Abstract.
Multiplex polymerase chain reaction (PCR) platforms have enhanced understanding of intestinal pathogens in low- and middle-income countries (LMICs). However, few such studies have been performed in Latin America, where poverty, poor sanitation, and undernutrition persist. Multiplex PCR (BioFire, Salt Lake City, UT) was used to identify viral, bacterial, and parasitic pathogens in stool collected on day 1 and 31 from children aged 6 to 35 months with acute, non-bloody diarrhea in two locations (rural and urban) in Guatemala. We analyzed correlation between pathogens and clinical, demographic, and socioeconomic variables; described patterns of pathogen acquisition, persistence, and clearance over the 30-day period; and calculated population attributable fractions (PAFs) for diarrheal causation for individual pathogens. We analyzed 316 subjects (144 urban; 172 rural) enrolled between March 2015 and January 2016. Rural subjects had significantly more malnutrition, animal exposure, and unimproved water/sanitation infrastructure. The majority of subjects had multiple pathogens/sample (4.8 rural and 2.7 urban). Few meaningful correlates were identified between individual pathogens and clinical, demographic, or environmental variables. Escherichia coli pathotypes, Shigella, Campylobacter, and Giardia had high rates of persistence between initial and 30-day follow-up. Statistically significant adjusted PAFs were identified for Campylobacter (14.9%, 95% CI: 3.2–23.1), norovirus (10.2%, 95% CI: 0.4–17.1), sapovirus (7.6%, 95% CI: 2.3–10.9), and adenovirus 40/41 (5.6%, 95% CI: 0.3–8.7). These observations further characterize the diversity and complexity of enteric pathogens in children in LMICs. Patterns of chronic symptomatic and asymptomatic infection among Latin American children are similar to those observed in other LMIC regions. Findings have direct implications for practitioners treating individuals with acute infectious diarrhea and should inform regional public health strategies.
INTRODUCTION
Diarrheal illness in children is a major contributor to the global burden of disease, with most of the impact occurring among children living in low- and middle-income countries (LMICs).1 Globally, an estimated 1.1 billion episodes of diarrhea in children younger than 5 years occur every year, resulting in approximately half a million childhood deaths.2 Furthermore, recurrent or persistent intestinal infections and chronic inflammation contribute to a cycle of malnutrition and delayed development.3,4
Despite the massive burden of pediatric diarrhea in LMICs, treatment options (e.g., zinc and antibiotics) remain limited in effectiveness, particularly when considering longer term or nutritional outcomes.5,6 From a public health perspective, the complexity of environmental, microbiologic, and human host factors leading to the acute and chronic sequelae of pediatric diarrhea poses significant challenges to the design and implementation of preventative strategies, including interventions designed to improve water, sanitation, and hygiene (WASH).7–11
Our understanding of the complexity of organisms that may cause acute diarrhea has been enhanced by utilization of multiplex PCR to simultaneously and comprehensively identify pathogens in both symptomatic and asymptomatic stools. Two recent prospective multicenter studies, the Global Enteric Multicenter Study (GEMS) and the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED), have greatly advanced our knowledge of the relative contribution of specific pathogens to acute diarrheal disease and highlighted the frequency of asymptomatic carriage of putative pathogens.12–14 However, underscoring the complexity and heterogeneity of pediatric diarrhea across populations, the two studies identified different pathogens with the highest population attributable fractions (PAFs).
Moreover, only one country in the GEMS and MAL-ED studies was in Latin America (Peru) and none were in Central America, where diarrheal disease remains a major problem despite the emergence of many national economies.15 We undertook an extensive analysis of children aged 6–35 months with acute diarrhea from two distinct environments (one urban and one rural) in Guatemala. Our objective was to identify correlations between specific bacterial, viral, and parasitic pathogens identified using multiplex PCR, and clinical, epidemiologic, demographic, and environmental characteristics of Guatemalan children.
METHODS
Study population.
Three hundred twenty-four study participants aged 6–35 months with severe or moderate diarrhea were enrolled in a randomized, double-blind, placebo-controlled clinical trial of a nutritional product, conducted at two investigational sites in Guatemala: the Center for Human Development in the rural southwest lowlands of Guatemala (rural) and Hospital Roosevelt and Hospital Infantil de Infectologia y Rehabilitacion in Guatemala City (urban). Inclusion criteria included acute non-bloody diarrhea of < 72 hours duration and more than three liquid stools in the previous 24 hours. Exclusion criteria included mild or improving condition, dysentery, weight-for-height z-score < −3 according to WHO standards, serious chronic illness, recent probiotics treatment, or immunodeficiency.16 Enrolled subjects were followed for 31 days. Parents or guardians of all subjects provided written informed consent before enrollment. Ethical approval was obtained from the Colorado Multiple Institutional Review Board, as well as from the ethics committees at the Guatemalan Ministry of Health, Hospital Roosevelt, and Universidad del Valle.
