Abstract
Background
The systemic treatment of metastatic melanoma has improved considerably with the introduction of new, targeted substances and immune checkpoint inhibitors. This article presents treatment options for advanced inoperable melanoma and in the setting of adjuvant treatment after complete metastasectomy.
Methods
The data for analysis were derived from a selective literature search in PubMed and a search for systematic reviews in the Cochrane Library.
Results
Immune checkpoint inhibitors, which target the cytotoxic T-lymphocyte antigen or the “programmed death” (PD) receptor, activate T-cells and other immune cells, so that the body’s own immune system attacks the melanoma. In unselected patients, immune checkpoint inhibition using nivolumab improved overall survival compared with dacarbazine (hazard ratio [HR]: 0.42; P<0.001). The antibody pembrolizumab also led to better overall survival than ipilimumab (HR 0.68; P<0.001). Combination treatment with anti-CTLA-4 and anti-PD-1 antibodies improved overall survival even more than ipilimumab monotherapy, albeit at the cost of greater toxicity (HR 0.55; P<0.001). Another treatment approach aims to inhibit intracellular signal transduction in the melanoma cells. For patients with a BRAF-V66 mutation, combination treatments with BRAF/MEK inhibitors led to a rapid response in most cases (64–75%). In principle, the novel treatments are also effective in patients with cerebral metastases. In the adjuvant setting, both immune checkpoint inhibitors and BRAF/MEK inhibitors reduced the risk of recurrence by about 50%.
Conclusions
High-quality studies show that the new substances are clinically effective in the palliative and adjuvant treatment of melanoma.
Up until just a few years ago, the prognosis for metastatic cutaneous melanoma was bleak. Before the therapeutic advances that have been made since the antibody ipilimumab, which targets the cytotoxic lymphocyte antigen (CTLA-4), was licensed in 2001, the five-year survival rate was about 5% and overall survival seven to eight months. Prolonged survival in metastatic melanoma was first achieved when immune checkpoint inhibitors were clinically tested, which since then have been successfully used to treat a multitude of malignancies (1).
Method
We conducted a selective literature search in PubMed. We aimed to identify phase III trials of adjuvant and palliative therapy of metastatic cutaneous malignant melanoma that had been published between 2013 and April 2018. For studies of cerebral metastases, we included phase II trials. Furthermore, we searched for systematic reviews in the Cochrane Library.
Immune checkpoint inhibition
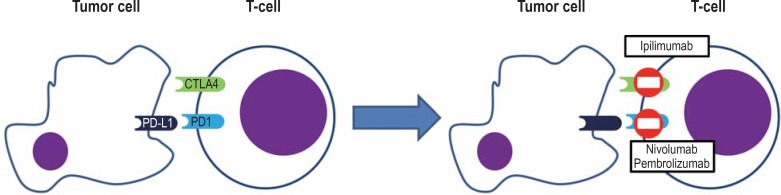
Immune checkpoints are defined as receptors and associated ligands that can modulate the immune reaction of T-cells but also other immune cells (figure 1). The first immune checkpoint with an inhibitory mechanism that could be successfully blocked in a therapeutically relevant setting is CTLA-4 (2). The antibody ipilimumab, which targets CTLA-4, results in enhanced stimulation and expansion of reactive T-cells and also suppresses the function of regulatory T-cells (3, 4).
Figure 1.
Mechanism of action of the immune checkpoint inhibitors
A clinical effect of the inhibition of CTLA-4 was confirmed in a phase III trial, in which ipilimumab was administered to patients with metastatic melanoma as monotherapy on the one hand, and on the other hand, in combination with a peptide vaccine (2). During the follow-up period, which lasted up to 10 years, a pooled analysis of data from 1861 patients showed that survival plateaued in 21% (5).
The second checkpoint to be therapeutically evaluated is PD-1. It binds to the PD ligands 1 and 2 (PD-L1, PD-L2) (1). PD-1 is expressed on activated T-cells, B-cells, and natural killer (NK) cells and is typically found on chronically stimulated and exhausted T-lymphocytes. The ligands, however, are expressed on many normal tissue cells and are crucially responsible for preventing autoimmune reactions. In the T-cell they produce a strong negative signal that may result in anergy or cell death.
Antibodies that bind to PD-1 or PD-L1 can trigger endogenous immune activation against the tumor cells across conditions in different malignant disorders (figure 1) (1). Nivolumab is a completely humanized IgG4 antibody which binds to PD-1, which in the CA209–037 trial in patients with metastatic melanoma that had previously been treated with ipilimumab was tested against chemotherapy with dacarbazine or paclitaxel/carboplatin, as selected by the investigators (table 1). The objective response rate (ORR)—the proportion of patients with complete and partial remission—was 31.7% compared with 10.6% in the chemotherapy arm (6).
