 0.2 to 0.7 μg/kg/h in our unit. The vasoactive drugs used during surgery included adrenaline, nitroglycerin, norepinephrine, and dopamine. The CPB apparatus used was equipped with oxygenators and a circuit used for cold blood cardioplegia (MAQUET, Rastatt, Germany). The acid–base balance was guaranteed under medium hypothermic CPB. Sevoflurane inhalation was provided through a Dräger vaporizer (Drägerwerk AG, Lübeck, Germany) attached to the oxygenator, started at approximately the same time as aortic declamping, and continued for approximately 20 min. A sevoflurane minimum alveolar concentration of 2.0 was maintained during postconditioning. Anesthesia maintenance was based on the combination of propofol and sufentanil, with the aim of maintaining a bispectral index of 40 to 60. After surgery, the patients were transferred to the ICU and sedated with propofol or sufentanil as required. The dose was controlled by the ICU physician.
The cTnI measurements were collected from an electronic database. The cTnI level was examined every 6 h for the first 24 h after surgery using an enzyme-linked immunosorbent assay kit (Life Diagnostics, West Chester, PA, USA) according to the manufacturer’s protocol. All available parameters for anesthetic and vasoactive agents were recorded from the perioperative procedure database. Perioperative hemodynamic data (mean arterial pressure, heart rate, cardiac index, and central venous pressure) were also recorded before induction, before CPB, 2 h after CPB, and 24 h after CPB.
The primary endpoint of this study was the peak cTnI level, which was defined as the highest average level among those determined at different time points. The following postoperative outcomes and complications were also reported: the length of ICU stay, time to extubation, length of hospital stay, and occurrence of renal failure, sepsis, and reoperation for bleeding.
Statistical methods
Continuous variables are expressed as mean ± standard deviation or median and interquartile range as appropriate. Categorical and rank variables are expressed as count (percentage). SPSS Statistics for Windows, Version 17.0 (SPSS Inc., Chicago, IL, USA) was used to conduct independent t-tests or chi-squared tests for continuous or categorical variables, and the Mann–Whitney U test was applied for non-normally distributed continuous variables. Linear regression was used with the univariate model. A stepwise method of multiple linear regression analysis was utilized to assess the associations between univariate factors and the peak cTnI level. Repeated-measures analysis of variance was applied to hemodynamic data and cTnI comparisons at different time points. All reported p values were two-sided, and p values of <0.05 were considered significant.
Results
Baseline and perioperative data
The study population comprised 100 patients (non-DEX group, n = 47; DEX group, n = 53). Demographic and preoperative data of all included patients are presented in Table 1. After screening, fully integrated data were found at only four time points for both groups. No significant differences were found in sex, body mass index, baseline cTnI level, creatine kinase isoenzyme MB level, creatinine level, left ventricular ejection fraction, NYHA class, surgery type, preoperative medication, coexisting diseases, cardioplegia dosage, surgery time, length of ICU stay, or extubation time between the two groups. Patients in the DEX group were older than those in the non-DEX group; moreover, lower cTnI levels and fewer instances of cardiac cardioversion were found in the non-DEX group (p < 0.01) (Table 1).
Table 1.
Demographic and perioperative data in both groups.
