Abstract
Mosquitoes transmit diseases such as dengue, chikungunya, Zika, and yellow fever to humans. Biological control methods are required for these insects because they can be environmentally friendlier, safer, and more cost-effective than chemical or physical methods currently available. The aim of this research is to identify fungi found in mosquito breeding containers that have the potential to control the population density of mosquitoes.
For the identification, water samples were taken from mosquito breeding containers situated in seven districts of Bangkok to obtain pure cultures. Deoxyribonucleic acid (DNA) was extracted from the cultures then sent for sequencing and analyzing. The results show that fourteen strains of fungi were isolated. The most common strain found was Aspergillus spp., which was present in 31 of the 78 fungi samples. The strains Metarhizium anisopliae and Penicilium citrinum were found to be interesting because they may have the potential to act as entomopathogenic fungi. The biological properties of these strains should be further investigated because they could help in the fight against mosquito-borne diseases.
Keywords: Microbiology, Zoology, Biological control, Entomopathogenic fungi, Mosquito
1. Introduction
Mosquitoes are small insects that carry arboviruses belonging to the following three families: Flaviviridae, which has been associated with dengue fever; Bunyaviridae, which has been linked to chikungunya; and Zika Togaviridae, which has been related to arthritis, encephalitis, and rubella [1, 2]. The Anopheles, Aedes and Culex genera, have been responsible for the majority of arbovirus transmission. Aedes aegypti is a highly anthropophilic species that has impacted significantly on public health by transmitting arboviruses such as dengue, Zika, chikungunya and yellow fever [3].
A reduction in dengue fever transmission could be achieved by controlling the population density of Aedes aegypti and ensuring it is maintained at a level below the critical threshold that signals an epidemic [4]. A variety of chemical, physical, and biological methods have been used to decrease the incidence of vector-borne diseases transmitted by mosquitoes [5]. However, the use of chemical insecticides has resulted in mosquitoes becoming resistant to treatment and beneficial non-target animals being harmed [6] Despite this, new nanobiotechnology materials such as selenium nanowires (Cr-SeNWs) [7, 8] and phytofabricated zinc oxide (ZnO) nanoparticles are still being developed [9].
In recent years, incidences of mosquito larvae becoming resistant to larvicide have been reported [10]. Therefore, attempts to exploit novel biocontrol agents and new strategies to control mosquito larvae are still necessary because they can minimize the use of synthetic chemical materials which often have negative effects on humans, animals, and the environment.
Biological control methods are environmentally friendlier, safer, and more cost-effective than chemical or physical methods that are time-consuming to implement at mosquito breeding sites [3, 4, 5]. Natural control techniques have utilized the larvae of Taxorhynchites, predators such as fish, copepods or parasitic organisms such as Bacillus thuringiensis israelensis, and Lysinibacillus sphaericus to target disease vectors [3]. As entomopathogenic fungi are mostly targeted towards adult mosquitoes, and because several different toxins produced during fungal infection are lethal to mosquitoes [11], selection pressure for resistance is likely to be less intense when compared to rapid-killing insecticides. Therefore, the evolution of fungus resistance is predicted to be much slower than the evolution of insecticide resistance [12]. The paucity of studies describing the effects of fungi on mosquito populations indicates further research is needed to determine the viability, infectivity, and persistence of fungal spores in the mosquito population [13].
Water collecting containers that serve as larval breeding habitats for mosquitoes, such as old tires, plastic cups, vases, flowerpots, water tanks, bowls pots, and coconut shells can be found throughout Bangkok. From the end of the monsoon season until the start of winter (collection period), these containers harbor still water that is suitable for egg-laying mosquitos [14]. In addition, heterotrophic fungi and pluricellular organisms that play an ecological role in degrading organic matter are important parasites that can occur, especially, in environments that have soil debris, insect remains, or dead leaves and plants [15]. Often, fungi share the same breeding habitat as mosquitoes.
The aim of this research is to identify fungi found in mosquito breeding containers from seven area of Bangkok that have the potential to control the population density of mosquitoes.
2. Materials and methods
2.1. Water sample collection
Water samples were collected from mosquito breeding containers situated in seven districts of Bangkok, Thailand (Bang Khen, Lat Krabang, Min Buri, Phra Khanong, Rat Burana, Taling Chan, and Thung Khru). The collection period was from the end of the monsoon season until the start of winter, when the temperature and relative humidity conditions were favorable for Aedes mosquitoes to breed. The samples were kept in 250 ml screw-capped sterilized bottles that were sealed and then stored at 4 °C prior to the isolation procedure [16, 17].
