Short abstract
Objective
Radiotherapy is reported to enhance immune responses in cancer, but appropriate doses and mechanisms remain to be investigated. This study explored whether autophagy is involved in the regulation of major histocompatibility complex class I (MHC-I) expression and CD8+ T cell infiltration at different radiation doses.
Methods
Non-small cell lung cancer (NSCLC) cell lines A549 and H1975 were exposed to different doses of radiation. The levels of autophagy and MHC-I expression were examined 6 hours after irradiation. The effects of the autophagy inhibitor chloroquine (CQ) on MHC-I expression were also investigated, as well as the relationship between autophagy and MHC-1 expression. Pathological specimens from 69 NSCLC patients were collected, and immunohistochemistry was used to detect MHC-1 expression and CD8+ T cell infiltration in tumors.
Results
Irradiation induced autophagy and MHC-I expression during a single radiation dose from 2 to 20 Gy in a dose-dependent manner. CQ downregulated MHC-I expression. Immunohistochemistry indicated that MHC-I levels were positively correlated with the infiltration of CD8+ T cells in NSCLC cells (R2 = 0.713).
Conclusions
Autophagy induced MHC-I expression and increased CD8+ T cell infiltration. A single radiation dose of 20 Gy induced the strongest CD8+ T cell infiltration.
Keywords: Radiotherapy, autophagy, MHC-I, CD8+ T, non-small cell lung cancer, immune response
Introduction
The ability of radiotherapy to enhance immunotherapy responses has attracted increasing attention.1 Radiotherapy and immunotherapy are expected to cooperate in the elimination of malignant tumors, but the mechanisms of their synergistic effects remain unclear. Previous studies of mouse tumor models indicated that radiation induced the cross-priming of T cells to relatively strong antigens and that T cells contributed to the therapeutic effects of radiation.2 Moreover, pre-existing tumoral and peritumoral immune infiltration, especially of functional CD8+ T cells, was shown to correlate with patient response to anti-programmed cell death protein-1 and anti-programmed death ligand 1 immunotherapy.3 The CD8+ T cell status has been proposed to impact the tumor response to radiation and immunotherapy, while only 18% of patients with advanced solid tumors were shown to respond to immune checkpoint inhibitors and radiaton.4 This might have resulted from less effective tumor antigens caused by inappropriate irradiation, as well as a less specific CD8+ T cell response.
Many studies have attempted to identify novel radiation-induced antigens. One of these, the DNA exonuclease Trex1, is an upstream regulator of radiation-induced anti-tumor immunity whose expression is dependent on the radiation dose. Previous results indicated that Trex1 levels were significantly increased by a single dose of 20 Gy and above, resulting in a decrease of cytosolic double-stranded DNA which is a vaccine that activates the immune system.5 LTX-315 is an oncolytic peptide with potent immunological properties,6 and transforming growth factor-β is another regulator of radiotherapy that has been used to generate an in situ tumor vaccine.7 Expression of the major histocompatibility complex class I (MHC-I) on CD8+ T cells is required to activate the immune response, regardless of the type of intracellular antigen.8 Therefore, an understanding of the regulation of MHC-I in tumor cells during radiotherapy is helpful to clarify the mechanism of CD8+ T cell infiltration.
Autophagy is fundamental to the maintenance of intracellular homeostasis in all types of human cells. Malignant cells harness autophagy to thrive, especially in adverse microenvironmental conditions, so the inhibition of autophagy is proposed as a strategy to kill and sensitize cancer cells. Autophagy is also critical for optimal immune function, and mediates cell-extrinsic homeostatic effects through its fundamental roles in danger signaling. Alternate-day feeding regimens and a 30% reduction in daily caloric intake, not resulting in dramatic weight loss, were reported to improve the ability of single-dose radiation therapy (6–8 Gy) to limit the local growth and metastatic dissemination of 4T1 and 67NR breast cancer cells implanted orthotopically into immunocompetent BALB/c mice.9 The roles of autophagy in the activation of anti-tumor adaptive immune responses are essential, and include regulation of the release of immunostimulatory danger signals.10 The inhibition of autophagy was reported to abolish cross-presentation almost completely, whereas its induction dramatically enhanced the cross-presentation of tumor antigens.11
We hypothesized that high-dose radiation activates the immune response by activating autophagy, inducing the expression of MHC-I, and increasing CD8+ T cell infiltration in lung cancers. Our findings provide important implications for the choice of radiation dose in the clinic to convert unresponsive patients into responders for immunotherapy.
Materials and methods
Cells
Non-small cell lung cancer (NSCLC) cell lines A549 and H1975 were purchased from The Cell Bank of Type Culture Collection, Chinese Academy of Sciences (Shanghai, China) and authenticated by Guangzhou Cellcook Biotech Co., Ltd. (Guangzhou, China). Cells were authenticated by morphology, phenotype, and growth. Cells were cultured in RPMI-H1640 medium supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, and 10% fetal bovine serum (all from Shanghai Lianshuo Biological Company, Shanghai, China).
