Abstract
Despite the lack of its toxicity evaluation, traditional herbal products are being widely used for various health indications. Geraniin, an ellagitannin, is a bioactive compound found in many traditional herbal medicines. In spite its numerous health benefits ranging from anti-inflammatory, anti-hyperglycaemic, hepatoprotective, anti-cancer and anti-microbial, no toxicity data on geraniin is available. The objective of this study is to evaluate the acute oral toxicity of geraniin and an enriched geraniin-extract of Nephelium lappaceum L rind. This study followed the guidelines of the OECD 423 acute oral toxicity test. Subsequent to a single oral administration of the test compounds, the rats were observed for 14 days for signs of toxicity and mortality. Following euthanasia, full blood count, biochemistry of blood and histopathology assessment of organs were carried out. All parameters analysed indicated insignificant difference compared to control. The LD50 cut-off values for both geraniin and geraniin-enriched extract was established to be 2000 mg/kg b. w., following a single oral dose. It was however observed that the hepatocytes of three geraniin-administered rats exhibited a ‘foamy appearance’. As such, the no-observed-adverse-effect level of geraniin is below 2000 mg/kg, while that of geraniin-enriched extract is up to 2000 mg/kg. Further detailed toxicity studies are required to establish geraniin or its enriched extract from Nephelium lappaceum L rind safe for human consumption.
Keywords: Toxicology, Nephelium lappaceum, Geraniin, Acute oral toxicity, Medicinal herbs, Ellagitannin
1. Introduction
Herbal products (HPs) are gaining popularity across the globe due to their various health promoting properties (WHO, 2004), and are therefore being widely sold in pharmacies, supermarkets [1] and online platforms [2]. The global market of HPs is expected to grow annually by 7.2% from 2017 to 2023, and is estimated to be valued at $111 billion by the year 2023 [3], pointing to the vast consumption of HPs.
HPs are often regarded to be safer compared to synthetic drugs. However, the possibility of adverse effects associated with the consumption of HPs cannot be discounted. Yet, most of the HPs are not evaluated for their toxic effects and information on safety margin lacks significantly [1].
Traditionally, geraniin-enriched plant extracts belonging to mainly the species of Geranium and Phyllanthus were consumed to treat certain disorders; G. thunbergii Sieb. et Zucc as an antidiarrhetic [4], Geranium carolinianum L in treating rheumatic arthritis [5] and Geranium sibiricum Linne in the treatment of dysentery and enteritis [6]. P. amarus and P. emblica for jaundice [7, 8]. Scientifically geraniin was shown to exhibit various bioactive properties ranging from anti-hypertensive [9], hepatoprotective [10], anti-hyperglycaemic [11] and anti-cancer [12]. In fact, P. niruri containing geraniin is also being commercially sold under various brand names such as Hepar R for its hepatoprotective activity.
Acute oral toxicity test (AOTT) provides valuable preliminary data that are useful in case of accidental ingestion of a lethal dose of a substance, the organs affected and dosage selection for subsequent toxicity studies such as sub-chronic test [13]. The generated data is also beneficial in establishing the therapeutic index of a compound. Subsequently, AOTT provides assurance on the safety of the tested substance. As described above, although geraniin have been shown to exhibit various biological properties, and is found in a number of traditional herbal preparation currently in the market, extensive toxicity data is lacking.
The acute and sub-chronic assessments of the N. lappaceum rind extracts in rats has however been reported by Subramaniam e al (2012) [14] and Thinkratok et al (2014) [22]. Subramaniam et al (2012) while using a crude preparation only reported histological examination of the liver and kidney while excluding all other major organs. Thinkratok and coworkers (2014) on the other hand, used a different preparation of the N. lappaceum crude extract and did not perform histological examination. A comprehensive and detailed acute oral toxicity of both geraniin and a standardised geraniin-enriched extract is warranted, in light of the growing interest in geraniin and its enriched extract.
2. Materials and methods
2.1. Materials and reagents
Reverse-phase C18 silica (particle size 50 μm, pore size 60 Å) (Davisil, GRACE), denatured ethanol, 95% (Systerm), methanol (Merck), acetonitrile (ACN) (Fisher), dichloromethane (DCM) (Merck), trifluoroacetic acid (TFA) (Merck), diethyl ether (Merck), formic acid (FA) (Sigma Aldrich, St. Louis, MO, USA).
