Short abstract
Objective
This study was performed to review the current evidence for the efficacy of shortwave and microwave diathermy in promoting nerve regeneration after peripheral nerve injuries in both animal models and human patients.
Methods
An extensive literature search was conducted without publication data restrictions. Studies including the intervention and outcome in animal or human models were selected. Non-English studies, reviews, letters, and case reports were excluded.
Results
Eleven articles were included in this study. Shortwave diathermy at the frequency of 27.12 or 40.68 MHz was used in six of seven animal studies, while only one study utilized microwave diathermy at 915 MHz. Seven animal experiments demonstrated that shortwave or microwave diathermy produces an increased myelinated nerve fiber number, myelin sheath thickness, and axon diameter as well as improved electrophysiological parameters and locomotion. A total of 128 patients (207 wrists) were enrolled in four clinical studies. The clinical use of diathermy in human patients with carpal tunnel syndrome showed positive effects on pain, hand function, and electrophysiological findings.
Conclusions
Shortwave or microwave diathermy can improve the electrophysiological parameters, myelinated fiber number, and axon diameter of the injured nerve.
Keywords: Peripheral nerve injury, peripheral nerve regeneration, physical therapy, microwave diathermy, shortwave diathermy, animal models
List of abbreviations
BDNF = brain-derived neurotrophic factor
VEGF = vascular endothelial growth factor
CTS = carpal tunnel syndrome
Introduction
Peripheral nerve injury is a commonly encountered clinical problem. Patients may benefit from surgical interventions such as nerve grafting, but these are delicate procedures and sometimes unsatisfactory in the case of severe injury.1 Although pharmacotherapeutics have also been used, functional recovery is often poor.2 Physical agents that influence healing of the injured nerve may be used to complement or supplement the aforementioned interventions. Physical agents can be categorized as thermal (e.g., deep heat, superficial heat, and cold), mechanical (e.g., traction, compression, water, and sound), or electromagnetic (e.g., electromagnetic fields and electrical currents).3 Some physical agents such as water and ultrasound can also have thermal and mechanical effects. Deep-heating agents, also called diathermies, include ultrasound, shortwave, and microwave. Therapeutic ultrasound is a method of stimulating the tissue beneath the skin’s surface using very high-frequency sound waves. Shortwave and microwave diathermy uses high-frequency electromagnetic energy to generate heat. The application of shortwave diathermy can reportedly accelerate peripheral nerve regeneration in rats and cats.4–7 The present study was performed to explore the current evidence for the efficacy of shortwave and microwave diathermy in promoting nerve regeneration after different nerve injuries in both animal models and human patients.
Materials and methods
We performed an online search through OVID (including EMBASE, MEDLINE, and BIOSIS Previews), PubMed, and Web of Science for English-language articles using the terms “diathermy/microwave diathermy/shortwave diathermy,” “pulsed electromagnetic field/pulsed magnetic field/pulsing electromagnetic field/pulsing magnetic field,” and “peripheral nerve/peripheral nerve injury/peripheral nervous system disease/nerve regeneration.” The inclusion criteria for articles were publication in the English language, an animal or human study, and reporting of detailed data, including the intervention and the outcome. Non-English studies, reviews, letters, and case repots were excluded. The full text was obtained for further study when necessary. Our search was last performed on 5 April 2019. Because this study was a literature review, the need for ethics approval was waived.
