Short abstract
Objectives
Calcium sensitizers have been shown to improve outcomes in patients with low cardiac output syndrome (LCOS) after cardiac surgery. We assessed the effects of levosimendan on LCOS in cardiac surgical patients to identify outcome predictors.
Methods
A total of 106 patients in whom LCOS persisted despite conventional therapy additionally received 0.1 µg/kg/min of levosimendan for 24 hours according to a defined treatment algorithm. Baseline and treatment data as well as hemodynamic and outcome parameters were compared between survivors and nonsurvivors, and a multivariate correlation and regression tree analysis was implemented.
Results
The ejection fraction significantly increased from 27% ± 4% to 38% ± 8% within 24 hours and to 45% ± 10% within 48 hours of starting levosimendan. These changes were accompanied by a significant increase in cardiac output from 5.2 ± 0.6 to 6.2 ± 0.7 L/min within 24 hours and significant dose reductions in vasopressors and inotropes. In contrast to nonsurvivors, survivors’ need for inotropic support decreased after the addition of levosimendan to the therapy.
Conclusion
In our patients, all of whom were treated according to the same algorithm, the response to levosimendan in terms of the post-levosimendan need for inotropes and vasopressors predicted survival.
Keywords: Postoperative low cardiac output syndrome, levosimendan, cardiac surgery, calcium sensitizers, outcome predictors, treatment algorithm
Introduction
Low cardiac output syndrome (LCOS) is a severe and potentially fatal complication after cardiac surgical procedures. Although the threshold of implementing mechanical circulatory assistance is not as high as in the past, pharmacological therapy is still the mainstay of therapy for LCOS. However, the range of available inotropic medications is limited and essentially consists of catecholamines and phosphodiesterase inhibitors.1–4
About two decades ago, these therapies were supplemented by levosimendan, an inodilator that exerts its calcium-sensitizing effect by specific interaction with the calcium-sensor troponin C molecule in cardiac myofilaments. Levosimendan also has a phosphodiesterase-inhibiting effect and causes activation of both ATP-sensitive sarcolemmal potassium channels of smooth muscle cells (which is reportedly a powerful vasodilator mechanism) and ATP-sensitive potassium channels in the mitochondria. Thus, levosimendan potentially extends the range of cellular actions toward the modulation of mitochondrial ATP production, implicating a pharmacological mechanism for cardioprotection. The neurohumoral alterations evoked by levosimendan suggest an immunomodulatory profile that may be capable of mobilizing a whole variety of cardioprotective mechanisms.5
Levosimendan is the most thoroughly investigated inotrope of the last 20 years, and its hemodynamic effect has been widely described in both randomized controlled and observational trials. Levosimendan essentially improves cardiac performance without increasing myocardial oxygen consumption or changing myocardial substrate utilization.6 However, its application in cardiac surgery is controversial because conflicting results have been reported from a multitude of studies, including randomized trials.
Rather than comparing patients receiving levosimendan with a control group, we examined the application of levosimendan as an integral component of a fixed treatment algorithm in patients with LCOS after cardiac surgery. In addition to investigating the effects of postoperative levosimendan treatment, we examined the differences between the response to levosimendan treatment in survivors and nonsurvivors to identify outcome predictors.