2.2. Preparation of N. lappaceum rind powder
N. lappaceum L. fruits were purchased in Kuala Lumpur, Malaysia, which was later authenticated by the Herbarium of the Forest Research Institute of Malaysia (FRIM). The rind was washed with distilled water and air dried at room temperature for around 2–3 h. The rind was completely dried in a circulation oven at 40 °C. The dried rind was then powderised using a blender and Fritsch dry miller.
2.3. Preparation of a standardised geraniin-enriched extract and geraniin from N. lappaceum rind
The geraniin-enriched extract was prepared as previously described [15]. The powdered rind was extracted with 95% denatured ethanol (1:10), filtered and the resulting filtrate was concentrated using rotary evaporator. This was followed by a freeze-drying process to obtain a dry geraniin-enriched extract ethanolic extract. The presence of geraniin and its quantification in the extract was confirmed on HPLC. Purification of geraniin from the geraniin-enriched extract was carried out using reverse phase chromatography as previously described [16]. Briefly, following column packing with Davisil C18 silica (160g), the geraniin-enriched extract (20g) was mixed thoroughly with 40 mL of distilled water (dH2O) and gently loaded onto the packed glass column. This was followed by elution with water to remove impurities and geraniin was purified using acetonitrile and water in a step-wise gradient. Ten percent acetonitrile fractions containing geraniin were evaporated and its purity was confirmed using HPLC.
2.4. Animal husbandry
Acute oral toxicity in Sprague Dawley (SD) rats were performed according to OECD 423 guidelines. Animal experimentation commenced soon after the receipt of animal ethics approval by the Monash University Monash Animal Research Platform Animal Ethics Committee (AEC approval No.: MARP/2017/01). Female Sprague Dawley rats of 8-weeks old were purchased from Monash Animal research Platform. Following one week of acclimatization, the rats were randomly divided into three groups. The control group was administered with distilled water (1mL/100g), while the treatment groups were gavaged with single doses of 2000 mg/kg of geraniin and geraniin-enriched extract respectively. Body weight, food consumption and fluid intake were recorded thrice a week. On day 14, the rats were anaesthetized by intraperitoneal injection of ketamine (100 mg/kg) and xylazine (10 mg/kg). Exsanguination was carried out by cardiac puncture and the rats were humanely killed and organs were removed for further analysis.
2.4.1. Observation – mortality and clinical signs
Following administration of geraniin and geraniin-enriched extract, the rats were observed hourly for the first 24 h and daily for a total of 2 weeks (14 days). Attention was given to changes in skin, fur, mucous membrane, eyes, respiration and behaviour patterns.
2.4.2. Hematology and biochemistry
Blood samples were delivered to Hematology Department, Faculty of Veterinary, University Putra Malaysia for full blood count and biochemical evaluation. Full blood count investigating red blood cell (RBC), hemaglobin (Hb), mean corpuscular volume (MCV), mean corpuscular hemaglobin concentration MCHC and white blood cell indices (WBC) were performed. While, biochemical analysis include aspartate transaminase (AST), alanine transaminase (ALT), alkaline phosphatase (ALP), gama-glutamyltransferase (GGT) total protein (TP), albumin (ALB), globulin (GLO), albumin to globulin ratio (A:G), creatinine (Cr), urea (U), glucose, direct bilirubin (Dbil), total bilirubin (Tbil), cholesterol, and triglyceride (TAG) evaluation.
2.4.3. Histopathology
Organs (liver, kidney, stomach, intestine, spleen, pancreas, lungs, heart, brain, ovaries) were fixed in 10% phosphate-buffered formalin. Following tissue processing, embedding and sectioning, the tissues were stained with haematoxylin and eosin stains. The stained slides were examined with the assistance of a pathologist. The tissue sections were given scores based on the architecture; ‘0‘ = absence of lesion; ‘1’ = <25% tissue affected; ‘2’ = 26–50% tissue affected; ‘3’ = 51–75% tissue affected and ‘4’ = 76–100% of tissue affected [17].
