Short abstract
Objective
It remains unknown whether insulin-like growth factor-1 (IGF-1) can attenuate myocardial ischemia/reperfusion (I/R) injury in vivo by activating the phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) pathway. This study investigated the possible interaction of IGF-1 with the PI3K/Akt pathway in cardioprotection against in vivo myocardial I/R injury in rats.
Methods
We established a myocardial I/R model in rats through left anterior descending artery ligation for 40 minutes followed by 6 hours reperfusion. The PI3K/Akt inhibitor, LY294002 (0.3 mg/kg), was injected through the caudal vein 30 minutes before myocardial ischemia, and IGF-1 (1 µg/kg or 5 µg/kg) was injected through the caudal vein 10 minutes before myocardial ischemia.
Results
IGF-1 treatment decreased myocardial infarct size; myocardial cell apoptosis; and serum lactate dehydrogenase, creatine kinase MB, and cardiac troponin I levels in rats with myocardial I/R in vivo. Moreover, IGF-1 treatment led to significant increases in expression levels of p-Akt (Ser473) and B cell lymphoma 2, while reducing expression levels of caspase-9 mRNA and cleaved caspase-9 protein in rats with myocardial I/R. However, pretreatment with LY294002 significantly reduced the cardioprotective effects of IGF-1.
Conclusion
Treatment with IGF-1 may confer cardiac protection against in vivo myocardial I/R injury via the PI3K/Akt pathway in rats.
Keywords: PI3K/Akt pathway, IGF-1, cardioprotection, myocardial I/R injury, caspase 9, troponin I, creatine kinase, apoptosis, Bcl-2, lactate dehydrogenase
Introduction
Cardiac ischemia/reperfusion (I/R) injury occurs in various cardiovascular diseases and therapies.1,2 Although early myocardial reperfusion is regarded as the most effective strategy to limit the size of a myocardial infarct and reduce mortality, restoration of blood flow to the ischemic myocardium induces I/R injury that may further damage the myocardium.3–5 Therefore, management of myocardial I/R injury may help to improve outcomes in patients with ischemic cardiomyopathy.6,7
Insulin-like growth factor-1 (IGF-1) is a multifunctional polypeptide growth factor—produced primarily by the liver—that plays important roles in regulating cell survival, proliferation, metabolism, and differentiation in several cell types.8,9 The biological functions of IGF-1 are mediated mainly through its binding of the plasma membrane IGF-1 receptor, which is widely expressed in various tissues.10–12 Activation of the IGF-1 receptor has been shown to attenuate I/R-induced cell death by activating the intracellular phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway, which is involved in the regulation of cell survival and can confer protection against I/R injury in various organs.13–16 Akt is a downstream phosphorylation target of PI3K, and is thus an important mediator of cell survival. The activation of PI3K—triggered by IGF-1—results in Akt phosphorylation and activation, which contributes significantly to improved cell survival.9,13 LY294002, a specific PI3K/Akt inhibitor, can inactivate PI3K by competitively inhibiting the ATP binding site in the PI3K subunit, thereby inhibiting Akt phosphorylation and blocking the IGF-1-triggered PI3K/Akt signaling pathway.17,18
IGF-1 is highly cardioprotective in the context of I/R, but the mechanism underlying its cardioprotective effects is not well-known.19,20 Thus far, few studies have reported that the PI3K/Akt pathway is involved in IGF-1 cardioprotection against myocardial I/R injury; all of those studies were conducted in isolated cardiomyocytes or in isolated Langendorff-perfused hearts,21–26 but not in vivo. It remains unknown whether IGF-1 can also attenuate myocardial I/R injury in vivo through activation of the PI3K/Akt pathway. Thus, in the present study, we aimed to elucidate the possible interaction of IGF-1 with the PI3K/Akt pathway in cardioprotection against in vivo myocardial I/R injury in a rat model.
Materials and methods
Animals and study groups
Male Sprague-Dawley rats (8–10 weeks of age), weighing 250 to 300 g, were obtained from the Laboratory Animal Center of the Southern Medical University (Guangzhou, China). This study was approved by the Institutional Animal Care and Use Committee of Sun Yat-Sen University (IACUC-DB-16-1105).