Stool sampling.
A 5–15-mL stool sample was collected at enrollment and again at study day 31. A detailed description of sample collection, storage, and processing has been published previously.17 Briefly, stool samples collected at the urban sites were stored initially at −20°C and transported daily on wet ice to a central laboratory in Guatemala City, where they were immediately processed and tested. Stool samples collected at the rural site were frozen at −80°C on site and transported twice monthly on dry ice to the central laboratory at the Universidad del Valle, Guatemala City, Guatemala. There, specimens were aliquoted into multiple cryovials (without melting previously frozen rural samples) and stored at −80°C. The raw samples were shipped on dry ice to the Microbiology Laboratory at Children’s Hospital Colorado, Aurora, CO, and stored at −80°C until tested for stool pathogens. Pathogen identification was performed using a multiplex PCR assay (FilmArrayTM GI-Panel, BioFire), which simultaneously detects 22 human diarrheal pathogens that include bacteria, viruses, and protozoa.
Statistical analysis.
Detailed demographic, household, environmental, and clinical data were collected for all subjects at enrollment. Height and weight measured at baseline were converted into z-scores using the WHO Version 3.2.2 2011 SAS Macros (Cary, NC).16 Height-for-age z-scores were modeled as continuous variables to explore the association of pathogens with chronic malnutrition, and height-for-weight z-scores were modeled as continuous variables to explore the association of pathogens with acute malnutrition. Demographic variables were compared between rural and urban sites using Student’s t-tests for continuous variables and chi-squared tests for categorical variables. Rates of pathogen acquisition, persistence, and clearance were calculated between initial and day-31 stool. To determine the PAF, we first calculated the odds ratio for the association between presence of diarrhea and each pathogen individually, stratified by site, using conditional logistic regression. The PAF was calculated as ([adjusted odds ratio−1] × prevalence of the pathogen in cases)/adjusted odds ratio, and the 95% CI were calculated by inserting the upper and lower confidence limits of the odds ratios into the same equation, in place of the odds ratios.18 The day-31 stool sample was used as the non-diarrheal control. Univariate and multivariable analysis to test for associations between pathogens and potential predictor variables were conducted using generalized linear models, with backwards intentional elimination used (only retaining adjustment variables that changed the beta estimate of the independent variable of interest by > 10%) to determine the final multivariable model. In multivariable analyses, “Site” was used as a proxy for the WASH variables because of the highly significant differences between the distribution of these variables by site (Table 1). Other adjustment variables explored included age, gender, household crowding, and number of children younger than 5 years in the household. SAS v9.4 was used for all statistical analyses.
Table 1.
Study subject demographics by rurality
| Rural (N = 172) | Urban (N = 144) | P-value* | |
|---|---|---|---|
| Age (months)† | 18.7 (7.8) | 16.5 (6.8) | 0.007 |
| Weight-for-age WHO z-score at enrollment† | −1.6 (1.1) | −0.8 (1.0) | < 0.0001 |
| Weight-for-length WHO z-score at enrollment† | −1.2 (1.0) | −0.7 (1.0) | < 0.0001 |
| Antibiotics prescribed at initial visit‡ | 24 (14%) | 16 (11%) | 0.45 |
| Zinc prescribed at initial visit‡ | 111 (65%) | 59 (41%) | < 0.0001 |
| Gender = Female‡ | 82 (48%) | 65 (45%) | 0.65 |
| Water supply to house‡ | < 0.0001 | ||
| Public or bottled water | 19 (11%) | 135 (94%) | |
| Rainwater or water tank | 1 (1%) | 6 (4%) | |
| Well or river | 152 (88%) | 3 (2%) | |
| Method of human waste disposal‡ | < 0.0001 | ||
| Indoor plumbing | 32 (19%) | 136 (94%) | |
| Latrine: septic tank | 2 (1%) | 3 (2%) | |
| Latrine: open pit | 138 (80%) | 5 (3%) |
* P-value obtained from t-test for continuous variables and by χ2 test for categorical variables.
† Estimates for symmetrical numeric variables are given as mean ± SD.
‡ Estimates for categorical variables are given as frequency, percent.
RESULTS
Study population.