2.5. Statistical analysis
Data are expressed as mean ± SEM. Parametric and non-parametric tests were carried out following test of normality using software Prism version 5.0 (GraphPad, San Diego, CA, USA), where p < 0.05 was considered as statistically significant. Two-way ANOVA with Bonferroni's post hoc, one-way Analysis of Variance (ANOVA) with LSD post-hoc, Kruskal-Wallis H and Mann-Whitney U tests were used to compare means among the groups.
3. Results
3.1. Standardised preparation of geraniin-enriched extract from N. lappaceum rind
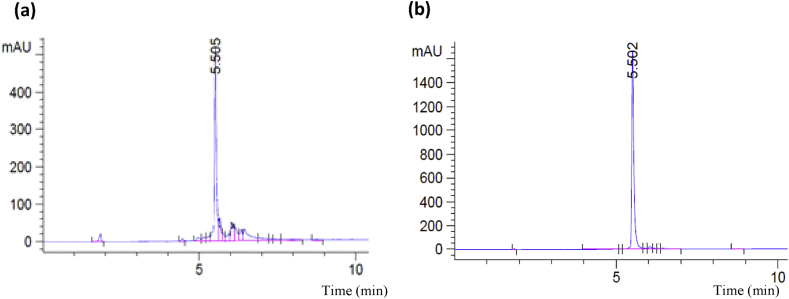
N.lappaceum rind powder yielded ∼13 g of crude extract for every 50g of the rind powder. The crude extract contained 45% geraniin (Fig. 1a) determined using calibrated pure geraniin.
Fig. 1.
(a) HPLC chromatogram of ethanolic extract of N. lappaceum rind. (b) HPLC chromatogram of geraniin crystals. Column - Chromolith Performance RP-18, mobile phase-water (Solvent A) and acetonitrile (Solvent B), flow rate- 0.5 mL/min, injection volume- 5μL, abs- 254 nm.
3.2. Preparation of geraniin from N.lappaceum rind
Twenty grams of crude generated ∼2 g of geraniin crystals (10 % yield) with a purity of >90 % (Fig. 1b). The geraniin crystals were concentrated in fractions 3, 4 and 5 which were eluted in 10:90 ACN:water.
3.3. Acute oral toxicity test
3.3.1. Observation
One rat died 48 h post-administration of geraniin. Necropsy of the rat revealed formation of blood clot around the thoracic region, indicating death due to flawed gavaging technique. All geraniin-administered rats had diarrhea for the initial 4 h. No other clinical signs were observed in all groups.
3.3.2. Necropsy
Gross pathological changes were absent in rats administered with geraniin and geraniin-enriched extract at a single dose of 2000 mg/kg. All organs exhibited normal architecture similar to the vehicle-administered group.
3.3.3. Body weight, food, fluid intake, relative organ weight
Body weight, water intake and relative organ weight of geraniin and geraniin-enriched extract groups were not significantly different compared to control. Geraniin-administered rats consumed significantly higher feed compared to control and crude.
3.3.4. Hematology and biochemical parameters
The full blood count (Table 1), liver function (Table 2), kidney function (Table 3), glucose and lipid profiles (Table 4) of the rats were not statistically affected by the administration of geraniin and geraniin-enriched extract.
Table 1.
Full blood count of control, geraniin and geraniin-enriched extract –administered rats.
| Parameters | Control | Geraniin | Geraniin-enriched extract |
|---|---|---|---|
| RBC (x1012/L) | 7.70 ± 0.30 | 7.85 ± 0.33 | 8.11 ± 0.23 |
| Hb (g/L) | 166.50 ± 2.40 | 163.40 ± 6.95 | 160.33 ± 3.30 |
| MCV (fL) | 59.50 ± 2.60 | 56.00 ± 1.30 | 54.50 ± 1.38 |
| MCHC (g/L) | 366.00 ± 6.48 | 372.20 ± 5.85 | 361.83 ± 6.34 |
| WBC (g/L) | 5.90 ± 0.88 | 5.90 ± 0.69 | 6.92 ± 0.79 |
Hb-hemaglobin, MCV - mean corpuscular volume, MCHC - mean corpuscular hemaglobin concentration, RBC - red blood cell, WBC -white blood cell indices.
