Abstract
Breast cancer is a heterogeneous disease comprising the estrogen receptor (ER)–positive luminal subtype which is subdivided into luminal A and luminal B and ER-negative breast cancer which includes the triple-negative subtype. This study has four aims: 1) to examine whether Minichromosome Maintenance (MCM)2, MCM4, and MCM6 can be used as markers to differentiate between luminal A and luminal B subtypes; 2) to study whether MCM2, MCM4, and MCM6 are highly expressed in triple-negative breast cancer, as there is an urgent need to search for surrogate markers in this aggressive subtype, for drug development purposes; 3) to compare the prognostic values of these markers in predicting relapse-free survival; and 4) to compare the three approaches used for scoring the protein expression of these markers by immunohistochemistry (IHC). MCM2, MCM4, MCM6, and MKI67 mRNA expression was first studied using in silico analysis of available breast cancer datasets. We next used IHC to evaluate their protein expression on tissue microarrays using three scoring methods. MCM2, MCM4, and MCM6 can help in distinction between luminal A and luminal B whose therapeutic management and clinical outcomes are different. MCM2, MCM4, MCM6, and Ki-67 are highly expressed in breast cancer of high histological grades that comprise clinically aggressive tumors such as luminal B, HER2-positive, and triple-negative subtypes. Low transcript expression of these markers is associated with increased probability of relapse-free survival. A positive relationship exists among the three scoring methods of each of the four markers. An independent validation cohort is needed to confirm their clinical utility.
Abbreviations: %, percentage score; DP, digital pathology; ER, estrogen receptor; FFPE, formalin-fixed, paraffin-embedded; HER2, human epidermal growth factor receptor 2; HPS, hematoxylinphloxine saffron; IHC, immunohistochemistry; IHS, Immunohistochemical score; MCM, Minichromosome Maintenance; SBR-EE, Scarff-Bloom-Richardson-Elston-Ellis; TCGA, The Cancer Genome Atlas; TMA, tissue microarray; Vis.Tis.S., Visiomorph Tissuemorph score
Introduction
Breast cancer is the most frequent neoplasia in women worldwide with an estimated 2.09 million new cases diagnosed in 2018 [1]. According to the American Cancer Society, it is estimated that 268,600 new cases of invasive breast cancer will be diagnosed among women in the United States in 2019, resulting in an estimated 41,760 deaths from breast cancer [2]. In Canada, it had been estimated that 26,300 new cases of breast cancer will be diagnosed among women in 2017 representing 26% of all new female cases [3]. Breast cancer is a complex and heterogeneous disease characterized by a wide range of clinical and pathological features, peculiar morphological characteristics, distinct molecular subtypes, and diverse responses to treatment [4].The different molecular subtypes of breast cancer, which were principally recognized by gene expression profiling, display characteristic gene expression patterns that translate into characteristic disease phenotypes and variable prognosis [5]. The immunohistochemical expression of four representative markers—ER, PR, HER2, and Ki-67—can, to a certain extent, be used to clinically approximate the molecular subtypes [6], [7], [8]. Using gene expression profiling, breast cancer was subgrouped into estrogen receptor–positive (ER+) luminal subtype and estrogen receptor–negative (ER−) which includes HER2-positive and triple-negative breast cancer [5]. Further studies revealed that the luminal subgroup can be stratified into two groups: luminal A and luminal B. These two types differ considerably at the molecular level as ER-related genes show the highest level of expression, while proliferation-related genes show the lowest level of expression in luminal A breast cancer, whereas luminal B shows the opposite pattern of gene expression [9].
The expression levels of cell-cycle regulated genes which control cell proliferation constitute the “proliferation signature” of tumor cells [10], a feature that has been detected in various types of malignancies [11], [12], [13], [14]. As the proliferative capacity of breast cancer exerts a strong influence on the clinical behavior, prognosis, and aggressiveness of the tumor, strict measurement of cell proliferation may guide the selection of the appropriate therapy [15]. Due to its utmost importance, proliferation constitutes the highest weight component in the Oncotype DX recurrence score [16]. Although measurement of tumor multigene expression proliferation signature in an automated and quantitative manner by DNA microarray or RNA-seq is an interesting option, it is not yet feasible in the daily clinical practice [10], [17]. There is a crucial need for an easy-to-use, histological-based proliferation assay for routine clinical assessment of breast cancer by the pathologist. Currently, proliferation is assessed in tissues by the mitotic index and by immunohistochemistry (IHC) of some proliferation-associated markers such as Ki-67 [15], [18].
Ki-67, encoded by MKI67 gene, is one of the most important cell proliferation-related markers. It has been proposed as a proliferative marker with the hope to distinguish luminal A from luminal B breast cancer [8], [19]. However, Ki-67 estimation in breast cancer has not yet succeeded to enforce itself as a powerful proliferative biomarker due to its lack of reproducibility and the disagreement in establishing an appropriate cutoff (i.e., 10%, 13.25%, 14%, 15%, and 25%) [8], [20], [21], [22], [23], [24]. In the current situation, it is indisputable that the identification of a potent breast cancer proliferation marker would be extremely useful clinically. MCM2, MCM4, and MCM6, whose roles in DNA replication are currently strongly established [25], [26], [27], appear to be attractive alternatives to Ki-67.
MCM2, MCM4, and MCM6 belong to the minichromosome maintenance (MCM) protein complex which consists of six highly conserved proteins (MCM2-7) collectively interacting to bring about initiation of DNA replication and DNA unwinding due to its replicative helicase activity [27]. MCM2-7 proteins are present in proliferating cells [25]. Cancers arising in different anatomic sites are also associated with MCM2, MCM4, and MCM6 overexpression [28], [29], [30], [31].
The determination of hormone receptor status in breast tumors is of considerable importance for therapy selection [32]. ER+ tumors of the luminal subtype may benefit from endocrine therapy, whereas HER2+ tumors may be treated with antibody or small tyrosine kinase-inhibitor drugs. In contrast, the only systemic treatment modality for triple-negative breast cancer remains to be chemotherapy [20]. The development of resistance during breast cancer treatment [33] highlights the urgent need for surrogate markers that may allow overcoming these resistances.
The present study has four goals. First, we wanted to examine whether MCM2, MCM4, and MCM6 can be used as markers to differentiate between luminal A and luminal B breast cancer subtypes. This is crucial since, in luminal A tumors, patients will receive endocrine therapy, while in luminal B tumors, all patients will receive both endocrine and cytotoxic therapy [20]. We hypothesize that MCM2, MCM4, and MCM6 are implicated in breast cancer cell proliferation and can be used as markers to differentiate luminal A and luminal B subtypes.
Second, we wanted to study whether MCM2, MCM4, and MCM6 are highly expressed in the triple-negative breast cancer, as there is an urgent need to search for surrogate markers in this aggressive subtype. We hypothesize that these proteins are highly expressed in the aggressive triple-negative breast cancer subtype. New inhibitors of the proliferation-related machinery may provide alternative anticancer agents to overcome treatment resistance. To achieve our first two aims, we first studied MCM2, MCM4, MCM6, and MKI67 mRNA expression using in silico analysis on available DNA microarray and RNA sequencing data of human breast cancer tissues. We next used IHC staining to evaluate the protein expression of MCM2, MCM4, MCM6, and Ki-67 on tissue microarrays (TMAs) constructed from a cohort of 249 breast cancer patients.
Third, we wanted to compare the prognostic values of MCM2, MCM4, MCM6, and Ki-67 in predicting relapse-free survival. Therefore, we accessed public database to evaluate how MCM2, MCM4, MCM6, and MKI67 mRNA expression affects the probability of relapse-free survival in breast cancer patients.
Our fourth aim was to compare the three approaches that were used in scoring of the protein expression of theses markers by IHC. The three scoring approaches used were percentage of positively stained nuclei, the IHC score, and computer-assisted automated scoring approach.
Here, we show that MCM2, MCM4, and MCM6 are highly expressed at the mRNA level in luminal B, HER2-enriched, and basal-like but not luminal A breast cancer. We identified a cutoff point that can distinguish between two distinct subgroups of low and high expression of MCM2, MCM4, and MCM6 in breast cancer. We also confirmed that MCM2, MCM4, and MCM6 expression can be detected in normal breast tissues. We found that higher levels of expression of MCM2, MCM4, and MCM6, compared to Ki-67, are associated with different stages of progression of breast cancer. Increased protein expression of MCM2, MCM4, and MCM6 is associated with luminal B, HER2-positive, and triple-negative breast cancer. Higher levels of protein expression of MCM2, MCM4, and MCM6 are associated with breast cancers of high histological grades. Low transcript expression of MCM2, MCM4, MCM6, and MKI67 is associated with increased probability of relapse-free survival. A positive relationship exists among the three approaches used in scoring of each of the four markers, and a positive relationship exists among the four markers using each of the three scoring approaches.
Materials and Methods
In Silico Analysis
The web application bc-GenExMiner database [34] was used to study the differential expression of MCM2, MCM4, MCM6, and MKI67 mRNA between different molecular subtypes of breast cancer using a microarray dataset comprising 5861 breast cancer patients. It was also used to examine the correlation of mRNA expression in breast cancer patients and within molecular subtypes and to study their expression among the different histological grades.
To validate results obtained from bc-GenExMiner database, a cohort from The Cancer Genome Atlas (TCGA) (RNAseq data of 754 breast cancer patients) using MiSTIC software tool [35] and The University of California Santa Cruz (UCSC) Cancer Genomics Browser (gene expression array of 597 patients (AgilentG4502A_07_3 array)) [36] was also used.
To examine the association between MCM2, MCM4, MCM6, and MKI67 expression and relapse-free survival, Kaplan-Meier Plotter, an online survival analysis tool, was used which includes microarray data of 3554 breast cancer patients.
Patients and Tissue Samples
The present study included a cohort of 249 female breast cancer patients comprising tumors of different histological grades. Formalin-fixed, paraffin-embedded (FFPE) samples containing tumor tissues were collected after surgery (lumpectomy or mastectomy). Tumor grades were confirmed using the Modified Scarff-Bloom-Richardson-Elston-Ellis grading system (SBR-EE) [37]. Normal and metastatic axillary lymph nodes were also included. Normal breast tissues (n = 21), from healthy women undergoing plastic surgery, were added to serve as internal controls. In addition, a number of extraneous tissues such as colon and thyroid were included in each TMA. All samples were obtained from Centre Hospitalier de l'Université de Montréal after the approval of the research ethical committee (SL 05.019). No individual patient consent was required since all donor blocks remained anonymous.
Tissue Microarray
Sections (4 μm) from each paraffin donor block were stained with hematoxylin phloxine saffron (HPS) stain and examined, and an area containing the lesion was identified. Core punches, 1 mm in diameter, were plucked from representative areas contained within each FFPE tumor blocks. Using a Manual Tissue Arrayer I (Beecher Instruments), each core was realigned in duplicate or triplicate into recipient blocks according to the intended design of the map. Blocks were next inverted and incubated overnight in the oven at 40°C over a glass slide. TMA blocks were allowed to cool until they could easily detach from the glass slide. Tissue sections from each TMA were prepared, and one slide from the block was stained with HPS to review the diagnosis and histological grades on all tissue samples. Additional representative sections from each block were submitted to IHC staining [38]. Table 1 shows the clinicopathological data of mammary gland and lymph node tissues used in the patient cohorts.
Table 1.
