Short abstract
Objectives
To investigate the effects of electroacupuncture in regulating astrocytes and oxidative stress in a rat model of postoperative cognitive dysfunction (POCD).
Methods
Male aged Sprague-Dawley rats were randomized to undergo left hepatic lobe resection to induce POCD, followed by either electroacupuncture or no treatment; or similar surgery without left lobe resection or electroacupuncture (sham). Postsurgical cognitive function, hippocampal astrocyte number and oxidative stress indicators were measured.
Results
At days 1, 3 and 7 following surgery, escape latency was significantly shorter and platform crossing frequency was increased with electroacupuncture versus other groups. At postoperative day 1, the electroacupuncture group showed significantly fewer glial fibrillary acidic protein (GFAP)-positive hippocampal astrocytes versus the POCD model group. In POCD rats, electroacupuncture significantly decreased serum S100 calcium binding protein B and neuron-specific enolase levels, and increased brain-derived neurotrophic factor and glial cell-derived neurotrophic factor levels, at days 1, 3 and 7. Electroacupuncture significantly attenuated the hippocampal POCD-induced increase in malondialdehyde and decreased superoxide dismutase levels at day 1 following surgery.
Conclusion
Electroacupuncture may improve cognitive function in rats with POCD by reducing hippocampal GFAP-positive astrocyte number and suppressing oxidative stress.
Keywords: Postoperative cognitive dysfunction (POCD), electroacupuncture (EA), partial hepatectomy, astrocyte, oxidative stress
Introduction
Postoperative cognitive dysfunction (POCD) is a disorder observed in the elderly following surgery, characterised by reduced cognitive functions, such as attention, memory and speech.1 In a study of coronary artery bypass grafting in adults, about 14% of elderly patients demonstrated reduced cognition within three months following surgery.2 POCD is thought to be associated with a variety of risk factors, including surgery-induced trauma, anaesthesia type, patient age, perioperative stress, and postoperative pain.3 Among these, general anaesthesia has been proposed to possibly cause POCD through affecting the neurological system, particularly in the case of inhalational anaesthetics, such as halothane and isoflurane, that can pass into the brain, leading to aggregation of amyloid peptides and neural cytotoxicity.3 Whether general anaesthesia is the direct promotor of POCD remains unclear, because major surgeries or the neural inflammatory response have also been proposed to accelerate the development of POCD.4
The pathological mechanisms of POCD are complex and unclear. In the hippocampus, a brain lobe with learning and memory functions, changes in signalling pathways have been shown, that are thought to act as the upstream mechanism of cognitive impairment in POCD induced by general anaesthetics.5 Isoflurane, a common inhaled anaesthetic, was reported to induce hippocampal neuroinflammation and subsequent cognitive impairment in aged rats, through various pathways such as calcineurin/nuclear factor-κB and hypoxia-inducible factor-1, indicating neuroinflammation as a general pathogenic mechanism in POCD.6,7 Oxidative stress may also be involved in the pathogenesis of POCD, as surgical trauma was shown to induce oxidative stress in the hippocampus of a rodent model of POCD.8 These mechanisms provide potential preventive or therapeutic targets for POCD, supported by the fact that inhibition of neuroinflammation or oxidative stress has been demonstrated to improve cognitive impairment in a mouse model of POCD.9,10
Astrocytes are a population of cells that regulate the neuronal energy supply. Surgery has been shown to increase astrocyte activation in a rat model of POCD, and to result in cognitive deficits.11 Brain mast cells are proposed to be the ‘first responders’ in neuroinflammation, with increased mast-cell numbers observed after surgery, followed by astrocyte activation, production of inflammatory factors, and subsequent cognitive deficits. Studies in rats have shown that a mast cell stabilizer can improve severe cognitive deficits after surgery, by reducing astrocyte activation and neuroinflammation.11,12
Acupuncture, a traditional Chinese medicine therapeutic technique using percutaneous thin needles, has been used to modulate homeostasis and treat many diseases.13 Electroacupuncture is an electrically driven acupuncture, in which the stimulation frequency and intensity can be regulated.14 A study by the present authors showed that electroacupuncture enhanced cognitive function in a rat model of POCD, by activating α7-nicotinic acetylcholine receptor and inhibiting neuroinflammation.15 However, the role of astrocytes and oxidative stress in the improved cognition associated with electroacupuncture in POCD remains unclear.
The present study aimed to explore the effects of electroacupuncture on cognitive function, astrocyte activation and oxidative stress in an aged rat model of POCD.
Materials and methods
Study population
This study was conducted at the Central Laboratory of Seventh People's Hospital of Shanghai University of TCM between January 2017 and December 2018. Adult male Sprague-Dawley rats (clean-grade; aged 18 months; weight, 500–600 g) were purchased from the SLRC Laboratory Animal Centre (Shanghai, China). All rats were housed in plastic cages, at 20–24°C/ 40–70% humidity, with a 12-h light/12-h dark cycle, and were given free access to standard food and water. A total of 72 rats who had been deemed successful in cognitive function training (as described below), were randomly divided into three groups using a random number table. The sham group received sham surgery (n = 24), the model group received left hepatic lobe resection (n = 24), and the electroacupuncture group received left hepatic lobe resection and electroacupuncture (n = 24), as described below. Rats in each group were further divided into three subgroups according to post-surgery time-point (day 1, 3, and 7; n = 8 per subgroup).
The study was approved by the Animal Care and Use Committee of Seventh People's Hospital of Shanghai University of TCM, and all experiments were conducted according to the Guidelines for Animal Experimentation of Shanghai University of TCM.