From March 9, 2015, to January 25, 2016, 324 children were enrolled in the study, of which 316 had sufficient data to be included in the analysis cohort: 144 children enrolled at the urban site and 172 at the rural site. Stool samples were available on study day 1 from 311 children (98.4%) and 298 children (92.0%) at study day 31. Clinical, demographic, and socioeconomic characteristics are presented in Table 1. There were notable differences between the sites in age, nutritional status, human waste disposal method, water supply, and animals in household. Percent dehydration at enrollment ([day 3 rehydrated weight−day 1 weight]/[Day 3 weight] × 100) was significantly greater in rural children than in urban children (P = 0.04).
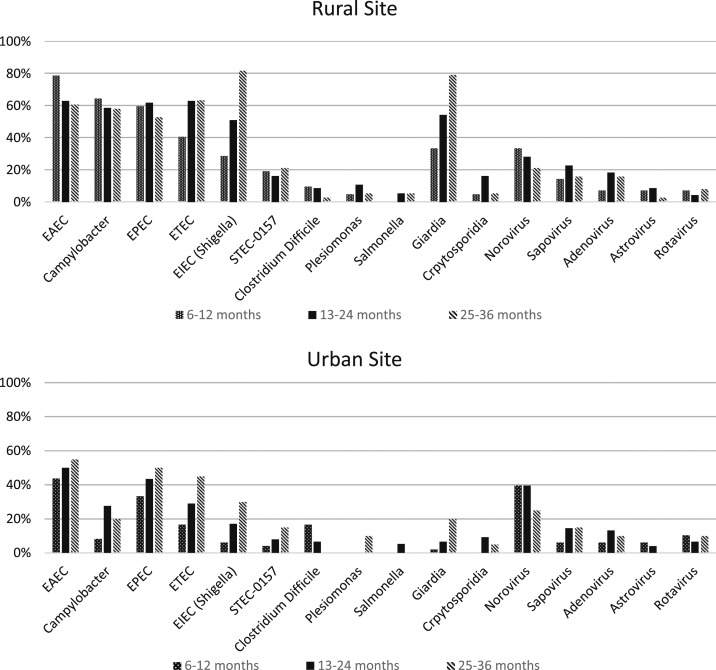
Stool results: enrollment (Figure 1).
Figure 1.
Spectrum of stool pathogens at baseline among Guatemalan children with acute non-bloody diarrhea, by age and rurality (% of children with each pathogen).
At least one pathogen was identified in stool at enrollment in 98% of subjects (Table 2). Pathotypes of Escherichia coli (enteroaggregative E. coli [EAEC], enteropathogenic E. coli [EPEC], and enterotoxigenic E. coli [ETEC]) were most common, occurring in 44.4–59.2% of subjects. The next most common organisms were Campylobacter and Shigella/enteroinvasive E. coli (EIEC). Among viral pathogens, norovirus was the most common (32.9%); other viruses, including rotavirus (7.1%), were much less frequently identified. Giardia (33.4%) was the most common parasitic pathogen, with fewer Cryptosporidia and no Entamoeba histolytica detected.19
Table 2.
Pathogen identification in diarrheal stool at enrollment among Guatemalan children, by rurality
| Individual pathogens detected at enrollment | Rural (N = 172) | Urban (N = 144)* | P-value |
|---|---|---|---|
| N (%) | N (%) | ||
| EAEC | 114 (66.3%) | 70 (50.4%) | 0.004 |
| EPEC | 102 (59.3%) | 59 (42.5%) | 0.003 |
| ETEC | 99 (57.6%) | 39 (28.1%) | < 0.0001 |
| Campylobacter | 103 (59.9%) | 29 (20.9%) | < 0.0001 |
| EIEC (Shigella) | 90 (52.3%) | 22 (15.8%) | < 0.0001 |
| Giardia | 94 (57.4%) | 10 (7.2%) | < 0.0001 |
| Norovirus | 48 (27.9%) | 54 (38.9%) | 0.04 |
| Sapovirus | 33 (19.2%) | 17 (12.2%) | 0.09 |
| STEC-0157 | 31 (18.0%) | 11 (7.9%) | 0.007 |
| Adenovirus | 26 (15.1%) | 15 (10.8%) | 0.26 |
| Cryptosporidia | 19 (11.1%) | 8 (5.8%) | 0.09 |
| Clostridium difficile | 13 (7.6%) | 13 (9.4%) | 0.57 |
| Rotavirus | 10 (5.8%) | 12 (8.6%) | 0.35 |
| Astrovirus | 12 (7.0%) | 6 (4.3%) | 0.31 |
| Plesiomonas | 14 (8.1%) | 2 (1.4%) | 0.004 |
| Salmonella | 7 (4.1%) | 4 (2.9%) | 0.57 |
| Cyclospora | 2 (1.2%) | 1 (0.7%) | 0.68 |
| Vibrio cholerae | 2 (1.2%) | 0 (0%) | 0.16 |
| Vibrio | 1 (0.6%) | 0 (0%) | 0.32 |
| Yersinia | 0 (0%) | 0 (0%) | N/A |
| Total pathogen count | 4.8 (1.8) | 2.7 (1.6) | < 0.0001 |
EAEC = enteroaggregative E. coli; EPEC = enteropathogenic E. coli; ETEC = enterotoxigenic E. coli; EIEC = Shigella/enteroinvasive E. coli; STEC = Shiga-like–toxin producing E. coli.