Western blot analysis
Proteins from A549 and H1975 cells were extracted in radioimmunoprecipitation assay buffer (Beyotime Biotechnology Co., Wuhan, China). Protein concentrations were determined using the BCA method. Proteins were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis and run at 80 V for the separating gel, then at 120 V for the resolving gel. Proteins were then transferred to an activated polyvinylidene fluoride membrane in an ice bath at 300 mA for 90 minutes. Membranes were probed with primary antibodies (anti-LC3, 1: 1000; anti-SQSTM1/p62, 1: 10000; and anti-GAPDH, 1: 1000; all Abcam, Cambridge, UK) at 4°C overnight. After washing three times with phosphate-buffered saline-Tween-20 for 15 minutes each, blots were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG secondary antibody (1:10000; Aspen Biotechnology Company, Wuhan, China) at room temperature for 2 hours. Chemiluminescence was used to visualize protein bands, and a Precision Plus Protein Dual Color standard (Bio-Rad Ltd., Hercules, CA, USA) was used to estimate molecular weight. Relative quantification was performed using ImageJ software (v.1.51; NIH) to determine the (LC3-II/LC3-I)/GAPDH and p62/GAPDH ratio for each sample.
Flow cytometry
Single cell suspensions were obtained from scraped tumor cells 6 hours after radiation. After blocking with 5% bovine serum albumin (Sigma-Aldrich, St Louis, MO, USA), the cells were incubated with an anti-human MHC-I antibody (Proteintech Company, Wuhan, China) at 4°C overnight. After washing with phosphate-buffered saline, the cells were incubated with fluorescein isothiocyanate at room temperature for 30 minutes. All samples were examined with a flow cytometer (Becton-Dickinson Ltd., Franklin Lakes, NJ, USA) and analyzed using FlowJo software (Tree Star, Inc., OR, USA).
Immunofluorescence
A549 and H1975 cells were plated in six-well plates at a density of 5 × 105 cells/mL. The next day, they were incubated with adenoviruses expressing the mCherry-GFP-LC3B fusion protein (Ad-mCherry-GFP-LC3B, Beyotime Biotechnology Company) for 24 hours. Cells were then irradiated with different doses of radiation from 2 to 20 Gy. One six-well plate was used as a negative control without irradiation but with adenoviral incubation. Cells were examined with a fluorescence microscope 6 hours after radiation. The multiplicity of infection was 20. The number of spots in 10 cells of each group were quantified and analyzed.
Immunohistochemistry
Pathological specimens from 69 NSCLC patients who received surgery at The First People’s Hospital of Jingzhou from January 1, 2017 to December 1, 2017 were collected. Oral consent was obtained from all patients. This study was approved by the Medical Ethics Committee of the First People’s Hospital Affiliated to Changjiang University (approval number 2016-10).
The MHC-I rabbit polyclonal antibody (1: 150) was used to detect MHC-I expression. After blocking with 15% swine/5% human serum for 12 hours, mouse EnVision secondary detection was used (DAKO, Glostrup, Denmark). Tumor cell membrane staining was evaluated by two experienced pathologists in a double-blinded manner. MHC-I expression was scored between 0 and 8 from the sum of a proportion score (0–5 scale reflecting the fraction of cells with any stain) and a staining intensity score (0–3 scale reflecting the strength of staining among positive cells).12 Average scores were further analyzed. Specimens were also immunostained for CD8 (clone C8/144B, 1: 100; DAKO). Four fields per sample were randomly selected and observed under a 40× microscope, and the number of positive cells in 100 lymphocytes of each field was counted to obtain the average number of CD8+ T cells.13 Non-nucleated small fragments were not counted.
Statistical analysis
Experiments were repeated at least three times, and results were presented as the mean ± standard deviation (SD). The difference between two measurements was analyzed by the unpaired Student’s t-test using GraphPad Prism 5.0 Software. Differences were considered significant when P<0.05.