Forty-eight rats were randomly assigned to six experimental groups (n = 8 per group): (1) Sham, in which the suture was passed through the left anterior descending artery (LAD) and was not tied; (2) I/R, in which rats experienced 40 minutes of myocardial ischemia followed by 6 hours reperfusion; (3) L-IGF-1 + I/R, in which a low dose of IGF-1 (1 µg/kg) was administered 10 minutes before myocardial ischemia; (4) LY + L-IGF-1 + I/R, in which LY294002 (0.3 mg/kg) was administered 30 minutes before myocardial ischemia and a low dose of IGF-1 (1 µg/kg) was administered 10 minutes before myocardial ischemia; (5) H-IGF-1 + I/R, in which a high dose of IGF-1 (5 µg/kg) was administered 10 minutes before myocardial ischemia; (6) LY + H-IGF-1 + I/R, in which LY294002 (0.3 mg/kg) was administered 30 minutes before myocardial ischemia and a high dose of IGF-1 (5 µg/kg) was administered 10 minutes before myocardial ischemia. In the present study, the specific PI3K/Akt inhibitor LY294002 (Cell Signaling Technology, Danvers, MA, USA) and recombinant human IGF-1 (Cell Signaling Technology) were injected through the caudal vein before myocardial ischemia at the specified time points.
Myocardial I/R model
Myocardial I/R injury was induced by ligating the LAD.27 Rats were anaesthetized with 10% chloral hydrate solution (3 ml/kg) by intraperitoneal injection. Following left thoracotomy, a 6-0 silk suture slipknot was tied around the LAD at 2 to 3 mm from its origin. Before LAD occlusion, electrocardiography was used to assess the S-T segment at baseline. The S-T segment became elevated throughout ischemia, compared with baseline. Myocardial ischemia was confirmed by visual cyanosis and immediate S-T segment elevation on electrocardiography. After 40 minutes of ischemia, reperfusion was allowed for 6 hours by releasing the slipknot. Sham group rats were subjected to the same surgical procedures, except that the suture around the LAD was not tied. At the completion of reperfusion, rats were sacrificed by an overdose with an intraperitoneal injection of 10% chloral hydrate solution (9 ml/kg), and the anterior wall of the left ventricular myocardium was obtained for further analysis.
Measurement of myocardial infarct size
After 6 hours reperfusion (described in the Myocardial I/R model subsection), the LAD was re-occluded and 2 ml of 3% Evans blue (Sigma-Aldrich, St. Louis, MO, USA) was injected via the jugular vein to delineate the area at risk (AAR). The AAR was the area not stained by Evans blue.28 Subsequently, rats were sacrificed by overdose with an intraperitoneal injection of 10% chloral hydrate (9 ml/kg), as described above; the heart was rapidly excised, washed with 0.9% saline, frozen at −20°C for 30 minutes, and then cut into five transverse slices of equal thickness (2 mm per slice) from the apex to the base. The slices were incubated at 37°C in 1% triphenyltetrazolium chloride (TTC) (TCI, Tokyo, Japan) for 10 minutes in the dark, then fixed in 4% paraformaldehyde (TCI) for 24 hours and photographed; images were quantified by planimetry using ImageJ software (version 1.45, National Institutes of Health, Bethesda, MD, USA) in a blinded manner. After TTC staining, the infarct area (Inf) was white, whereas the viable myocardium in the AAR was red. Myocardial infarct size was expressed as the percentage of infarct area (Inf) relative to the AAR (Inf/AAR × 100%).
Measurements of serum lactate dehydrogenase (LDH), creatine kinase MB (CK-MB), and cardiac troponin I (cTnI) levels
The levels of LDH, CK-MB, and cTnI in cardiomyocytes are higher than those in serum.29,30 During myocardial I/R, the cardiomyocyte LDH, CK-MB, and cTnI leak into the blood, resulting in abnormally high LDH, CK-MB, and cTnI levels in serum.31 In the present study, the serum levels of LDH, CK-MB, and cTnI were used as indicators of myocardial I/R injury. After 6 hours reperfusion, blood samples (2 ml) were obtained from the inferior vena cava, stored for 12 hours at 4°C, and then centrifuged at 2500 × g for 15 minutes at 4°C; the resulting supernatant was collected and stored at −80°C until further analysis. Serum levels of LDH, CK-MB, and cTnI were measured using commercial kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China), in accordance with the manufacturer’s instructions.