Table 1. Phase III trials of PD-1 inhibition in metastatic melanoma.
|
Study (publication) |
Treatment arm | Number of patients | Median follow-up | Proportion of‧patients with complete/partial remission | Progression free survival – hazard ratio | Overall survival hazard ratio | Patients with raised LDH at the time of inclusion in the study | Proportion of M1c- patients*1 |
| CA209–037 (6) | Nivolumab 3 mg/kg, q14*2 | 272 | 8.4 months | 3.3/28.3%*3 | 0.82 (99.99 %-CI 0.32 to 2.05) | 51% | 75% | |
| Dacarbazine 1000 mg/m2 q21*2 or paclitaxel 175 mg/m2 + carboplatin AUC 6, q21 | 133 | 0/10.6%*3 | 35% | 77% | ||||
| CA209–066*4 (7) | Nivolumab 3 mg/kg, q14 | 210 | 5.2–16.7 months*5 | 7.6/32.4% | 0.43 (95%- CI: 0.34 to 0.56; P <0.001) | 0.42 (99.79%- CI 0.25 to 0.73; P <0.001) | 37,6% | 61.0% |
| Dacarbazine 1000 mg/m2, q21 | 208 | 1.0/13.0% | 35,6% | 61.1% | ||||
| KEYNOTE-006 (8) | Pembrolizumab 10 mg/kg, q14 | 279 | 22.9 months | 12/25% | 0.61 (95%- CI 0.50 to 0.75; P <0.0001)*6 | 0.68 (95% CI 0.53 to 0.87; P = 0.0009)*7 | 29,0% | 64.2% |
| Pembrolizumab 10 mg/kg, q21 | 277 | 13/23% | 0.68 (95% CI 0.53 to 0.86; P = 0.0008)*7 | 35,4% | 68.2% | |||
| Ipilimumab 3 mg/mg, q21 for 4 cycles | 278 | 5/8% | 32,7% | 63.7% | ||||
| CA209–067 (9) | Nivolumab 3 mg/kg, q14 | 316 | 35.7 months | 16/28% | 0.55 (95% CI 0.5 to 0.66; P <0.001)*8 | 0.63 (98% CI 0.48 to 0.81; P <0.001)*7 | 35% | 58% |
| Nivolumab 1 mg/kg + Ipilimumab 3 mg/mg, q21 for 4 cycles, followed bynivolumab 3 mg/kg, q14 | 314 | 38.0 months | 19/39% | 0.43 (95% CI 0.35 to 0.52; P <0.001)*5 | 0.55 (98% CI 0.42 to 0.72; P <0.001)*7 | 36% | 58% | |
| Ipilimumab 3 mg/mg, q21 for 4 cycles | 315 | 18.6 months | 5/14% | 37% | 58% |
Some studies reported unusual confidence intervals (for example,. 99.99%; 99.79%; 98%), which can be explained by their methodological approach.
*1 Distant visceral metastases including the brain or any localization with raised LDH
*2 q14: every two weeks, q21: every three weeks
*3 Data refer to the first 120 patients
*4 Only BRAF-wildtype patients
*5 Publication reports only timespan, no median
*6 Both study arms with pembrolizumab vs ipilimumab
*7 versus ipilimumab; *8 versus ipilimumab
AUC, area under the curve; CI, confidence interval; LDH, lactate dehydrogenase
The double blinded CA209–066 trial compared nivolumab as first-line treatment with chemotherapy using dacarbazine (7). The ORR for nivolumab was 40% versus 13.9% in the dacarbazine group. Pembrolizumab is another completely humanized IgG4 antibody targeting PD-1, which in the KEYNOTE-006 trial was compared in two doses against ipilizumab (8). Because of the superiority of pembrolizumab regarding overall survival, the trial was stopped early.
The combination of nivolumab and ipilimumab in the CA-209–067 trial was found to be highly effective in previously untreated patients with metastatic melanoma. The trial compared monotherapy with ipilimumab, monotherapy with nivolumab, and combination treatment using ipilimumab and nivolumab in overall four combinations, and subsequent monotherapy with nivolumab (9). The study design was such that ipilimumab was compared with the two nivolumab arms (+/- ipilimumab) with regard to the endpoints progression free survival and overall survival. Monotherapy with ipilimumab was clearly inferior to both nivolumab arms, the ORRs were 19% (ipilimumab with 3 mg/kg body weight), 43.7% (monotherapy with nivolumab at 3 mg/kg body weight), and 57.6% (ipilimumab 3 mg/kg body weight + nivolumab 1 mg/kg body weight). The rate of severe adverse effects for the combination was roughly three times that of monotherapy with nivolumab (59% versus 21% treatment associated adverse effects of grade 3 and 4) (9). Currently the combination of ipilimumab and pembrolizumab is being trialed—but at other dosages—with the objective of possibly reaching a more favorable profile of effectiveness versus adverse effects. Currently, the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA) have licensed ipilimumab, nivolumab, and nivolumab as monotherapies, and combination therapy with ipilimumab and nivolumab.
Oncolytic virus therapy using Talimogene Laherparepvec (T-VEC) is licensed for use in inoperable metastases and those that are suitable for injection; this has been found to be effective as monotherapy especially in locally advanced melanoma at stages IIIB, IIIC, and IVA, when compared with granulocyte-macrophage colony-stimulating factor (GM-CSF) (10). This is the first virolytic therapy that has been introduced into clinical use. The virus is injected directly into the tumor lesion or the affected lymph nodes. Response rates were 26.4% versus 5.7% for GM-CSF.
