Short abstract
Objective
To study the effects of D-methionine in a mouse model of noise-induced hearing loss (NIHL).
Methods
We investigated changes in auditory function and microscopic cochlear structure in a mouse model of NIHL, and carried out 4-hydroxynonenal (4-HNE) immunostaining and terminal deoxynucleotidyl transferase dUTP nick-end labeling, and examined expression levels of connexins 26 and 30 by western blot.
Results
The auditory brainstem response threshold was significantly increased by noise exposure. Noise exposure also damaged the inner and particularly the outer hair cells in the cochlear basement membrane, while histochemistry demonstrated only scattered loss of hair cells in the basement membrane in mice treated with D-methionine before or after noise exposure. D-methionine inhibited apoptosis in the cochlear basement membrane, stria vascularis, and spiral ligament. 4-HNE expression in the basement membrane, stria vascularis, and spiral collateral ligament was increased by noise exposure, but this increase was attenuated by D-methionine. Connexin 26 and connexin 30 expression levels were reduced by noise exposure, and this effect was similarly attenuated by D-methionine administered either before or after noise exposure.
Conclusion
D-methionine administered before or after noise exposure could rescue NIHL by protecting cochlear morphology, inhibiting apoptosis, and maintaining connexin 26 and 30 expression.
Keywords: D-methionine, noise-induced hearing loss, oxidative stress, connexin 26, connexin 30, cochlea
Introduction
Noise-induced hearing loss (NIHL) may result from excessive noise exposure in recreational, occupational, and military settings. Exposure to excessive noise is the major cause of permanent hearing loss. Intense noise generally leads to permanent hearing loss, while noise at lower intensities may cause temporary hearing loss.1 Hearing protection measures, such as the use of protective earplugs and earmuffs, are effective ways to prevent NIHL. Several factors may contribute to the mechanism of NIHL, including reduced cochlear blood flow, glutamate excitoxicity, altered calcium homeostasis, and the generation of oxidative stress.2,3 Hearing loss reflects oxidative stress and structural changes in the hearing organ.4 Oxidative stress plays a critical role in auditory dysfunction and hearing loss by activating cochlear cell death pathways after exposure to noise.5,6 Noise can also induce metabolic disorders in cells, alter their redox state, and produce excessive oxygen and nitrogen free radicals, which can in turn destroy cellular lipids, proteins, and nucleotides, thus positively regulating the apoptotic pathway. Oxidative stress has been identified as a major contributor to noise-related damage,7 while the use of antioxidants has been reported to reduce NIHL.8,9
D-methionine (D-met) is an essential amino acid in the human body, and is a natural component of cheese and yogurt. A prior study showed that D-met protected against both drug-induced ototoxicity and NIHL,10 and against permanent NIHL and ototoxic deafness caused by cisplatin, carboplatin, and aminoglycosides.11 D-met may serve as a free radical scavenger, and can be reversibly oxidized12 and support cellular antioxidant systems by preventing the efflux of glutathione from cells,13 elevating cellular glutathione levels14 and protecting levels of the antioxidant enzymes superoxide dismutase, catalase, and glutathione reductase. The otoprotective ability of D-met in NIHL may be secondary to its direct and indirect antioxidant effects, which mitigate the oxidative stress generated by excessive noise exposure, thus helping to prevent hearing loss and sensory cell death.15 In this study, we explored the preventive and therapeutic effects of D-met on auditory function, cochlear morphology, oxidative stress, and apoptosis in a mouse model of NIHL.