Clinicopathological Data of Mammary Gland Tissues Used in the Patient Cohorts
| Variables | Number of Cores (%) 528 |
|---|---|
| Mammary gland | 483 (100%) |
| Normal breast tissue | 21 (4.4%) |
| Benign breast tumors | 3 (0.6%) |
| In situ carcinoma | 6 (1.2%) |
| Invasive breast cancer | 423 (87.6%) |
| Grades | 393 (100%) |
| I | 55 (14.0%) |
| II | 99 (25.2%) |
| III | 239 (60.8%) |
| Molecular subtypes | 393 (100%) |
| Luminal A | 131 (33.3%) |
| Luminal B | 66 (16.8%) |
| Her2-positive | 65 (16.6%) |
| Triple-negative | 131 (33.3%) |
| Nonrepresentative cores | 30 (6.2%) |
| Axillary lymph nodes | 26 |
| Other tissues (placenta, colon, thyroid, intestine) | 19 |
Immunohistochemistry
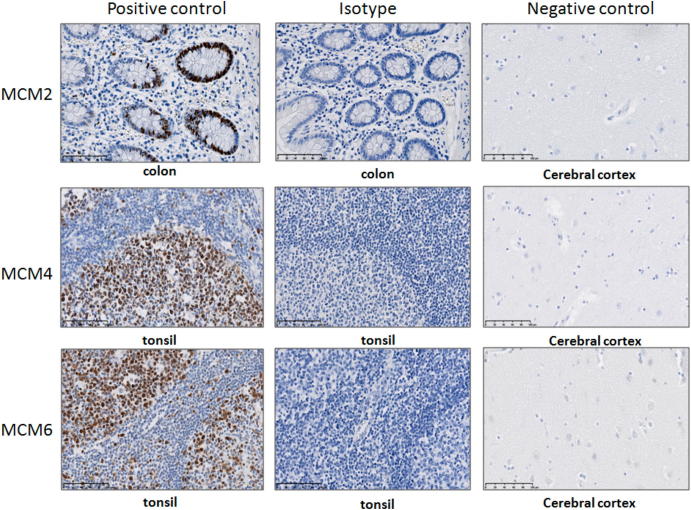
IHC assays were performed on FFPE tissues obtained from each TMA. These assays were carried out according to the manufacturer recommendations on an automated immune-stainer (Discovery XT system, Ventana Medical Systems, Roche). IHC analysis was performed using the following antibodies: MCM2 [mouse monoclonal; #12079, dilution 1/100, TRIS EDTA pH 8 (sCC1), Cell Signaling], MCM4 [rabbit monoclonal, # 12973, dilution 1/50, TRIS EDTA pH 8 (sCC1), Cell Signaling], MCM6 [rabbit monoclonal; (EPR17686) ab201683, dilution 1/500, TRIS EDTA pH 8 (sCC1), Abcam], and Ki-67 [rabbit monoclonal; CRM325A, dilution 1/100, TRIS EDTA pH 8 (sCC1), Biocare Medical]. The chromogen used was 3,3′-diaminobenzidine. Specificities of anti-MCM2, anti-MCM4, and anti-MCM6 antibodies were confirmed using normal colonic mucosa and normal tonsil as positive controls, respectively, and normal cerebral cortex as negative control based on the expression data in the Human Protein Atlas database [39]. Isotype control antibodies were used to estimate the nonspecific binding of target primary antibodies due to Fc receptor binding or other protein-protein interaction. The isotype control antibodies used were mouse [#5415S (G3A1) IgG1 Isotype Control mAb, Cell Signaling] and rabbit [#3900S (DA1E) IgG Isotype Control mAb, Cell Signaling]. An isotype control antibody should have the same immunoglobulin type and be used at the same concentration as the test antibody (Figure 1). ER, PR, and HER2 status of the FFPE breast cancer tissues were retrieved from the pathology reports.
Figure 1.
Optimization of anti-MCM2, anti-MCM4, and anti-MCM6 monoclonal antibodies used in IHC.
Specificity of the anti-MCM2, anti-MCM4, and anti-MCM6 monoclonal antibodies is confirmed using normal colonic mucosa and normal tonsil, respectively, as positive control and normal cerebral cortex as negative control based on the expression data in the Human Protein Atlas database [37]. Isotype control antibodies are used to estimate the nonspecific binding of target primary antibodies due to Fc receptor binding or other protein–protein interaction. An isotype control antibody should have the same immunoglobulin type and be used at the same concentration as the test antibody.
Digital Scanning of Stained Slides
The stained slides were next subjected to digital slide scanning that converts glass slides into high-resolution digital data by high-speed scanning using the NanoZoomer Digital Pathology (NDP) 2.0-HT digital slide scanner (Hamamatsu, Japan).Using digital microscopy for scoring of scanned TMA images has advantages over the conventional light microscopic method. These include ease of handling and linking of cores to the predefined TMA “map” which ensures that each core/case is accurately identified and recorded. Moreover, the samples can be accessed and evaluated via any computer without the requirement for availability of a conventional light microscope [40].
Scoring of Stained Slides
The scoring systems used for each antibody are listed in Table 2. The expression of MCM2, MCM4, MCM6, and Ki-67 in breast epithelium was studied. A representative core must contain at least 50 tumor cells per core to be included in the study. Any cores which contained inadequate number of tumor cells, folded sections, or nonrepresentation cores were not scored. Three different approaches of scoring were used: 1) a visual method using the digital scanned slides by calculating the percentage of positively stained nuclei (range 0%-100%); 2) immunohistochemical score (IHS): a visual method using the digital scanned slides by multiplying the percentage of positively stained cells by the intensity of staining (range 0-12); and 3) computer-assisted automated scoring method using Visiomorph Tissuemorph Digital Pathology (DP) software (range 0-1). Compared to visual scoring, automated MCM2, MCM4, MCM6, and Ki-67 scorings were carried out resulting in a much more rapid readout. Visiomorph DP has the distinct advantage of leaving out stromal cells from the analysis, retaining only cancer cells in the region of interest (ROI). As for Tissuemorph, it allows accurate counting of the positive and negative nuclei in the ROI. Ki-67 was also used as a surrogate marker, in addition to ER, PR, and HER2, to subclassify breast cancers into different molecular subtypes as listed in Table 3.
Table 2.
The Immunohistochemical Scoring System Used in Scoring of the Markers' Protein Expression
| Marker | Subcellular Localization | Scoring System | Total Score | Criteria | Results |
|---|---|---|---|---|---|
| MCM2 | Nuclei | % of positively stained nuclei | 0%-100% | % of positively stained tumor cells among the total number of malignant cells assessed | MCM2 low = 0%-<35% MCM2 high ≥35%-100% |
| IHS | 0-12 | Combining an estimate of the percentage of immune-reactive cells (quantity score) with an estimate of staining intensity (staining intensity score), by multiplying both scores. Quantity score: 1 (no staining or <10%); 2 (10%-50%); 3 (50%-70%); 4 (70%-100%). Staining intensity score: 0 = negative; 1 = weak; 2 = moderate; 3 = strong. | IHS >3 was considered as positive expression. | ||
| Visiomorph Tissuemorph | 0-1 | Visiomorph retains only cancer cells in the ROI. Tissuemorph allows accurate counting of the positive and negative nuclei in the ROI. | |||
| MCM4 | Nuclei | % of positively stained nuclei | 0%-100% | % of positively stained tumor cells among the total number of malignant cells assessed | MCM4 low = 0%-<40% MCM4 high ≥40%-100% |
| IHS | 0-12 | Quantity score × staining intensity score | IHS >3 was considered as positive expression. | ||
| Visiomorph Tissuemorph | 0-1 | Visiomorph retains only cancer cells in the ROI. Tissuemorph allows accurate counting of the positive and negative nuclei in the ROI. | |||
| MCM6 | Nuclei | % of positively stained nuclei | 0%-100% | % of positively stained tumor cells among the total number of malignant cells assessed | MCM6 low = 0%-<55% MCM6 high ≥55%-100% |
| IHS | 0-12 | Quantity score × staining intensity score | IHS >3 was considered as positive expression. | ||
| Visiomorph Tissuemorph | 0-1 | Visiomorph retains only cancer cells in the ROI. Tissuemorph allows accurate counting of the positive and negative nuclei in the ROI. | |||
| Ki-67 | Nuclei | % of positively stained nuclei | 0%-100% | % of positively stained tumor cells among the total number of malignant cells assessed | Ki-67 low = 0%-<14% Ki-67 high ≥14%-100% |
| IHS | 0-12 | Quantity score × staining intensity score | IHS >3 was considered as positive expression. | ||
| Visiomorph Tissuemorph | 0-1 | Visiomorph retains only cancer cells in the ROI. Tissuemorph allows accurate counting of the positive and negative nuclei in the ROI. | |||
| ER and PR | Nuclei | Allred score | 0-8 | Sum of the proportion and average intensity scores of positive tumor cells | Negative = 0-2; positive = 3-8 |
| HER-2 | Membrane | CAP-approved scoring system | 0, 1+, 2+, 3+ | 0 = no immunostaining or membrane staining which is incomplete or barely perceptible within ≤10% of the invasive tumor cells. 1 + = incomplete membrane or barely perceptible staining within >10% of invasive tumor cells 2 + = circumferential membrane staining that is incomplete and/or weak/moderate within >10% of the invasive tumor cells or complete membranous staining that is intense within ≤10% of the invasive tumor cells. 3 + = circumferential membranous staining that is complete and intense in >10% of tumor cells. | 0-1+ are negative; 2+ is equivocal; 3+ is positive |
Table 3.
Surrogate Definitions of Intrinsic Subtypes of Breast Cancer (Derived from Goldhirsch et al., 2013 [20])
| Intrinsic Subtype | Clinicopathologic Surrogate Definition |
|---|---|
| Luminal A |
‘Luminal A-like’ ER positive PR positive HER2 negative Ki-67 ‘low’; <14% |
| Luminal B | ‘Luminal B-like (HER2 negative)’ ER positive PR ‘negative or low’ HER2 negative Ki-67 ‘high’; >14% ‘Luminal B-like (HER2 positive)’ ER positive Any PR HER2 overexpressed or amplified Any Ki-67 |
| Erb-B2 overexpression | ‘HER2 positive (nonluminal)’ HER2 overexpressed or amplified ER and PR absent |
| ‘Basal like’ | ‘Triple negative (ductal)’ ER and PR absent HER2 negative |
Statistical Analyses
The statistical analysis was carried out using XLSTAT (http://www.xlstat.com/en/). The receiver-operating characteristic (ROC) curve was used to detect the optimal cutoff point, which simultaneously reached maximum sensitivity and specificity values for each of MCM2, MCM4, and MCM6. Using each of these cutoff points, continuous variables could then be treated as dichotomous variables (low and high MCM2, MCM4, and MCM6 expression) [41]. Distribution of MCM2, MCM4, MCM6, and Ki-67 in breast cancer subtypes and grades is displayed using histograms. Mann-Whitney test was used to compare the scores among the different molecular subtypes. Kandall rank correlation was used to examine the correlation between the three approaches used during scoring of the protein expression of each of the four markers. Intraclass correlation coefficient was used to examine the correlation between the four markers on comparing each of the three scoring approaches. P value <.05 was considered significant.
Results
MCM2, MCM4, and MCM6 mRNA expression
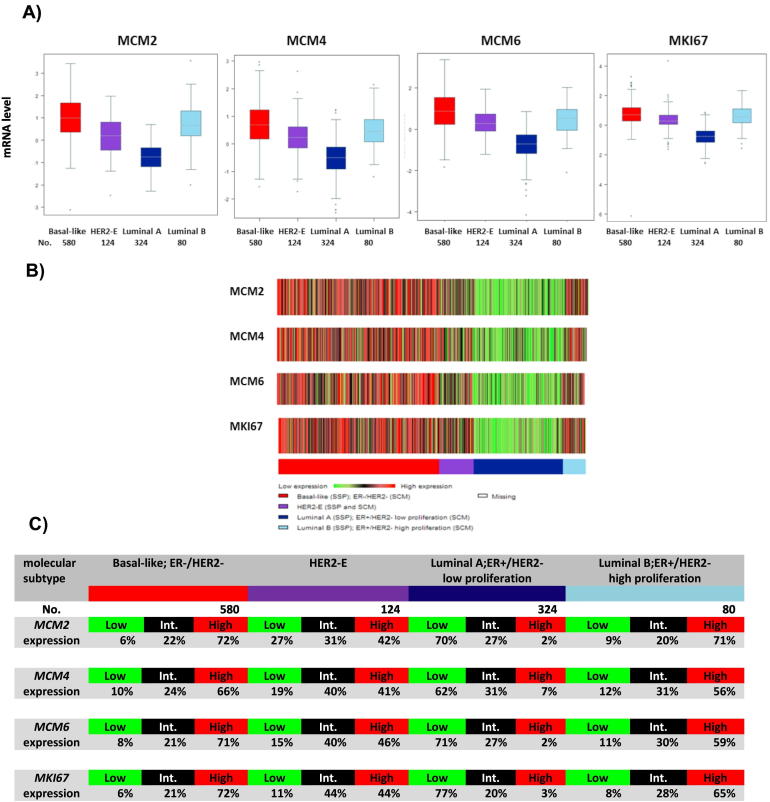
The web application bc-GenExMiner v3.2 was used to compare the mRNA levels within breast cancer molecular subtypes on a dataset comprising 5861 breast cancer patients [34]. Luminal B, HER2-enriched, and basal-like breast cancers show higher expression of MCM2, MCM4, MCM6, and MKI67 mRNA levels in comparison with luminal A (P < .0001) (Figure 2). Notably, there is no overlap between individual boxes in the boxplot when luminal A breast cancers were compared to luminal B breast cancers (Figure 2A). The percentage of patients with low, intermediate, and high levels of MCM2, MCM4, MCM6, and MKI67 expression in each molecular subtype of breast cancer is depicted in Figure 2C. In this microarray data set, 72% of basal-like (total no. = 580), 71% of luminal B (total no. = 80), and 42% of HER2-enriched (total no. = 124) breast cancer patients show high level of MCM2 mRNA expression. In contrast, only 2% of luminal A (total no. = 324) breast cancers show high level of MCM2 mRNA expression. Similar figures were observed in the different molecular subtypes of breast cancer using MCM4, MCM6, and MKI67 mRNA expression data (Figure 2C). By looking at the percentages of high expressers in the luminal A group, we would conclude that MCM2 and MCM6 are slightly superior to MCM4 (2%, 2%, vs. 7%, respectively) (Figure 2C), although it may not be significant at the biological level. The heat map produced from the UCSC Cancer Genomic Browser shows similar results as to the expression of MCM2, MCM4, MCM6, and MKI67 among the different molecular subtypes (Figure 3). This shows that the pattern of gene expression of these four genes is similar among the different breast cancer molecular subtypes.