| Variables | Non-DEX group (n=47) | DEX group (n=53) | p-value |
|---|---|---|---|
| Age, years | |||
| Median (IQR) | 46 (29) | 57 (21) | 0.041* |
| Sex | |||
| Female | 22 (47) | 30 (57) | |
| Male | 25 (53) | 23 (43) | 0.328 |
| BMI, kg/m2 | |||
| Median (IQR) | 24 (13) | 26 (11) | 0.609 |
| Baseline cTnI, ng/mL | |||
| Median (IQR) | 0.08 (0.079) | 0.07 (0.060) | 0.709 |
| Average highest cTnI, ng/mL | |||
| Median (IQR) | 16.5 (5.4) | 13.4 (5.8) | <0.01** |
| CK-MB, U/L | |||
| Median (IQR) | 24 (9) | 24 (7) | 0.461 |
| Creatinine level, μmol/L | |||
| Median (IQR) | 87 (37) | 77 (43) | 0.785 |
| Coexisting diseases | |||
| Hypertension | 43 (91) | 45 (85) | 0.312 |
| Diabetes | 12 (26) | 8 (15) | 0.193 |
| Renal failure | 3 (6) | 2 (4) | 0.550 |
| Pulmonary hypertension | 12 (26) | 10 (19) | 0.422 |
| Atrial fibrillation | 6 (13) | 9 (17) | 0.556 |
| Congestive heart failure | 4 (9) | 3 (6) | 0.577 |
| Hypertrophic cardiomyopathy | 0 (0) | 1 (2) | 0.344 |
| Sepsis | 1 (2) | 0 (0) | 0.286 |
| LVEF | |||
| ≥50% | 31 (66) | 39 (74) | |
| <50% | 16 (34) | 14 (26) | 0.406 |
| NYHA classification | |||
| I | 6 (13) | 10 (19) | |
| II | 28 (59) | 32 (60) | |
| III | 13 (28) | 11 (21) | 0.305 |
| Surgery type | |||
| 1 valve | 10 (21) | 9 (17) | 0.585 |
| 2 valves | 22 (47) | 24 (45) | 0.879 |
| 3 valves | 15 (32) | 20 (38) | 0.542 |
| Preoperative medication | |||
| ACEI | 6 (13) | 8 (15) | 0.738 |
| Beta blocker | 10 (21) | 15 (28) | 0.418 |
| Calcium blocker | 1 (2) | 2 (4) | 0.630 |
| Nitrate drug | 2 (4) | 3 (6) | 0.748 |
| Diuretic | 46 (98) | 51 (96) | 0.630 |
| Spironolactone | 46 (98) | 51 (96) | 0.630 |
| Digoxin | 18 (38) | 20 (38) | 0.954 |
| Perioperative parameters | |||
| Blood transfusion | |||
| Yes | 34 (72) | 43 (81) | |
| No | 13 (28) | 10 (19) | 0.297 |
| CPB time | |||
| ≥120 minutes | 16 (34) | 23 (43) | |
| <120 minutes | 31 (66) | 30 (57) | 0.339 |
| Cross-clamping time | |||
| ≥60 minutes | 30 (64) | 34 (64) | |
| <60 minutes | 17 (36) | 19 (36) | 0.973 |
| Cardioplegia dosage | |||
| ≥25 ml/kg | 33 (70) | 38 (72) | |
| <25 ml/kg | 14 (30) | 15 (28) | 0.870 |
| Cardiac cardioversion | |||
| Yes | 36 (77) | 29 (55) | |
| No | 11 (23) | 24 (45) | 0.022* |
Data are presented as median (IQR) for continuous variables and n (%) for categorical data. BMI, body mass index; cTnI, cardiac troponin I; CK-MB, creatine kinase isoenzyme MB; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; ACEI, angiotensin-converting enzyme inhibitors; CPB, cardiopulmonary bypass; DEX, dexmedetomidine. *p<0.05, **p<0.01.
DEX was injected at an average infusion rate of 0.5 µg/kg/h, and the overall amount was 28 µg (interquartile range, 13 µg). The doses of sufentanil (118±19 vs. 183±26 µg) and propofol (1243±104 vs. 1754±143 mg) were markedly lower in the DEX than non-DEX group (p < 0.01), but these dose differences were not associated with the cTnI level. There were no differences in sevoflurane or midazolam consumption between the two groups. We could not calculate the use of vasoactive drugs because of the variation of use of these drugs in the ICU according to the patients’ different needs.
The perioperative hemodynamic data (mean arterial pressure, heart rate, cardiac index, and central venous pressure) are shown in Table 2, and the postoperative outcomes and complications are shown in Table 3. No obvious differences were found between the DEX and non-DEX groups.
Table 2.
Perioperative hemodynamic data in both groups.
| Before induction | Before CPB | 2 hours after CPB | 24 hours after CPB | ||
|---|---|---|---|---|---|
| MAP, mmHg | |||||
| DEX group | 90±18 | 69±12* | 80±17* | 93±24 | |
| Non-DEX group | 91±20 | 71±10* | 83±21 | 92±21 | |
| HR, beats/minute | |||||
| DEX group | 86±13 | 75±12* | 90±24 | 95±24* | |
| Non-DEX group | 82±15 | 77±11 | 93±25* | 93±26* | |
| CVP, mmHg | |||||
| DEX group | 9±2 | 8±3 | 7±3# | ||
| Non-DEX group | 10±3 | 9±3 | 8±3# | ||
| CI, L/min/m2 | |||||
| DEX group | 3.58±0.75 | 2.78±0.54# | 3.49±0.82 | ||
| Non-DEX group | 3.40±0.78 | 2.74±0.65# | 3.25±0.75 | ||
Values are presented as mean ± standard deviation. DEX, dexmedetomidine; CPB, cardiopulmonary bypass; MAP, mean arterial pressure; HR, heart rate; CVP, central venous pressure; CI, cardiac index. *p<0.05 compared with before induction; #p<0.05 compared with before CPB.