Materials and methods
Animals
Forty healthy female Kunming mice (weight 25–30 g) were provided by the Laboratory Animal Center (Hebei Medical University, China). This study was approved by the Animal Care and Ethics Committee of the Third Hospital of Hebei Medical University, Shijiazhuang, China. Mice were randomly divided into four groups: control group (CG; mice without noise exposure, with intraperitoneal (i.p.) injection of normal saline (400 mg/kg) twice a day for 3 consecutive days); noise-exposed group (NG; i.p. injection of normal saline twice a day for 3 days followed by exposure to noise (100 dB of sound pressure level (SPL)) twice a day for 3 days, for 8 hours each); pre D-met group (PreG; i.p. injection of D-met (400 mg/kg; M9375-5G, Meilunbio, Shijiazhuang, China) twice a day for 3 days, followed by exposure to noise (100 dB SPL) twice a day for 3 days, for 8 hours each); and post D-met group (PostG; exposure to noise (100 dB SPL) twice a day for 3 days, for 8 hours each, followed by i.p. injection of D-met (400 mg/kg) twice a day for 3 days). The treatment schedules are shown in Table 1.
Table 1.
Dosing time and noise exposure in experimental mice.
| Group | Dosing time (3 consecutive days) | Noise exposure time (3 consecutive days) |
|---|---|---|
| Control group | normal saline (8:00, 20:00) | None |
| Noise-exposed group | normal saline (8:00, 20:00) | 8:00–20:00 |
| Pre D-met group | D-met (8:00, 20:00) | 8:00–20:00 |
| Post D-met group | 8:00–20:00 (noise time) | D-met (8:00, 20:00) |
D-met, D-methionine.
Noise exposure
Mice were exposed to 4 kHz narrow-band noise at 100 dB SPL. The noise was created using Sony Pictures Digital Sound Forge 8.0 software (Sony Pictures Digital, Culver City, CA, USA) and delivered by a set of loudspeakers driven by a Sony type D-NE005 player (Sony Corporation, Beijing, China) and LANEY CPX-115 active loudspeaker (Laney Amplification, Birmingham, UK) to obtain a diffuse acoustic field. The noise parameters were monitored during exposure by a system comprising a Brüel & Kjaer type 4190 microphone (Brüel & Kjaer Sound & Vibration Measurement, Denmark), a Svantek microphone preamplifier type SV03, and a Svantek type SVAN 912 sound analyzer (Svantek, Poland). The uniformity of the sound pressure distribution within the exposure chamber was confirmed by multiple location measurements. The microphone was positioned above the cage. Mice in the control group were simultaneously placed in an exposure chamber without noise.
Auditory brainstem response (ABR) test
Mice were assessed by ABR test before and after noise exposure. In the exposure chamber, mice were injected i.p. with 5% chloral hydrate (400 mg/kg) and placed in thermal bags at room temperature on the test bench. A differential active needle electrode was placed subcutaneously below the test ear, a reference electrode at the vertex, and a ground electrode was positioned just above the hind limb. A click sound was used as the ABR stimulus in this experiment. The sound stimulus consisted of 12.5-ms tone bursts with a rise–fall time of 1 ms at a frequency of 8 kHz, generated by a Centor-O audio generator (Deltamed, Racia-Alvar, Le Bouscat Cedex, France). ABR data were collected using an Intelligent Hearing Systems evoked potential unit (Intelligent Hearing Systems Corp., Miami, FL, USA). The stimuli were presented to the external auditory meatus at an intensity high enough to allow easy identification of the ABR peaks, and the sound intensity was then decreased in 5-dB intervals near the threshold. A total of 1000 tone presentations were delivered at 10 per second and averaged to obtain a waveform, using a data acquisition system and a microcomputer with Racia software. The hearing threshold was defined as the lowest intensity of stimulation that yielded a repeatable waveform with an one or two identifiable peaks.3
Cochlear basement membrane
After noise exposure for 3 days, mice were injected i.p. with 5% chloral hydrate (400 mg/kg) and placed in a supine position on a dissection table. The thoracic cavity was opened to expose the heart, and a 0.3-cm incision was made on the right atrium using ophthalmology scissors. Normal saline at 40°C was then injected from the left ventricular apex until clear fluids flowed from the right atrium, followed by injection of 4% paraformaldehyde solution. The mice were then decapitated and the temporal bone was extracted and put into 0.01 M phosphate-buffered saline (PBS). The acoustic vesicle was opened and the stapes was removed under a microscope. The round and oval window membranes were broken, a hole was punched in the tip of the cochlea, and 4% paraformaldehyde was poured in at 4°C overnight. The cochlear tissue was then washed with 0.01 M PBS and 10% EDTA was added for decalcification. After thorough decalcification, the cochlear tissue was dehydrated through graded alcohol solutions and embedded in paraffin. Finally, 4-µm tissue slices were cut along the direction of the modiolus.