TUNEL assay
Myocardial apoptosis was determined by the terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) assay using an in situ apoptosis detection kit (TaKaRa Bio, Dalian, China). The anterior wall of the left ventricular myocardium was used, cut into 6-μm-thick heart tissue transverse sections. Apoptotic nuclei in the sections were identified by TUNEL staining (green); all nuclei were counterstained with 4, 6-diamino-2-phenylindole (DAPI; Beyotime, Nantong, China) (blue). For each rat, six fields per section were randomly chosen and then photographed at 400× magnification using a fluorescence microscope (DMI4000B, Leica, Wetzlar, Germany). The apoptotic index was expressed as the proportion of apoptotic myocytes relative to the total number of myocytes, as determined using ImageJ software (version 1.45, National Institutes of Health); these analyses were performed in a blinded manner.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the anterior wall of the left ventricular myocardium using Trizol reagent (Generay Biotech, Shanghai, China) and then reverse transcribed into cDNA using a PrimeScript™ RT Master Mix Kit (TaKaRa Bio), in accordance with the manufacturers’ instructions. qRT-PCR was performed on the ABI 7500 Fast Real-Time PCR system (Thermo Fisher Scientific, Waltham, MA, USA) using the SYBR Fast qPCR Mix kit (TaKaRa Bio). Melting curve analysis was performed to verify the authenticity of qRT-PCR amplification. Primers for real-time PCR were synthesized by Generay Biotech, with the following sequences: Bcl-2, forward 5′-CTCAGGCTGGAAGGAGAAGAT-3′ and reverse 5′-AGAGGGGCTACGAGTGGGATA-3′; caspase-9, forward 5′-AGGGTGTATGCCATATCTGCA-3′ and reverse 5′-TGGGACTCAAATCAAAGGAGC-3′; and GAPDH, forward 5′-GGAAAGCTGTGGCGTGAT-3′, and reverse 5′-AAGGTGGAAGAATGGGAGTT-3′. GAPDH was used as the internal control. Relative mRNA expression levels of Bcl-2 and caspase-9 were calculated using the 2−ΔΔCt method.32
Western blot analysis
Total protein was extracted from the anterior wall of the left ventricular myocardium using RIPA lysis buffer (Beyotime, Nantong, China) supplied with 1% phosphatase protein inhibitors (Beyotime) and 1 mM phenylmethanesulfonyl fluoride (Beyotime), in accordance with the manufacturer’s instructions; protein concentrations were determined using the bicinchoninic acid protein assay kit (Beyotime). Protein samples were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% skim milk in Tris-buffered saline with 0.1% Tween 20 (TBST) at room temperature for 1 hour. They were then incubated overnight at 4°C (in TBST buffer containing 5% skim milk) with primary antibodies against Bcl-2 (1:5000; Cat. No. 625509, R&D Systems, Minneapolis, MN, USA), Akt (1:2000; Cat. No. 2920, Cell Signaling Technology), p-Akt (Ser473) (1:1000; Cat. No. 4051, Cell Signaling Technology), cleaved caspase-9 (1:1000; Cat. No. 9508, Cell Signaling Technology), and β-actin (1:1000; Cat. No. AF0003, Beyotime; β-actin was used as a loading control). Subsequently, membranes were washed four times (5 minutes each) in TBST buffer and then incubated with horseradish peroxidase-conjugated secondary antibody (1:1000; Cat. No. A0216, Beyotime) for 1 hour at room temperature. Membranes were again washed four times (5 minutes each) in TBST buffer and protein bands were detected by enhanced chemiluminescence (Beyotime). Immunoblot band intensity was measured using ImageJ software (version 1.45, National Institutes of Health). The results were expressed as fold change relative to expression levels in the Sham group.
Statistical analysis
All data are shown as means ± standard deviations. Student’s t-test or one-way analysis of variance with a Student–Newman–Keuls post hoc test was used to evaluate differences between groups. Statistical analyses were performed using SPSS software (version 17.0, SPSS, Inc., Chicago, IL, USA). Differences with P < 0.05 were considered to be statistically significant.
Results
IGF-1 attenuates myocardial I/R injury by activating the PI3K/Akt pathway
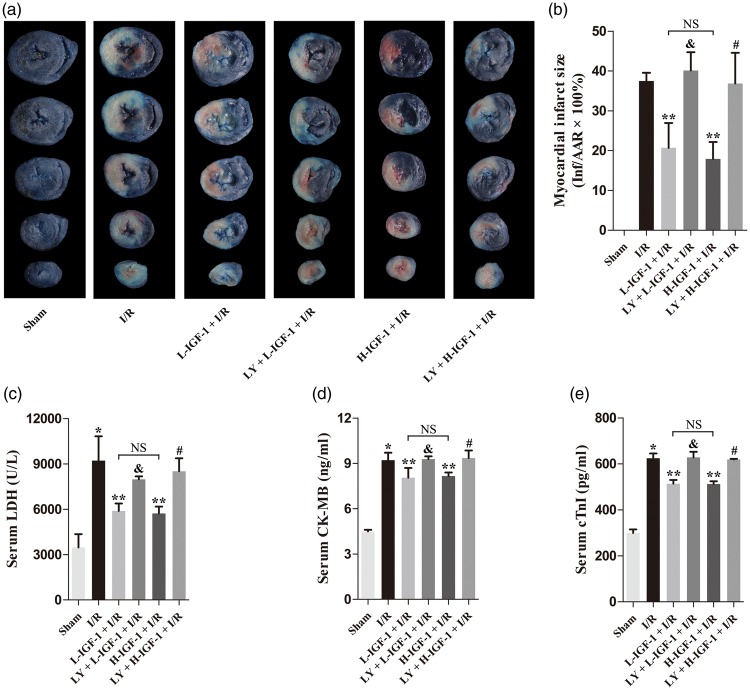
Myocardial infarction was not detectable in the Sham group (Figure 1a, b). Compared with the I/R group, administration of IGF-1 to rats with myocardial I/R significantly reduced myocardial infarct size in both L-IGF-1 + I/R and H-IGF-1 + I/R groups (P < 0.05; Figure 1a, b). Next, the PI3K/Akt inhibitor, LY294002, was used to further investigate the roles of the PI3K/Akt signaling pathway in IGF-1 cardioprotection against myocardial I/R injury. Notably, pretreatment with LY294002 abrogated the infarct-limiting effect of IGF-1 (P < 0.05; Figure 1a, b). In addition, we found no significant difference in myocardial infarct size between the L-IGF-1 + I/R and H-IGF-1 + I/R groups (Figure 1a, b).