Establishment of POCD model
The rat model of POCD was established on day 0, using left hepatic lobe resection, as previously described.16 Rats were anaesthetized with 1% pentobarbital sodium (30 mg/kg) through intraperitoneal (i.p.) injection, then placed in the supine position before undergoing abdominal disinfection. A small incision was then cut into the upper abdominal midline and the left hepatic lobe (approximately one third of the whole liver) was resected. The incision was sutured to close the abdominal cavity, and covered with sterile gauze. The duration of surgery was approximately 90 min per rat. The sham group received similar anaesthesia and surgical procedure, but the left hepatic lobe was not resected.
Electroacupuncture
Rats in the electroacupuncture group received electroacupuncture using the following acupoints: Baihui (GV20, located in the central point of the head midline), Neiguan (PC6, located on the anterior forelimbs between the wrist and elbow, approximately 3 mm from the wrist and between the radius and ulnar), and bilateral Hegu (LI4, located dorsally between the first and second metacarpal of the forelimb). At each acupoint, a stainless-steel needle (0.35 mm × 13 mm) was inserted to approximately 2.5 mm at a vertical angle. The three acupoints (on five sites of the rat body surface) were then connected using a HANS-200A acupoint nerve stimulator (Huayunante Co., Beijing, China), and stimulation was conducted with a continuous dilatational wave of 2/100 Hz, 4 mA intensity.15 Following anaesthesia with 1% pentobarbital sodium (30 mg/kg; i.p.), rats in the electroacupuncture group received electroacupuncture for a continuous 30 min each day at day 0 (prior to POCD induction surgery), and post-surgery day 1 to day 7. Rats in the sham and model groups received the same daily anaesthesia but without electroacupuncture stimulation.
Cognitive function measurements
Cognitive function was evaluated using the Morris water-maze test, as described previously.17 Briefly, the rats were trained for 5 days, four times daily, before undergoing left hepatic lobe resection. A transparent platform was placed in a pool below the water surface, and rats were trained to find it, and rest on the platform for 30 min. When rats could swim and climb to the platform within 60 s on three consecutive occasions, the rats were regarded as successfully trained and were selected for study inclusion and further cognitive testing. Cognitive function was measured using a positioning navigation test and space exploration test at day 1, 3 and 7 following surgery. In the positioning navigation test, a platform was placed within a ½ radius of one quadrant. A rat was placed at the farthest quadrant opposite to the platform, and the duration taken to find and climb onto the platform was recorded as escape latency. A maximal value of 60 s was recorded when a rat failed to find the platform, and each rat was tested at four quadrants to acquire a mean value for escape latency. In the space exploration test, the platform was removed and the same rat was then placed into the water to allow free swimming. The swimming path was observed for 60 s to count the number of times the rat crossed over the original platform position, which was recorded as number of platform crossings.
Immunofluorescence staining
On day 1 (24 h) following surgery to induce POCD, rats were anaesthetized with 1% pentobarbital sodium (30 mg/kg, i.p) and then perfused with 0.9% saline for 15 min, followed by 4% paraformaldehyde for 20 min. The hippocampus was then collected and post-fixed with paraformaldehyde overnight at 4°C, followed by dehydration with 30% sucrose at 4°C for 72 h and incubation with xylene. The tissue was then embedded in paraffin using standard techniques before being serially cut into 20-µm coronal sections. Antigen retrieval was performed by submerging sections into sodium citrate buffer (0.01 mol/l, pH 6.0), and heating in a microwave oven for 10 min. Sections were then blocked by incubating with 0.1 mol/l phosphate buffered saline (PBS) containing 3% bovine serum albumin (BSA) at room temperature for 3 h, and washed in PBS. Next, sections were incubated with rabbit anti-glial fibrillary acidic protein (GFAP) antibody (1:100 dilution; Cat No. MAB360; EMD Millipore, Billerica, MA, USA) at 4°C for 24 h, washed three times in 0.05% PBS-Tween-20 (5 min), then further incubated with goat anti-rabbit IgG conjugated with Alexa Fluor 488 (1:400 dilution; Thermo Fisher Scientific, Waltham, MA, USA) in the dark for 2 h at room temperature. Following antibody incubation, sections were washed in 0.05% PBS-Tween-20, and mounted using mounting medium containing DAPI. Images were then obtained using an OLS5000 laser confocal fluorescence microscope (Olympus [Shanghai], Beijing, China). For each rat, five random fields were selected from tissue sections (hippocampal CA1 region) to count the number of GFAP-positive cells (red astrocytes) and the number DAPI-labelled cells (blue nuclei marker of all cells). The percentage of GFAP-positive astrocytes was then calculated from the proportion of red stained cells out of all DAPI-stained blue nuclei for each rat, as previously described.18
Cytokine analysis
Blood samples (1 ml per rat) were collected from the tail vein of rats in the POCD model and electroacupuncture groups, at day 0 (before surgery), and at day 1, 3 and 7 following POCD induction surgery, into tubes without anticoagulant. Blood was allowed to clot, then serum was immediately separated by centrifugation at 2 000 × g for 15 min at 4°C and stored at –80°C prior to use. Enzyme-linked immunosorbent assay (ELISA) kits were used to measure serum levels of S100 calcium binding protein B (S-100β; Cat No. E-EL-R0868c; Elabscience, Wuhan, China), neuron-specific enolase (NSE; Cat No. E-EL-R0058c; Elabscience), brain-derived neurotrophic factor (BDNF; Cat No. KA0330, R&D Systems; Minneapolis, MN, USA) and glial cell-derived neurotrophic factor (GDNF; Shanghai Sunred Biological Technology Co., Ltd, Shanghai, China) according to the manufacturers’ instructions.