Results
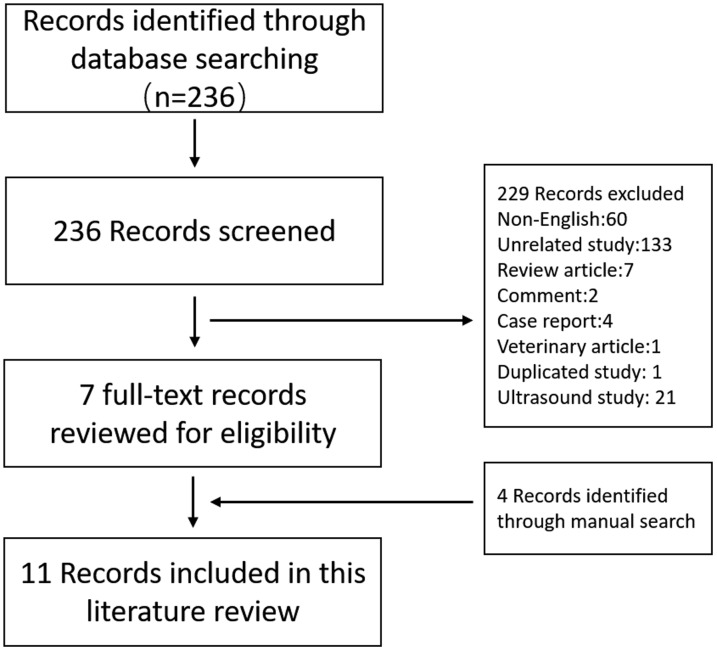
In total, 236 articles were identified through the search of PubMed. Studies including the use of shortwave or microwave diathermy for nerve injuries in animal models or human patients were selected. In the screening phase, articles not written in English (n = 60), irrelevant studies (n = 133), review articles (n = 7), comments (n = 2), case reports (n = 4), and duplicate articles (n = 1) were excluded based only on the title. The abstracts of the remaining articles were screened, and veterinary articles (n = 1) and ultrasound studies (n = 21) were excluded. The reference lists of the included articles were then manually searched to identify additional articles of interest, and four articles were included. Finally, 11 articles were enrolled in the review (Figure 1).
Figure 1.
Flow diagram of the identification and selection of the studies included in the analysis.
Animal models
Seven animal studies showed that the application of shortwave diathermy and microwave diathermy after peripheral nerve injury increased the myelinated nerve fiber number, myelin sheath thickness, and axon diameter. Moreover, electrophysiological parameters and locomotion were also improved, indicating that shortwave or microwave diathermy may accelerate nerve regeneration (Table 1). In detail, Wilson and Jagadeesh6 and Wilson et al.7 divided the median and ulnar nerves in a rat model. The nerves were treated using a Diapulse machine (Diapulse Corporation of American, Great Neck, NY, USA) at a frequency of 27.12 MHz for 15 minutes beginning 2 hours after surgery and then daily for 15 minutes. At 30 days, the nerves in the treated animals had progressed further toward recovery than the nerves of the untreated animals at 60 days. Nerve conduction studies indicated that function was restored to normal after 45 days in the treated group; in contrast, the untreated nerves required 60 days to recover. Raji4 and Bowden5 designed experiments with standardized operative, histological, cytological, and morphometric techniques to assess the effect of diathermy (Diapulse machine at a frequency of 27.12 MHz) on lesions of the common peroneal nerve in rats. The animals underwent 15 minutes of diathermy daily for periods ranging from 3.5 days to 8 weeks after injury. Wallerian degeneration was more rapid in animals treated with diathermy. Compared with untreated group, the diameter of the myelinated fibers and axons at and below the level of the lesions was significantly larger in the diathermy-treated than untreated group (P < 0.05).
Table 1.
Setup of shortwave or microwave diathermy in animal experiments.