* Five children did not have pathogen information at enrollment.
There were notable differences in pathogen number and spectrum between the rural and urban sites. Subjects in the rural area had a mean number of pathogens of 4.8/subject, compared with 2.7/subject in the urban population (P < 0.0001). More than half of children in the rural area had Giardia in their stool, whereas it was found in less than 10% of urban subjects. Conversely, norovirus was the only pathogen that was significantly higher (38.9% versus. 27.9%, P = 0.04) in the urban population. Evolution of pathogen frequency with increasing age depended on the specific pathogen. For example, Giardia and EIEC had clear increases with each age interval in both rural and urban sites.
Clinical/pathogen association.
In multivariable analysis adjusted for site, few clear patterns were observed between clinical characteristics or symptoms at presentation and the presence of individual pathogens in the stool; fever within 72 hours before enrollment (which occurred in 42% of subjects) was associated with EIEC (P = 0.038) and inversely associated with ETEC (P = 0.02). No unique organism was associated with increased severity of dehydration. The most notable associations were observed between specific pathogens and malnutrition: acute malnutrition was associated with the presence of C-diff (P = 0.05), Cryptosporidium (P = 0.017), and norovirus (P = 0.03) and inversely associated with the presence of Shigella (P = 0.03). Chronic malnutrition was associated with the presence of Shigella (P = 0.002) and Giardia (P = 0.05).
Environmental/pathogen correlation.
There were strong differences between the presence of specific pathogens at enrollment by site (urban versus rural) (Table 2). In multivariable analysis adjusted for site, the only statistically significant association between household animals/foul/livestock and pathogens measured at presentation was lower rates of ETEC and Shiga-like toxin–producing E. coli (STEC) O157 in households containing dogs. There was no association between household size and presence of specific pathogens. Despite distinct wet and dry seasons in Guatemala, no clear temporal pattern in pathogen occurrence was visually discernible across the 11-month study period.
Comparison of initial and 30-day follow-up stool sample (Table 3).
Table 3.
Pathogen acquisition, persistence, and clearance among Guatemalan children
| Pathogen | Negative to negative (n) | Positive to negative (n) | Positive to positive (n) | Negative to positive (n) | Persistence (%) | Acquisition (%) | Prevalence |
|---|---|---|---|---|---|---|---|
| EAEC | 51 | 55 | 124 | 68 | 69.3% | 57.1% | 60.1% |
| EPEC | 72 | 63 | 92 | 71 | 59.4% | 49.7% | 52.0% |
| ETEC | 91 | 52 | 81 | 74 | 60.9% | 44.8% | 44.6% |
| Campylobacter | 140 | 56 | 72 | 30 | 56.3% | 17.6% | 43.0% |
| EIEC (Shigella) | 164 | 23 | 85 | 26 | 78.7% | 13.7% | 36.2% |
| Giardia | 167 | 21 | 82 | 28 | 79.6% | 14.4% | 34.6% |
| Norovirus | 162 | 60 | 38 | 38 | 38.8% | 19.0% | 32.9% |
| Sapovirus | 233 | 38 | 8 | 19 | 17.4% | 7.5% | 15.4% |
| STEC-0157 | 215 | 29 | 11 | 42 | 27.5% | 16.3% | 13.5% |
| Adenovirus | 243 | 31 | 6 | 18 | 16.2% | 6.9% | 12.4% |
| Cryptosporidia | 247 | 16 | 11 | 24 | 40.7% | 8.9% | 9.1% |
| Clostridium difficile | 249 | 13 | 13 | 23 | 50.0% | 8.5% | 8.7% |
| Rotavirus | 264 | 15 | 4 | 15 | 21.1.% | 5.4% | 6.4% |
| Astrovirus | 258 | 15 | 2 | 23 | 11.8% | 8.2% | 5.7% |
| Plesiomonas | 269 | 15 | 1 | 13 | 6.3% | 4.6% | 5.4% |
| Salmonella | 284 | 8 | 3 | 3 | 27.3% | 1.0% | 3.7% |
| Cyclospora | 295 | 2 | 1 | 0 | 33.3% | 0.0% | 1.0% |
| Vibrio cholerae | 295 | 1 | 1 | 1 | 50% | 0.3% | 0.7% |
| Vibrio | 295 | 1 | 0 | 2 | 0.0% | 0.7% | 0.3% |
| Yersinia | 296 | 0 | 0 | 2 | N/A | 0.7% | 0.0% |
EAEC = enteroaggregative E. coli; EPEC = enteropathogenic E. coli; ETEC = enterotoxigenic E. coli; EIEC = Shigella/enteroinvasive E. coli; STEC = Shiga-like–toxin producing E. coli.
