Short abstract
Objective
To investigate the role of promoter and pre-rRNA antisense (PAPAS) long noncoding (Lnc) RNA in cancer biology.
Methods
Tumour and tumour-adjacent healthy tissue biopsies from patients with triple-negative breast cancer (TNBC), and plasma samples from these patients plus healthy controls, were assessed for PAPAS and microRNA (miR)-34a. Effects of PAPAS and miR-34a overexpression were also investigated in vitro.
Results
PAPAS was upregulated in tumour tissues of patients with TNBC versus tumour-adjacent healthy tissues. Plasma PAPAS levels were also upregulated in patients with TNBC versus healthy controls. Levels of PAPAS in tumour tissue was significantly positively correlated with PAPAS levels in plasma from patients with TNBC. MiR-34a was downregulated in tumour tissues versus adjacent healthy tissues, and was significantly correlated with PAPAS in tumour tissues. PAPAS overexpression in vitro was associated with miR-34a inhibition, while miR-34a failed to significantly affect PAPAS levels. PAPAS overexpression promoted in vitro migration and invasion of TNBC cells, while miR-34a overexpression was inhibitory. MiR-34a overexpression decreased the enhanced cell migration and invasion associated with PAPAS overexpression. PAPAS overexpression showed no significant effects on cancer-cell proliferation.
Conclusion
LncRNA PAPAS may promote TNBC by downregulating miR-34a.
Keywords: Triple-negative breast cancer, lncRNA PAPAS, miR-34a
Introduction
Genomic biology studies have revealed that most transcriptions in the human genome are not related to protein coding and 98% of the genome is transcribed as non-protein-coding RNA transcripts.1,2 Long noncoding (lnc) RNAs are defined as non-protein-coding RNA transcripts that are longer than 200 nucleotides,3 and many lncRNAs are reported to be expressed in unique types of tissues or cells, indicating their potential involvement in specific biological processes.4 With unique patterns of expression, different lncRNAs have been shown to participate in different human diseases, such as different types of cancer.5,6 To date, the functions of most lncRNAs remain unknown.
Triple-negative breast cancer (TNBC) is a major breast-cancer subtype that is characterized by the lack of progesterone and oestrogen receptors, and human epidermal growth factor receptor 2.7 Compared with other subtypes, TNBC is more aggressive and prognosis is usually poor,8 thus, there is always high demand for the identification of a novel therapeutic target to improve the survival of patients with TNBC.9 Bioinformatic methods have been implemented to identify molecular pathways that relate to TNBC.10 Regulation of the expression of some lncRNAs, such as lncRNA DANCR, has shown potential in the inhibition of TNBC.11 The lncRNA promoter and pre-rRNA antisense (PAPAS) is a recently identified lncRNA with a critical regulatory role in rRNA synthesis,12 however, its involvement in cancer biology remains unknown.
A preliminary transcriptome analysis by the present authors revealed an upregulated expression pattern for PAPAS in TNBC tissues (data not shown). Thus, the aim of the present study was to investigate the role of PAPAS lncRNA in cancer biology, by studying levels of PAPAS lncRNA and microRNA (miR)-34a, and their interactions, in tumour and tumour-adjacent healthy tissue from patients with TNBC, and in plasma from this patient population compared with healthy controls. The effects of upregulated expression of PAPAS and miR-34a in TNBC cell lines were also investigated.
Patients and methods
Study population and specimens
Female patients (aged > 18 years) with TNBC who were admitted to the Forth Hospital Affiliated to Hebei Medical University between May 2016 and July 2018 were sequentially enrolled into the study. Inclusion criteria were: (1) diagnosis of TNBC by pathological biopsies, confirmed by experienced patholgosits; and (2) patient’s willingness to participate. Exclusion criteria comprised: (1) other types of breast cancer or diagnosis complicated by other diseases; and (2) patients who had been treated prior to admission or who had been transferred from other hospitals. Tumour and tumour-adjacent healthy tissue biopsies were obtained from each patient. Blood (5ml) was extracted from each patient from the elbow vein under fasting conditions, before any treatments began. Blood (5ml) was also extracted from a group of healthy controls, recruited during physical health examinations at the Forth Hospital Affiliated to Hebei Medical University, also under fasting conditions. Blood was transferred to an ethylenediaminetatra-acetic acid-treated tube, followed by centrifugation at 1200 x g for 10 min to prepare plasma. Plasma samples were then stored in liquid nitrogen before use.
