Short abstract
Objective
Aberrant expression of microRNAs is a key regulator of tumorigenesis and progression in endometrial cancer. We assessed the effect of microRNA-29b (miR-29b) on proliferation, chemosensitivity, migration, and invasion of endometrial cancer cells.
Methods
The proliferation of endometrial cancer cells was examined by water-soluble tetrazolium (WST)-1 assay. The effects of miR-29b on migration and invasion were evaluated by transwell migration and Matrigel invasion assays. Western blotting was used to assess protein expression levels after altered expression of miR-29b. The effect of miR-29b on cisplatin-induced apoptosis was examined by Caspase-Glo 3/7 assay.
Results
miR-29b inhibited proliferation and decreased migration and invasion of endometrial cancer cells. It also enhanced the sensitivity of endometrial cancer cells to cisplatin and increased cisplatin-induced apoptosis by regulating expression of BAX and Bcl-2. Moreover, miR-29b changed the expression level of phosphatase and tensin homolog (PTEN) and p-AKT by directly binding to the 3′ untranslated region of PTEN.
Conclusion
miR-29b played important roles in proliferation and progression in endometrial cancer cells by direct regulation of PTEN. It might be used as a biomarker to predict chemotherapy response and prognosis in endometrial cancer.
Keywords: MicroRNA-29b, phosphatase and tensin homolog (PTEN), AKT, endometrial cancer, cisplatin, chemoresistance
Introduction
Endometrial cancer is a malignant neoplasm with the ability to invade or metastasize. It occurs mainly in menopausal and postmenopausal women, with an average age of 60 years at the time of diagnosis.1 In the United States, endometrial cancer is the most common cancer of the female reproductive organs. It is estimated that about 63,230 new cases will be diagnosed and about 11,350 women will die from endometrial cancer in the United States in 2018.2 Endometrial cancers are often diagnosed at an early stage, with a 5-year relative survival rate of about 82%.3 Depending on the grade and stage of the disease, the standard treatment of endometrial cancer includes surgery followed by chemotherapy with or without radiotherapy.4 Unfortunately, some patients with advanced stage endometrial cancer or recurrent disease do not respond very well to the standard therapies. Development of resistance to chemotherapy remains a problem during treatment.5 A variety of mechanisms are involved in chemotherapy resistance, including apoptotic pathway,6 PI3K/AKT pathways,7 and hormone receptor signaling pathways.8 Therefore, it is necessary to find alternative strategies to promote sensitivity and decrease resistance to chemotherapy.
MicroRNAs (miRNAs) are small RNA molecules, about 22 nucleotides long, with the biological ability to induce gene silencing.9,10 Emerging evidence has shown that miRNAs are involved in various biological activities, including cell differentiation, metabolism, apoptosis, and tumorigenesis.11–13 Recent studies have shown differences in miRNA expression between normal endometrium and endometrial cancer cells. Down-regulation of miR-34a is associated with a good outcome in endometrial cancer patients; miR-34a inhibits proliferation by regulating expression of Notch1 in endometrial cancer cells.14 Another study showed that miR-34b is involved in methylation of the promoter region in endometrial cancer.15 Some miRNAs have diagnostic and prognostic value. Torres et al.16 showed that increased levels of miR-99a, miR-100, and miR-199b in serum of patients with endometrioid cancer might be correlated with prognosis. miR-29b has been shown to be expressed in a variety of normal tissues and is associated with different diseases. The expression of miR-29 is decreased in brain tissue of patients with Alzheimer disease17 and associated with neural cell death and infarct size in patients with acute ischemic stroke.18 In addition, miR-29b expression is down-regulated in different cancers due to direct binding of Gli to the miR-29 promoter.19 Garzon et al.20 reported that miR-29 acts as a tumor suppressor in acute myeloid leukemia by targeting apoptosis, cell cycle, and proliferation pathways. Ru et al.21 showed that miR-29b inhibits metastasis in prostate cancer cells by regulating epithelial-mesenchymal transition signaling.
Here, we examined the effect of miR-29b in cell proliferation, invasion, and migration of endometrial cancer cell lines.
Materials and methods
Ethics approval was not required because no humans or animals were used in this study.
Cell lines
The HEC-1-B and Ishikawa endometrial cancer cell lines were purchased from Shanghai cell bank, Chinese Academy of Sciences (Shanghai, China). These cancer cells were cultured in Dulbecco’s modified Eagle’s medium (Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 µg/mL streptomycin (Invitrogen), in a humidified atmosphere containing 5% CO2 at 37°C.
Cell transfection
The endometrial cancer cells were seeded in 24-well plates and transfection was performed using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s protocol. miR-29b and its negative control as well as miR-29b inhibitor and its negative control (Invitrogen) were transfected into endometrial cancer cells and these cells were used for further studies.
Real-time quantitative reverse transcription-PCR
TRIzol reagent (Invitrogen) was used to extract total RNA from the transfected endometrial cancer cells according to the manufacturer’s instructions. The miRNA analysis was performed using TaqMan MicroRNA Reverse Transcription kit (Thermo Fisher Scientific, Waltham, MA, USA). The PCR conditions were 95°C for 5 minutes, followed by 34 application cycles of 95°C for 15 s and 60°C for 30 s, followed by a dissociation stage. The primer sequences for real-time quantitative-PCR were as follows: miR-29b forward: 5′-GCCCTAG CACCATTTGAAA-3′; reverse: 5′-TGGT ATCCTTGAGGGATTGGTTC-3′. U6 was used as the endogenous control. All independent experiments were repeated three times in triplicate samples.
WST-1 assay
To examine the effect of miR-29b on the proliferation of endometrial cancer cells, the water-soluble tetrazolium (WST)-1 assay (Roche, Branford, CT, USA) was carried out. Briefly, HEC-1-B and Ishikawa endometrial cancer cells transfected with miR-29b mimics, miR-29b inhibitor, and scrambled negative control RNAs were placed in 96-well plates at a density of 1 × 104 cells/well and grown overnight. Then, the culture medium was removed and replaced with growth medium containing different concentrations of cisplatin (0, 1, 2, or 4 µg/mL). After 1, 3, 5, and 7 days, the medium was removed and 20 µL of WST-1 solution was added to each well and incubated for 1 hour at 37°C. Absorbance was measured at 490 nm on a microplate reader (BioTek Instruments GmbH, Bad Friedrichshall, Germany). All independent experiments were repeated three times in triplicate samples.