Table 3.
Postoperative outcomes and complications in both groups.
| Variables | Non-DEX group (n=47) | DEX group (n=53) | p-value |
|---|---|---|---|
| Length of ICU stay, days | 34 (10) | 33 (7) | 0.214 |
| Time to extubation, hours | 22 (14) | 21 (9) | 0.174 |
| Length of hospital stay, days | 10 (4) | 11 (3) | 0.309 |
| Renal failure (n, %) | 4 (8) | 5 (9) | 1.000 |
| Sepsis (n, %) | 2 (4) | 2 (4) | 1.000 |
| Reoperation for bleeding (n, %) | 3 (6) | 2 (4) | 0.664 |
Values are presented as median (interquartile range) for continuous variables and n (%) for categorical data. DEX, dexmedetomidine; ICU, intensive care unit. *p<0.05, **p<0.01.
Univariate analysis of peak cTnI level in both groups
In the univariate linear regression model, which included age, NYHA class, CPB time, cross-clamping time, cardiac cardioversion, DEX use, and cTnI level, dependent variables (NYHA class, CPB time, and DEX use) had a potential influence on the induced variables (p < 0.01) (Table 4). For each unit increase in the NYHA class and CPB time, the cTnI level increased by 1.436 and 0.035 ng/ml, respectively. Conversely, the cTnI level was 4.035 ng/ml lower in the DEX than non-DEX group (p < 0.01).
Table 4.
Linear regression models (univariate) to identify the associations with the cTnI level.
| Variables | R2 | B | p-value |
|---|---|---|---|
| Age (years) | 0.015 | −0.033 | 0.224 |
| NYHA class (I, II, III) | 0.042 | 1.436 | 0.040* |
| CPB time (minutes) | 0.053 | 0.035 | 0.021* |
| Cross-clamping time (minutes) | 0.013 | 0.020 | 0.264 |
| Cardioversion (yes, no) | 0.018 | 1.219 | 0.188 |
| DEX use (yes, no) | 0.211 | −4.035 | <0.001* |
cTnI, cardiac troponin I; NYHA, New York Heart Association; CPB, cardiopulmonary bypass; DEX, dexmedetomidine. *p<0.1.
Multivariable analysis of peak cTnI level in both groups
Among the meaningful influential variables found in the univariable analysis, the NYHA class, DEX use, and CPB time were included in the regression model. The established regression equation was significant (F(3,96) = 12.582, p < 0.01) through elimination of the NYHA class (B = 0.780), but lost significance in the multivariate linear regression model (R = 0.531, R2 = 0.282, adjusted R2 = 0.26) (Table 5). DEX use and CPB time were independent factors that contributed to cTnI changes (DEX use: B = −4.097; 95% confidence interval, −5.609 to −2.584, p < 0.01 and CPB time: B = 0.038; 95% confidence interval, 0.011–0.064; p < 0.01). Moreover, the use of DEX exerted a more significant influence on the cTnI level than did the CPB time (standardized B for DEX and CPB time, −0.466 and 0.244, respectively). DEX decreased the cTnI level by 4.097 µmol/L when we controlled for other dependent variables.
Table 5.
Results of multivariable linear models for potential factors influencing the cTnI level.
|
Unstandardized |
Standardized |
95% CI of B |
||||
|---|---|---|---|---|---|---|
| Factor | B | Std error | Beta | p-value | Lower bound | upper bound |
| Constant | 11.573 | 1.839 | <0.001* | 9.923 | 15.222 | |
| DEX | −3.989 | 0.765 | −0.454 | <0.001* | −5.507 | −2.471 |
| CPB time | 0.034 | 0.014 | 0.220 | 0.015* | 0.007 | 0.061 |
| NYHA class | 0.780 | 0.622 | 0.112 | 0.213 | −0.455 | 2.015 |
cTnI, cardiac troponin I; CI, confidence interval; DEX, dexmedetomidine; CPB, cardiopulmonary bypass; NYHA, New York Heart Association; Std error, standard error. The independent variables are DEX and CPB. The dependent variable is the cTnI level. A negative standardized beta value indicates that the use of DEX is associated with a lower cTnI level. *p<0.05.