No patient had clinically defined (> 3 liquid stools in 24 hours before collection) diarrhea at the 31-day time point; 31-day stools were characterized by study personnel as formed for 204 subjects, soft for 100 subjects and liquid for five subjects. Escherichia coli pathotypes (EIEC, EPEC, and ETEC), EIEC/Shigella and Campylobacter that were most common in the initial stool overall, also had high rates of persistence from day 1 to day 31 (all > 55%), whereas Shiga toxin–producing E. coli, including 0157:H7, was less likely to persist (27.5%). Giardia was highly likely to persist from day 1 to day 31 (79.6%). Viral pathogens were overall less likely to remain positive over the 30-day period, although norovirus persisted in 38.8% of stools.
Escherichia coli pathotypes were commonly acquired between day 1 and 31, and to a lesser extent norovirus (19.0%), Campylobacter (17.6%), and Giardia (14.4%). Day-1 prevalence of each pathogen was strongly correlated with new acquisition rates in other subjects (R2 = 0.818, P < 0.01).
Population attributable fractions.
Four pathogens were noted to have a statistically significant positive PAF: in the rural site, Campylobacter (PAF 25.9%, 95% CI: 1.8–40.0) and sapovirus (12.1%, 95% CI: 4.5–15.8); and in the urban site, norovirus (19.7%, 95% CI: 2.0–29.0) and rotavirus (7.6%, 95% CI: 1.1–8.5). See Supplemental Table for stratified PAFs for all pathogens with overall prevalence in diarrheal stools > 3%.
DISCUSSION
This study of 316 children with acute non-bloody diarrhea in rural and urban Guatemala underscores the complexity of enteric infection in LMICs. Stool samples from both symptomatic and asymptomatic episodes were characterized by multiple potential enteric pathogens, particularly in the rural area, where hygiene and sanitation conditions promote heavy and frequent exposure. Our results are, to our knowledge, the largest published study of diarrheal pathogens identified by multiplex PCR in Central America and indicate that the pathogen burden and spectrum in this region (particularly in the rural area) are consistent with those observed in Africa and Asia.12–14,20–22 In addition, although the overall pathogen burden was less in the urban population with improved water and sanitation infrastructure, the samples were nevertheless characterized by the presence of multiple pathogens and a spectrum of enteropathic bacteria similar to that observed in the rural subjects.
Significant PAFs in our study for viral pathogens and Campylobacter are consistent with the pathogens with the highest PAFs in the GEMS and MAL-ED studies, with some notable exceptions. In the MAL-ED populations, norovirus, rotavirus, and astrovirus represented three of the top four etiologies contributing to diarrheal disease, along with Campylobacter.13 In the initial analysis of GEMS subjects that did not use multiplex PCR, the pathogens with the highest contribution to acute diarrheal disease included rotavirus, ST-ETEC, Cryptosporidium, and Shigella, but subsequent reanalysis using multiplex PCR identified significantly higher incidence for most pathogens. In particular, adenovirus was five times more common in the reanalysis and became the third highest attributable pathogen, after Shigella spp. and rotavirus.12,14
However, most pathogens in both the diarrheal and asymptomatic stools in our population were not associated with diarrhea, consistent with the GEMS, MAL-ED studies, and others that use multiplex PCR.20 Rather, they may be more likely to contribute to pathology through the cycle of environmental enteropathy, in which recurrent or chronic infection leads to a pathologic inflammatory state in the gut, which prevents adequate growth and nutrition, which in turn may result in increased susceptibility to new and prolonged reinfection.4 Little is known about the role that specific individual or coinfecting pathogens may play in this cycle, and in our study, no clear pattern of correlation between specific pathogen and malnutrition or post-diarrheal weight gain were observed.
