Short abstract
Objective
Insulin-like growth factor-1 (IGF-1) signaling is inhibited in end-stage dilated cardiomyopathy (DCM), and recombinant IGF-1 improves cardiac function in DCM patients. Long non-coding (lnc)RNA HAND2-AS1 was previously shown to be involved in cancer development, but its role in DCM is unknown. The present study investigated the involvement of IGF-1 and HAND2-AS1 in DCM.
Methods
Plasma HAND2-AS1 was detected by quantitative real-time PCR. IGF-1 plasma levels were measured by a human IGF-1 enzyme-linked immunosorbent assay. All experiments were performed in triplicate. Pearson’s correlation coefficient analyzed correlations between levels of IGF-1 and HAND2-AS1. Patients were divided into high and low IGF-1 or lncRNA HAND2-AS1 groups, and survival curves were plotted and compared by the Kaplan–Meier method and log-rank test, respectively.
Results
Plasma levels of IGF-1 and lncRNA HAND2-AS1 were significantly lower in end-stage DCM patients than in healthy controls, and were only positively correlated in end-stage DCM patients. A follow-up study revealed that low levels of IGF-1 or lncRNA HAND2-AS1 were significantly associated with poor survival. In the human cardiomyocyte cell line AC16, lncRNA HAND2-AS1 overexpression failed to alter IGF-1 levels and IGF-1 overexpression did not affect lncRNA HAND2-AS1 expression.
Conclusions
LncRNA HAND2-AS1 may participate in end-stage dilated cardiomyopathy.
Keywords: End-stage dilated cardiomyopathy, insulin-like growth factor-1, lncRNA HAND2-AS1, survival, cardiac function, tumor suppressor
Introduction
As one of the most common types of chronic cardiovascular disease, dilated cardiomyopathy (DCM) is characterized by the dilation of both ventricles or left ventricle alone.1 Patients with early-stage DCM usually lack obvious symptoms, so a considerable portion of patients are diagnosed at the end stages of disease.2 However, in the absence of timely and effective treatment, end-stage DCM leads to heart failure or even death.3 Although previous studies have shown that genetic factors play central roles in the pathogenesis of DCM,4,5 molecular mechanisms of the development and progression of DCM remain largely unknown, leading to difficulties in its prevention and treatment.
Insulin-like growth factor 1 (IGF-1), also known as somatomedin C, is a hormone with a similar molecular structure to insulin that has critical functions in childhood growth and anabolic effects in adults.6 IGF-1 signaling was reported to be inhibited in end-stage DCM,7 and recombinant IGF-1 improves cardiac function in DCM patients.8 Therefore, IGF-1 is a promising treatment target for end-stage DCM. IGF-1 participates in human diseases through interactions with different signaling molecules including long non-coding (lnc)RNAs.9 LncRNA HAND2-AS1 is a characterized tumor suppressor in several types of human cancers.10–12 In the present study we found that lncRNA HAND2-AS1 may participate in end-stage dilated cardiomyopathy by interacting indirectly with IGF-1.
Materials and methods
Patients
Our study included 48 patients with end-stage DCM who were admitted and treated at Renmin Hospital of Wuhan University from May 2013 to May 2015. Inclusion criteria were: 1) DCM diagnosed at end stage; 2) no treatment performed within 60 days before admission; and 3) completed treatment and a 3-year follow-up period. Exclusion criteria were: 1) presence of other diseases; 2) death from other causes during follow-up; and 3) secondary or familial DCM. Based on diagnostic criteria established by the New York Heart Association, all patients were diagnosed with class III or IV congestive heart failure. Radionuclide angiography and left ventricle (LV) angiography showed that all patients had an ejection fraction of less than 25%, which is defined as LV systolic dysfunction.
The patient group included 28 males and 20 females with an age range of 39 to 66 years (mean age, 51.2 ± 6.7 years). Forty-two healthy volunteers were enrolled from the Renmin Hospital of Wuhan University to serve as the control group. This included 26 males and 16 females with an age range of 39 to 64 years (mean age, 50.8 ± 5.9 years). Similar age and gender distributions were observed in both groups. Blood was extracted from each patient and control on the day of admission. All patients were followed up for 3 years after admission to record survival conditions, and were monitored monthly. All individuals provided their written informed consent for participation, and the study was approved by the Ethics Committee of Renmin Hospital of Wuhan University.