Figure 2.
In silico analysis of MCM2, MCM4, MCM6, and MKI67 mRNA expression as represented by bc-GenExMiner database v3.2
(A and B) Basal-like, HER2-enriched, and luminal B breast cancers show higher expression of MCM2, MCM4, MCM6, and MKI67 mRNA levels in comparison with luminal A (P < .0001), as shown in the boxplots and the heat maps. Notably, there is no overlap between individual boxes in the boxplot when luminal A breast cancers were compared to luminal B breast cancers. (C) Gene expression values were used to define three equal groups (low, intermediate, and high expression) to examine their distribution in the different breast cancer molecular subtypes. The percentage of patients with low, intermediate, and high levels of MCM2, MCM4, MCM6 and MKI67 expression in each molecular subtype of breast cancer is depicted in the tables.
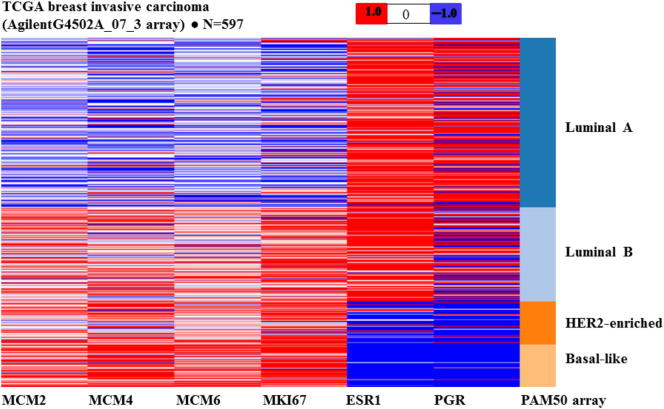
Figure 3.
In silico analysis of MCM2, MCM4, MCM6, MKI67, ESR1, and PGR mRNA expression in PAM50 molecular subtypes of 597 breast cancers obtained from UCSC Cancer Genomics Browser.
The heat map produced from the UCSC Cancer Genomic Browser displays the expression of different genes using distinct sets of colors, with red representing data values >0, blue values <0, and white values = 0. Luminal B, HER2-enriched, and basal-like breast cancers show higher expression of MCM2, MCM4, MCM6, and MKI67 mRNA levels in comparison with luminal A. ESR1 mRNA levels are higher in luminal A and luminal B compared to HER2-enriched and basal-like breast cancers. PGR mRNA levels are higher in luminal A compared to luminal B, while levels are low in HER2-enriched and basal-like breast cancers.
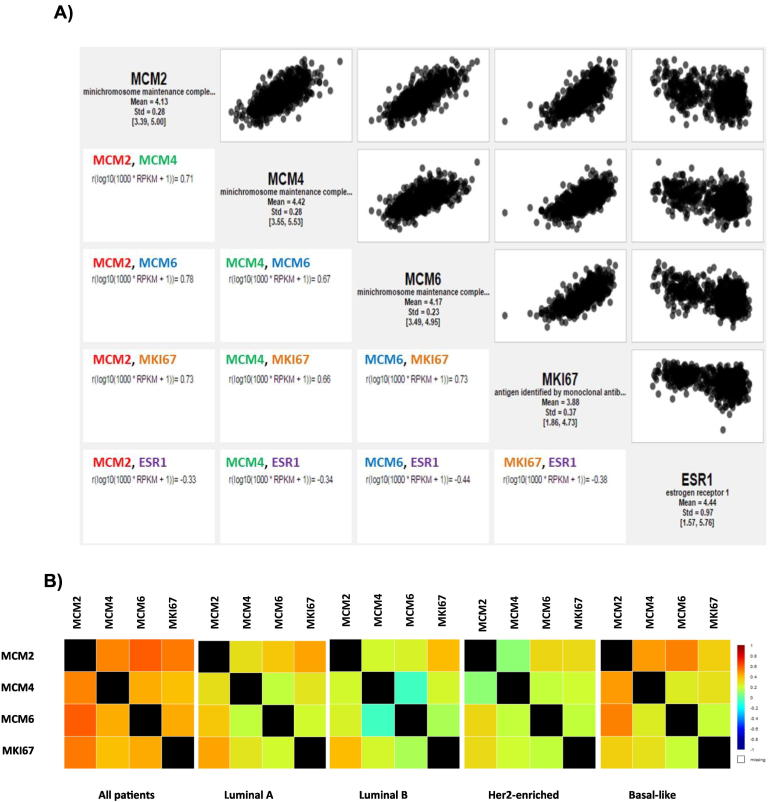
MCM2, MCM4, and MCM6 were next correlated with MKI67 and ESR1 in breast cancers based on RNA-sequencing data derived from TCGA using MiSTIC, a software tool developed at the Institute for Research in Immunology and Cancer. A strong positive correlation could be detected between each of MCM2, MCM4, and MCM6 and MKI67 (r = 0.73, 0.66, and 0.73, respectively), whereas a negative correlation was observed between ESR1 and each of MCM2, MCM4, MCM6, and MKI67(r = −0.33, −0.34, −0.44, and −0.38, respectively) (Figure 4A).These results show that the MCM transcript expression is positively correlated with that of MKI67 and negatively correlated with ESR1. This is consistent with previous findings where ER status was inversely correlated with Ki-67 expression, indicating that tumors having the highest rates of ER positivity show the lowest proliferative activity [23].To validate these correlation results, we further used bc-GenExMiner database to perform targeted correlation analysis of MCM2, MCM4, MCM6, and MKI67 in all breast cancer patients and within molecular subtypes. The results were consistent with those produced from MiSTIC, as highest correlation was between MCM2 and MCM6, followed by MCM2 and MCM4, and finally MCM4 and MCM6 in all patients. These correlation values were different within each molecular subtype as shown in Figure 4B.
Figure 4.
Correlation analysis of MCM2, MCM4, MCM6, and MKI67 in breast cancer patients.
(A) Correlation diagrams using MiSTIC visualization tool to correlate MCM2, MCM4, and MCM6 with MKI67 and ESR1 in breast cancers based on RNA-sequencing data derived from TCGA. A strong positive correlation could be detected between each of MCM2, MCM4, and MCM6 and MKI67 (r = 0.73, 0.66, and 0.73, respectively), whereas a negative correlation was observed between ESR1 and each of MCM2, MCM4, MCM6, and MKI67(r = −0.33, −0.34, −0.44, and −0.38, respectively). (B) Targeted correlation analysis of MCM2, MCM4, MCM6, and MKI67 in all breast cancer patients and within molecular subtypes as represented by bc-GenExMiner database v4.0. The correlation values were different within each molecular subtype.
MCM2, MCM4, and MCM6 cutoff points
A separate ROC curve was used to set the optimal cutoff point based on the percentage of positively stained nuclei data set for each marker: MCM2, MCM4, and MCM6. When the accuracy and the sum of sensitivity and specificity were taken into account, the optimal cutoff point corresponded to a value of 35% for MCM2, 37.5% for MCM4, and 55% for MCM6. Using these cutoff values, the sensitivity, specificity, and accuracy observed are shown in Table 4. For MCM4, we used 40% rather than 37.5% as it would be easier in practice. In other words, tumors with scores ranging from 0% to <40% could be considered to have low MCM4 expression, while those with scores equal to and exceeding 40% were considered to have high MCM4 expression. The same applies for MCM2 and MCM6 with their respective cutoff values. As for Ki-67, we maintained the approved 14% threshold used in literature [8].
Table 4.
Cutoff Values of the Three Markers and Their Associated Sensitivity, Specificity, Accuracy, and Area Under the Curve
| Marker | Cutoff Value | Sensitivity | Specificity | Accuracy | Area Under the Curve |
|---|---|---|---|---|---|
| MCM2 | 35% | 0.63 | 0.91 | 0.86 | 0.800 |
| MCM4 | 37.5% | 0.83 | 0.80 | 0.81 | 0.842 |
| MCM6 | 55% | 0.85 | 0.80 | 0.81 | 0.865 |
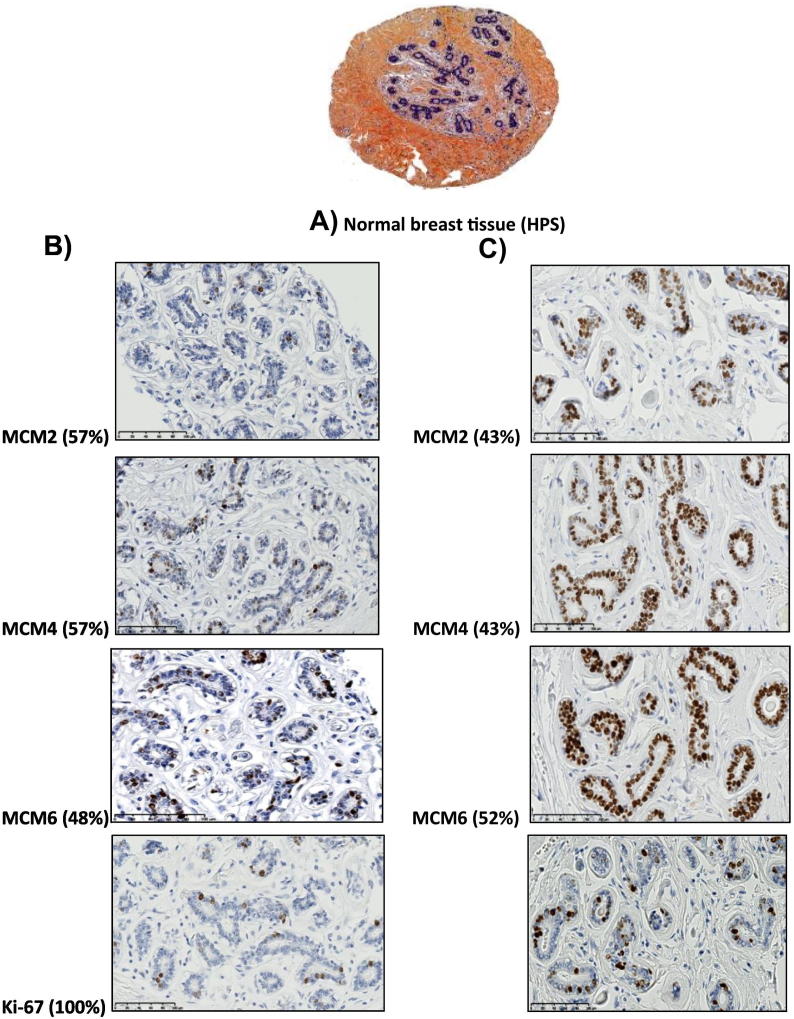
Distinct patterns of MCM2, MCM4, and MCM6 expression in normal breast tissues
MCM2, MCM4, and MCM6 nuclear labeling in only a few scattered luminal cells lining the terminal duct lobular units was noticed in 57%, 57%, and 48% of normal breast tissue samples respectively (Figure 5B). This pattern of expression was also found in 100% of normal breast tissues using Ki67 (Figure 5B). However, a proportion of normal breast tissues demonstrated a strong MCM2, MCM4, and MCM6 nuclear labeling in most if not all of the luminal cells lining the normal breast ducts (Figure 5C). Of note, there was no MCM or Ki67 labeling either in the myoepithelial cell layer or in the surrounding stromal cells. Also, we never observed cytoplasmic or membranous staining in any of the labeled cells. MCM2, MCM4, and MCM6 were expressed at higher levels in normal breast tissue compared to Ki-67.
Figure 5.
Patterns of MCM2, MCM4, MCM6, and Ki-67 expression in normal breast tissue.
(A) A tissue core showing normal breast tissue and stained with HPS. (B) MCM2, MCM4, and MCM6 nuclear labeling in only a few scattered luminal cells lining the terminal duct lobular units was noticed in 57%, 57%, and 48% of normal breast tissue samples, respectively. This pattern of expression was also found in 100% of normal breast tissues using Ki67. (C) A proportion of normal breast tissues demonstrated a strong MCM2, MCM4, and MCM6 nuclear labeling in most if not all of the luminal cells lining the normal breast ducts (43%, 43%, and 52% of normal breast tissue samples, respectively).