| Animal model | Intervention | Results | Functional tests | Reference |
|---|---|---|---|---|
| Nerve division and suture (Wistar rats) | 27.12 MHz for 15 min/day, 45 days | Faster wound healing, less scarring and fibrosis | Earlier biphasic nerve potential | Wilson et al. (1974)7 |
| Partial nerve removal and suture (Wistar rats) | 27.12 MHz for 15 min/day, 30 days | Fewer adhesions, more regenerating fibers | Earlier biphasic nerve potential | Wilson and Jagadeesh (1976)6 |
| Nerve crush, cut, and suture (Lewis’s rats) | 27.12 MHz for 15 min/day, 7 days/week, 8 weeks | Increased myelinated nerve fiber diameter, myelinated axon diameter, epineurium thickness, and luminal CSA of endoneurial vessels | More rapid functional recovery | Raji and Bowden (1983)5 |
| Nerve cut and suture (Lewis rats) | 27.12 MHz for 15 min/day, 7 days/week, 8 weeks | Increased nerve fiber number, luminal CSA of intraneural vessels, and epineurium thickness | Earlier functional recovery | Raji (1984)4 |
| Acellular nerve allografts (Wistar rats) | 40.68 MHz for 7 min/day, 7 days/week, 12 weeks | Increase in BDNF and VEGF, myelinated nerve fiber number, myelin sheath thickness, and axon diameter | Increased SFI | Pang et al. (2013)10 |
| Acellular nerve allografts (Wistar rats) | 40.68 MHz for 7 min/day, 20 times/course, 3 courses | Increase in VEGF, myelinated nerve number, and axon diameter | Increased NCV | Zhang et al. (2008)9 |
| Nerve compression (Sprague-Dawley rats) | 915 MHz for 10 min/day, 7 days/week, 12 weeks | Increased expression of S-100 protein, increased number and diameter of myelinated nerve fibers | Increased MNCV and SFI | Zhao et al. (2013)13 |
Abbreviations: CSA, cross-sectional area; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; SFI, sciatic function index; NCV, nerve conduction velocity; MNCV, motor nerve conduction velocity.
Two of the seven studies used an acellular nerve allograft model in Wistar rats. Leitgeb et al.,8 Zhang et al.,9 and Pang et al.10 repaired the sciatic nerve of rats with acellular nerve allografts, followed by a 7-minute treatment with shortwave diathermy at 40.68 MHz within 24 hours of the operation and then daily for up to 12 weeks. The authors demonstrated that the myelinated fiber number, myelin sheath thickness, and axon diameter were significantly higher in the shortwave diathermy-treated than untreated group (P < 0.05). Electrophysiological analysis showed that the shortwave diathermy-treated group had a faster conduction velocity, shorter latent period, and higher wave amplitude than the untreated group. The sciatic function index was used for the functional evaluation of sciatic nerve regeneration.11,12 Pang et al.10 reported that the sciatic function index was significantly higher in the shortwave diathermy-treated group than in the untreated group at 2, 4, 8, and 12 weeks (P < 0.05).
One of the seven studies used a nerve compression model in Sprague–Dawley rats. Zhao et al.13 demonstrated that microwave diathermy at 915 MHz could effectively inhibit the inflammatory reaction after injury as well as improve the local circulation of the injured nerve and consequently reduce adhesion between the injured nerves. They also reported that microwave diathermy was effective in promoting the proliferation of Schwann cells.
Human patients
Our literature search revealed limited evidence from four studies focusing on the clinical use of shortwave or microwave diathermy for peripheral nerve injury in human patients with carpal tunnel syndrome (CTS) (Table 2). Shortwave or microwave diathermy produced pain relief and function improvement in patients with CTS. In addition, the median distal motor latency, distal sensory latency, and sensory nerve conduction velocity were improved after application of shortwave diathermy. Boyaci et al.14 reported the effects of 27.12-MHz shortwave diathermy in humans with mild or moderate idiopathic CTS. Patients with CTS were treated for 20 minutes per day, 5 days/week, for 3 weeks and showed significant improvements in terms of pain, median nerve distal motor latency, median nerve distal sensory latency, and median sensory nerve conduction velocity. In contrast, the placebo group showed improvements only in median nerve distal motor latency. The shortwave diathermy group showed significant improvements in median nerve distal sensory latency and median sensory nerve conduction velocity compared with the placebo group. Improvements in median nerve sensory velocity were also reported by Ozcete et al.15 after application of shortwave diathermy for 20 minutes per day, 5 days a week, for 2 weeks versus sham therapy in patients with mild to moderate CTS. A subsequent study by Incebiyik et al.16 using the same shortwave apparatus confirmed significant improvements in pain and hand function in the shortwave diathermy group following treatment; in contrast, no significant improvement was seen in the placebo group. Frasca et al.17 reported the benefits of a superficial cooling system and a deep heating source with a microwave power generator at 434 MHz on CTS after administration of 20-minute treatments at two sessions per week for 3 weeks. The microwave diathermy group experienced a significant improvement in pain and functional status relative to baseline, while no improvements in pain intensity or functionality were observed in the sham-treated group. The changes in pain severity between baseline and the end of treatment were larger in the microwave diathermy group than in the sham-treated group. However, no intra-group or between-group differences were observed in the median nerve distal motor latency or median sensory nerve conduction velocity.
