Abstract
Background
Segmental progeroid syndromes (SPS) are rare hereditary diseases in which the affected individuals show signs of premature aging in more than one organ or type of tissue. We review the clinical and genetic features of some of these syndromes and discuss the extent to which their study affords a complementary opportunity to study aging processes in general.
Methods
This review is based on publications retrieved by a selective search in PubMed.
Results
Segmental progeroid syndromes are a clinically and genetically heterogeneous group of hereditary diseases. They can be categorized, for example, by the age of onset of manifestations (congenital vs. infantile vs. juvenile/adult forms). They are diagnosed on clinical grounds supplemented by genetic testing on the basis of next-generation sequencing, which is of central importance in view of the marked heterogeneity and complexity of their overlapping clinical features. The elucidation of the genetic and molecular causes of these diseases can lead to causally directed treatment, as shown by the initial clinical trials in Hutchinson–Gilford progeria syndrome. The molecular features of SPS are identical in many ways to those of “physiological” aging. Thus, studying the molecular mechanisms of SPS may be helpful for the development of molecularly defined treatment approaches for age-associated diseases in general.
Conclusion
Segmental progeroid syndromes are a complex group of diseases with overlapping clinical features. Current research efforts focus on the elucidation of the molecular mechanisms of these diseases, most of which are very rare. This should enable the development of treatments that might be applicable to general processes of aging as well.
Cellular and organismic aging is a complex biological process, affecting every human being. Due to the rise in life expectancy, age-related diseases are a challenge of ever increasing proportions for both society and healthcare systems. A targeted, molecularly defined drug intervention for the prevention or treatment of age-associated diseases is indeed an attractive perspective, but unachievable at present due to our incomplete understanding of the molecular mechanisms underlying the regulation of aging processes.
One possible approach to shed light on the molecular basis of aging processes is to study monogenic premature aging syndromes. Segmental progeroid syndromes (SPS) is the term used for a group of disorders characterized by signs of premature aging in more than one organ or tissue (1). By contrast, a disorder is classified as unimodal progeroid, if premature aging is limited to one organ or one tissue, e.g. monogenic hereditary colorectal cancer syndromes or early-onset forms of Alzheimer’s disease). Among the typical signs of premature aging in SPS is the premature onset of the following symptoms or disorders:
Graying/loss of hair
Hearing loss
Cataract
Scleroderma-like skin changes
Type 2 diabetes mellitus
Osteoporosis
Atherosclerosis and coronary heart disease
Various malignant tumors.
These signs typically occur in the general population only at a more advanced age. An additional characteristic feature of SPS is growth retardation, because many of the genes involved in the pathogenesis of these syndromes play a role in various aspects of cell viability; consequently, these patients stop growing as the result of significant functional impairment of these genes. Even though the usually hereditary SPS are very rare—the overall incidence worldwide is estimated to be approximately 1:50 000—they can be regarded as paradigms for research into aging processes and age-related disorders in general. However, it should be noted that just like in a healthy person not all organ systems age at the same speed, patients with SPS do not show signs of premature aging in all organs—for this reason, these syndromes are referred to as “segmental“ syndromes.
More than 100 syndromes with clinical signs of premature aging have so far been described in the scientific literature, but only in recent years have the genetic defects causing these syndromes been identified by analyses performed on at times very few patients, using high-throughput sequencing (next-generation sequencing, NGS) (2– 5). Segmental progeroid syndromes are both clinically and genetically a very heterogeneous group of diseases. Based on the age at onset of the disease, it is differentiated between congenital, infantile, and juvenile/adult forms of SPS.
Given the significant advances that have been made in recent years in identifying the causes of SPS and a first success in the development of a causative treatment, this review is intended to provide an overview of this rapidly evolving field. Based on a selective search of the PubMed database, examples of SPS typical for each age of onset will be described and special clinical and genetic features of these syndromes will be highlighted in order to enable the reader to identify these variable and pleiotropic diseases as early as possible. Building on this information, it will be shown, using the Hutchinson–Gilford progeria syndrome as an example, how discovering the genetic cause of a rare syndrome paved the way for the first successful use of a targeted and causative treatment. Finally, it will be discussed how identifying the underlying genetic and molecular mechanisms as well as developing targeted treatment strategies for rare diseases can also help to improve the management of age-related diseases in general.