This study was approved by ethics committee of the Forth Hospital Affiliated Hebei Medical University (No. FHHMU201603093223), and all participants provided written informed consent.
Cell lines, vectors and transfection of PAPAS and miR-34a
Two human TNBC cell lines, MDA-MB-157 and BT-549 (both from the ATCC; Manassas, VA, USA), were used to investigate the effects of PAPAS and miR-34a overexpression. Cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Cat No. ATCC® 30-2001™; ATCC, Manassas, VA, USA) supplemented with 10% fetal bovine serum (FBS; Cat No. ATCC® 30-2020™; ATCC, Manassas, VA, USA). Vectors expressing PAPAS were designed and synthesized by Sangon (Shanghai, China). Negative control miRNA and miR-34a mimic were purchased from Sigma-Aldrich (St. Louis, MO, USA). Lipofectamine 2000 reagent (Cat No. 11668-019; Invitrogen, Carlsbad, CA, USA) was used for all cell transfections, according to the manufacturer’s instructions, with 10 nM vectors and 40 nM miRNA. The control group (C) included cells that had not undergone transfection, and the negative control group (NC) comprised cells transfected with empty vector or transfected with negative control miRNA. The NC group transfections were performed using lipofectamine 2000 reagent, according to the manufacturer’s instructions. Cells were collected at 24 h following transfection to use in subsequent experiments.
RNA extraction and real-time quantitative reverse transcription PCR (qRT-PCR)
In order to detect PAPAS expression in TNBC tumour and tumour-adjacent healthy tissues, in plasma from patients with TNBC and healthy controls, and in TNBC cell lines, or Lnc-ovarian cancer (OC)1 expression in TNBC cell lines, total RNA was extracted from tissue (15 mg), plasma (0.2 ml) or cell lines (105 cells) using RNAzol reagent (Sigma-Aldrich) according to the manufacturer’s instructions. Applied Biosystems™ High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems [ThermoFisher Scientific], Waltham, MA, USA) was used to synthesize cDNA, and LightCycler® 480 SYBR Green I Master (Roche Life Science, Penzberg, Germany) was used to perform all PCR reactions. Primer sequences for PAPAS amplification were: 5′-CGCTGC TGCTGG CGCCTGGC-3′ (forward) and 5′-CGTGT GGGCTGTGAAAGACC-3′ (reverse); and for Lnc-OC1 amplification, were: 5′-GGC CTGTGTGTTGAATGCT G-3′ (forward) and 5′-CTGTGGTCACAA AGGCCTGA-3′ (reverse). The cycling programme involved preliminary denaturation at 95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 20 s, annealing at 55°C for 20 s, and elongation at 72°C for 30s.
In order to detect miR-34a in TNBC tumour and tumour-adjacent healthy tissues, and in TNBC cell lines, miRNA extraction was performed using a PureLink™ miRNA Isolation Kit (ThermoFisher Scientific), according to the manufacturer’s instructions. Reverse transcription was performed using a qScript microRNA cDNA Synthesis Kit (Quantabio, Beverly, MA, USA), according to the manufacturer’s instructions. Subsequent amplification of the cDNA was performed using the miScript SYBR Green PCR Kit (QIAGEN, Hilden, Germany) according to the manufacturer’s instructions. MiR-34a primer sequences were: 5′-GTGC AGGGTCCGAGGT-3′ (forward) and 5′-G CCGCTGGCAGTGTCTTAG-3′ (reverse). The cycling programme involved preliminary denaturation at 95°C for 1 min, followed by 40 cycles of denaturation at 95°C for 20 s, annealing at 54°C for 20 s, and elongation at 72°C for 10s.
All PCR reactions were performed on an ABI 7500 Real-Time PCR System (ThermoFisher Scientific). GAPDH was used as the internal control for PAPAS PCR, and U6 was used as the internal control for miR-34a PCR. All data were normalized using the 2-ΔΔCT method.