Hematoxylin and eosin (HE) staining
Tissue sections were deparaffinized in xylene, rehydrated in graded alcohol solutions (70%, 85%, and 95%), and washed briefly in distilled water. The sections were then stained with Harris hematoxylin solution (Hebei Bio-High Technology, China) for 5 minutes, washed in tap water, and counterstained in eosin-phloxine solution for 2 minutes. The sections were then observed and photographed under a light microscope (Olympus, Japan).
Myosin VI staining
Tissue sections were immersed in 0.25% Triton X-100 for 5 minutes and 4% goat serum phosphate buffer for 30 minutes, and then immersed in Myosin-ССС VI phosphate buffer (1:200, Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. After two washes with 0.01 M PBS (5 minutes each), the sections were immersed in Alexa Fluor 555 (goat anti-rabbit fluorescent antibody II, 1:200; Invitrogen, Carlsbad, CA, USA) and incubated at room temperature for 1 hour. After three washes with 0.01 M PBS, the sections were mounted with 50% glycerol phosphate buffer and observed under a confocal laser scanning microscope (400×; Leica, Germany).
Immunohistochemical staining
The sections were dehydrated with alcohol and xylene and washed three times with 0.01 M PBS for 5 minutes each. The sections were then repaired with antigen repair solution (Abcam, Cambridge, MA, USA) for 2.5 minutes. The sections were blocked with endogenous peroxidase for 10 minutes, washed three times with 0.01 M PBS, and then blocked with goat serum for 10 minutes. After drying, the sections were incubated with 4-hydroxynonenal antibodies (4-HNE, 1:300; Abcam) at 4°C overnight and washed three times with 0.01 M PBS. The sections were then incubated with second antibody for 10 minutes and with reagent chain affinity labeling horseradish peroxidase (HRP) (ZSGB-BIO, Beijing, China) for 10 minutes, followed by staining with DAB (ZSGB-BIO) and HE. The sections were examined under a light microscope (200×) to visualize and quantify the positive areas.
Terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay
Cell apoptosis was detected by TUNEL assay. Tissue sections were dehydrated in alcohol and xylene, washed three times with 0.01 M PBS, repaired with antigen repair solution for 2.5 minutes, and washed a further three times with 0.01 M PBS. The sections were then permeabilized with 0.25% Triton X-100 for 8 minutes and TUNEL reagent (enzyme:stain, 1:9; Roche, Switzerland) was added and incubated without light at 37°C for 1 hour. After three washes with PBS, the sections were mounted with 50% glycerol/PBS and examined under a confocal laser scanning microscope (200×) (Leica) to observe apoptosis.
Western blotting analysis
Basement membrane proteins were extracted by centrifugation and stored in liquid nitrogen. The protein concentration was measured using a BCA protein analysis kit (Pierce Company, Thermo Fisher Scientific, Rockford, IL, USA) according to the manufacturer’s protocol. The samples were boiled for 7 minutes and then separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The separated proteins were transferred to polyvinylidene difluoride membranes (Thermo Fisher Scientific, Waltham, MA, USA) and blocked with TBST (NaCl 500 mM, Tris 20 mM, pH 7.5) containing 5% skim milk for 1 hour, and then incubated with primary antibodies (rabbit connexin 26 and connexin 30 polyclonal antibodies, 1:1000; Invitrogen; mouse β-actin monoclonal antibody, 1:2000; ZSGB-BIO) overnight at 4°C. The membranes were then washed with TBST for 10 minutes (total four times) and incubated with HRP-labeled secondary antibody (1:5000; ZSGB-BIO) for 1 hour at room temperature. The membranes were washed three times with TBST for 5 minutes each after each incubation. Finally, the membranes were treated with enhanced chemiluminescence color solution (Beyotime, Shanghai, China) and scanned using image analysis software (BandScan 5.0) for semi quantitative analysis.