2.2. Isolation of filamentous fungi
First, the water samples were filtered through a 47 mm diameter sterile 0.45 μm membrane cellulose nitrate filter (Whatman, 7141-104; Whatman International Ltd, UK) using Millipore vacuum apparatus in a laminated flow chamber. Then, the filter was thoroughly washed for 30 seconds in a petri dish containing 4 ml of sterile water. Next, 1 ml of sterile water was cultured on Potato Dextrose Agar (PDA, Difco, BBL/USA) supplemented with 50 mg/l of Choloramphenicol and 25 mg/l of Gentamycine. Three replicates were used for each water sample to ensure the accuracy of the results. The agar plates were incubated at 28 °C ± 2 °C for 7–10 days and then monitored daily for the appearance of fungal colonies. Finally, the fungal isolates were subcultured separately to obtain pure cultures on PDA for identification [18, 19, 20, 21].
2.3. Identification of filamentous fungi
Colony descriptions were based on PDA observations under ambient daylight conditions. Microscopic observations and measurements were logged from preparations that were mounted in lactic acid. The morphological characteristics of the fungi were septation of hypha, formation, morphology, conidiophores, and the branching frequency of the fruiting bodies. Characteristics of the colony such as structure, size, and color were also observed and recorded. Filamentous fungi were identified at the generic level according to morphological characteristics described in fungal atlases.
2.4. The sequencing and analyzing of fungal strains by ITS
The DNA was extracted from the isolated fungi using a fungal DNA extraction kit (PrestoTM Mini gDNA Yeast kit; Geneaid, New Taipei City, Taiwan). Purified DNA specimens were amplified in a PCR reaction with universal primers of ITS1: 5′-TCCGTAGGTGAACCTGCGC-3′ and ITS4: 5′-TCCTCCGCTTATTGATATGC-3’ [21]. The PCR reaction contained 0.4 μM of ITS1 and ITS4 primers, 0.2 mM dNTPs, 1.5 mM MgCl2, 1xPCR buffer (50 mM KCl, 10 mM Tris-HCl), and 1.25 units of Taq DNA polymerase (New England Biolabs). The reaction was carried out in a BIO-RAD MJ Mini Personal Thermal Cycler. The cycle conditions consisted of a single initial denaturation at 94 °C for 5 min followed by 35 cycles at 94 °C for 30 sec, 50 °C for 30 sec, 72 °C for 1 min, and a final extension step at 72 °C for 5 min. The PCR product size was about 550 bp. It was sent to Solutions for Genetic Technologies in South Korea for sequencing.
First, the resulting sequences were checked and aligned using the BioEdit 7.0 sequence alignment editor (Isis Pharmaceuticals, Inc., Carlsbad, CA, USA). Then, they were compared with a homologous sequence stored on the GenBank database. Finally, the BLAST program, downloaded from the National Center for Biotechnology Information (NCBI) website, was used to evaluate the sequences.
3. Results
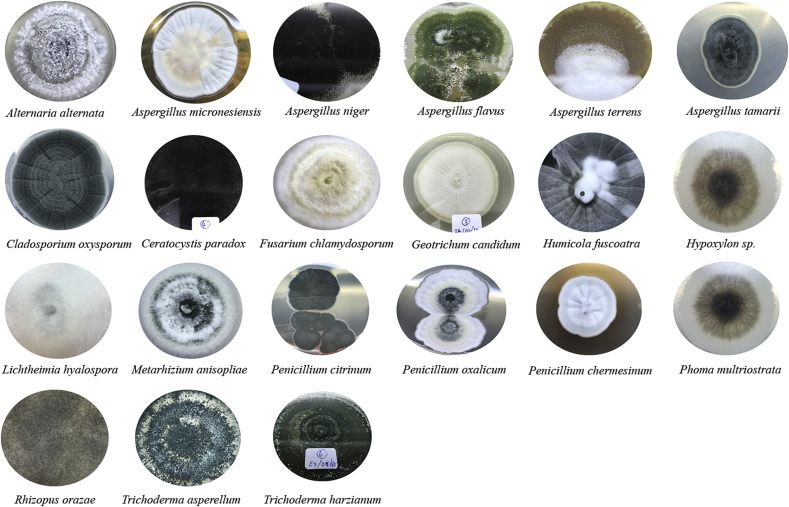
Seventy-eight fungal isolates were found in water samples collected from seven districts around Bangkok. The results of the rDNA-ITS sequencing show that 550 bp of the PCR product was sequenced from the isolated strains. The sequences were compared with the 18S rDNA BLAST sequence stored on the GenBank database using the BLAST tool. Twenty-one strains of fungi were isolated, as shown in Fig. 1.