Transwell migration and invasion assay
Serum-free ATCC-formulated RPMI-1640 medium was used to prepare single-cell suspensions of MDA-MB-157 and BT-549 cells, that were adjusted to a cell density of 3 × 104 cells/ml. A 0.1 ml volume of cell suspension was transferred to the upper chamber of each well of a 96-well cell-culture insert (Transwell®; CLS3384, Corning Co., Corning, NY, USA) that was either uncoated (for the migration assay) or that had been pre-coated with Matrigel (356234; Millipore, Billerica, MA, USA) for the invasion assay. RPMI-1640 Medium (with 20% FBS) was added to the lower chamber. After incubation at 37°C with 5% CO2 for 2 h, the upper chamber membrane was stained for 15 min with 0.5% crystal violet (Sigma-Aldrich) at 22°C. Stained cells were counted using a BX43 light microscope (Olympus, Tokyo, Japan) and the results were expressed as a proportion of the cells counted in the control (untransfected) group.
Measurement of cell proliferation rates
RPMI-1640 medium (with 10% FBS) was used to prepare single-cell suspensions of MDA-MB-157 and BT-549 cells, at a density of 3 × 104 cells/ml. Cells were seeded into 96-well cell-culture plates using 0.1 ml of cell suspension per well, and cells were cultured at 37°C with 5% CO2. Cell counting kit-8 solution (10 µl; Sigma-Aldrich) was added to each well every 24 h, up to 96 h. Cells were then cultured for an additional 4 h, before adding 10 µl DMSO into each well. The optical density (OD) values were measured at 450 nm using an ELx808™ Absorbance Microplate Reader (BioTek, Winoski, VT, USA) to evaluate cell proliferation.
Statistical analyses
Data are presented as mean ± SD, and mean values were calculated using data from three biological replicates. Comparisons of PAPAS and miR-34a expression levels between two types of TNBC tissues were performed using paired t-test. One-way analysis of variance (ANOVA) and Tukey’s t-test were used to analyse the differences between the cell transfection groups. Pearson’s correlation coefficient was performed to investigate the correlations between expression levels of PAPAS and miR-34a in tumour tissues and adjacent healthy tissues and the correlations of PAPAS levels between biopsies and plasma. All data analyses were performed using GraphPad Prism 6 (GraphPad, San Diego, CA, USA) and a P value < 0.05 was considered statistically significant.
Results
Study population
A total of 78 female patients with TNBC were enrolled. According to the American Joint Committee on Cancer (AJCC; 8th edition) staging, there were 18 cases of stage I, 30 cases of stage II, 22 cases of stage III and 8 cases of stage IV TNBC. Patient age ranged between 30 and 68 years (mean age, 47.7 ± 4.8 years). Plasma samples were obtained from healthy volunteers (demographic data not shown).
PAPAS and miR-34a showed opposite expression patterns in TNBC tumour tissues
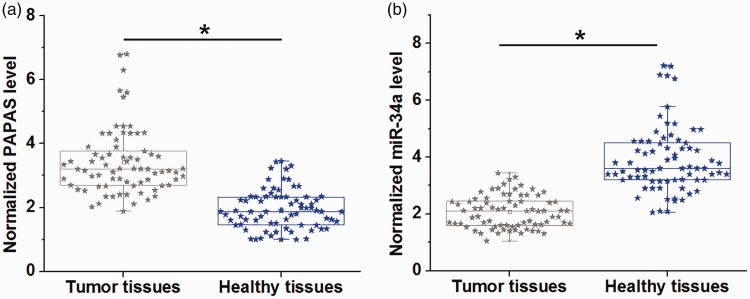
Expression of PAPAS and miR-34a in tumour and adjacent healthy tissues from 78 patients with TNBC was analysed by RT-qPCR. Compared with tumour-adjacent healthy tissues, the tumour tissues showed significantly higher PAPAS levels (Figure 1a; P <0.05), and significantly lower miR-34a levels (Figure 1b; P <0.05).
Figure 1.
Box-whisker plots with scatterplot overlay, showing expression patterns of long noncoding RNA promoter and pre-rRNA antisense (PAPAS) and microRNA (miR)-34a in tumour and adjacent healthy tissues from patients with triple-negative breast cancer (TNBC). Reverse-transcription quantitative PCR showed that (a) levels of PAPAS were significantly higher, and (b) levels of miR-34a were significantly lower in tumour versus healthy tissues (*P <0.05; paired t-test). Central line, median; extremities of the box, 25th and 75th percentiles; error bars, minimum and maximum values.