Patients and methods
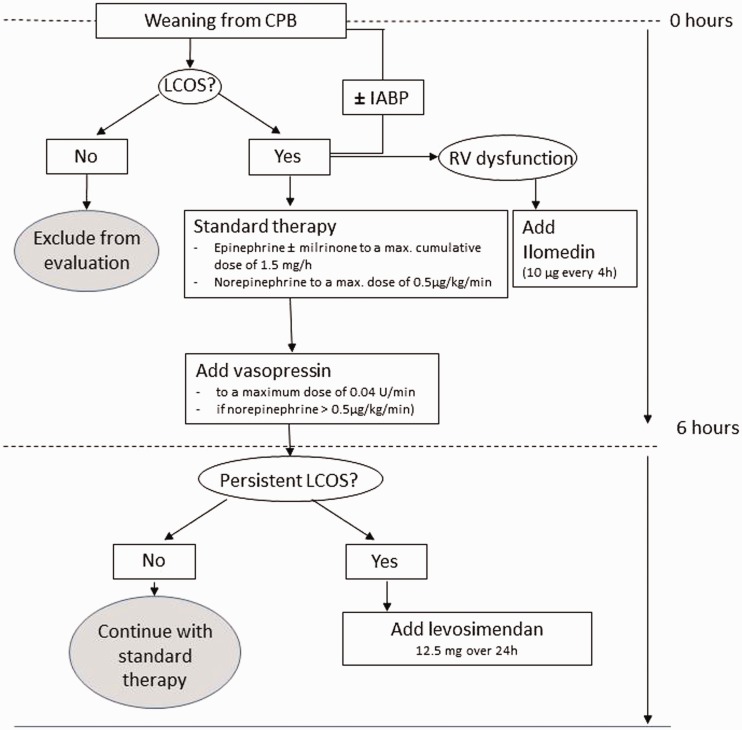
We identified all patients who received levosimendan for LCOS during a 1-year period according to the treatment algorithm of our institution’s cardiac surgical intensive care unit (ICU). Hemodynamics, pharmacological therapy, and outcomes were retrospectively analyzed. Postoperative therapy with inotropes and vasopressors was guided by echocardiography and hemodynamic measurements. The first-choice therapy consisted of a combination of epinephrine, milrinone, and norepinephrine. Vasopressor support was supplemented by vasopressin (maximum dosage of 0.04 U/min) if the norepinephrine dosage exceeded 0.5 µg/kg/min. Iloprost inhalation (10 µg every 4 h) was used in patients with right ventricular dysfunction as proven by echocardiography or pulmonary artery catheterization. Pharmacological therapy was escalated as needed during the first 6 hours after weaning from cardiopulmonary bypass (CPB). Volume and electrolyte optimization were an integral part of treatment. Continuous infusion of levosimendan at a rate of 0.52 mg/hour was started without a loading dose and continued for 24 hours in patients in whom LCOS persisted after a cumulative dosage of epinephrine and/or milrinone of ≥1.5 mg/hour (i.e., approximately 0.3 µg/kg/min for a 75-kg patient) had been administered for 6 hours after weaning from CPB (Figure 1).
Figure 1.
Treatment algorithm. CPB, cardiopulmonary bypass; IABP, intra-aortic balloon pump; LCOS, low cardiac output syndrome; RV, right ventricular.
LCOS was defined as hemodynamic compromise with evidence of organ dysfunction and/or a cardiac index of <2.0 L/min/m2. Patients with a postoperative left ventricular ejection fraction (EF) of ≥40% were excluded.
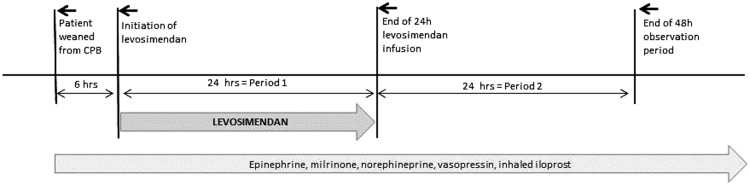
Hemodynamic monitoring was performed by means of a pulmonary artery catheter or the PiCCO® system (Pulsion Medical Systems SE, Munich, Germany) in all patients. The central venous pressure, mean arterial pressure (MAP), and mean pulmonary arterial pressure (MPAP) were continuously monitored. Cardiac output (CO), the cardiac index, systemic vascular resistance, pulmonary vascular resistance, and the pulmonary capillary wedge pressure were measured as needed but at least once every 2 hours. Echocardiography was performed as needed. Echocardiographic determination of left ventricular function was performed at least twice during the initial 6-h period (i.e., upon admission to the ICU and before commencement of levosimendan infusion) and at least once every 12 hours during the administration of levosimendan and the subsequent 24-h observation period (Figure 2). Assessment of the EF was performed by experienced senior physicians.
Figure 2.
Treatment and evaluation periods.
For the purposes of this study, the parameters obtained from 6 hours before to 48 hours after the initiation of treatment with levosimendan were collected and evaluated. Medication doses documented throughout the periods shown in Figure 2 were evaluated. For vasopressors and inotropes, the maximum doses recorded during the respective periods were indicated. For iloprost, which was administered according to a fixed schedule, the cumulative doses administered during the respective periods were indicated.