We also explored the converse hypothesis that malnutrition may itself promote susceptibility to persistence of particular pathogens over the 30-day period (particularly the more prevalent organisms Campylobacter, E. coli pathotypes, and Giardia) but again did not observe any clear association between measures of malnutrition or short term weight gain and persistence of any pathogen.23 If nutritional status was not a key driver of persistence of specific pathogens, our data hint at additional factors that may be responsible for repeat positive stool samples for some organisms. For instance, the strong correlation between rates of persistence and rates of new pathogen acquisition (representing transmission in the household/community) for the E. coli pathotypes EPEC, EAEC, and ETEC suggests that a high environmental/household exposure burden and reinfection may be driving repeat positive samples for these organisms. By contrast, lower new acquisition rates for Campylobacter and Giardia suggest that repeat positive testing may be related to true persistence of these organisms in the intestine, perhaps because of intrinsic characteristics of these organisms, gut inflammation, and associated immune responses.24,25 At present, these observations remain speculative; future studies using pathogen genomic data could more clearly discriminate persistence from re-acquistion.
One practical implication of observations that environmental enteropathy is not driven by a single pathogen or distinct diarrheal episodes should be a dampening of enthusiasm for the use of multiplex PCR diagnostics in present form in daily clinical practice in LMICs. Results with multiple pathogens from a single patient may have very little utility for practicing clinicians to make treatment decisions because it may be difficult or impossible to ascertain which pathogen is the cause of an acute illness.26 Identification of multiple pathogens in stool samples risks antibiotic over-utilization and increased cost, adverse events, and antimicrobial resistance for little long-term benefit.27 Future iterations of multiplex PCR incorporating quantitative determination of individual pathogens (such as those that have been useful in recent epidemiologic studies) may eventually provide more relevant results for medical decision-making, but this is not yet widely available in LMIC clinical settings.28–30
More generally, a transition from a focus on acute infections and individual organisms to chronic polymicrobial colonization, such as our observations suggest, has important implications for development of both therapeutic and public health approaches. Therapeutic interventions for individual children, including nonpathogen–targeted antibiotic administration (e.g., azithromycin, rifaximin), probiotics, prebiotics, and gut anti-inflammatory medications, have generally proven unsuccessful in clinical trials.31 At present, treatment options are generally limited to nutritional rehabilitation through support of breast-feeding, and supplementation with macro- and micronutrients.32 To contribute to a better understanding of pathologic mechanisms that may elucidate more effective therapeutic interventions, we plan future work with our study population to identify perturbations in the microbiome that correlate with clinical outcomes, intestinal inflammation, nutritional status, and both individual and combinations of pathogens.
The high burden and diverse spectrum of intestinal pathogens observed in our study also underscores the significant challenge of interrupting the cycle of environmental enteropathy from a public health perspective. Given the large number of pathogens that may be involved in driving chronic enteric inflammation and challenges determining the contribution of individual pathogens to the overall burden of disease, public health strategies which target individual pathogens may not be as impactful in the near term as those more generally targeted. Most prominent among such strategies is vaccination, and yet among common enteric pathogens, only rotavirus, typhoid, and cholera vaccines are currently licensed.33 Vaccines against ETEC, Shigella, norovirus, and Campylobacter are still in early or developmental stages, and benefits that may eventually result from a spectrum of enteric vaccines are likely many years away.34 Thus, programs such as WASH, designed to more broadly limit pathogen exposure would appear to be more compelling public health strategies in the near term. However, despite the clear logic of improving infrastructure and hygiene literacy for communities with high burdens of enteric infections, WASH interventions have not been universally effective.7 Notably, recent vigorous randomized clinical trials incorporating both WASH and nutritional interventions have failed to demonstrate clear sustainable benefits.9,11 Even in our study, the challenges of reducing the enteric infection through WASH improvements are exemplified by the reduced, yet persistent pathogen burden in the urban subjects. Furthermore, introducing and sustaining WASH interventions on a global scale will require a large and sustained investment, one inextricably linked with economic development in LMICs.