Statistical analysis
All data were analyzed using SPSS 21.0 (IBM Corp., Armonk, NY, USA). The results were expressed as mean ± standard deviation. Differences among multiple groups were analyzed by one-way ANOVA with post hoc comparisons (Bonferroni test). Values of P < 0.05 were considered statistically significant.
Results
General observations
Mice in the CG had glossy fur, showed increased weight, and were sensitive to sound, while mice in the NG had less-glossy fur, showed decreased weight, and were insensitive to sound. Mice in the PreG had intermediately glossy fur, no weight loss, and were sensitive to sound, while mice in the PostG also had intermediately glossy fur, slight weight decrease, and were sensitive to sound.
ABR test
There were large interindividual differences in hearing thresholds among the mice, ranging from 15 to 40 dB before noise exposure, and from 20 to 50 dB after noise exposure. There was a 22-dB threshold shift after noise exposure. The ABR threshold shift after noise exposure for 3 days was significantly decreased by D-met at a dose of 400 mg/kg. There were no significant differences in ABR threshold values among the four groups prior to noise exposure. ABR threshold values were significantly increased after noise exposure for 3 days in the NG, PreG, and PostG (all P < 0.05) (Figure 1). However, there was no significant difference in ABR threshold values between the PreG and PostG (Figure 1).
Figure 1.
Assessment of auditory brainstem response in experimental mice. *P < 0.05 compared with control group; #P < 0.05 compared with noise group.
HE and myosin-VI staining
The inner and outer hair cells in the CG were arranged in an orderly fashion at the basement membrane, with clear structure and no signs of degeneration or defects, as shown by HE staining. However, the inner and outer hair cells in the NG showed degeneration and structural abnormalities compared with the CG. In addition, the inner hair cells in the PreG and PostG showed clear structures, but the outer hair cells showed scattered losses compared with the NG (Figure 2a). Specifically, the numbers of inner and outer hair cells indicated that noise exposure mainly damaged the outer hair cells, especially the cells in the basal turn (Figure 2b). A similar pattern was observed with myosin-VI staining. However, only PreG and PostG hair cells showed disorganization (Figure 2c).
Figure 2.
Changes in hair cells in experimental mice. (a) Hematoxylin-eosin staining of hair cells in the basement membrane (400×). (b) Quantization of inner and outer hair cells in the basement membrane. *,#,&P < 0.05 compared with control group; a,b,cP < 0.05 compared with noise group. (c) Myosin-VI staining of hair cells in the basement membrane (400×). There were obvious defects in the hair cells of the cochlear basement membrane in the noise group, but no significant changes in the structures of the inner and outer hair cells in the pre- and post D-methionine groups. i: control group; ii: noise group; iii: pre D-methionine group; iv: post D-methionine group. IHC, inner hair cell; OHC, outer hair cell.
4-HNE staining
4-HNE was barely expressed in the CG, while strong 4-HNE immunoreactivity was detected in the NG. 4-HNE immunoreactivity was significantly decreased after treatment with D-met compared with the matching NG (Figure 3a). The changes in 4-HNE staining were quantified (Figure 3b), and protein expression was significantly increased in the NG compared with the CG (P < 0.05). However, treatment with D-met significantly decreased the expression of 4-HNE in the PreG and PostG (both P < 0.05). Interestingly, there was no significant difference between the PreG and PostG with regard to 4-HNE expression.