Migration and invasion assays
Endometrial cancer cells transfected with either miR-29b mimic or miR-29b inhibitor were suspended in medium without FBS and seeded in the upper chamber of a transwell (BD Bioscience, San Jose, CA, USA) at a density of 3 × 104 cells/well. Endometrial cancer cells transfected with scrambled negative control RNAs were used as a negative control. Medium containing 5% FBS was added to the lower chamber. The difference between invasion and migration assay is that the upper chambers were pre-coated with Matrigel (BD Bioscience) for the invasion assay but not for the migration assay. Then, endometrial cancer cells were cultured overnight in a humidified atmosphere containing 5% CO2 at 37°C. The non-invading cells were removed and the invading cells were stained with Diff Quik solution (Polysciences Inc., Warrington, PA, USA). The invading cells were counted in 5 random visual fields under light microscope. The percentage invasion was expressed as the ratio of invading cells over the cell number normalized on day 2 of the growth curve. All independent experiments were repeated three times in triplicate.
Apoptosis analysis
To study the effect of miR-29b on cisplatin-induced apoptosis of endometrial cancer cells, the activity of caspase 3/7 was used for apoptosis analysis according to the manufacturer’s protocol (Thermo Fisher Scientific). HEC-1-B and Ishikawa cells transfected with either miR-29b mimic or scrambled negative control RNAs were suspended in growth medium and placed in 24-well plates at a density of 2 × 105 cells/well. The cells were grown overnight and then the culture medium was removed and replaced with medium containing different concentrations of cisplatin (0, 1, 2, or 4 µg/mL). The cells were cultured for an additional 48 h, and then the Caspase-Glo reagent (Promega, Madison, WI, USA) was added to each well and incubated for 8 hours at room temperature with gentle shaking. Caspase 3/7 activity was detected using a 1-min lag time and 0.5 second/well read time with a luminometer (Thermo Labsystems/Thermo Fisher Scientific). All independent experiments were repeated three times in triplicates.
Luciferase reporter assays
To examine the target of miR-29b, we used the In-Fusion Dry-Down PCR Cloning Kit (Takara Bio USA Inc., Mountain View, CA, USA) to amplify the fragment of the 3′ untranslated region (UTR) of phosphatase and tensin homolog (PTEN) containing the putative miR-29b binding site from genomic DNA of HEK293T cells. Then, the amplified fragments were cloned into the XbaI site of the pmirGLO Dual-Luciferase miRNA Target Expression Vector (Promega). HEC-1B cells were co-transfected with Luc-PTEN and miR-29b in 24-well plates using Lipofectamine RNAiMAX following the manufacturer’s protocol (Thermo Fisher Scientific). Then, luciferase activities were detected using the Dual-Luciferase Reporter Assay system (Promega). All experiments were performed three times in triplicates.
Western blot analysis
Transfected endometrial cancer cells were lysed with RIPA buffer (Abcam, Cambridge, MA, USA), and the protein concentration was measured using a bicinchoninic acid (BCA) kit (Thermo Fisher Scientific) according the manufacturer’s protocol. Thirty micrograms of protein was separated by sodium dodecyl sulfate-PAGE in a 10% gel and transferred to polyvinylidene difluoride membranes (Sigma, St. Louis, MO, USA). The membranes were incubated with nonfat powdered milk in PBS for 1 hour at room temperature with gentle agitation. Then, the primary antibodies (Table 1) were added and incubated with the membranes overnight at 4°C with gentle agitation. The membranes were incubated with secondary antibody for 1 hour at room temperature with gentle agitation. Enhanced chemiluminescence reagent was used to develop the signal, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as the loading control.
Table 1.
Antibodies used in western blot analysis.
| Antibody | Vendor | Cat no./clone | Dilution |
|---|---|---|---|
| BAX | Cell Signaling Technology, Danvers, MA, USA | #2772 | 1:500 |
| BCL-2 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | sc-7382 | 1:300 |
| p-AKT | Abcam, Cambridge, MA, USA | ab3844 | 1:500 |
| PTEN | Cell Signaling Technology | #9552 | 1:500 |
| AKT | Cell Signaling Technology | #9272 | 1:300 |
| GAPDH | Cell Signaling Technology | #2118 | 1:2000 |
Statistical analysis
All statistical calculations were conducted using SPSS statistical software (version 18.0; IBM Corp. Chicago, IL, USA). Differences were analyzed using one-way analysis of variance (Tukey, ANOVA), and p-values < 0.05 were considered statistically significant.
Results
miR-29b decreased proliferation of endometrial cancer cells and enhanced cisplatin sensitivity
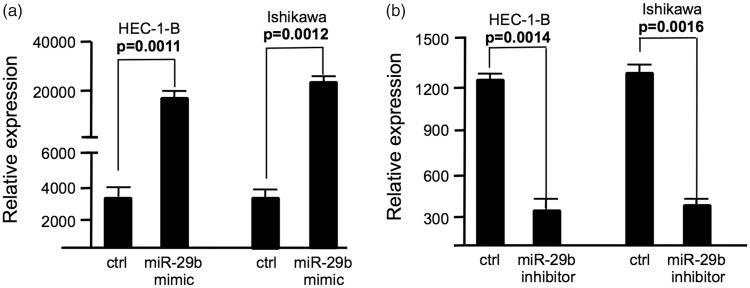
To examine the effect of miR-29b on cell proliferation and cisplatin sensitivity on endometrial cancer cells, miR-29b mimic or miR-29b inhibitor was transfected to endometrial cancer cells using Lipofectamine 2000. Scrambled negative control RNAs were used as negative controls and the WST-1 assay was carried out using HEC-1-B and Ishikawa cells. As shown in Figure 1a, expression of miR-29b was significantly increased in HEC-1-B and Ishikawa cells after transfection of miR-29b mimic. In contrast, expression of miR-29b was decreased in HEC-1-B and Ishikawa cells after transfection of miR-29b inhibitor (Figure 1b).