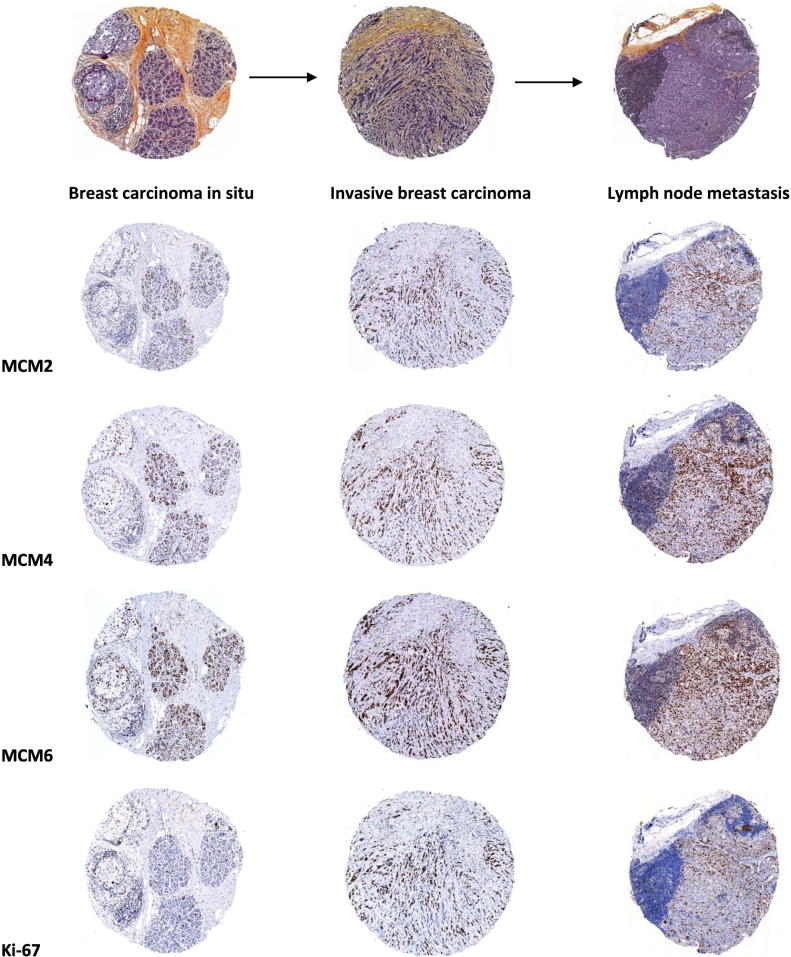
MCM2, MCM4, and MCM6 expression in the different stages of progression of breast cancer
The expression of MCM2, MCM4, MCM6, and Ki-67 was then examined in the different stages of progression of breast cancer (i.e., breast carcinoma in situ, invasive breast carcinoma, and axillary lymph node metastasis). It is noticeable that MCM2, MCM4, and MCM6 were always expressed at higher levels in breast carcinoma in situ, invasive breast carcinoma, and axillary lymph node metastasis compared to Ki-67 (Figure 6). One explanation to this observation might be that Ki67 is expressed from late G1 to M phase, while MCMs are expressed in all phases of the cell cycle. As a result, the fraction of cells at early G1 phase of the cell cycle is missed.
Figure 6.
MCM2, MCM4, and MCM6 expression in the different stages of progression of breast cancer.
Three representative cores showing three different stages of breast cancer (i.e., breast carcinoma in situ, invasive breast carcinoma, and lymph node metastasis) stained with HPS. Levels of MCM2, MCM4, and MCM6 expression exceed that of Ki-67 in the different stages of progression of breast cancer (the same tissue cores were stained with the four antibodies in consecutive sections prepared from the tissue microarray).
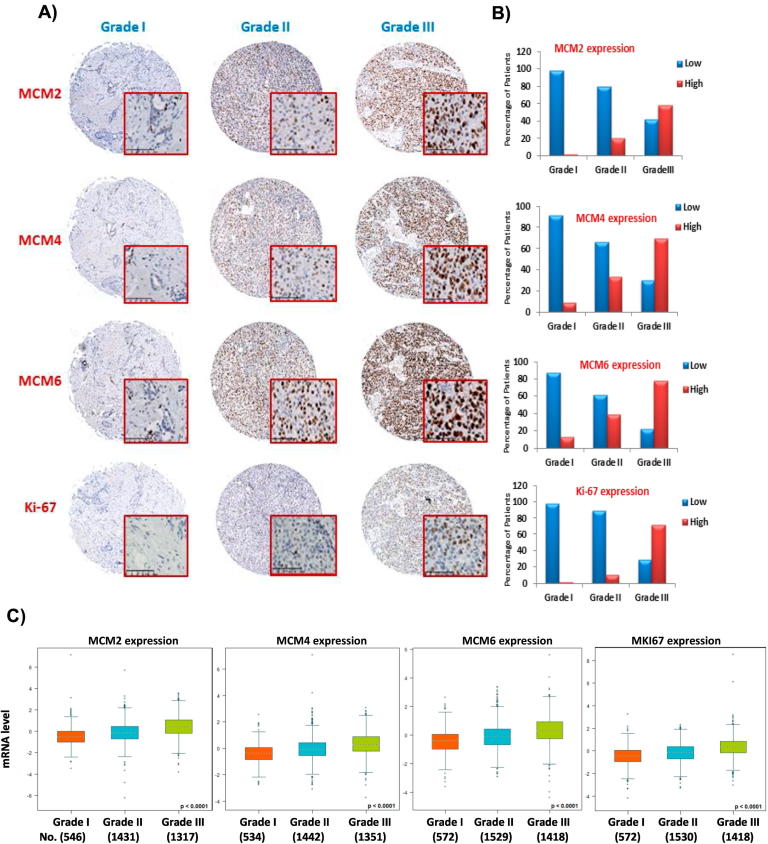
MCM2, MCM4, and MCM6 expression in the different histological grades of breast cancers
We next wanted to study whether the expression of MCM2, MCM4, MCM6, and Ki-67 differs in breast cancers of various histological grades. Based on the previously defined cutoff values (35%, 40%, 55%, and 14%) for MCM2, MCM4, MCM6, and Ki-67, respectively, our results demonstrated that 98.2%, 91%, 87%, and 98.1% of grade I breast cancers expressed low levels of MCM2, MCM4, MCM6, and Ki-67, respectively. On the other hand, high levels of MCM2, MCM4, and MCM6 were detected in 20%, 34%, and 39% of grade II, respectively, and 58%, 70%, and 77% of grade III, respectively (Figure 7B). We also found higher levels of expression of Ki-67 in grade II and grade III breast cancers in comparison to grade I tumors. This shows that expression of MCM2, MCM4, MCM6, and Ki-67 correlates closely with histological grade. As far as the cellular localization is concerned, MCM2, MCM4, MCM6, and Ki-67 reactivity was restricted to the nuclei of cancer cells. None of the four markers was detected in the adjacent stromal cells. Again, levels of MCM2, MCM4, and MCM6 expression in breast cancers exceeded that of Ki-67 (Figure 7A). Furthermore, we performed an in silico analysis of MCM2, MCM4, MCM6, and MKI67 mRNA expression in the three SBR grades using bc-GenExMiner database v4.0. As shown in Figure 7C, the mRNA expression of these four genes increased in high SBR grades, and the difference is statistically significant, (P < .0001).
Figure 7.
Overexpression of MCM2, MCM4, and MCM6 is associated with high histological grade of breast cancer.
(A) Protein overexpression of MCM2, MCM4, MCM6, and Ki-67 is associated with high histological grade of breast cancer. Levels of MCM2, MCM4, and MCM6 expression exceed that of Ki-67 in different grades of breast cancer (the same tissue cores were stained with the four antibodies in consecutive sections prepared from the tissue microarray). The whole tissue cores are shown, and a magnified area is shown inside the red square (magnification 30×). (B) Histograms displaying the percentage of patients with low and high MCM2, MCM4, MCM6, and Ki-67 expression in breast cancer of different histological grades (cutoff values are 35%, 40%, 55%, and 14% for MCM2, MCM4, MCM6, and Ki-67, respectively). Most of grade I breast cancer patients expressed low levels of MCM2, MCM4, MCM6, and Ki-67. In contrast, high levels of MCM2, MCM4, MCM6, and Ki-67 expression were detected in the majority of grade III patients. (C) In silico analysis of MCM2, MCM4, MCM6, and MKI67 mRNA expression in the three SBR grades, as represented by bc-GenExMiner database v4.0.
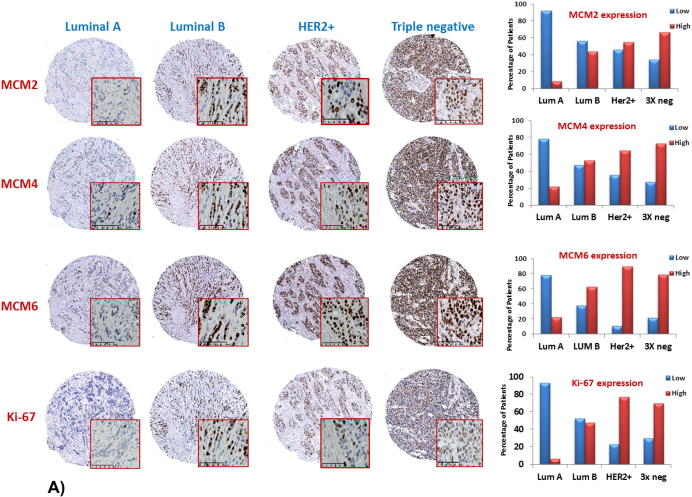
MCM2, MCM4, and MCM6 expression in different molecular subtypes of breast cancer
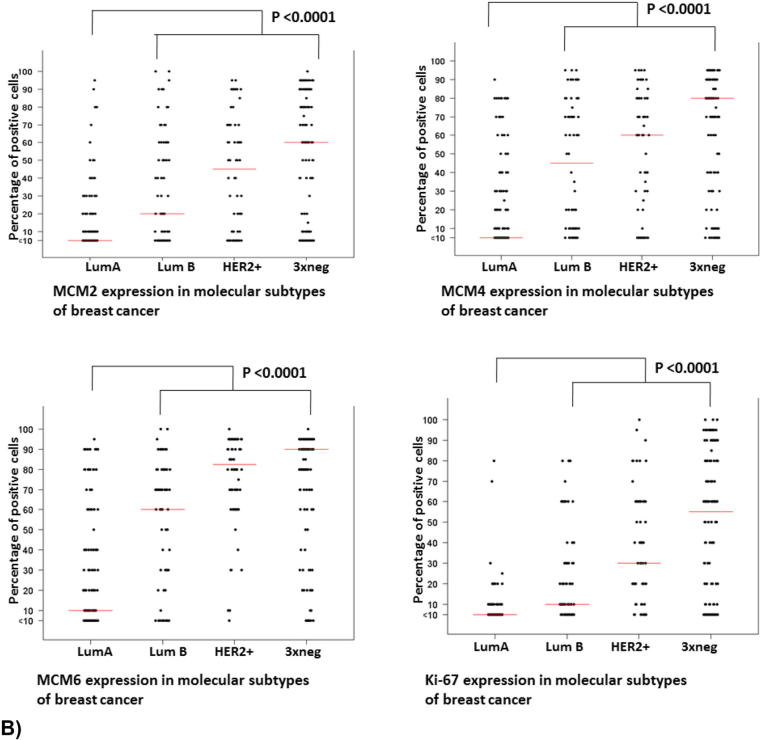
To validate the results of the in silico analysis in breast tumors, we studied the expression of MCM2, MCM4, MCM6, and Ki-67 at the protein level in breast cancers of different molecular subtypes and assessed their respective cellular and subcellular localization. Molecular subtypes were defined using the following four surrogate markers: ER, PR, HER2, and Ki-67 as shown in Table 3. Low levels of MCM2, MCM4, and MCM6 were detected in 91.7%, 78.3%, and 78% of luminal A breast cancers, respectively (Figure 8A). In contrast, high levels of MCM2, MCM4, and MCM6 expression were detected in luminal B, HER2-positive, and triple-negative breast cancers. For each of MCM2, MCM4, and MCM6, all molecular subtypes display significant differences (P < .0001) when compared to luminal A breast cancers (Figure 8B). Similarly, significant differences (P < .0001) could be detected between Ki-67 expression in luminal B, HER2-positive, and triple negative breast cancers when compared to luminal A subtype (Figure 8B). As mentioned earlier, levels of MCM2, MCM4, and MCM6 expression are constantly higher than those of Ki-67 for each molecular subtype.
Figure 8.
Increased expression of MCM2, MCM4, and MCM6 is associated with luminal B, HER2-positive, and triple-negative breast cancer.
(A) Elevated levels of protein expression of MCM2, MCM4, MCM6, and Ki-67 were detected in luminal B, HER2-positive, and triple-negative breast cancer. Levels of MCM2, MCM4, and MCM6 protein expression exceed that of Ki-67 in the different molecular subtypes of breast cancer (the same tissue cores were stained with the four antibodies in consecutive sections prepared from the tissue microarray). The whole tissue cores are shown, and a magnified area is shown inside the red square (magnification 30×). Histograms displaying the percentage of patients with low and high MCM2, MCM4, MCM6, and Ki-67 expression in breast cancer of different molecular subtypes (cutoff values are 35%, 40%, 55%, and 14% for MCM2, MCM4, MCM6, and Ki-67, respectively). Most of luminal A breast cancer patients expressed low levels of MCM2, MCM4, MCM6, and Ki-67. In contrast, high levels of MCM2, MCM4, MCM6, and Ki-67 expression were detected in the majority of Her2-positive and triple-negative subtypes. (B) Significant differences (P < .0001) were detected between these three subtypes and luminal A breast cancer.