Figure 3.
4-Hydroxynonenal (4-HNE) staining in the basement membrane, stria vascularis, and spiral ligament of the cochlea (200×). (a) Immunohistochemical staining of 4-HNE. 4-HNE staining was significantly decreased in the post D-methionine group compared with the noise group, but there was no difference in staining between the pre and post D-methionine groups. (b) Quantitative analysis of 4-HNE staining. *,#,&P < 0.05 compared with control group; a,b,cP < 0.05 compared with noise group. BM, basilar membrane; SV, stria vascularis; SL, spiral ligament of cochlea. i: control group; ii: noise group; iii: pre D-methionine group; iv: post D-methionine group. *P < 0.05 compared with control group; #P < 0.05 compared with noise group.
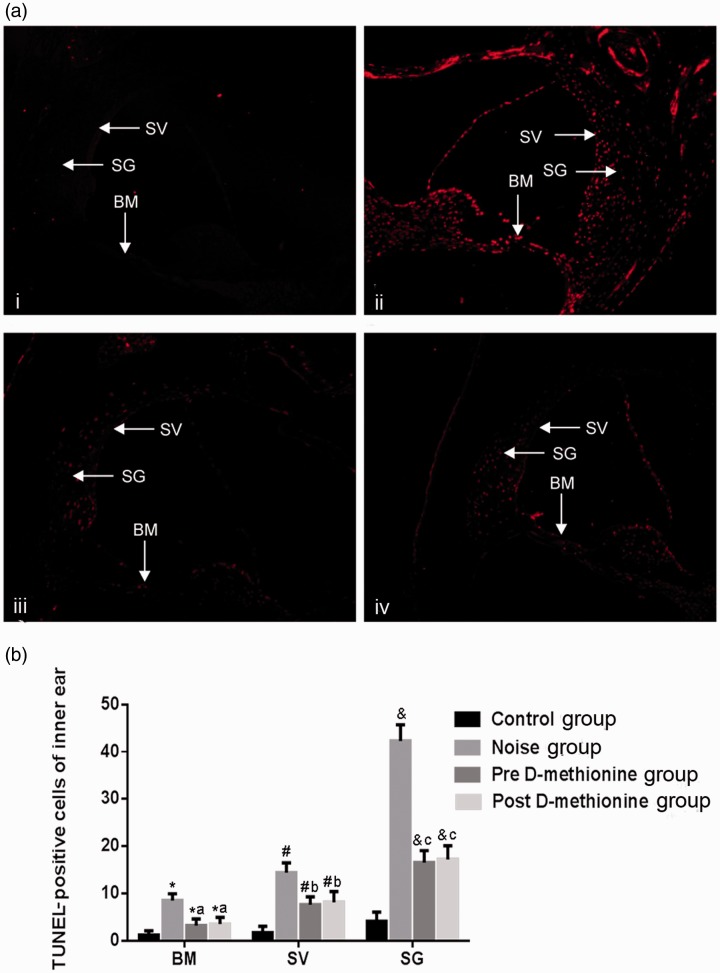
TUNEL assay
TUNEL staining was significantly greater in the NG compared with the CG. Few apoptotic cells were present on the spiral ligament of the cochlea in the CG, while the numbers of apoptotic cells in the cochlear basement membrane, stria vascularis, and spiral ligament of the cochlea were significantly higher in the NG compared with the CG (P < 0.05) (Figure 4a and 4b). Apoptotic cells in the PreG and PostG were mainly distributed on the stria vascularis and spiral ligament of the cochlea, and were significantly more frequent than in the CG (P < 0.05) (Figure 4a and 4b), but significantly less frequent than in the NG (P < 0.05). However, there was no significant difference with regard to apoptotic cell numbers between the PreG and PostG.
Figure 4.