Stratification analysis of both groups
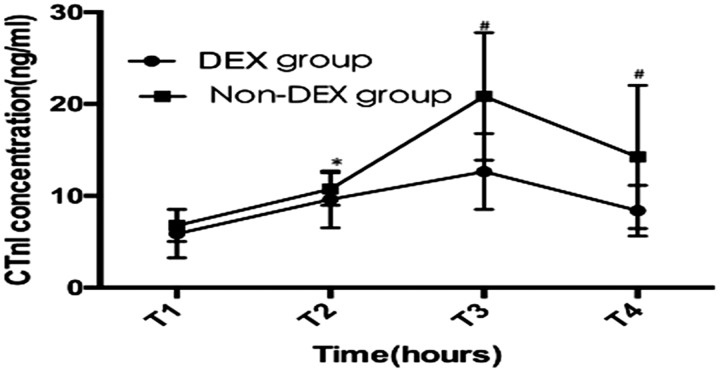
Over time, the cTnI level showed significant fluctuations in both groups (p < 0.01) (Figure 1). The peak cTnI level appeared at approximately 12 h after surgery. DEX use had an interactive influence on the cTnI level at every time point (p < 0.01). Based on the multivariate analysis of variance, the cTnI levels at 6, 12, and 24 h after surgery were significantly lower in the DEX than non-DEX group (p < 0.01, p < 0.01, and p = 0.020, respectively) (Table 6).
Figure 1.
cTnI values at different time points after surgery. DEX, dexmedetomidine; Non-DEX, without dexmedetomidine; cTnI, cardiac troponin I; T1, end of surgery; T2, 6 hours after surgery; T3, 12 hours after surgery; T4, 24 hours after surgery. The line chart with bar shows the mean ± standard deviation. *p=0.028, #p<0.01.
Table 6.
cTnI levels at different time points.
| Time points | D group | C group | C-D value | p-value | 95% CI |
|---|---|---|---|---|---|
| T1 | 5.87±2.64 | 6.75±1.75 | 0.88±0.46 | 0.055 | −0.21, 1.78 |
| T2 | 9.60±3.10 | 10.74±1.77 | 1.14±0.51 | 0.028* | 0.12, 2.16 |
| T3 | 12.65±4.13 | 20.48±6.95 | 7.83±1.13 | 0.000** | 5.59, 10.07 |
| T4 | 8.38±2.76 | 14.24±7.80 | 5.86±1.80 | 0.002** | 2.28, 9.44 |
Values are presented as mean ± standard deviation. D group, DEX group; C group, non-DEX group; C-D value, difference between the C and D groups; CI, confidence interval; T1, end of surgery; T2, 6 hours after surgery; T3, 12 hours after surgery; T4, 24 hours after surgery. *p<0.05, **p<0.01.
Discussion
In the present study, DEX infusion was correlated with changes in the cTnI level after valve replacement surgery with sevoflurane postconditioning. Additionally, DEX infusion led to decreased cTnI levels. DEX infusion may protect against myocardial injury at 6, 12, and 24 h after cardiac valve surgery.
Elevated cTnI levels have always been associated with adverse outcomes and poor short- and mid-term survival.6,9 Despite the high sensitivity and specificity of cTnI, the underlying cause of elevated cardiac cTnI levels after cardiac surgery has generally remained unclear because of the lack of high-throughput assay standardization, confounding factors, or high variation among different studies, such as the timing of testing and confounding diseases. Thus far, the specific mechanism remains unknown. Identifying the cause of these elevated levels and establishing precise methods with which to address this dilemma are urgently needed. In the present study, we adopted the highest average cTnI level as the primary outcome instead of the cTnI level at a single time point to avoid confounding factors.