Prognostic value of MCM2, MCM4, MCM6, and MKI67
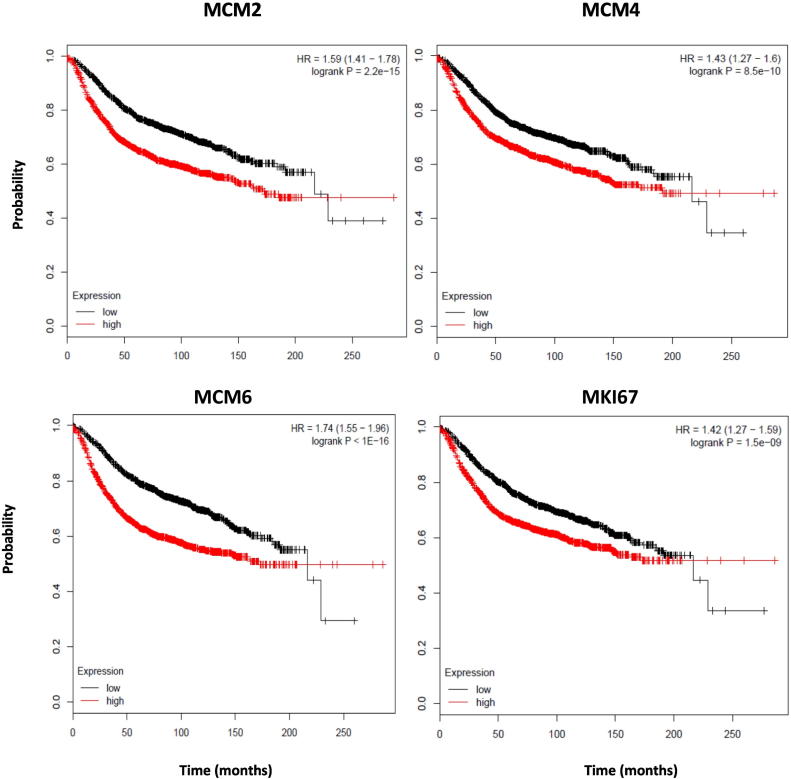
We next wanted to sort out the association between MCM2, MCM4, MCM6, and MKI67 expression and relapse-free survival using Kaplan-Meier Plotter, an online survival analysis tool which includes microarray data of 3554 breast cancer patients (2014 version) [42]. At first, we assessed this association among all breast cancer patients (n = 3554 patients) without stratification. Low expression of each of MCM2, MCM4, MCM6, and MKI67 is associated with increased probability of relapse-free survival (P < .001) (Figure 9).Therefore, MCM2, MCM4, MCM6, and MKI67 have a prognostic value.
Figure 9.
Low expression of MCM2, MCM4, MCM6, and MKI67 is associated with increased probability of relapse-free survival [using the online survival analysis tool Kaplan-Meier Plotter (Gyorffy et al., 2010)].
The association between MCM2, MCM4, MCM6, and MKI67 expression and relapse-free survival of breast cancer patients was studied using the online survival analysis tool Kaplan-Meier Plotter. MCM2, MCM4, MCM6, and MKI67 mRNA expression was stratified into high or low expression using the median mRNA expression level as the cutoff point. Kaplan-Meier survival curve for MCM expression and the P value for log-rank test are shown alongside for each of the studied transcript expression (P < .0001).
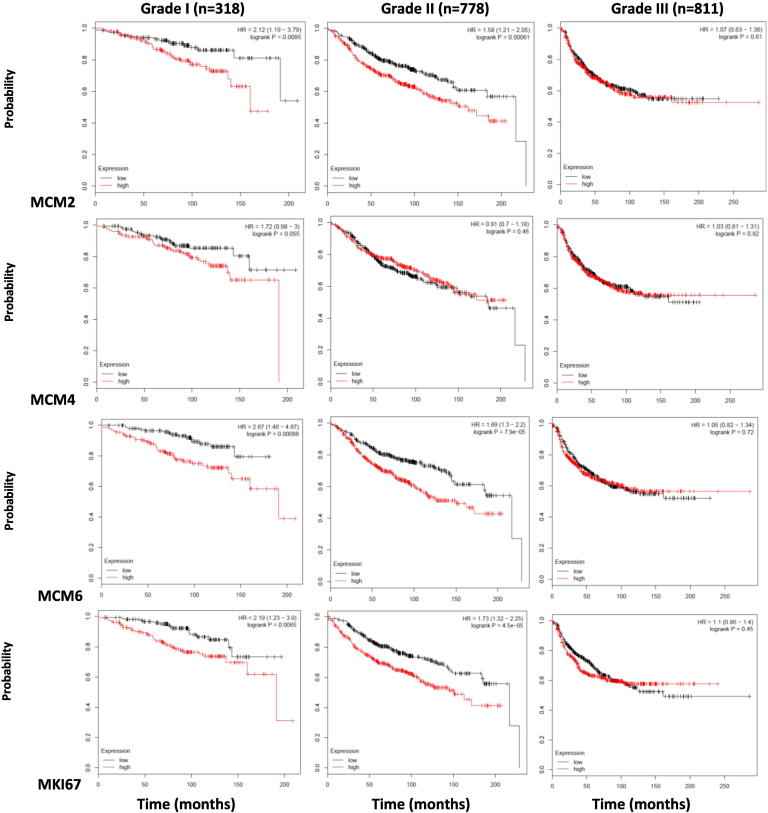
We also investigated this association based on breast cancer histological grade. We found that in grade I breast cancer, low expression of each of MCM2, MCM4, MCM6, and MKI67 was associated with increased probability of relapse-free survival (P = .0095, .055, .00088, and .0065, respectively) (Figure 10). In grade II breast cancer, this association was still observed for MCM2, MCM6, and MKI67 (P = .0006, <.001, and <.001, respectively) but not for MCM4 (P = .46). In grade III breast cancer, none of the four markers' expression maintained this association (Figure 10). Taken together, low levels of MCM2, MCM4, MCM6, and MKI67expression are associated with relapse-free survival in low histological grades. This also shows that MCM2, MCM6, and MKI67exceed MCM4 in terms of relapse-free survival in grade II tumors.
Figure 10.
Low expression of MCM2, MCM4, MCM6, and MKI67 is associated with increased probability of relapse-free survival in grade I breast cancer using the online survival analysis tool Kaplan-Meier Plotter (Gyorffy et al., 2010).
The association between MCM2, MCM4, MCM6, and MKI67 expression and relapse-free survival of breast cancer patients, when they were stratified according to the histological grade, was studied using the online survival analysis tool Kaplan-Meier Plotter. MCM2, MCM4, MCM6, and MKI67 mRNA expression was stratified into high or low expression using the median mRNA expression level as the cutoff point. In grade I breast cancer, low expression of each of MCM2, MCM4, MCM6, and MKI67 was associated with increased probability of relapse-free survival (P = .0095, .055, .00088, and .0065, respectively). In grade II breast cancer, this association was still observed for MCM2, MCM6, and MKI67 (P = .0006, <.001, and <.001, respectively) but not for MCM4 (P = .46). In grade III breast cancer, none of the four markers' expression maintained this association.
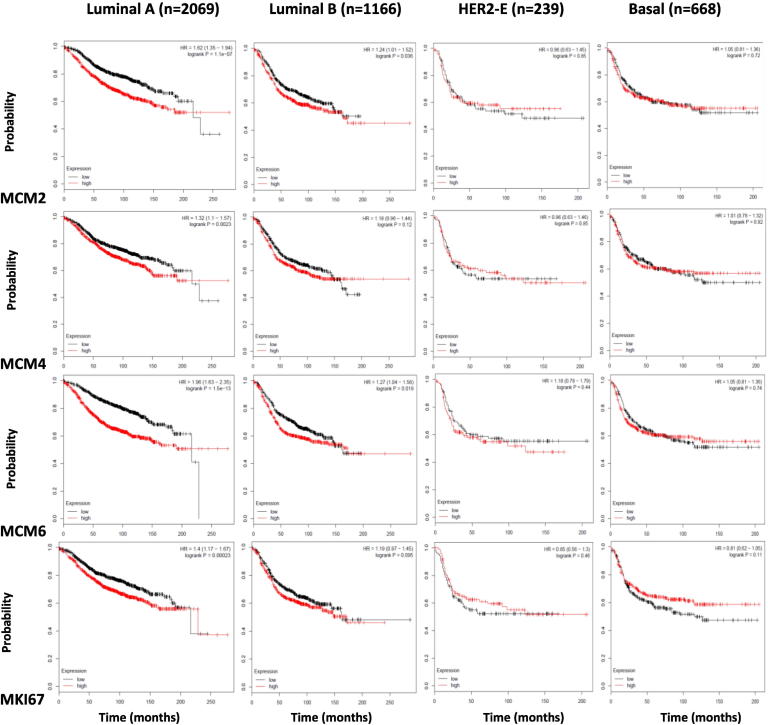
We then wanted to study the association of the expression of the four markers with the probability of relapse-free survival when breast cancer patients were sub-stratified according to the molecular subtype. Low expression of each of MCM2, MCM4, MCM6, and MKI67 was associated with increased probability of relapse-free survival in luminal A breast cancer (P = <.001). MCM6 and MCM2expression shows a significant association in luminal B breast cancer (P = .036 and .019, respectively), while MCM4 and MKI67expression does not (P = .12 and .095, respectively). None of the four markers show this association in HER2-positive and basal-like breast cancer (Figure 11). This shows that low expression of MCM2, MCM4, MCM6, and MKI67is associated with relapse-free survival in less aggressive luminal A breast cancer subtype. This also shows that MCM6 and MCM2 are superior to MCM4 and MKI67 as their expression is associated with relapse-free survival in the more aggressive luminal B breast cancer subtype.
Figure 11.
Low expression of MCM2, MCM4, MCM6, and MKI67 is associated with increased probability of relapse-free survival in luminal A breast cancer molecular subtype. Kaplan-Meier Plotter (Gyorffyet al, 2010).
The association between MCM2, MCM4, MCM6, and MKI67 expression and relapse-free survival of breast cancer patients, when they were stratified according to the molecular subtype, was studied using the online survival analysis tool Kaplan-Meier Plotter. MCM2, MCM4, MCM6, and MKI67 mRNA expression was stratified into high or low expression using the median mRNA expression level as the cutoff point. Low expression of each of MCM2, MCM4, MCM6, and MKI67 was associated with increased probability of relapse-free survival in luminal A breast cancer (P <.001). MCM6 and MCM2expression shows a significant association in luminal B breast cancer (P = .036 and .019, respectively), while MCM4 and MKI67expression does not (P = .12 and .095, respectively). None of the four markers show this association in HER2-positive and basal-like breast cancer.
Positive relationship among the three scoring approaches of each of the four markers
As each of the three scoring methods used has strengths and weaknesses, we wanted to examine whether they could reliably assess the protein expression of these markers in breast cancer and could be used interchangeably. We wanted to examine if there is a relationship among the three approaches we used during scoring the protein expression of the four studied markers by IHC, specifically because each approach has a different range. Using Kandall rank correlation, we observed a positive relationship between the MCM2 protein expression score detected by the percentage of positively stained nuclei approach with that detected by the immunohistochemical score (IHS) approach and also with that detected by the Visiomorph Tissuemorph DP software approach. The same applies to MCM4, MCM6, and Ki-67 (Table 5). This shows that each of these three approaches can be reliably used to assess the protein expression of these markers in breast cancer patients in the lab.
Table 5.
Correlation between the Three Scoring Approaches Used during Scoring of the Protein Expression of Each of the Four Markers Using Kandall Rank Correlation
| MCM2 IHS | MCM2% | |
|---|---|---|
| MCM2% | 0.777 (<.0001) | |
| MCM2 Vis.Tis.S. | 0.702 (<.0001) | 0.714 (<.0001) |
| MCM4 IHS | MCM4% | |
| MCM4% | 0.778 (<.0001) | |
| MCM4 Vis.Tis.S. | 0.689 (<.0001) | 0.745 (<.0001) |
| MCM6 IHS | MCM6% | |
| MCM6% | 0.801 (<.0001) | |
| MCM6 Vis.Tis.S. | 0.722 (<.0001) | 0.777 (<.0001) |
| Ki-67 IHS | Ki-67% | |
| Ki-67% | 0.708 (<.0001) | |
| Ki-67 Vis.Tis.S. | 0.609 (<.0001) | 0.650 (<.0001) |
%: percentage score. r (P value).