Cell apoptosis in the basement membrane, stria vascularis, and spiral ligament of the cochlea. (a) TUNEL staining of basement membrane, stria vascularis, and spiral ligament of the cochlea (200×). (b) Quantitative analysis of TUNEL-positive cells in inner ear. *,#,&P < 0.05 compared with control group; a,b,cP < 0.05 compared with noise group. BM, basilar membrane; SV, stria vascularis; SL, spiral ligament of the cochlea. i: control group; ii: noise group; iii: pre D-methionine group; iv: post D-methionine group.
Western blotting
Connexin 26 and connexin 30 expression were detected by western blot (Figure 5). Expression levels of connexin 26 and connexin 30 were significantly lower in the NG compared with the CG (P < 0.05), and were significantly higher in the PreG/PostG than in the NG (both P < 0.05). However, there were no significant differences in either connexin between the PreG and PostG, and no significant differences between the CG and PreG/PostG (Figure 5).
Figure 5.
Connexin 26 and connexin 30 protein expression. (a) Connexin 26 and connexin 30 protein expression detected by western blotting. (b) Quantitative analyses of connexin 26 and connexin 30 protein expression levels. CG, control group; NG, noise group; PreG, pre D-methionine group; PostG, post D-methionine group. *P < 0.05 compared with CG group; #P < 0.05 compared with NG group. Cx26, connexin 26; Cx30, connexin 30.
Discussion
D-met is an oral protective agent with the ability to prevent NIHL in animal experiments.16 Numerous animal and human experiments have showed that D-met administration before noise exposure can improve the rate of hair cell loss.1 The results of the current study confirmed that D-met protected the inner and outer hair cells of the cochlear basement membrane. Many experimental studies have examined NIHL,17,18 but its complex molecular pathological mechanism means that its precise pathogenesis remains unclear. The main pathogenic mechanism is considered to involve mechanical injury and metabolic damage,12 and loss of cochlear hair cells is a characteristic of NIHL. Cochlear hair cells in mammals are non-renewable, and it is therefore important to protect against NIHL. The current HE staining and immunohistochemistry results demonstrated the obvious changes associated with auditory function in mice after noise exposure for 3 consecutive days, including the degeneration and death of inner and outer hair cells in the basement membrane. The main manifestations were an unclear cellular structure, disordered cell arrangement, and reduced cell numbers in the basement membrane. Furthermore, the most serious outer hair cell loss occurred in the basal turn and the lightest loss in the apical turn. Our findings were consistent with the results of a previous study,19 which also demonstrated that noise mainly damaged the outer hair cells. The ABR was used as an objective detection index in this study and revealed no significant difference in ABR thresholds among the groups before noise exposure, but the ABR increased in all groups exposed to noise. However, the ABR threshold was smaller in the PreG, suggesting that D-met protected against NIHL.
Previous studies showed that cochlear tissue can produce excessive reactive oxygen species (ROS) after noise exposure.20,21 Under physiological conditions, the body’s oxidation and antioxidant systems are maintained in dynamic balance; however, disruption of the balance under pathological conditions may result in excessive ROS, which may in turn promote the rapid proliferation of polyunsaturated fatty acids on biological membranes, leading to cell degeneration and apoptosis. Compared with other tissues, the cochlea has a high metabolic rate, and energy for its various functions is generated by the mitochondria and organ of Corti. The process of energy generation results in the production of a large number of oxygen free radicals.7 A reduction in the generation of ROS after noise exposure may thus improve NIHL. The current study revealed high 4-HNE staining on the basement membrane, vascular stria, and spiral ligament of the cochlea in the NG, and TUNEL assay confirmed apoptosis at these sites. These findings suggested that noise caused oxidative stress and excessive ROS production, leading to apoptosis. The antioxidant D-met antagonized 4-HNE and thereby reduced the occurrence of apoptosis, consistent with the auditory and morphological tests. These findings suggest that D-met exerts a protective effect against NIHL by reducing oxidative stress and apoptosis.