Figure 1.
Insulin-like growth factor-1 (IGF-1) attenuates myocardial ischemia/reperfusion (I/R) injury through activation of the PI3K/Akt pathway. (a) Representative photographs of triphenyltetrazolium chloride (TTC) and Evans blue-stained slices of rat heart. Blue color indicates nonischemic area, the area at risk (AAR) contains no blue dye, white color indicates infarct area (Inf), and red color indicates uninfarcted area. (b) Myocardial infarct size expressed as percentage of infarct area (Inf) over the AAR. (c) Serum lactate dehydrogenase (LDH) level. (d) Serum creatine kinase MB (CK-MB) level. (e) Serum cardiac troponin I (cTnI) level. Data are presented as means ± standard deviations (n = 4). *P < 0.05 vs. Sham group; **P < 0.05 vs. I/R group; &P < 0.05 vs. L-IGF-1 + I/R group; #P < 0.05 vs. H-IGF-1 + I/R group; NS, not significant.
After 6 hours of reperfusion, serum levels of LDH, CK-MB, and cTnI were significantly higher in the I/R group than in the Sham group (P < 0.05; Figure 1c–e). Compared with the levels in the I/R group, serum levels of LDH, CK-MB, and cTnI were decreased in the L-IGF-1 + I/R and H-IGF-1 + I/R groups (P < 0.05; Figure 1c–e). These effects of IGF-1 were blocked by pretreatment with LY294002 (P < 0.05; Figure 1c–e). No significant differences were found in serum levels of LDH, CK-MB, or cTnI between the L-IGF-1 + I/R and H-IGF-1 + I/R groups (Figure 1c–e).
IGF-1 reduces myocardial cell apoptosis by activating the PI3K/Akt pathway
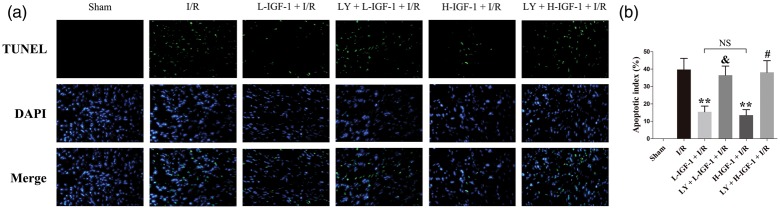
The TUNEL assay was used to compare myocardial apoptosis among the rats. In the Sham group, apoptotic myocardial cells were not detectable (Figure 2a, b). IGF-1 treatment significantly reduced the apoptotic indices of myocardial cells in the L-IGF-1 + I/R and H-IGF-1 + I/R groups, compared with the apoptotic index in the I/R group (P < 0.05; Figure 2a, b). The apoptotic indices of myocardial cells did not differ between the L-IGF-1 + I/R and H-IGF-1 + I/R groups (Figure 2a, b). Moreover, pretreatment with LY294002 significantly inhibited the anti-apoptotic effect of IGF-1 (P < 0.05; Figure 2a, b).
Figure 2.
Insulin-like growth factor-1 (IGF-1) reduces myocardial cell apoptosis through activation of the PI3K/Akt pathway. (a) Representative images of immunofluorescent TUNEL staining (green color) and 4, 6-diamino-2-phenylindole staining (DAPI, blue color) taken with a fluorescence microscope (magnification, 400×). (b) Apoptotic index, expressed as the proportion of apoptotic myocytes relative to the total number of myocytes. Data are presented as means ± standard deviations (n = 4). **P < 0.05 vs. I/R group; &P < 0.05 vs. L-IGF-1 + I/R group; #P < 0.05 vs. H-IGF-1 + I/R group; NS, not significant.