Congenital segmental progeroid syndromes
Wiedemann–Rautenstrauch syndrome
The Wiedemann–Rautenstrauch syndrome (WRS) was described by Thomas Rautenstrauch (1977) and Hans Rudolph Wiedemann (1979). Since then, about 50 patients with WRS-similar phenotype have been described (6). These patients showed prenatal growth retardation, resulting in reduced weight and length at birth. Typical clinical features include sparse hair, prominent scalp veins, triangular shape of face, sunken eyes, microstomia with maxillary hypoplasia, natal teeth, and a prominent chin (table). Perinatal respiratory problems are common among these patients. In the further course of the disease, WRS is characterized by growth delays, thin and atrophic skin, generalized lipodystrophy with local fat pads, joint contractures, progressive ataxia and tremor, as well as a global developmental delay (6). Clinical signs of premature aging include thin and atrophic skin, progressive ataxia and tremor, as well as lipodystrophy with associated cachectic appearance. This autosomal recessive syndrome is caused by mutations in the POLR3A gene which encodes a subunit of RNA polymerase III (7– 10). WRS-causing mutations are often located in introns, i.e. in non-coding regions inside a gene, and bring about defective splicing of pre-mRNA, resulting in loss of RNA-Polymerase III function. The life expectancy in patients with WRS is not yet known, but is likely to be strongly associated with the severity of perinatal respiratory problems.
Table. Clinical characteristics of selected segmental progeroid syndromes.
| Wiedemann–Rautenstrauch syndrome | PYCR1-related cutis laxa | Hutchinson–Gilford progeria syndrome | MDPL syndrome | Werner syndrome | Myotonic dystrophy type 1 | |
| Congenital abnormalities | ||||||
| Intrauterine growth retardation | + | + | − | − | − | − |
| Natal teeth | + | − | − | − | − | − |
| Perinatal respiratory problems | + | − | − | − | − | − |
| Physical abnormalities | ||||||
| Graying of scalp hair | − | − | − | − | + | − |
| Hair loss | + | + | + | − | + | + |
| Prominent scalp veins | + | + | + | − | − | − |
| Hearing loss | − | − | + | + | − | + |
| Cataract | − | − | − | − | + | + |
| Mandibular hypoplasia | + | + | + | + | + | − |
| Triangular shape of face | + | + | − | − | − | − |
| Microcephaly | − | + | − | − | − | − |
| Short stature | + | + | + | − | + | − |
| Joint contractures | + | − | + | − | − | −* |
| Hypermobility of joints | − | + | − | − | − | − |
| Dermatological abnormalities | ||||||
| Thin/atrophic skin | + | + | + | + | + | − |
| Wrinkled skin | − | + | − | − | − | − |
| Telangiectasia | − | − | − | + | − | − |
| Skin pigment disorders | − | − | ± | − | + | − |
| Ulcers | − | − | − | − | + | − |
| Hyperkeratosis | − | − | − | − | + | − |
| Metabolic abnormalities | ||||||
| Type 2 diabetes mellitus | − | − | − | + | + | + |
| Lipodystrophy | + | + | + | + | + | − |
| Hypogonadism | − | − | − | + | + | + |
| Neurological abnormalities | ||||||
| Intellectual disability | ± | + | − | − | − | −* |
| Muscle weakness | − | + | + | + | + | + |
| Myotonia | − | − | − | − | − | + |
| Other abnormalities | ||||||
| Atherosclerosis | − | − | + | − | + | − |
| Arrhythmia | − | − | − | − | − | + |
| Osteopenia | − | + | − | − | + | − |
| Osteoporosis | − | − | + | − | + | − |
| Increased cancer risk | − | − | − | − | + | + |
| Causative altered gene | POLR3A | PYCR1 | LMNA | POLD1 | WRN | DMPK |
MDPL, mandibular hypoplasia, deafness, progeroid features, and lipodystrophy;
+, typically abnormal finding; -, typically normal finding; ±, in some patients; *, only with the congenital form
PYCR1-related cutis laxa
Cutis laxa syndromes are a heterogeneous group of rare (incidence < 1:1 000 000) connective tissue disorders with the shared characteristic of wrinkled, redundant and inelastic skin, resulting in a progeroid appearance. The phenotypic variability of autosomal recessive PYCR1-related cutis laxa is considerable, but patients typically show prenatal growth retardation (11). In addition, many patients present with poor sucking ability, sparse hair, thin and transparent skin with prominent veins, mandibular hypoplasia, and lipodystrophy (table). Later, these patients may develop a global developmental delay (in some cases with cerebral malformations) with mild to moderate intellectual disability, microcephaly, and muscular hypotonia. Other clinical signs that typically manifest in the first two years after birth include osteopenia or osteoporosis, finger contractures, cataract, corneal opacity, and movement disorders. Significant progression of these signs is rare in children older than 2 years of age. The life expectancy of patients with this syndrome also remains unknown to date.