Cell lines
Human cardiomyocyte cell line AC16 cells were purchased from EMD Millipore (Billerica, MA, USA) and cultured in cardiomyocyte growth medium (5901D; ScienCell, Carlsbad, CA, USA) at 37°C with 5% CO2 according to the supplier’s instructions. pIRSE2 vectors expressing lncRNA HAND2-AS1 were designed and synthesized by GenePharma (Shanghai, China). Lipofectamine 2000 reagent (11668-019; Invitrogen, Carlsbad, CA, USA) was used to transfect 15 nM pIRSE2 vectors into 3 × 105 cells according to the manufacturer’s instructions. Cells treated only with Lipofectamine 2000 reagent were control cells and cells transfected with empty vectors were negative control cells.
Real-time quantitative PCR (RT-qPCR)
The Maxwell® 16 Total RNA Purification Kit (Promega, Madison, WI, USA) was used to perform all RNA extractions, and the RNA quality was determined by agarose gel electrophoresis. The RevertAid RT Reverse Transcription Kit (Thermo Fisher Scientific, Inc., Rockford, IL, USA) was used to synthesize cDNA using RNA samples as templates. PCR of cDNA was performed using the SuperScript III Platinum One-Step qRT-PCR Kit (Thermo Fisher Scientific, Inc.) on the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). Reaction conditions were: 95°C for 55 s, followed by 40 cycles of 95°C for 15 s and 58.5°C for 30 s. Primer sequences were: 5′-GGGTGTTTACGTAGACCAGAACC-3′ (forward) and 5′-CTTCCAAAAGCCTTCTGCCTTAG-3′ (reverse) for HAND2-AS1; 5′-GACCTCTATGCCAACACAGT-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′ (reverse) for human β-actin. The 2−ΔΔCT method was used for all data analysis.
Enzyme-linked immunosorbent assay (ELISA)
The Human IGF-1 ELISA Kit (ab100545; Abcam, Cambridge, MA, USA) was used to measure plasma levels of IGF-1 which were converted to ng/mL.
Western blotting
Western blotting was performed using standard methods. In brief, protein was extracted from cells using a Total Protein Extraction Kit (NBP2-37853; Novus Biologicals, Centennial, CO, USA), then protein samples were denatured by boiling in water for 8 minutes and subjected to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis. Following gel transfer to polyvinylidene fluoride membranes, these were blocked in 5% non-fat milk for 2 hours at room temperature. They were then incubated with rabbit anti-IGF1 (1: 2000, ab40657, Abcam) and rabbit anti-GAPDH (1: 2000, ab9485, Abcam) primary antibodies for 18 hours at 4°C followed by a goat anti-rabbit IgG-horseradish peroxidase secondary antibody (1:1000, MBS435036; MyBioSource, San Diego, CA, USA) for 2 hours at 24°C. After signal development using ECL Western Blotting Substrate (Promega), signals were processed using Image J v1.46 software.
Statistical analysis
All experiments were performed in triplicate, and data were processed by Graphpad Prism 6 software as means ± standard deviation. The Pearson’s correlation coefficient was used to analyze correlations between levels of IGF-1 and HAND2-AS1. According to the Youden index, patients were divided into high (n=22) and low (n=26) lncRNA HAND2-AS1 expression groups, and high (n=25) and low (n=23) IGF-1 expression groups. Survival curves of different groups of patients were plotted using the Kaplan–Meier method and were compared by the log-rank test. Diagnostic values of plasma HAND2-AS1and IGF-1 for end-stage DCM were analyzed by the receiver operating characteristic (ROC) curve with end-stage DCM patients as true positive cases and healthy controls as true negative cases. Comparisons between the two groups were performed by the pairwise t test. Comparisons among multiple groups were performed using parametric analysis of variance (ANOVA) tests, followed by the Tukey test. P<0.05 was considered statistically significant.