Cochlear connexin plays an important role in the cochlear microenvironment, which includes tight junction, gap junction, and adherent junction proteins.22 Connexins 26 and 30 are involved in normal hearing function in humans and are important subunits of the cochlear gap junction.23 Recent studies demonstrated reduced expression of connexins 26 and 30 in the cochlear lateral wall in NIHL mice, suggesting that these connexins may be related to the pathogenesis of NIHL.24,25 Gap junction proteins are crucial for the stability of the inner environment and intercellular biochemical substance exchange. The current study indicated that D-met could prevent decreased expression of connexin 26 and connexin 30 in the lateral wall of the cochlea in NIHL mice, suggesting that these proteins may be involved in the pathogenesis of NIHL. However, the mechanism by which NIHL regulates connexin 26 and connexin 30 requires more in-depth investigation.
Conclusion
NIHL mainly affects the inner and particularly the outer hair cells of the cochlear basement membrane. ROS, apoptosis, and connexins 26 and 30 may be involved in the mechanism of NIHL, and D-met, administered either before or after noise exposure, may affect auditory function, cochlear morphology, ROS, apoptosis, and connexin 26 and 30 expression levels to protect mice against NIHL.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the Natural Science Foundation of Hebei (No: H2014206141)
References
- 1.Claussen AD, Fox DJ, Yu XC, et al. D-methionine pre-loading reduces both noise-induced permanent threshold shift and outer hair cell loss in the chinchilla. Int J Audiol 2013; 52: 801–807. DOI: 10.3109/14992027.2013.840933. [DOI] [PubMed] [Google Scholar]
- 2.Oishi N, Schacht J. Emerging treatments for noise-induced hearing loss. Expert Opin Emerg Drugs 2011; 16: 235–245. DOI: 10.1517/14728214.2011.552427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rewerska A, Pawelczyk M, Rajkowska E, et al. Evaluating D-methionine dose to attenuate oxidative stress-mediated hearing loss following overexposure to noise. Eur Arch Otorhinolaryngol 2013; 270: 1513–1520. DOI: 10.1007/s00405-012-2265-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stucken EZ, Hong RS. Noise-induced hearing loss: an occupational medicine perspective. Curr Opin Otolaryngol Head Neck Surg 2014; 22: 388–393. DOI: 10.1097/moo.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 5.Vetter DE. Cellular signaling protective against noise-induced hearing loss - A role for novel intrinsic cochlear signaling involving corticotropin-releasing factor? Biochem Pharmacol 2015; 97: 1–15. DOI: 10.1016/j.bcp.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ivory R, Kane R, Diaz RC. Noise-induced hearing loss: a recreational noise perspective. Curr Opin Otolaryngol Head Neck Surg 2014; 22: 394–398. DOI: 10.1097/moo.0000000000000085. [DOI] [PubMed] [Google Scholar]
- 7.Henderson D, Bielefeld EC, Harris KC, et al. The role of oxidative stress in noise-induced hearing loss. Ear Hear 2006; 27: 1–19. DOI: 10.1097/01.aud.0000191942.36672.f3. [DOI] [PubMed] [Google Scholar]
- 8.Halliwell B. Free radicals and antioxidants: updating a personal view. Nutr Rev 2012; 70: 257–265. DOI: 10.1111/j.1753-4887.2012.00476.x. [DOI] [PubMed] [Google Scholar]
- 9.Minami SB, Yamashita D, Ogawa K, et al. Creatine and tempol attenuate noise-induced hearing loss. Brain Res 2007; 1148: 83–89. DOI: 10.1016/j.brainres.2007.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell KC, Meech RP, Rybak LP, et al. The effect of D-methionine on cochlear oxidative state with and without cisplatin administration: mechanisms of otoprotection. J Am Acad Audiol 2003; 14: 144–156. [PubMed] [Google Scholar]