According to the mechanism of action of immune checkpoint inhibitors, the adverse effects of these medications arise primarily through autoimmune processes (11, 12). PD-1 and PD-L1 antibodies are characterized by an overall lower adverse effect profile, dominated by fatigue, thyroid function disorders, and adverse cutaneous effects. More rarely, pulmonary adverse effects may develop, in the form of pneumonitis. Administration of the anti-CTLA-4 antibody ipilimumab, by contrast, triggers more strongly pronounced adverse effects that often affect—in addition to skin and thyroid—the gut (diarrhea and colitis), liver (hepatitis), and pituitary gland (hypophysitis). The combination of CTLA-4 antibodies and PD-1 antibodies again triggers a notable increase in adverse effects (9). The adverse effects can become life threatening, especially if antibody therapy is continued in spite of adverse effects or if early immunosuppressive therapy is not initiated in severe and/or dynamic rapidly progressing immune-associated adverse effects. The patients will have to be informed and instructed thoroughly with regard to developing adverse effects.
The strength of expression of PD-1 on tumor cells and immune cells is a potential biomarker that can distinguish between response and non-response (13). In case of low or non-existent expression of PD-L1, a trend was observed towards greater overall survival and improved responsiveness of patients receiving combination treatment with ipilimumab and nivolumab compared with monotherapy with nivolumab (9). Especially patients with a high mutational burden in the tumor benefited from immune checkpoint inhibition using anti-CTLA-4 and anti-PD-1/PD-L1 antibodies (14). This indicates the particular importance of neoantigens as target structures for the patient’s own immune system (15).
Targeted therapy
Modified signaling molecules that can be medically inhibited and that are the result of therapy-relevant mutations in the tumor genome have so far been discovered for melanoma in the V600 codon of the BRAF gene (the rate at which this mutation occurs is 35–50%), in the Q61 or the NRAS gene (10–25%), and for the c-kit gene (2%); further potential target genes have been identified (16, 17). No licensed therapies exist for NRAS-mutated melanoma (18). Only for acral-lentiginous cutaneous and mucosal melanoma, molecular testing for c-kit mutations in the exons 11 and 13 is recommended. Therapy with a c-kit inhibitor, such as imatinib, is possible only off label, but it is justifiable in individual cases.
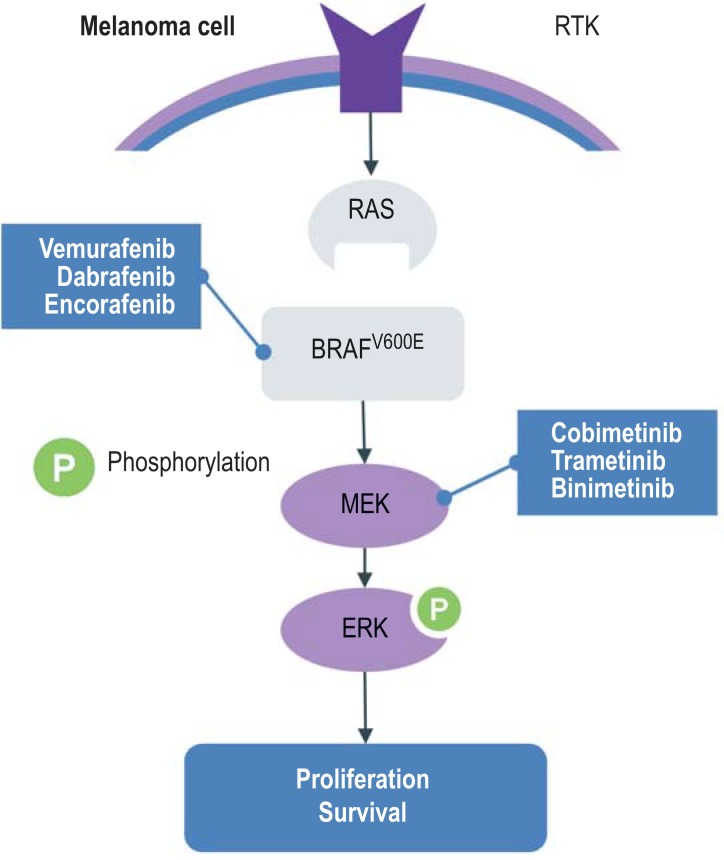
In the scenario of the BRAF-V600 mutation, the mitogen-activated protein kinase (MAPK) signaling pathway is continually activated (figure 2). For patients with a confirmed BRAF-V600 mutation, monotherapy using the BRAF inhibitor vemurafenib in the BRIM-3 trial (19) and the BRAF inhibitor dabrafenib in the BREAK-3 trial improved the main outcome measure progression-free survival compared with dacarbazine, with a hazard ratio (HR) of 0.26 or 0.3, respectively (20). The effect of the BRAF inhibitors set in rapidly and comprehensively. Secondary resistance against BRAF inhibitor monotherapy limited the effectiveness, however. These were explained mainly with the paradoxical reactivation of the MAPK signaling pathway by MEK.
Figure 2.
Inhibition of the MAPK(RAS-RAF-MEK-ERK) signaling pathway by BRAF inhibitors and MEK inhibitors
MAPK, mitogen-activated protein kinase; RTK, receptor-tyrosine-kinase
Because of the improved response rate, progression-free survival, and overall survival as a result of combined inhibition of mutated BRAF-V600 and MEK, three combination therapies are currently licensed. The combination of dabrafenib and trametinib improved progression-free survival and overall survival in patients with the BRAF-V500E/K mutation who had inoperable metastatic melanoma, compared with dabrafenib monotherapy (COMBI-d trial) (21) and vemurafenib in the COMBI-v trial (table 2) (22). The safety profile was consistent over both studies; in particular it showed fewer cases of squamous cell carcinoma and keratoacanthoma, as well as fewer follicular and palmoplantar keratoses, which often develop under monotherapy with BRAF inhibitors. Pyrexia—one or repeated episodes of a fever higher than 38.5 °C that could not be explained with an infection—was common and more severe under combination treatment.