Results
Comparison of lncRNA HAND2-AS1 and IGF-1 plasma levels between end-stage DCM patients and healthy controls
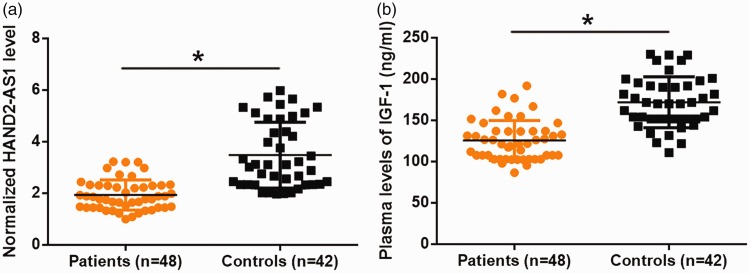
RT-qPCR results showed that, compared with healthy controls, end-stage DCM patients had significantly reduced lncRNA HAND2-AS1 plasma levels (Figure 1a, P<0.05). Similarly, IGF-1 plasma levels were significantly lower in end-stage DCM patients than in healthy controls (Figure 1b, P<0.05). It is notable that embolism occurred in 12 patients by the time of admission, but that no significant difference in the expression of lncRNA HAND2-AS1 and IGF-1 was found in patients with and without embolism (data not shown).
Figure 1.
Plasma IGF-1 and lncRNA HAND2-AS1 were downregulated in end-stage DCM patients compared with healthy controls.
Plasma levels of HAND2-AS1 and IGF-1 were measured by ELISA and RT-qPCR, respectively, and differences were analyzed by the unpaired t test. Compared with healthy controls, end-stage DCM patients had significantly reduced HAND2-AS1 (a) and IGF-1(b) levels (*, P<0.05).
Diagnostic values of plasma lncRNA HAND2-AS1 and IGF-1 for end-stage DCM patients
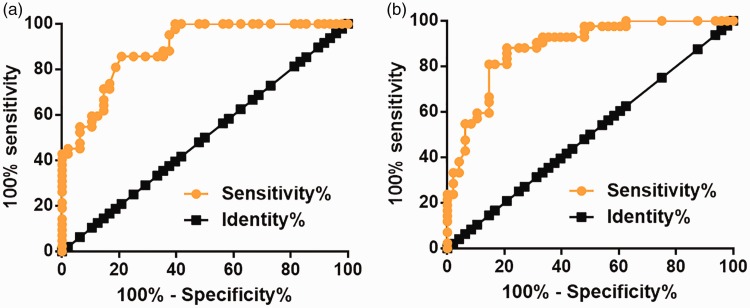
Diagnostic values of plasma lncRNA HAND2-AS1 and IGF-1 for end-stage DCM were analyzed by the ROC curve, with end-stage DCM patients as true positive cases and healthy controls as true negative cases. For plasma HAND2-AS1, the area under the curve was 0.8916 (standard error: 0.03216; 95% confidence interval [CI]: 0.8286–0.9547; P<0.0001, Figure 2a). For plasma IGF-1, the area under the curve was 0.8822 (standard error: 0.03505; 95% CI: 0.8135–0.9509; P<0.0001, Figure 2b).
Figure 2.
Downregulated plasma lncRNA HAND2-AS1 and IGF-1 separated end-stage DCM patients from healthy controls.
ROC curve analysis was performed using end-stage DCM patients as true positive cases and healthy controls as true negative cases. Downregulated plasma lncRNA HAND2-AS1 (a) and IGF-1 (b) separated end-stage DCM patients from healthy controls.
Correlation between plasma levels of IGF-1 and lncRNA HAND2-AS1
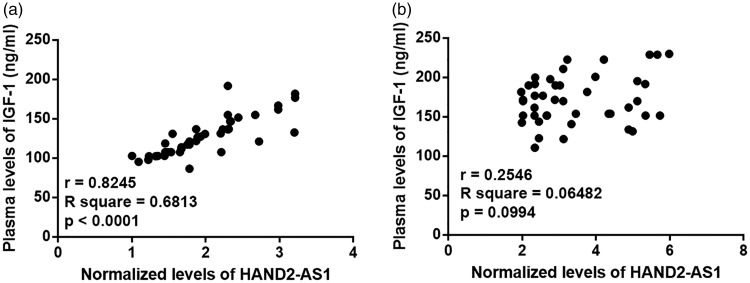
Pearson’s correlation coefficient was used to analyze correlations between the levels of IGF-1 and lncRNA HAND2-AS1. Plasma levels of IGF-1 and lncRNA HAND2-AS1 were positively correlated in end-stage DCM patients (Figure 3a), but not in healthy controls (Figure 3b).
Figure 3.
Plasma levels of IGF-1 and lncRNA HAND2-AS1 were positively correlated in end-stage DCM patients but not in healthy controls.
Pearson’s correlation coefficient revealed that plasma levels of IGF-1 and lncRNA HAND2-AS1 were positively correlated in end-stage DCM patients (a) but not in healthy controls (b).