- 11.Campbell KC, Meech RP, Klemens JJ, et al. Prevention of noise- and drug-induced hearing loss with D-methionine. Hear Res 2007; 226: 92–103. DOI: 10.1016/j.heares.2006.11.012. [DOI] [PubMed] [Google Scholar]
- 12.Vogt W. Oxidation of methionyl residues in proteins: tools, targets, and reversal. Free Radic Biol Med 1995; 18: 93–105. [DOI] [PubMed] [Google Scholar]
- 13.Ghibelli L, Fanelli C, Rotilio G, et al. Rescue of cells from apoptosis by inhibition of active GSH extrusion. FASEB J 1998; 12: 479–486. DOI: 10.1096/fasebj.12.6.479. [DOI] [PubMed] [Google Scholar]
- 14.Lu SC. Regulation of hepatic glutathione synthesis: current concepts and controversies. FASEB J 1999; 13: 1169–1183. [PubMed] [Google Scholar]
- 15.Campbell K, Claussen A, Meech R, et al. D-methionine (D-met) significantly rescues noise-induced hearing loss: timing studies. Hear Res 2011; 282: 138–144. DOI: 10.1016/j.heares.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Vogt W. Oxidation of methionyl residues in proteins: Tools, targets, and reversal. Free Radic Biol Med 1995; 18: 93–105. [DOI] [PubMed] [Google Scholar]
- 17.Kujawa SG, Liberman MC. Synaptopathy in the noise-exposed and aging cochlea: primary neural degeneration in acquired sensorineural hearing loss. Hear Res 2015; 330: 191–199. DOI: 10.1016/j.heares.2015.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tziridis K, Korn S, Ahlf S. Protective effects of Ginkgo biloba extract EGb 761 against noise trauma-induced hearing loss and tinnitus development. Neural plasticity. 2014; 2014: 427298. DOI: 10.1155/2014/427298. [DOI] [PMC free article] [PubMed]
- 19.Yang W, Vethanayagam RR, Dong Y, et al. Activation of the antigen presentation function of mononuclear phagocyte populations associated with the basilar membrane of the cochlea after acoustic overstimulation. Neuroscience 2015; 303: 1–15. DOI: 10.1016/j.neuroscience.2015.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao G, Liu Y, Zhou CH, et al. Solid lipid nanoparticles loaded with edaravone for inner ear protection after noise exposure. Chin Med J (Engl) 2015; 128: 203–209. DOI: 10.4103/0366-6999.149202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molina SJ, Miceli M, Guelman LR. Noise exposure and oxidative balance in auditory and extra-auditory structures in adult and developing animals. Pharmacological approaches aimed to minimize its effects. Pharmacol Res 2016; 109: 86–91. DOI: 10.1016/j.phrs.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 22.Forge A, Becker D, Casalotti S, et al. Gap junctions in the inner ear: comparison of distribution patterns in different vertebrates and assessment of connexin composition in mammals. J Comp Neurol 2003; 467: 207–231. DOI: 10.1002/cne.10916. [DOI] [PubMed] [Google Scholar]
- 23.Scott CA, Kelsell DP. Key functions for gap junctions in skin and hearing. Biochem J 2011; 438: 245–254. DOI: 10.1042/bj20110278. [DOI] [PubMed] [Google Scholar]
- 24.Yamaguchi T, Nagashima R, Yoneyama M, et al. Disruption of ion-trafficking system in the cochlear spiral ligament prior to permanent hearing loss induced by exposure to intense noise: possible involvement of 4-hydroxy-2-nonenal as a mediator of oxidative stress. PLoS One 2014; 9: e102133. DOI: 10.1371/journal.pone.0102133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abitbol JM, Kelly JJ, Barr K, et al. Differential effects of pannexins on noise-induced hearing loss. Biochem J 2016; 473: 4665–4680. DOI: 10.1042/bcj20160668. [DOI] [PubMed] [Google Scholar]