According to institutional guidelines, approval by an ethics review committee was not required because our study was limited to a retrospective data analysis. For this reason, the patients were not required to provide consent.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation, and categorical variables are expressed as number and percentage. Patients were categorized into survivors and nonsurvivors as well as into subgroups depending on the type of procedure they had undergone. For evaluation of between-group differences, parametric and nonparametric variables were compared using Student’s t-test and the Mann–Whitney U-test, respectively. Binary variables were analyzed using the chi-square test. Changes in parameters across time (e.g., dosage of catecholamines) were analyzed by means of repeated-measures analysis of variance using the time point as a within-subject variable and survival as a between-subject variable. Parameters for which statistically significant differences were shown were additionally included in a multivariate analysis using a correlation and regression tree (CART) model, which is capable of dividing collectives into meaningful subsets, thus identifying the most important discriminating factor between two groups and providing a predictive capability rather than a simple association. All analyses were performed using SPSS software version 18.0 for Windows (SPSS, Inc., Chicago, IL, USA). A p-value of <0.05 was considered statistically significant.
Results
In total, 106 cardiac surgical patients (mean age, 62.6 ± 13.6 years; 75.5% male) were enrolled in this retrospective analysis. Their mean EuroSCORE I was 10.7 ± 4.2, and their mean preoperative left ventricular EF was 36.2% ± 17%. A total of 28 (26.4%) patients underwent aortic valve surgery, 26 (24.5%) underwent coronary artery bypass grafting (CABG), 19 (17.9%) underwent heart transplantation, 13 (12.3%) underwent double valve surgery, 11 (10.4%) underwent mitral valve surgery, 3 (2.8%) underwent tricuspid valve surgery, and 6 (5.7%) underwent other procedures. The cohort comprised a considerable proportion of high-risk patients as evidenced by EuroSCOREs of 6.7 ± 4.0 to 12.5 ± 8.0 and American Society of Anesthesiologists (ASA) scores of 3.4 ± 0.5 to 4.1 ± 0.3 among the different subgroups. Demographic and baseline data for these groups are shown in Table 1.
Table 1.
Demographic and preoperative baseline characteristics.
| AVS28 (26.4%) | CABG 26 (24.5%) | HTX 19 (17.9%) | DVS 13 (12.3%) | MVS 11 (10.4%) | TVS3 (2.8%) | Other6 (5.7%) | |
|---|---|---|---|---|---|---|---|
| Age, years | 66.7 ± 12.7 | 65.9 ± 8.3 | 48.2 ± 13.4 | 70.7 ± 9.3 | 64.6 ± 12.9 | 69.7 ± 10.3 | 51.0 ± 11.1 |
| EuroSCORE, points | 10.5 ± 3.5 | 11.0 ± 2.8 | 10.8 ± 4.3 | 11.9 ± 4.9 | 9.0 ± 5.0 | 6.7 ± 4.0 | 12.5 ± 8.0 |
| ASA class, points | 3.6 ± 0.6 | 3.8 ± 0.6 | 4.1 ± 0.3 | 3.6 ± 0.7 | 3.4 ± 0.5 | 3.7 ± 0.6 | 3.8 ± 0.9 |
| NYHA class, points | 3.0 ± 0.5 | 3.3 ± 0.5 | 3.6 ± 0.5 | 3.2 ± 0.6 | 3.0 ± 0.6 | 3.0 ± 0.0 | 3.3 ± 0.6 |
| EF, % | 40 ± 15 | 33 ± 12 | 19 ± 8 | 43 ± 16 | 46 ± 21 | 61 ± 10 | 25 ± 22 |
| Bilirubin, mg/dl | 1.2 ± 0.6 | 0.8 ± 0.3 | 1.3 ± 1.0 | 2.0 ± 1.0 | 0.9 ± 0.3 | 1.5 ± 0.5 | 1.5 ± 0.9 |
| Creatinine, mg/dl | 1.6 ± 1.7 | 1.2 ± 0.3 | 2.0 ± 1.9 | 1.4 ± 1.0 | 1.6 ± 0.6 | 1.5 ± 0.7 | 1.6 ± 0.7 |
| Female, % | 25.0 | 19.2 | 15.8 | 46.2 | 27.3 | 0.0 | 33.3 |
| Diabetes, % | 28.6 | 46.2 | 10.5 | 23.2 | 0.0 | 33.3 | 0.0 |
| Arterial hypertension, % | 67.9 | 65.4 | 10.5 | 53.8 | 63.6 | 66.7 | 16.7 |
Data are presented as mean ± standard deviation or percentage of patients. AVS, aortic valve surgery; CABG, coronary artery bypass grafting; HTX, heart transplantation; DVS, double valve surgery; MVS, mitral valve surgery; TVS, tricuspid valve surgery; ASA, American Society of Anesthesiologists; NYHA, New York Heart Association; EF, ejection fraction.