Fig. 1.
Colony and conidia of isolated fungi on PDA agar medium.
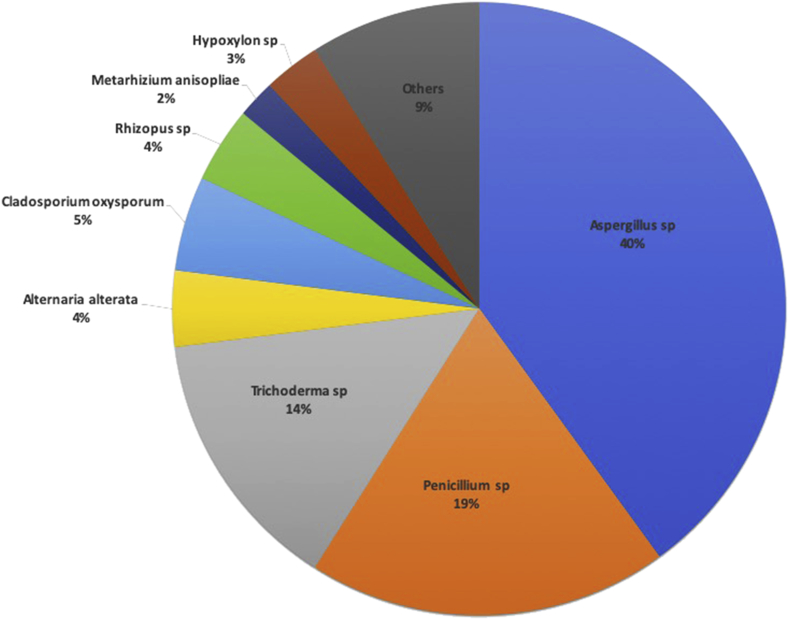
Aspergillus spp. was the most dominant of the isolated strains, it was found in all the districts of Bangkok that were sampled for this research, as shown in Table 1. The following six species of Aspergillus were identified: Aspergillus terrus, Aspergillus niger, Aspergillus oryzae, Aspergillus alterata, Aspergillus flavus, and Aspergillus tamarii. These species of Aspergillus consisted of 31 isolates. Six isolates of this strain accounted for 40 % of the total population, as shown in Fig. 2. Penicillium spp. was found in all the districts, except Min Buri. The following three species of Penicillium were identified: P. oxalicum, P. citrinum, and P. chermesinum. These species of Penicillium consisted of 15 isolates, which accounted for 19% of the total population. The following two species of Trichoderma spp. were identified: T. asperellum and T. harzianum. These species of Trichoderma consisted of 11 isolates, which accounted for 14 % of the total population. Four isolates of Cladosporium oxysporum were identified, which accounted for about 5 % of the total population. In addition, the following minor species with only one or two isolates were identified: Geotrichum candidum, Ceratocystis paradox, Fusarium chlamydosporum, Metarhizium anisopliae, Phuma multirostrata, Hypoxylon sp, Humicola fuscoatra, and Lichtheimia hyalospora.
Table 1.
The collection district, the genus of the isolated fungi and the number of isolated fungi identified.