Results
Irradiation induced autophagy and autophagy flux
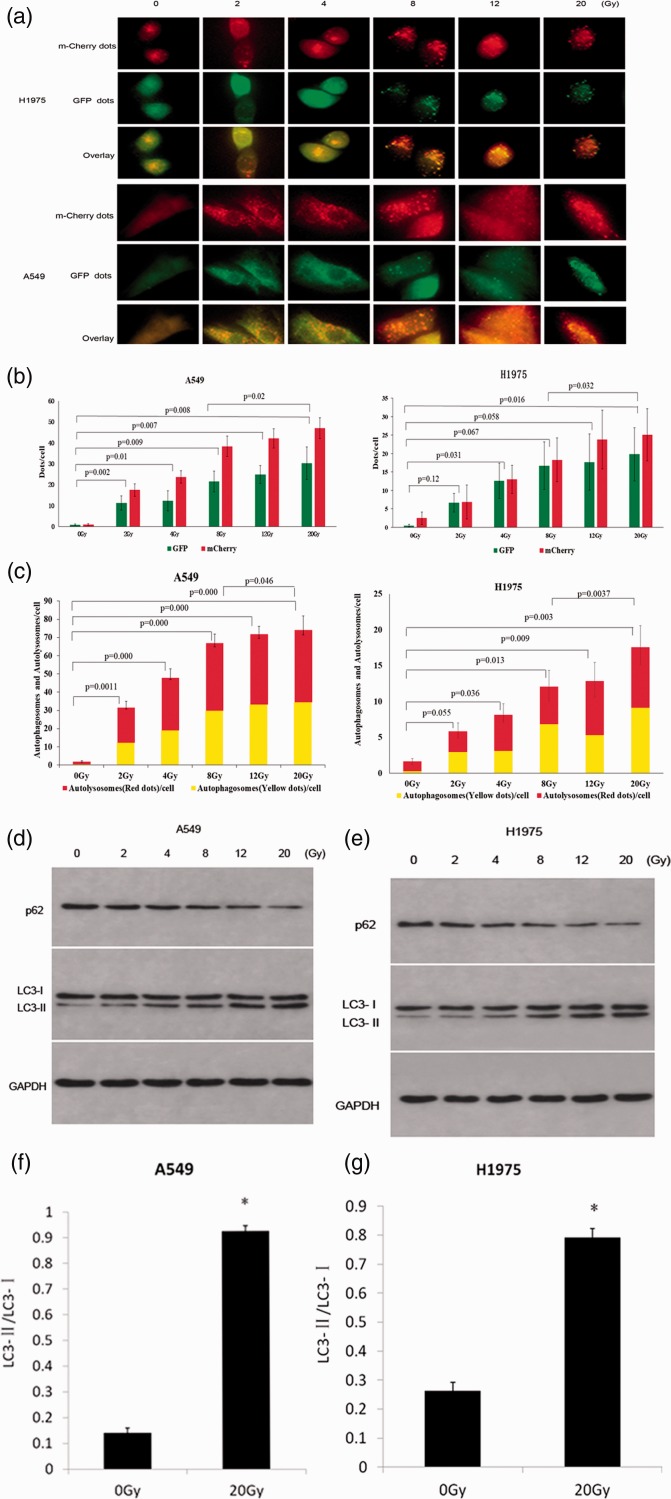
Several stresses, including nutrient deprivation and chemotherapeutic agents, were reported to induce autophagy in cancer cells. To determine whether autophagy was also induced by radiotherapy in NSCLC cells, we used fluorescence microscopy to detect the formation of autophagosomes and autolysosomes in A549 and H1975 cells 6 hours after exposure to radiation. Multiple green and red fluorescent proteins were observed in irradiated cells, suggesting that the adenoviruses expressing mCherry-GFP-LC3B fusion protein had invaded the cells (Figure 1a, b). Green GFP fluorescence quenched when autophagosomes were fused with acidic lysosomes because GFP is highly sensitive to acid. Therefore, the yellow and red spots represented autophagosomes and autolysosomes, respectively, suggesting that autophagic flow was unobstructed. In overlay images, the yellow and red spots indicated that autophagic flux was induced, while there were few autophagosomes in the control group (Figure 1c).
Figure 1.
Irradiation induced autophagy in A549 and H1975 cells. (a) A549 and H1975 cells were transfected by adenoviruses expressing mCherry-GFP-LC3B fusion protein. After 24 hours, cells were irradiated with different doses of radiation, and autophagy was detected with a fluorescence microscope (400×)6 hours later. (b) GFP and mCherry dots per cell were quantified. Data are represented as mean ± SD. The sum of GFP and mCherry was analyzed, and a significant difference was detected between groups. (c) Autophagosome and autolysosome dots per cell were quantified at different doses of radiation in A549 and H1975 cells. The sum of autolysosomes and autophagosomes was analyzed, and a significant difference was detected between groups. (d, e) Autophagy-related proteins were detected by western blot analysis in A549 and H1975 cells treated with different irradiation doses. (f, g) LC3-II/LC3-I was increased by radiation in both A549 and H1975 cells. ∗, P < 0.05.
Western blot was used to detect the expression of LC3 protein, which is an important component of autophagosome membranes. Additionally, the LC3-I to LC3-II shift was reported to be associated with the formation of autophagy.11 The ratio of LC3-II to LC3-I was significantly increased in both A549 and H1975 cells 6 hours after irradiation compared with the untreated group (P<0.05; Figure 1d–g). p62 is selectively incorporated into autophagosomes through binding to LC3, and is rapidly degraded during autophagy.14 Thus, the total expression of p62 is inversely correlated with autophagic activity. We found that the production of p62 was significantly decreased in irradiated groups compared with the control group (P<0.05; Figure 1d), suggesting that irradiation induced autophagy and autophagy flux in both A549 and H1975 cells.