Infantile segmental progeroid syndromes
Hutchinson–Gilford progeria syndrome
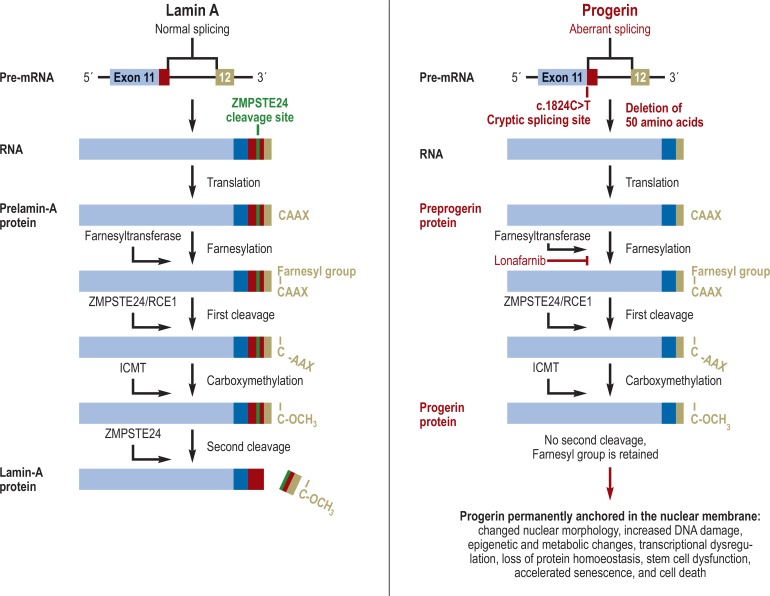
The Hutchinson–Gilford progeria syndrome (HGPS, incidence approximately 1:4 000 000) was described in 1886/1897 by Sir Jonathan Hutchinson and Hastings Gilford. While affected children have a normal appearance at birth, they typically develop failure to thrive in their first year of life. During the first, second, and third year after birth, the facial characteristics of HGPS—a hypoplastic mandible and a thin nose with a “beaked” tip—become increasingly apparent. In addition, patients experience progressive alopecia, loss of eyebrows and eyelashes, postnatal growth retardation, scleroderma-like skin changes, lipodystrophy, progressive joint contractures, and nail dystrophy (table). Over time, conductive hearing loss, osteoporosis, and incomplete dentition can also develop; in addition, some patients show insulin resistance without manifest diabetes mellitus or develop Raynaud’s syndrome. Motor and intellectual development is usually normal. Patients typical die of complications of atherosclerosis after myocardial infarction or stroke; the average life expectancy is approximately 15 years (12). The genetic cause is a recurrent, synonymous, heterozygous de novo mutation c.1824C>T (p.Gly608Gly) in exon 11 of the lamin A/C (LMNA) gene which encodes a cell membrane component (13, for details on pathogenesis, see the Figure).
Figure.