Figure 1.
The expression level of miR-29b in endometrial cancer cells after transfection. (a) miR-29b expression level in endometrial cancer cells transfected with miR-29b mimic; (b) miR-29b expression level in endometrial cancer cells transfected with miR-29b inhibitor. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
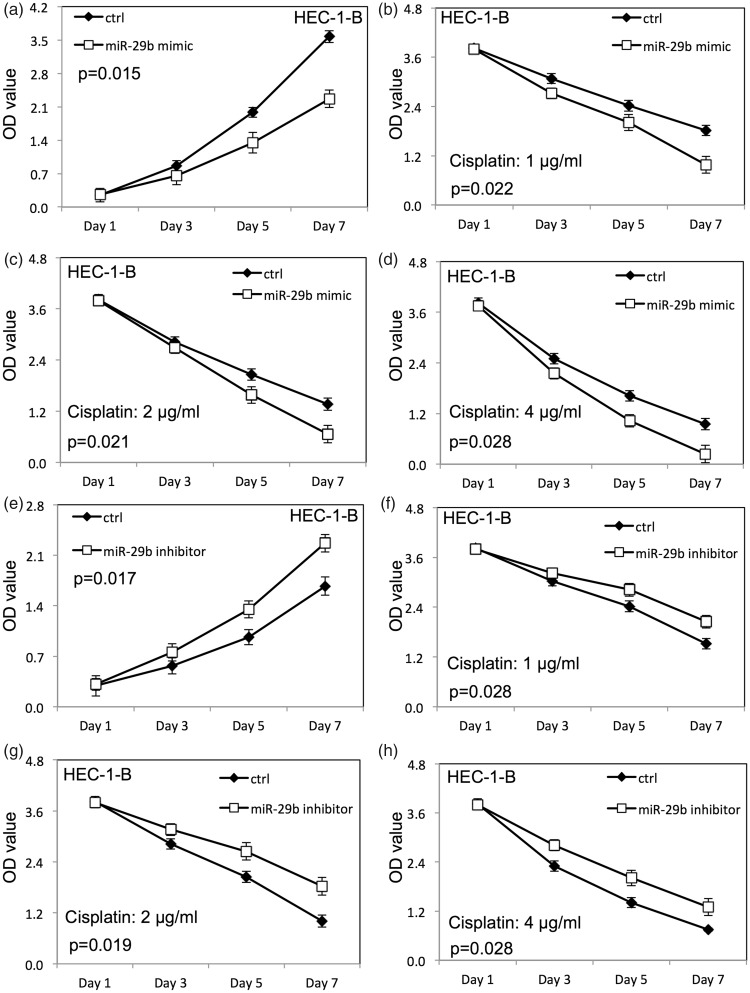
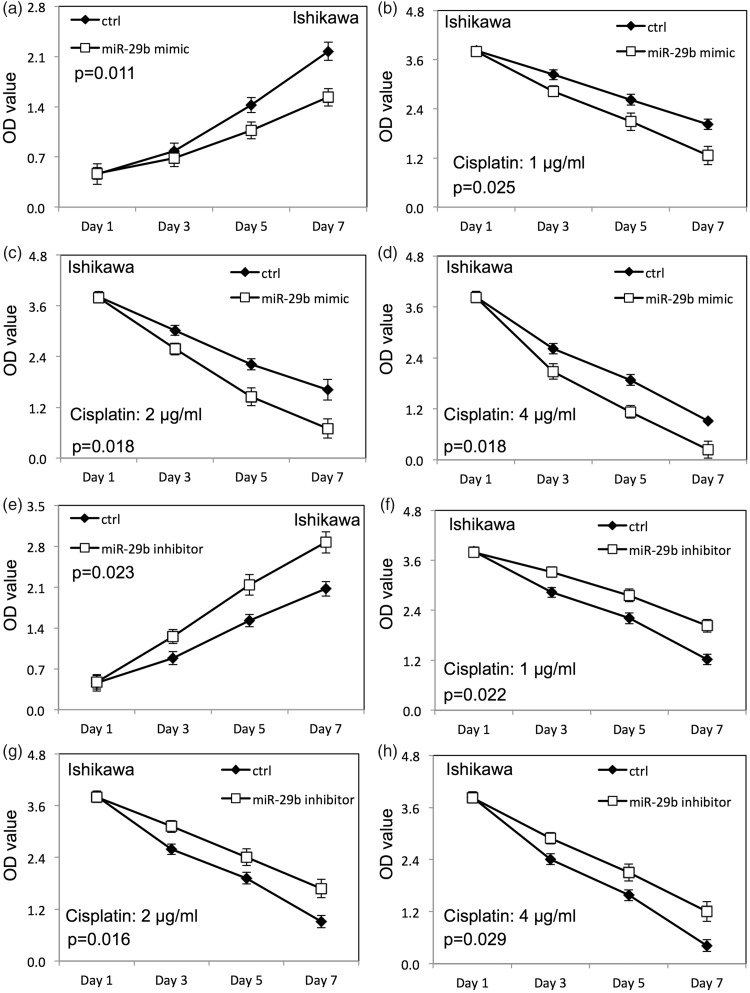
Up-regulation of miR-29b inhibited proliferation of HEC-1-B (Figure 2a) and Ishikawa cells (Figure 3a) compared with cells transfected with negative control RNAs (p < 0.05). The survival rate of HEC-1-B (Figure 2b–d) and Ishikawa (Figure 3b–d) cells was decreased after up-regulation of miR-29b when HEC-1-B and Ishikawa cells were treated with cisplatin compared with the negative control group (p < 0.05). Down-regulation of miR-29b increased proliferation of HEC-1-B (Figure 2e) and Ishikawa (Figure 3e) cells compared with cells transfected with negative control RNAs. Meanwhile, the survival rate of HEC-1-B (Figure 2f–h) and Ishikawa (Figure 3f–h) cells was increased after down-regulation of miR-29b when HEC-1-B and Ishikawa cells were treated with cisplatin compared with the negative control group (p < 0.05).