CPB and aortic cross-clamping trigger I/R injury in cardiac surgery. “Conditioning” the myocardium may be an effective solution. Sevoflurane postconditioning, a form of pharmacological conditioning, exhibits the same efficacy as ischemic conditioning. Several clinical studies and meta-analyses have demonstrated the contribution of a potential cardioprotective strategy by sevoflurane postconditioning.17,22,23,40 Related fundamental research assumed the involvement of the protein kinase C, PI3K/Akt, and extracellular signal-related kinase 1/2 pathways.23 The safety and efficacy of DEX in perioperative cardiac surgery have been confirmed.24 Furthermore, the organ-protective characteristics of DEX have been verified by an increasing number of studies in which the signaling pathways are the same as those influenced by sevoflurane.41,42 However, not every study has shown advantages; one study revealed important abnormalities, such as bradycardia or hypotension,34 that were mostly caused by DEX infusion of different loading and maintenance doses. Additionally, in a study by Chi et al.,43 the extubation time and length of stay were prolonged in patients who underwent off-pump CABG with high-dose DEX infusion (loading dose, 1 μg/kg; maintenance dose, 0.6 μg/kg/h) despite significant cardiac protection. Thus, DEX can enhance the protective effect if an appropriate dose or complex strategy is applied, such as sevoflurane “conditioning.” For this reason, we applied DEX preconditioning combined with sevoflurane “conditioning” in the present study.
The influencing factors that lead to cardiac injury range from anesthetic factors to operative factors. In the present study, the CPB time, NYHA class, and DEX use were all correlated with myocardial injury. As the only meaningful anesthetic factor in this study, DEX was negatively correlated with the cTnI level (r = −0.454, p < 0.001), and this was actually equivalent to the well-known myocardial protection of DEX in cardiac surgery.24,44 Based on entering or removing the probability of F≤0.05 and F≥0.100, both DEX use and the CPB time contributed to the occurrence of myocardial injury in the multivariable analysis, and DEX use was a greater contributor to myocardial injury (0.454>0.220).
To the best of our knowledge, the present study is the first to reveal the influence of DEX preconditioning on sevoflurane postconditioning in cardiac surgery. cTnI was selected as the only outcome with which to predict myocardial injury and was measured at 6-h intervals after surgery in our hospital. The average peak cTnI level was 14.7 ng/ml at approximately 12 to 24 h after surgery, indicating obvious myocardial injury, despite the contributions of multiple factors. This result is similar to the results reported by Croal et al.,13 who analyzed many different factors involved in myocardial injury in patients undergoing cardiac surgery and detected extremely elevated cTnI levels 24 h after CPB. In a study by Shen et al.,32 lower cTnI levels were observed following DEX intervention at 48 h after surgery than following the control intervention; the 48-h postsurgery cTnI levels were also lower than the 24-h postsurgery cTnI levels. Studies by Sedighinejad et al.45 and Chi et al.43 also indicated that DEX significantly decreased the cTnI level after CABG surgery. Compared with the abovementioned outcomes, the protective events against cardiac injury appeared sooner in our study than in other studies, although different surgical backgrounds and DEX usages were found in some studies. Furthermore, compared with non-DEX use, DEX infusion decreased the average cTnI level by approximately 4 ng/ml (p < 0.001), and the greatest decrease was 7.8 ng/ml at 12 h after surgery. A similar trend of the changes in cTnI at different time points in the DEX group was observed in a study by Chen et al.,36 in which CABG under CPB was carried out and DEX was infused throughout the entire surgical period. Thus, the difference in surgery type and DEX use resulted in a further decrease in cTnI, but the consequence was identical to that in the current study.
Generally, the peak cTnI concentration, which depends on the type of surgery or the subsequent degree of myocardial trauma, is most often observed 24 h after CPB, regardless of other factors. In the present study, the peak cTnI level appeared 12 h after CPB, which differs from the results of the study by Croal et al.13 This difference may be due to the recruitment of patients who underwent valve surgery only; the direct result was a higher cTnI concentration observed sooner after valve surgery than after CABG or other surgery types.
Another risk factor that led to high cTnI levels was the CPB time, which is the main cause of myocardial injury in surgery and is similar to the conclusion regarding risk factors reported by Paparella et al.,6 showing that a long CPB time is one of the contributors to myocardial damage.