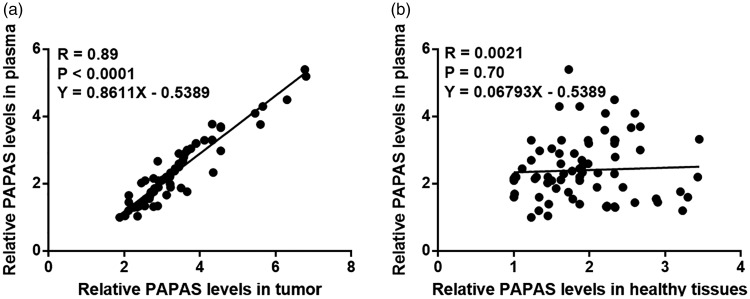
Tumour tissue PAPAS expression was significantly positively correlated with plasma PAPAS levels
PAPAS levels in plasma (measured by RT-qPCR) were also found to be upregulated in patients with TNBC versus healthy controls (P < 0.05; data not shown). Pearson’s correlation coefficient was performed to assess the associations between PAPAS expression levels in biopsies and in plasma from patients with TNBC. The analyses showed that PAPAS expression in tumour tissue was significantly positively correlated with plasma PAPAS levels (R = 0.89, P < 0.001; Figure 2a) and PAPAS expression in tumour-adjacent healthy tissue was not correlated with plasma PAPAS level (Figure 2b).
Figure 2.
Correlation between long noncoding RNA promoter and pre-rRNA antisense (PAPAS) expression in tumour and tumour-adjacent healthy tissue, and plasma PAPAS levels, in patients with triple-negative breast cancer: Pearson’s correlation coefficient analysis showed that PAPAS expression in (a) tumour tissue, but not in (b) tumour-adjacent healthy tissue, was significantly and positively correlated with plasma PAPAS levels.
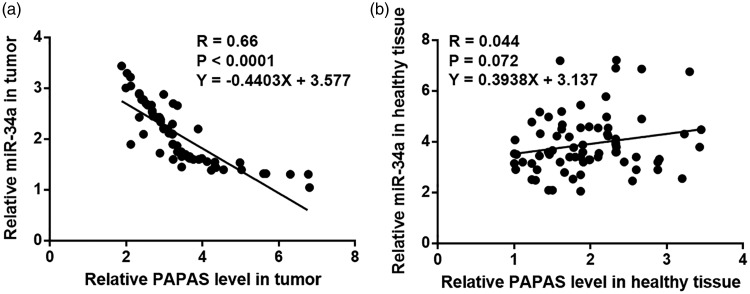
PAPAS and miR-34a were inversely correlated in tumour tissues
Pearson’s correlation coefficient analyses to investigate the correlations between PAPAS and miR-34a showed that, in tumour tissues, PAPAS and miR-34a were significantly inversely correlated (R = 0.66, P < 0.001; Figure 3a). However, PAPAS and miR-34a were not significantly correlated in tumour-adjacent tissues (R = 0.044, P = 0.07; Figure 3b).
Figure 3.
Correlations between long noncoding RNA promoter and pre-rRNA antisense (PAPAS) and miR-34a levels in tumour and tumour-adjacent healthy tissue from patients with triple-negative breast cancer. Pearson’s correlation coefficient analyses showed that PAPAS and miR-34a were (a) significantly inversely correlated in tumour tissues, but (b) were not correlated in adjacent healthy tissues.
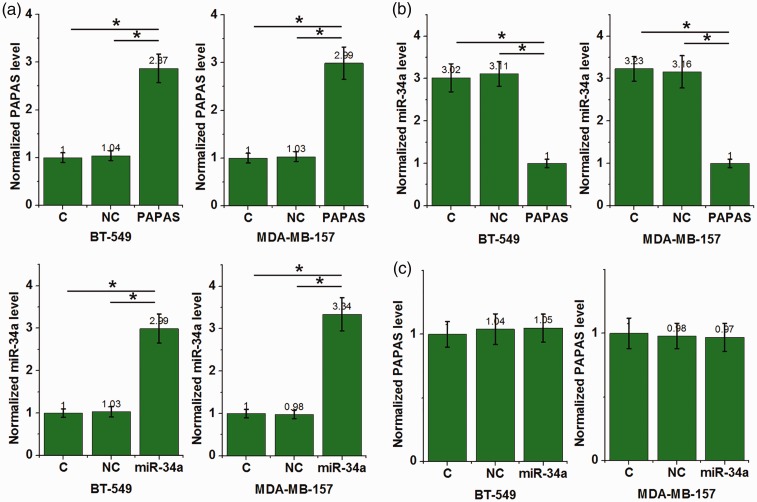
PAPAS is an upstream negative regulator of miR-34a in TNBC cells
The TNBC cell lines, BT-549 and MDA-MB-157, were transfected with PAPAS and miR-34a mimic to further investigate potential interactions between PAPAS and miR-34a. PAPAS and miR-34a were shown to be overexpressed in these cell lines (P < 0.05; Figure 4a) versus the control group (C) with untransfected cells, and the negative control group (NC) transfected with negative control miRNA or empty vectors. Compared with the two control groups, PAPAS overexpression was associated with inhibition of miR-34a in both cell lines (P <0.05; Figure 4b). Overexpression of miR-34a did not significantly affect PAPAS levels in either cell line (P >0.05; Figure 4c).