Figure 2.
miR-29b altered the proliferation (measured as optical density, OD) and cisplatin sensitivity of HEC-1-B endometrial cancer cells. (a) Proliferation of HEC-1-B cells after miR-29b overexpression; (b–d) proliferation of HEC-1-B cells transfected with miR-29b mimic with different doses of cisplatin; (e) proliferation of HEC-1-B cells after miR-29b down-regulation; (f–h) proliferation of HEC-1-B cells transfected with miR-29b inhibitor with different doses of cisplatin. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
Figure 3.
miR-29b altered the proliferation (measured as optical density, OD) and cisplatin sensitivity of Ishikawa endometrial cancer cells. (a) Proliferation of Ishikawa cells after miR-29b overexpression; (b–d) proliferation of Ishikawa cells transfected with miR-29b mimic with different doses of cisplatin; (e) proliferation of Ishikawa cells after miR-29b down-regulation; (f–h) proliferation of Ishikawa cells transfected with miR-29b inhibitor with different doses of cisplatin. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
miR-29b increased cisplatin-induced apoptosis of endometrial cancer cells
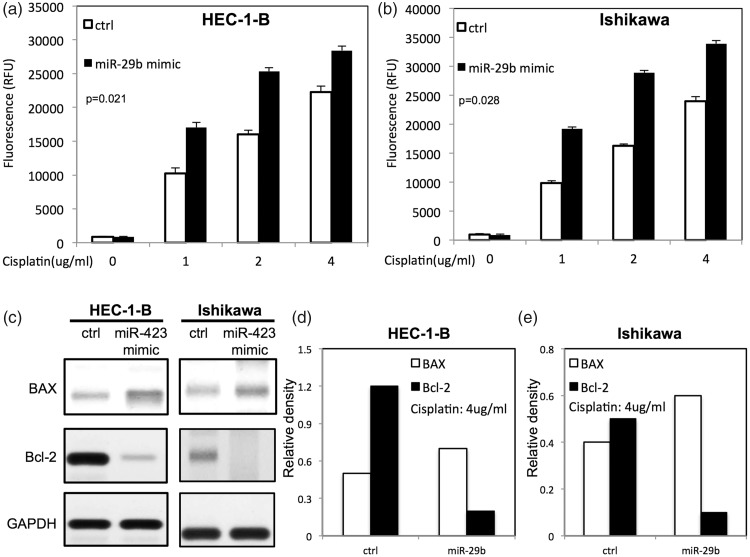
To examine the function of miR-29b on chemotherapy-induced apoptosis, transfected HEC-1-B and Ishikawa cells were treated with different doses of cisplatin. Up-regulation of miR-29b promoted cisplatin-induced apoptosis by increasing activities of caspase 3/7 (Figure 4a and b). To study the expression of apoptosis-related proteins on transfected endometrial cells after cisplatin treatment, Western blotting was performed. As shown in Figure 4c–e, up-regulation of miR-29b increased expression of BAX and decreased expression of Bcl-2 in endometrial cancer cells.
Figure 4.
miR-29b enhanced cisplatin-induced apoptosis in endometrial cancer cells. (a) Caspase 3/7 activity in HEC-1-B cells transfected with miR-29b after different doses of cisplatin treatment; (b) caspase 3/7 activity in Ishikawa cells transfected with miR-29b after different doses of cisplatin treatment; (c) levels of apoptotic proteins (BAX and Bcl-2) in HEC-1B cells and Ishikawa cells after cisplatin treatment; (d and e) relative expression of apoptotic proteins in HEC-1-B and Ishikawa cells after transfection with miR-29b mimic by densitometric analysis. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
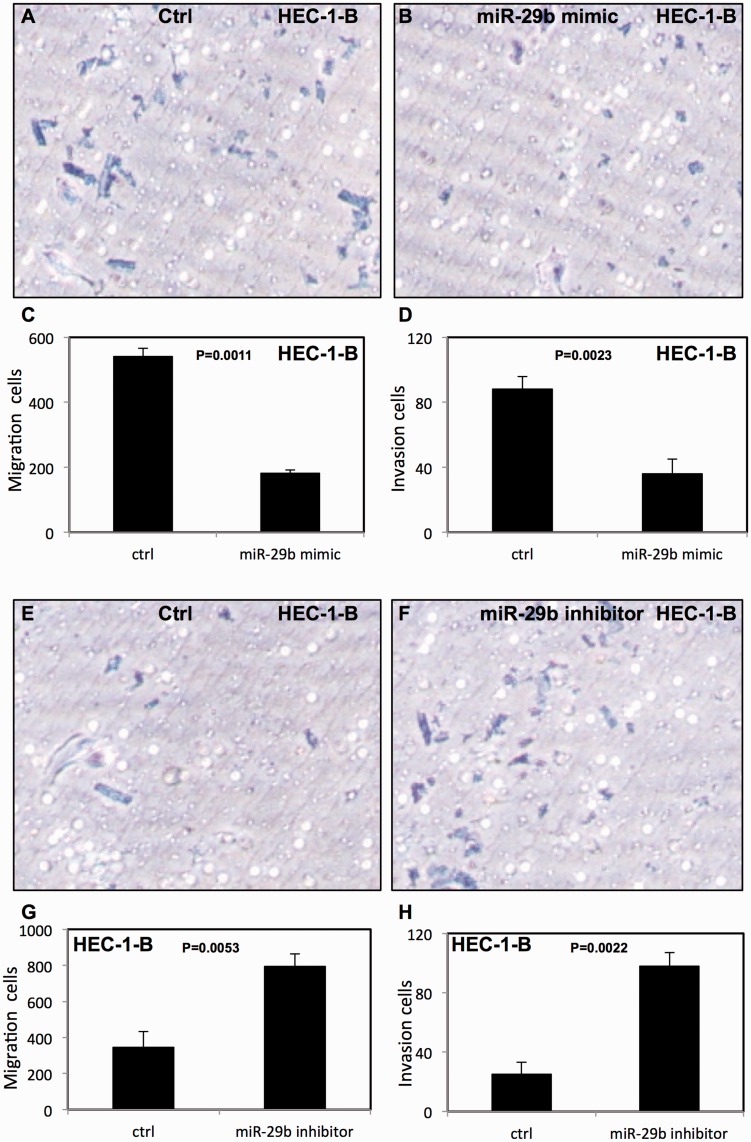
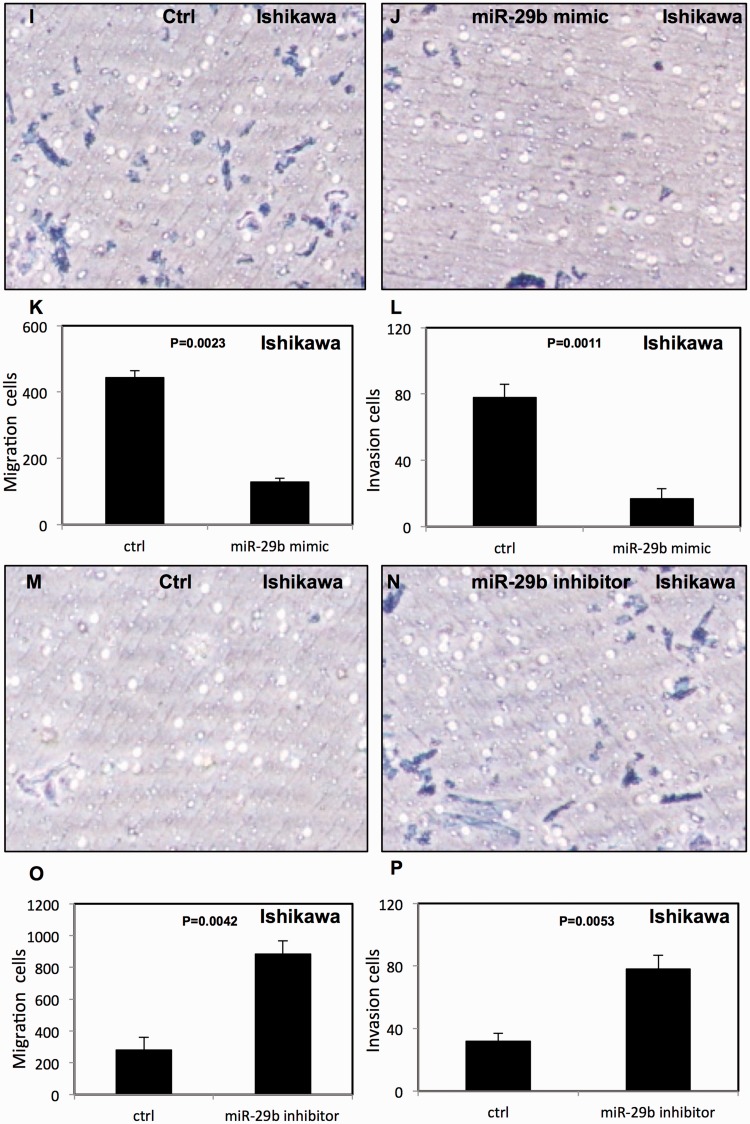
miR-29b decreased migration and invasion in endometrial cancer cells