Table 2. Phase III trials of targeted therapy in metastatic melanoma with a BRAF mutation.
| Study (publication) | Treatment arm | Tumor stages | Number of patients | Median follow-up | Response rates |
Median progression free survival |
Median overall survival | Patients with raised LDH at the time of inclusion in the study | Proportions of M1c |
| COMBI-d (21) | Dabrafenib 150 mg BID/trametinib 2 mg/d | IIIC-IV BRAF-V600 mutation |
211 | 36 months | 68% | 11.0 months | 25.1 months | 36% | 67% |
| Dabrafenib 150 mg BID/placebo | 212 | 55% | 8.8 months | 18.7 months | 33% | 65% | |||
| COMBI-v (22) | Dabrafenib 150 mg BID/trametinib 2 mg/d | IIIC-IV BRAF-V600 mutation |
352 | Not known | 64% | 11.4 months | Not known | 34% | 63% |
| Vemurafenib 960 mg BID | 352 | 51% | 7.3 months | Not known | 32% | 59% | |||
| coBRIM (23) | Cobimetinib 60 mg/d Vemurafemib 960 mg BID | IIIC-IV BRAF-V600 mutation |
247 | 14.2 months |
70% | 12.3 months | 22. | 46% | 59% |
| PlaceboVemurafenib 960 mg BID | 248 | 50% | 7.2 months | 17.4 months | 43% | 62% | |||
| COLUMBUS (26, 27) | Encorafenib 450 mg/d Binimetinib 45 mg BID | IIIB-IV BRAF-V600 mutation |
192 | 16.6 months |
75% | 14.9 months | 33.6 months | 29% | 64% |
| Encorafenib 300 mg/d | 194 | 58% | 9.6 months | 23.5 months | 24% | 62% | |||
| Vemurafenib 960 mg BID | 191 | 49% | 7.3 months | 16.9 months | 27% | 65% |
BID, twice daily; LDH, lactate dehydrogenase; M1c, distant metastases in a visceral location including the brain or any localization with raised LDH
Combination treatment using vemurafenib and the MEK inhibitor cobimetinib brought about improved responses and prolonged progression-free survival and overall survival compared with vemurafenib monotherapy in the coBRIM trial (23). Of note in this targeted therapeutic combination was the lesser extent of skin toxicity. However, photosensitivity and raised creatine kinase were higher than for monotherapy.
Patients with a generally good prognosis, characterized by a serum concentration of lactate dehydrogenase (LDH) below the upper normal limit and fewer than three affected organs, also had the best chance of long-term benefit under targeted therapy (table 2). For example, patients in whom both criteria were positive, had reached a progression-free survival rate after 2 years of 46% and a 2-year survival rate of 75%. If the criteria were not met and the LDH serum level was raised to twice the upper normal limit, the progression-free survival rate was 2% and the 2-year survival rate only 7% (24). These data were recently also confirmed for combination therapy using cobimetinib and vemurafenib: LDH serum concentrations, Eastern Cooperative Oncology Group Performance Scale (ECOG-PS), and the sum of the diameter of the metastases were the key determinants (25).
The third treatment to be tested was the combination of the high affinity BRAF inhibitor encorafenib with the MEK inhibitor binimetinib. The three-arm phase III trial, the comparison of the combination versus monotherapy with vemurafenib or, ditto, encorafenib showed improved progression-free survival of 14.9 months (vemurafenib 7.3 months) (26) and overall survival of 33.6 months (vemurafenib 16.9 months) (27), and the adverse effect profile was more favorable. Since fewer patients with raised LDH were included, the patient population treated had tendentially more promising characteristics than the patients in both other combination treatment arms (table 2). Typical further adverse effects of all combinations of BRAF/MEK inhibitors included raised liver enzyme levels, ophthalmological toxicities (serous retinopathy owing to neurosensory detachment), and cardiac toxicities (heart failure, prolonged QT interval), which required relevant monitoring and provision of information to the patient/patient education.
Patients with inoperable metastatic melanoma should be tested for a BRAF-V600E/K mutation, and if they test positive, they should be treated with a combination of BRAF/MEK. Currently ongoing clinical trials are investigating the question of whether this treatment should be given as first line therapy. The recommendation is that patients in whom symptomatic metastases necessitate rapid remission should be given targeted treatment as the first-line treatment. A raised serum concentration of LDH or cerebral metastases are, however, not an unequivocal criterion to start immediate targeted treatment.
The treatment should not be stopped since some 50% of patients will experience a recurrence after stopping. However, it is possible to treat recurrences anew with targeted combination therapy, so that making use of the drug holiday concept might be considered. After resistance has developed, re-induction seems to be of only transient benefit (28).