Pathogenesis of Hutchinson–Gilford progeria syndrome. The LMNA gene, which encodes lamin A (a structural protein of the nuclear membrane), contains 12 exons. The correctly spliced pre-messenger RNA (mRNA) results in lamin A which contains a detection site and cleavage site for the ZMPSTE24 enzyme within exon 11. The c.1824C>T mutation in exon 11, which is the cause of Hutchinson–Gilford progeria syndrome, activates a cryptic splicing site and causes an aberrant splicing event, removing 150 nucleotides (i.e. exactly 50 amino acids) from the mRNA. The post-translational protein modification normally consists of four steps: (1) A farnesyl group is attached by farnesyl transferase to the cysteine of the C-terminal CAAX box. (2) The last three amino acid residues are proteolytically cleaved by ZMPSTE24. (3) Carboxymethylation by ICMT. (4) The 15 C-terminal amino acids, including the farnesylated and carboxymethylated cysteine, are cleaved by ZMPSTE24. Since progerin lacks the detection site and cleavage site for the ZMPSTE24 enzyme, the second cleavage cannot occur; thus, the last 15 C-terminal amino acids, including the farnesylated cysteine are retained. As the result, progerin is permanently anchored in the nuclear membrane, influencing various cellular processes. Important progerin-associated cellular effects are shown in the Figure. Lonafarnib, a farnesyltransferase inhibitor, blocks the first step of posttranslational modification, the farnesylation. Therefore, progerin generally remains in the nucleoplasm and is hardly bound to the nuclear membrane; consequently, its effects are mitigated (adapted from [24]).
Mandibular hypoplasia, deafness, progeroid features, and lipodystrophy syndrome
Mandibular hypoplasia, deafness, progeroid features, and lipodystrophy syndrome (MDPL) is a rare (incidence <1:1 000 000) syndrome which has been characterized only recently (14). Similar to HGPS, affected children appear normal at birth. The first clinical signs rarely manifest before the fifth year of life; then, patients develop unusually thin arms and legs with broad trunk and mandibular hypoplasia. Starting at the age of 10 years, progressive lipodystrophy, dental crowding with irregular positioning of teeth, joint contractures, general muscle wasting, hearing loss, and a high-pitched voice are observed (table). Later, patients frequently develop type 2 diabetes, telangiectasia, and osteopenia or osteoporosis. The oldest described patient was 62 years old at the time of the last follow-up examination (14). The genetic cause of MDPL are heterozygous mutations in the POLD1 gene which encodes the catalytic subunit of DNA polymerase delta; the majority of patients carry a recurrent deletion of a single amino acid (p.Ser605del) (14, 15). Interestingly, other heterozygous POLD1 germline mutations are associated with an increased risk of early-onset colorectal cancer and endometrial cancer (polymerase proofreading–associated polyposis, PPAP) (16), which can be regarded as a unimodal progeroid syndrome. However, patients with MDPL appear not to have a (significantly) increased tumor risk; thus, a good genotype–phenotype correlation can be assumed for this gene (14). Some additional congenital and infantile SPS are listed in the Box.
The clinical perspective.
A segmental progeroid syndrome (SPS) should be included in the differential diagnosis of newborns presenting with a combination of growth retardation, sparse scalp hair, and wrinkled, very thin skin with prominent scalp veins. In the further course of the disease, premature (for the respective age) occurrence of age-related signs or disorders (e.g. graying/loss of hair, hearing loss, cataract, skin changes, lipodystrophy, type 2 diabetes mellitus, osteopenia, cardiovascular abnormalities, or malignancy) in more than one tissue can be strongly indicative of SPS. However, the initial signs and symptoms of SPS are frequently nonspecific, making it difficult to relate them to a specific syndrome. Additionally, more than 100 disease genes for different SPS have so far been identified; thus, it appears advisable to perform an NGS-based genetic analysis. A definite diagnosis supported by the identification of causative genetic changes enables gene-specific personalized preventive care and aftercare in an increasing number of cases. Currently, a causally defined experimental treatment is available for patients with Hutchinson–Gilford progeria syndrome. The primary strategy for the management of other SPS is to prevent or treat signs and symptoms or complications as early as possible in order to improve survival and quality of life. In many cases, interdisciplinary and regular cardiovascular, dermatological, orthopedic, ophthalmological, and endocrinological care plays a decisive role; physiotherapy and—in patients with increased cancer risk—additional oncological care may be beneficial as well in individual cases.