Prognostic values of lncRNA HAND2-AS1 and IGF-1 for end-stage DCM
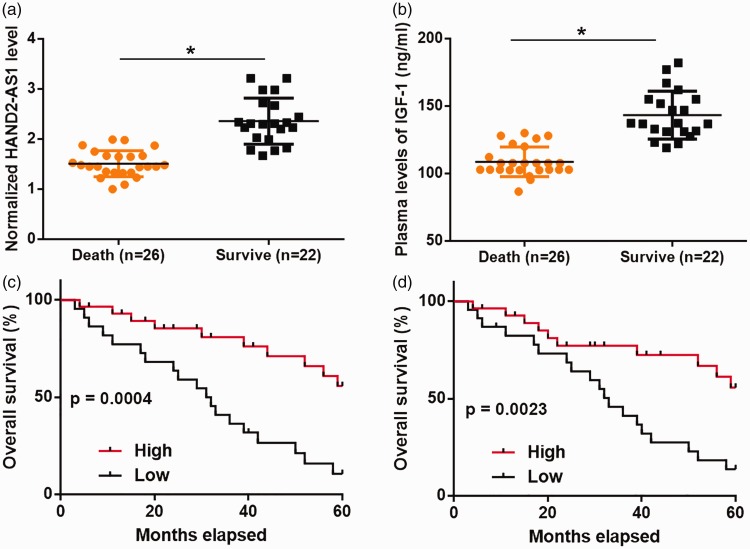
During the 3-year follow-up period, 26 patients died and the mortality rate was 54.2%. Compared with patients who died, those who survived had significantly higher levels of lncRNA HAND2-AS1 (Figure 4a, P<0.05) and IGF-1 (Figure 4b, P<0.04). Compared with the high lncRNA HAND2-AS1 expression group, patients in the low lncRNA HAND2-AS1 expression group had a significantly lower overall survival rate (Figure 4c). Similarly, compared with the high IGF-1 expression group, patients in the low IGF-1 expression group had a significantly lower overall survival rate (Figure 4d).
Figure 4.
Low levels of lncRNA HAND2-AS1 and IGF-1 were significantly correlated with poor survival.
Plasma levels of IGF-1 and lncRNA HAND2-AS1 as measured by ELISA and RT-qPCR were analyzed by the unpaired t test. Patients who survived the follow-up period (n=22) had significantly higher levels of HAND2-AS1 (a) and IGF-1 (b) than those who died (n=26) (*, P<0.04). Survival analysis showed that low levels of lncRNA HAND2-AS1 (c) and IGF-1 (d) were significantly correlated with poor survival in patients with end-stage DCM.
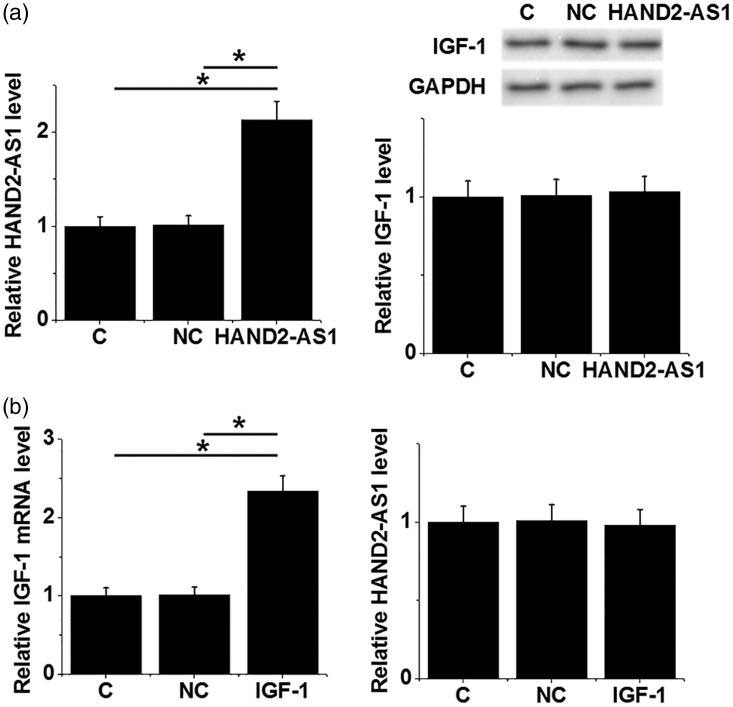
Possible interaction between lncRNA HAND2-AS1 and IGF-1 in AC16 human cardiomyocyte cells
To investigate the possible interaction between lncRNA HAND2-AS1 and IGF-1, lncRNA HAND2-AS1 and IGF-1 expression vectors were transfected into AC16 human cardiomyocyte cells and the expression of lncRNA HAND2-AS1 and IGF-1 was detected by RT-qPCR and western blot, respectively. Compared with control (C) and negative control (NC) groups, lncRNA HAND2-AS1 overexpression failed to alter IGF-1 expression AC16 cells (Figure 5a). IGF-1 overexpression also failed to affect lncRNA HAND2-AS1 expression in these cells (Figure 5b).