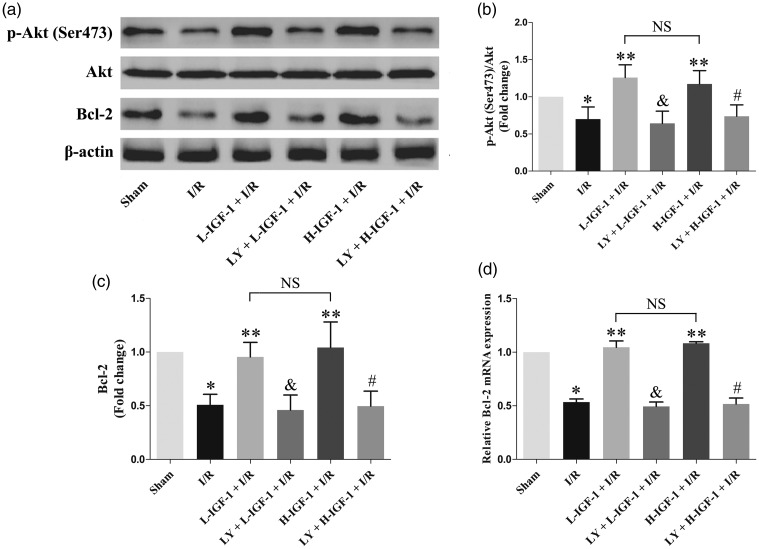
IGF-1 increases the protein expression level of p-Akt (Ser473) and the protein and mRNA expression levels of Bcl-2 in rats with myocardial I/R
The results of western blot and qRT-PCR analyses suggested that the protein expression level of p-Akt (Ser473) and the protein and mRNA expression levels of Bcl-2 in the I/R group were lower than those in the Sham group (P < 0.05; Figure 3a–d). Treatment with IGF-1 significantly increased the protein expression level of p-Akt (Ser473), as well as the protein and mRNA expression levels of Bcl-2 in rats with myocardial I/R (P < 0.05; Figure 3a–d). However, pretreatment with LY294002 significantly inhibited the effects of IGF-1, thus decreasing the protein expression level of p-Akt (Ser473), as well as the protein and mRNA expression levels of Bcl-2 ( P < 0.05; Figure 3a–d). There were no significant differences in the protein expression level of p-Akt (Ser473) or in the protein and mRNA expression levels of Bcl-2 between L-IGF-1 + I/R and H-IGF-1 + I/R groups (Figure 3a–d).
Figure 3.
Insulin-like growth factor-1 (IGF-1) increases p-Akt (Ser473) protein expression and Bcl-2 protein and mRNA expression in rats with myocardial ischemia/reperfusion (I/R). (a) Representative western blots of p-Akt (Ser473) and Bcl-2 protein expression. (b) Quantification of p-Akt (Ser473) protein expression. (c) Quantification of Bcl-2 protein expression. (d) Quantification of relative Bcl-2 mRNA expression, as detected by qRT-PCR. Data are presented as means ± standard deviations (n = 4). *P < 0.05 vs. Sham group; **P < 0.05 vs. I/R group; &P < 0.05 vs. L-IGF-1 + I/R group; #P < 0.05 vs. H-IGF-1 + I/R group; NS, not significant.
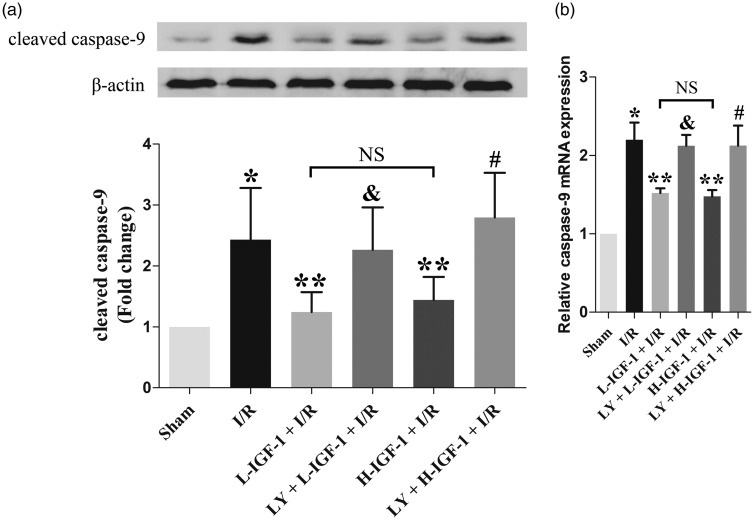
IGF-1 reduces the protein expression level of cleaved caspase-9 and the mRNA expression level of caspase-9 in rats with myocardial I/R
Western blot and qRT-PCR analyses showed that the protein expression level of cleaved caspase-9 and the mRNA expression level of caspase-9 were higher in the I/R group than in the Sham group (P < 0.05; Figure 4a, b). Compared with the levels in the I/R group, treatment with IGF-1 significantly lowered the protein expression level of cleaved caspase-9 and the mRNA expression level of caspase-9 in the L-IGF-1 + I/R and H-IGF-1 + I/R groups (P < 0.05; Figure 4a, b). Notably, IGF-1-induced reduction of the cleaved caspase-9 protein expression level and the caspase-9 mRNA expression level was reversed by pretreatment with LY294002 (P < 0.05; Figure 4a, b). No significant differences were found in the protein expression level of cleaved caspase-9 or the mRNA expression level of caspase-9 between L-IGF-1 + I/R and H-IGF-1 + I/R groups (Figure 4a, b).