Radiation dose-dependently increased autophagy in A549 and H1975 cell, which was inhibited by chloroquine
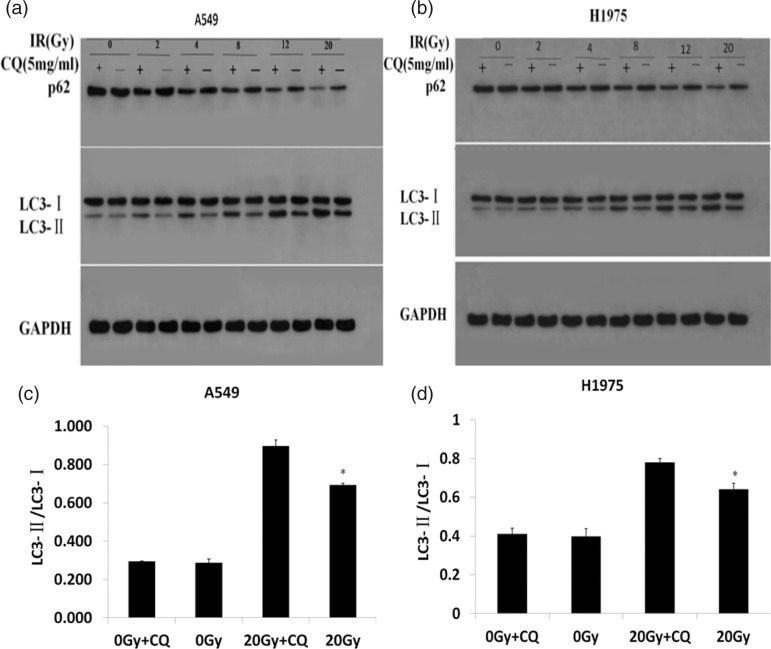
The numbers of autophagosomes and autolysosomes at different doses of radiotherapy were counted, and shown to increase significantly in line with irradiation doses (P<0.05, except between 0 Gy and 2 Gy in H1975 cells) (Figure 1c). To determine the optimal radiation dose for autophagy induction, a radiotherapy dose ramp test was performed. We found that protein levels of p62 and LC3-I decreased significantly with increasing doses from 0 to 20 Gy, while LC3-II and the ratio of LC3-II/LC3-I increased significantly (Figure 1d–g). Previous studies suggested that autophagy has a cytoprotective role in cancer treatment, and that autophagy inhibition enhanced therapy-induced cell death.15 CQ is a protease inhibitor that prevents autophagy lysosome formation during autophagy, and which was reported to increase LC3-II levels through the aggregation of autophagosomes.16 Our results showed that CQ significantly upregulated the levels of LC3-II and p62 because autophagosomes could not degrade by binding to lysosomes (P<0.05; Figure 2a–d), suggesting that CQ inhibited autophagy in A549 and H1975 cell lines. These results indicated that CQ inhibited radiation-induced autophagy in a dose-dependent manner.
Figure 2.
CQ increased LC3-II levels through the aggregation of autophagosome. (a, b) A549 and H1975 cells were treated in the presence or absence of 5 mg/ml CQ for 12 hours. Six hours after irradiation with different doses, protein levels of LC3-I, LC3-II, and p62 were examined by western blot analysis. (c, d) LC3-II/LC3-I was increased by CQ, especially at 20 Gy. ∗, P < 0.05 vs 20 Gy + CQ.