Positive relationship among the three scoring methods of each of the four markers within each molecular subtype and within each grade
Next, we wanted to examine if this positive relationship among the three scoring approches used during scoring of the protein expression of each of the four markers would still exist within each molecular subtype and also within each tumor grade. Using Kandall rank correlation, this positive relationship did exist within each molecular subtype and also within each tumor grade (Table 6, Table 7). This adds to the strength of the relationship among these three approaches and shows that this positive relatioship was not specific to a molecular subtype or a histological grade.
Table 6.
Correlation between the Three Scoring Approaches Used during Scoring of the Protein Expression of Each of the Four Markers in Each Molecular Subtype Using Kandall Rank Correlation
| Luminal A |
Luminal B |
||||
|---|---|---|---|---|---|
| MCM2 IHS | MCM2% | MCM2 IHS | MCM2% | ||
| MCM2% | 0.709 (<.0001) | MCM2% | 0.791 (<.0001) | ||
| MCM2 Vis.Tis.S. | 0.561 (<.0001) | 0.587 (<.0001) | MCM2 Vis.Tis.S. | 0.702 (<.0001) | 0.734 (<.0001) |
| MCM4 IHS | MCM4% | MCM4 IHS | MCM4% | ||
| MCM4% | 0.702 (<.0001) | MCM4% | 0.860 (<.0001) | ||
| MCM4 Vis.Tis.S. | 0.540 (<.0001) | 0.697 (<.0001) | MCM4 Vis.Tis.S. | 0.652 (<.0001) | 0.667 (<.0001) |
| MCM6 IHS | MCM6% | MCM6 IHS | MCM6% | ||
| MCM6% | 0.763 (<.0001) | MCM6% | 0.824 (<.0001) | ||
| MCM6 Vis.Tis.S. | 0.622 (<.0001) | 0.737 (<.0001) | MCM6 Vis.Tis.S. | 0.768 (<.0001) | 0.756 (<.0001) |
| Ki-67 IHS | Ki-67% | Ki-67 IHS | Ki-67% | ||
| Ki-67% | 0.526 (<.0001) | Ki-67% | 0.692 (<.0001) | ||
| Ki-67 Vis.Tis.S. | 0.497 (<.0001) | 0.491 (<.0001) | Ki-67 Vis.Tis.S. | 0.571 (<.0001) | 0.623 (<.0001) |
| HER2-positive | Triple-negative | ||||
| MCM2 IHS | MCM2% | MCM2 IHS | MCM2% | ||
| MCM2% | 0.818 (<.0001) | MCM2% | 0.757 (<.0001) | ||
| MCM2 Vis.Tis.S. | 0.784 (<.0001) | 0.700 (<.0001) | MCM2 Vis.Tis.S. | 0.730 (<.0001) | 0.749 (<.0001) |
| MCM4 IHS | MCM4% | MCM4 IHS | MCM4% | ||
| MCM4% | 0.788 (<.0001) | MCM4% | 0.789 (<.0001) | ||
| MCM4 Vis.Tis.S. | 0.783 (<.0001) | 0.745 (<.0001) | MCM4 Vis.Tis.S. | 0.748 (<.0001) | 0.757 (<.0001) |
| MCM6 IHS | MCM6% | MCM6 IHS | MCM6% | ||
| MCM6% | 0.685 (<.0001) | MCM6% | 0.761 (<.0001) | ||
| MCM6 Vis.Tis.S. | 0.617 (<.0001) | 0.682 (<.0001) | MCM6 Vis.Tis.S. | 0.682 (<.0001) | 0.707 (<.0001) |
| Ki-67 IHS | Ki-67% | Ki-67 IHS | Ki-67% | ||
| Ki-67% | 0.611 (<.0001) | Ki-67% | 0.721 (<.0001) | ||
| Ki-67 Vis.Tis.S. | 0.512 (<.0001) | 0.538 (<.0001) | Ki-67 Vis.Tis.S. | 0.612 (<.0001) | 0.680 (<.0001) |
%: percentage score.
Table 7.
Correlation between the Three Scoring Approaches Used during Scoring of the Protein Expression of Each of the Four Markers in Each Tumor Grade Using Kandall Rank Correlation
|
Grade I |
Grade II |
Grade III |
||||||
|---|---|---|---|---|---|---|---|---|
| MCM2 IHS | MCM2% | MCM2 IHS | MCM2% | MCM2 IHS | MCM2% | |||
| MCM2% | 0.553 (<.0001) | MCM2% | 0.738 (<.0001) | MCM2% | 0.795 (<.0001) | |||
| MCM2 Vis.Tis.S. | 0.577 (<.0001) | 0.483 (<.0001) | MCM2 Vis.Tis.S. | 0.538 (<.0001) | 0.600 (<.0001) | MCM2 Vis.Tis.S. | 0.763 (<.0001) | 0.734 (<.0001) |
| MCM4 IHS | MCM4% | MCM4 IHS | MCM4% | MCM4 IHS | MCM4% | |||
| MCM4% | 0.565 (<.0001) | MCM4% | 0.742 (<.0001) | MCM4% | 0.790 (<.0001) | |||
| MCM4 Vis.Tis.S. | 0.374 (0.0002) | 0.633 (<.0001) | MCM4 Vis.Tis.S. | 0.619 (<.0001) | 0.673 (<.0001) | MCM4 Vis.Tis.S. | 0.721 (<.0001) | 0.724 (<.0001) |
| MCM6 IHS | MCM6% | MCM6 IHS | MCM6% | MCM6 IHS | MCM6% | |||
| MCM6% | 0.693 (<.0001) | MCM6% | 0.768 (<.0001) | MCM6% | 0.736 (<.0001) | |||
| MCM6 Vis.Tis.S. | 0.506 (<.0001) | 0.686 (<.0001) | MCM6 Vis.Tis.S. | 0.667 (<.0001) | 0.778 (<.0001) | MCM6 Vis.Tis.S. | 0.692 (<.0001) | 0.687 (<.0001) |
| Ki-67 IHS | Ki-67% | Ki-67 IHS | Ki-67% | Ki-67 IHS | Ki-67% | |||
| Ki-67% | 0.498 (<.0001) | Ki-67% | 0.503 (<.0001) | Ki-67% | 0.669 (<.0001) | |||
| Ki-67 Vis.Tis.S. | 0.448 (<.0001) | 0.299 (.008) | Ki-67 Vis.Tis.S. | 0.471 (<.0001) | 0.557 (<.0001) | Ki-67 Vis.Tis.S. | 0.560 (<.0001) | 0.593 (<.0001) |
%: percentage score.
Positive relationship among the four markers using each of the three scoring methods
Then, we wanted to study the relationship between the protein expressions of each two of the four markers using the same scoring approach. We thus used intraclass correlation coefficient to examine the protein expression of markers using a scoring approach of the same scale. Using IHS, MCM6 shows almost perfect agreement with MCM4 and strong agreement with Ki-67. MCM2 shows moderate agreement with both MCM6 and MCM4. Ki-67 shows moderate to strong agreement with MCM4 and fair agreement with MCM2 (Table 8, A). Using the percentage scoring method, the protein expression of each of the four markers showed a strong to almost perfect agreement with those of the other three markers (Table 8, B). Using Visiomorph Tissuemorph scoring approach, the protein expression of each of the four markers showed a strong to almost perfect agreement with those of the other three markers (Table 8, C). This shows that the protein expression of each two markers showed agreement on using the same scoring approach and that this agreement is superior in both the percentage scoring method and Visiomorph Tissuemorph scoring method compared to the IHS.
Table 8.
Correlation between the Four Markers on Comparing Each of the Three Scoring Schemes Using Intraclass Correlation Coefficient
| A. Immunohistochemical Scoring (IHS) | |||
|---|---|---|---|
| MCM4 IHS | MCM2 IHS | Ki-67 IHS | |
| MCM6 IHS | 0.828 [0.792-0.858] (<.0001) | 0.530 [0.434-0.611] (<.0001) | 0.73 [0.674-0.777] (<.0001) |
| MCM4 IHS | 0.514 [0.413-0.597] (<.0001) | 0.664 [0.594-0.723] (<.0001) | |
| MCM2 IHS | 0.386 [0.259-0.492] (<.0001) | ||
| B. Percentage Scoring (%) | |||
|---|---|---|---|
| MCM4% | MCM2% | Ki-67% | |
| MCM6% | 0.911 [0.892-0.926] (<.0001) | 0.861 [0.833-0.885] (<.0001) | 0.787 [0.742-0.823] (<.0001) |
| MCM4% | 0.864 [0.836-0.888] (<.0001) | 0.781 [0.735-0.819] (<.0001) | |
| MCM2% | 0.828 [0.793-0.858] (<.0001) | ||
| C. Visiomorph Tissuemorph Scoring (Vis. Tis. S.) | |||
|---|---|---|---|
| MCM4 Vis.Tis.S | MCM2 Vis.Tis.S | Ki-67 Vis.Tis.S | |
| MCM6 Vis.Tis.S | 0.913 [0.895-0.928] (<.0001) | 0.850 [0.819-0.875] (<.0001) | 0.741 [0.688-0.786] (<.0001) |
| MCM4 Vis.Tis.S | 0.846 [0.815-0.873] (<.0001) | 0.769 [0.720-0.809] (<.0001) | |
| MCM2 Vis.Tis.S | 0.715 [0.656-0.764] (<.0001) | ||
IHS: immunohistochemical score, %: percentage score, Vis. Tis. S.: Visiomorph Tissuemorph score.
ICC can be interpreted as follows:
0.0-0.2 indicates poor agreement;
0.3-0.4 indicates fair agreement;
0.5-0.6 indicates moderate agreement;
0.7-0.8 indicates strong agreement; and
>0.8 indicates almost perfect agreement.
Discussion
In the present study, we sought to examine whether MCM2, MCM4, and MCM6 can be used as alternative markers to Ki-67 in differentiating between luminal A and luminal B breast cancer subtypes. We have compared the expression levels of MCM2, MCM4, MCM6, and Ki-67 as a means to assess cellular proliferation in a large cohort of patients diagnosed with breast cancer of different molecular subtypes and different grades. We also compared the prognostic value of these four markers in predicting the relapse-free survival using Kaplan-Meier Plotter, an online survival analysis tool which includes microarray data of 3554 breast cancer patients [42]. Finally, we compared the three scoring approaches used during scoring of the protein expression of these four markers.
Our findings confirmed that two distinct subgroups among luminal breast cancer (ER+) could be detected using an MCM2 labeling index of 35%, an MCM4 labeling index of 40%, and an MCM6 labeling index of 55%. MCM2, MCM4, MCM6, and Ki-67 are highly expressed in higher histological grade tumors especially in the clinically aggressive breast cancers such as luminal B, HER2-positive, and triple-negative subtype tumors. To our knowledge, this is the first study which examines the MCM6 protein expression by IHC in breast cancer.
Using TMAs comprising normal human breast tissue and breast cancers of different molecular subtypes and histological grades, we found higher levels of MCM2, MCM4, and MCM6 protein expression when compared to Ki-67 in normal breast tissue and in breast cancer. This is in accordance with previous observations by others reporting higher protein expression of MCM2 when compared to Ki-67 using IHC in normal breast tissue and breast carcinoma [43], [44], [45]. We speculate that this indicates that MCM2, MCM4, and MCM6 labeling is able to detect subgroups of proliferating mammary epithelial cells that cannot be detected by Ki-67 alone. This is in agreement with the results of a previous study [46] that confirmed the complete absence of Ki-67 in the initial G1 phase of the cell cycle. Alternatively, we cannot exclude the possibility that Ki-67 is present but remains undetected. This might be due to preanalytical condition such as fixation or because of altered biological properties of Ki-67 such as stable interactions with other proteins or conformational changes [47].
The dual pattern of MCM2, MCM4, and MCM6 expression in normal breast tissue (n = 21) is interesting. Whereas a proportion of normal breast tissue displays MCM2, MCM4, and MCM6 nuclear labeling in only a few scattered cells lining the terminal duct units, a significant proportion of normal tissue shows MCM2, MCM4, and MCM6 expression in the vast majority of normal breast luminal cells. The biological significance of these two subpopulations with distinct patterns of MCM2, MCM4, and MCM6 expression is currently unknown and needs further clarification. It has been hypothesized that these cells reside in a “licensed for DNA replication” state and thus have proliferative potential but not synthesizing DNA [25]. Another proposed hypothesis is that the hormonal stimulation might influence the MCM complex chromatin binding. For instance, progesterone-stimulated proliferation of normal human breast cells may occur through targeting the DNA replication licensing machinery [48]. At this point, one can only hypothesize that the highly proliferative group reflects the state of hormonal stimulation in a given patient at the time of surgery. Whether or not it results in a higher susceptibility to neoplastic transformation is an unresolved question [49]. Obviously, further elaborate investigations are required with larger cohorts of normal breast tissues and their follow-up data to clarify this issue.