Hippocampus homogenate was obtained from the three groups at day 1 following POCD induction, and centrifuged at 10 000 × g for 10 min at 4°C to obtain the supernatant. ELISA was then used to measure hippocampal levels of the proinflammatory cytokines, interleukin (IL)-1β (cat. No. E-EL-R0012c, Elabscience) and tumour necrosis factor (TNF)-α (R&D Systems), according to the manufacturers’ instructions.
All ELISA results were obtained by measuring optical density (OD) values at 450 nm using an ELISA plate reader (ELx800, Bio-Rad Laboratories, Inc., Hercules, CA, USA). Mean values were calculated from at least three experiments.
Oxidative activity evaluation
Hippocampus homogenate was obtained from the three groups at day 1 following surgery to induce POCD, and separated by centrifugation at 10 000 g for 10 min at 4°C to obtain the supernatant. Proteins associated with oxidative activity, namely, malondialdehyde (MDA) and superoxide dismutase (SOD), were measured using spectrophotometric assay kits (Nanjing Jiancheng Bioengineering Research Institute, Nanjing, China), according to the manufacturer’s instructions. A Bio-Rad ELx800 microplate reader was used to determine MDA activity by measuring absorbance at 532 nm (nmol/mg protein), and SOD activity was determined at 560 nm absorbance (U/mg protein).
Statistical analyses
All quantitative data are presented as mean ± SD, and statistical analyses were performed using SPSS software, version 19.0 (IBM, Armonk, NY, USA). Between-group differences in S-100β, NSE, BDNF and GDNF were compared using repeated measures one-way analysis of variance (ANOVA) followed by Scheffe's post hoc comparisons. Between-group differences in other parameters were analysed by one-way ANOVA, followed by Student-Newman-Keuls test (Q value) for mutual between-group comparisons. A P value <0.05 was considered to be statistically significant.
Results
Electroacupuncture improved cognitive dysfunction in aged rats following surgery
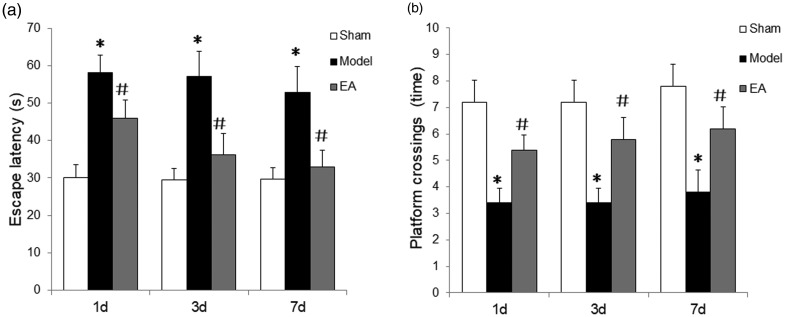
The rat model of POCD was established by partial hepatectomy to resect the left hepatic lobe (one third of the whole liver), and rats were observed following surgery, to evaluate their physiological functions. All rats displayed normal eating and active movement, and their vital signs, heart rate, blood pressure and breathing rates were within normal range, with no obvious signs of distress. The place navigation test revealed that escape latency was prolonged in the POCD model group versus sham control on days 1, 3 and 7 following surgery, and this prolonged escape latency was attenuated at each time-point by electroacupuncture stimulation (P<0.05; Figure 1a). In the space exploration test, the number of platform crossings in the POCD model group were reduced on days 1, 3 and 7 following surgery versus the sham control (P<0.05; Figure 1b), and electroacupuncture stimulation attenuated the reduction in platform crossings induced by POCD (P<0.05; Figure 1b).
Figure 1.
Electroacupuncture (EA) improved cognitive ability in rats with postoperative cognitive dysfunction (POCD) on days 1, 3 and 7 following left hepatic lobe resection (n = 8 per group): (a) Escape latency was increased in POCD model rats versus the sham group, and electroacupuncture significantly attenuated the escape latency time (place navigation test); and (b) the number of platform crossings (space exploration test) were significantly reduced in POCD model rats versus the sham group, and electroacupuncture significantly increased the number of crossings. Model, POCD model group; EA, POCD electroacupuncture group; Sham, sham surgery group without electroacupuncture. Data presented as mean ± SD; *P<0.05 versus sham group; #P < 0.05 versus model group (one-way analysis of variance).