| District | Genus | Isolate |
|---|---|---|
| Lat Krabang (total 22 isolates) | Aspergillus spp. | 5 isolates |
| Penicillium spp. | 4 isolates | |
| Rhizopus spp. | 1 isolate | |
| Trichoderma spp. | 5 isolates | |
| Geotrichum spp. | 1 isolate | |
| Cladosporium spp. | 3 isolates | |
| Fusarium spp. | 1 isolate | |
| Metarhizium spp. | 1 isolate | |
| Alternaria spp. | 1 isolate | |
| Rat Burana (total 6 isolates) | Aspergillus spp. | 1 isolate |
| Penicillium spp. | 3 isolates | |
| Trichoderma spp. | 1 isolate | |
| Cladosporium spp. | 1 isolate | |
| Thung Khru (total 20 isolates) | Aspergillus spp. | 10 isolates |
| Penicillium spp. | 3 isolates | |
| Trichoderma spp. | 1 isolate | |
| Cladosporium spp. | 3 isolates | |
| Ceratocystis spp. | 1 isolate | |
| Phoma spp. | 1 isolate | |
| Hypoxylon spp. | 1 isolate | |
| Phra Khanong (total 16 isolates) | Aspergillus spp. | 10 isolates |
| Penicillium spp. | 1 isolate | |
| Rhizopus spp. | 2 isolates | |
| Trichoderma spp. | 1 isolate | |
| Humicola spp. | 1 isolate | |
| Lichtheimia spp. | 1 isolate | |
| Taling Chan (total 8 isolates) | Aspergillus spp. | 2 isolates |
| Penicillium spp. | 3 isolates | |
| Trichoderma spp. | 2 isolates | |
| Cladosporium spp. | 1 isolate | |
| Min Buri (total 3 isolates) | Aspergillus spp. | 1 isolate |
| Rhizopus spp. | 1 isolate | |
| Trichoderma spp. | 1 isolate | |
| Bang Khen (total 3 isolates) | Aspergillus spp. | 2 isolates |
| Penicillium spp. | 1 isolate |
Fig. 2.
The percentage of isolated strains of fungi gathered from the water samples.
4. Discussion
Female Aedes mosquitoes breed in various artificial containers such as discarded tires and old flower pots. Uniquely, they lay their eggs in a variety of habitats to spread them around and increase their offsprings’ survival probability. This leads to an abundance of mosquitoes that have the potential to transmit dengue, chikungunya, Zika and other viral infections [22]. In their immature stages, they are non-selective filter-feeders of organic particles such as bacteria, protozoa, and fungi that are suspended in water [23]. Generally, fungi live in the alimentary canal of insects as symbiotic microorganisms, however, some fungi can attack and kill their hosts.
Monitoring the diversity of filamentous fungi as part of mosquito vector management could considerably benefit public health. This is the first study to isolate and identify fungi gathered from mosquito breeding containers situated in Bangkok. The results of the BLAST tests show that two strains of the entomopathogenic fungi Metarhizium anisopliae and Penicillium citrinum may have the potential to biologically control mosquitoes.
Prior research [24, 25] has shown that Aspiragilus spp., Rhizopus spp., Geotrichum spp., and Saccharomyces were present in the water of breeding containers and larval food. The results demonstrated that all the mosquito larvae were exposed to either oral or cuticle fungal infection [24]. Some fungi have developed symbiotic relationships or can be pathogenic due to toxin production [25]. There may be naturally occurring fungi at mosquito breeding sites that have the potential to kill insects. Research has been published that investigated the interactions between fungi and Culicidae larvae [26, 27]. Smittium morbosum were found to be lethal to mosquito larvae, and they have been known to invade ovaries and produce cysts which are "oviposited" by females instead of eggs, thus reducing the fertility in populations of those kinds of aquatic insects. The results suggested that mosquitoes might have the potential to serve as a type of biological control [28, 29, 30]. Entomopathogenic fungi have shown promise as biological control agents of mosquito vectors of tropical diseases [31].
M. anisopliae or Entomophthora anisopliae is a fungus found in nature throughout the world which has caused disease in various insects by acting as a parasitoid [13]. M. anisopliae has infected mosquitoes in the genera of Aedes and Culex [32, 33]. A histological study has reported that the ingestion of conidia eventually leads to blockage of the anatomic structure [34]. Conidia attach to the cuticle, germinate, and penetrate the cuticle. Once in the hemocoel, the mycelium grows throughout the host, forming hyphal bodies called blastospores. Death of the insect is often due to a combination of the action of fungal toxins, physical obstruction of blood circulation, nutrient depletion, and invasion of the organs. After the host has died, hyphae usually emerge from the cadaver and, under suitable abiotic conditions, conidia are produced on the exterior of the host. Then, they are dispersed by wind or water [35]. The gut of mosquito larvae was found to contain high concentrations of conidia, suggesting that it is usually ingested [33]. Experiments have shown that the mortality of mosquito larvae is related to stress-induced apoptosis [36]. Blastospores produced by the Metarhizium species have potential for field applications because they exhibit a high degree of host specificity and cause mortalities in mosquito species rapidly after ingestion [3].