Cerebral metastases
The prognosis for patients with cerebral metastases was regarded as poor in the chemotherapy era (that is, the time before targeted and immune therapies). In principle, all therapies that are used in patients with exclusively extracerebral metastases can also be used in patients with cerebral metastases (etable) (29– 32). The response will be better the fewer cerebral metastases are present. In selected cohorts (for example, asymptomatic patients with a cerebral; metastasis), intracranial response rates of more than 50% can be achieved by using targeted therapies as well as immune checkpoint therapy without radiotherapy. In advanced findings, the disease can be stabilized at least in a substantial proportion of patients.
eTable. Studies of the treatment of cerebral metastases.
| Studie | Treatment arm | Cohort under study | Number of patients | Follow-up | Number of cerebral target lesions | Intracranial response (patients/percentages) | ||
| (29) | Dabrafenib 150 mg BID + trametinib 2 mg/d | BRAFV600E mutation, asymptomatic cerebral metastases, without previous cerebral local therapy, ECOG status 0/1 | 76 | 8.5 months (median) | 1 2 3 4 5 |
41 (54%) 20 (26%) 7 (9%) 4 (5%) 4 (5%) |
CR PR SD PD Not evaluable |
3 (4%) 41 54%) 15 20%) 14 18%) 3 (4%) |
| BRAFV600E mutation, asymptomatic cerebral metastases, previous cerebral local therapy, ECOG status 0/1 | 16 | 20.0 months (median) | 1 2 3 4 5 |
7 (44%) 7(44%) 2 (13%) 0 0 |
CR PR SD PD Not evaluable |
1 (6%) 8 (50%) 4 (31%) 1 (6%) 1 (6%) |
||
| BRAFV600D/K/R mutation, previous cerebral local therapy permitted, ECOG status 0/1 | 16 | 9.5 months (median) | 1 2 3 4 5 |
7 (44%) 6 (38%) 2 (13%) 0 1 (6%) |
CR PR SD PD Not evaluable |
0 7 (44%) 5 (31%) 4 (25%) 0 |
||
| BRAFV600D/E/K/R mutation, asymptomatic cerebral metastases, previous cerebral local therapy permitted, ECOG status 0–2 | 17 | 11.0 months (median) | 1 2 3 4 5 |
7 (41%) 7 (41%) 1 (6%) 1 (6%) 1 (6%) |
CR PR SD PD Not evaluable |
1 (6%) 9 (53%) 4 (24%) 3 (18%) 0 |
||
| (30) | Vemurafenib 960 mg BID | Therapy naive patients regarding cerebral metastases, previous systemic therapy permitted (excluding BRAF- or MEK- inhibitors) | 90 | 9.6 months (median) | 1 2–4 >4 |
40 (44%) 37 (41%) 13 (14%) |
CR PR SD PD Not evaluable |
2 (2%) 24 (27%) 36 (40%) 25 (28%) 3 (3%) |
| Previous treatment with stereotactic surgery, whole brain radiotherapy, or cerebral metastasectomy, measurable cerebral disease progression | 56 | 1 2–4 >4 |
11 (20%) 35 (63%) 10 (18%) |
CR PR SD PD Not evaluable |
0 13 (23%) 30 (54%) 11 (20%) 2 (4%) |
|||
| (31) | NIVO/IPI*1 | Asymptomatic cerebral metastases, no previous metastasectomy, stereotactic surgery or whole brain radiotherapy | 36 | 17 months (median) | 1 2–4 >4 |
11 (31%) 10 (29%) 14 (40%) |
CR PR SD PD Not evaluable |
6 (17%) 10 (29%) 4 (11%) 14 (40%) 1 (3%) |
| Nivolumab 3 mg/kg body weight, q14 | Asymptomatic cerebral metastases, no previous metastasectomy, stereotactic surgery or whole brain radiotherapy | 27 | 1 2–4 >4 |
6 (24%) 14 (56%) 5 (20%) |
CR PR SD PD Not evaluable |
3 (12%) 2 (8%) 0 19 (76%) 1 (4%) |
||
| Symptomatic cerebral metastasis or progressive or new cerebral metastases after local pre-treatment or leptomeningeal disease or the combination of these | 16 | 1 2–4 >4 |
1 (6%) 7 (44%) 8 (50%) |
CR PR SD PD Not evaluable |
0 1 (6%) 2 (13%) 13 (81%) 0 |
|||
| (32) | NIVO/IPI*1 | Asymptomatic cerebral metastases, no previous metastasectomy, stereotactic surgery or whole brain radiotherapy | 94*2 | 14 months (median) | 1 2 ≥ 3 |
49 23 22 |
CR PR SD (≥ 6 months) PD Not evaluable |
24 (26%) 28 (30%) 2 (2%) 31 (33%) 9 (10%) |
*1 Nivolumab 1 mg/kg body weight+ ipilimumab 3 mg/kg body weight q21 for 4 cycles, followed by nivolumab 3 mg/kg body weight; q14: every two weeks;
*2 a patient without measurable cerebral lesions
BID, twice per day; CR, complete remission; PR, partial remission; PD, progressive disease; SD, stable disease
Adjuvant therapy
A recent meta-analysis of 15 studies including more than 9000 patients calculated for adjuvant therapy using interferon-a a risk reduction of 14% (HR 0.86; 95% confidence interval [0.81; 0.91]; P<0.0001) regarding event-free survival and of 10% (HR 0.90; [0.85; 0.97]; P=0.003) regarding overall survival (table 3) (33). No indications were found that the following variables affected the benefit of interferon-a:
Table 3. Adjuvant therapy in metastatic melanoma.