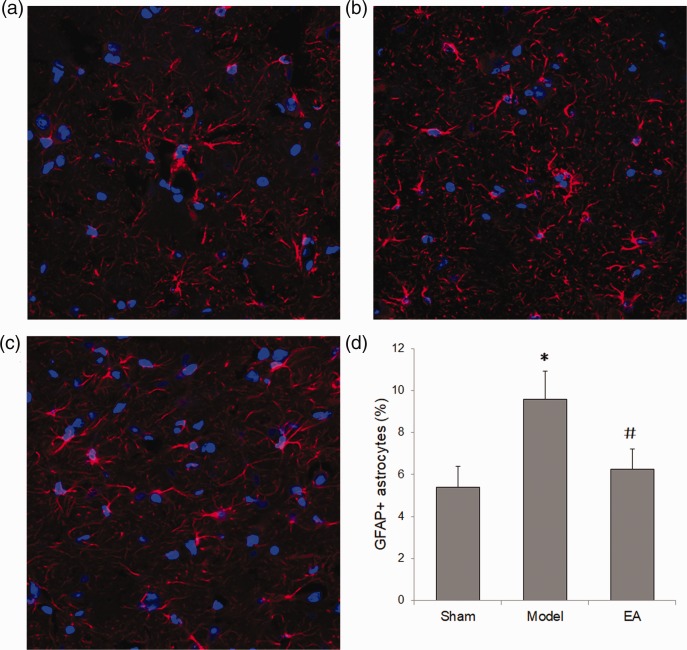
Electroacupuncture decreased hippocampal astrocytes in rats with POCD
The number of astrocytes in hippocampal tissue was evaluated by GFAP immunofluorescence staining in rats with POCD, at day 1 following POCD induction surgery. GFAP-positive astrocytes were observed in the hippocampus CA1 area in all groups (Figure 2a, 2b and 2c), with the POCD model group showing the brightest fluorescence. Quantitative summary data showed that GFAP-positive astrocytes were significantly increased in rats with POCD (model) versus the sham group, and electroacupuncture significantly reversed this increase (Figure 2d).
Figure 2.
Electroacupuncture (EA) reduced the astrocyte number in the hippocampus of rats with postoperative cognitive dysfunction (POCD). Rats were sacrificed on day 1 following surgery to obtain hippocampal tissue for immunofluorescence staining with anti-glial fibrillary acidic protein (GFAP) antibody. Representative photomicrographs show GFAP-positive astrocytes and DAPI-stained nuclei (n = 8 per group): (a) Sham group; (b) POCD Model group; and (c) electroacupuncture group; (d) summary data showing that electroacupuncture treatment significantly attenuated the increased GFAP-positive astrocytes seen in the hippocampus of rats with POCD. Data presented as mean ± SD; *P < 0.05 versus sham group; #P < 0.05 versus model group (one-way analysis of variance); original magnification × 200.
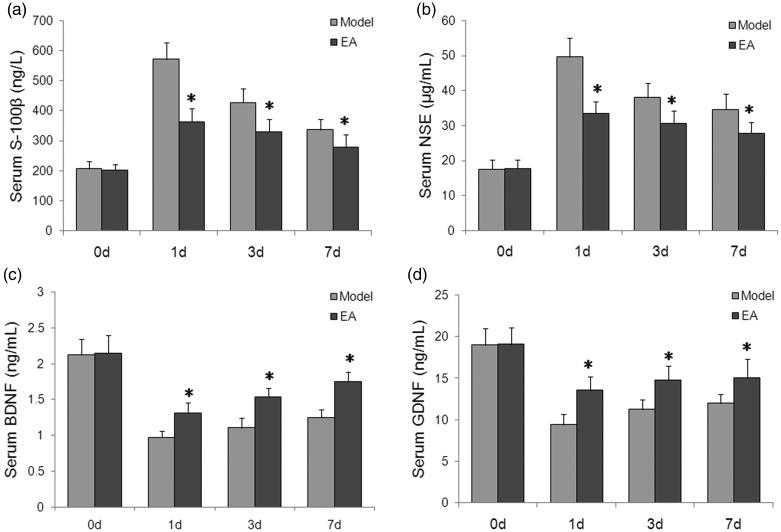
Effects of electroacupuncture on serum cytokine levels in rats with POCD
Serum S-100β and NSE levels were significantly increased at days 1, 3 and 7 following POCD induction compared with day 0 (p with the peak increase seen at 1 day following surgery for both S-100β and NSE (all P < 0.05 versus day 0). Electroacupuncture significantly attenuated the increased serum S-100β and NSE levels in rats with POCD at day 1, 3 and 7 (P < 0.05; Figure 3a and 3b). Furthermore, serum BDNF and GDNF levels were significantly decreased at days 1, 3 and 7 after POCD induction compared with day 0 (all P < 0.05), and these changes were significantly attenuated by electroacupuncture stimulation for all time-points (P < 0.05; Figure 3c and 3d).
Figure 3.
Effects of electroacupuncture (EA) on serum cytokine levels in rats with postoperative cognitive dysfunction (POCD): (a) Serum S-100β and (b) serum NSE levels were significantly increased at days 1, 3, and 7 following POCD induction; and (c) serum BDNF and (d) serum GDNF levels were significantly decreased at days 1, 3, and 7 following POCD induction. Electroacupuncture significantly attenuated the changes in all four cytokines versus the POCD model group. Data presented as mean ± SD; n = 8 per group; *P < 0.05 versus day 0 (pre-surgery) in the same group; #P < 0.05 versus model group at the same time points (one-way analysis of variance). S-100β, S100 calcium binding protein B; NSE, neuron-specific enolase; BDNF, brain-derived neurotrophic factor; GDNF, glial cell-derived neurotrophic factor.