In agreement with our findings, it has been noticed that antibodies against the MCM complex proteins consistently label a higher proportion of tumor cells in comparison to Ki-67 in different types of malignancies. MCM2 showed expression in a greater proportion of tumor cells than Ki-67 in breast cancer [40], [45]. MCM6 index (61%) was significantly higher than Ki-67 (19.8%) in mantle cell lymphoma [50], as well as in meningioma (42% vs. 10%, respectively) [51]. It has been hypothesized that this reflects the existence of two cell populations within the neoplastic dynamic cell populations: actively proliferating cells and cells with proliferative potential [25].
Histological grade is an important prognostic marker that takes into account the mitotic rate [40].Our results indicate that MCM2, MCM4, MCM6, and Ki-67 are highly expressed in breast cancer of higher histological grades. This is consistent with the findings of previous studies [31], [40], [52], [53] where a significant correlation between the proliferative markers MCM2, MCM4, and Ki-67 and breast cancer grades was reported. Kwok et al. [31] also reported, using in silico analysis of four breast cancer datasets (n = 777 patients), that grade III breast cancer tumors had a significantly higher level of MCM4 transcript expression when compared to grade I or grade II breast cancer. Our study is the first to examine the protein expression of MCM6 and breast cancer histological grades. In agreement with our findings, MCM6 labeling index was significantly higher in grade II than in grade I meningioma [51].
Our results coming from both in silico analyses and IHC also support the belief that MCM2, MCM4, and MCM6, similar to Ki-67 [54], are highly expressed at both the mRNA and protein levels in subgroups of luminal B, HER2-positive, and triple-negative subtypes of breast cancer when compared to luminal A breast cancer. To our knowledge, with the exception of a recent study [45], there has been no previous report in the literature that specifically correlated MCM2, MCM4, and MCM6 expression with individual breast cancer molecular subtypes. Our findings are in agreement with recent studies that reported that, compared to ER+ tumors, ER− tumors show high MCM2, MCM4, and Ki-67 protein expression in breast cancer [31], [40].
Results of in silico analysis of microarray data have shown that MCM2, MCM4, MCM6, and MKI67 mRNA expression is associated with relapse-free survival. This is in agreement with previous studies reporting that MCM2 and Ki-67 were prognostic markers associated with breast cancer specific survival [40] and with disease-free survival and overall survival in breast cancer [43]. Kwok et al. [31] have reported that high levels of MCM4 mRNA expression were strongly associated with a shorter survival time. When breast cancer patients were substratified according to the molecular subtypes, our findings show that the association between MCM4 expression and survival was observed in the ER+ luminal A molecular subtype but not in luminal B, HER2+, or triple-negative subtypes. This is partially in agreement with previous reports [31], [40] that the association between MCM2 and MCM4 and survival was strongly observed in patients whose breast tumors were ER positive.
As for the prognostic value of MCM6, and in agreement with our findings, high MCM6 mRNA expression was significantly associated with shorter breast cancer patient survival [31]. MCM6 protein expression showed increased labeling in bad prognosis histological subtypes of non–small cell lung cancer, and MCM6 proliferation index correlated with a poor overall survival [55]. MCM6 labeling index more than 70% would translate to an 80% recurrence rate in meningioma [51].
Although the MCM is a heterohexameric complex, it is composed of six independent subunits, each serving a distinct role [56]. In S. cerevisiae, MCM ATPase activity depends upon coordinate interactions among all six subunits. Mcm4/6/7p is responsible for most of the ATP hydrolysis, while Mcm2/3/5p performs a regulatory role [56]. The distinct biological role of each member of the MCM complex in the human breast cell is still to be unraveled, and the ongoing research is trying to discover the diagnostic, prognostic, and predictive values of each member.
The role of MCM4 in breast cancer is interesting. The causative role of an MCM4 mutation in breast cancer development was revealed for the first time in 2007 [57]. A hypomorphic mutation in the Mcm4 gene in mice, Chaos3 (chromosome aberrations occurring spontaneously 3), was found to increase the risk of breast cancer and caused mammary adenocarcinomas in approximately 80% of homozygous females [57]. Furthermore, MCM4 appears to have a predictive value. Turnbull et al. [58] have reported that MCM4 was among a four-gene signature that proved to be efficient in predicting response to aromatase inhibitors in ER+ postmenopausal breast cancer patients [58].
In this study, we provide important supplementary information regarding comparisons between three scoring approaches. In the IHS, the intensity of staining is assessed visually and has a major influence on the score, whereas the percentage scoring method and Visiomorph Tissuemorph DP score assess the quantity of positively stained nuclei. Nevertheless, the three scoring methods showed good correlation when they were used for scoring of the protein expression of each of the four markers, and this good correlation existed when the patients were substratified according to the molecular subtype or the histological grade. This denotes that the three scoring methods are comparable, and since each method has its own strengths and weaknesses, each lab has to choose the most suitable method to be adopted. Our results are consistent with previous studies reporting the comparison of manual and automated methods for scoring the protein expression of different markers by IHC in different malignancies [59], [60], [61], although different scoring methods and software were used.
Our results have shown that there is an agreement between the protein expression of each two markers on using the same scoring approach and that this agreement is superior in both the percentage scoring method and Visiomorph Tissuemorph scoring method compared to the IHS. This might be due to the influence of the staining intensity which impacts IHS and not the two other scoring methods. This agreement is also superior among the three studied MCM proteins compared to agreement between Ki-67 and any of the studied MCMs. It is noticeable that almost perfect agreement exists between MCM4 and MCM6 using any of the three methods, followed by agreement between MCM2 and each of MCM4 or MCM6. This shows that the protein expression of the three studied MCM markers is highly correlated as they are members of the same complex, and this correlation is superior to correlation between Ki-67 and any of the studied MCMs.
IHC was considered as state of the art for clinical routine by the St. Gallen Conference 2011 and 2013 [62], [63]. The scoring of proliferation marker-stained scanned images was performed by human observers and by digital image analysis software to increase objectivity [64]. Interobserver variation is an important issue with IHC in the routine setting [40]. We did not evaluate interobserver variation in our study, although a general agreement regarding the score existed between the first and last author. TMA technology has been broadly used in research [40]. However, there are some unsettled issues such as the extraction of a per-sample score from values obtained from multiple cores (average or maximum) and the optimum number of cores to be evaluated [40]. Whole sections rather than TMA cores are needed to check on the robustness of MCMs especially in regions found to be heterogeneous using Ki-67. We have also not compared the cost of using each of the three scoring methods or the pathologist's fatigue and time consumption. Future studies comparing all aspects of using these scoring methods elaborately will give us a clearer vision as to the strengths and weaknesses of each method.
Conclusion and Perspectives
MCM2, MCM4, and MCM6 labeling seems to outperform Ki67 as tools to assess cellular proliferation in breast cancer. The onset of sustained Ki67 expression occurs only in late G1 phase, while MCM can label all proliferative cells during the active phases of cell cycle. This theoretically makes MCM2, MCM4, and MCM6 much more sensitive markers of proliferation since they detect cells which are “licensed to proliferate” and capable of initiating DNA replication [25]. The role of MCM in DNA replication is now firmly established, whereas the mechanism of action of Ki-67 is poorly understood [65]. The cutoff values for the MCM proteins are more convenient to use than the canonical 14% cutoff value of Ki-67. Further experiments including larger number of patient samples with their full clinical and follow-up data, in addition to a validation cohort to validate the obtained cutoff values, are required before definitive conclusions can be reached as to whether these MCM proteins could be alternatives to Ki-67. Examination of a large–sample size cohort which includes normal breast tissues with their follow-up data is required to understand the significance of the two distinct patterns of MCM labeling observed in our study as well as in others. Moreover, examination of the association between MCM scoring and the size of nuclei, mitotic index, tubular formation, and TNM staging would be important.
Acknowledgements
We thank Mrs. Julie Hinsinger for help with Visiomorph Tissuemorph Digital Pathology software, Mrs. Micheline Fortine and Mirela Birlea for their expert technical assistance, Mr. Romain Sabina for the biobanking facility, and Mr. Christian Charbonneau for assistance with the preparation of the histological pictures.
Footnotes
This work was supported by funds from the Perseverance Award, the Institute for Research in Immunology and Cancer, to M. I. The above-mentioned parties had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declarations of interest: none.
References
- 1.F. Bray, J. Ferlay, I. Soerjomataram, R. Siegel, L. Torre, A. Jemal, Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. doi: 10.3322/caac.21492. [DOI] [PubMed]
- 2.American Cancer Society . American Cancer Society; Atlanta: 2019. Cancer Facts & Figures 2019. [Google Scholar]
- 3.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Society; Toronto, ON: 2017. Canadian Cancer Statistics 2017.cancer.ca/Canadian-Cancer-Statistics-2017-EN.pdf Available at: [Google Scholar]
- 4.Viale, G., The current state of breast cancer classification. Ann Oncol, 2012. 23 Suppl 10: p. x207–10. [DOI] [PubMed]
- 5.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 6.Geyer FC, Rodrigues DN, Weigelt B, Reis-Filho JS. Molecular classification of estrogen receptor-positive/luminal breast cancers. Adv Anat Pathol. 2012;19(1):39–53. doi: 10.1097/PAP.0b013e31823fafa0. [DOI] [PubMed] [Google Scholar]
- 7.Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheang MC, Chia SK, Voduc D, Gao D, Leung S, Snider J, Watson M, Davies S, Bernard PS, Parker JS. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitfield ML, George LK, Grant GD, Perou CM. Common markers of proliferation. Nat Rev Cancer. 2006;6(2):99–106. doi: 10.1038/nrc1802. [DOI] [PubMed] [Google Scholar]
- 11.Wistuba II, Behrens C, Lombardi F, Wagner S, Fujimoto J, Raso MG, Spaggiari L, Galetta D, Riley R, Hughes E. Validation of a proliferation-based expression signature as prognostic marker in early stage lung adenocarcinoma. Clin Cancer Res. 2013;19(22):6261–6271. doi: 10.1158/1078-0432.CCR-13-0596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrisani OM, Studach L, Merle P. Gene signatures in hepatocellular carcinoma (HCC) Semin Cancer Biol. 2011;21(1):4–9. doi: 10.1016/j.semcancer.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cuzick J, Swanson GP, Fisher G, Brothman AR, Berney DM, Reid JE, Mesher D, Speights VO, Stankiewicz E, Foster CS. Prognostic value of an RNA expression signature derived from cell cycle proliferation genes in patients with prostate cancer: a retrospective study. Lancet Oncol. 2011;12(3):245–255. doi: 10.1016/S1470-2045(10)70295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cancer Genome Atlas N. Comprehensive molecular portraits of human breast tumours. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Diest PJ, van der Wall E, Baak JP. Prognostic value of proliferation in invasive breast cancer: a review. J Clin Pathol. 2004;57(7):675–681. doi: 10.1136/jcp.2003.010777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein ME, Dabbs DJ, Shuai Y, Brufsky AM, Jankowitz R, Puhalla SL, Bhargava R. Prediction of the Oncotype DX recurrence score: use of pathology-generated equations derived by linear regression analysis. Mod Pathol. 2013;26(5):658–664. doi: 10.1038/modpathol.2013.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chibon F. Cancer gene expression signatures — the rise and fall? Eur J Cancer. 2013;49(8):2000–2009. doi: 10.1016/j.ejca.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 18.Beresford MJ, Wilson GD, Makris A. Measuring proliferation in breast cancer: practicalities and applications. Breast Cancer Res. 2006;8(6):216. doi: 10.1186/bcr1618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yerushalmi R, Woods R, Ravdin PM, Hayes MM, Gelmon KA. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol. 2010;11(2):174–183. doi: 10.1016/S1470-2045(09)70262-1. [DOI] [PubMed] [Google Scholar]