Nonetheless, the present study had several limitations. First, additional outcome variables were not studied. Moreover, DEX intervention has not been confirmed as an independent influencing factor; thus, further research is warranted. Second, the sample size was small, and the peak cTnI level served as the only parameter. In other studies, the cTnI level varied among different valve surgeries, and some evidence even indicated that no apparent effect was observed in some types of valve surgery. Therefore, a subgroup analysis in terms of different surgeries is needed in future studies. Third, this study contained selection bias due to the DEX dosage and utility time, which were determined by the anesthetist. Therefore, future studies should highlight the influence of these factors on related outcomes. These factors will be further explored in future research if the plasma concentration of DEX is considered. Finally, there was no subgroup analysis of the effect of different doses of DEX on the outcome.
Conclusion
In conclusion, we found a significant decrease in myocardial injury at 6, 12, and 24 h after valve replacement surgery when DEX was infused with sevoflurane postconditioning. However, more prospective, multicenter, randomized controlled trials are needed to verify the reliability of these results and mechanisms.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Zhejiang Science and Technology Project for Medical and Health (grant no. 2015KYB388) and the Jiaxing Municipal Science and Technology Project (grant no. 2016BY28019).
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Association. Circulation 2015; 131: e29–e322. [DOI] [PubMed] [Google Scholar]
- 2.Nashef SA, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012; 41: 734–744; discussion 44–45. [DOI] [PubMed] [Google Scholar]
- 3.Bustamante A, Díaz-Fernández B, Pagola J, et al. Admission troponin-I predicts subsequent cardiac complications and mortality in acute stroke patients. Eur Stroke J 2016; 1: 205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boeddinghaus J, Nestelberger T, Twerenbold R, et al. High-sensitivity cardiac troponin I assay for early diagnosis of acute myocardial infarction. Clin Chem 2019; 65. PMID: 30988172. [DOI] [PubMed] [Google Scholar]
- 5.Al-Riyami AZ, Al-Khabori M, Baskaran B, et al. Impact of blood transfusion on troponin I levels and outcomes after cardiac surgery: a cohort study. Oman Med J 2019; 34: 14–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paparella D, Guida P, Caparrotti S, et al. Myocardial damage influences short- and mid-term survival after valve surgery: a prospective multicenter study. J Thorac Cardiovasc Surg 2014; 148: 2373–2379.e1. [DOI] [PubMed] [Google Scholar]
- 7.van Geene Y, van Swieten HA, Noyez L. Cardiac troponin I levels after cardiac surgery as predictor for in-hospital mortality. Interact Cardiovasc Thorac Surg 2010; 10: 413–416. [DOI] [PubMed] [Google Scholar]
- 8.Tzimas P, Baikoussis NG, Kalantzi K, et al. Is early assessment of cardiac troponin I a valuable predictor of mortality after cardiac surgery? Interact Cardiovasc Thorac Surg 2010; 10: 416–417. [DOI] [PubMed] [Google Scholar]
- 9.Lurati Buse GA, Koller MT, Grapow M, et al. The prognostic value of troponin release after adult cardiac surgery - a meta-analysis. Eur J Cardiothorac Surg 2010; 37: 399–406. [DOI] [PubMed] [Google Scholar]
- 10.Nesher N, Alghamdi AA, Singh SK, et al. Troponin after cardiac surgery: a predictor or a phenomenon? Ann Thorac Surg 2008; 85: 1348–1354. [DOI] [PubMed] [Google Scholar]
- 11.Oshima K, Kunimoto F, Takahashi T, et al. Postoperative cardiac troponin I (cTnI) level and its prognostic value for patients undergoing mitral valve surgery. Int Heart J 2010; 51: 166–169. [DOI] [PubMed] [Google Scholar]
- 12.Adabag AS, Rector T, Mithani S, et al. Prognostic significance of elevated cardiac troponin I after heart surgery. Ann Thorac Surg 2007; 83: 1744–1750. [DOI] [PubMed] [Google Scholar]