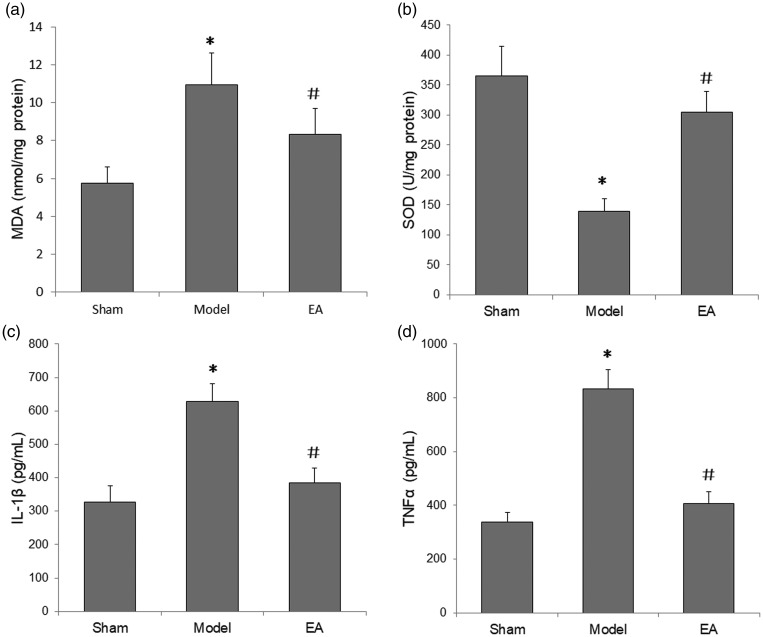
Electroacupuncture inhibited oxidative stress and inflammation in rats with POCD
Oxidative activity and inflammation were measured in the hippocampal tissue of rats with POCD at day 1 following POCD induction surgery. Compared with sham rats, model rats showed significantly higher MDA and lower SOD levels in the hippocampus (P < 0.05), however, electroacupuncture was observed to significantly attenuate these changes in hippocampal MDA and SOD levels (P < 0.05; Figure 4a and 4b).
Figure 4.
Effects of electroacupuncture (EA) on oxidative stress and neuroinflammation in the hippocampus of rats with postoperative cognitive dysfunction (POCD) at 1 day following POCD induction surgery: (a) malondialdehyde (MDA) levels were significantly increased, and (b) superoxide dismutase (SOD) levels were significantly decreased in the POCD model versus sham groups, and these changes were significantly attenuated by electroacupuncture; (c) interleukin (IL)-1β and (d) tumour-necrosis factor (TNF)-α levels were significantly increased in the POCD model versus sham group and these increases were significantly inhibited by electroacupuncture. Data presented as mean ± SD (n = 8); *P < 0.05 versus sham group; #P < 0.05 versus POCD Model group (one-way analysis of variance).
Inflammatory cytokine levels in the hippocampus were measured to evaluate the extent of neuroinflammation at 1-day post-surgery. POCD induction significantly increased the hippocampal levels of IL-1β and TNF-α versus the sham group (P<0.05), and these increases in IL-1β and TNF-α levels were reversed by electroacupuncture stimulation (P < 0.05; Figure 4c and 4d).
Discussion
In the present study, electroacupuncture was found to improve cognitive impairment and inhibit astrocyte activation and oxidative stress in the hippocampus of aged rats (aged 18 months) with POCD induced by left hepatic lobe resection. The Morris water-maze test showed that escape latency was significantly increased and platform crossings were significantly reduced in the POCD model group versus sham controls, and electroacupuncture treatment significantly attenuated these changes on postoperative days 1, 3 and 7. Furthermore, immunofluorescence staining showed a significant increase in the number of GFAP-positive astrocytes in the hippocampus of rats with POCD compared with sham rats, on postoperative day 1, and this effect was shown to be reversed by electroacupuncture. Electroacupuncture was associated with significantly reduced serum S-100β and NSE, and increased serum BDNF and GDNF in rats with POCD, and thus, may attenuate the brain damage induced by surgery and promote nerve regeneration. Electroacupuncture also suppressed oxidative stress in the hippocampal tissue of rats with POCD, as evidenced by significantly lower MDA and higher SOD levels with electroacupuncture treatment. Finally, electroacupuncture decreased levels of the inflammatory cytokines IL-1β and TNF-α in the hippocampal tissue of rats with POCD, suggesting an inhibitory effect on neuroinflammation in POCD. In summary, the results indicate that cognitive impairment was successfully produced in the present rat model of POCD, and abnormal astrocyte activation, oxidative stress, and inflammatory cytokines in the hippocampus may be associated with POCD pathogenesis and the effect of electroacupuncture stimulation on improved cognition.
Cognitive function in rats was evaluated in the present study using the Morris water maze test (a procedure that requires experimental animals, such as rats and mice, to swim and learn to find platforms hidden in the water).19 The Morris water maze test is mainly used to assess the learning and memory skills of spatial position and direction in experimental animals, and is widely used in studies on learning and memory, senile dementia, hippocampus/external hippocampus research, intelligence and aging, and new drug development. The basis of this experiment is that rodents have a strong incentive to escape from water, and will escape from the water environment in the quickest and most direct way. Learning ability is reflected by the process of learning to escape the water environment; and spatial memory ability is reflected by spatial positioning according to the surrounding environment, and purposeful swimming to a safe place (platform) in the water.19 Escape latency was used to evaluate the learning ability of rats, and shorter escape latency indicated higher performance. The number of platform crossings was used to evaluate spatial memory ability, with a higher number of platform crossings indicating higher performance levels. Therefore, the present results demonstrated that electroacupuncture stimulation improved the learning and spatial memory abilities of rats with POCD.