Juvenile/adult segmental progeroid syndromes
Werner syndrome
Werner syndrome (WS), described by Otto Werner in his medical dissertation at the University of Kiel in 1904, is a prototypical example of juvenile SPS. WS manifests during adolescence; a characteristic feature is the absence of the growth spurt seen early in puberty. Additional clinical features, such as graying/thinning of scalp hair, scleroderma-like skin changes, regional atrophy of the subcutaneous fat, and development of a high-pitched voice, usually manifest after the 20th year of life. In the further course of the disease, patients often develop bilateral cataract, type 2 diabetes, skin ulcers (typically around the ankle), osteoporosis (especially of the long bones), calcification in the Achilles tendon, hypogonadism, atherosclerosis of the coronary arteries with increased risk of myocardial infarction, as well as cancers, such as soft-tissue sarcoma, osteosarcoma, melanoma, and thyroid cancer, which are rather rare in the general population (table). The most common causes of death are cardiovascular disease and cancer; the average life expectancy is 54 years (17, 18). The genetic cause of Werner syndrome are autosomal recessive mutations in the WRN helicase gene, which encodes a RecQ-type helicase, a DNA repair protein.
Myotonic dystrophy type 1
Myotonic dystrophy type 1 (DM1) is a progressive multisystem disorder, primarily characterized by muscular dystrophy. Although there is a very rare congenital form—characterized by muscular hypotonia and very poor sucking ability, global developmental delay (frequently associated with respiratory insufficiency), and early death—, classical DM1 typically manifests no earlier than about age 20 years. As an early clinical sign, an initially distal weakness of the muscles is noted. Typically, patients present with myotonia, but may in addition develop primarily frontal alopecia, cataract, sensorineural hearing loss, dysarthria and dysphagia, cardiac arrhythmia, hypogonadism, Type 2 diabetes, and hypothyroidism (table). Genetically, the disease is caused by autosomal dominant inherited expansions of unstable CTG repeats in the DMPK gene, which encodes a kinase. The average life expectancy is 48 to 55 years (19).
First clinical studies
Clinical trials on rare diseases are difficult to conduct as there are few patients available to participate. The progressive course of the disease can be a further challenge, because conducting a placebo-controlled and randomized, double-blind study can be difficult for ethical reasons. Understanding the molecular basis of SPS is essential for developing a personalized treatment. Studies on Hutchinson–Gilford progeria syndrome led to the identification of progerin as a likely cause. This assumption was also supported by the identification of individuals with milder clinical courses who produced less progerin compared to patients with typical HGPS (20). Lamin A is modified by farnesylation and other reactions (figure) (21). Therefore, it was assumed that farnesyltransferase inhibitors (FTI), originally developed as anti-cancer agents, should, in principle, have a therapeutic potential which was then substantiated in subsequent studies using HGPS cell and mouse models (21). The first clinical study evaluating the FTI lonafarnib in 25 HGPS patients showed after a treatment period of 2 years a reduction in joint stiffness, moderate weight gain, an improvement in bone structure (22), and a survival benefit of 1.6 years (95% confidence interval: [0.8; 2.4]; p <0.001) (23). Side effects included mild diarrhea, fatigue, nausea, vomiting, anorexia, transient elevation of liver enzymes, and transient reduction in serum hemoglobin levels (22, 23). Likewise, the only recently published data from a lonafarnib-based clinical study with 63 patients demonstrated a reduction in mortality after a median treatment period of 2.2 years (hazard ratio: 0.12 [0.01; 0.93]; p = 0.04) (24).
These studies show that a drug originally developed for another indication can potentially be used for the treatment of a distinct rare disease. This approach is based on deciphering the genetic and pathophysiological roots of this disease and will have to be confirmed and/or optimized by follow-up studies.
The significance of these findings for our understanding of aging processes
The identification of the genetic causes of SPS and the functional characterization of the identified mutations in cell-/animal-based models have helped to clarify the molecular characteristics of monogenic premature aging syndromes. These findings showed in particular the significance of telomere dysfunction, genomic instability, mitochondrial dysfunction, exhaustion of the stem cell pool, cellular senescence, deregulation of the cell cycle, as well as epigenetic changes and inflammatory processes (25). Interestingly, these features of SPS are almost identical with known molecular characteristics of the “normal aging“ process (26). For example, the normal aging process is also associated with progerin accumulation (27), HGPS-like defects of the nuclear membrane, changes to histone modifications, increased genomic instability (28), and reduced expression of specific marker proteins, such as PRKDC, Ku70 and Ku80 (29). The increased epigenetic age in fibroblasts of patients with Werner syndrome is yet another example (30). Sharing not only clinical signs, but also molecular and cellular characteristics with “normal aging”, SPS represent a suitable model for research into general aging processes. However, it is important to emphasize that SPS do not represent simple phenocopies of normal aging; for example, osteoporosis in patients with Werner syndrome affects primarily the long bones, while osteoporosis in the general population primarily affects the spine (17). In addition, there are other characteristic signs of SPS, such as mandibular hypoplasia or growth retardation, which are not typical for normal aging. Nevertheless, it can already be seen that our improved understanding of the mechanism underlying SPS contribute to the development of senolytics which, for example, are designed to delay the onset of age-related disorders and thus to increase the healthy period of life by inhibiting senescence and/or apoptosis (31, 32). A recently published pilot study showed mobility improvements in participants after treatment with a senolytic; however, due to the small sample size the results should be interpreted with caution and further randomized studies are needed (33).