Table 2.
Clinical use of shortwave or microwave diathermy in patients with carpal tunnel syndrome.
| Number of patients | Management |
Outcome |
Reference | ||
|---|---|---|---|---|---|
| Pain | Hand function | Electrophysiological test | |||
| 30 (55 wrists) | 27.12 MHz diathermy for 20 min/day, 5 days/week, 3 weeks | Decreased VAS score | Decreased BCTQ score | Improved mDML, mDSL, and mSNCV | Boyaci et al. (2014)14 |
| 45 (60 wrists) | 27.12 MHz diathermy for 20 min/day, 5 days/week, 2 weeks | n/a | Increase in grip and pinch strength | Improved mSNCV | Ozcete et al. (2013)15 |
| 31 (58 wrists) | 27.12 MHz diathermy for 15 min/day, 5 days/week, 3 weeks | Decreased VAS score | Decreased BCTQ score | n/a | Incebiyik et al. (2015)16 |
| 22 (34 wrists) | 434 MHz diathermy for 20 min/session, 2 sessions/week, 3 weeks | Decreased VAS score | Decreased BCTQ score | No change in mDML or mSNCV | Frasca et al. (2011)17 |
Abbreviations: VAS, visual analog scale; BCTQ, Boston Carpal Tunnel Questionnaire; mDML, median distal motor latency; mDSL, median distal sensory latency; mSNCV, median sensory nerve conduction velocity; n/a, not available.
Discussion
Diathermy and nerve regeneration
Diathermy, from the Greek words dia meaning “through” and therma meaning “heating,”18 is the application of shortwave (frequency of about 3–300 MHz) or microwave (frequency of 300 MHz to 300 GHz) electromagnetic energy and ultrasound (acoustic vibration with a frequency of 0.8–3.0 MHz) to produce heat and other physiological changes within tissues (Table 3). Both shortwave and microwave radiation are nonionizing. Shortwave diathermy devices have been allocated the three frequency bands centered on 13.56, 27.12, and 40.68 MHz,19 of which the 27.12-MHz band is the most commonly used modality. Microwave diathermy devices operate at three frequencies: 433.92, 915, and 2045 MHz.8,19–21
Table 3.
Classification of diathermy.
| Ultrasound | Shortwave diathermy |
Microwave diathermy |
||||||
|---|---|---|---|---|---|---|---|---|
| Decimeter wave | Centimeter wave | Millimeter wave | ||||||
| Wavelength | / | 22.12 m | 11.06 m | 7.37 m | 0.69 m | 0.33 m | 0.1225 m | 0.008 m |
| Frequency | 0.8–3.0 MHz | 13.56 MHz | 27.12 MHz | 40.68 MHz | 433.92 MHz | 915 MHz | 2450 MHz | 37.5 GHz |
Despite the ability of peripheral axons to regenerate and form functional connections, the recovery of function is frequently disappointing after primary surgical repair. This is especially true in patients with traumatic peripheral nerve injuries because axon regeneration often must extend over much longer distances in humans than in mice.22 The major reasons for the poor functional recovery are the restricted timeframe of opportunity for nerve regeneration and the slow growth of regenerating axons.23 Four studies in this review demonstrated that shortwave diathermy at the frequency of 27.12 MHz induced regeneration of myelinated axons histologically and functionally, whether in crushed nerve models or nerve transection models.4–7 Moreover, microwave diathermy at 915 MHz facilitated neural regeneration with inhibition of the inflammatory reaction and improvement of local blood circulation. Both shortwave and microwave diathermy can be delivered in a continuous or pulsed mode and, when delivered at a sufficient intensity, can generate heat in the treated area.24–26 Heat is generated by eddy currents induced within the body and by the movement of ions and distortion of molecules and crystal lattices within the field.27 The physiological effects of increasing tissue temperature include amelioration of local blood flow, alteration of the sensory nerve response, an increased rate of nerve conduction, and elevation of the pain threshold, which may be related to faster nerve regeneration. Furthermore, the thermal effects produce an increase in the nutrients and oxygen in the treated region. Both nutrients and oxygen have a pivotal role in all of the anabolic processes that take place in an organism, and they are necessary for nerve repair.28
Molecular basis of promotion of nerve regeneration by diathermy