In the present study, the Baihui (GV20), Neiguan (PC6) and Hegu (LI4) acupoints were selected to perform electroacupuncture, all of which are associated with cognitive function, and may serve as potential therapeutic targets for POCD. For example, electroacupuncture at the Baihui acupoint has been shown to alleviate cognitive impairment and exert neuroprotective effects in cerebral ischemia-reperfusion injury, and regulate gene expression in hippocampal tissues.20,21 Electroacupuncture at the Neiguan acupoint may improve the neurological injury induced by cerebral ischemia-reperfusion, via the suppression of neuroinflammation and hippocampal neuron death.22 Acupuncture at the Hegu acupoint in patients with mild cognitive impairment was shown to alter activities in brain regions associated with memory and cognition, such as the frontal and temporal lobes.23
In the present study, rats were anaesthetized with 1% pentobarbital sodium (30 mg/kg) i.p. As an anaesthetic, pentobarbital itself can injure the hippocampus and impair spatial learning and memory.24,25 Thus, care was taken to ensure that pentobarbital showed only a slight influence on cognition in the present study rats, and all three study groups received i.p. pentobarbital, to diminish any between-group disparity in potential hippocampal injury.
Electroacupuncture was shown to have neuroprotective effects in the present study, and may reduce brain damage and promote nerve regeneration, as evidenced by decreased serum S-100β and NSE levels and increased serum BDNF and GDNF levels. Increased serum levels of S-100β (an acidic Ca2+ binding protein present in glial cells) and NSE (a marker of neuronal damage in neural cells) indicate injury in brain and neural cells, and can be used as biochemical indicators of brain damage. Increased serum S-100β and NSE levels have been found in patients with POCD compared with patients without POCD and healthy subjects.26 Electroacupuncture was shown to reduce serum S-100β and NSE in patients with cerebral infarction, while reducing hemiplegia, supporting the notion that electroacupuncture mitigated brain damage that may be caused by neuroinflammation in rats with POCD in the present study.27,28 BDNF and GDNF are two secreted neurotrophic factors that display important roles in the growth, differentiation, myelination and survival of neuronal cells,29,30 and reduced serum BDNF and GDNF concentrations have been found in cognitively impaired patients.31 Electroacupuncture has been shown to promote neurotrophic factor secretion and increase serum BDNF and GDNF levels in rats with cerebral ischemia-reperfusion injury, thereby enhancing hippocampal neural stem cell proliferation.32 The mechanisms underlying neural regeneration by electroacupuncture remain largely unknown, and the association between electroacupuncture and cognition warrants further study.
The present study showed decreased GFAP-positive hippocampal astrocytes in response to electroacupuncture, suggesting that electroacupuncture reduced astrocyte activation in the hippocampus of aged rats with POCD. Astrocytes are a major type of glial cell, that are recognized to promote neuroinflammation when activated, and have been shown to play a critical role in the aetiology and progression of POCD.11 Astrocytes can be activated by amyloid-beta peptide, promoting the secretion of the inflammatory cytokines IL-6, TNF-α, and IL-1β, and subsequent neurotoxicity.33 Thus, astrocytes appear to be attractive potential target cells in the treatment of POCD. Inhibiting astrocyte activation, shown by reduced levels of hippocampal GFAP, may improve hepatectomy-induced cognitive impairment in aged mice, and may also mediate neuroprotective effects in rats with traumatic brain injury.34,35 The present authors considered that astrocyte activation is a mechanism in cognitive dysfunction and an early event, and astrocyte activation is shown to be suppressed as early as 1 day following electroacupuncture treatment.36 Electroacupuncture has also been reported to attenuate aberrant astrocyte activation in animal models of central poststroke pain and traumatic brain injury,37,38 thus astrocyte number was measured at day 1 following electroacupuncture in the present study, administered as an inhibitor of astrocytes in the rat model of POCD. Locally increased hippocampal TNF-α has been shown to activate astrocyte TNF receptor type 1, resulting in hippocampal synaptic alteration and impairment in learning and memory,39 providing further evidence that astrocyte activation may mediate neuroinflammation-induced cognitive impairment. Consistent with these data, the present results showed that electroacupuncture was associated with reduced IL-1β and TNF-α protein, in conjunction with reduced astrocyte activation, in the hippocampus of rats with POCD. However, the regulatory mechanisms between neuroinflammation and astrocytes remain unclear.
Electroacupuncture was shown to be associated with decreased hippocampal MDA and increased SOD levels in the present study, suggesting that electroacupuncture inhibited oxidative stress in the hippocampus of aged rats with POCD. Surgical trauma is reported to induce oxidative stress in the hippocampus of rodents with POCD, thereby participating in POCD pathogenesis.8 Age is also a risk factor for POCD, and the aging hippocampus shows a variety of neurobiological alterations, including increased oxidative stress, which is regarded as a promoter of age-related cognitive decline.40 Therefore, oxidative stress, particularly in the hippocampus, may be a potential therapeutic target for the treatment of POCD. Electroacupuncture has been shown to inhibit oxidative stress by attenuating the abnormally increased levels of reactive oxygen species and MDA in the hippocampus of rats with cerebral ischemia-reperfusion injury, thereby improving the learning and memory ability.19 In addition, treatment with electroacupuncture significantly increased serum glutathione content and glutathione peroxidase activity in rats with cerebral ischemia and reperfusion injury, and oxidative stress was shown to be an early event with rapid response to electroacupuncture treatment,41 thus, markers of oxidative stress were measured in the hippocampus at day 1 in the present study. Consistent with previous publications, the present results showed that oxidative stress was suppressed by electroacupuncture in the hippocampus of rats with POCD. Inhibiting hippocampal oxidative injury may also normalize the stress-induced production of pro-inflammatory cytokines, such as IL-1β and TNF-α.42 Indeed, oxidative stress is negatively regulated by the nuclear factor erythroid 2-related factor 2 (Nrf2)-antioxidant response element (ARE) signalling pathway, and electroacupuncture has shown anti-oxidative action through Nrf2-ARE signalling in a mouse model of Parkinson's disease.43 Whether hippocampal Nrf2-ARE signalling is regulated by electroacupuncture in patients with POCD requires further investigation.