Outlook
SPS comprise a clinically and genetically highly heterogeneous group of hereditary disorders which resemble “normal aging” in many aspects. The various syndromes often show overlapping signs and symptoms, and in many cases significant experience is required for the clinical classification of the different syndromes. Given the marked heterogeneity, variability and complexity of the overlapping conditions (2, 3, 14, 34), an NGS-based analysis— described in greater detail by us elsewhere (35)—should be performed as the first diagnostic step in a specialized center. Early diagnosis prevents a long diagnostic journey, results in a differentiated evaluation of the risk of recurrence for parents and other relatives, and enables the creation of a personalized screening program for an increasing number of affected persons, currently especially patients with Hutchinson–Gilford progeria syndrome and Werner syndrome. In addition, a genetic diagnosis is the foundation for a hopefully increasing number of clinical trials and treatment approaches. In the long term, exploring and discovering the basis of these rare diseases will also help to obtain a better understanding of normal aging and age-related diseases, potentially paving the way for a targeted positive influence on aging.
BOX. Other segmental progeroid syndromes.
Besides the two congenital segmental progeroid syndromes (SPS) described here, there are other early-onset progeria syndromes; however, these are not discussed in detail in this article. They include Bloom syndrome, Rothmund–Thomson syndrome, dyskeratosis congenita, Hoyeraal–Hreidarsson syndrome, restrictive dermopathy, marfanoid–progeroid–lipodystrophy syndrome, CAV1-associated neonatal progeroid syndrome, LEMD2-assoziated progeroid syndrome, and Berardinelli–Seip syndrome, among others (4, 25, 36– 38).
Likewise, besides the two infantile SPS discussed here, there are numerous other syndromes with childhood onset, which could not be described in detail, again for lack of space. These include Fanconi anemia, xeroderma pigmentosum, Cockayne syndrome, ataxia teleangiectatica, trichothiodystrophy, Néstor–Guillermo syndrome, Ruijs–Aalfs syndrome, and MDM2-associated progeroid syndrome, among others (2, 3, 5, 25, 37).
Key Messages.
Segmental progeroid syndromes are rare genetic disorders characterized by signs of premature aging affecting more than one organ or tissue.
Given the variability and complexity of the partly overlapping conditions, a next generation sequencing (NGS)–based analysis performed at a specialized center should be offered as the first diagnostic step.
An early diagnosis allows a specific evaluation of the recurrence risk and can enable the creation of a personalized screening program.
The discovery of the pathophysiological basis of rare diseases can pave the way for the development of a personalized treatment, as shown using the Hutchinson–Gilford progeria syndrome as an example.
The clinical signs and molecular basis of SPS are often similar to “normal aging” and thus are a suitable model for research into general aging processes.
Acknowledgments
Translated from the original German by Ralf Thoene, MD.
Footnotes
Conflict of interest statement
The authors declare that no conflict of interest exists.