After an injury occurs in the peripheral nerve system, Schwann cells proliferate, transdifferentiate, and become repair cells.22 Regeneration-associated genes are expressed, including those encoding neurotrophic factors such as brain-derived neurotrophic factor (BDNF), nerve growth factors, glial-derived neurotrophic factor, and pleiotrophin.29 BNDF can promote axon regeneration and functional recovery after nerve injury,32 playing an important role in the formation of synapses and charging of motor function for the motor neurons of the spinal anterior horn. Therefore, the BDNF level is regarded as an index of the nerve regeneration status. Shortwave diathermy upregulates the expression of BDNF in the spinal cord and muscles,10 suggesting that BDNF expression may be involved in the process of nerve regeneration treated by shortwave diathermy. Zhang et al.9 and Pang et al.10 also found that shortwave diathermy can promote peripheral nerve regeneration by upregulating vascular endothelial growth factor (VEGF) mRNA expression in the spinal cord and muscle at the operated site. VEGF reportedly stimulates neurogenic, protective, and neurotrophic activities, including proliferation of astrocytes and Schwann cells.33–35 Hobson et al.34 found that VEGF increased angiogenesis in a silicone sciatic nerve chamber, enhanced Schwann cell proliferation and migration, and played a significant role in peripheral nerve regeneration. Therefore, VEGF upregulation in the spinal cord can be anterogradely transported to the injured nerve to promote nerve regeneration by direct action. However, VEGF upregulation in the muscle can improve the nutrition of the target tissue, lighten muscle atrophy, and be retrogradely transported to the injury site to accelerate nerve regeneration.36 Previous studies showed that S-100 protein expression was limited in Schwann cells in the peripheral nervous system and that its expression was absent in axons.37–42 Therefore, high levels of S-100 protein indicate active proliferation of Schwann cells, which has been shown to promote nerve regeneration.43–47 Using S-100 protein as a Schwann cell marker, Zhao et al.13 found that microwave diathermy induced S-100 protein expression in the regenerated nerves. In conclusion, the mechanism by which diathermy promotes nerve regeneration may involve upregulation the BNDF and VEGF expression in the spinal cord and muscle and S-100 protein expression in the injured nerve.
Application of diathermy in clinical practice
During the last few years, shortwave and microwave diathermy has been proven effective in the management of skeletal muscle injuries.28,48 However, only a few randomized controlled studies have focused on diathermy in the treatment of peripheral nerve injuries, primarily CTS. The efficacy of diathermy is related to the increase in heat in the deep tissue. The effect includes an increase in vasodilatation and soft tissue elasticity, amelioration of local blood flow, and reduction of muscle spasms. This mechanism might be relevant in the treatment of CTS, given that the positive effect of diathermy on local ischemia plays a pivotal role in the pathogenesis of CTS. Although all four studies14–17 were double-blind, randomized, and sham-controlled trials, their small sample sizes (n = 22, 45, 30, and 31) and short-term follow-ups might be limitations. Moreover, diathermy at 27.12 MHz improved the distal motor and sensory latency14,15 and nerve conduction velocity of the median nerve in patients with CTS, while diathermy at 434 MHz caused no change in these electrodiagnostic parameters.17 These conflicting findings deserve further investigation. Notably, most cases of CTS are idiopathic; however, various etiologies have been reported, such as diabetes mellitus, rheumatoid arthritis, hypothyroidism, pregnancy, and tenosynovitis.16 The pathophysiology of CTS is different from that of traumatic peripheral nerve injury. Despite the encouraging initial results in patients with compressive pathology following CTS, much more evidence is needed to support the widespread use of diathermy for peripheral nerve injury in humans.