All 106 patients received levosimendan according to our treatment algorithm when standard treatment failed. By the time levosimendan was added to their therapies, 42 (40%) patients had been undergoing intra-aortic balloon pump therapy for up to 6 hours without a sufficient effect. Intra-aortic balloon pump therapy was continued throughout the 48-h period following the commencement of levosimendan infusion. The dosages of both vasopressors and inotropes could be substantially reduced within 48 hours from initiation of therapy with levosimendan, as shown in Table 2.
Table 2.
Changes in inotrope, vasopressor, and iloprost dosages during periods 1 and 2.
| Pre-levosimendan | Period 1 | Period 2 | |
|---|---|---|---|
| Epinephrinea, mg/h | 1.3 ± 1.2 | 0.4 ± 0.4* | 0.3 ± 0.4* |
| Norepinephrinea, mg/h | 1.0 ± 0.8 | 0.3 ± 0.3* | 0.15 ± 0.2* |
| Milrinonea, mg/h | 1.5 ± 0.6 | 0.9 ± 0.7* | 0.8 ± 0.9* |
| Iloprostb, µ/d | 54 ± 10 | 43 ± 11* | 32 ± 20* |
| IABP | 42 (40) | 42 (40) | 42 (40) |
Data are presented as mean ± standard deviation or n (%).
Period 1: within 24 hours from the initiation of treatment with levosimendan; Period 2: >24 to <48 hours from the initiation of treatment with levosimendan; IABP, intra-aortic balloon pump.
aMaximum doses used during the respective period.
bCumulative doses used during the respective period.
*p < 0.05 in comparison with pre-levosimendan values.
The EF significantly improved from 27% ± 4% to 38% ± 8% within 24 hours of starting levosimendan and to 45% ± 10% within 48 hours of starting levosimendan (p < 0.05). The MAP also showed a continuous and statistically significant increase throughout the 48-hour period from 67 ± 10 to 80 ± 9 mmHg and to 83 ± 9 mmHg (p < 0.05). The MPAP simultaneously decreased from 31 ± 7 mmHg before levosimendan to 24 ± 6 and 23 ± 6 mmHg during the first and second 24-hour period after initiation of levosimendan, respectively. These differences were not statistically significant. The CO showed a statistically significant increase from 5.2 ± 0.6 L/min at initiation of treatment to 6.2 ± 0.7 L/min during the first 24 hours (p < 0.05), then slightly decreased to 5.9 ± 0.6 L/min during the second 24 hours (Table 3). Notably, these improvements occurred despite the above-described tapering of inotrope and vasopressor dosages.
Table 3.
Hemodynamic changes during periods 1 and 2.
| Pre-levosimendan | Period 1 | Period 2 | |
|---|---|---|---|
| Ejection fraction, % | 27 ± 4 | 38 ± 8* | 45 ± 10* |
| Cardiac output, L/min | 5.2 ± 0.6 | 6.2 ± 0.7* | 5.9 ± 0.6 |
| MAP, mmHg | 67 ± 10 | 80 ± 9* | 83 ± 9* |
| MPAP, mmHg | 31 ± 7 | 24 ± 6 | 23 ± 6 |
Data are presented as mean ± standard deviation.
Period 1: within 24 hours from the initiation of treatment with levosimendan; Period 2: >24 to <48 hours from the initiation of treatment with levosimendan. MAP, mean arterial pressure; MPAP, mean pulmonary arterial pressure.
*p < 0.05 in comparison with pre-levosimendan values.
The overall 30-day mortality rate was 34.9% (n = 37) and was not associated with a poor preoperative EF (<35%). No significant differences in age; the preoperative left ventricular EF; the EuroSCORE; or the Canadian Cardiovascular Society, New York Heart Association, or ASA classification scores were found between survivors and nonsurvivors.