In conclusion, electroacupuncture significantly improved cognitive impairment in rats with POCD, possibly through suppression of astrocyte activation and oxidative stress in the hippocampus. These results suggest that electroacupuncture may be a potential therapeutic strategy for patients with POCD. Further study is required to explore the mechanisms of astrocyte suppression and the associations between electroacupuncture and astrocyte-mediated regulation of oxidative stress and neuroinflammation in POCD.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This study was funded by Projects of Academic Leaders, Shanghai Pudong New Area Health System (PWRd2016-17), and by the Important Weak Subject Construction Project of Pudong Health and Family Planning Commission of Shanghai (Grant No. PWZbr 2017-19).
References
- 1.Bekker AY, Weeks EJ. Cognitive function after anaesthesia in the elderly. Best Pract Res Clin Anaesthesiol 2003; 17: 259–272. [DOI] [PubMed] [Google Scholar]
- 2.Norkiene I, Samalavicius R, Misiuriene I, et al. Incidence and risk factors for early postoperative cognitive decline after coronary artery bypass grafting. Medicina (Kaunas) 2010; 46: 460–464. [PubMed] [Google Scholar]
- 3.Shoair OA, Grasso Ii MP, Lahaye LA, et al. Incidence and risk factors for postoperative cognitive dysfunction in older adults undergoing major noncardiac surgery: a prospective study. J Anaesthesiol Clin Pharmacol 2015; 31: 30–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou YQ, Li XB, Yang ZX, et al. Impact of inhalational anesthetics on postoperative cognitive function: study protocol of a systematic review and meta-analysis. Medicine (Baltimore) 2018; 97: e9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cascella M, Bimonte S. The role of general anesthetics and the mechanisms of hippocampal and extra-hippocampal dysfunctions in the genesis of postoperative cognitive dysfunction. Neural Regen Res 2017; 12: 1780–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li Z, Ni C, Xia C, et al. Calcineurin/nuclear factor-κB signaling mediates isoflurane-induced hippocampal neuroinflammation and subsequent cognitive impairment in aged rats. Mol Med Rep 2017; 15: 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao Y, Li Z, Ma L, et al. Isoflurane-induced postoperative cognitive dysfunction is mediated by hypoxia‑inducible factor‑1α‑dependent neuroinflammation in aged rats. Mol Med Rep 2018; 17: 7730–7736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An LN, Yue Y, Guo WZ, et al. Surgical trauma induces iron accumulation and oxidative stress in a rodent model of postoperative cognitive dysfunction. Biol Trace Elem Res 2013; 151: 277–283. [DOI] [PubMed] [Google Scholar]
- 9.Zhou H, Luo T, Wei C, et al. RAGE antagonism by FPS‑ZM1 attenuates postoperative cognitive dysfunction through inhibition of neuroinflammation in mice. Mol Med Rep 2017; 16: 4187–4194. [DOI] [PubMed] [Google Scholar]
- 10.Zhao WX, Zhang JH, Cao JB, et al. Acetaminophen attenuates lipopolysaccharide-induced cognitive impairment through antioxidant activity. J Neuroinflammation 2017; 14: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Yao H, Qian Q, et al. Cerebral mast cells participate in postoperative cognitive dysfunction by promoting astrocyte activation. Cell Physiol Biochem 2016; 40: 104–116. [DOI] [PubMed] [Google Scholar]
- 12.Liu Y, Yin Y. Emerging roles of immune cells in postoperative cognitive dysfunction. Mediators Inflamm 2018; 2018: 6215350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao L, Chen J, Liu CZ, et al. A review of acupoint specificity research in China: status quo and prospects. Evid Based Complement Alternat Med 2012; 2012: 543943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mayor D. An exploratory review of the electroacupuncture literature: clinical applications and endorphin mechanisms. Acupunct Med 2013; 31: 409–415. [DOI] [PubMed] [Google Scholar]
- 15.Liu PR, Zhou Y, Zhang Y, et al. Electroacupuncture alleviates surgery-induced cognitive dysfunction by increasing α7-nAChR expression and inhibiting inflammatory pathway in aged rats. Neurosci Lett 2017; 659: 1–6. [DOI] [PubMed] [Google Scholar]
- 16.Cao XZ, Ma H, Wang JK, et al. Postoperative cognitive deficits and neuroinflammation in the hippocampus triggered by surgical trauma are exacerbated in aged rats. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1426–1432. [DOI] [PubMed] [Google Scholar]