Limitations
A limitation of this review is the absence of blinding to the origins of the articles. In addition, because studies of the application of shortwave or microwave diathermy in nerve regeneration are insufficient, four animal studies performed in the 1970s and 1980s were included in the current review.4–7 Finally, only one animal study13 demonstrated an increase in S-100 protein and two studies9,10 showed increased VEGF after shortwave or microwave diathermy; therefore, the underlying molecular mechanisms remain incompletely understood.
Conclusions
The positive efficacy of shortwave and microwave diathermy in promoting nerve regeneration in animal models has been verified in several independent studies.5–7,9,10,13 The mechanisms responsible for diathermy have yet to be fully elucidated and adequately reported. Clinical interest in the use of diathermy for peripheral nerve injury in humans has primarily involved CTS. The preliminary studies performed with a shortwave device working at 27.12 MHz or a microwave device working at 434 MHz have shown encouraging results. However, at the time of this writing, we were unaware of any studies examining the effects of diathermy in humans after peripheral nerve transection or more extensive studies of compressive nerve pathology. More large-scale randomized controlled trials with longer follow-ups are needed to investigate the efficacy of shortwave or microwave diathermy in improving regenerative outcomes.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Lee SK, Wolfe SW. Peripheral nerve injury and repair. J Am Acad Orthop Sur 2000; 8: 243–252. [DOI] [PubMed] [Google Scholar]
- 2.Reynolds E. Vitamin B12, folic acid, and the nervous system. Lancet Neurol 2006; 5: 949–960. [DOI] [PubMed] [Google Scholar]
- 3.Cameron MH. Physical agents in rehabilitation: from research to practice. 4th ed St Louis: Elsevier/Saunders, 2013. [Google Scholar]
- 4.Raji AM. An experimental study of the effects of pulsed electromagnetic field (Diapulse) on nerve repair. J Hand Surg Br 1984; 9: 105–112. [PubMed] [Google Scholar]
- 5.Raji AR, Bowden RE. Effects of high-peak pulsed electromagnetic field on the degeneration and regeneration of the common peroneal nerve in rats. J Bone Joint Surg Br 1983; 65: 478–492. [DOI] [PubMed] [Google Scholar]
- 6.Wilson DH, Jagadeesh P. Experimental regeneration in peripheral nerves and the spinal cord in laboratory animals exposed to a pulsed electromagnetic field. Paraplegia 1976; 14: 12–20. [DOI] [PubMed] [Google Scholar]
- 7.Wilson DH, Jagadeesh P, Newman PP, et al. The effects of pulsed electromagnetic energy on peripheral nerve regeneration. Ann N Y Acad Sci 1974; 238: 575–585. [DOI] [PubMed] [Google Scholar]
- 8.Leitgeb N, Omerspahic A, Niedermayr F. Exposure of non-target tissues in medical diathermy. Bioelectromagnetics 2010; 31: 12–19. [DOI] [PubMed] [Google Scholar]
- 9.Zhang LX, Tong XJ, Sun XH, et al. Experimental study of low dose ultrashortwave promoting nerve regeneration after acellular nerve allografts repairing the sciatic nerve gap of rats. Cell Mol Neurobiol 2008; 28: 501–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pang CJ, Tong L, Ji LL, et al. Synergistic effects of ultrashort wave and bone marrow stromal cells on nerve regeneration with acellular nerve allografts. Synapse 2013; 67: 637–647. [DOI] [PubMed] [Google Scholar]
- 11.Hare GM, Evans PJ, Mackinnon SE, et al. Walking track analysis: a long-term assessment of peripheral nerve recovery. Plast Reconstr Surg 1992; 89: 251–258. [PubMed] [Google Scholar]