Figure 4.
Long noncoding RNA promoter and pre-rRNA antisense (PAPAS) and micro (miR)-34a levels in triple-negative breast cancer cell lines, BT-549 and MDA-MB-157, transfected with either PAPAS or miR-34a: (a) PAPAS and miR-34a were overexpressed in both cell lines at 24 h following transfection; (b) PAPAS overexpression was associated with significantly lower miR-34a levels in both cell lines versus controls; and (c) miR-34a overexpression did not significantly affect PAPAS levels in either cell line; C, control group with untransfected cells; NC, negative control group transfected with negative control miRNA or empty vectors; (*P <0.05, versus control groups; one-way ANOVA and Tukey’s t-test).
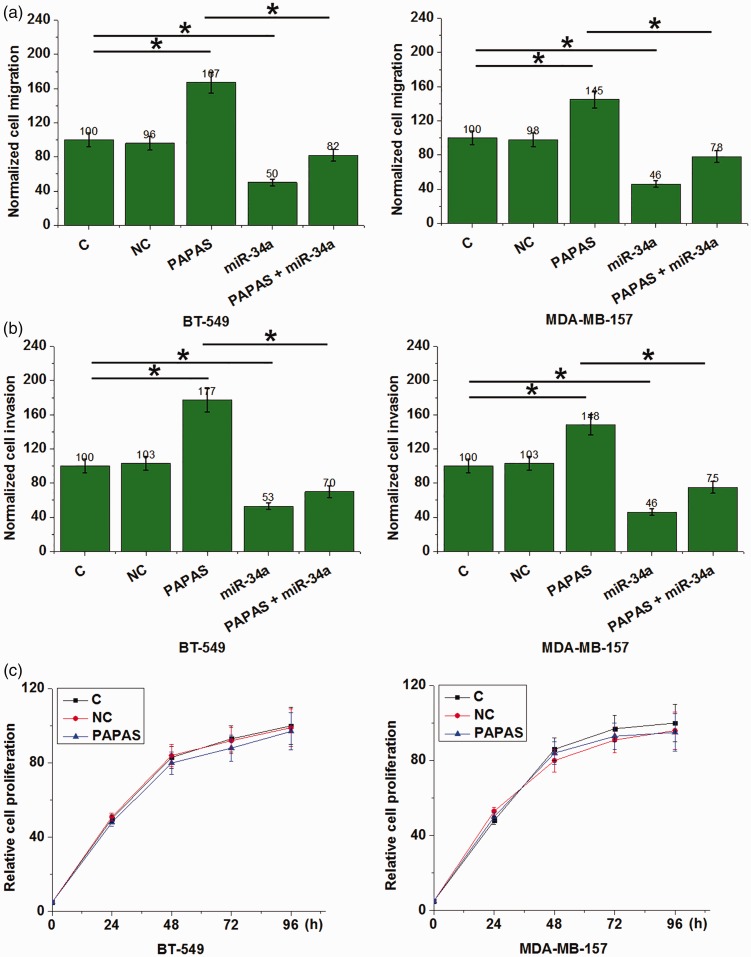
PAPAS overexpression promoted TNBC cell migration and invasion but not proliferation
PAPAS overexpression in BT-549 and MDA-MB-157 cells was associated with increased cell migration (Figure 5a) and invasion (Figure 5b) compared with the control groups (C and NC; P < 0.05). Conversely, overexpression of miR-34a significantly inhibited migration (Figure 5a) and invasion (Figure 5b) in both TNBC cell lines (all P <0.05 versus control groups). In addition, the inhibition of migration and invasion associated with miR-34a overexpression was attenuated by overexpression of PAPAS (P <0.05). Finally, PAPAS overexpression showed no statistically significant effects on cell proliferation in either TNBC cell line (P >0.05; Figure 5c).