|
Study (publication) |
Treatment arm | Tumor stages | Number of patients | Number of events | Follow-up | 12 month recurrence-free survival rate | 18 month recurrence- free survival rate |
Hazard ratio for recurrence/death [confidence interval] |
| Interferon-α, pegylated interferon-α | ||||||||
| Meta-analysis (33) | 15 studies included | I–IV (III main emphasis) | 5826 (PFS) 7699 (OS) |
3706 (PFS) 3899 (OS) |
40.8–202.8 months, depending on study |
HR for PFS0.86; (95%- CI [0.81; 0.91]; P <0.0001) HR for death 0.90; (95%- CI [0.85; 0.97]; P = 0.003) |
||
| Ipilimumab versus placebo | ||||||||
| EORTC 18071 (34) |
Ipilimumab 10 mg/kg q21 for 4 cycles, then every 12 weeks for 3 years | IIIA (>1 mm) – IIIC, no in-transit metastases | 475 | 264 | 63,6 months (median) | 63.5% | 51.5% (2-Y-PFS)40,8% (5-Y-PFS) | 0.76; (95% CI [0.64; 0.89]; P <0.001 hr for death 0.72; (95% ci [0.58; 0.88]; p = 0.001) |
| Placebo | 476 | 323 | 56.1% | 43.8% (2-Y-PFS)30,3% (5-Y-PFS) | ||||
| Pembrolizumab versus placebo | ||||||||
| EORTC 1325 (37) | Pembrolizumab 200 mg, q21 | IIIA (>1 mm) – IIIC, no in-transit metastases | 514 | 135 | 15 months (median) | 75.4% (95% CI 71.3 to 78.9) | 71.4% (95% CI 66.8 to 75.4) | 0.57; (98.4% CI [0.43; 0.74]); P <0.001 |
| Placebo | 505 | 216 | 61.0%(95% CI 56.5 to 65.1) | 53.2% (95% CI 47.9 to 58.2) | ||||
| Nivolumab versus ipilimumab | ||||||||
| CA209–238 (36) |
Nivolumab 3 mg/kg body weight, q14 | IIIB–IV (NED) | 453 | 154 | 18 months (minimum) | 70.5% (95% CI 66.1 to 74.5) | 66.4% (95% CI 61.8 to 70.6) | 0.65; (97.5%-CI [0.51; 0.83]; P <0.001) |
| Ipilimumab 10 mg/kg q21 for 4 cycles, then every 12 weeks | 453 | 206 | 60.8% (95% CI 56.0 to 65.2) | 52.7% (95% CI 47.8 to 57.4) | ||||
| Dabrafenib + trametinib versus pacebo | ||||||||
| COMBI-AD (39) |
Dabrafenib 150 mg BID Trametinib 2 mg/d |
IIIA–IIIC | 438 | 163 (recurrence) 60 (death) |
30 months (minimum) | 88% 97% (1-Y-OS) |
67% (2-Y-PFS) 91% (2-Y-OS) |
0.47; (95% CI [0.39; 0.58]; P <0.001) hr for death 0.57; (95% ci [0.42; 0.79]; p = 0.0006) |
| Placebo | 432 | 247 (recurrence) 93 (death) |
56% 94% (1-Y-OS) |
44% (2-Y-PFS) 83% (2-Y-OS) |
||||
Some studies reported unusual confidence intervals (for example, 97.5%; 98.4%), which can be explained by their methodological approach..
BID, twice daily; HR, hazard ratio; CI, confidence interval; NED, no evidence of disease; PFS, progression free survival; OS, overall survival;
q14: every two weeks, q21: every three weeks; Y, year
Dosage or duration of treatment
Age
Sex
Location of primary tumor
Cancer stage
Tumor thickness according to Breslow
Presence and number of lymph node macrometastases.
Only for ulceration of the primary tumor was there an indication of an interaction, in the sense that patients with ulcerated primary tumors seem to benefit notably more from interferon-a.
Adjuvant treatment with ipilimumab 10 mg/kg body weight was found to yield a prognostic advantage compared with placebo (EORTC 18071 trial, Table 3). After a median follow-up period of 5.3 years, patients who had been treated with ipilimumab had a significant advantage in terms of 5 year survival rates for recurrence-free survival of 10.5% and overall survival of 10% precisely (34, 35).
Two recent randomized phase III trials studied adjuvant therapy at stage III with anti-PD-1 antibodies (36, 27). One of the trials included patients with stage IV cancer after complete metastasectomy (36). In the CA209–238 trial, nivolumab 3 mg/kg was evaluated versus ipilimumab 10 mg/kg. Recurrence-free survival rates after 18 months in patients treated with nivolumab was 13.4|% better than in those treated with ipilimumab. This corresponds to a risk reduction for recurrence or death of 35%. In the second trial (EORTC 1352/KEYNOTE-054) pembrolizumab 200 mg was compared with placebo treatment (37). On the one hand, patients with stage IIIA melanoma (micrometastases) were included whose metastases in the affected lymph node at stage N1a were found to have a minimum diameter of >1 mm. On the other hand, patients at stage IIIB/C were included in whom no in-transit metastases were found. Recurrence-free survival in patients who had been treated with pembrolizumab was longer than that in patients who had received the placebo treatment and corresponded to a risk reduction for recurrence or death of 43% (37). No data for overall survival are available yet for either study. The proportion of patients with BRAF-V600 mutations in the studies was about 40%. No difference was found for treatment effectiveness for patients with the BRAF mutation or BRAF wildtype patients.