- 13.Croal BL, Hillis GS, Gibson PH, et al. Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 2006; 114: 1468–1475. [DOI] [PubMed] [Google Scholar]
- 14.Swaanenburg JC, Loef BG, Volmer M, et al. Creatine kinase MB, troponin I, and troponin T release patterns after coronary artery bypass grafting with or without cardiopulmonary bypass and after aortic and mitral valve surgery. Clin Chem 2001; 47: 584–587. [PubMed] [Google Scholar]
- 15.Eagle KA, Guyton RA, Davidoff R, et al. ACC/AHA 2004 guideline update for coronary artery bypass graft surgery: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1999 Guidelines for Coronary Artery Bypass Graft Surgery). Circulation 2004; 110: 1168–1176. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Shen Z, Liu Y, et al. Sevoflurane protects against intestinal ischemia-reperfusion injury partly by phosphatidylinositol 3 kinases/Akt pathway in rats. Surgery 2015; 157: 924–933. [DOI] [PubMed] [Google Scholar]
- 17.Bettex DA, Wanner PM, Bosshart M, et al. Role of sevoflurane in organ protection during cardiac surgery in children: a randomized controlled trial. Interact Cardiovasc Thorac Surg 2015; 20: 157–165. [DOI] [PubMed] [Google Scholar]
- 18.Peng S, Kalikiri P, Mychaskiw G, 2nd, et al. Sevoflurane postconditioning ameliorates oxygen-glucose deprivation-reperfusion injury in the rat hippocampus. CNS Neurosci Ther 2011; 17: 605–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kong HY, Zhu SM, Wang LQ, et al. Sevoflurane protects against acute kidney injury in a small-size liver transplantation model. Am J Nephrol 2010; 32: 347–355. [DOI] [PubMed] [Google Scholar]
- 20.Annecke T, Chappell D, Chen C, et al. Sevoflurane preserves the endothelial glycocalyx against ischaemia-reperfusion injury. Br J Anaesth 2010; 104: 414–421. [DOI] [PubMed] [Google Scholar]
- 21.Xie H, Zhu J, Liu LX, et al. Sevoflurane post-conditioning protects isolated rat hearts against ischemia-reperfusion injury via activation of the ERK1/2 pathway. Acta Pharmacol Sin 2014; 35: 1504–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elena B, Marina P, Francesco DS, et al. Volatile anaesthetics added to cardiopulmonary bypass are associated with reduced cardiac troponin. Perfusion 2017; 32: 547–553. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine S, Tritapepe L, Hanouz JL, et al. The mechanisms of cardio-protective effects of desflurane and sevoflurane at the time of reperfusion: anaesthetic post-conditioning potentially translatable to humans? Br J Anaesth 2016; 116: 456–475. [DOI] [PubMed] [Google Scholar]
- 24.Ye CH, Jia Y, Lei Z, et al. Molecular targets and mechanism of action of dexmedetomidine in treatment of ischemia/reperfusion injury (Review). Mol Med Rep 2014; 9: 1542–1550. [DOI] [PubMed] [Google Scholar]
- 25.Zhang JJ, Peng K, Zhang J, et al. Dexmedetomidine preconditioning may attenuate myocardial ischemia/reperfusion injury by down-regulating the HMGB1-TLR4-MyD88-NF-small ka, CyrillicB signaling pathway. PloS One 2017; 12: e0172006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan F, Fu H, Sun K, et al. Effect of dexmedetomidine on cerebral ischemia-reperfusion rats by activating mitochondrial ATP-sensitive potassium channel. Metab Brain Dis 2017; 32: 539–546. [DOI] [PubMed] [Google Scholar]
- 27.Yeda X, Shaoqing L, Yayi H, et al. Dexmedetomidine protects against renal ischemia and reperfusion injury by inhibiting the P38-MAPK/TXNIP signaling activation in streptozotocin induced diabetic rats. Acta Cir Bras 2017; 32: 429–439. [DOI] [PubMed] [Google Scholar]
- 28.Yang YF, Peng K, Liu H, et al. Dexmedetomidine preconditioning for myocardial protection in ischaemia-reperfusion injury in rats by downregulation of the high mobility group box 1-toll-like receptor 4-nuclear factor kappaB signalling pathway. Clin Exp Pharmacol Physiol 2017; 44: 353–361. [DOI] [PubMed] [Google Scholar]