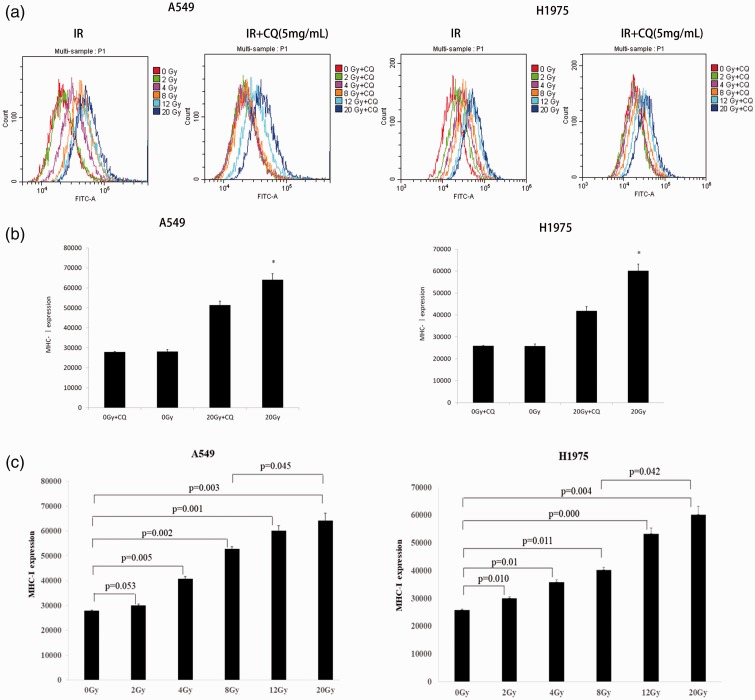
Radiation dose-dependently increased MHC-I expression and CQ reversed its effects in A549 and H1975 cells
Irradiation induces the oxidization of proteins and signal molecules, resulting in the antigen presentation pathway response.17 We exposed A549 and H1975 cell lines to different doses of X-ray radiation, and detected MHC-I complexes on the cell surface by flow cytometry 6 hours later. Consistent with previous reports,18 radiation induced the expression levels of MHC-I in a dose-dependent manner, with 20 Gy identified as optimal to maximize MHC-I expression (Figure 3a–c). To examine the effects of autophagy inhibition on MHC-I expression, A549 and H1975 cell lines were treated with CQ after irradiation at different radiation doses. Inhibition of autophagy shifted the highest peak in the flow chart to the left, suggesting a decrease in MHC-1 expression following the inhibition of autophagy (Figure 3a, b).
Figure 3.
CQ decreased irradiation-induced MHC-I expression in lung cancer cells. (a) MHC-I expression was measured by flow cytometry in A549 and H1975 cells. (b) MHC-I expression increased with increasing doses of irradiation. CQ reversed the effects of irradiation (∗, P < 0.05 vs 20 Gy + CQ). (c) MHC- I expression levels were quantified.
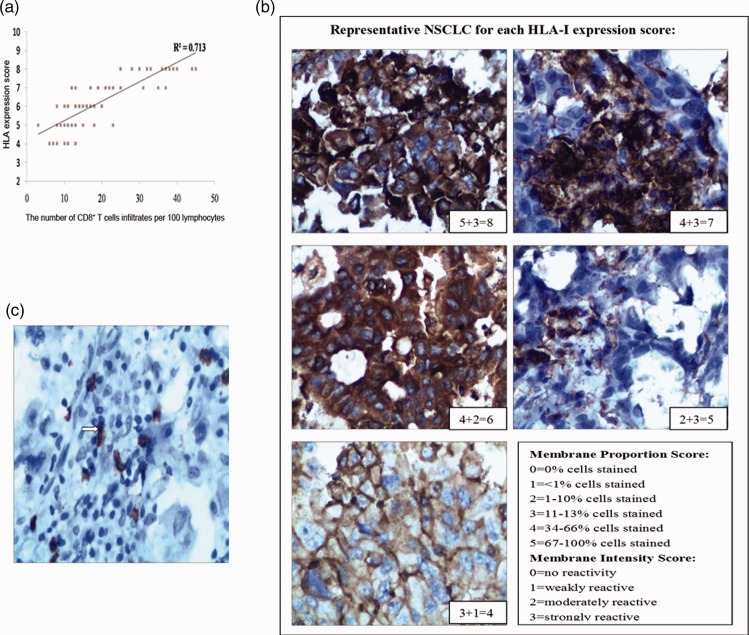
Expression of MHC-I positively correlated with the infiltration of CD8+ T cells
Increased numbers of CD8+ T cells are crucial to the response to immunotherapy19 and are associated with excellent clinical outcomes.20 Detailed patient information is listed in Table 1. MHC-I expression and CD8+ T cell infiltration were determined by immunohistochemistry (Figure 4b, c), and shown to be highly correlated: R2 = 0.713 (Figure 4a). These results suggested that MHC-1 expression is upregulated in tumor-induced CD8+ T cell infiltration.
Table 1.
Characteristics of 69 patients with NSCLC.
| All patients (n=69) | |
|---|---|
| Median age (years) | 56.0 (41.0–66.0) |
| Sex | |
| Female | 28 (40.58%) |
| Male | 41 (59.42%) |
| Histological type | |
| Adenocarcinoma | 59 (85.51%) |
| Squamous | 8 (11.59%) |
| Others | 2 (2.9%) |
| Differentiation | |
| Well | 34 (49.28%) |
| Moderate | 14 (20.29%) |
| Poor | 7 (10.14%) |
| Others (not reported) | 14 (20.29%) |
| Smoking (before operation) | |
| No | 38 (55.07%) |
| Yes | 31 (44.93%) |
| AJCC stage* | |
| I | 13 (18.84%) |
| II | 21 (30.43%) |
| III | 34 (49.28%) |
| IV | 1 (1.45%) |
*AJCC: American Joint Committee on Cancer. The stage was postoperative staging based on the AJCC Cancer Staging Manual, Eighth Edition.