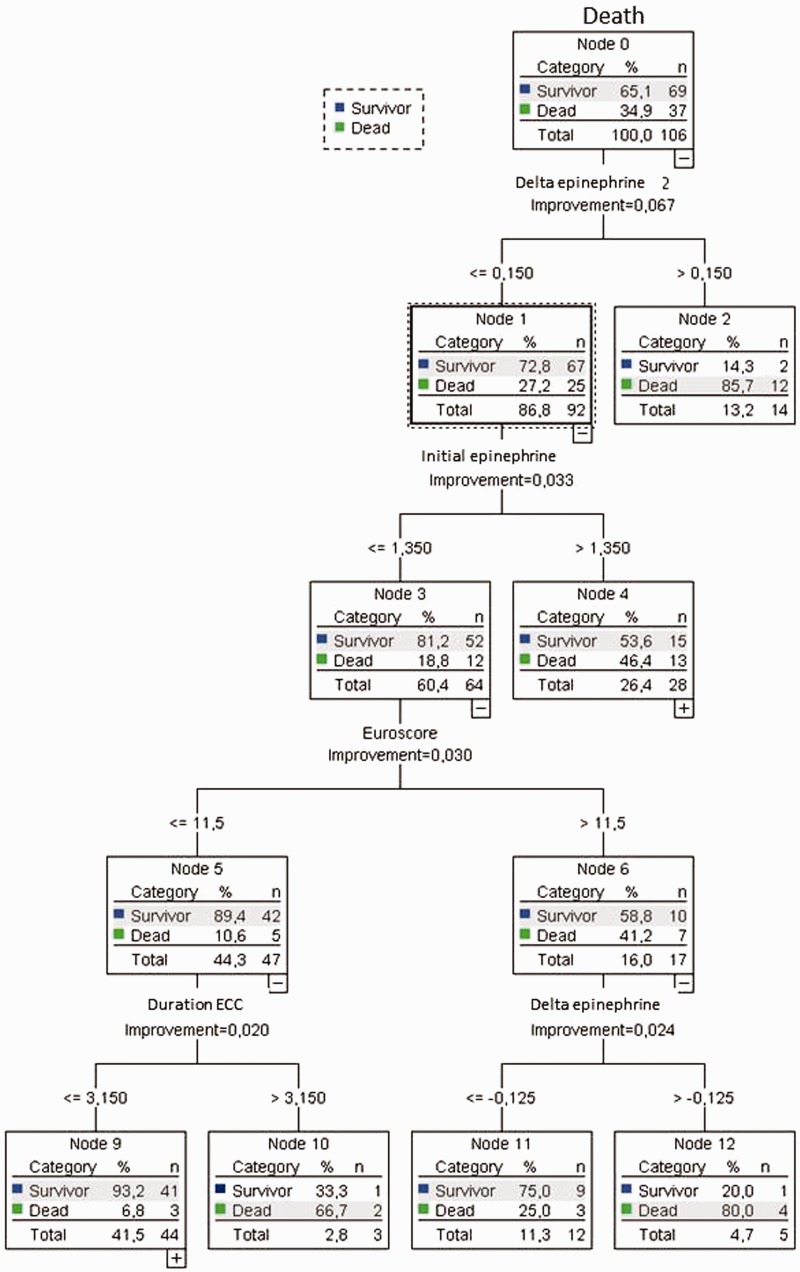
The CART analysis (Figure 3) showed that the response to levosimendan treatment significantly differed between survivors and nonsurvivors in that the need for inotropic support with epinephrine after termination of the levosimendan infusion was significantly lower in survivors (p < 0.05). Patients in whom the need for inotropic support with epinephrine (delta epinephrine) increased by >0.15 mg/hour compared with the dosage at the beginning of levosimendan treatment within 24 hours after termination of the levosimendan infusion had a mortality rate of 85.7%; in contrast, the mortality rate of those in whom such an increase in the need for inotropic support was not present was only 27.2%.
Figure 3.
Multivariate correlation and regression tree analysis of survivors versus nonsurvivors.
Within the group of patients in whom a >0.15-mg/hour post-levosimendan increase in the epinephrine dosage did not occur, a subgroup with a particularly low mortality rate was identified: those in whom the initial dosage of epinephrine (initial epinephrine) was <1.3 mg/hour had a mortality rate of only 18.8%, while those with an initial epinephrine dosage of >1.3 mg/hour had a mortality rate of 46.4%.
Further stratification of patients with an initial epinephrine dosage of <1.3 mg/hour and a post-levosimendan increase in the need for inotropes of <0.15 mg/hour yielded a cut-off in the preoperative EuroSCORE. Mortality in those with a EuroSCORE of 11.5 was 41.2%, while mortality in those with a EuroSCORE of ≤11.5 was 10.6%.
In patients receiving levosimendan for LCOS in the framework of a defined treatment algorithm, a post-levosimendan increase in the epinephrine dosage by >0.15 mg/hour and an initial dosage of epinephrine of <1.3 mg/hour were associated with particularly high and low mortality, respectively.