- 12.Mimura T, Dezawa M, Kanno H, et al. Peripheral nerve regeneration by transplantation of bone marrow stromal cell-derived Schwann cells in adult rats. J Neurosurg 2004; 101: 806–812. [DOI] [PubMed] [Google Scholar]
- 13.Zhao F, He W, Zhang Y, et al. Electric stimulation and decimeter wave therapy improve the recovery of injured sciatic nerves. Neural Regen Res 2013; 8: 1974–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boyaci A, Tutoğlu A, Koca İ, et al. Comparison of the short-term effectiveness of short-wave diathermy treatment in patients with carpal tunnel syndrome: a randomized controlled trial. Turkish Journal of Rheumatology 2014; 29: 298–303. [Google Scholar]
- 15.Ozcete ZA, Ozturk C, On AY, et al. Effect of short wave therapy in idiopathic carpal tunnel syndrome: a randomized double-blind controlled study. Ftr Turkiye Fiziksel Tip Ve Rehabilitasyon Dergisi 2013; 59: 103–107. [Google Scholar]
- 16.Incebiyik S, Boyaci A, Tutoglu A. Short-term effectiveness of short-wave diathermy treatment on pain, clinical symptoms, and hand function in patients with mild or moderate idiopathic carpal tunnel syndrome. J Back Musculoskelet Rehabil 2015; 28: 221–228. [DOI] [PubMed] [Google Scholar]
- 17.Frasca G, Maggi L, Padua L, et al. Short-term effects of local microwave hyperthermia on pain and function in patients with mild to moderate carpal tunnel syndrome: a double blind randomized sham-controlled trial. Clin Rehabil 2011; 25: 1109–1118. [DOI] [PubMed] [Google Scholar]
- 18.Fee WDM. Diathermy in medicine. Br Med J 1933; 2: 873. [Google Scholar]
- 19.Low J, Reed AS. Electrotherapy explained: principles and practice. 2nd ed Oxford: Butterworth-Heinemann, 1994. [Google Scholar]
- 20.Lerman Y, Caner A, Jacubovich R, et al. Electromagnetic fields from shortwave diathermy equipment in physiotherapy departments. Physiotherapy 1996; 82: 456–458. [Google Scholar]
- 21.Martin CJ, Mccallum HM, Strelley S, et al. Electromagnetic fields from therapeutic diathermy equipment: a review of hazards and precautions. Physiotherapy 1991; 77: 3–7. [Google Scholar]
- 22.Scheib J, Hoke A. Advances in peripheral nerve regeneration. Nat Rev Neurol 2013; 9: 668–676. [DOI] [PubMed] [Google Scholar]
- 23.Willand MP, Nguyen MA, Borschel GH, et al. Electrical stimulation to promote peripheral nerve regeneration. Neurorehabil Neural Repair 2016; 30: 490–496. [DOI] [PubMed] [Google Scholar]
- 24.Conradi E, Pages IH. Effects of continuous and pulsed microwave irradiation on distribution of heat in the gluteal region of minipigs. A comparative study. Scand J Rehabil Med 1989; 21: 59–62. [PubMed] [Google Scholar]
- 25.Draper DO, Knight K, Fujiwara T, et al. Temperature change in human muscle during and after pulsed short-wave diathermy. J Orthop Sports Phys Ther 1999; 29: 13–18, 19–22. [DOI] [PubMed] [Google Scholar]
- 26.Silverman DR, Pendleton L. A comparison of the effects of continuous and pulsed short-wave diathermy on peripheral circulation. Arch Phys Med Rehabil 1968; 49: 429–436. [PubMed] [Google Scholar]
- 27.Goats GC. Pulsed electromagnetic (short-wave) energy therapy. Br J Sports Med 1989; 23: 213–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Giombini A, Giovannini V, Cesare AD, et al. Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Brit Med Bull 2007; 83: 379–396. [DOI] [PubMed] [Google Scholar]
- 29.Gordon T, English AW. Strategies to promote peripheral nerve regeneration: electrical stimulation and/or exercise. Eur J Neurosci 2016; 43: 336–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marcol W, Kotulska K, Larysz-Brysz M, et al. Extracts obtained from predegenerated nerves improve functional recovery after sciatic nerve transection. Microsurg 2005; 25: 486–494. [DOI] [PubMed] [Google Scholar]