Figure 4.
Detection of MHC-I expression and infiltration of interstitial CD8+ T cells in NSCLC. (a) Interstitial lymphocyte infiltration and MHC-I expression on NSCLC cells were positively correlated (R2 = 0.713). (b) Representative MHC-I immunohistochemistry of NSCLC cells (400×). Images were scored for the proportion of cells expressing MHC-I and the intensity of expression. (c) Representative image of infiltrating interstitial CD8+ T cells. White arrows indicate CD8+ T cells (400×).
Discussion
NSCLC is the most common fatal malignancy worldwide. Great progress has been made in NSCLC treatment during recent decades, with a combination of immunotherapy and radiotherapy seen as one of the most promising current treatments. Evidence has shown that a single high dose of radiation increased the release of immunogenic antigen, and achieved better results in conjunction with immunotherapy than conventional fraction radiotherapy.21 Lehrer et al. reported that the synergy of stereotactic radiosurgery and immune checkpoint inhibitors reduced the probability of new brain lesions occurring by 40% in patients with brain metastases.22
Compared with 10 × 3 Gy fractions, one dose of 30 Gy was reported to induce more CD8+ T cell infiltration and fewer myeloid-derived suppressor cells in CT26 and MC38 colon tumors.23 Moreover, advances in image-guided radiotherapy now enable single radiation doses as high as 30 Gy to be safely delivered to tumor sites.23 However, Vanpouillebox et al. found that abscopal responses were only seen in mice irradiated with 3 × 8 Gy fractions plus anti-CTLA4, and that a single dose of 20 Gy was ineffective.5 Indeed, they showed that radiation doses above 12 Gy induced DNA exonuclease Trex1, which downregulated cytosolic DNA and activated the cyclic GMP–AMP synthase/stimulator of interferon genes pathway. Golden et al. investigated the outcome of patients with metastatic solid tumors treated with concurrent radiotherapy at 10 × 3.5 Gy fractions to one metastatic site and the subcutaneous injection of granulocyte–macrophage colony-stimulating factor (125 µg/m2) daily for 2 weeks. They found that metastatic lesions outside the field shrank in 11 of 41 patients (26.8%, 95% confidence interval 14.2–42.9).24 Radiation (≤3 Gy) was reported to induce abscopal responses outside the radiation field,25 while Marconi et al. suggested that the probability of the abscopal effect was 50% for radiotherapy with equivalent biological doses over 60 Gy, regardless of the single radiation dose and number of fractions.26 However, the optimal radiotherapy regimen for the strongest immune response remains controversial, and the mechanisms of how radiotherapy affects inflammation and immunity are still to be explored.
Autophagy is also induced by radiation and delivers cytoplasmic constituents for lysosomal degradation. It was reported to provide a substantial source of intra- and extracellular antigens for MHC presentation to T cells, which impacted on tumor-specific immune responses.27 In adaptive immunity, the autophagy pathway was shown to be essential to antigen presentation,28 while MHC-I downregulation was reported to be involved directly or indirectly in the mechanism of immune escape.29 Previous studies revealed the regulatory mechanism of MHC-I by increasing its expression to avoid immune escape.30 Patients with Merkel cell carcinoma demonstrated strong MHC-I expression after interferon (IFN) treatment,12 while IFN production by lymphocytes was triggered through other signals released during radiation-induced immunogenic cell death.31 In the present study, we used A549 and H1975 cell lines to investigate autophagy induction by irradiation and its effects on MHC-I expression. We showed that autophagy and MHC- I expression reached the highest levels when cells received a single dose of 20 Gy irradiation. However, MHC-I expression decreased after inhibition of autophagy by CQ, indicating that cellular autophagy regulates MHC-I expression.
The mechanism of autophagic regulation of the MHC-I could involve the release of immunostimulatory danger signals induced by irradiation, such as high-mobility group protein B1 (HMGB1), ATP, and calreticulin.32 Indeed, Golden et al.33 reported that HMGB1, ATP, and calreticulin were highly stimulated by ionizing radiation at 20 Gy. Additionally, autophagy is known to be involved in the release of HMGB1, a non-histone chromatin-binding protein involved in the perception of cancer cell death as immunogenic.34 Both extranuclear and extracellular pools of HMGB1 previously promoted robust autophagic responses, indicating the existence of bidirectional crosstalk between autophagy and danger signaling.35
We demonstrated that autophagy and MHC-I increased in line with ionizing radiation increases up to 20 Gy, and that a single irradiation dose of 20 Gy activated the strongest autophagy with expression of the highest level of MHC-I. This finding differed from that of Zhang et al.36 who showed that 3 × 8 Gy induced the strongest IFN-β secretion. Although a variety of radiation doses and delivery schedules have been used to induce anti-tumor T cells in different mouse tumor models,1 the optimal strategy to achieve this remains to be defined. To enable repair of healthy tissue, radiation is usually administered in multiple fractions, typically of 2 Gy each time, but this dose might result in inefficient activation of the immune response. However, using drugs that activate autophagy may achieve similar goals as high-dose radiotherapy, such as activation of the immune response.