Figure 5.
No interaction between HAND2-AS1 and IGF-1 was observed in AC16 human cardiomyocyte cells.
LncRNA HAND2-AS1 and IGF-1 expression in AC16 cells was detected by RT-qPCR and western blot, respectively. Expression data were analyzed by one-way ANOVA and the Tukey test. LncRNA HAND2-AS1 overexpression failed to alter IGF-1 expression in AC16 cells (a). IGF-1 overexpression also failed to affect lncRNA HAND2-AS1 expression in these cells (b).
Discussion
The functionality of lncRNA HAND2-AS1 has been well characterized in several types of human cancers,10–12 but its role in other human diseases is largely unknown. The present study showed that plasma-circulating lncRNA HAND2-AS1 was downregulated in patients with end-stage DCM and that inhibited expression of lncRNA HAND2-AS1 had diagnostic and prognostic values for this disease. LncRNA HAND2-AS1 was also suggested to participate in the pathology of end-stage DCM.
The development of DCM is accompanied by changes in the expression pattern of a large set of lncRNAs.13 Some, such as lncRNA MIAT, were shown to have critical functions in the development and progression of DCM.14 As a tumor suppressor lncRNA, HAND2-AS1 is downregulated in several types of human cancers.10–12 In the current study, we showed that HAND2-AS1 was also downregulated in end-stage DCM, indicating its involvement in the disease.
A previous study reported that IGF-1 signaling was inhibited in patients with end-stage DCM.7 In another study, Komamura showed that treatment with recombinant IGF-1 improved cardiac function in DCM patients.8 Consistent with previous studies, we observed significantly reduced plasma levels of IGF-1 in end-stage DCM compared with healthy controls, further confirming its involvement in this disease.
The development of human diseases is usually accompanied by changes in certain substances of the circulating system, and the detection of these changes may provide guidance for the diagnosis and prognosis of disease.15,16 Circulating biomarkers such as circulating miR-489 were previously reported to have predictive values for the development and progression of DCM.17 In the present study we showed that downregulated plasma lncRNA HAND2-AS1 and IGF-1 separated end-stage DCM patients from healthy controls. Additionally, a follow-up study showed that low levels of lncRNA HAND2-AS1 and IGF-1 were significantly correlated with poor survival. Therefore, circulating lncRNA HAND2-AS1 and IGF-1 may act as diagnostic and prognostic biomarkers for end-stage DCM. However, clinical trials are required to confirm this.
We observed a significant and positive correlation between plasma levels of lncRNA HAND2-AS1 and IGF-1 in end-stage DCM patients, indicating the existence of potential interactions between them. However, this correlation was not observed in healthy controls. Moreover, based on overexpression data, no interactions between lncRNA HAND2-AS1 and IGF-1 were observed in human cardiomyocyte AC16 cells. Therefore, it is possible that pathological mediators exist to mediate interactions between lncRNA HAND2-AS1 and IGF-1. Regarding this, lncRNA HAND2-AS1 was reported to achieve its function by interacting with hypoxia-inducible factor (HIF)1α,10 which has critical functions in DCM.18 Additionally, IGF-1 participates in cross-talk with HIF1α.19 Therefore, it is conceivable that HIF1α acts as a mediator between HAND2-AS1 and IGF-1. We will test this possibility in future studies.
End-stage DCM patients are often affected by multiple complications including poor blood flow and heart failure, although it is unclear whether they affect lncRNA HAND2-AS1 expression. We also aim to investigate this in our future studies.