Table 2.
Liver function profile of control, geraniin and geraniin-enriched extract –administered rats.
| Parameters | Control | Geraniin | Geraniin-enriched extract |
|---|---|---|---|
| ALT (U/L) | 43.40 ± 3.82 | 44.60 ± 3.54 | 39.83 ± 2.01 |
| ALP (U/L) | 150.60 ± 15.62 | 190.80 ± 22.92 | 147.17 ± 9.97 |
| AST (U/L) | 115.25 ± 17.72 | 110.83 ± 10.05 | 103.80 ± 9.08 |
| GGT (U/L) | <2 | <2 | <2 |
| TP (g/L) | 72.04 ± 1.61 | 74.40 ± 1.16 | 71.65 ± 1.64 |
| ALB (g/L) | 31.48 ± 0.432 | 30.68 ± 0.60 | 30.00 ± 0.50 |
| GLO (g/L) | 40.56 ± 1.39 | 43.72 ± 1.31 | 41.65 ± 1.53 |
| A:G | 0.78 ± 0.04 | 0.68 ± 0.04 | 0.72 ± 0.03 |
| DBIL (mmol/L) | 0.82 ± 0.15 | 1.12 ± 0.06 | 0.80 ± 0.13 |
| TBIL (mmol/L) | 1.64 ± 0.19 | 1.72 ± 0.15 | 1.35 ± 0.17 |
A:G-albumin to globulin ratio, ALP-Alkaline phosphatase, ALB-albumin, ALT -Alanine transaminase, GGT - Gama-glutamyltransferase, GLO-globulin, TP - total protein, Dbil - direct bilirubin, Tbil - total bilirubin.
Table 3.
Kidney function test of control, geraniin and geraniin-enriched extract –administered rats.
| Parameters | Control | Geraniin | Geraniin-enriched extract |
|---|---|---|---|
| Creatinine (μmol/L) | 56.60 ± 0.87 | 52.60 ± 2.67 | 45.00 ± 1.92 |
| Urea (μmol/L) | 6.56 ± 0.47 | 6.48 ± 0.62 | 5.90 ± 0.54 |
Table 4.
Glucose and lipid profile of control, geraniin and geraniin-enriched extract –administered rats.
| Parameters | Control | Geraniin | Geraniin-enriched extract |
|---|---|---|---|
| Glucose (mmol/L) | 11.82 ± 1.28 | 13.88 ± 0.43 | 13.08 ± 0.93 |
| Cholesterol (mmol/L) | 1.84 ± 0.14 | 1.94 ± 0.11 | 1.87 ± 0.14 |
| TAG (mmol/L) | 0.82 ± 0.14 | 0.90 ± 0.16 | 0.83 ± 0.20 |
TAG - triglyceride (TAG).
3.3.5. Histopathology
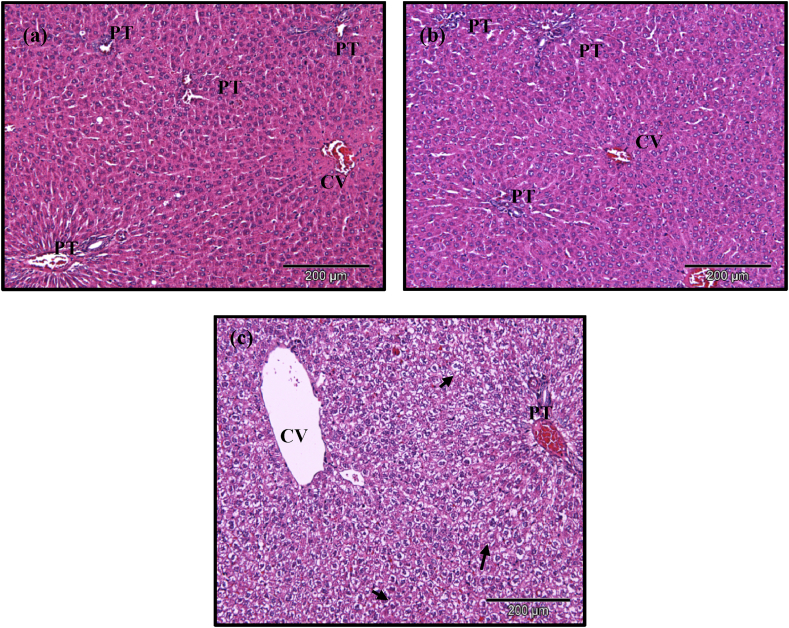
Histopathological examination of all the major organs revealed intact architecture following administration of geraniin and geraniin-enriched extract. However, the livers of three rats administered with geraniin showed significant presence of ‘foamy appearance’ of the hepatocytes (Fig. 2) compared to the control and crude.