T cells recognize antigens presented in the form of MHC-antigen complex molecules, which is pivotal for the identification of infected or cancerous cells. Following antigen processing by the MHC, tumor-associated antigens are cross-presented to CD8+ T or CD4+ T cells, leading to the activation of T cell populations.30 Thus, MHC molecules and T cells are thought to be closely linked. Romagnoli et al. demonstrated the presence of MHC-II molecules on activated murine CD8+ T cells in vitro as well as in vivo.37 When complexed with antigenic peptides, MHC-I molecules initiate CD8+ T cell responses via interaction with the T cell receptor and co-receptor CD8.38 In the present study, we found that the expression of MHC-I was positively correlated with the infiltration of CD8+ T cells.
An infiltration of CD8+ T cells is required for good responses to immunotherapy. Matsutani et al.39 reported that an evaluation of CD8+ tumor-infiltrating lymphocyte density in rectal cancer during pretreatment biopsy could be used as a predictor of effectiveness of neoadjuvant treatment. The evolution of a local immune response may be triggered by the irradiation-mediated release of tumor antigens, creating a tumor microenvironment for cytotoxic T cell infiltration. This might indicate a role for irradiation driven by CD8+ T cells in anti-tumor immune responses.
Conclusion
We concluded that autophagy induction should be used to guide the selection of radiation doses and fractionation to boost CD8+ T cell infiltration. High-dose radiation therapy activated immune responses by stimulating autophagy and increasing MHC-I expression.
Author contributions
All authors were involved in research design, data collection, results analysis, and manuscript preparation.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was supported by the National Natural Science Foundation of China (81372498, 81572967, 81773236, and 81800429), the National Project for Improving the Ability of Diagnosis and Treatment of Difficult Diseases, the National Key Clinical Specialty Construction Program of China ([2013]544), Fundamental Research Funds for the Central Universities (2042018kf0065 and 2042018kf1037), the Health Commission of Hubei Province Scientific Research Project (WJ2019H002 and WJ2019Q047), Wuhan City Huanghe Talents Plan, and Zhongnan Hospital of Wuhan University Science, Technology and Innovation Seed Fund (znpy2016050, znpy2017001, znpy2017049, and znpy2018028).
References
- 1.Pfannenstiel LW, McNeilly C, Xiang C, et al. Combination PD-1 blockade and irradiation of brain metastasis induces an effective abscopal effect in melanoma. Oncoimmunology 2018; 8: e1507669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood 2009; 114: 589–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature 2017; 541: 321–330. [DOI] [PubMed] [Google Scholar]
- 4.Twymansaint VC, Rech AJ, Maity A, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 2015; 520: 373–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vanpouillebox C, Alard A, Aryankalayil MJ, et al. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat Commun 2017; 8: 15618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nestvold J, Wang MY, Camilio KA, et al. Oncolytic peptide LTX-315 induces an immune-mediated abscopal effect in a rat sarcoma model. Oncoimmunology 2017; 6: e1338236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vanpouillebox C, Diamond JM, Pilones KA, et al. TGFβ is a master regulator of radiation therapy-induced anti-tumor immunity. Cancer Res 2015; 75: 2232–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sijts EJAM, Kloetzel P-M. The role of the proteasome in the generation of MHC class I ligands and immune responses. Cell Mol Life Sci 2011; 68: 1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simone BA, Dan T, Palagani A, et al. Caloric restriction coupled with radiation decreases metastatic burden in triple negative breast cancer. Cell Cycle 2016; 15: 2265–2274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khateri M, Abdoli A, Motevalli F, et al. Evaluation of autophagy induction on HEV 239 vaccine immune response in a mouse model. IUBMB Life 2018; 70: 207–214. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wang LX, Yang G, et al. Efficient cross-presentation depends on autophagy in tumor cells. Cancer Res 2008; 68: 6889–6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulson KG, Tegeder A, Willmes C, et al. Downregulation of MHC-I expression is prevalent but reversible in Merkel cell carcinoma. Cancer Immunol Res 2014; 2: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shinto E, Hase K, Hashiguchi Y, et al. CD8+ and FOXP3+ tumor-infiltrating T cells before and after chemoradiotherapy for rectal cancer. Ann Surg Oncol 2014; 21: 414–421. [DOI] [PubMed] [Google Scholar]