Conclusions
LncRNA HAND2-AS1 and IGF-1 were both downregulated in patients with end-stage DCM, indicating that lncRNA HAND2-AS1 participates in end-stage DCM pathology.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- Weintraub RG, Semsarian C, Macdonald P. Dilated cardiomyopathy. Lancet 2017; 390: 400–414. [DOI] [PubMed] [Google Scholar]
- Japp AG, Gulati A, Cook SA, et al. The diagnosis and evaluation of dilated cardiomyopathy. J Am Coll Cardiol 2016; 67: 2996–3010. [DOI] [PubMed] [Google Scholar]
- Merlo M, Cannatà A, Gobbo M, et al. Evolving concepts in dilated cardiomyopathy. Eur J Heart Fail 2018; 20: 228–239. [DOI] [PubMed] [Google Scholar]
- Garfinkel AC, Seidman JG, Seidman CE. Genetic pathogenesis of hypertrophic and dilated cardiomyopathy. Heart Fail Clin 2018; 14: 139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Bao D, Dong W, et al. Dkk3 prevents familial dilated cardiomyopathy development through Wnt pathway. Lab Invest 2016; 96: 239–248. [DOI] [PubMed] [Google Scholar]
- LeRoith D, Yakar S. Mechanisms of disease: metabolic effects of growth hormone and insulin-like growth factor 1. Nat Clin Pract Endocrinol Metab 2007; 3: 302–310. [DOI] [PubMed] [Google Scholar]
- Baán JA, Varga ZV, Leszek P, et al. Myostatin and IGF-1 signaling in end-stage human heart failure: a qRT-PCR study. J Transl Med 2015; 13: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komamura K. Recombinant insulin-like growth factor-1 improves cardiac function and symptoms in the patients on the waiting list for heart transplantation with end-stage dilated cardiomyopathy. Eur Heart J 2017; 38: ehx502.P1105. [Google Scholar]
- Ellis BC, Graham LD, Molloy PL. CRNDE, a long non-coding RNA responsive to insulin/IGF signaling, regulates genes involved in central metabolism. Biochim Biophys Acta 2014; 1843: 372–386. [DOI] [PubMed] [Google Scholar]
- Kang Y, Zhu X, Xu Y, et al. Energy stress-induced lncRNA HAND2-AS1 represses HIF1α-mediated energy metabolism and inhibits osteosarcoma progression. Am J Cancer Res 2018; 8: 526–537. [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Lin J, Zhang H, et al. LncRNA HAND2-AS1 sponging miR-1275 suppresses colorectal cancer progression by upregulating KLF14. Biochem Biophys Res Commun 2018; 503: 1848–1853. [DOI] [PubMed] [Google Scholar]
- Yang X, Wang CC, Lee WYW, et al. Long non-coding RNA HAND2-AS1 inhibits invasion and metastasis in endometrioid endometrial carcinoma through inactivating neuromedin U. Cancer Lett 2018; 413: 23–34. [DOI] [PubMed] [Google Scholar]
- Li H, Chen C, Fan J, et al. Identification of cardiac long non-coding RNA profile in human dilated cardiomyopathy. Cardiovasc Res 2018; 114: 747–758. [DOI] [PubMed] [Google Scholar]
- Frade AF, Laugier L, Ferreira LR, et al. Myocardial infarction–associated transcript, a long noncoding RNA, is overexpressed during dilated cardiomyopathy due to chronic chagas disease. J Infect Dis 2016; 214: 161–165. [DOI] [PubMed] [Google Scholar]
- López B, González A, Ravassa S, et al. Circulating biomarkers of myocardial fibrosis: the need for a reappraisal. J Am Coll Cardiol 2015; 65: 2449–2456. [DOI] [PubMed] [Google Scholar]
- Zafari S, Backes C, Meese E, et al. Circulating biomarker panels in Alzheimer's disease. Gerontology 2015; 61: 497–503. [DOI] [PubMed] [Google Scholar]
- Ishiguro T, Hayashi M, Sugishita Y, et al. Circulating miR-489 could be a new generation biomarker for dilated cardiomyopathy. J Card Fail 2016; 22: S178. [Google Scholar]
- Mazelin L, Panthu B, Nicot AS, et al. mTOR inactivation in myocardium from infant mice rapidly leads to dilated cardiomyopathy due to translation defects and p53/JNK-mediated apoptosis. J Mol Cell Cardiol 2016; 97: 213–225. [DOI] [PubMed] [Google Scholar]
- Sinha S, Koul N, Dixit D, et al. IGF-1 induced HIF-1α-TLR9 cross talk regulates inflammatory responses in glioma. Cell Signal 2011; 23: 1869–1875. [DOI] [PubMed] [Google Scholar]