Fig. 2.
H&E stained liver sections of (a) control, (b) geraniin-enriched extract, and (c) geraniin groups. Control and geraniin-enriched extract groups show intact liver architecture while 60% of the animals in the geraniin group exhibited foamy hepatocytes (a few has been highlighted). CV-central vein, PT-portal triad. x100.
4. Discussion
The OECD 423 is a guideline that assess the mortality or the moribund state of test animals following administration of a compound. Based on the criteria established within this standard, the geraniin and geraniin-enriched extract at a single oral dose of 2000 mg/kg did not cause any mortality. Thus, the LD50 cut-off value for the geraniin used in our study, which has 90 % purity, is above 2000 mg/kg b. w. Therefore, we estimate that pure geraniin would have a LD50 cut-off value of 1800 mg/kg b. w. Similarly, the LD50 cut-off value for the geraniin-enriched extract was also found to be above 2000 mg/kg b. w.
Administration of geraniin and geraniin-enriched extract did not affect body weight, though the daily food intake of geraniin-administered group was significantly higher compared to control and geraniin-enriched extract. The relative organ weights were also not significantly affected compared to control. This indicates, geraniin and the enriched extract did not affect any vital organs of the rats. Nonetheless, all geraniin-administered rats had diarrhea for the first 4 h which ceased soon after food intake. Microscopic examination of stomach and intestines, however, revealed intact histology. The exact mechanism underlying this effect cannot be derived from this study. Nevertheless, a substance is identified to cause diarrhea if it acts as an intestinal mucosal irritant leading to increased peristaltic activity [18], it has been proven to be poorly bioavailable if the consumed substance is indigestible [19] or absence of intestinal enzymes for the digestion of the ingested substance [20].
Negative modulation of hematology markers in animal studies is a significant predictor of adverse reactions in human [21]. None of the blood parameters assessed were affected by geraniin or geraniin-enriched extract. Normal range in RBC, Hb, MCV, MCHC and WBC indicate geraniin and geraniin-enriched extract did not interfere with the hematopoietic process. A similar finding was observed by Thinkratok et al (2014) [22], whereby a 30 d administration of 2000 mg/kg of N. lapppaceum crude extract did not affect the full blood count profile.
Histopathological examination of the liver showed presence of foamy hepatocytes in three out of the five rats. Foamy hepatocytes, also referred as feathery degeneration often seen in high fat diet induced model [23]. In our study however, the plasma total cholesterol and TAG levels were within normal range. In addition, the rats were fed a standard chow and not the high fat diet. Therefore, the foamy appearance is unrelated to the diet or fat deposition in the liver. We were unable to do oil red O staining to confirm if the observation was indeed attributed to fat deposition as the liver sections were not snap frozen. The foamy appearance was only observed in the geraniin supplemented group and not in the enriched-extract group, as such we postulate that this is due to the higher concentration of geraniin itself and not its impurities. However, although foamy appearance was observed in the geraniin treated group, other markers indicative of hepatotoxicity were not observed (ALT, AST, ALP, GGT). It is however important to note that geraniin at lower doses (50–200 mg/kg) have been shown to have hepatoprotective ability [11, 24, 25, 26].