- 17.Barnhart CD, Yang D, Lein PJ. Using the Morris water maze to assess spatial learning and memory in weanling mice. PLoS One 2015; 10: e0124521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes-Haro D, Labrada-Moncada FE, Varman DR, et al. Anorexia reduces GFAP+ cell density in the rat hippocampus. Neural Plast 2016; 2016: 2426413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc 2006; 1: 848–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin R, Lin Y, Tao J, et al. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Mol Med Rep 2015; 12: 6807–6814. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Lin R, Tao J, et al. Electroacupuncture improves cognitive ability following cerebral ischemia reperfusion injury via CaM-CaMKIV-CREB signaling in the rat hippocampus. Exp Ther Med 2016; 12: 777–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han B, Lu Y, Zhao H, et al. Electroacupuncture modulated the inflammatory reaction in MCAO rats via inhibiting the TLR4/NF-κB signaling pathway in microglia. Int J Clin Exp Pathol 2015; 8: 11199–11205. [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Z, Nie B, Li D, et al. Effect of acupuncture in mild cognitive impairment and Alzheimer disease: a functional MRI study. PLoS One 2012; 7: e42730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W, Tan T, Tu M, et al. Acute pentobarbital treatment impairs spatial learning and memory and hippocampal long-term potentiation in rats. Physiol Behav 2015; 149: 169–173. [DOI] [PubMed] [Google Scholar]
- 25.Wang W, Tan T, Yu Y, et al. Inhibition of AMPAR endocytosis alleviates pentobarbital-induced spatial memory deficits and synaptic depression. Behav Brain Res 2018; 339: 66–72. [DOI] [PubMed] [Google Scholar]
- 26.Chi YL, Li ZS, Lin CS, et al. Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia. Eur Rev Med Pharmacol Sci 2017; 21: 1346–1354. [PubMed] [Google Scholar]
- 27.Zhang H, Kang T, Li L, et al. Electroacupuncture reduces hemiplegia following acute middle cerebral artery infarction with alteration of serum NSE, S-100B and endothelin. Curr Neurovasc Res 2013; 10: 216–221. [DOI] [PubMed] [Google Scholar]
- 28.Pleines UE, Morganti-Kossmann MC, Rancan M, et al. S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J Neurotrauma 2001; 18: 491–498. [DOI] [PubMed] [Google Scholar]
- 29.Fletcher JL, Murray SS and Xiao J. Brain-derived neurotrophic factor in central nervous system myelination: a new mechanism to promote myelin plasticity and repair. Int J Mol Sci 2018; 19: pii: E4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibáñez CF and Andressoo JO. Biology of GDNF and its receptors - Relevance for disorders of the central nervous system. Neurobiol Dis 2017; 97: 80–89. [DOI] [PubMed] [Google Scholar]
- 31.Forlenza OV, Miranda AS, Guimar I, et al. Decreased neurotrophic support is associated with cognitive decline in non-demented subjects. J Alzheimers Dis 2015; 46: 423–429. [DOI] [PubMed] [Google Scholar]
- 32.Tao J, Chen B, Gao Y, et al. Electroacupuncture enhances hippocampal NSCs proliferation in cerebral ischemia-reperfusion injured rats via activation of notch signaling pathway. Int J Neurosci 2014; 124: 204–212. [DOI] [PubMed] [Google Scholar]
- 33.Zhang L, Xia R, Jia J, et al. Oleanolic acid protects against cognitive decline and neuroinflammation-mediated neurotoxicity by blocking secretory phospholipase A2 IIA-activated calcium signals. Mol Immunol 2018; 99: 95–103. [DOI] [PubMed] [Google Scholar]
- 34.Jin WJ, Feng SW, Feng Z, et al. Minocycline improves postoperative cognitive impairment in aged mice by inhibiting astrocytic activation. Neuroreport 2014; 25: 1–6. [DOI] [PubMed] [Google Scholar]
- 35.Liu ZK, Ng CF, Shiu HT, et al. Neuroprotective effect of Da Chuanxiong Formula against cognitive and motor deficits in a rat controlled cortical impact model of traumatic brain injury. J Ethnopharmacol 2018; 217: 11–22. [DOI] [PubMed] [Google Scholar]
- 36.Liang Y, Qiu Y, Du J, et al. Inhibition of spinal microglia and astrocytes contributes to the anti-allodynic effect of electroacupuncture in neuropathic pain induced by spinal nerve ligation. Acupunct Med 2016; 34: 40–47. [DOI] [PubMed] [Google Scholar]
- 37.Tian GH, Tao SS, Chen MT, et al. Electroacupuncture treatment alleviates central poststroke pain by inhibiting brain neuronal apoptosis and aberrant astrocyte activation. Neural Plast 2016; 2016: 1437148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang WC, Hsu YC, Wang CC, et al. Early electroacupuncture treatment ameliorates neuroinflammation in rats with traumatic brain injury. BMC Complement Altern Med 2016; 16: 470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Habbas S, Santello M, Becker D, et al. Neuroinflammatory TNFα impairs memory via astrocyte signaling. Cell 2015; 163: 1730–1741. [DOI] [PubMed] [Google Scholar]
- 40.Bettio LEB, Rajendran L, Gil-Mohapel J. The effects of aging in the hippocampus and cognitive decline. Neurosci Biobehav Rev 2017; 79: 66–86. [DOI] [PubMed] [Google Scholar]
- 41.Shen MH, Zhang CB, Zhang JH, et al. Electroacupuncture attenuates cerebral ischemia and reperfusion injury in middle cerebral artery occlusion of rat via modulation of apoptosis, inflammation, oxidative stress, and excitotoxicity. Evid Based Complement Alternat Med 2016; 2016: 9438650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HY, Lee JS, Kim HG, et al. The ethanol extract of Aquilariae Lignum ameliorates hippocampal oxidative stress in a repeated restraint stress mouse model. BMC Complement Altern Med 2017; 17: 397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lv E, Deng J, Yu Y, et al. Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson's disease. Free Radic Res 2015; 49: 1296–1307. [DOI] [PubMed] [Google Scholar]