Discussion
The use of levosimendan in cardiac surgical patients is not a recent idea as evidenced by a considerable number of studies investigating levosimendan versus either placebo or other inotropes (summarized, for example, by Toller et al.7). Prophylactic levosimendan did not reduce the rates of death, renal replacement therapy, perioperative myocardial infarction, or use of mechanical cardiac assist devices in patients with a low left ventricular EF undergoing on-pump cardiac surgery.8 Additionally, a trial comparing low-dose levosimendan administered in addition to standard care in patients requiring hemodynamic care after cardiac surgery versus placebo was even stopped due to futility because no significant differences were found between levosimendan and placebo in terms of 30-day mortality, the duration of mechanical ventilation, the length of hospital stay, or the rates of hypotension and cardiac arrhythmia.9 Similarly, the findings of a randomized trial comparing levosimendan versus placebo in patients with poor left ventricular function undergoing CABG did not support the use of levosimendan in this setting.10
In contrast, a meta-analysis of 177 randomized trials comprising >28,000 patients showed a reduction in mortality associated with inotrope therapy in the setting of cardiac surgery and demonstrated that levosimendan was the only substance associated with a significant improvement in survival.11 The authors of the 2015 European Expert Opinion on the Preoperative and Perioperative Use of Levosimendan in Cardiac Surgery recommend the preoperative use of levosimendan in patients with compromised myocardial function, including poor right ventricular function.12
Previous recommendations for the use of levosimendan in patients undergoing cardiac surgery (e.g., avoidance of bolus administration) are reflected by our treatment algorithm, which also requires volume and electrolyte optimization, simultaneous norepinephrine administration, optimization of diuretics, and discontinuation of β-blockers. However, our approach is specific in that levosimendan was used as part of the postoperative treatment only. Rather than randomizing patients to treatment with levosimendan versus conventional inotropes, we treated all patients according to the same fixed algorithm that was established as part of our standard treatment of LCOS and required levosimendan to be initiated when LCOS persisted after treatment with conventional inotropes, vasopressors, and volume and electrolyte optimization for 6 hours after weaning from CPB.
This approach was established in accordance with the recommendation expressed by Toller et al.7 to prevent levosimendan from being used as a last resort; i.e., from being delayed until all other strategies have failed and organ failure is already present. Additionally, our treatment algorithm ensures application of levosimendan in a controlled and well-established fashion within a relatively short period from the onset of postoperative LCOS. This not only prevents delays in the initiation of treatment with and uncontrolled use of levosimendan, but also improves the comparability of data between patients and eliminates differences in outcomes that might otherwise have occurred due to differences in the timing of therapy with levosimendan. Additionally, our approach with simultaneous administration of vasopressors and conventional inotropes reflects clinical reality and allows us to monitor and evaluate the reductions of catecholamines, phosphodiesterase inhibitors, and vasopressor doses enabled by levosimendan as parameters reflecting its effectiveness.
The significant improvement in our patients’ EF that occurred during and after administration of levosimendan resembles the findings of several other studies involving cardiac surgical patients. However, it must be acknowledged that the hemodynamic conditions associated with recovering from cardiac surgery are complex, and caution must be exerted in attributing the improvement in the EF to one single component of a complex treatment algorithm.
The significant reductions in the inotrope and vasopressor dosages that were achieved in our patients during and after administration of levosimendan, however, reflect the findings of previous studies such as those by De Hert et al.13 and Levin et al.14 These authors compared levosimendan with milrinone and dobutamine, respectively, in patients undergoing CABG and reported a significantly lower need for inotropes and vasopressors in patients treated with levosimendan. In the course of these two studies, during which levosimendan was administered after releasing the cross clamp13 and in which LCOS was diagnosed within the first 6 hours after surgery,14 respectively, the additional benefits of levosimendan were a significantly higher stroke volume index despite similar filling pressures and shorter mechanical ventilation times in the levosimendan than milrinone group13 and significantly lower postoperative mortality in the levosimendan than dobutamine group.14
In contrast, Gandham et al.15 reported findings that suggested an increased requirement of inotropes and particularly vasopressors in cardiac surgical patients receiving levosimendan. In their study, the effects of levosimendan and dobutamine administered for weaning from CPB were compared in patients undergoing mitral valve surgery.15 These findings, especially with regard to the vasopressor dosage, also contrast the findings in our study population, in which the decrease in systemic vascular resistance was not statistically significant while a significant increase in MAP, accompanied by a reduction in vasopressors and inotropes, occurred from the initiation of therapy with levosimendan and lasted until the end of the 48-h observation period.