There are several notable limitations in this analysis, many of which relate to the constraints of the clinical trial. These include exclusion of dysentery and severe malnutrition (preventing analysis of pathogen patterns associated with these conditions), a relatively short follow-up time for analyzing longitudinal pathogen trends in single individuals and a relatively low sample size limiting analysis of uncommon pathogens. The original trial was a randomized, placebo-controlled intervention, and thus, approximately half of subjects received 3 days of a nutritional product that had a demonstrable impact on diarrheal duration among subjects with product-targeted organisms (rotavirus, enterotoxigenic E. coli, STEC, and Salmonella) in the enrollment stool.17 However, in the main analysis, there were no differences in demographic, environmental, or clinical characteristics, including weight gain between study intervention groups, initial stool samples were collected before product administration, and treatment group was included in all presented multivariable analyses.35 An additional limitation arises because of the study enrollment period of only 10½ months, which may have impacted our inability to identify all temporal trends in etiology. Although the use of multiplex PCR was a significant advantage in the study, the assay is still limited in granular discrimination between subtypes of pathogens, which may dilute the precision of associations with diarrhea. For example, the assay does not discriminate between heat-labile and heat-stable STEC or between norovirus genogroups that have differing associations with acute diarrhea.13,36 Finally, although additional treatments (zinc, antibiotics, and probiotics) were not controlled in the context of the clinical trial, these variables were included in the multivariable analyses.
In conclusion, stool samples collected both during acute diarrheal episode and asymptomatic follow-up are characterized by the presence of multiple enteric pathogens among Guatemalan children, particularly in the rural area. Few clear correlations between individual pathogens and environmental, clinical, or nutritional outcomes were observed. Both individual therapeutic and public health strategies to reduce the burden of enteric infection and environmental enteropathy among children in LMICs need to account for a complex and chronic polymicrobial milieu.
Supplemental table
Note: Supplemental table appears at www.ajtmh.org.
REFERENCES
- 1.Liu L, et al. Child Health Epidemiology Reference Group of WHO and UNICEF , 2012. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet 379: 2151–2161. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Diarrhoeal Disease Collaborators , 2018. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of diarrhoea in 195 countries: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis 18: 1211–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richard SA, et al. Childhood Malnutrition and Infection Network , 2014. Catch-up growth occurs after diarrhea in early childhood. J Nutr 144: 965–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McCormick BJJ, Lang DR, 2016. Diarrheal disease and enteric infections in LMIC communities: how big is the problem? Trop Dis Travel Med Vaccines 2: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhutta ZA, Nizami SQ, Isani Z, 1999. Zinc supplementation in malnourished children with persistent diarrhea in Pakistan. Pediatrics 103: e42. [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization , 2005. The Treatment of Diarrhoea: A Manual for Physicians and Other Senior Health Workers. Geneva, Switzerland: WHO. [Google Scholar]
- 7.Dangour AD, Watson L, Cumming O, Boisson S, Che Y, Velleman Y, Cavill S, Allen E, Uauy R, 2013. Interventions to improve water quality and supply, sanitation and hygiene practices, and their effects on the nutritional status of children. Cochrane Database Syst Rev 8: CD009382. [DOI] [PubMed] [Google Scholar]
- 8.Larsen DA, Grisham T, Slawsky E, Narine L, 2017. An individual-level meta-analysis assessing the impact of community-level sanitation access on child stunting, anemia, and diarrhea: evidence from DHS and MICS surveys. PLoS Negl Trop Dis 11: e0005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Null C, et al. 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health 6: e316–e329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luby SP, et al. 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health 6: e302–e315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart CP, et al. 2018. Effects of water quality, sanitation, handwashing, and nutritional interventions on child development in rural Kenya (WASH benefits Kenya): a cluster-randomised controlled trial. Lancet Child Adolesc Health 2: 269–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kotloff KL, et al. 2013. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 382: 209–222. [DOI] [PubMed] [Google Scholar]