References
- 1.Martin GM. Genetic modulation of senescent phenotypes in homo sapiens. Cell. 2005;120:523–532. doi: 10.1016/j.cell.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 2.Lessel D, Wu D, Trujillo C, et al. Dysfunction of the MDM2/p53 axis is linked to premature aging. J Clin Invest. 2017;127:3598–3608. doi: 10.1172/JCI92171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lessel D, Vaz B, Halder S, et al. Mutations in SPRTN cause early onset hepatocellular carcinoma, genomic instability and progeroid features. Nat Genet. 2014;46:1239–1244. doi: 10.1038/ng.3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schrauwen I, Szelinger S, Siniard AL, et al. A frame-shift mutation in CAV1 is associated with a severe neonatal progeroid and lipodystrophy syndrome. PLoS One. 2015;10 doi: 10.1371/journal.pone.0131797. e0131797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Puente XS, Quesada V, Osorio FG, et al. Exome sequencing and functional analysis identifies BANF1 mutation as the cause of a hereditary progeroid syndrome. Am J Hum Genet. 2011;88:650–656. doi: 10.1016/j.ajhg.2011.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paolacci S, Bertola D, Franco J, et al. Wiedemann-Rautenstrauch syndrome: a phenotype analysis. Am J Med Genet A. 2017;173:1763–1772. doi: 10.1002/ajmg.a.38246. [DOI] [PubMed] [Google Scholar]
- 7.Jay AM, Conway RL, Thiffault I, et al. Neonatal progeriod syndrome associated with biallelic truncating variants in POLR3A. Am J Med Genet A. 2016;170:3343–3346. doi: 10.1002/ajmg.a.37960. [DOI] [PubMed] [Google Scholar]
- 8.Paolacci S, Li Y, Agolini E, et al. Specific combinations of biallelic POLR3A variants cause Wiedemann-Rautenstrauch syndrome. J Med Genet. 2018;55:837–846. doi: 10.1136/jmedgenet-2018-105528. [DOI] [PubMed] [Google Scholar]
- 9.Wambach JA, Wegner DJ, Patni N, et al. Bi-allelic POLR3A loss-of-function variants cause autosomal-recessive Wiedemann-Rautenstrauch syndrome. Am J Hum Genet. 2018;103:968–975. doi: 10.1016/j.ajhg.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lessel D, Ozel AB, Campbell SE, et al. Analyses of LMNA-negative juvenile progeroid cases confirms biallelic POLR3A mutations in Wiedemann-Rautenstrauch-like syndrome and expands the phenotypic spectrum of PYCR1 mutations. Hum Genet. 2018;137:921–939. doi: 10.1007/s00439-018-1957-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dimopoulou A, Fischer B, Gardeitchik T, et al. Genotype-phenotype spectrum of PYCR1-related autosomal recessive cutis laxa. Mol Genet Metab. 2013;110:352–361. doi: 10.1016/j.ymgme.2013.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Gordon LB, Brown WT, Collins FS. Adam MP, Ardinger HH, Pagon RA, et al., editors. Hutchinson-Gilford progeria syndrome 2003 Dec 12 [updated 2019 Jan 17] GeneReviews [Internet] Seattle, University of Washington 1993-2019. [PubMed] [Google Scholar]
- 13.Eriksson M, Brown WT, Gordon LB, et al. Recurrent de novo point mutations in lamin A cause Hutchinson-Gilford progeria syndrome. Nature. 2003;423:293–298. doi: 10.1038/nature01629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lessel D, Hisama FM, Szakszon K, et al. POLD1 germline mutations in patients initially diagnosed with Werner Syndrome. Hum Mutat. 2015;36:1070–1079. doi: 10.1002/humu.22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weedon MN, Ellard S, Prindle MJ, et al. An in-frame deletion at the polymerase active site of POLD1 causes a multisystem disorder with lipodystrophy. Nat Genet. 2013;45:947–950. doi: 10.1038/ng.2670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Palles C, Cazier JB, Howarth KM, et al. Germline mutations affecting the proofreading domains of POLE and POLD1 predispose to colorectal adenomas and carcinomas. Nat Genet. 2013;45:136–144. doi: 10.1038/ng.2503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lessel D, Oshima J, Kubisch C. [Werner syndrome A prototypical form of segmental progeria.] Med Genet. 2012;24:262–267. doi: 10.1007/s11825-012-0360-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oshima J, Martin GM, Hisama FM, et al. Adam MP, Ardinger HH, Pagon RA, editors. Werner syndrome 2002 Dec 2 [updated 2016 Sep 29] GeneReviews. Seattle, University of Washington 1993-2019. [PubMed] [Google Scholar]