The effect of miR-29b on migration and invasion of endometrial cancer cells was assessed using transwell migration and Matrigel invasion assays. We found that overexpression of miR-29b decreased migration and invasion of HEC-1-B cells (Figure 5a–d) and Ishikawa cells (Figure 5i–l). In contrast, down-regulation of miR-29b enhanced the migration and invasion of HEC-1-B cells (Figure 5e–h) and Ishikawa cells (Figure 5m–p).
Figure 5.
miR-29b decreased migration and invasion of endometrial cancer cells. (a–d) miR-29b increased migration and invasion of HEC-1B cells; (e–h) migration and invasion of HEC-1-B cells were decreased after treatment with miR-29b inhibitor; (i–l) miR-29b increased migration and invasion in Ishikawa cells; (m–p) migration and invasion of Ishikawa cells were decreased after treatment with miR-29b inhibitor. All experiments were performed three times in triplicate. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
miR-29b activated AKT pathway in endometrial cancer cells
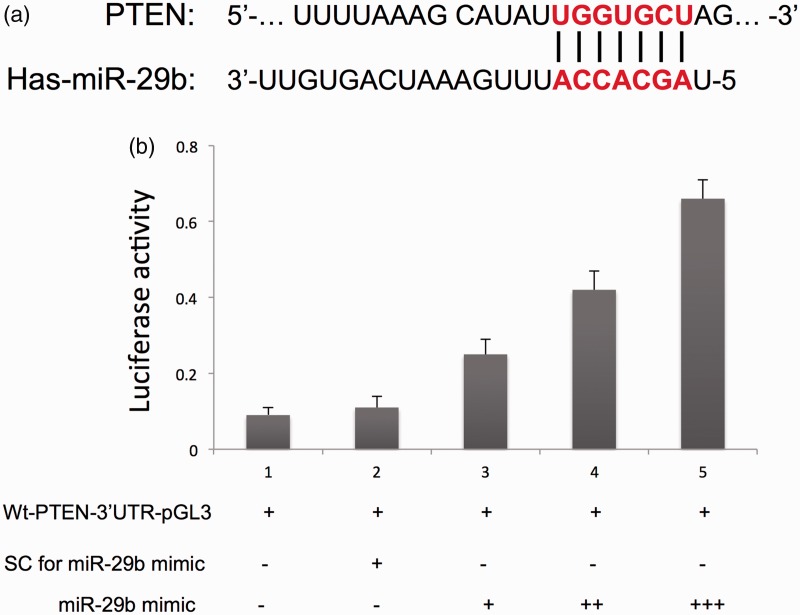
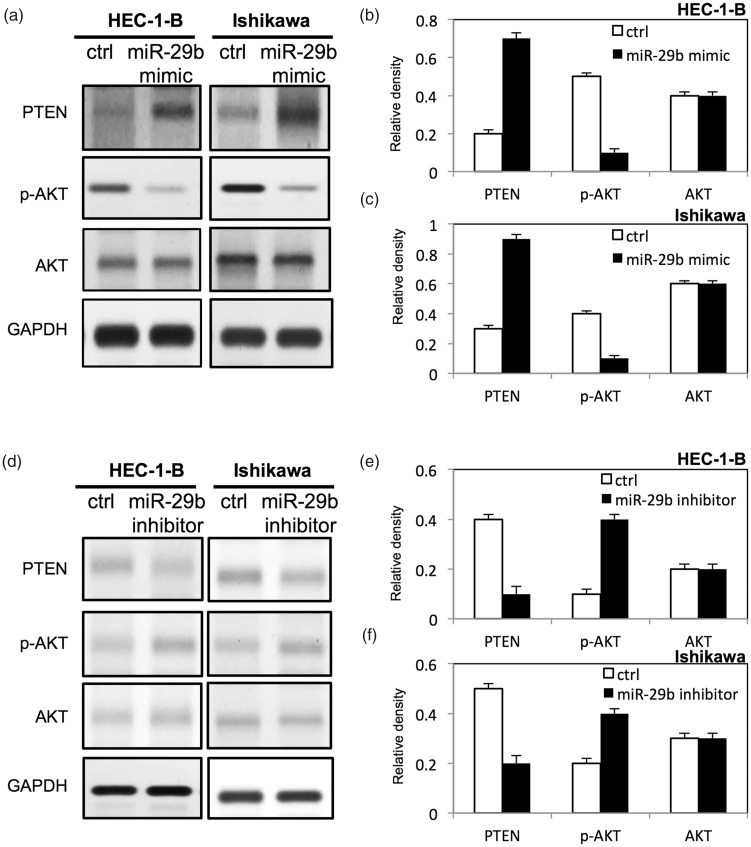
To further study the molecular mechanisms underlying miR-29b–mediated cell proliferation and apoptosis in endometrial cancer, we examined multiple signaling pathways. The target of miR-29b was predicted using the miRDB online database (http://mirdb.org). As shown in Figure 6a, the potential binding site of miR-29b in the 3′-UTR of PTEN was predicted. To validate the potential direct binding site, we performed a dual-luciferase reporter assay. As shown in Figure 6b, the expression of luciferase of Luc-PTEN was upregulated compared with that of the negative control group (p < 0.05). In addition, up-regulation of miR-29b increased the expression of PTEN and decreased the expression of p-AKT in HEC-1-B and Ishikawa endometrial cancer cells (Figure 7a–c). In contrast, down-regulation of miR-29b reduced the expression of PTEN and increased the expression of p-AKT in both HEC-1-B and Ishikawa endometrial cancer cells (Figure 7d–f).
Figure 6.
The potential binding site of miR-29b in the 3′-UTR of PTEN (a) and luciferase activity (b). UTR, untranslated region; PTEN, phosphatase and tensin homolog. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
Figure 7.