- 13.Platts-Mills JA, et al. MAL-ED Network Investigators , 2015. Pathogen-specific burdens of community diarrhoea in developing countries: a multisite birth cohort study (MAL-ED). Lancet Glob Health 3: e564–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu J, et al. 2016. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet 388: 1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan American Health Organization , 2017. Health in the Americas: Children’s Health. Available at: https://www.paho.org/salud-en-las-americas-2017/?tag=infant-mortality. Accessed August 24, 2018. [Google Scholar]
- 16.World Health Organization , 2018. Child Growth Standards: WHO Anthro (Version 3.2.2, January 2011) and Macros. Available at: https://www.who.int/childgrowth/software/en/. Accessed June 15, 2018. [Google Scholar]
- 17.Gaensbauer JT, Melgar MA, Calvimontes DM, Lamb MM, Asturias EJ, Contreras-Roldan IL, Dominguez SR, Robinson CC, Berman S, 2017. Efficacy of a bovine colostrum and egg-based intervention in acute childhood diarrhoea in Guatemala: a randomised, double-blind, placebo-controlled trial. BMJ Glob Health 2: e000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bruzzi P, Green SB, Byar DP, Brinton LA, Schairer C, 1985. Estimating the population attributable risk for multiple risk factors using case-control data. Am J Epidemiol 122: 904–914. [DOI] [PubMed] [Google Scholar]
- 19.Kamidani S, Melgar M, Robinson C, Asturias E, Berman S, Gaensbauer J, 2018. No detection of Entamoeba histolytica by multiplex polymerase chain reaction in children with acute non-bloody diarrhea in Guatemala. Pediatr Infect Dis J 37: e107–e108. [DOI] [PubMed] [Google Scholar]
- 20.Pelkonen T, Dos Santos MD, Roine I, Dos Anjos E, Freitas C, Peltola H, Laakso S, Kirveskari J, 2018. Potential diarrheal pathogens common also in healthy children in Angola. Pediatr Infect Dis J 37: 424–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen H, et al. 2016. The 12 gastrointestinal pathogens spectrum of acute infectious diarrhea in a Sentinel Hospital, Shenzhen, China. Front Microbiol 7: 1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adam MA, Wang J, Enan KA, Shen H, Wang H, El Hussein AR, Musa AB, Khidir IM, Ma X, 2018. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front Microbiol 9: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, Guerrant RL, Lima AA, 2010. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology 139: 1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnee AE, Petri WA, Jr., 2017. Campylobacter jejuni and associated immune mechanisms: short-term effects and long-term implications for infants in low-income countries. Curr Opin Infect Dis 30: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bartelt LA, Sartor RB, 2015. Advances in understanding Giardia: determinants and mechanisms of chronic sequelae. F1000Prime Rep 7: 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platts-Mills JA, Operario DJ, Houpt ER, 2012. Molecular diagnosis of diarrhea: current status and future potential. Curr Infect Dis Rep 14: 41–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eibach D, Krumkamp R, Hahn A, Sarpong N, Adu-Sarkodie Y, Leva A, Käsmaier J, Panning M, May J, Tannich E, 2016. Application of a multiplex PCR assay for the detection of gastrointestinal pathogens in a rural African setting. BMC Infect Dis 16: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Boer RF, Ott A, Kesztyus B, Kooistra-Smid AM, 2010. Improved detection of five major gastrointestinal pathogens by use of a molecular screening approach. J Clin Microbiol 48: 4140–4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Platts-Mills JA, et al. 2018. Use of quantitative molecular diagnostic methods to assess the aetiology, burden, and clinical characteristics of diarrhoea in children in low-resource settings: a reanalysis of the MAL-ED cohort study. Lancet Glob Health 6: e1309–e1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, et al. 2014. Development and assessment of molecular diagnostic tests for 15 enteropathogens causing childhood diarrhoea: a multicenter study. Lancet Infect Dis 14: 716–724 [DOI] [PubMed] [Google Scholar]
- 31.Watanabe K, Petri WA, Jr., 2016. Environmental enteropathy: elusive but significant subclinical abnormalities in developing countries. EBioMedicine 10:25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization , 2013. Essential Nutrition Actions: Improving Maternal, Newborn, Infant and Young Child Health and Nutrition. Geneva, Switzerland: WHO. [PubMed] [Google Scholar]
- 33.Riddle MS, Chen WH, Kirkwood CD, MacLennan CA, 2018. Update on vaccines for enteric pathogens. Clin Microbiol Infect 24: 1039–1045. [DOI] [PubMed] [Google Scholar]
- 34.Holmgren J, Parashar UD, Plotkin S, Louis J, Ng SP, Desauziers E, Picot V, Saadatian-Elahi M, 2017. Correlates of protection for enteric vaccines. Vaccine 35: 3355–3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gaensbauer J, Melgar M, Lamb MM, Calvimontes DM, Asturias EJ, Contreras-Roldan I, Dominguez S, Robinson CC, Berman S, 2017 Efficacy of a novel nutritional product in acute childhood diarrhea in Guatemala: secondary and exploratory analyses of a randomized, double-blind, placebo-controlled trial. Open Forum Infect Dis 4: S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vega E, Barclay L, Gregoricus N, Shirley SH, Lee D, Vinjé J, 2014. Genotypic and epidemiologic trends of norovirus outbreaks in the United States, 2009 to 2013. J Clin Microbiol 52: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.