P. citrinum, which was also isolated, may have the potential to control mosquitoes biologically. This fungus has been found to cause mortalities in Culex quinquefasciatus or the southern house mosquito [37], which is a carrier of the West Nile and Japanese encephalitis virus [38]. Light transmission electron micrographs showed that the fungal conidia were ingested by the larvae and deposited in the gut [39].
The results of this research show that the most abundant genus found was Aspergillus spp. This may be because these fungi grow as saprophytes on decaying vegetation, soil debris, insect remains, dead leaves or plants, and organic compost piles that are commonly found in artificial mosquito breeding containers. Six of the thirty isolated fungi were found to be of the P. citrinum species. Further research should evaluate whether a combination of M. anisopliae and P. citrinum could be used to manage mosquito populations.
Some bacteria such as Bacillus thuringiensis israelensis and Lysinibacillus sphaericus have been used to control mosquito populations. The toxins produced by Bti and Ls have the advantage of being highly effective as larvicides for various vector species of arboviruses. Bti has been broadly effective against mosquitoes in the genera of Aedes, Culex, and Anopheles, whereas the toxicity of Ls has been limited to Culex and Anopheles [40].
Research into the application of microorganism insecticides has found that mosquitofish or Gambusia affinis were compatible with the simultaneous use of other chemical or biological control tools [41]. An experiment in the rice fields of California, that mainly focused on Culex tarsalis, evaluated the release of G. affinis followed by treatment with Bacillus thuringiensis israelensis [42].
In the future, we hope to further investigate the isolates of M. anisopliae and P. citrinum to determine whether they have biological control properties that could be used to manage the mosquito populations.
5. Conclusions
In this research, several genera of isolated fungi were identified from water samples taken from artificial containers situated in districts around Bangkok, however, only two possible entomopathogenic fungi, M. anisopliae and P. citrinum, were identified. Our future research will focus on these fungi to determine whether their biological control properties could be used to manage mosquito populations.
Declarations
Author contribution statement
Ladawan Wasinpiyamongkol: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Panan Kanchanaphum: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by a grant from Research Institute of Rangsit University, Thailand (Grant no. 63/2560).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We would sincerely like to thank Mr. Stewart Miller for proofreading and language editing.
References
- 1.Dutta P., Prakash A., Bhattacharyya D.R., Khan S.A., Gogoi P.R., Sharma C.K., Mahanta J. Mosquito biodiversity of Dibru-Saikhowa biosphere reserve in Assam, India. J. Environ. Biol. 2010;31:695–699. [PubMed] [Google Scholar]
- 2.Marcondes C.B., Ximenes Mde F. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev. Soc. Bras. Med. Trop. 2016;49:4–10. doi: 10.1590/0037-8682-0220-2015. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y.S., Higgs S., Vanlandingham D.L. Biological control strategies for mosquito vectors of arboviruses. Insects. 2017;8(1) doi: 10.3390/insects8010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Focks D.A., Chadee D.D. Pupal survey: an epidemiologically significant surveillance method for Aedes aegypti: an example using data from Trinidad. Am. J. Trop. Med. Hyg. 1997;56:159–167. doi: 10.4269/ajtmh.1997.56.159. [DOI] [PubMed] [Google Scholar]