miR-29b inactivated AKT in endometrial cancer cells. (a) Expression of p-AKT, AKT, and PTEN in HEC-1-B and Ishikawa cells after transfection with miR-29b mimic; (b, c) relative expression level of proteins in HEC-1-B and Ishikawa cells after transfection with miR-29b mimic by densitometric analysis; (d) expression of p-AKT, AKT, and PTEN in HEC-1-B and Ishikawa cells after transfection with miR-29b inhibitor; (e, f) relative expression level of proteins in HEC-1-B and Ishikawa cells after transfection with miR-29b inhibitor by densitometric analysis. PTEN, phosphatase and tensin homolog. Ctrl, cancer cells transfected with negative control miRNA. Values are expressed as mean ± standard deviation.
Discussion
miR-29b is known to be down-regulated in endometrial cancer and to be correlated with poor prognosis.22 Chen et al.23 found that miR-29b was involved in angiogenesis in endometrial cancer by targeting MAPK/ERK and PI3K/Akt signaling pathways. Gene expression analysis showed that overexpression of miR-29b inhibited proliferation of acute myeloid leukemia (AML) cells and osteosarcoma cells by targeting cyclin-dependent kinase 6 (CDK6).20,24 Wang et al. reported that upregulation of miR-29b reduced proliferation of RH30 rhabdomyosarcoma cells by regulation of nuclear factor (NF)-κB and YY1.25 In our study, miR-29b inhibited proliferation of endometrial cancer cells, a result consistent with previous findings.
Apoptosis plays important roles in tumorigenesis. It has been shown that miR-29b inhibits apoptosis by activating caspase-3, increasing BAX, and decreasing Bcl-2.26 miR-29b induces apoptosis in various tumors including AML cells,20 malignant KMCH cholangiocarcinoma (CCA) cells,27 hepatocellular carcinoma cells28 by regulating myeloid cell leukemia-1 (Mcl-1) protein and Bcl-2. Cisplatin is an effective anticancer drug that has been used to treat a variety of malignant tumors including endometrial cancer.29,30 Chemoresistance remains a major challenge despite extensive efforts to develop new chemotherapeutic agents. Therefore, a strategy to increase chemosensitivity and reverse resistance is urgently needed. Studies have shown that miRNAs are associated with cancer chemosensitivity.31 Okamoto et al. reported that miR-29b enhanced chemosensitivity to gemcitabine in a cholangiocarcinoma cell line HuH28.32 Studies have also demonstrated that miR-29b improves chemosensitivity in OVCAR-3 and multiple myeloma cells by increasing paclitaxel-induced and bortezomib-induced apoptosis, respectively.33,34 Further studies revealed that miR-29b could act as a predictive marker for the clinical response to chemotherapy.35,36 Our study showed that overexpression of miR-29b promoted cisplatin-induced apoptosis. Up-regulation of miR-29b increased expression of BAX and decreased that of Bcl-2 in endometrial cancer cells.