It can be concluded the LD50 cut-off value for the geraniin used in our study, which has 90 % purity, is above 2000 mg/kg b. w. Therefore, we estimate that pure geraniin would have a LD50 cut-off value of 1800 mg/kg b. w. Similarly, the LD50 cut-off value for the geraniin-enriched extract was also found to be above 2000 mg/kg b. w. The No-Observed-Adverse-Effect Level (NOAEL) for geraniin is below 2000 mg/kg, which corresponds to 1800 mg/kg based on the 90% purity of the geraniin, while for the geraniin-enriched extract, the NOAEL is up to 2000 mg/kg. It is therefore important that a sub-chronic toxicity study with geraniin to be carried out in order to elucidate the liver pathology.
Declarations
Author contribution statement
Mohanambal Moorthy: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Khoo Joon Joon: Analyzed and interpreted the data; Wrote the paper.
Uma D. Palanisamy: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Ministry of Higher Education, FRGS/1/2017/SKK08/MUSM/02/2, Monash University, Tropical Medicine and Biology (TMB) Platform.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
References
- 1.Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2013;4(177):1–10. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Owens C., Baergen R., Puckett D. Online sources of herbal product information. Am. J. Med. 2014;127(2):109–115. doi: 10.1016/j.amjmed.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 3.Reuters. Future Trend of Herbal Medicine Market 2018 Scope | at a CAGR of ∼ 7.2 % during 2017 to 2023 | Increasing Demand for Safe Therapies 2018 [Available from: https://www.reuters.com/brandfeatures/venture-capital/article?id=32992.
- 4.Okabe S., Suganuma M., Imayoshi Y., Taniguchi S., Yoshida T., Fujiki H. New TNF-areleasing inhibitors, geraniin and corilagin, in leaves of acer nikoense, megusurino-ki. Biol. Pharm. Bull. 2001;24(10) doi: 10.1248/bpb.24.1145. 1145—8. [DOI] [PubMed] [Google Scholar]
- 5.Li J., Huang H., Zhou W., Feng M., Zhou P. Anti-hepatitis B virus activities of Geranium carolinianum L. Extracts and identification of the active components. Biol. Pharm. Bull. 2008;31(4):743–747. doi: 10.1248/bpb.31.743. [DOI] [PubMed] [Google Scholar]
- 6.Yang Y.-C., Li J., Zu Y.-G., Fu Y.-J., Luo M., Wu N. Optimisation of microwave-assisted enzymatic extraction of corilagin and geraniin from Geranium sibiricum Linne and evaluation of antioxidant activity. Food Chem. 2010;122(1):373–380. [Google Scholar]
- 7.Notka F., Meier G., Wagner R. Inhibition of wild-type human immunodeficiency virus and reverse transcriptase inhibitor-resistant variants by Phyllanthus amarus. Antivir. Res. 2003;58(2):175–186. doi: 10.1016/s0166-3542(02)00213-9. [DOI] [PubMed] [Google Scholar]
- 8.Yokozawa T., Kim H., Kim H., Tanaka T., Sugino H., Okubo T. Amla (Emblica officinalis gaertn.) attenuates age-related renal dysfunction by oxidative stress. J. Agric. Food Chem. 2007;55(19):7744–7752. doi: 10.1021/jf072105s. [DOI] [PubMed] [Google Scholar]
- 9.Moreno E., Gayosso J.A., Montejano J.R., Almaguer G., Vázquez N., Cruz C. Geraniin is a diuretic by inhibiting the Na+-K+-2Cl− cotransporter NKCC2. Am. J. Physiol. Renal. Physiol. 2018;314(2):F240–F250. doi: 10.1152/ajprenal.00221.2017. [DOI] [PubMed] [Google Scholar]
- 10.Xiao F., Zhai Z., Jiang C., Liu X., Li H., Qu X. Geraniin suppresses RANKL-induced osteoclastogenesis in vitro and ameliorates wear particle-induced osteolysis in mouse model. Exp. Cell Res. 2015;330(1):91–101. doi: 10.1016/j.yexcr.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Chung A.P.Y.S., Ton S.H., Gurtu S., Palanisamy U.D. Ellagitannin geraniin supplementation ameliorates metabolic risks in high-fat diet-induced obese Sprague Dawley rats. J. Funct. Foods. 2014;9:173–182. [Google Scholar]