The use of levosimendan in patients with coronary artery disease has been investigated in a substantial number of studies that have suggested benefits beyond a mere positive inotropic effect. These benefits include restoration of ventriculoarterial coupling, increases in tissue perfusion, anti-stunning and anti-inflammatory effects,16,17 reductions in the size of hypokinetic segments,17 reductions in the incidence of myocardial infarction,18 and anti-ischemic,19 anti-remodeling,20 and anti-apoptotic effects.21 Levosimendan has also been variously used and recommended to treat primary graft failure after heart transplantation.22–24 The reported effects in this highly specific patient subset included significant improvements in CO, MAP, and MPAP; significant increases in EF; reductions in the need for inotropic support;25 and improved reno-protective properties.15,26
In our study, worse survival was associated with the development of a need for inotropic support following levosimendan infusion. This finding was not unexpected because an increasing need for inotropic support is a sign of a poor response not only to levosimendan but also to the accompanying inotropes. Therefore, an increasing need for inotropic support after administration of levosimendan must be interpreted as not only non-response to levosimendan but also a surrogate of the patient’s poor response to all inotropes administered. In this sense, it certainly heralds a poor outcome, which is evidenced by the mortality rate of >85% versus 27% we found in those in whom inotropic support increased by >0.15 mg/hour compared with the dosage at the beginning of levosimendan treatment within 24 hours after termination of the levosimendan infusion.
Our study has several limitations. First, our patient cohort was very heterogeneous in terms of the procedures performed. However, this reflects the conditions prevailing in the average cardiac surgical ICU. Comparison between survivors and nonsurvivors was enabled by the fact that levosimendan was applied according to the same treatment algorithm in all patients. A control group was not included because the study focused on illustrating the function of a clinical treatment algorithm comprising the administration of levosimendan at a predefined stage and highlighting the different responses to treatment in survivors and nonsurvivors.
Conclusions
Our results, supported by the findings of recent reviews and meta-analyses, suggest that levosimendan is a useful component of an algorithm for the treatment of LCOS after cardiac surgery. A post-levosimendan increase in the need for inotropes should be considered an indicator of a poor prognosis. In a given patient, such an increase in the need for inotropes during and after treatment with levosimendan suggests that the effect of the therapy is not as favorable as expected and may aid intensivists in decision-making.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Kheterpal S, Fellahi JL. Less is more. A superior clinical strategy? Anesthesiology 2014; 120: 1067–1068. [DOI] [PubMed] [Google Scholar]
- 2.Arrigo M, Mebazaa A. Understanding the differences among inotropes. Intensive Care Med 2015; 41: 912–915. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen DV, Hansen MK, Johnsen SP, et al. Health outcomes with and without use of inotropic therapy in cardiac surgery: results of a propensity score-matched analysis. Anesthesiology 2014; 120: 1098–1108. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard CB, Dzavik V. Inotropes and vasopressors. Review of physiology and clinical use in cardiovascular disease. Circulation 2008; 118: 1047–1056. [DOI] [PubMed] [Google Scholar]
- 5.Papp Z, Edes I, Fruhwald S, et al. Levosimendan: molecular mechanisms and clinical implications. Consensus of experts on the mechanisms of action of levosimendan. Int J Cardiol 2012; 159: 82–87. [DOI] [PubMed] [Google Scholar]
- 6.Lilleberg J, Nieminen MS, Akkila J, et al. Effects of a new calcium sensitizer, levosimendan, on haemodynamics, coronary blood flow and myocardial substrate utilization early after coronary artery bypass grafting. Eur Heart J 1998; 19: 660–668. [DOI] [PubMed] [Google Scholar]
- 7.Toller W, Algotsson L, Guarracino F, et al. Perioperative use of levosimendan: best practice inoperative settings. J Cardiothorac Vas Anesth 2013; 27: 361–366. [DOI] [PubMed] [Google Scholar]