- 5.Smith D.S. Mentoring about vector-borne disease control. Perm. J. 2010;14(3):85. doi: 10.7812/tpp/10.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scholte E.J., Takken W., Knols B.G. Infection of adult Aedes aegypti and Ae. albopictus mosquitoes with the entomopathogenic fungus Metarhizium anisopliae. Acta Trop. 2007;102(3):151–158. doi: 10.1016/j.actatropica.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 7.Rekha R., Vaseeharan B., Vijayakumar S., Abinaya M., Govindarajan M., Alharbi N.S. Crustin-capped selenium nanowires against microbial pathogens and Japanese encephalitis mosquito vectors - insights on their toxicity and internalization. J. Trace Elem. Med. Biol. 2019;51:191–203. doi: 10.1016/j.jtemb.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Abinaya M., Vaseeharan B., Rekha R., Shanthini S., Govindarajan M., Alharbi N.S. Microbial exopolymer-capped selenium nanowires - towards new antibacterial, antibiofilm and arbovirus vector larvicides? J. Photochem. Photobiol., B. 2019;192:55–67. doi: 10.1016/j.jphotobiol.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 9.Yazhiniprabha M., Vaseeharan B., Sonawane A., Behera A. In vitro and in vivo toxicity assessment of phytofabricated ZnO nanoparticles showing bacteriostatic effect and larvicidal efficacy against Culex quinquefasciatus. J. Photochem. Photobiol., B. 2019;192:158–169. doi: 10.1016/j.jphotobiol.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 10.Sébastien M., Somsanith C., Phoutmany T., Paul T.B. Alternative insecticides for larval control of the dengue vector Aedes aegypti in Lao PDR: insecticide resistance and semi-field trial study. Parasites Vectors. 2018;11:616. doi: 10.1186/s13071-018-3187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knols B.G., Bukhari T., Farenhorst M. Entomopathogenic fungi as the next-generation control agents against malaria mosquitoes. Future Microbiol. 2010;5(3):339–341. doi: 10.2217/fmb.10.11. [DOI] [PubMed] [Google Scholar]
- 12.Mnyone L.L., Kirby M.J., Lwetoijera D.W., Mpingwa M.W., Simfukwe E.T., Knols B.G. Tools for delivering entomopathogenic fungi to malaria mosquitoes: effects of delivery surfaces on fungal efficacy and persistence. Malar. J. 2010;9:246. doi: 10.1186/1475-2875-9-246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharma V.P., Sharma R.C., Gautam A.S. Bio-environmental control of malaria in Nadiad, Kheda district, Gujarat. Indian J. Malariol. 1986;23:95–117. [PubMed] [Google Scholar]
- 14.Chareonviriyaphap T., Akratanakul P., Nettanomsak S., Huntamai S. Larval habitats and distribution patterns of Aedes aegypti (Linnaeus) and Aedes albopictus (Skuse), in Thailand. Southeast Asian J. Trop. Med. Public Health. 2003;34:529–535. [PubMed] [Google Scholar]
- 15.Standard Method 9060 . Standard Methods for the Examination of Water and Wastewater. American Water Works Association; Washington, DC: 1995. Sample collection procedure for microbiological examination of water samples. [Google Scholar]
- 16.Pitt J.I., Hocking A.D. Academic press; Sydney: 1997. Fungi and Food Spoilage. [Google Scholar]
- 17.Dogget M.S. Characterization of fungal biofilms within a municipal water distribution system. Appl. Environ. Microbiol. 2000;66:1249e1251. doi: 10.1128/aem.66.3.1249-1251.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Samson R.A., Hoekstra E.S., Frisvad J.C. Centraalbureau voor Schimmel cultures; Utrecht, The Netherlands: 2004. Introduction to Food and Airborne Fungi. [Google Scholar]
- 19.Pereira V.J., Fernandes D., Carvalho G., Benoliel M.J., San Romão M.V., Barreto Crespo M.T. Assessment of the presence and dynamics of fungi in drinking water sources using cultural and molecular methods. Water Res. 2010:4850–4859. doi: 10.1016/j.watres.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 20.Lichtwardt R.W. Springer-Verlag; New York: 1986. The Trichomycetes, Fungal Associates of Arthropods; p. 343p. [Google Scholar]
- 21.Lu Y., Chen C., Chen H., Zhang J., Chen W. Isolation and identification of endophytic fungi from actinidia macrosperma and investigation of their bioactivities. Evid. Based Complement Altern. Med. 2012:382742. doi: 10.1155/2012/382742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dejene G., Habte T., Teshome G.M., Meshesha B., Akalu M. Breeding sites of Aedes aegypti: potential dengue vectors in dire dawa, east Ethiopia. Interdiscipl. Perspec. Infect. Dis. 2015 doi: 10.1155/2015/706276. Article ID 706276, 8 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forattini O.P. Editora USP; São Paulo: 2002. Culicidologia, Médica; p. 860p. v 2. [Google Scholar]