- 12.Wang Y., Wan D., Zhou R., Zhong W., Lu S., Chai Y. Geraniin inhibits migration and invasion of human osteosarcoma cancer cells through regulation of PI3K/Akt and ERK1/2 signaling pathways. Anti Canccer Drugs. 2017;28(9):959–966. doi: 10.1097/CAD.0000000000000535. [DOI] [PubMed] [Google Scholar]
- 13.Asare G.A., Addo P., Bugyei K., Gyan B., Adjei S., Otu-Nyarko L.S. Acute toxicity studies of aqueous leaf extract of Phyllanthus niruri. Interdiscip. Toxicol. 2011;4(4):206–210. doi: 10.2478/v10102-011-0031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Subramaniam S., Chakravathi S.K., Palanisamy U., Radhakrishnan A., Haleagrahara N. Acute and sub chronic oral toxicity assessment of the ethanolic extract from the rind of Naphelium lappaceum in rats. J. Pharmacol. Toxicol. 2012;7(8):378–385. [Google Scholar]
- 15.Palanisamy U., Cheng H.M., Masilamani T., Subramaniam T., Ling L.T., Radhakrishnan A.K. Rind of the rambutan, Nephelium lappaceum, a potential source of natural antioxidants. Food Chem. 2008;109:54–63. doi: 10.1016/j.foodchem.2007.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Perera A., Appleton D., Loh H.Y., Elendran S., Palanisamy U.D. Large scale purification of geraniin from Nephelium lappaceum rind waste using reverse-phase chromatography. Separ. Purif. Technol. 2012;98:145–149. [Google Scholar]
- 17.Gibson-Corley K.N., Olivier A.K., Meyerholz D.K. Principles for valid histopathologic scoring in research. Vet. Pathol. 2013;50(6):1007–1015. doi: 10.1177/0300985813485099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choudhary G. Antidiarrhoeal activity of ethanolic extract of onosma bracteatum wall. Int. J. Adv. Pharm. Biol. Chem. 2012;1(3):402–405. [Google Scholar]
- 19.Baldi F., Bianco M.A., Nardone G., Pilotto A., Zamparo E. Focus on acute diarrhoeal disease. World J. Gastroenterol. 2009;15(27):3341–3348. doi: 10.3748/wjg.15.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Podewils L.J., Mintz E.D., Nataro J.P., Parashar U.D. Acute, infectious diarrhea among children in developing countries. Semin. Pediatr. Infect. Dis. 2004;15(3):155–168. doi: 10.1053/j.spid.2004.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Olson H., Betton G., Robinson D., Thomas K., Monro A., Kolaja G. Concordance of the toxicity of pharmaceuticals in humans and in animals. Regul. Toxicol. Pharmacol. 2000;32(1):56–67. doi: 10.1006/rtph.2000.1399. [DOI] [PubMed] [Google Scholar]
- 22.Thinkratok A., Suwannaprapha P., Srisawat R. Safety assessment of hydroethanolic rambutan rind extract: acute and sub-chronic toxicity studies. Indian J. Exp. Biol. 2014;52:989–995. [PubMed] [Google Scholar]
- 23.Greaves P. third ed. 2007. Liver and Pancreas. Histopathology of Preclinical Toxicity Studies: Interpretation and Relevance in Drug Safety Evaluation; pp. 1–953. [Google Scholar]
- 24.Aayadi H., Mittal S.P.K., Deshpande A., Gore M., Ghaskadbi S.S. Protective effect of geraniin against carbon tetrachloride induced acute hepatotoxicity in Swiss albino mice. Biochem. Biophys. Res. Commun. 2017;487(1):62–67. doi: 10.1016/j.bbrc.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 25.Ambrose S., Solairaj P., Subramoniam A. Hepatoprotective activity of active fractions of Thespesia lampas Dalz and Gibs (Malvaceae) J. Pharmacol. Pharmacother. 2012;3(4):326–328. doi: 10.4103/0976-500X.103691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Madrigal-Santillán E., Bautista M., Gayosso-De-Lucio J.A., Reyes-Rosales Y., Posadas-Mondragón A., Morales-González A. Hepatoprotective effect of Geranium schiedeanum against ethanol toxicity during liver regeneration. World J. Gastroenterol. 2015 July 7;21(25):7718–7729. doi: 10.3748/wjg.v21.i25.7718. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]