- 8.Mehta RH, Leimberger JD, van Diepen S, et al. Levosimendan in patients with left ventricular dysfunction undergoing cardiac surgery. N Engl J Med 2017; 376: 2032–2042. [DOI] [PubMed] [Google Scholar]
- 9.Landoni G, Lomivorotov VV, Alvaro G, et al. Levosimendan for hemodynamic support after cardiac surgery. N Engl J Med 2017; 376: 2021–2031. [DOI] [PubMed] [Google Scholar]
- 10.Cholley B, Caruba T, Grosjean S, et al. Effect of levosimendan on low cardiac output syndrome in patients with low ejection fraction undergoing coronary artery bypass grafting with cardiopulmonary bypass. JAMA 2017; 318: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belletti A, Castro ML, Silvetti S, et al. The effect of inotropes and vasopressors on mortality: a meta-analysis of randomized clinical trials. Br J Anaesth 2015; 115: 656–675. [DOI] [PubMed] [Google Scholar]
- 12.Toller W, Heringlake M, Guarracino F, et al. Preoperative and perioperative use of levosimendan in cardiac surgery: European expert opinion. Int J Cardiol 2015; 184: 323–336. [DOI] [PubMed] [Google Scholar]
- 13.De Hert SG, Lorsomradee S, Cromheecke S, et al. The effects of levosimendan in cardiac surgery patients with poor left ventricular function. Anesth Analg 2007; 104: 766–773. [DOI] [PubMed] [Google Scholar]
- 14.Levin RL, Degrange MA, Porcile R, et al. The calcium sensitizer levosimendan gives superior results to dobutamine in postoperative low cardiac output syndrome. Rev Esp Cardiol 2008; 61: 471–479. [PubMed] [Google Scholar]
- 15.Gandham R, Syamasundar A, Ravulapalli H, et al. A comparison of hemodynamic effects of levosimendan and dobutamine in patients undergoing mitral valve repair/replacement for severe mitral stenosis. Ann Card Anaesth 2013; 16: 11–15. [DOI] [PubMed] [Google Scholar]
- 16.Nieminen MS, Buerke M, Cohen-Solál A, et al. The role of levosimendan in acute heart failure complicating acute coronary syndrome: a review and expert consensus opinion. Int J Cardiol 2016; 218: 150–157. [DOI] [PubMed] [Google Scholar]
- 17.Sonntag S, Sundberg S, Lehtonen LA, et al. The calcium sensitizer levosimendan improves the function of stunned myocardium after percutaneous transluminal coronary angioplasty in acute myocardial ischemia. J Am Coll Cardiol 2004; 43: 2177–2182. [DOI] [PubMed] [Google Scholar]
- 18.Tarki M, Stark C, Haavisto M, et al. Effect of levosimendan therapy on myocardial infarct size and left ventricular function after acute coronary occlusion. Heart 2016; 102: 465–471. [DOI] [PubMed] [Google Scholar]
- 19.Levijoki J, Pollesello P, Kaheinen P, et al. Improved survival with simendan after experimental myocardial infarction in rats. Eur J Pharmacol 2001; 419: 243–248. [DOI] [PubMed] [Google Scholar]
- 20.Louhelainen M, Vahtola E, Kaheinen P, et al. Effects of levosimendan on cardiac remodelling and cardiomyocyte apoptosis in hypertensive Dahl/Rapp rats. Br J Pharmacol 2007; 150: 851–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maytin M, Colucci WS. Cardioprotection: a new paradigm in the management of acute heart failure syndromes. Am J Cardiol 2005; 96: 26G–31G. [DOI] [PubMed] [Google Scholar]
- 22.Barisin S, Djuzel V, Barisin A, et al. Levosimendan reverses right-heart failure in a 51-year-old patient after heart transplantation. Wien Klin Wochenschr 2014; 126: 495–499. [DOI] [PubMed] [Google Scholar]
- 23.Beiras-Fernandez A, Weis F, Fuchs H, et al. Levosimendan treatment after primary organ failure in heart transplantation: a direct way to recovery? Transplantation 2006; 82: 1101–1103. [DOI] [PubMed] [Google Scholar]
- 24.Beiras-Fernandez A, Weis FC, Kur F, et al. Primary graft failure and Ca2+ sensitizers after heart transplantation. Transpl Proc 2008; 40: 951–952. [DOI] [PubMed] [Google Scholar]
- 25.Weis F, Beiras-Fernandez A, Kaczmarek I, et al. Levosimendan: a new therapeutic option in the treatment of primary graft dysfunction after heart transplantation. J Heart Lung Transpl 2009; 28: 501–504. [DOI] [PubMed] [Google Scholar]
- 26.Knezevic I, Poglajen G, Hrovat E, et al. The effects of levosimendan on renal function early after heart transplantation: results from a pilot randomized trial. Clin Transplant 2014; 28: 1105–1111. [DOI] [PubMed] [Google Scholar]