- 29.Kucukebe OB, Ozzeybek D, Abdullayev R, et al. Effect of dexmedetomidine on acute lung injury in experimental ischemia-reperfusion model. Braz J Anesthesiol 2017; 67: 139–146. [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Wu S, Yu X, et al. Dexmedetomidine protects rat liver against ischemia-reperfusion injury partly by the alpha2A-adrenoceptor subtype and the mechanism is associated with the TLR4/NF-kappaB pathway. Int J Mol Sci 2016; 17: pii: E995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng L, Chen H, Wei N, et al. The cardioprotective effect of dexmedetomidine on regional ischemia/reperfusion injury in type 2 diabetic rat hearts. Microvasc Res 2019; 123: 1–6. [DOI] [PubMed] [Google Scholar]
- 32.Shen J, Sun Y, Han D, et al. [Effects of dexmedetomidine on perioperative cardiac adverse events in elderly patients with coronary heart disease]. Zhong Nan Da Xue Xue Bao Yi Xue Ba 2017; 42: 553–557. [DOI] [PubMed] [Google Scholar]
- 33.Bulow NM, Colpo E, Pereira RP, et al. Dexmedetomidine decreases the inflammatory response to myocardial surgery under mini-cardiopulmonary bypass. Braz J Med Biol Res 2016; 49: e4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tosun Z, Baktir M, Kahraman HC, et al. Does dexmedetomidine provide cardioprotection in coronary artery bypass grafting with cardiopulmonary bypass? A pilot study. J Cardiothorac Vasc Anesth 2013; 27: 710–715. [DOI] [PubMed] [Google Scholar]
- 35.Ren JJ, Huang LN, Liu Y, et al. Protective effect of dexmedetomidine in coronary artery bypass grafting surgery. Exp Ther Med 2013; 6: 497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen SL, Lu J, Jiang Y, et al. Effect of dexmedetomidine on myocardial ischemia-reperfusion injury. Int J Clin Exp Med 2015; 8: 21166–21172. [PMC free article] [PubMed] [Google Scholar]
- 37.Wang HS, Zhang SD, Xu SY, et al. The efficacy and mechanism of dexmedetomidine in myocardial apoptosis via the renin–angiotensin– aldosterone system. J Renin Angiotensin Aldosterone Syst 2015; 16: 1274–1280. [DOI] [PubMed] [Google Scholar]
- 38.Cheng XY, Gu XY, Gao Q, et al. Effects of dexmedetomidine postconditioning on myocardial ischemia and the role of the PI3K/Akt-dependent signaling pathway in reperfusion injury. Mol Med Rep 2016; 14: 797–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Patsias I, Swanson B, Hudson M, et al. The (Dis) utility of a change in troponin i for diagnosis of non-ST-segment elevation myocardial infarction in an observation unit. Crit Pathw Cardiol 2017; 16: 105–108. [DOI] [PubMed] [Google Scholar]
- 40.van der Baan A, Kortekaas KA, van Es E, et al. Sevoflurane-enriched blood cardioplegia: the intramyocardial delivery of a volatile anesthetic. Perfusion 2015; 30: 295–301. [DOI] [PubMed] [Google Scholar]
- 41.Zhang X, Zhao X, Wang Y. Dexmedetomidine: a review of applications for cardiac surgery during perioperative period. J Anesth 2015; 29: 102–111. [DOI] [PubMed] [Google Scholar]
- 42.Sun Y, Jiang C, Jiang J, et al. Dexmedetomidine protects mice against myocardium ischemic/reperfusion injury by activating an AMPK/PI3K/Akt/eNOS pathway. Clinical and experimental pharmacology & physiology 2017; 44(9): 946–953. [DOI] [PubMed] [Google Scholar]
- 43.Chi X, Liao M, Chen X, et al. Dexmedetomidine attenuates myocardial injury in off-pump coronary artery bypass graft surgery. J Cardiothorac Vasc Anesth 2016; 30: 44–50. [DOI] [PubMed] [Google Scholar]
- 44.Xue FS, Li RP, Liu GP, et al. Assessing renoprotective effect of perioperative dexmedetomidine in cardiac surgery patients. Kidney Int 2016; 89: 1163–1164. [DOI] [PubMed] [Google Scholar]
- 45.Sedighinejad A, Mohammadzadeh Jouryabi A, Imantalab V, et al. Efficacy of dexmedetomidine in coronary artery bypass graft surgery under cardiopulmonary bypass: a randomized, double-blind clinical trial. Iranian Red Crescent Med J 2018; 20: e67738. [DOI] [PMC free article] [PubMed] [Google Scholar]