- 14.Jiang X, Huang Y, Liang X, et al. Metastatic prostate cancer-associated P62 inhibits autophagy flux and promotes epithelial to mesenchymal transition by sustaining the level of HDAC6. Prostate 2018; 78: 426–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu X, Si S, Wang H, et al. Blocking autophagy improves the anti-tumor activity of afatinib in lung adenocarcinoma with activating EGFR mutations in vitro and in vivo. Sci Rep 2017; 7: 4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ryabaya OO, Inshakov AN, Egorova AV, et al. Autophagy inhibitors chloroquine and LY294002 enhance temozolomide cytotoxicity on cutaneous melanoma cell lines in vitro. Anticancer Drugs 2017; 28: 307–315. [DOI] [PubMed] [Google Scholar]
- 17.Salama AK, Postow MA, Salama JK. Irradiation and immunotherapy: From concept to the clinic. Cancer 2016; 122:1659–1671. [DOI] [PubMed] [Google Scholar]
- 18.Reits EA, Hodge JW, Herberts CA, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med 2006; 203: 1259–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takahashi Y, Yasui T, Minami K, et al. Radiation enhances the efficacy of antitumor immunotherapy with an immunocomplex of interleukin-2 and its monoclonal antibody. Anticancer Res 2017; 37: 6799–6806. [DOI] [PubMed] [Google Scholar]
- 21.Postow MA, Callahan MK, Barker CA, et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 2012; 366: 925–931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lehrer EJ, McGee HM, Peterson JF, et al. Stereotactic radiosurgery and immune checkpoint inhibitors in the management of brain metastases. Int J Mol Sci 2018; 19: 3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filatenkov A, Baker J, Mueller AM, et al. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015, 21: 3727–3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golden EB, Chhabra A, Chachoua A, et al. Local radiotherapy and granulocyte-macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 2015; 16: 795–803. [DOI] [PubMed] [Google Scholar]
- 25.Chandra RA, Wilhite TJ, Balboni TA, et al. A systematic evaluation of abscopal responses following radiotherapy in patients with metastatic melanoma treated with ipilimumab. Oncoimmunology 2015; 4: e1046028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marconi R, Strolin S, Bossi G, et al. A meta-analysis of the abscopal effect in preclinical models: is the biologically effective dose a relevant physical trigger? PLoS One 2017; 12: e0171559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.You L, Mao L, Wei J, et al. The crosstalk between autophagic and endo-/exosomal pathways in antigen processing for MHC presentation in anticancer T cell immune responses. J Hematol Oncol 2017; 10: 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.You L, Jin S, Zhu L, et al. Autophagy, autophagy-associated adaptive immune responses and its role in hematologic malignancies. Oncotarget 2016; 8: 12374–12388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park IA, Hwang SH, Song IH, et al. Expression of the MHC class II in triple-negative breast cancer is associated with tumor-infiltrating lymphocytes and interferon signaling. Plos One 2017; 12: e0182786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brea EJ, Oh CY, Manchado E, et al. Kinase regulation of human MHC class I molecule expression on cancer cells. Cancer Immunol Res 2016; 4: 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Deng L, Liang H, Xu M, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity 2014; 41: 843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galluzzi L, Buqué A, Kepp O, et al. Immunogenic cell death in cancer and infectious disease. Nat Rev Immunol 2017; 17: 97–111. [DOI] [PubMed] [Google Scholar]
- 33.Golden EB, Frances D, Pellicciotta I, et al. Radiation fosters dose-dependent and chemotherapy-induced immunogenic cell death. Oncoimmunology 2014; 3: e28518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacqueline T, Henrick H, Jasmina R, et al. Autophagy regulates selective HMGB1 release in tumor cells that are destined to die. Cell Death Differ 2009; 16: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou W, Zhang Q, Yan Z, et al. Strange attractors: DAMPs and autophagy link tumor cell death and immunity. Cell Death Dis 2013; 4: e966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang I, Formenti SC, Knisely J. Immunotherapy plus stereotactic radiosurgery: building on the promise of precision medicine for CNS malignancies-part 1: principles of combined treatment. Oncology 2018; 32: e28. [PubMed] [Google Scholar]
- 37.Romagnoli PA, Premenko-Lanier MF, Loria GD, et al. CD8 T cell memory recall is enhanced by novel direct interactions with CD4 T cells enabled by MHC class II transferred from APCs. PLoS One 2013; 8: e56999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geng J, Altman JD, Krishnakumar S, et al. Empty conformers of HLA-B preferentially bind CD8 and regulate CD8+ T cell function. eLife 2018; 7: e36341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Matsutani S, Shibutani M, Maeda K, et al. The significance of tumor‐infiltrating lymphocytes before and after neoadjuvant therapy for rectal cancer. Cancer Sci 2018; 109: 966–979. [DOI] [PMC free article] [PubMed] [Google Scholar]