- 19.Bird TD. Adam MP, Ardinger HH, Pagon RA, et al., editors. Myotonic dystrophy type 1 1999 Sep 17 [updated 2018 Dec 6] GeneReviews. Seattle, University of Washington 1993-2019 [Google Scholar]
- 20.Hisama FM, Lessel D, Leistritz D, et al. Coronary artery disease in a Werner syndrome-like form of progeria characterized by low levels of progerin, a splice variant of lamin A. Am J Med Genet A. 2011;155A:3002–3006. doi: 10.1002/ajmg.a.34336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gordon LB, Rothman FG, Lopez-Otin C, Misteli T. Progeria: a paradigm for translational medicine. Cell. 2014;156:400–407. doi: 10.1016/j.cell.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordon LB, Kleinman ME, Miller DT, et al. Clinical trial of a farnesyltransferase inhibitor in children with Hutchinson-Gilford progeria syndrome. Proc Natl Acad Sci USA. 2012;109:16666–16671. doi: 10.1073/pnas.1202529109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon LB, Massaro J, D‘Agostino RB Sr, et al. Impact of farnesylation inhibitors on survival in Hutchinson-Gilford progeria syndrome. Circulation. 2014;130:27–34. doi: 10.1161/CIRCULATIONAHA.113.008285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gordon LB, Shappell H, Massaro J, et al. Association of lonafarnib treatment vs no treatment with mortality rate in patients with Hutchinson-Gilford progeria syndrome. JAMA. 2018;319:1687–1695. doi: 10.1001/jama.2018.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carrero D, Soria-Valles C, Lopez-Otin C. Hallmarks of progeroid syndromes: lessons from mice and reprogrammed cells. Dis Model Mech. 2016;9:719–735. doi: 10.1242/dmm.024711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McClintock D, Ratner D, Lokuge M, et al. The mutant form of lamin A that causes Hutchinson-Gilford progeria is a biomarker of cellular aging in human skin. PLoS One. 2007;2 doi: 10.1371/journal.pone.0001269. e1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Scaffidi P, Misteli T. Lamin A-dependent nuclear defects in human aging. Science. 2006;312:1059–1063. doi: 10.1126/science.1127168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu GH, Barkho BZ, Ruiz S, et al. Recapitulation of premature ageing with iPSCs from Hutchinson-Gilford progeria syndrome. Nature. 2011;472:221–225. doi: 10.1038/nature09879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maierhofer A, Flunkert J, Oshima J, Martin GM, Haaf T, Horvath S. Accelerated epigenetic aging in Werner syndrome. Aging (Albany NY) 2017;9:1143–1152. doi: 10.18632/aging.101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD. The clinical potential of senolytic drugs. J Am Geriatr Soc. 2017;65:2297–2301. doi: 10.1111/jgs.14969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhu Y, Tchkonia T, Pirtskhalava T, et al. The Achilles‘ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell. 2015;14:644–658. doi: 10.1111/acel.12344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Justice JN, Nambiar AM, Tchkonia T, et al. Senolytics in idiopathic pulmonary fibrosis: results from a first-in-human, open-label, pilot study. EBioMedicine. 2019;40:554–563. doi: 10.1016/j.ebiom.2018.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lessel D, Saha B, Hisama F, et al. Atypical Aicardi-Goutieres syndrome: is the WRN locus a modifier? Am J Med Genet A. 2014;164A:2510–2513. doi: 10.1002/ajmg.a.36664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mahler EA, Johannsen J, Tsiakas K, et al. Exome sequencing in children. Dtsch Arztebl Int. 2019;116:197–204. doi: 10.3238/arztebl.2019.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Passarge E, Robinson PN, Graul-Neumann LM. Marfanoid-progeroid-lipodystrophy syndrome: a newly recognized fibrillinopathy. Eur J Hum Genet. 2016;24:1244–1247. doi: 10.1038/ejhg.2016.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kipling D, Davis T, Ostler EL, Faragher RG. What can progeroid syndromes tell us about human aging? Science. 2004;305:1426–1431. doi: 10.1126/science.1102587. [DOI] [PubMed] [Google Scholar]
- 38.Marbach F, Rustad CF, Riess A, et al. The discovery of a LEMD2-associated nuclear envelopathy with early progeroid appearance suggests advanced applications for aI-driven facial phenotyping. Am J Hum Genet. 2019;104:749–757. doi: 10.1016/j.ajhg.2019.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]