Figure 4.
Insulin-like growth factor-1 (IGF-1) suppresses cleaved caspase-9 protein expression and caspase-9 mRNA expression in rats with myocardial ischemia/reperfusion (I/R). (a) Representative western blot and corresponding quantification of cleaved caspase-9 protein expression. (b) Quantification of relative caspase-9 mRNA expression, as detected by qRT-PCR. Data are presented as means ± standard deviations (n = 4). *P < 0.05 vs. Sham group; **P < 0.05 vs. I/R group; &P < 0.05 vs. L-IGF-1 + I/R group; #P < 0.05 vs. H-IGF-1 + I/R group; NS, not significant.
Discussion
In the past few decades, the beneficial roles of PI3K/Akt pathway in IGF-1 cardioprotection against myocardial I/R injury have been reported in isolated cardiomyocytes or in isolated perfused hearts,21–26 but not using in vivo models. The present study was performed to elucidate the possible interaction of IGF-1 with the PI3K/Akt pathway in cardioprotection against myocardial I/R injury in vivo. Using an in vivo rat model of myocardial I/R injury, we observed that 40 minutes ischemia followed by 6 hours reperfusion caused myocardial injury—evidenced by increased myocardial infarct size, as well as increased serum levels of LDH, CK-MB, and cTnI. These effects were significantly attenuated by IGF-1 administration 10 minutes prior to myocardial ischemia. However, pretreatment with the PI3K/Akt inhibitor, LY294002, significantly abrogated the protective effects of IGF-1. Our results indicate that IGF-1 can protect ischemic myocardium from I/R injury by activating the PI3K/Akt pathway in vivo.
The PI3K/Akt pathway is one of the most important survival signaling pathways, which promotes cell survival by inhibiting cell apoptosis. Activation of PI3K catalyzes the production of phosphatidylinositol (3, 4, 5)-trisphosphate, which accumulates at the membrane and activates Akt by phosphorylation.33,34 p-Akt (Ser473) then increases the expression of the anti-apoptotic protein, Bcl-2, and mediates the phosphorylation and inhibition of the pro-apoptotic protein, caspase-9; these functions of p-Akt (Ser473) appear to be closely associated with PI3K-mediated modulation of cell survival.35,36 As an inhibitor of PI3K, LY294002 can inhibit the phosphorylation and activation of Akt.18 In the current study, we observed that treatment with IGF-1 significantly increased the protein expression level of p-Akt (Ser473) in rats with myocardial I/R, this increase in p-Akt (Ser473) could be eliminated by treatment with LY294002. Moreover, we found that IGF-1 treatment significantly increased the protein and mRNA expression levels of Bcl-2, whereas it reduced the protein expression level of cleaved caspase-9 and the mRNA expression level of caspase-9 in rats with myocardial I/R. Bcl-2 and caspase-9 are closely related to apoptosis and are downstream effector signaling molecules in the PI3K/Akt pathway; Bcl-2 is an anti-apoptotic protein, whereas caspase-9 is a pro-apoptotic protein (cleaved caspase-9 is its activated form).37–39 Furthermore, the TUNEL assay demonstrated that, in rats with myocardial I/R, treatment with 1 µg/kg or 5 µg/kg IGF-1 significantly decreased myocardial apoptosis. Thus, the results showed that IGF-1 could protect myocardial cells from I/R injury by inhibiting myocardial apoptosis in vivo. However, the anti-apoptotic effects of IGF-1 were reversed by pretreatment with the PI3K/Akt inhibitor, LY294002, suggesting that the anti-apoptotic effects of IGF-1 against myocardial I/R injury were mediated by activation of the PI3K/Akt pathway.