Increasing evidence indicates that miR-29b plays important roles in cancer cell metastasis and invasion. Up-regulation of miR-29b inhibits metastasis in prostate cancer cells by targeting pro-metastatic matrix molecules, including Mcl-1, collagen (COL)1A1, COL4A1, and matrix metalloproteinase-2 (MMP-2).37 miR-29b is also involved in angiogenesis and extracellular matrix signaling including vascular endothelial growth factor A (VEGFA), angiopoietin-like 4 (ANGPTL4), platelet-derived growth factor (PDGF), lysyl oxidase (LOX), and matrix metalloproteinase-9 (MMP-9).38 Our study showed that miR-29b inhibited migration and invasion of endometrial cancer cells and indicated that high expression level of miR-29b was associated with favorable outcomes in patients with endometrial cancer.
Phosphatase and tensin homolog (PTEN) is a well-known tumor suppressor gene and it regulates multiple biological processes, including apoptosis, cell proliferation, invasion, adhesion, and metabolism.39 PTEN negatively regulates AKT activity by dephosphorylating phosphatidylinositol-trisphosphate (PIP3) to PIP2.40 Mutations in PTEN have been found in 55% of precancerous lesions, up to 80% of endometrioid endometrial cancers, and up to 90% of high-grade tumors.41 Loss of PTEN and activation of AKT is also correlated with resistance to small molecule compound treatment in endometrial cancer. Jia et al.42 found that overexpression of miR-29b decreased the proliferation, migration, invasion, and cell-cycle progression of tongue squamous cell cancer cells through the PTEN-AKT signaling pathway. We found that miR-29b enhanced the expression of PTEN in endometrial cancer cells by directly binding to the 3′-UTR of PTEN. These results suggested that miR-29b acted as a key regulator in proliferation and progression of endometrial cancer cells by targeting PTEN.
Conclusion
miR-29b played important roles in tumorigenesis and malignant progression of endometrial cancer. The present study indicates that miR-29b might be used as a biomarker to predict the chemotherapy response and prognosis in endometrial cancer.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Prat J. Pathology of cancers of the female genital tract. Int J Gynaecol Obstet 2015; 131: S132–S145. DOI: 10.1016/j.ijgo.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL Miller KD, andJemal A.. Cancer statistics, 2018. CA Cancer J Clin 2018; 68: 7–30. DOI: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Dinkelspiel HE, Wright JD, Lewin SN, et al. Contemporary clinical management of endometrial cancer. Obstet Gynecol Int 2013; 2013: 583891. DOI: 10.1155/2013/583891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colombo N, Preti E, Landoni F, et al. Endometrial cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013; 24: vi33–vi38. DOI: 10.1093/annonc/mdt353. [DOI] [PubMed] [Google Scholar]
- 5.Davidson BA, Foote J, Clark LH, et al. Tumor grade and chemotherapy response in endometrioid endometrial cancer. Gynecol Oncol Rep 2016; 17: 3–6. DOI: 10.1016/j.gore.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohammad RM, Muqbil I, Lowe L, et al. Broad targeting of resistance to apoptosis in cancer. Semin Cancer Biol 2015; 35: S78–S103. DOI: 10.1016/j.semcancer.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.West KA Castillo SS, andDennis PA.. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 2002; 5: 234–248. [DOI] [PubMed] [Google Scholar]
- 8.Johnston SR. Enhancing endocrine therapy for hormone receptor-positive advanced breast cancer: cotargeting signaling pathways. J Natl Cancer Inst 2015; 107: pii: djv212. DOI: 10.1093/jnci/djv212. [DOI] [PubMed] [Google Scholar]
- 9.Catalanotto C, Cogoni C, Zardo G. MicroRNA in control of gene expression: an overview of nuclear functions. Int J Mol Sci 2016; 17: pii: E1712. DOI: 10.3390/ijms17101712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wahid F, Shehzad A, Khan T, et al. MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 2010; 1803: 1231–1243. DOI: 10.1016/j.bbamcr.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane LA, Murphy PR. MicroRNA: biogenesis, function and role in cancer. Curr Genomics 2010; 11: 537–561. DOI: 10.2174/138920210793175895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pillai RS. MicroRNA function: multiple mechanisms for a tiny RNA? RNA 2005; 11: 1753–1761. DOI: 10.1261/rna.2248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peng Y, Croce CM. The role of MicroRNAs in human cancer. Signal Transduct Target Ther 2016; 1: 15004. DOI: 10.1038/sigtrans.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Wang W, Huang K, et al. Micro RNA-34a inhibits cells proliferation and invasion by downregulating Notch1 in endometrial cancer. Oncotarget 2017; 8: 111258–111270. DOI: 10.18632/oncotarget.22770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hiroki E, Suzuki F, Akahira J, et al. MicroRNA-34b functions as a potential tumor suppressor in endometrial serous adenocarcinoma. Int J Cancer 2012; 131: E395–E404. DOI: 10.1002/ijc.27345. [DOI] [PubMed] [Google Scholar]