Figure 5.
Migration, invasion and proliferation of triple-negative breast cancer cell lines, BT-549 and MDA-MB-157, transfected with long noncoding RNA promoter and pre-rRNA antisense (PAPAS), micro (miR)-34a, or both. PAPAS overexpression promoted (a) migration and (b) invasion in both cell lines, whereas miR-34a overexpression inhibited (a) migration and (b) invasion in both cell lines. PAPAS overexpression attenuated the inhibition of migration and invasion associated with miR-34a overexpression (a and b); and (c) PAPAS overexpression did not affect proliferation in either cell line (*P <0.05; one-way ANOVA and Tukey’s t-test).
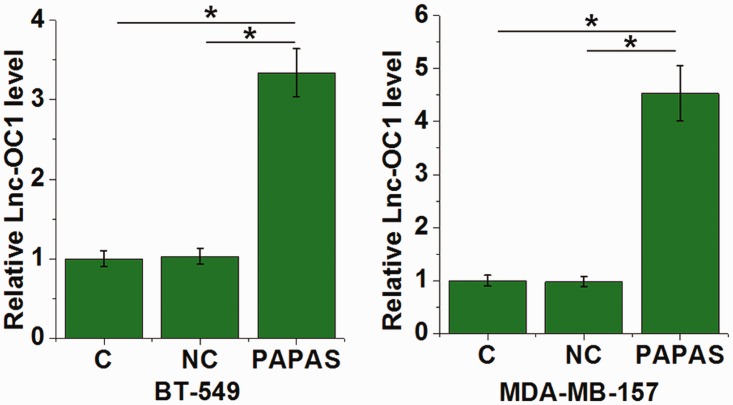
PAPAS overexpression was associated with increased Lnc-OC1 levels in TNBC cells
Levels of lnc-OC1 in BT-549 and MDA-MB-157 cells were evaluated by RT-qPCR. Lnc-OC1 levels were upregulated in cells that overexpressed PAPAS compared with control cells (C and NC) in both cell lines (P <0.05; Figure 6; one-way ANOVA and Tukey’s t-test).
Figure 6.
Levels of long noncoding RNA (lnc)-ovarian cancer (OC)1 in triple-negative breast cancer cell lines, BT-549 and MDA-MB-157, transfected with long noncoding RNA promoter and pre-rRNA antisense (PAPAS). Compared with control cells (C and NC), PAPAS overexpression was associated with upregulated Lnc-OC1 in both cell lines (*P <0.05; one-way ANOVA and Tukey’s t-test); C, control group with untransfected cells; NC, negative control group transfected with negative control miRNA or empty vectors.
Discussion
Long noncoding RNAs are potential therapeutic targets for cancer treatment. The present study found that PAPAS was upregulated in tumour tissues and plasma of patients with TNBC, and promoted TNBC cell migration and invasion, possibly by downregulating miR-34a, which was found to suppress migration and invasion in TNBC cell lines.
Previous studies have revealed the tumour suppressive role of miR-34a in different types of cancer.13,14 For example, miR-34a has been shown to participate in cancer biology by interacting with downstream oncogenic or tumour suppressor pathways, such as interleukin (IL)-6 receptor/signal transducer and activator of transcription 3 (STAT3) signalling, E2F transcription factor 3 (E2F3) and survivin.14,15 MiR-34a is downregulated in TNBC tissues, and overexpression of miR-34a has been shown to attenuate tumour growth by silencing c-SRC.16 In concurrence, the present study showed downregulation of miR-34a in the tumour tissues of patients with TNBC. The present study also showed that miR-34a overexpression was associated with inhibited cancer cell migration and invasion, however, the upstream regulators of miR-34a remain largely unknown. PAPAS overexpression was shown to inhibit miR-34a, and the inhibition of miR-34a by PAPAS was involved in the regulation of TNBC cell migration and invasion. Thus, the present findings have enriched our understanding of the pathogenesis of TNBC.
RNAs synthesized in tumour tissues may enter the blood circulatory system. Therefore, measurement of plasma levels of circulating RNAs may reflect the occurrence of cancer.17,18 The present study showed that expression of PAPAS in tumour tissues from patients with TNBC was positively correlated with plasma levels of PAPAS, but no such correlation with plasma PAPAS was seen in adjacent healthy tissues from these patients. The present authors surmise that PAPAS may enter the plasma to systemically regulate gene expression, and thus, detection of PAPAS in plasma may reflect the development of TNBC.