Furthermore, we observed that serum LDH, CK-MB, and cTnI levels; infarct size; and apoptotic index did not differ in rats with myocardial I/R in vivo, based on the doses of IGF-1 used in this study (1 µg/kg and 5 µg/kg). We also found that the expression levels of p-Akt (Ser473), Bcl-2, and caspase-9/cleaved caspase-9 did not differ based on the dose of IGF-1 in rats with I/R. These results suggested that the protective effects of 1 µg/kg IGF-1 against myocardial I/R injury were the same as those of IGF-1 at 5 µg/kg. Currently, there is insufficient data to explain why protective effects were similar regardless of the dose of IGF-1. Therefore, further studies are needed to determine whether the protective effect of IGF-1 against myocardial I/R injury is correlated in some manner with the dose of IGF-1.
In summary, our study demonstrated that treatment with IGF-1 could confer cardioprotection against myocardial I/R injury through the PI3K/Akt pathway in a rat model in vivo. Although further studies are needed, administration of IGF-1 might be an effective therapeutic strategy for the treatment of ischemic cardiomyopathy.
Declaration of conflicting interest
The authors declare that there is no conflict of interest.
Funding
The study was supported by grants from the National Natural Science Foundation of China (NO. 81600396), and the Natural Science Foundation of Guangdong Province (NO. 2016A030310190).
References
- 1.Davani EY, Brumme Z, Singhera GK, et al. Insulin-like growth factor-1 protects ischemic murine myocardium from ischemia/reperfusion associated injury. Crit Care 2003; 7: R176–R183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gonzalez Arbelaez LF, Ciocci Pardo A, Fantinelli JC, et al. Cardioprotection and natural polyphenols: an update of clinical and experimental studies. Food Funct 2018; 9: 6129–6145. [DOI] [PubMed] [Google Scholar]
- 3.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med 2007; 357: 1121–1135. [DOI] [PubMed] [Google Scholar]
- 4.Eltzschig HK, Eckle T. Ischemia and reperfusion–from mechanism to translation. Nat Med 2011; 17: 1391–1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong Q, Dai L, Wang Y, et al. HSPA12B attenuated acute myocardial ischemia/reperfusion injury via maintaining endothelial integrity in a PI3K/Akt/mTOR-dependent mechanism. Sci Rep 2016; 6: 33636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ibanez B, Heusch G, Ovize M, et al. Evolving therapies for myocardial ischemia/reperfusion injury. J Am Coll Cardiol 2015; 65: 1454–1471. [DOI] [PubMed] [Google Scholar]
- 7.Koeppen M, Lee JW, Seo SW, et al. Hypoxia-inducible factor 2-alpha-dependent induction of amphiregulin dampens myocardial ischemia-reperfusion injury. Nat Commun 2018; 9: 816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dai W, Kloner RA. Cardioprotection of insulin-like growth factor-1 during reperfusion therapy: what is the underlying mechanism or mechanisms? Circ Cardiovasc Interv 2011; 4: 311–313. [DOI] [PubMed] [Google Scholar]
- 9.Troncoso R, Ibarra C, Vicencio JM, et al. New insights into IGF-1 signaling in the heart. Trends Endocrinol Metab 2014; 25: 128–137. [DOI] [PubMed] [Google Scholar]
- 10.Kim C, Park S. IGF-1 protects SH-SY5Y cells against MPP(+)-induced apoptosis via PI3K/PDK-1/Akt pathway. Endocr Connect 2018; 7: 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lara-Diaz VJ, Castilla-Cortazar I, Martin-Estal I, et al. IGF-1 modulates gene expression of proteins involved in inflammation, cytoskeleton, and liver architecture. J Physiol Biochem 2017; 73: 245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salzano A, Marra AM, D'Assante R, et al. Growth hormone therapy in heart failure. Heart Fail Clin 2018; 14: 501–515. [DOI] [PubMed] [Google Scholar]
- 13.Westermeier F, Bustamante M, Pavez M, et al. Novel players in cardioprotection: insulin like growth factor-1, angiotensin-(1-7) and angiotensin-(1-9). Pharmacol Res 2015; 101: 41–55. [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Zhang JQ, Meng FM, et al. Dexmedetomidine protects against lung ischemia-reperfusion injury by the PI3K/Akt/HIF-1alpha signaling pathway. J Anesth 2016; 30: 826–833. [DOI] [PubMed] [Google Scholar]
- 15.Liu T, Zhang Q, Mo W, et al. The protective effects of shikonin on hepatic ischemia/reperfusion injury are mediated by the activation of the PI3K/Akt pathway. Sci Rep 2017; 7: 44785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu D, Shi J, Elmadhoun O, et al. Dihydrocapsaicin (DHC) enhances the hypothermia-induced neuroprotection following ischemic stroke via PI3K/Akt regulation in rat. Brain Res 2017; 1671: 18–25. [DOI] [PubMed] [Google Scholar]