Two phase III trials investigated adjuvant treatment with BRAF inhibitors and MEK inhibitors (38, 39). One study investigated combined administration of the BRAF inhibitors and MEK inhibitors dabrafenib and trametinib (39), and a second study investigated adjuvant therapy using the BRAF inhibitor vemurafenib, both copared with placebo treatment (38). In the first study, patients at stages IIIA-IIIC with a BRAF-V6000E/K mutation received dabrafenib 150 mg twice daily (BID) and trametinib 2 mg/ once daily or a comparable placebo treatment for a total of 12 months (39). After a median follow-up period of 2.8 years, the 3 year probability for recurrence free survival in the treatment arm was 58% and for the placebo arm, 39%. The three year probability for overall survival was 86% for the treatment arm and 77% for the placebo arm.
In the second study, patients with stage IIC melanoma (tumor thickness >4 mm with ulceration), IIIA and IIIB, or IIIC with a BRAF-V600 mutation were given vemurafenib 960 mg BID without a MEK inhibitor or placebo therapy for a total of 52 weeks (38). This study was overall rated as negative.
Conclusions
Thanks to the new substances, the systemic therapy of melanoma has improved substantially. Giving patients PD-1 inhibitors has yielded 3-year survival rates of 50–52%; giving the immune combination has resulted in 3-year survival of a hitherto unsurpassed 58%, and for dabrafenib and trametinib, of 44% (21). However, many patients are not helped in the long term by these new therapies. Making use of clinical research services in skin cancer centers therefore continues to be an important option.
Key Messages.
Antibodies targeting the PD-1 molecule are the therapeutic standard in inoperable metastatic melanoma. Nivolumab and pembrolizumab are licensed for monotherapy.
Combination treatment using ipilimumab and nivolumab is more effective in melanomas with low or absent PD-L1 expression than anti-PD1 monotherapy, but is often associated with severe immunological adverse effects.
Patients with inoperable metastatic melanoma and a confirmed BRAF-V600 mutation in the tumor can be treated with combination therapy including BRAF/MEK inhibitors.
Patients who have had a complete metastasectomy (stages III/IV) can be offered nivolumab as adjuvant therapy; those in whom lymph node metastases have been completely removed at stage III can be given pembrolizumab.
After complete metastasectomy, patients with a confirmed BRAF-V66 mutation in the tumor at stage III can be offered adjuvant combination therapy using dabrafenib and trametinib.
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
PD Dr Terheyden received lecture honoraria from BMS, Novartis, Pierre-Fabre, CureVac, and Roche, as well as consultancy fees from BMS, Merck, Novartis, Pierre-Fabre, Merck Serono, Sanofi, and Roche. He was reimbursed travel expenses and conference delegate fees by BMS, Pierre-Fabre, and Roche. He received study funding/support (third party funding/support) from BMS, Roche, and Novartis.
Prof Krackhardt received consultancy fees from BMS, Sanofi, Novartis, Roche, Pierre-Fabre, Vaccibody, and Zelluna. She was paid lecture honoraria by BMS and Roche. Travel expenses were paid on her behalf by BMS, Sanofi, Pierre-Fabre, and Janssen. She received study funding/ support (third party funding/support) from BMS, Kiadis, Vaccibody, and Zelluna.
Prof Eigentler received lecture honoraria from BMS, Novartis, Roche, and Amgen, as well as consultancy fees from BMS, Novartis, Roche, Amgen, MSD, Pierre-Fabre, and Sanofi. He received study funding/support from BMS, Roche, CureVac, Sanofi, and MSD.
References
- 1.Ribas A, Wolchok JD. Cancer immunotherapy using checkpoint blockade. Science. 2018;359:1350–1355. doi: 10.1126/science.aar4060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hodi FS, O‘Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 4.Arce Vargas F, Furness AJS, Litchfield K, et al. Fc effector function contributes to the activity of human anti-CTLA-4 antibodies. Cancer Cell. 2018;33:649–663 e4. doi: 10.1016/j.ccell.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schadendorf D, Hodi FS, Robert C, et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J Clin Oncol. 2015;33:1889–1894. doi: 10.1200/JCO.2014.56.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weber JS, D‘Angelo SP, Minor D, et al. Nivolumab versus chemotherapy in patients with advanced melanoma who progressed after anti-CTLA-4 treatment (CheckMate 037): a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2015;16:375–384. doi: 10.1016/S1470-2045(15)70076-8. [DOI] [PubMed] [Google Scholar]
- 7.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372:320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 8.Schachter J, Ribas A, Long GV, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390:1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 9.Wolchok JD, Chiarion-Sileni V, Gonzalez R, et al. Overall survival with combined nivolumab and ipilimumab in advanced Melanoma. N Engl J Med. 2017;377:1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Andtbacka RH, Kaufman HL, Collichio F, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 11.Heinzerling L , De Toni E, Schett G, Hundorfean G, Zimmer L. Checkpoint inhibitors—the diagnosis and treatment of side effects. Dtsch Arztebl Int. 2019;116:119–126. doi: 10.3238/arztebl.2019.0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haanen J, Carbonnel F, Robert C, et al. Management of toxicities from immunotherapy: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Onco. 2017;28:iv119–iv142. doi: 10.1093/annonc/mdx225. [DOI] [PubMed] [Google Scholar]