- 31.Silverman WF, Krum JM, Mani N, et al. Vascular, glial and neuronal effects of vascular endothelial growth factor in mesencephalic explant cultures. Neuroscience 1999; 90: 1529–1541. [DOI] [PubMed] [Google Scholar]
- 32.Sondell M, Lundborg G, Kanje M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J Neurosci 1999; 19: 5731–5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sondell M, Lundborg G, Kanje M. Regeneration of the rat sciatic nerve into allografts made acellular through chemical extraction. Brain Res 1998; 795: 44–54. [DOI] [PubMed] [Google Scholar]
- 34.Hobson MI, Green CJ, Terenghi G. VEGF enhances intraneural angiogenesis and improves nerve regeneration after axotomy. J Anat 2000; 197: 591–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sondell M, Sundler F, Kanje M. Vascular endothelial growth factor is a neurotrophic factor which stimulates axonal outgrowth through the flk-1 receptor. Eur J Neurosci 2000; 12: 4243–4254. [DOI] [PubMed] [Google Scholar]
- 36.Liu H, Kim Y, Chattopadhyay S, et al. Matrix metalloproteinase inhibition enhances the rate of nerve regeneration in vivo by promoting dedifferentiation and mitosis of supporting schwann cells. J Neuropathol Exp Neurol 2010; 69: 386–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kobayashi M, Ishibashi S, Tomimitsu H, et al. Proliferating immature Schwann cells contribute to nerve regeneration after ischemic peripheral nerve injury. J Neuropathol Exp Neurol 2012; 71: 511–519. [DOI] [PubMed] [Google Scholar]
- 38.Matsuse D, Kitada M, Kohama M, et al. Human umbilical cord-derived mesenchymal stromal cells differentiate into functional Schwann cells that sustain peripheral nerve regeneration. J Neuropathol Exp Neurol 2010; 69: 973–985. [DOI] [PubMed] [Google Scholar]
- 39.McGrath AM, Novikova LN, Novikov LN, et al. BD PuraMatrix peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res Bull 2010; 83: 207–213. [DOI] [PubMed] [Google Scholar]
- 40.Torigoe K, Hashimoto K, Lundborg G. A role of migratory Schwann cells in a conditioning effect of peripheral nerve regeneration. Exp Neurol 1999; 160: 99–108. [DOI] [PubMed] [Google Scholar]
- 41.Kerns JM, Fakhouri AJ, Weinrib HP, et al. Electrical stimulation of nerve regeneration in the rat: the early effects evaluated by a vibrating probe and electron microscopy. Neuroscience 1991; 40: 93–107. [DOI] [PubMed] [Google Scholar]
- 42.Patodia S, Raivich G. Role of transcription factors in peripheral nerve regeneration. Front Mol Neurosci 2012; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rajaram A, Chen XB, Schreyer DJ. Strategic design and recent fabrication techniques for bioengineered tissue scaffolds to improve peripheral nerve regeneration. Tissue Eng Part B Rev 2012; 18: 454–467. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt A. [Nerve compression syndromes in the area of the elbow joint in patients with chronic polyarthritis. Review of the literature]. Handchir Mikrochir Plast Chir 1993; 25: 75–79. [PubMed] [Google Scholar]
- 45.Zanakis MF. Differential effects of various electrical parameters on peripheral and central nerve regeneration. Acupunct Electrother Res 1990; 15: 185–191. [DOI] [PubMed] [Google Scholar]
- 46.Shields N, Gormley J, O'Hare N. Short-wave diathermy: a review of existing clinical trials. Physical Therapy Reviews 2001; 6: 101–118. [Google Scholar]