- 17.Toledo LM, Lydon NB, Elbaum D. The structure-based design of ATP-site directed protein kinase inhibitors. Curr Med Chem 1999; 6: 775–805. [PubMed] [Google Scholar]
- 18.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat 2002; 5: 234–248. [DOI] [PubMed] [Google Scholar]
- 19.Tejada T, Tan L, Torres RA, et al. IGF-1 degradation by mouse mast cell protease 4 promotes cell death and adverse cardiac remodeling days after a myocardial infarction. Proc Natl Acad Sci U S A 2016; 113: 6949–6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suleiman MS, Singh RJ, Stewart CE. Apoptosis and the cardiac action of insulin-like growth factor I. Pharmacol Ther 2007; 114: 278–294. [DOI] [PubMed] [Google Scholar]
- 21.Matsui T, Li L, del Monte F, et al. Adenoviral gene transfer of activated phosphatidylinositol 3'-kinase and Akt inhibits apoptosis of hypoxic cardiomyocytes in vitro. Circulation 1999; 100: 2373–2379. [DOI] [PubMed] [Google Scholar]
- 22.Fujio Y, Nguyen T, Wencker D, et al. Akt promotes survival of cardiomyocytes in vitro and protects against ischemia-reperfusion injury in mouse heart. Circulation 2000; 101: 660–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mehrhof FB, Muller FU, Bergmann MW, et al. In cardiomyocyte hypoxia, insulin-like growth factor-I-induced antiapoptotic signaling requires phosphatidylinositol-3-OH-kinase-dependent and mitogen-activated protein kinase-dependent activation of the transcription factor cAMP response element-binding protein. Circulation 2001; 104: 2088–2094. [DOI] [PubMed] [Google Scholar]
- 24.Vivar R, Humeres C, Varela M, et al. Cardiac fibroblast death by ischemia/reperfusion is partially inhibited by IGF-1 through both PI3K/Akt and MEK-ERK pathways. Exp Mol Pathol 2012; 93: 1–7. [DOI] [PubMed] [Google Scholar]
- 25.Otani H, Yamamura T, Nakao Y, et al. Insulin-like growth factor-I improves recovery of cardiac performance during reperfusion in isolated rat heart by a wortmannin-sensitive mechanism. J Cardiovasc Pharmacol 2000; 35: 275–281. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita K, Kajstura J, Discher DJ, et al. Reperfusion-activated Akt kinase prevents apoptosis in transgenic mouse hearts overexpressing insulin-like growth factor-1. Circ Res 2001; 88: 609–614. [DOI] [PubMed] [Google Scholar]
- 27.Zhao Y, Zheng ZN, Pi YN, et al. Cardioprotective effects of transfusion of late-phase preconditioned plasma may be induced by activating the reperfusion injury salvage kinase pathway but not the survivor activating factor enhancement pathway in rats. Oxid Med Cell Longev 2017; 2017: 8526561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li J, Ren Y, Shi E, et al. Inhibition of the let-7 family microRNAs induces cardioprotection against ischemia-reperfusion injury in diabetic rats. Ann Thorac Surg 2016; 102: 829–835. [DOI] [PubMed] [Google Scholar]
- 29.Danese E and Montagnana M.. An historical approach to the diagnostic biomarkers of acute coronary syndrome. Ann Transl Med 2016; 4: 194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soetkamp D, Raedschelders K, Mastali M, et al. The continuing evolution of cardiac troponin I biomarker analysis: from protein to proteoform. Expert Rev Proteomics 2017; 14: 973–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun XQ, Chen S, Wang LF, et al. Total flavones of rhododendron simsii planch flower protect isolated rat heart from ischaemia-reperfusion injury and its mechanism of UTR-RhoA-ROCK pathway inhibition. J Pharm Pharmacol 2018; 70: 1713–1722. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 33.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell 2017; 169: 381–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghigo A, Li M. Phosphoinositide 3-kinase: friend and foe in cardiovascular disease. Front Pharmacol 2015; 6: 169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell 2007; 129: 1261–1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matsui T, Rosenzweig A. Convergent signal transduction pathways controlling cardiomyocyte survival and function: the role of PI 3-kinase and Akt. J Mol Cell Cardiol 2005; 38: 63–71. [DOI] [PubMed] [Google Scholar]
- 37.Riedl SJ, Shi Y. Molecular mechanisms of caspase regulation during apoptosis. Nat Rev Mol Cell Biol 2004; 5: 897–907. [DOI] [PubMed] [Google Scholar]
- 38.Galluzzi L, Vitale I, Aaronson SA, et al. Molecular mechanisms of cell death: recommendations of the nomenclature committee on cell death 2018. Cell Death Differ 2018; 25: 486–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gustafsson AB, Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol 2007; 292: C45–C51. [DOI] [PubMed] [Google Scholar]