- 24.Agarwala S.P., Sagar S.K., Sehgal S.S. Use of mycelial suspension and metabolites of Paecilomyces lilacinus (Fungi: Hyphomycetes) in control of Aedes aegypti larvae. J. Commun. Dis. 1999;31:193–196. [PubMed] [Google Scholar]
- 25.Pereira E.S., Ferreira R.L.M., Hamada N., Lichtwardt R.W. Trichomycete fungi (Zygomycota) associated with mosquito larvae (Diptera: Culicidae) in natural and artificial habitats in Manaus, AM Brazil. Neotrop. Entomol. 2005;34:325–329. [Google Scholar]
- 26.Misra J.K. Trichomycetes-fungi associated with arthropods: review and world literature. Symbiosis. 1998;24(2):179–220. [Google Scholar]
- 27.Tajedin L., Hashemi J., Abaei M.R., Hosseinpour L., Rafei F., Basseri H.R. Study on fungal flora in the midgut of the larva and adult of the different populations of the malaria vector Anopheles stephensi. Iran. J. Arthropod-Borne Dis. 2009;3:36–40. [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtwardt R.W. Springer-Verlag; New York: 1986. The Trichomycetes: Fungal Associates of Arthropods. [Google Scholar]
- 29.Lichtwardt R.W., Cafaro M.J., White M.M. University of Kansas; the Kansas City: 2001. The Trichomycetes: Fungal Associates of Arthropods.http://www.nhm.ku.edu/ Available at: [Google Scholar]
- 30.Lichtwardt R.W., White M.M., Cafaro M.J. Freshwater Trichomycetes and their arthropod hosts. Fungal Divers. Res. Ser. 2003;10:81–100. [Google Scholar]
- 31.Scholte E.J., Knol B.G.J., Samson R.A., Takken W. Entomopathogenic fungi for mosquito control: a review. J. Insect Sci. 2004;4:1–24. doi: 10.1093/jis/4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davise H.L. fourth ed. American Society for Microbiology Press; Washington, D.C.: 2002. Medically Important Fungi: A Guide to Identification. 409 pp., illustrated. [Google Scholar]
- 33.Colin K.C., Elizabeth M.J., David W.W. second ed., Kindle ed. Wiley-Blackwell; 2013. Identification of Pathogenic Fungi. [Google Scholar]
- 34.Frazzon A.P., da Silva Vaz Junior I., Masuda A., Schrank A., Vainstein M.H. In vitro assessment of Metarhizium anisopliae isolates to control the cattle tick Boophilus microplus. Vet. Parasitol. 2000;94:117–125. doi: 10.1016/s0304-4017(00)00368-x. [DOI] [PubMed] [Google Scholar]
- 35.Riba G., Keita A., Soares G.G., Jr., Ferron P. Comparative studies of Metarhizium anisopliae and Tolypocladium cylindrosporum as pathogens of mosquito larvae. J. Am. Mosq. Control Assoc. 1986;2:469–473. [PubMed] [Google Scholar]
- 36.Agudelo-Silva F., Wassink H. Infectivity of a Venezuelan strain of Metarhizium anisopliae to Aedes aegypti larvae. J. Invertebr. Pathol. 1984;43:435–436. doi: 10.1016/0022-2011(84)90094-6. Piasecka, J., Eastwood, D.C., 2013 Metarhizium anisopliae pathogenesis of mosquito larvae: a verdict of accidental death. PLoS One. 8:e81686. [DOI] [PubMed] [Google Scholar]
- 37.Maketon M., Amnuaykanjanasin A., Kaysorngup A. A rapid knockdown effect of Penicillium citrinum for control of the mosquito Culex quinquefasciatus in Thailand. World J. Microbiol. Biotechnol. 2014;30:727–736. doi: 10.1007/s11274-013-1500-4. [DOI] [PubMed] [Google Scholar]
- 38.Turell M.J. Members of the Culex pipiens complex as vectors of viruses. J. Am. Mosq. Control Assoc. 2012;28:123–126. doi: 10.2987/8756-971X-28.4.123. [DOI] [PubMed] [Google Scholar]
- 39.Scholte E.J., Knols B.G., Samson R.A., Takken W. Entomopathogenic fungi for mosquito control: a review. J. Insect Sci. 2004;4:19. doi: 10.1093/jis/4.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bellow T.S., Fisher T.W. Academic Press; San Diego, CA, USA: 1999. Handbook of Biological Control: Principle and Applications of Biological Control. [Google Scholar]
- 41.Bay E.C. Mosquito control by fish: a present-day appraisal. WHO Chron. 1967;21:415–423. [PubMed] [Google Scholar]
- 42.Haq S., Prasad R.N., Prasad H., Shukla R.P., Sharma V.P. Gambusia affinis: dispersal due to floods and its failure to colonize new water bodies in Shahjahanpur District (U.P.) Indian Malariol. 1992;29:113–118. [PubMed] [Google Scholar]