- 16.Torres A, Torres K, Pesci A, et al. Deregulation of miR-100, miR-99a and miR-199b in tissues and plasma coexists with increased expression of mTOR kinase in endometrioid endometrial carcinoma. BMC Cancer 2012; 12: 369. DOI: 10.1186/1471-2407-12-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hebert SS, Horre K, Nicolai L, et al. Loss of microRNA cluster miR-29a/b-1 in sporadic Alzheimer's disease correlates with increased BACE1/beta-secretase expression. Proc Natl Acad Sci U S A 2008; 105: 6415–6420. DOI: 10.1073/pnas.0710263105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S, Rink C, Ghoorkhanian R, et al. Loss of miR-29b following acute ischemic stroke contributes to neural cell death and infarct size. J Cereb Blood Flow Metab 2013; 33: 1197–1206. DOI: 10.1038/jcbfm.2013.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mott JL, Kurita S, Cazanave SC, et al. Transcriptional suppression of mir-29b-1/mir-29a promoter by c-Myc, hedgehog, and NF-kappaB. J Cell Biochem 2010; 110: 1155–1164. DOI: 10.1002/jcb.22630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garzon R, Heaphy CE, Havelange V, et al. MicroRNA 29b functions in acute myeloid leukemia. Blood 2009; 114: 5331–5341. DOI: 10.1182/blood-2009-03-211938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ru P, Steele R, Newhall P, et al. miRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther 2012; 11: 1166–1173. DOI: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- 22.Hiroki E, Akahira J, Suzuki F, et al. Changes in microRNA expression levels correlate with clinicopathological features and prognoses in endometrial serous adenocarcinomas. Cancer Sci 2010; 101: 241–249. DOI: 10.1111/j.1349-7006.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen HX, Xu XX, Tan BZ, et al. MicroRNA-29b inhibits angiogenesis by targeting VEGFA through the MAPK/ERK and PI3K/Akt signaling pathways in endometrial carcinoma. Cell Physiol Biochem 2017; 41: 933–946. DOI: 10.1159/000460510. [DOI] [PubMed] [Google Scholar]
- 24.Zhu K, Liu L, Zhang J, et al. MiR-29b suppresses the proliferation and migration of osteosarcoma cells by targeting CDK6. Protein Cell 2016; 7: 434–444. DOI: 10.1007/s13238-016-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang H, Garzon R, Sun H, et al. NF-kappaB-YY1-miR-29 regulatory circuitry in skeletal myogenesis and rhabdomyosarcoma. Cancer Cell 2008; 14: 369–381. DOI: 10.1016/j.ccr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li P, Guo W, Du L, et al. microRNA-29b contributes to pre-eclampsia through its effects on apoptosis, invasion and angiogenesis of trophoblast cells. Clin Sci (Lond) 2013; 124: 27–40. DOI: 10.1042/CS20120121. [DOI] [PubMed] [Google Scholar]
- 27.Mott JL, Kobayashi S, Bronk SF, et al. mir-29 regulates Mcl-1 protein expression and apoptosis. Oncogene 2007; 26: 6133–6140. DOI: 10.1038/sj.onc.1210436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jin F, Wang Y, Li M, et al. MiR-26 enhances chemosensitivity and promotes apoptosis of hepatocellular carcinoma cells through inhibiting autophagy. Cell Death Dis 2017; 8: e2540. DOI: 10.1038/cddis.2016.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol 2014; 740: 364–378. DOI: 10.1016/j.ejphar.2014.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moxley KM, McMeekin DS. Endometrial carcinoma: a review of chemotherapy, drug resistance, and the search for new agents. Oncologist 2010; 15: 1026–1033. DOI: 10.1634/theoncologist.2010-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng F, Henson R, Lang M, et al. Involvement of human micro-RNA in growth and response to chemotherapy in human cholangiocarcinoma cell lines. Gastroenterology 2006; 130: 2113–2129. DOI: 10.1053/j.gastro.2006.02.057. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto K Miyoshi K, andMurawaki Y.. miR-29b, miR-205 and miR-221 enhance chemosensitivity to gemcitabine in HuH28 human cholangiocarcinoma cells. PloS One 2013; 8: e77623. DOI: 10.1371/journal.pone.0077623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan B, Guo Q, Fu FJ, et al. The role of miR-29b in cancer: regulation, function, and signaling. Onco Targets Ther 2015; 8: 539–548. DOI: 10.2147/OTT.S75899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amodio N, Di Martino MT, Foresta U, et al. miR-29b sensitizes multiple myeloma cells to bortezomib-induced apoptosis through the activation of a feedback loop with the transcription factor Sp1. Cell Death Dis 2012; 3: e436. DOI: 10.1038/cddis.2012.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blum W, Garzon R, Klisovic RB, et al. Clinical response and miR-29b predictive significance in older AML patients treated with a 10-day schedule of decitabine. Proc Natl Acad Sci U S A 2010; 107: 7473–7478. DOI: 10.1073/pnas.1002650107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blum W, Schwind S, Tarighat SS, et al. Clinical and pharmacodynamic activity of bortezomib and decitabine in acute myeloid leukemia. Blood 2012; 119: 6025–6031. DOI: 10.1182/blood-2012-03-413898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steele R Mott JL, andRay RB.. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer 2010; 1: 381–387. DOI: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melo SA, Kalluri R. miR-29b moulds the tumour microenvironment to repress metastasis. Nat Cell Biol 2013; 15: 139–140. DOI: 10.1038/ncb2684. [DOI] [PubMed] [Google Scholar]
- 39.Xie Y, Naizabekov S, Chen Z, et al. Power of PTEN/AKT: molecular switch between tumor suppressors and oncogenes. Oncol Lett 2016; 12: 375–378. DOI: 10.3892/ol.2016.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu W, Yang Z, Zhou SF, et al. Posttranslational regulation of phosphatase and tensin homolog (PTEN) and its functional impact on cancer behaviors. Drug Des Devel Ther 2014; 8: 1745–1751. DOI: 10.2147/DDDT.S71061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Djordjevic B, Hennessy BT, Li J, et al. Clinical assessment of PTEN loss in endometrial carcinoma: immunohistochemistry outperforms gene sequencing. Mod Pathol 2012; 25: 699–708. DOI: 10.1038/modpathol.2011.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jia LF, Huang YP, Zheng YF, et al. miR-29b suppresses proliferation, migration, and invasion of tongue squamous cell carcinoma through PTEN-AKT signaling pathway by targeting Sp1. Oral Oncol 2014; 50: 1062–1071. DOI: 10.1016/j.oraloncology.2014.07.010. [DOI] [PubMed] [Google Scholar]