MiR-34a is known to inhibit TNBC cell proliferation. In the present study, PAPAS inhibited miR-34a expression, but showed no significant effects on TNBC cell proliferation. This controversial observation may indicate that PAPAS interacts with multiple downstream effectors to achieve a regulatory effect on TNBC cell behaviours.
The lncRNA, Lnc-OC1, was recently reported to serve as an miR-34a sponge to regulate cancer cell behaviours in ovarian cancer.19 In the present study, upregulated Lnc-OC1 was observed in TNBC cells following PAPAS overexpression. Therefore, PAPAS may downregulate miR-34a by upregulating its sponge, Lnc-OC1.
It should be noted that the results of the present study may be limited by the fact that the sample size was relatively small and in vivo animal model experiments were not performed. Future studies should be designed to overcome such limitations.
In conclusion, PAPAS was overexpressed in patients with TNBC and PAPAS may promote the migration and invasion of TNBC cells by downregulating miR-34a.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Mattick JS, Rinn JL. Discovery and annotation of long noncoding RNAs. Nat Struct Mol Biol 2015; 22: 5–7. [DOI] [PubMed] [Google Scholar]
- 2.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem 2012; 81: 145–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nat Rev Genet 2009; 10: 155–159. [DOI] [PubMed] [Google Scholar]
- 4.Iyer MK, Niknafs YS, Malik R, et al. The landscape of long noncoding RNAs in the human transcriptome. Nat Genet 2015; 47: 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi X, Sun M, Liu H, et al. Long non-coding RNAs: a new frontier in the study of human diseases. Cancer Lett 2013; 339: 159–166. [DOI] [PubMed] [Google Scholar]
- 6.Gibb EA, Brown CJ, Lam WL. The functional role of long non-coding RNA in human carcinomas. Mol Cancer 2011; 10: 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. N Engl J Med 2010; 363: 1938–1948. [DOI] [PubMed] [Google Scholar]
- 8.Bianchini G, Balko JM, Mayer IA, et al. Triple-negative breast cancer: challenges and opportunities of a heterogeneous disease. Nat Rev Clin Oncol 2016; 13: 674–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denkert C, Liedtke C, Tutt A, et al. Molecular alterations in triple-negative breast cancer–the road to new treatment strategies. Lancet 2017; 389: 2430–2442. [DOI] [PubMed] [Google Scholar]
- 10.Zhang W, Wan YW, Allen GI, et al. Molecular pathway identification using biological network-regularized logistic models. BMC Genomics 2013; 14: S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sha S, Yuan D, Liu Y, et al. Targeting long non-coding RNA DANCR inhibits triple negative breast cancer progression. Biol Open 2017; 6: 1310–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Z, Sentürk N, Song C, et al. lncRNA PAPAS tethered to the rDNA enhancer recruits hypophosphorylated CHD4/NuRD to repress rRNA synthesis at elevated temperatures. Genes Dev 2018; 32: 836–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, Li J, Dong K, et al. Tumor suppressor miR-34a targets PD-L1 and functions as a potential immunotherapeutic target in acute myeloid leukemia. Cell Signal 2015; 27: 443–452. [DOI] [PubMed] [Google Scholar]
- 14.Rokavec M, Öner MG, Li H, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest 2014; 124: 1853–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geng D, Song X, Ning F, et al. MiR-34a inhibits viability and invasion of human papillomavirus-positive cervical cancer cells by targeting E2F3 and regulating survivin. Int J Gynecol Cancer 2015; 25: 707–713. [DOI] [PubMed] [Google Scholar]
- 16.Adams BD, Wali VB, Cheng CJ, et al. miR-34a silences c-SRC to attenuate tumor growth in triple-negative breast cancer. Cancer Res 2016; 76: 927–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silva JM, Rodriguez R, Garcia JM, et al. Detection of epithelial tumour RNA in the plasma of colon cancer patients is associated with advanced stages and circulating tumour cells. Gut 2002; 50: 530–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qi P, Zhou X, Du X. Circulating long non-coding RNAs in cancer: current status and future perspectives. Mol Cancer 2016; 15: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tao F, Tian X, Lu M, et al. A novel lncRNA, Lnc-OC1, promotes ovarian cancer cell proliferation and migration by sponging miR-34a and miR-34c. J Genet Genomics 2018; 45: 137–145. [DOI] [PubMed] [Google Scholar]