- 20.Goldhirsch A, Winer EP, Coates AS, Gelber RD, Piccart-Gebhart M, Thürlimann B, Senn HJ. Panel members. Personalizing the treatment of women with early breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann Oncol. 2013;24(9):2206–2223. doi: 10.1093/annonc/mdt303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colozza M, Sidoni A, Piccart-Gebhart M. Value of Ki67 in breast cancer: the debate is still open. Lancet Oncol. 2010;11(5):414–415. doi: 10.1016/S1470-2045(10)70089-9. [DOI] [PubMed] [Google Scholar]
- 22.Aleskandarany MA, Rakha EA, Macmillan RD, Powe DG, Ellis IO, Green AR. MIB1/Ki-67 labelling index can classify grade 2 breast cancer into two clinically distinct subgroups. Breast Cancer Res Treat. 2011;127(3):591–599. doi: 10.1007/s10549-010-1028-3. [DOI] [PubMed] [Google Scholar]
- 23.Inwald EC, Klinkhammer-Schalke M, Hofstädter F, Zeman F, Koller M, Gerstenhauer M, Ortmann O. Ki-67 is a prognostic parameter in breast cancer patients: results of a large population-based cohort of a cancer registry. Breast Cancer Res Treat. 2013;139(2):539–552. doi: 10.1007/s10549-013-2560-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spyratos F, Ferrero-Poüs M, Trassard M, Hacène K, Phillips E, Tubiana-Hulin M, Le Doussal V. Correlation between MIB-1 and other proliferation markers: clinical implications of the MIB-1 cutoff value. Cancer. 2002;94(8):2151–2159. doi: 10.1002/cncr.10458. [DOI] [PubMed] [Google Scholar]
- 25.Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, Williams ED, Williams GH. DNA replication licensing and human cell proliferation. J Cell Sci. 2001;114(Pt 11):2027–2041. doi: 10.1242/jcs.114.11.2027. [DOI] [PubMed] [Google Scholar]
- 26.Ohtani K, Iwanaga R, Nakamura M, Ikeda M, Yabuta N, Tsuruga H, Nojima H. Cell growth-regulated expression of mammalian MCM5 and MCM6 genes mediated by the transcription factor E2F. Oncogene. 1999;18(14):2299–2309. doi: 10.1038/sj.onc.1202544. [DOI] [PubMed] [Google Scholar]
- 27.Forsburg SL. Eukaryotic MCM proteins: beyond replication initiation. Microbiol Mol Biol Rev. 2004;68(1):109–131. doi: 10.1128/MMBR.68.1.109-131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das M, Prasad SB, Yadav SS, Govardhan HB, Pandey LK, Singh S, Pradhan S, Narayan G. Over expression of minichromosome maintenance genes is clinically correlated to cervical carcinogenesis. PLoS One. 2013;8(7) doi: 10.1371/journal.pone.0069607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishimi Y, Okayasu I, Kato C, Kwon HJ, Kimura H, Yamada K, Song SY. Enhanced expression of Mcm proteins in cancer cells derived from uterine cervix. Eur J Biochem. 2003;270(6):1089–1101. doi: 10.1046/j.1432-1033.2003.03440.x. [DOI] [PubMed] [Google Scholar]
- 30.Karimi S, Mohammadi F, Khodadad K, Emami H, Seyfollahi L. High expression of minichromosome maintenance protein 6 in classic Hodgkin's lymphoma points to a cell cycle arrest in G1 phase. Arch Iran Med. 2008;11(5):532–538. [PubMed] [Google Scholar]
- 31.Kwok HF, Zhang SD, McCrudden CM, Yuen HF, Ting KP, Wen Q, Khoo US, Chan KY. Prognostic significance of minichromosome maintenance proteins in breast cancer. Am J Cancer Res. 2015;5(1):52–71. [PMC free article] [PubMed] [Google Scholar]
- 32.Moy B, Goss PE. Estrogen receptor pathway: resistance to endocrine therapy and new therapeutic approaches. Clin Cancer Res. 2006;12(16):4790–4793. doi: 10.1158/1078-0432.CCR-06-1535. [DOI] [PubMed] [Google Scholar]
- 33.Bektas N, Haaf At, Veeck J, Wild PJ, Lüscher-Firzlaff J, Hartmann A, Knüchel R, Dahl E. Tight correlation between expression of the Forkhead transcription factor FOXM1 and HER2 in human breast cancer. BMC Cancer. 2008;8:42. doi: 10.1186/1471-2407-8-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jezequel P, Campone M, Gouraud W, Guérin-Charbonnel C, Leux C, Ricolleau G, Campion L. bc-GenExMiner: an easy-to-use online platform for gene prognostic analyses in breast cancer. Breast Cancer Res Treat. 2012;131(3):765–775. doi: 10.1007/s10549-011-1457-7. [DOI] [PubMed] [Google Scholar]
- 35.Lemieux S, Sargeant T, Laperrière D, Ismail H, Boucher G, Rozendaal M, Lavallée VP, Ashton-Beaucage D, Wilhelm B, Hébert J. MiSTIC, an integrated platform for the analysis of heterogeneity in large tumour transcriptome datasets. Nucleic Acids Res. 2017;45(13):e122. doi: 10.1093/nar/gkx338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosenbloom KR, Armstrong J, Barber GP, Casper J, Clawson H, Diekhans M, Dreszer TR, Fujita PA, Guruvadoo L, Haeussler M. The UCSC Genome Browser database: 2015 update. Nucleic Acids Res. 2015;43(Database issue):D670–D681. doi: 10.1093/nar/gku1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tavassoli FA. D.P.e., World health organization classification of tumours. In: Pathology and genetics tumours of the breast and female genital organs. IARC Press: Lyon. France. 2003;2003:19–23. [Google Scholar]
- 38.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4(7):844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 39.The human protein atlas. http://wwwproteinatlasorg Available at.
- 40.Joshi S, Watkins J, Gazińska P, Brown JP, Gillett CE, Grigoriadis A, Pinder SE. Digital imaging in the immunohistochemical evaluation of the proliferation markers Ki67, MCM2 and Geminin, in early breast cancer, and their putative prognostic value. BMC Cancer. 2015;15:546. doi: 10.1186/s12885-015-1531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh G. Determination of Cutoff Score for a Diagnostic Test. Internet J Lab Med. 2006;2:1–4. [Google Scholar]
- 42.Gyorffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, Li Q, Szallasi Z. An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat. 2010;123(3):725–731. doi: 10.1007/s10549-009-0674-9. [DOI] [PubMed] [Google Scholar]
- 43.Gonzalez MA, Pinder SE, Callagy G, Vowler SL, Morris LS, Bird K, Bell JA, Laskey RA, Coleman N. Minichromosome maintenance protein 2 is a strong independent prognostic marker in breast cancer. J Clin Oncol. 2003;21(23):4306–4313. doi: 10.1200/JCO.2003.04.121. [DOI] [PubMed] [Google Scholar]
- 44.Shetty A, Loddo M, Fanshawe T, Prevost AT, Sainsbury R, Williams GH, Stoeber K. DNA replication licensing and cell cycle kinetics of normal and neoplastic breast. Br J Cancer. 2005;93(11):1295–1300. doi: 10.1038/sj.bjc.6602829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yousef E, Furrer D, Laperriere D, Tahir MR, Mader S, Diorio C, Gaboury LA. MCM2:An alternative to Ki-67 for measuring breast cancer cell proliferation. Mod Pathol. 2017;30:682–697. doi: 10.1038/modpathol.2016.231. [DOI] [PubMed] [Google Scholar]
- 46.Lopez F, Belloc F, Lacombe F, Dumain P, Reiffers J, Bernard P, Boisseau MR. Modalities of synthesis of Ki67 antigen during the stimulation of lymphocytes. Cytometry. 1991;12(1):42–49. doi: 10.1002/cyto.990120107. [DOI] [PubMed] [Google Scholar]
- 47.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182(3):311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 48.Graham JD, Mote PA, Salagame U, van Dijk JH, Balleine RL, Huschtscha LI, Reddel RR, Clarke CL. DNA replication licensing and progenitor numbers are increased by progesterone in normal human breast. Endocrinology. 2009;150(7):3318–3326. doi: 10.1210/en.2008-1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alison MR, Hunt T, Forbes SJ. Minichromosome maintenance (MCM) proteins may be pre-cancer markers. Gut. 2002;50(3):290–291. doi: 10.1136/gut.50.3.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schrader C, Janssen D, Klapper W, Siebmann JU, Meusers P, Brittinger G, Kneba M, Tiemann M, Parwaresch R. Minichromosome maintenance protein 6, a proliferation marker superior to Ki-67 and independent predictor of survival in patients with mantle cell lymphoma. Br J Cancer. 2005;93(8):939–945. doi: 10.1038/sj.bjc.6602795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gauchotte G, Vigouroux C, Rech F, Battaglia-Hsu SF, Soudant M, Pinelli C, Civit T, Taillandier L, Vignaud JM, Bressenot A. Expression of minichromosome maintenance MCM6 protein in meningiomas is strongly correlated with histologic grade and clinical outcome. Am J Surg Pathol. 2012;36(2):283–291. doi: 10.1097/PAS.0b013e318235ee03. [DOI] [PubMed] [Google Scholar]
- 52.Ali HR, Dawson SJ, Blows FM, Provenzano E, Pharoah PD, Caldas C. Aurora kinase A outperforms Ki67 as a prognostic marker in ER-positive breast cancer. Br J Cancer. 2012;106(11):1798–1806. doi: 10.1038/bjc.2012.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wojnar A, Kobierzycki C, Krolicka A, Pula B, Podhorska-Okolow M, Dziegiel P. Correlation of Ki-67 and MCM-2 proliferative marker expression with grade of histological malignancy (G) in ductal breast cancers. Folia Histochem Cytobiol. 2010;48(3):442–446. doi: 10.2478/v10042-010-0069-0. [DOI] [PubMed] [Google Scholar]
- 54.Ferguson NL, Bell J, Heidel R, Lee S, Vanmeter S, Duncan L, Munsey B, Panella T, Orucevic A. Prognostic value of breast cancer subtypes, Ki-67 proliferation index, age, and pathologic tumor characteristics on breast cancer survival in Caucasian women. Breast J. 2013;19(1):22–30. doi: 10.1111/tbj.12059. [DOI] [PubMed] [Google Scholar]
- 55.Vigouroux C, Casse JM, Battaglia-Hsu SF, Brochin L, Luc A, Paris C, Lacomme S, Gueant JL, Vignaud JM, Gauchotte G. Methyl(R217)HuR and MCM6 are inversely correlated and are prognostic markers in non small cell lung carcinoma. Lung Cancer. 2015;89(2):189–196. doi: 10.1016/j.lungcan.2015.05.008. [DOI] [PubMed] [Google Scholar]
- 56.Schwacha A, Bell SP. Interactions between two catalytically distinct MCM subgroups are essential for coordinated ATP hydrolysis and DNA replication. Mol Cell. 2001;8(5):1093–1104. doi: 10.1016/s1097-2765(01)00389-6. [DOI] [PubMed] [Google Scholar]
- 57.Shima N, Alcaraz A, Liachko I, Buske TR, Andrews CA, Munroe RJ, Hartford SA, Tye BK, Schimenti JC. A viable allele of Mcm4 causes chromosome instability and mammary adenocarcinomas in mice. Nat Genet. 2007;39(1):93–98. doi: 10.1038/ng1936. [DOI] [PubMed] [Google Scholar]
- 58.Turnbull AK, Arthur LM, Renshaw L, Larionov AA, Kay C, Dunbier AK, Thomas JS, Dowsett M, Sims AH, Dixon JM. Accurate Prediction and Validation of Response to Endocrine Therapy in Breast Cancer. J Clin Oncol. 2015;33(20):2270–2278. doi: 10.1200/JCO.2014.57.8963. [DOI] [PubMed] [Google Scholar]
- 59.Vijayashree, R., P. Aruthra, and K.R. Rao, A comparison of manual and automated methods of quantitation of oestrogen/progesterone receptor expression in breast carcinoma. J Clin Diagn Res, 2015. 9(3): p. EC01–5. [DOI] [PMC free article] [PubMed]
- 60.Rizzardi AE, Johnson AT, Vogel RI, Pambuccian SE, Henriksen J, Skubitz AP, Metzger GJ, Schmechel SC. Quantitative comparison of immunohistochemical staining measured by digital image analysis versus pathologist visual scoring. Diagn Pathol. 2012;7:42. doi: 10.1186/1746-1596-7-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rizzardi AE, Zhang X, Vogel RI, Kolb S, Geybels MS, Leung YK, Henriksen JC, Ho SM, Kwak J, Stanford JL. Quantitative comparison and reproducibility of pathologist scoring and digital image analysis of estrogen receptor beta2 immunohistochemistry in prostate cancer. Diagn Pathol. 2016;11(1):63. doi: 10.1186/s13000-016-0511-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gnant M, Harbeck N, Thomssen C. St. Gallen 2011: summary of the consensus discussion. Breast Care (Basel) 2011;6(2):136–141. doi: 10.1159/000328054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Untch M, Gerber B, Harbeck N, Jackisch C, Marschner N, Möbus V, von Minckwitz G, Loibl S, Beckmann MW, Blohmer J-U. 13th st. Gallen international breast cancer conference 2013: primary therapy of early breast cancer evidence, controversies, consensus - opinion of a german team of experts (zurich 2013) Breast Care (Basel) 2013;8(3):221–229. doi: 10.1159/000351692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Laurinavicius A, Laurinaviciene A, Ostapenko V, Dasevicius D, Jarmalaite S, Lazutka J. Immunohistochemistry profiles of breast ductal carcinoma: factor analysis of digital image analysis data. Diagn Pathol. 2012;7:27. doi: 10.1186/1746-1596-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jonat W, Arnold N. Is the Ki-67 labelling index ready for clinical use? Ann Oncol. 2011;22(3):500–502. doi: 10.1093/annonc/mdq732. [DOI] [PubMed] [Google Scholar]