- 13.Buder-Bakhaya K, Hassel JC. Biomarkers for clinical benefit of immune checkpoint inhibitor treatment—a review from the melanoma perspective and beyond. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Snyder A, Makarov V, Merghoub T, et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verdegaal EM, de Miranda NF, Visser M, et al. Neoantigen landscape dynamics during human melanoma-T cell interactions. Nature. 2016;536:91–95. doi: 10.1038/nature18945. [DOI] [PubMed] [Google Scholar]
- 16.Hayward NK, Wilmott JS, Waddell N, et al. Whole-genome landscapes of major melanoma subtypes. Nature. 2017;545:175–180. doi: 10.1038/nature22071. [DOI] [PubMed] [Google Scholar]
- 17.Cancer Genome Atlas Network. Cancer Genome Atlas: Genomic classification of cutaneous melanoma. Cell. 2015;161:1681–1696. doi: 10.1016/j.cell.2015.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dummer R, Schadendorf D, Ascierto PA, et al. Binimetinib versus dacarbazine in patients with advanced NRAS-mutant melanoma (NEMO): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017;18:435–445. doi: 10.1016/S1470-2045(17)30180-8. [DOI] [PubMed] [Google Scholar]
- 19.Chapman PB, Hauschild A, Robert C, et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med. 2011;364:2507–2516. doi: 10.1056/NEJMoa1103782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hauschild A, Grob JJ, Demidov LV, et al. Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2012;380:358–365. doi: 10.1016/S0140-6736(12)60868-X. [DOI] [PubMed] [Google Scholar]
- 21.Long GV, Flaherty KT, Stroyakovskiy D, et al. Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: long-term survival and safety analysis of a phase 3 study. Ann Oncol. 2017;28:1631–1639. doi: 10.1093/annonc/mdx176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robert C, Karaszewska B, Schachter J, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med. 2015;372:30–39. doi: 10.1056/NEJMoa1412690. [DOI] [PubMed] [Google Scholar]
- 23.Ascierto PA, McArthur GA, Dreno B, et al. Cobimetinib combined with vemurafenib in advanced BRAF(V600)-mutant melanoma (coBRIM): updated efficacy results from a randomised, double-blind, phase 3 trial. Lancet Oncol. 2016;17:1248–1260. doi: 10.1016/S1470-2045(16)30122-X. [DOI] [PubMed] [Google Scholar]
- 24.Long GV, Grob JJ, Nathan P, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17:1743–1754. doi: 10.1016/S1470-2045(16)30578-2. [DOI] [PubMed] [Google Scholar]
- 25.Hauschild A, Larkin J, Ribas A, et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-Mutated Metastatic Melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol. 2018;4:1382–1388. doi: 10.1001/jamaoncol.2018.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dummer R, Ascierto PA, Gogas HJ, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19:603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 27.Dummer R, Ascierto PA, Gogas HJ, et al. Overall survival in patients with BRAF-mutant melanoma receiving encorafenib plus binimetinib versus vemurafenib or encorafenib (COLUMBUS): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2018;19:1315–1327. doi: 10.1016/S1470-2045(18)30497-2. [DOI] [PubMed] [Google Scholar]
- 28.Valpione S, Carlino MS, Mangana J, et al. Rechallenge with BRAF-directed treatment in metastatic melanoma: a multi-institutional retrospective study. Eur J Cancer. 2018;91:116–124. doi: 10.1016/j.ejca.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 29.Davies MA, Saiag P, Robert C, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18:863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McArthur GA, Maio M, Arance A, et al. Vemurafenib in metastatic melanoma patients with brain metastases: an open-label, single-arm, phase 2, multicentre study. Ann Oncol. 2017;28:634–641. doi: 10.1093/annonc/mdw641. [DOI] [PubMed] [Google Scholar]
- 31.Long GV, Atkinson V, Lo S, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol. 2018;19:672–681. doi: 10.1016/S1470-2045(18)30139-6. [DOI] [PubMed] [Google Scholar]
- 32.Tawbi HA, Forsyth PA, Algazi A, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. N Engl J Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ives NJ, Suciu S, Eggermont AMM, et al. International melanoma meta-analysis collaborative G Adjuvant interferon-alpha for the treatment of high-risk melanoma: an individual patient data meta-analysis. Eur J Cancer. 2017;82:171–183. doi: 10.1016/j.ejca.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 34.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. doi: 10.1016/S1470-2045(15)70122-1. [DOI] [PubMed] [Google Scholar]
- 35.Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Prolonged survival in stage III melanoma with ipilimumab adjuvant therapy. N Engl J Med. 2016;375:1845–1855. doi: 10.1056/NEJMoa1611299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weber J, Mandala M, Del Vecchio M, et al. Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med. 2017;377:1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 37.Eggermont AMM, Blank CU, Mandala M, et al. Adjuvant pembrolizumab versus placebo in resected stage III melanoma. N Engl J Med. 2018;378:1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 38.Maio M, Lewis K, Demidov L, et al. Adjuvant vemurafenib in resected, BRAF(V600) mutation-positive melanoma (BRIM8): a randomised, double-blind, placebo-controlled, multicentre, phase 3 trial. Lancet Oncol. 2018;19:510–520. doi: 10.1016/S1470-2045(18)30106-2. [DOI] [PubMed] [Google Scholar]
- 39.Long GV, Hauschild A, Santinami M, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med. 2017;377:1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]